Abstract
Genetic factors that might influence susceptibility or resistance in naive individuals and early-stage pathology in schistosomiasis are difficult to study in clinical trials, since in areas where the disease is endemic the first contact with the parasite occurs most often at very early ages. Therefore, four strains (DR1.Aβ°, DR2.Aβ°, DQ8.Aβ°, and DQ6.Aβ°) of major histocompatibility complex class II-deficient mice (Aβ°), transgenic for different HLA alleles, have been used to evaluate the potential role of HLA class II polymorphism in the onset of the infection by Schistosoma mansoni. The survival rates and parasitological and immunological parameters after infection were evaluated and compared against the control values obtained with Aβ° mice. All four mouse strains used in this study were able to generate a specific immune response against S. mansoni antigens (cytokine production and antibody production). However, only mice expressing DR alleles survived until the chronic stage of the infection and were able to mount protective granulomatous response avoiding hepatic damage, presenting predominant gamma interferon production. In contrast, strains expressing DQ alleles revealed an impairment in generating effective granulomas, resulting in earlier death, which was associated with an impaired hepatic granulomatous response and liquefactic necrosis, reflecting the influence of HLA polymorphism in the establishment of protective response in the early stage of infection.
Several associations between various pathologies and specific HLA antigens have been reported (36). However, the role of HLA polymorphism in infectious diseases has not yet been fully explored. Presently, the only valuable association, to our knowledge, between an infectious disease and HLA class II molecules is that of tuberculoid leprosy with HLA-DRA/B1∗0301 (25).
In schistosomiasis, it is difficult to estimate the development of acquired resistance to reinfection, which is age and sex related (31) but also depends on daily exposure to the parasite. Recently, a genomic region involved in resistance has been described (21, 22). This locus is positioned on chromosome 5q31-q33, a region encoding several candidate genes involved in the regulation of the immune response to pathogens, namely, colony-stimulating factor-1 receptor, interleukin-3 (IL-3), IL-4, IL-5, and IL-13 (5, 33).
Most of the epidemiological studies focused on the factors involved in progression of fibrosis and development of severe hepatic disease. Lethal disease is a consequence of portal hypertension, which progressively leads to hematemesis and heart failure. In its early stage, fibrosis is part of the healing process that follows the acute inflammatory reaction around parasite eggs trapped in presinusoidal venules. Chronical hepatosplenomegaly is a consequence of extended fibrosis in the hepatoportal spaces. Severe hepatoportal disease was noted in certain families, while others living in the same environmental and hygienic conditions were less affected (10). The group of Salam et al. (32) was the first to describe a linkage between progression toward hepatosplenomegaly and HLA class I antigens. A study of an Egyptian population (15) showed a negative association of DR2 with severe disease. Secor et al. (34), in a study on Brazilian patients, could not confirm this finding, but showed that HLA-DQB1∗0201 was associated with an increased risk in developing severe hepatosplenic forms of disease. Unfortunately, the HLA-DQB1 frequencies in healthy populations were not evaluated.
In areas where the parasite is endemic, the first contact with the parasite occurs most often at very early ages, and little is known about genetic factors involved in the induction of protective response. In the initial stages of infection in mice, granulomas have a protective role against the diffusion of toxins released by the parasite eggs. This was shown in SCID, nude, or T-cell-depleted mice, which are unable to develop granulomas and die as a consequence of hepatocellular necrosis (2, 11, 28). Egg-induced granulomas have been characterized as a CD4+ T-cell-mediated delayed-type hypersensitivity response (23, 37). In mice, the early stage of Schistosoma mansoni infection is dominated by a type 1 response, which is then progressively replaced, from the onset off egg laying, by a type 2 response that becomes maximal at 8 weeks of infection (27).
Whereas the influence exerted by CD4+ T cells has been well studied, little is known about the impact of the HLA polymorphism on the early stage of the immune response induced against S. mansoni. In order to tackle this problem, we took advantage of the facts that in mice this parasite can fully develop and that this experimental model is currently used to explore cellular mechanisms involved in the granulomatous reaction. Therefore, HLA transgenic mice expressing different HLA alleles (36) were chosen. These mice were generated or backcrossed on an Aβ° background (i.e., deficient for murine major histocompatibility complex [MHC] class II molecules) (8). As a consequence of the absence of endogenous murine MHC class II molecules, the class II response is restricted to the HLA transgene, allowing the contribution of each of the HLA alleles to be assessed independently. This is of special interest since several HLA-DR and -DQ molecules are cross-linked, enhancing the difficulty in evaluating the specific role of each. It has to be stressed that the pool of CD4+ T cells is reconstituted in HLA.Aβ° transgenic mice due to thymic selection restricted to the transgene (36). This model was shown to be valuable in the study of several autoimmune pathologies such as collagen-induced arthritis (4, 14), experimental encephalitis (1, 16), autoimmune thyroiditis (17), and in “Der p” (extract of Dermatophagoides pteronyssinus)-induced allergy (24). In our study, we used four strains of mice expressing HLA class II molecules: two DR (DR1 and DR2) and two DQ (DQ6 and DQ8) strains. Survival rates, liver histology, and parasitological and immunological parameters were evaluated after S. mansoni infection, in comparison to nontransgenic Aβ° mice. We discuss the diversity of responses obtained in these different strains as a consequence of HLA polymorphism.
MATERIALS AND METHODS
Mice.
Mice used in this study (Table 1) were bred and maintained in pathogen-free conditions in the animal unit of the Pasteur Institute of Lille (France). Mice expressing different HLA alleles (HLA-DR2,-DQ6, and -DQ8) and deficient in murine class II molecules (HLA.Aβ°) were a kind gift from C. David (Mayo Clinic, Rochester, Minn.) (36). Mice expressing HLA-DR1 transgene on an FVB/N background were kindly provided by D. Altmann (1). To eliminate murine endogenous MHC class II molecules, DR1 mice were backcrossed with Aβ° mice for two generations. Thus, in the case of DR1.Aβ° mice the influence of the mixed background on parasitic and immune parameters cannot be excluded. Prior to experimentations, CD4+ T-cell levels in DR1.Aβ° were verified: 33.4% of cells present in lymph nodes expressed CD4+ (compared to 41.6% in DR1/FVB mice), while 16.1% of splenic cells were CD4+ (compared to 27.55% in DR1/FVB mice). Aβ° mice were provided by D. Mathis and C. Benoist (LGME/CNRS, Strasbourg, France) (8), and immunologically intact C57BL/6 mice were purchased from Iffa Credo (l'Arbesle, France). Five- to seven-week-old mice were used in our experiments.
TABLE 1.
HLA-transgenic mice used in this study, with references characterizing the strains indicated
| Strain | Transgenes expressed | Reference |
|---|---|---|
| DR1 | DRA/DRB1∗0101 | 1 |
| DR2.Aβ° | I-Ekα/DRB1∗1502 | 14 |
| DQ6.Aβ° | DQA1∗0103/DQB1∗0601 | 4 |
| DQ8.Aβ° | DQA1∗0301/DQB1∗0302 | 7 |
Parasite life cycle.
A Puerto Rican strain of S. mansoni was maintained in Biomphalaria glabrata snails as intermediate hosts and in Mesocricetus auratus golden hamsters as definitive hosts. Cercariae for experimental infections were used within 1 h of collection and enumeration, from 1-month-infected snails exposed to light and to a temperature of 30°C for 1 h.
Infection Protocol.
Mice were infected percutaneously by exposing the abdominal skin to 50 cercariae of S. mansoni as previously described (35).
Sera were collected by retro-orbital bleeding 24 h before infection (day 0) and 14, 28, and 42 days after infection and stored at −80°C for immunoglobulin and cytokine quantifications. At 42 days after infection, the parasite burden was evaluated by total perfusion. Following perfusion, the liver was recovered and the parasite eggs retained in the tissue were counted, after an alkaline digestion with a 4% KOH solution for 24 h at 37°C. Results are expressed as the mean number of eggs/worm pair/gram of liver ± the standard deviation.
Prior to KOH digestion, one hepatic lobe was removed, fixed in Bouin liquor (saturated solution of picric acid-formaldehyde-acetic acid, 15:2:1 [vol/vol/vol]) and used for histological analysis.
Parasite antigen preparations.
Schistosomulum antigen (SOM) was prepared by sonication of frozen-thawed mechanically prepared schistosomula (30) and centrifuged for 20 min at 10,000 × g. Soluble worm antigenic product (SWAP) was prepared by homogenizing adult worms by a 5-min sonication (Labsonic U, B. Braun, Templemars, France) and centrifuged for 20 min at 10,000 × g. Schistosome egg antigen (SEA) was prepared from homogenized eggs isolated from livers of 40-day S. mansoni-infected hamsters. Frozen eggs were disrupted by eight passages through an X-press (A. B. Biot, Jarfalla, Sweden), and the soluble fraction was collected after centrifugation for 15 min at 10,000 × g. The antigenic preparations were filtered through a 0.22-μm (pore-size) membrane (Millipore, Bedford, Mass.) and stored at −20°C until use. Protein concentrations were determined using the BCA Protein Assay Reagent (Pierce, Rockford, Ill.).
Histological study.
Liver sections (6 μm thick), prepared from paraffin-embedded samples, were stained using Masson Trichrome stain (Sigma Chemical Co, St. Louis, Mo.) and then examined using a Leitz Diaplan microscope (Wild Leitz, Rueil-Malmaison, France).
To measure collagen deposition, the colorimetric method described by Lopez de-Leon et al. (18) was employed. Liver sections 10 μm thick were placed on slides, deparaffinized, and incubated with a saturated solution of picric acid in distilled water, containing 0.1% fast green FCF (Sigma) which stains noncollagenous proteins, and 0.1% sirius red F3B (Gurr BDH Chemicals, Ltd., Poole, United Kingdom) staining specifically collagen. Sections were kept in the dark and incubated at room temperature for 2 h under agitation. They were then rinsed with distilled water, and the retained stain was eluted from the section using 1 ml of 0.1 N NaOH in absolute methanol (1:1 [vol/vol]). Fluids were carefully withdrawn, and the absorbance was measured at 540 nm (maximal absorbance of sirius red) and 620 nm (maximal absorbance of fast green) by using a multichannel spectrophotometer (Titertek Multiskan MCC/340; Labsystem, Helsinki, Finland). For each individual, three consecutive sections of the liver were used. The amount of collagen deposition in hepatic tissue was calculated using the formula described previously (18). Data were expressed as micrograms of collagen/milligrams of noncollagenous proteins.
In vitro stimulation assay.
Spleens from infected mice or healthy controls were removed aseptically 42 days after infection. Cells were cultured in 24-well plates (Nune, Intermed S.A., Roskilde, Denmark) in 1 ml per well of cell culture medium (ML-10) consisting of RPMI 1640 (Gibco, Courbevoie, France) supplemented with 50 μM β-mercaptoethanol (Merck, Darmstadt, Germany), 2 mM l-glutamine (Merck), 1 mM sodium pyruvate (Gibco), 10% heat-inactivated fetal calf serum (Gibco), and 50 μg of gentamicin (Gibco) per ml at 37°C in a 5% CO2 atmosphere. Cells (2 × 106 cells/ml) were specifically stimulated with 50 μg of SEA per ml. Mitogenic stimulation with concanavalin A (5 μg/ml) was used as positive control. At 48 h after antigenic stimulation, cell culture supernatants were harvested and tested for cytokine release.
Cytokine quantification.
Using the enzyme-linked immunosorbent assay (ELISA) method, gamma interferon (IFN-γ) and IL-4 production were evaluated in sera or in culture supernatants of in vitro-stimulated splenic cells. Briefly, Maxisorp plates (Nunc) were coated overnight at 4°C with 0.05 μg of purified rat anti-mouse monoclonal antibody (MAb) R4-6A2 (for IFN-γ) or 11B11 (for IL-4) (PharMingen, San Diego, Calif.) per well, respectively, diluted in 0.1 M sodium carbonate-bicarbonate buffer (pH 8.6). After a washing with phosphate-buffered saline (PBS; 0.15 M NaCl, 10 mM PBS; pH 7.2)–0.1% Tween 20 (T-PBS), a blocking step was performed using 100 μl of a 3% bovine serum albumin (BSA; Sigma) solution in PBS for 2 h at room temperature (RT). After additional washings, sera or supernatants were added and incubated overnight at 4°C. Sera were tested at 1:10 to 1:20 dilutions, while cell culture supernatants were used in serial dilutions of from 1:1 to 1:8 dilution in PBS–3% BSA. After the washing, 100 μl of 1-μg/ml dilution of the biotinylated rat anti-mouse MAb XMG1-2 (IFN-γ) or BVD6-24G2 (IL-4) (PharMingen) per well, respectively, were added for 1 h at RT. After a renewed washing, peroxidase-labeled streptavidin conjugate (PharMingen) was added at a 1:10,000 dilution in PBS for 30 min at RT. After three washes with T-PBS, plates were incubated with 100 μl of o-phenylenediamine dihydrochloride substrate (Sigma) at 1 mg/ml diluted in sodium phosphate-citrate buffer (0.1 mM, pH 5.5) containing 0.03% H2O2 for 10 min at RT. The reaction was stopped by the addition of 50 μl of HCl at 1 N per well. The absorbance at 492 nm was measured using a multichannel spectrophotometer (Labsystem). The results are expressed as the mean absorbance values of duplicate wells after subtraction of the background. Samples were tested individually, and the results are expressed as the mean ± the standard deviation.
Specific antibody quantification.
Detection of specific antibodies in sera was performed by ELISA on Maxisorp plates, coated overnight at 4°C with 50 μl of a 15-μg/ml solution of the parasite antigens SOM, SWAP, or SEA diluted in sodium carbonate buffer per well. After a washing with T-PBS and a blocking step, 100 μl of mouse serum in PBS–3% BSA buffer was incubated overnight at 4°C. After additional washes, 100 μl of peroxidase-labeled anti-mouse IgG(H+L) (Sanofi Diagnostics Pasteur, Marnes-la-Coquette, France) diluted 1:10,000 in the same buffer was added for 1 h at RT, followed by washing and o-phenylenediamine substrate addition under the same conditions mentioned above for the cytokine quantification.
Statistics.
Statistical significance was determined by using Student's t test, with P values of <0.05.
RESULTS
Mortality and liver pathology in HLA.Aβ° infected mice.
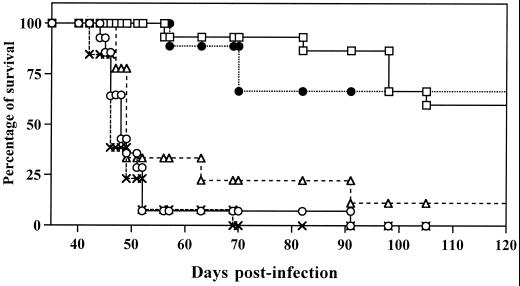
As shown in Fig. 1, DR1.Aβ° and DR2.Aβ° mice survived more than 90 days after infection with 50 cercariae of S. mansoni, whereas the survival of the DQ6.Aβ° and of the Aβ° control mice was limited to ca. 50 days postinfection (p.i.). Mortality in DQ8.Aβ° mice at 50 days p.i. was slightly lower than in Aβ° mice but significantly higher than in DR1.Aβ° and DR2.Aβ° mice.
FIG. 1.
Survival of HLA.Aβ° transgenic mice compared to Aβ° mice, exposed percutaneously to 50 cercariae of S. mansoni. Cumulative results of two independent experiments are represented. The number of mice used for each strain were: 14 for Aβ° (○), 15 for DR1.Aβ° (□), 9 for DR2.Aβ° (●), 13 for DQ6.Aβ° (×) and 9 for DQ8.Aβ° (▵).
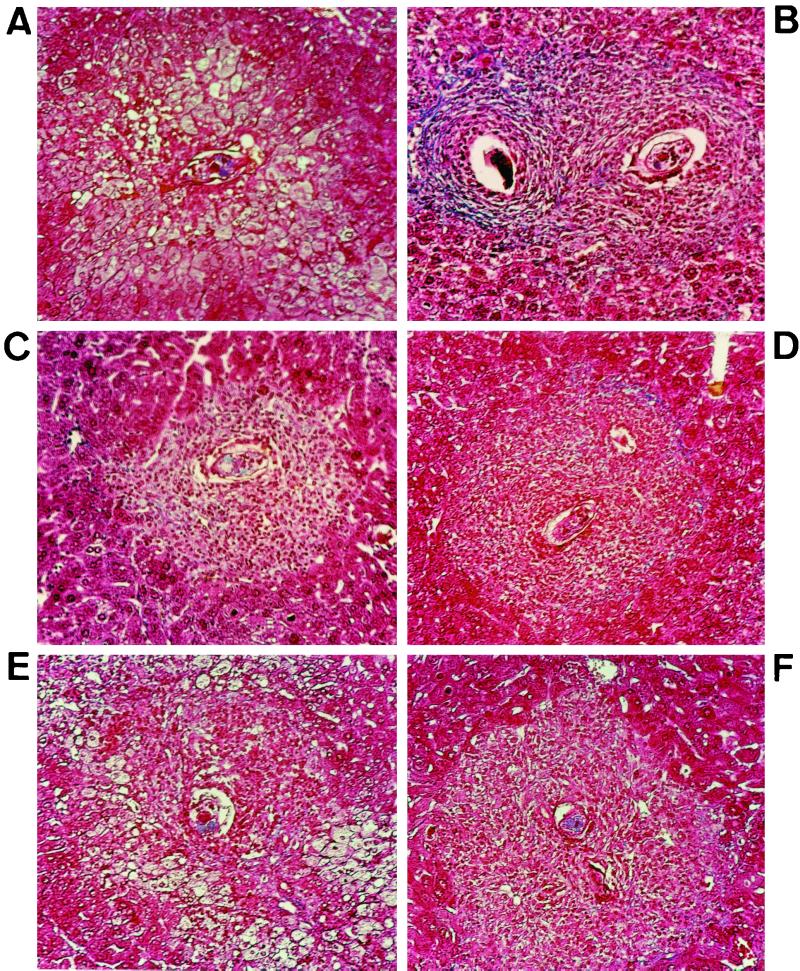
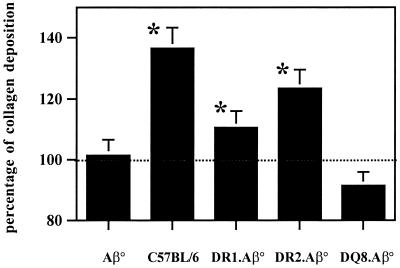
Since the survival of S. mansoni-infected mice is related to their ability to generate granulomas around the parasite eggs, histological analysis of the hepatic tissue was performed at 42 days p.i. Figure 2B shows the histological appearance of classical granulomas in immunocompetent infected C57BL/6 mice with the same background as Aβ° mice. As already described (3), Masson's trichrome staining showed lack of granulomatous reaction in Aβ° mice (Fig. 2A), with few cellular aggregates around certain eggs without collagen deposition. The absence of a granulomatous reaction was associated with a profound hepatic damage in the vicinity of the parasite eggs, indicating a hepatotoxic effect. DR1.Aβ° and DR2.Aβ° mice were able to mount efficient granulomatous reactions (Fig. 2C and D). DQ6.Aβ° mice showed the same histological pattern as Aβ° mice (Fig. 2E), whereas DQ8.Aβ° mice presented a cellular reaction devoid of any tissular damage (Fig. 2F). Quantification of collagen content in the liver at 42 days p.i. showed that both DR1.Aβ° and DR2.Aβ° mice displayed collagen deposition unlike DQ8.Aβ° mice (Fig. 3), thus being concordant with the histological aspect. However, in both DR-expressing mice, collagen deposition is somewhat lower than in immunologically intact C57BL/6 mice. Unfortunately, due to the severe hepatic damage, this study was not feasible in DQ6.Aβ° mice.
FIG. 2.
Histological analysis of hepatic tissues at 42 days p.i. stained with Masson trichrome stain (nuclei appear purple, cytoplasma is red, collagen is blue). (A) Control Aβ° mice. (B) Immunocompetent C57BL/6 mice. (C) DR1.Aβ° mice. (D) DR2.Aβ° mice. (E) DQ6.Aβ° mice. (F) DQ8.Aβ° mice. In panel A, hepatocytolysis is obvious, compared to the classical aspect of the granulomatous reaction in the immunocompetent control mice in panel B. DR1.Aβ° and DR2.Aβ° mice present typical granulomas, while DQ6.Aβ° mice show results similar to those of Aβ° mice. DQ8.Aβ° mice show a small but effective cellular reaction. Magnification, ×200.
FIG. 3.
Collagen deposition at 42 p.i., expressed as a percentage of collagen increase in infected mice compared to healthy mice of the same age. Eight to ten mice/group were tested individually. The dotted line indicates collagen in uninfected individuals of the same age. The results are expressed as the mean ± the standard deviation and are representative of two experiments. ∗, Statistical analysis using the Student's t test gave P = 0.0056, P = 0.029, and P = 0.044, indicating a significant difference when comparing C57BL/6, DR1.Aβ°, and DR2.Aβ° mice, respectively, with Aβ° mice. No significant difference was observed between DQ8.Aβ° and Aβ° mice (P = 0.724).
Thus, a graduation in the intensity of pathology could be established, from the DR1.Aβ° and DR2.Aβ° strains that mounted protective granulomatous responses, to the DQ6.Aβ° and Aβ° mice that died rapidly, while DQ8.Aβ° presented intermediate survival and granulomatous reactions.
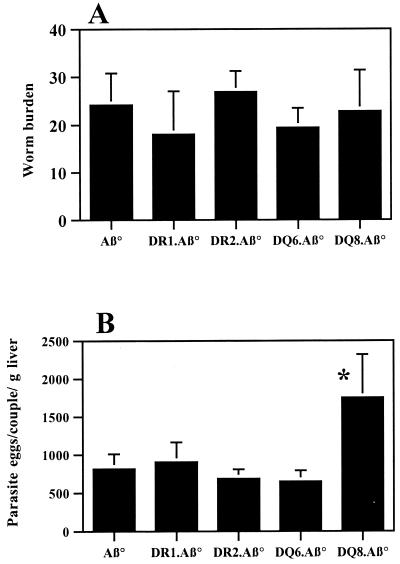
Parasite burden in HLA.Aβ° infected mice.
At 42 days p.i., the adult worm burden was assessed by blood perfusion (Fig. 4A), and the numbers of parasite eggs/worm pair were counted in the liver (Fig. 4B). No significant difference in parasite burden among the different transgenic and Aβ° mice strains could be seen. Similarly, no significant differences were observed in the numbers of parasite eggs trapped in the liver, except between the DQ8.Aβ° strain and the control mice (P value of Student's t test = 0.0239).
FIG. 4.
Parasite burden (A) and egg count (B) in the liver (expressed as eggs/worm pair/gram of liver) at 42 days p.i. Five to six mice/group were individually tested. The results are expressed as mean ± the standard deviation. ∗, Statistical analysis using the Student's t test gave P = 0.0239, indicating a significant difference between DQ8.Aβ° and Aβ° mice. No significant differences were observed in the adult worm burden or the numbers of parasite eggs trapped in the liver for the other transgenic strains.
Cellular response to parasite antigens.
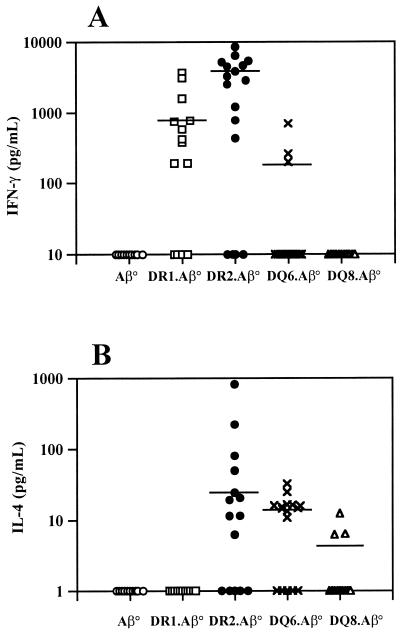
Levels of IFN-γ and IL-4 were determined in sera 42 days p.i. As shown in Fig. 5, transgenic mice were able to produce IFN-γ, IL-4, or both cytokines, unlike the Aβ° mice. DR2.Aβ° and DQ6.Aβ° mice produced both cytokines. However, only 18% of DQ6.Aβ° produced IFN-γ, while 72% DR2.Aβ° mice produced this cytokine. In addition, the concentrations of IFN-γ in DQ6.Aβ° were significantly lower than in DR2.Aβ° mice sera (P = 0.026). The proportion of mice producing IL-4 in DQ6.Aβ° mice was 65% compared to 56% of DR2.Aβ°, but the amounts of IL-4 detected in DQ6.Aβ° mice were significantly lower than those found in DR2.Aβ° mouse sera (P < 0.01). Fifty-eight percent of the DR1.Aβ° mice secreted IFN-γ in amounts comparable to the DR2.Aβ° mice but were unable to secrete detectable levels of IL-4. In contrast, DQ8.Aβ° mice, unable to systemically produce IFN-γ, secreted low but detectable levels of IL-4, with 23% of the sera tested being positive for IL-4.
FIG. 5.
Cytokine production showing IFN-γ (A) and IL-4 (B) levels in the sera of infected transgenic and control Aβ° mice at 42 days p.i. Mice were individually tested. Cumulative results of three independent experiments are represented. The numbers of mice used for each strain were as follows: 11 for Aβ° mice (○), 19 for DR1.Aβ° mice (□), 18 for DR2.Aβ° mice (●), 17 for DQ6.Aβ° mice (×), and 12 for DQ8.Aβ° mice (▵). The choices of 10 pg/ml (IFN-γ) and 1 pg/ml (IL-4) is an arbitrary representation of sera detected below the cutoff of our ELISA test, calculated as the concentration at (mean ± 3 standard deviations) of the background. Statistical analysis using the Student's t test gave concentrations of IFN-γ in DQ6.Aβ° mice significantly lower than those in DR2.Aβ° mice sera (P = 0.026). Also, the amounts of IL-4 detected in DQ6.Aβ° mice were significantly lower than in DR2.Aβ° mice sera (P < 0.01).
We also tested the in vitro response to SEA of splenic cells of the infected transgenic mice. Cells from Aβ° mice were not able to respond (3), whereas transgenic mice cells produced both cytokines when stimulated with SEA (Table 2). In all transgenic strains, the production of IFN-γ was predominant, the IL-4 levels being relatively low. DR2.Aβ° mice secreted the highest amounts of both cytokines, DR1.Aβ° and DQ6.Aβ° mice produced significant, but intermediate quantities, while DQ8.Aβ° mice produced the lowest level. It can be thus concluded that all of the transgenes tested were capable of presenting antigenic fragments to CD4+ murine T cells.
TABLE 2.
IFN-γ and IL-4 production in supernatants after in vitro stimulation of splenocytes at 42 days p.i. with SEA
| Strain | Cytokine production (pg/ml)a
|
|
|---|---|---|
| IFN-γ | IL-4 | |
| Aβ° | <39 | <0.15 |
| DR1.Aβ° | 1,265 | 4.53 |
| DR2.Aβ° | 7,500 | 19.6 |
| DQ6.Aβ° | 634 | 5.08 |
| DQ8.Aβ° | 78 | 0.48 |
Results are expressed as the means of the concentrations obtained and are representative of two independent experiments. Lightface text represents values below the background (cut offs were at 39 pg/ml for IFN-γ and at 0.15 pg/ml for IL-4 and were calculated as the mean concentration [+ 3 standard deviations] of the background). Boldface indicates significant production.
Humoral response to parasite antigens.
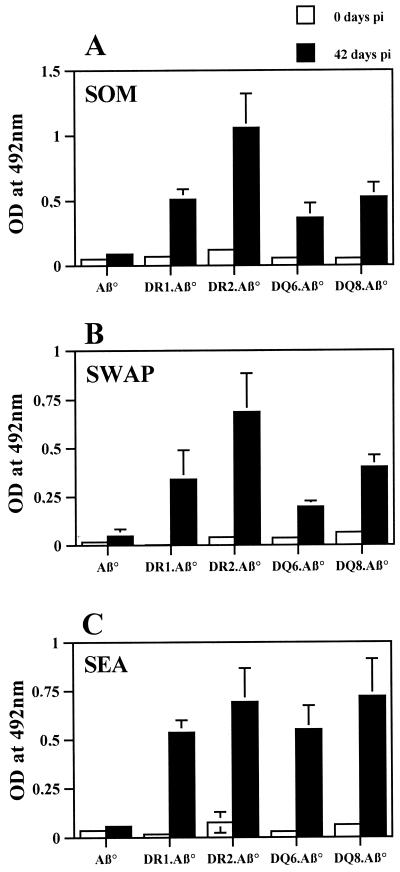
Aβ° mice are unable to generate antibodies against thymodependent antigens (8, 20). In order to verify the effectiveness of T-B cellular cooperation in HLA transgenic strains, specific IgG antibodies to SOM, SWAP, and SEA antigens were analyzed. As shown in Fig. 6, all of the transgenic strains were able to produce antibodies against the three antigens tested, similar to the findings with immunocompetent mice (data not shown). Nevertheless, some variations in the amounts of specific antibodies to SOM (A) and SWAP (B) were observed among the strains, with DR2.Aβ° mice producing the highest and DQ6.Aβ° mice producing the lowest concentrations.
FIG. 6.
Specific IgG(H+L) antibodies production in sera (dilution, 1:100) of infected transgenic and control Aβ° mice to parasitic antigens: SOM (A), SWAP (B), and SEA (C). Seven to eight mice/group were individually tested. The results are expressed as the mean ± the standard deviation and are representative of two independent experiments.
DISCUSSION
Among the parameters we studied, the first finding was the striking difference in mortality, according to the expressed transgene. Indeed, only DR-expressing mice (DR1.Aβ° and DR2.Aβ°) survived until the chronic stage. Additionally, preliminary results in DR3.Aβ° mice show similar trends (data not shown). In contrast, DQ6.Aβ° mice have the same mortality profile as the Aβ° mice. Thus, it seems that the expression of the sole DQ6 allele is unable to confer protection to the initial stage of infection. DQ8.Aβ° transgenic mice survived longer than the Aβ° mice, but survival was significantly shorter than with the DR-expressing mice. To further determine the origin of this variability, parasitic parameters were evaluated at 42 days p.i. corresponding to the onset of parasite egg laying. No difference could be detected in the parasite burdens among the strains, suggesting that parasites may develop equally well in all of them. In general, worm fecundity in transgenic mice was similar to that in Aβ° mice, with the sole exception of the DQ8.Aβ° mice, which presented a significantly higher egg count than the other HLA.Aβ° strains.
As previously reported (3), mortality in Aβ° mice is due to hepatotoxic lysis induced by the parasite eggs in the absence of granulomatous reaction. DR1.Aβ° and DR2.Aβ° mice are able to mount typical granulomas comparable to the responses of immunocompetent mice. These reactions seem to be effective, since the surrounding hepatic tissue appears intact. On the contrary, DQ6.Aβ° mice present a lack of granulomatous response accompanied by diffuse hepatocytolysis, like that observed in Aβ° mice. The DQ8.Aβ° strain shows a limited cellular reaction around parasite eggs that is nevertheless sufficient to protect the hepatic tissue against egg toxins. Collagen deposition measured by the method of Lopez-de Leon et al. (18) was in accord with the histological aspects, showing a lack of collagen deposition in DQ8.Aβ° mice, unlike the DR-expressing mice. Thus, we shown that the variability in mortalities of the various transgenic strains is strongly linked to the intensity of the granulomatous reaction. In the case of DQ8.Aβ° mice, with hepatic tissue appearing unaffected at 42 days p.i., mortality could be related to a higher egg count. Nevertheless, the incomplete granulomatous reaction around deposited eggs might also contribute to the fatal outcome.
Since the early granulomatous reaction is mainly CD4+ dependent and thus MHC class II restricted, we wanted to investigate whether the revealed differences in pathology are related to the capacity to mount specific class II-restricted responses. While Aβ° mice are unable to produce cytokines against parasitic antigens, the cellular response is reconstituted in all tested HLA-Aβ° strains. Moreover, the in vitro proliferation of splenic cells restimulated with SEA was similar in the four strains (data not shown). Some variations in the amount of the produced cytokines are evident and could explain the differences in the intensity of the granulomatous reaction. DR2.Aβ° mice were the most potent in producing cytokines systemically and after in vitro restimulation. Interestingly, DR1.Aβ° mice produced only IFN-γ (and no IL-4) in sera, but they secreted this cytokine when splenic cells were restimulated in vitro. This suggests that during infection IL-4 is probably produced locally. DQ6.Aβ° mice produced both cytokines, though at lower levels than DR-expressing mice. In general, DQ8.Aβ° mice produced very low amounts of either cytokine, with values being close to the cutoff value. IL-10 detection yielded negative results with all of the transgenic strains mice (data not shown).
However, a certain influence of a mixed background in DR1.Aβ° mice on parasitic and immune parameters cannot be excluded, in our study. However, compared to DR2.Aβ° mice (on an H-2b background), the response appears qualitatively close, suggesting that in our model the influence of the genetic background on the outcome of the pathology is of low importance. In this sense, it should be noted that DQ6.Aβ° and DQ8.Aβ° mice with the same background as the DR2.Aβ° mice, reveal striking differences during infection, which are more important than those between the two DR-allele-expressing mice (DR1 and DR2). Therefore, in this study we concluded that the differences we observed are mainly due to the transgene expressed. Backcrossing DR1.Aβ° mice until the genetic background becomes similar to the other transgenic mice would certainly strengthen our results but would probably not change the information obtained.
The observed quantitative and qualitative variations might also be a consequence of differences in the expression of the transgenes or due to thymic selection of a preferential T-cell-receptor repertoire. Thus, in the DQ6.Aβ° strain, the interspecific interaction between the DQ6 transgene and the corresponding murine CD4 receptor is effective but not efficient in the protection against the parasite S. mansoni. This finding notwithstanding, the DQ6.Aβ° strain was shown to be functional in other experimental models, such as rheumatoid arthritis (4), recognition of human pre-pro-insulin peptides (29), and allergy (6).
The profile of cytokine secretion by transgenic mice is also a parameter to consider. Previous studies in mice showed a protective role for IFN-γ in reducing fibrosis at the chronic stage (9, 19, 26). A recent epidemiological study in Sudan revealed an association between resistance to severe hepatosplenic disease and a genomic region located on 6q22 to 6q23, coding for IFN-γ receptor 1 (10). In our study, we found a predominant IFN-γ production in all strains, but the most effective were DR-allele-expressing mice, which also mounted an efficient granulomatous response. In general, IL-4 production is low: DR2.Aβ° mice secrete the highest levels among the transgenic strains, representing about 50% of the levels secreted by immunocompetent C57BL/6 mice.
Strikingly, DR1.Aβ° mice were unable to produce detectable amounts of IL-4 in the sera but generated granulomas. In contrast, DQ6.Aβ° mice, producing low levels of both cytokines in sera and with an IL-4 level in supernatants comparable to that of DR1.Aβ° mice, showed an impaired cellular reaction. It is thus tempting to conclude that in the initial stage, IFN-γ is necessary for an efficient granulomatous response, whereas IL-4 seems to be less important. Recently, Fallon et al. (12), using IL-13-deficient mice, showed that IL-13 has a profibrotic effect during the chronic stage of infection. It would therefore be interesting to evaluate in our transgenic model the production of this cytokine. However, it is difficult to establish any direct correlation between the intensity of cellular reaction and the levels of produced cytokines. For example, DQ8.Aβ° mice produced fewer cytokines than DQ6.Aβ° mice but survived slightly longer. The mechanisms involved in generating a granulomatous response are complex and cannot be reduced exclusively to the class II-restricted response. Other fibrogenic factors could also be implicated, such as tumor necrosis factor alpha (2). However, in general it seems that high levels of IFN-γ are linked to efficient granulomas, while low amounts indicate an inability to induce granulomas.
All transgenic strains are, unlike Aβ° mice, able to generate specific antibodies against all of the three parasite stages (3). The variation in quantity of antibodies among the transgenic strains against SOM and SWAP might be a consequence of the diversity in the repertoire of CD4+ T cells selected by each transgene. This may influence the B-T cellular cooperation, which is necessary to induce antibodies against thymodependent antigens.
Thus, the four strains used in this study were able to generate specific immune responses against the parasite S. mansoni. However, only DR-expressing mice were able to mount a protective response. Between the two DR alleles studied, the DR2 seems to be more effective in our system. It should be noted that DR2.Aβ° mice express a murine alpha chain I-Eαk, which might influence the efficiency of the interaction between MHC class II molecules and the murine T-cell receptor. An epidemiological study implicating the DR2 allele (15) showed that this allele might be linked to resistance to severe forms of schistosomiasis, but this finding was not confirmed by others (34). It is difficult to compare our results to those of epidemiological studies since the mechanisms implicated in the initial stage of granuloma induction and those effective in the chronic stages are different. Nevertheless, it is interesting to mention that Geluk et al. (13), using DR3 transgenic mice, identified the same immunodominant determinants to hsp65 (heat shock protein) of Mycobacterium tuberculosis as those recognized by DR3 human T cells. Unfortunately, we could not acquire mice expressing HLA-DQB1∗0201, an allele which was associated with an increased risk in developing severe hepatosplenic forms of schistosomiasis (34).
It is known that DRB1 are the main MHC class II molecules expressed on cells (representing about 40 to 50%), while DQ molecules are represented in lower amounts (about 15 to 20% of the pool). Accordingly, it is tempting to conclude that the induction of the immune response observed in schistosomiasis (i.e., a granulomatous reaction) is mainly due to DR responses, while DQ molecules are less effective. However, the relative effect of HLA-DR versus HLA-DQ interactions should further be investigated. Based on the finding that DQ6 is transmitted cross-linked with DR2, it would be interesting to study the consequences of S. mansoni infection in double transgenic mice in order to establish whether DQ6 molecules are ineffective in presenting schistosomal antigens.
In conclusion, all of the four strains used in this study: DR1.Aβ°, DR2.Aβ°, DQ8.Aβ°, and DQ6.Aβ° mice were able to generate specific immune response against S. mansoni antigens, although with some quantitative and qualitative differences. Mice expressing the DR alleles (DR1 and DR2) showed effective granulomas, surviving until the chronic stage of infection and mounting cellular reaction with predominant IFN-γ production. In contrast, mouse strains expressing DQ alleles showed an impairment in generating granulomas, while mounting a lower specific immune response against the parasite than had the DR mice. Thus, we conclude that DR alleles might play an important role in inducing the granulomatous response, whereas DQ alleles seem to be less effective, reflecting the influence of HLA polymorphism in the establishment of protective response. However, the panel of alleles studied should be enlarged, and the relative importance of each one of these alleles should be further investigated.
ACKNOWLEDGMENTS
This study was financially supported by CNRS, the Pasteur Institute of Lille, and the Région Nord-Pas de Calais (Ph.D. studentship to G.A.).
We are indebted to Chella S. David and Daniel Altmann for providing transgenic mice. We are also grateful to Julie Hanson and Michelle Smart for technical advice.
REFERENCES
- 1.Altmann D, Douek D, Frater A, Hetherington C, Inoko H, Elliott J. The T cell response of HLA-DR transgenic mice to human basic protein and other antigens in the presence and absence of human CD4. J Exp Med. 1995;181:867–875. doi: 10.1084/jem.181.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amiri P, Locksley R, Parslow T, Sadick M, Rector E, Ritter D, McKerrow J. Tumor necrosis factor α restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature. 1992;356:604–607. doi: 10.1038/356604a0. [DOI] [PubMed] [Google Scholar]
- 3.Angyalosi G, Pancré V, Herno J, Auriault C. Immunological response of major histocompatibility complex class II-deficient (Aβ°) mice infected by the parasite Schistosoma mansoni. Scand J Immunol. 1998;48:159–169. doi: 10.1046/j.1365-3083.1998.00372.x. [DOI] [PubMed] [Google Scholar]
- 4.Bradley D, Nabozny G, Cheng S, Zhou P, Griffiths M, Luthra H, David C S. HLA-DQB1 polymorphism determines incidence, onset and severity of collagen-induced arthritis in transgenic mice. Implications in human rheumatoid arthritis. J Clin Investig. 1997;100:2227–2234. doi: 10.1172/JCI119760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandrasekharappa S, Rebelsky R M, Firak T A, Le Beau M M, Westbrook C A. A long-range map of the interleukin-4 and interleukin-5 linkage group on chromosome. Genomics. 1990;6:94–99. doi: 10.1016/0888-7543(90)90452-z. [DOI] [PubMed] [Google Scholar]
- 6.Chapoval S P, Nabozny G, Marietta E L, Raymond E L, Krco C J, Andrews A G, David C S. Short ragweed allergen induces eosinophilic lung disease in HLA-DQ transgenic mice. J Clin Investig. 1999;103:1707–1717. doi: 10.1172/JCI6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng S, Baisch J, Krco C, Savarirayan S, Hanson J, Hodgson K, Smart M, David C. Expression and function of HLA-DQ8 (DQA1∗0301/DQB1∗0302) genes in transgenic mice. Eur J Immunogenet. 1996;23:15–20. doi: 10.1111/j.1744-313x.1996.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 8.Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Mice lacking MHC class II molecules. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 9.Czaja M, Weiner F, Takahashi S, Giambrone M, van der Meide P, Schellekens H, Biempica L, Zern M. γ-IFN treatment inhibits collagen deposition in murine schistosomiasis. Hepatology. 1989;10:795–800. doi: 10.1002/hep.1840100508. [DOI] [PubMed] [Google Scholar]
- 10.Dessein A, Hillaire D, Elwali N E, Marquet S, Mohammed-Ali Q, Mirghani A, Henri S, Abdelhameed A A, Saed O K, Magzoub M M, Abel L. Severe hepatic fibrosis in Schistosoma mansoni infection is controlled by a major locus that is closely linked to the interferon-gamma receptor gene. Am J Hum Genet. 1999;65:709–721. doi: 10.1086/302526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doenhoff M J, Pearson S, Dunne D W, Bickle Q, Lucas S, Bain J, Musallam R, Hassounah O. Immunological control of hepatotoxicity and parasite egg excretion in S. mansoni infections: stage specificity of the reactivity of immune serum in T-cell-deprived mice. Trans R Soc Trop Med Hyg. 1981;75:41–53. doi: 10.1016/0035-9203(81)90012-2. [DOI] [PubMed] [Google Scholar]
- 12.Fallon P G, Richarsdon E J, McKenzie G J, McKenzie A N J. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol. 2000;164:2585–2591. doi: 10.4049/jimmunol.164.5.2585. [DOI] [PubMed] [Google Scholar]
- 13.Geluk A, Taneja V, van Meijgaarden K E, Zanelli E, Abou-Zeid C, Thole J E R, de Vries R R P, David C S, Ottenhoff T. Identification of HLA class II-restricted determinants of Mycobacterium tuberculosis-derived proteins by using HLA-transgenic, class II-deficient mice. Proc Natl Acad Sci USA. 1998;95:10797–10802. doi: 10.1073/pnas.95.18.10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzales-Gay M A, Zanelli E, Khare S D, Krco C J, Zhou P, Inoko H, Griffiths M M, Luthra H S, David C S. Human leukocyte antigen DRB1∗1502 (DR2DW12) transgene reduces incidence and severity of arthritis in mice. Hum Immunol. 1996;50:54–60. doi: 10.1016/0198-8859(96)00123-1. [DOI] [PubMed] [Google Scholar]
- 15.Hafez M, Aboul Hassan S, el-Tahan H, el-Shennawy F, Khashaba M, al-Tonbary Y, el-Morsi Z, el-Sallab S, el-Desoky I, el-Shazly A. Immunogenetic susceptibility for post-schistosomal hepatic fibrosis. Am J Trop Med Hyg. 1991;44:424–433. doi: 10.4269/ajtmh.1991.44.424. [DOI] [PubMed] [Google Scholar]
- 16.Kawamura K, Yamamura T, Yokoyama K, Chui D-H, Fukui Y, Sasazuki T, Inoko H, David C S, Tabira T. HLA-DR2-restricted responses to proteolipid protein 95-116 peptide cause autoimmune encephalitis in transgenic mice. J Clin Investig. 2000;105:997–984. doi: 10.1172/JCI8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong Y C M, Lomo L, Motte R, Giraldo A A, Baisch J, Strauss G, Hammerling G J, David C S. HLA-DRB1 polymorphism determines susceptibility to autoimmune thyroiditis in transgenic mice: definitive association with HLA-DRB1∗0301 (DR3) gene. J Exp Med. 1996;184:1167–1172. doi: 10.1084/jem.184.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez-de Leon A, Rojkind M. A simple micromethod for collagen and total protein determination in formalin-fixed paraffin-embedded sections. J Histochem Cytochem. 1985;33:737–743. doi: 10.1177/33.8.2410480. [DOI] [PubMed] [Google Scholar]
- 19.Lortat-Jacob H, Baltzer F, Desmoulière A, Peyrol S, Grimaud J-A. Lobular—but not periovular–inhibition of collagen deposition in the liver of S. mansoni-infected mice using interferon-gamma. J Hepatol. 1997;26:894–903. doi: 10.1016/s0168-8278(97)80258-9. [DOI] [PubMed] [Google Scholar]
- 20.Markowitz J S, Rogers P R, Grusby M J, Parker D C, Glimcher L H. B lymphocyte development and activation independent of MHC class II expression. J Immunol. 1993;150:1223–1233. [PubMed] [Google Scholar]
- 21.Marquet S, Abel L, Hillaire D, Dessein H, Dessein A. Full results of the genome-wide scan which localises a locus controlling the intensity of infection by Schistosoma mansoni on chromosome 5q31–q33. Eur J Hum Genet. 1999;7:88–97. doi: 10.1038/sj.ejhg.5200243. [DOI] [PubMed] [Google Scholar]
- 22.Marquet S, Abel L, Hillaire D, Dessein H, Kalil J, Feingold J, Weissenbach J, Dessein A. Genetic localization of a locus controlling the intensity of infection by Schistosoma mansoni on chromosome 5q31–q33. Nat Genet. 1996;14:181–184. doi: 10.1038/ng1096-181. [DOI] [PubMed] [Google Scholar]
- 23.Mathew R, Boros D. Anti-L3T4 antibody treatment suppresses hepatic granuloma formation and abrogates antigen-induced interleukin 2 production in Schistosoma mansoni infection. Infect Immun. 1986;54:820–826. doi: 10.1128/iai.54.3.820-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neeno T, Krco C, Harders J, Baisch J, Cheng S, David C. HLA-DQ8 transgenic mice lacking endogenous class II molecules respond to house dust allergens. J Immunol. 1996;156:3191–3195. [PubMed] [Google Scholar]
- 25.Ottenhoff T H M, Haanen J B A G, Geluk A, Mutis T, Kale A B, Thole J E R, van Shooten W C A, van den Elsen P J, de Vries R R P. Regulation of mycobacterial heat-shock protein reactive T cells by HLA class II molecules: lessons from leprosy. Immunol Rev. 1991;121:171–191. doi: 10.1111/j.1600-065x.1991.tb00828.x. [DOI] [PubMed] [Google Scholar]
- 26.Pancré V, Wolowczuk I, Guerret S, Copin M, Delanoye A, Capron A, Auriault C. Protective effect of Sm28GST-specific T cells in schistosomiasis: role of gamma interferon. Infect Immun. 1994;62:3723–3730. doi: 10.1128/iai.62.9.3723-3730.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearce E, James S, Dalton J, Barrall A, Ramos C, Strand M, Sher A. Down regulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips S, DiConza J, Gold J, Reid W. Schistosomiasis in the congenitally athymic (nude) mouse. I. Thymic dependency of eosinophilia, granuloma formation, and host morbidity. J Immunol. 1977;118:594–599. [PubMed] [Google Scholar]
- 29.Raju R, Munn S, David C S. T cell recognition of human pre-proinsulin peptides depends on the polymorphism at HLA DQ locus: a study using HLA DQ8 and DQ6 transgenic mice. Hum Immunol. 1997;1997:21–29. doi: 10.1016/s0198-8859(97)00212-7. [DOI] [PubMed] [Google Scholar]
- 30.Ramalho-Pinto G, Gazzinelli G, Howells R E, Mota-Santos T A, Figueiredo E A, Pellegrino J. Schistosoma mansoni: defined system for stepwise transformation of cercariae to schistosomula in vitro. J Exp Parasitol. 1974;36:360. doi: 10.1016/0014-4894(74)90076-9. [DOI] [PubMed] [Google Scholar]
- 31.Remoue F, Rogerie F, Gallisot M-C, Guyatt H L, Neyrinck J-L, Diakkhate M-M, Niang M, Butterworth A E, Auriault C, Capron A, Riveau G. Sex-dependent neutralizing humoral response to Schistosoma mansoni 28GST antigen in infected human populations. J Infect Dis. 2000;181:1855–1859. doi: 10.1086/315454. [DOI] [PubMed] [Google Scholar]
- 32.Salam E A, Ishaac S, Mahmoud A A F. Histocompatibility-linked susceptibility for hepatosplenomegaly in human schistosomiasis mansoni. J Immunol. 1979;123:1829–1831. [PubMed] [Google Scholar]
- 33.Saltman D L, Dolganov G, Warrington J A, Wasmuth J J, Lovett M A. A physical map of 15 loci on human chromosome 5q23–q33 by two-color fluorescence in situ hybridization. Genomics. 1990;16:726–732. doi: 10.1006/geno.1993.1254. [DOI] [PubMed] [Google Scholar]
- 34.Secor E, del Corral H, dos Reis M, Ramos E A, Zimon A E, Peixoto Matos E, Reis E A G, do Carmo T M A, Hirayama K, David R A, David J R, Harn D A. Association of hepatosplenic schistosomiasis with HLA-DQB1∗0201. J Infect Dis. 1996;174:1131–1135. doi: 10.1093/infdis/174.5.1131. [DOI] [PubMed] [Google Scholar]
- 35.Smithers S R, Terry R J. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology. 1969;55:695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- 36.Taneja V, David C S. HLA class II transgenic mice as models of human diseases. Immunol Rev. 1999;169:67–79. doi: 10.1111/j.1600-065x.1999.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 37.Warren K S, Domingo E O, Cowan R B T. Granuloma formation around schistosome eggs as a manifestation of delayed hypersensitivity. Am J Pathol. 1967;51:735–756. [PMC free article] [PubMed] [Google Scholar]