Abstract
Obesity is a major threat to health, but the etiology of obesity is incompletely understood. Phthalates, synthetic chemicals ubiquitous in the environment, are suspected to have obesogenic effects, but the relationship of phthalates and obesity in humans remains uncertain. We examined whether phthalate exposure was associated with body fat gain in midlife women. We analyzed data from 1369 women in the Study of Women’s Health Across the Nation Multi-Pollutant Study. Eleven phthalate metabolites measured in spot urine samples at baseline (1999/2000) were standardized with covariate-adjusted creatinine. Body weight (BW), fat mass (FM) from dual-energy X-ray absorptiometry (DXA), and body fat percentage (BF%) from DXA were measured near-annually until 2016/2017. For each metabolite, linear mixed effects models with time and log2(metabolite) interactions were examined, adjusting for demographic, lifestyle, and menopause-related factors. Analyses were conducted overall and stratified by baseline obesity status. As sensitivity analyses, all analyses were repeated using a second set of metabolites measured in 2002/2003. Higher levels of all metabolites except mono-carboxy-isononyl phthalate were associated with faster increases in BF%. Per doubling of metabolite concentrations, differences in five-year BF% change ranged from 0.03 percentage point (ppt) (95% confidence interval (CI): −0.03, 0.09) for mono-isobutyl phthalate to 0.09 ppt (95% CI: 0.02, 0.16) for mono(3-carboxypropyl) phthalate. Results were similar for FM change, but associations with BW change were mostly null. In stratified analyses by baseline obesity status, positive associations were strongest in women who were normal/underweight at baseline. When metabolites from 2002/2003 were used as exposures, most associations were attenuated and not statistically significant, but they remained positive for normal/underweight women. In conclusion, phthalate metabolites were associated with more rapid body fat gain in midlife women, but our results need confirmation given attenuation of estimates in the sensitivity analyses.
Keywords: Phthalates, body composition, body fat percentage, environmental obesogen, obesity
Introduction
Obesity affects nearly 1 in 2 women in the United States (Hales, 2020) and is a major threat to health because it increases the risk of leading causes of death and disability (Ryan, 2007). Preventing obesity requires a thorough understanding of its etiology, but the current understanding is incomplete (Schwartz et al., 2017). Some environmental chemicals are hypothesized to have obesogenic effects given their potential to interfere with nutrient and energy metabolism pathways in experimental models (Grün and Blumberg, 2006; Heindel and Blumberg, 2019). Investigating the relationship between chemical exposure and measures of obesity is critical to understanding the pathophysiology of obesity to appropriately identify targets for prevention.
Phthalates, diesters of 1, 2-benzenedicarboxylic acid, are among the chemicals suspected to promote body fat gain and contribute to obesity (Gore et al., 2015). Since the 1930s, phthalates have been added to numerous industrial and consumer products (Warner and Flaws, 2018). Low-molecular-weight (LMW) phthalates are often added to personal care products as solvents and are frequently found in fragrance, shampoo, and cosmetics (Zota et al., 2014). High-molecular-weight (HMW) phthalates are often added to polyvinyl chloride plastics (PVC) as plasticizers and are found in many PVC applications, including flooring, cables, wires, clothing, food processing equipment, food packaging, and some medical devices (National Research Council, 2008). Human exposure to phthalates occurs mainly through ingesting food contaminated during handling, processing, and storage (Smith et al., 2021), dermal absorption by use of personal care products (Koniecki et al., 2011), and ingestion or inhalation of contaminated indoor dust (Andersen et al., 2018; Yang et al., 2020). Exposure to phthalates is widespread; the metabolites of many were detected in over 90% of urine samples in biomonitoring studies in the US and elsewhere in the past 30 years (Bai et al., 2015; Jung et al., 2022; Saravanabhavan et al., 2013; Wittassek et al., 2007; Zota et al., 2014).
Mechanistic support for the hypothesis of obesogenic effects of phthalates comes from observations that some phthalate metabolites, such as mono-ethyl phthalate (MEP), mono(2-ethylhexyl) phthalate (MEHP), and monobenzyl phthalate (MBzP), activate peroxisome proliferator-activated receptor gamma (PPAR-γ), a nuclear receptor critical to the differentiation and survival of adipocytes, promoting adipogenesis in vitro (Bility et al., 2004; Feige et al., 2007; Hurst and Waxman, 2003). Furthermore, mice fed di(2-ethylhexyl) phthalate (DEHP) for 5–10 weeks gained more body weight than controls (Schmidt et al., 2012; Lv et al., 2016; Majeed et al., 2017). To date, however, epidemiologic studies have yet to confirm whether phthalate exposure predicts excess body fat gain in humans. A recent systematic review on the metabolic effects of phthalates concludes that the current body of epidemiologic evidence is inadequate to determine whether phthalates are linked to obesity, mainly because most studies have been cross-sectional (Radke et al., 2019). Only seven studies have examined the associations between phthalates and longitudinal changes in adiposity in adults (Díaz Santana et al., 2019; Haggerty et al., 2021; Perng et al., 2020; Philips et al., 2020; Rodríguez-Carmona et al., 2019; Song et al., 2014; van der Meer et al., 2021). In these studies, some phthalate metabolites, such as MEP, DEHP metabolites, and MBzP, have been associated with faster body weight gain, but not consistently. Further, insights from these studies are limited because most have used body weight or body mass index (BMI) as the primary outcomes, rather than specific measures of body fat. These proxies for body fat may not be sensitive and specific enough to detect associations between phthalate metabolites and body fat, especially in older individuals whose loss of muscle mass may mask gains in body fat (Kuk et al., 2009).
In this study, we investigated whether urinary phthalate metabolites predicted faster increases in body weight (BW), fat mass (FM), and body fat percentage (BF%) in a group of midlife women followed for up to 18 years. Because previous studies suggest that obesity status may modify the associations between phthalates and changes in body weight (Philips et al., 2020; Song et al., 2014), we additionally conducted stratified analyses by baseline obesity status. We aimed to expand the evidence base on phthalates’ potential obesogenic effects, particularly for midlife women, as the menopause – marked by profound changes in endocrine pathways relevant for energy balance (Greendale et al., 2019) – may be a sensitive window for environmental obesogens (Heindel et al., 2015).
Methods
Study population
Participants were drawn from the Study of Women’s Health Across the Nation (SWAN), an ongoing cohort study of mid-life women’s health. Details about SWAN are available on the study’s website (https://www.swanstudy.org/about/about-swan/). Briefly, since 1996/1997, women from seven study sites (Oakland, CA, Los Angeles, CA, Chicago, IL, Detroit-area, MI, Pittsburgh, PA, Boston, MA and Newark, NJ) have been followed near-annually through interviews and clinical examinations. Eligibility criteria at cohort inception included 1) self-identifying as White, Black, Chinese, Japanese, or Hispanic, 2) aged between 42 and 52 years, 3) having an intact uterus, at least one ovary, and at least one menstrual period in the past 3 months, and 4) not having used any exogenous reproductive hormone in the past 3 months. In total, 3302 women met these eligibility criteria and enrolled in SWAN. The study protocols of SWAN were approved by institutional review boards at each study site, and all participants provided informed consent to participate in the study at each study visit.
The SWAN Multi-pollutant Study (SWAN-MPS) is an ancillary study that selected SWAN participants for environmental chemical exposure assessments using banked biospecimens from the 1999/2000 and 2002/2003 study visits. Of the 2694 women who participated in the SWAN 1999/2000 study visit, the SWAN-MPS excluded all 646 women from the Chicago and Newark sites because neither site collected urine samples necessary for environmental chemical measurements. An additional 648 women were excluded because they had insufficient blood or urine samples for environmental chemical measurements. The SWAN-MPS thus included 1400 women; of those, all had phthalate metabolite measurements from 1999/2000 samples and 1,387 also had phthalate metabolite measurements from 2002/2003 samples.
We used phthalate metabolite data from 1999/2000 for our primary analyses because this allowed us to fully utilize outcomes data collected since 1999/2000. Of the 1400 SWAN-MPS women, we excluded 15 women with missing data on urinary creatinine or its predictors (age, race/ethnicity, BMI, height, and diabetes). We further excluded 16 women missing key covariates (education, calorie intake, menopausal status, hormone therapy (HT) use, physical activity, and smoking). The analytic sample thus included 1369 women. All of these women had at least one adiposity measure. Participants were followed for a maximum of 18 years including a maximum of 13 study visits. The median follow-up time was 16 years (Interquartile range (IQR): 13, 17), and the median number of observations per woman was 11 for body weight (interquartile range (IQR): 9, 12) and 10 for fat mass and body fat percentage (IQR: 8, 12).
Phthalate metabolites
Women provided spot urine samples during in-person visits in 1999/2000 and 2002/2003. Urine was collected in polyethylene tubes and transferred to −80 °C freezers for long-term storage. In 2017/2018, urine samples were thawed, and phthalate metabolites were measured using on-line solid phase extraction (SPE) coupled to high-performance liquid chromatography-isotope dilution tandem mass spectroscopy (HPLC-MS). Twelve phthalate metabolites were measured, which can be grouped into three categories based on their parents’ similarity in structure and sources (Wolff et al., 2008): 1) LMW phthalate metabolites: mono-ethyl phthalate (MEP), mono-n-butyl phthalate (MnBP), and mono-isobutyl phthalate (MiBP); 2) DEHP metabolites: mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), and mono(2-ethyl-5-carboxypentyl) phthalate (MECPP); and 3) Other HMW phthalate metabolites: monobenzyl phthalate (MBzP), mono-isononyl phthalate (MiNP), mono-carboxyoctyl phthalate (MCOP), mono-carboxy-isononyl phthalate (MCNP), and mono(3-carboxypropyl) phthalate (MCPP). The lower limits of detection of each phthalate metabolite are available in Table S1 in Appendix A. Within each batch of assays, duplicate quality control samples of three concentrations were also analyzed. The coefficients of variation (CV, in %) of these quality control samples ranged from 3.3% (MCPP) to 20.5% (MCOP) at high concentrations; 4.3% (MECPP) to 21.4% (MCOP) at medium concentrations; and 4.1% (MEHP) to 23.6% (MCOP) at low concentrations. We excluded mono-isononyl phthalate (MiNP) from all analyses because it was detected in less than 1% of urine samples.
Body weight, fat mass, and body fat percentage
Body weight and body composition were measured near-annually between 1999/2000 and 2016/2017 at the Michigan, Boston, and Los Angeles sites. For the Oakland site, body weight was measured until 2015/2016, and body composition was measured until 2012/2013. For the Pittsburgh site, body weight and body composition were measured until 2015/2016. We used all available data in our analyses.
Body weight was measured in light clothing and without shoes using a calibrated scale and recorded to the nearest 0.1 kg. Body composition measures were acquired using a Hologic dual-energy X-ray absorptiometry (DXA) instruments (Hologic Inc.). Different models of DXA were used throughout follow-up and across sites; calibration studies were conducted any time there was a change in DXA machinery. For this analysis, all body composition measures were calibrated to the Hologic QDR-4500 model under “NHANES” tissue-type calibration. All body composition measures excluded the head. Details of DXA instruments used, DXA measurement protocols, and calibration methods can be found in Greendale et al. (Greendale et al., 2019). Body fat percentage was calculated as the ratio of fat mass and the sum of fat mass and the mass of lean soft tissues (i.e., body fat percentage = fat mass/ [fat mass + (total lean mass – bone mineral content)]. In the denominator, bone mineral content was subtracted from total lean mass because accurate bone mineral content measurements could not be obtained for all women due to the presence of metals in the body or the wearing of jade jewelry.
Other variables
Creatinine, used to account for hydration status, was measured in urine from the 1999/2000 and 2002/2003 visits with a Cobas Mira analyzer (Horiba ABX, Montpellier, France). Time was calculated as date of visit minus date of sample collection for phthalate assay. Age was calculated as date of visit minus date of birth. Race/ethnicity (White, Black, Chinese, Japanese) and educational attainment (high school or less, some college, college degree, postgraduate studies) were self-reported in 1996/1997. Height (cm) was measured with a stadiometer at each visit. BMI was calculated as body weight (kg)/height (m2). Obesity was defined using race-specific BMI cut-points (Joslin Diabetes Center, 2016). For White and Black women, normal/underweight was defined as BMI < 25 kg/m2; overweight, 25 kg/m2 ≤ BMI < 30 kg/m2; and obese, BMI ≥ 30 kg/m2. For Chinese and Japanese women, normal/underweight was defined as BMI < 23 kg/m2; overweight, 23 kg/m2 ≤ BMI < 27 kg/m2; and obese, BMI ≥ 27 kg/m2. Dietary energy intake (kcal/day) was estimated with a modified Block Food Frequency Questionnaire (FFQ) in 1996/1997 and 2001/2002 (Block et al., 1986). Dietary energy intake in 1996/1997 was used to approximate dietary energy intake in 1999/2000. Physical activity across three domains, including leisure-time sports, active living, and household activities, was quantified by an index derived from the Kaiser Physical Activity Survey (Sternfeld et al., 1999). Physical activity was assessed six times over the 18 years of follow-up. For visits where physical activity data were not available (38% of observations), we set the physical activity index to its most recent value. Smoking status (never, former, or current) and current use of hormone therapy (HT) (yes, no) was self-reported at each visit. Menopausal status at each visit was determined based on self-reported bleeding frequency, history of oophorectomy and hysterectomy, and use of HT. Diabetes status at each visit was defined as self-reported anti-diabetic medication use, self-reported physician’s diagnosis of diabetes, or having a fasting glucose value at or above 126 mg/dL. Physician’s diagnosis of cancer was self-reported at each visit.
Statistical methods
To facilitate log2-transformation, we replaced 7 negative observations of MiBP, 2 negative observations of MEHP, and 1 negative observation of MCPP with each metabolite’s median concentration below its limit of detection. All other metabolite concentrations were used as output by the assay, including those that were below limits of detection. All urinary phthalate metabolite concentrations were adjusted for hydration using the covariate-adjusted creatinine standardization method (O’Brien et al., 2016). Each phthalate metabolite concentration was divided by the ratio of observed to predicted urinary creatinine. Predictors of creatinine were identified from the literature (Barr et al., 2005; Buckley et al., 2019) and included age, race/ethnicity, BMI, height, and diabetes. We also calculated the molar sums of hydration-adjusted LMW phthalate metabolites (“∑LMW phthalates”), DEHP metabolites (“∑DEHP”), and all HMW metabolites (“∑HMW phthalates”, which includes all DEHP metabolites, MBzP, MCOP, MCNP, and MCPP) to evaluate the impact of aggregate exposure.
Descriptive statistics (median (1st quartile, 3rd quartile) for continuous variables and count (proportion) for categorical variables) of the analytic sample in 1999/2000 were calculated. To understand the distributions and potential correlates of phthalates, median (1st quartile, 3rd quartile) values of phthalate metabolites were calculated overall, by baseline obesity status, and by covariates. Phthalate metabolite concentrations by baseline obesity status and covariate levels were compared using the Kruskal-Wallis test. To understand the correlation between metabolites, Spearman correlation coefficients were calculated between metabolites at baseline. To understand the within-person correlation of metabolites, we calculated the intraclass correlation coefficient (ICC) of each metabolite using phthalate metabolite data from 1999/2000 and 2002/2003. The ICCs were estimated using linear mixed effects models that predicted each log2-transformed metabolite with random intercepts and no fixed effects.
The trajectories of BW, FM, and BF% overall and by baseline obesity status were modeled with linear mixed effects models. Each model included time, age at baseline, race/ethnicity, site, educational attainment at baseline, baseline dietary energy intake, and time-varying menopausal status, HT use, smoking status, and physical activity as predictors. Time was modeled with a linear spline with a knot at time (T) = 6 years for BW and as a linear term for FM and BF%. These functional forms were selected based on smoothing plots from generalized additive mixed models (GAMM) (Figs S1 and S2 in Appendix A). Specifically, the smoothing plot of BW showed that among all women, BW increased and peaked somewhere between T = 5 and T = 12 years before decreasing (Fig S1 in Appendix A). A linear spline for time was thus appropriate. The knot at T = 6 was selected because this knot produced the smallest Akaike Information Criterion (AIC) among a series of models with knots at each year between T = 5 to 12 years. For FM and BF%, the smoothed trajectory within each study site was near-linear, suggesting a linear term for time was adequate, although there were substantial differences in FM and BF% trajectories by site (Fig S2 in Appendix A). All models included random intercepts and random slopes for time to account for within-woman correlation of multiple observations.
To test whether phthalate exposure was associated with differences in the rates of change of each outcome, for each metabolite, we added to each outcome’s trajectory model the main effect term of the metabolite and the interaction term between the metabolite and time. Phthalate metabolites were log2-transformed due to right-skewness. Models for the outcome of BW also included a time by race/ethnicity interaction, and models for FM and BF% also included a time by site interaction. We included these interaction terms because race/ethnicity- and site-specific smoothing plots from GAMMs showed that BW trajectories differed by race/ethnicity, while FM and BF% trajectories differed by site (Figs S1 and S2 in Appendix A). For each outcome, we obtained the main effect term of each phthalate metabolite and the interaction term between the phthalate metabolite and time. To facilitate interpretation, we scaled all phthalate metabolite by time interaction terms by five years. The scaled interaction terms can be interpreted as differences in the change in an adiposity outcome over five years per doubling of phthalate metabolite concentrations. The main effect term of the phthalate metabolite can be interpreted as the difference in an adiposity measure at baseline per doubling of metabolite concentration. The main effect terms are of secondary interests and are reported in supplementary tables in Appendix A only.
To visualize adiposity trajectories associated with different levels of phthalate exposure, we plotted the least-squared means of BW, FM, and BF% at baseline, Year 6, and Year 10 for women at high (75th percentile) vs. low (25th percentile) levels of exposure to each phthalate metabolite. We calculated the adjusted differences in each outcome between exposure levels at each time point and the adjusted differences in the (annualized) changes in each outcome between exposure levels. All analyses were conducted overall and by baseline obesity status.
We conducted a series of sensitivity analyses to examine the robustness of our findings. First, all models were additionally adjusted for the total intake frequency (times/week) of food items previously reported to be associated with phthalate exposure. These food items included red meat, poultry, liver, processed meat, dairy, margarine, refined grains, salty snacks, desserts, meat substitutes, pizza, salad dressing, and salsa (Colacino et al., 2010; Trasande et al., 2013; Serrano et al., 2014; Bai et al., 2015; Zota et al., 2016; Varshavsky et al., 2018; Buckley et al., 2019). Second, because the onset of cancer or diabetes may impact body weight and body composition, we re-fitted all models after censoring data at the time of cancer or diabetes onset. Finally, because phthalate metabolites in spot urine samples may not accurately reflect habitual exposure, all analyses were repeated using phthalate metabolite data from 2002/2003 and the within-person mean of phthalate metabolite concentrations from 1999/2000 and 2002/2003. The baseline for these analyses was 2002/2003. Dietary energy intake from 2001/2002 was used to approximate energy intake in 2002/2003. For BW, the knot for the linear spline term for time was set at T = 3 years to be consistent with primary analyses.
All statistical analyses were performed in R version 4.0.3 using packages mgcv (version 1.8–33), nlme (version 3.1 – 151), and emmeans (version 1.5.5–1). Statistical significance was defined as two-sided p-value < 0.05.
Results
At baseline (1999/2000), women had a median age of 49.4 years (quartile (Q) 1 and Q3: 47.4, 51.5) (Table 1). Approximately 50% of the participants were White, 22% Black, 13% Chinese, and 15% Japanese. Approximately half of the participants did not have a college degree. Most women were pre- or peri- menopausal at baseline in 1999/2000 (71%) and approximately 30% and 34% of women had overweight and obesity, respectively.
Table 1.
Participant characteristics in 1999/2000 (N = 1369)
| Median (Q1, Q3)a | |
|---|---|
| Age (years) | 49.4 (47.4, 51.5) |
| Body weight (kg) | 68.9 (58.6, 83.8) |
| Fat mass (kg) | 25.3 (19.1, 34.3) |
| Body fat percentage (%) | 40.4 (35.7, 45.1) |
| N (%)b | |
| Site | |
| Detroit area, MI | 247 (18%) |
| Boston, MA | 227 (16.6%) |
| Oakland, CA | 306 (22.4%) |
| Los Angeles, CA | 359 (26.2%) |
| Pittsburgh, PA | 230 (16.8%) |
| Race/ethnicity | |
| White | 695 (50.8%) |
| Black | 294 (21.5%) |
| Chinese | 176 (12.9%) |
| Japanese | 204 (14.9%) |
| Education | |
| High school or less | 248 (18.1%) |
| Some college | 438 (32%) |
| College degree | 336 (24.5%) |
| Postgraduate | 347 (25.3%) |
| Smoking | |
| Never | 863 (63%) |
| Past | 364 (26.6%) |
| Current | 142 (10.4%) |
| Menopausal status | |
| Pre- or peri- menopausal | 969 (70.8%) |
| Natural/surgical menopause | 198 (14.5%) |
| Unknown due to hormone therapy | 202 (14.8%) |
| Currently on hormone therapy | |
| No | 1089 (79.5%) |
| Yes | 280 (20.5%) |
| Obesity status | |
| Normal/underweight | 502 (36.7%) |
| Overweight | 407 (29.7%) |
| Obese | 460 (33.6%) |
“Q1” stands for 1st quartile and “Q3” stands for 3rd quartile.
Percentages may not sum to 100 due to rounding.
The detection frequencies of phthalate metabolites, defined as percentages of observations above the limits of detection, ranged from 84.4% for MEHP to nearly 100% for the other metabolites (Table 2). The median concentrations of metabolites ranged from 2.61 ng/mL (Q1 and Q3: 1.55, 4.48) for MiBP to 81.8 ng/mL (Q1 and Q3: 36.42, 210.47) for MEP. The concentrations of most phthalate metabolites were higher in women who were younger, from Michigan, Black, or current smokers (Tables S2 – S4 in Appendix A). Overweight and obesity were positively associated with the urinary concentrations of most phthalate metabolites (Table 2).
Table 2.
Phthalate metabolite concentrations in 1999/2000, overall and by obesity status
| Overall (N = 1369) |
Normal/under-weight (N = 502) |
Overweight (N = 407) |
Obese (N = 460) |
p-valueb | |||
|---|---|---|---|---|---|---|---|
| Group | Phthalate metabolitea | N (%) detected | Median (Q1, Q3) |
Median (Q1, Q3) |
Median (Q1, Q3) |
Median (Q1, Q3) |
|
| Low-molecular-weight (LMW) phthalate metabolites | MEP (ng/mL) | 1368 (99.9%) | 81.8 (36.42, 210.47) |
68.99 (33.16, 148.83) |
72.63 (33.78, 185.69) |
106.72 (47.89, 292.99) |
<0.0001 |
| MnBP (ng/mL) | 1369 (100%) | 18.5 (11.69, 32.79) |
17.32 (10.42, 27.61) |
19.34 (11.74, 34.58) |
19.27 (13.04, 36.36) |
0.0003 | |
| MiBP (ng/mL) | 1342 (98%) | 2.61 (1.55, 4.48) |
2.53 (1.5, 4.26) |
2.68 (1.5, 4.68) |
2.64 (1.66, 4.5) |
0.39 | |
| ∑LMW phthalatesc (nmol/mL) | -- | 0.57 (0.29, 1.31) |
0.50 (0.26, 0.94) |
0.52 (0.27, 1.2) |
0.72 (0.37, 1.77) |
<0.0001 | |
| Di(2-ethylhexyl) phthalate (DEHP) metabolites | MEHP (ng/mL) | 1156 (84.4%) | 3.07 (1.59, 6.03) |
2.98 (1.5, 6.04) |
3.13 (1.71, 5.72) |
3.11 (1.6, 6.41) |
0.68 |
| MEHHP (ng/mL) | 1368 (99.9%) | 16.13 (8.5, 30.51) |
13.08 (6.85, 26.6) |
15.29 (7.85, 29.91) |
19.48 (11.2, 38.06) |
<0.0001 | |
| MEOHP (ng/mL) | 1367 (99.9%) | 9.63 (5.17, 18.68) |
8.05 (4.19, 16) |
8.93 (4.7, 18.02) |
11.36 (6.69, 21.87) |
<0.0001 | |
| MECPP (ng/mL) | 1369 (100%) | 16.85 (9.82, 31.33) |
14.01 (8.43, 26.31) |
15.70 (9.54, 29.48) |
20.52 (12.75, 38.12) |
<0.0001 | |
| ∑DEHPd (nmol/mL) |
-- | 0.16 (0.09, 0.29) |
0.13 (0.08, 0.26) |
0.15 (0.08, 0.28) |
0.19 (0.11, 0.38) |
<0.0001 | |
| Other High-molecular-weight (HMW) phthalate metabolites | MBzP (ng/mL) | 1366 (99.8%) | 10.43 (5.8, 18.53) |
8.78 (4.66, 15.64) |
10.00 (5.87, 17.23) |
12.28 (7.49, 21.7) |
<0.0001 |
| MCOP (ng/mL) | 1365 (99.7%) | 4.41 (2.62, 7.88) |
3.66 (2.37, 6.63) |
4.40 (2.63, 6.93) |
5.38 (3.22, 9.61) |
<0.0001 | |
| MCNP (ng/mL) | 1365 (99.7%) | 2.69 (1.52, 5.01) |
2.19 (1.31, 4.07) |
2.41 (1.41, 4.32) |
3.66 (1.99, 6.16) |
<0.0001 | |
| MCPP (ng/mL) | 1351 (98.7%) | 2.65 (1.7, 4.27) |
2.47 (1.55, 4.07) |
2.57 (1.62, 3.95) |
2.94 (2.01, 4.71) |
<0.0001 | |
| ∑HMW phthalatese (nmol/mL) |
-- | 0.27 (0.16, 0.47) |
0.23 (0.13, 0.4) |
0.25 (0.16, 0.47) |
0.31 (0.2, 0.55) |
<0.0001 | |
All phthalate metabolites were adjusted for hydration using the “covariate-adjusted creatinine standardization” method. Median and the 1st (“Q1”) and 3rd (“Q3”) quartiles are reported.
p-values were obtained from Kruskal-Wallis tests.
∑LMW phthalates: molar sum of low-molecular-weight phthalate metabolites, including MEP, MnBP, and MiBP.
∑DEHP: molar sum of DEHP metabolites, including MEHP, MEHHP, MEOHP, and MECPP.
∑HMW phthalates: molar sum of MEHP, MEHHP, MEOHP, MECPP, MBzP, MCOP, MCNP, and MCPP
At baseline, the least-squared means of BW, FM, and BF% were 70.7 kg (95% confidence interval (CI): 69.3, 72.0), 26.3 kg (95% CI: 25.5, 27.1), and 39.7% (95% CI: 39.2, 40.2), respectively (Table 3 and Fig S4 in Appendix A). On average, BW increased by 0.17 kg/year (95% CI: 0.099, 0.24) in the first six years, followed by an average loss of 0.079 kg/year (95% CI: −0.13, −0.028) thereafter. FM and BF% increased at a rate of 0.015 kg/year (95% CI: −0.010, 0.041) and 0.030 percentage points (ppt)/year (95% CI: 0.011, 0.049), respectively. There was substantial heterogeneity in these growth rates by baseline obesity status (Table 3 and Fig S4 in Appendix A). Women who were normal/underweight at baseline had the most rapid increases in BW prior to stabilization, FM, and BF%. In contrast, women who were obese at baseline primarily experienced decreases in BW, FM, and BF% over time.
Table 3.
Baseline levels and rates of change in adiposity measures, overall and by obesity status
| Least-squared means of adiposity measures at baselinea (95% CI) | ||||
|---|---|---|---|---|
| Allb | Normal/underweight | Overweight | Obese | |
| Body weight (kg) | 70.7 (69.3, 72.0) |
56.2 (55.3, 57.0) |
67.2 (66.2, 68.2) |
87.4 (84.9, 89.9) |
| Fat mass (kg) | 26.3 (25.5, 27.1) |
17.9 (17.3, 18.5) |
24.0 (23.4, 24.6) |
36.6 (35.3, 38.0) |
| Body fat percentage (%) | 39.7 (39.2, 40.2) |
34.9 (34.2, 35.6) |
39.1 (38.6, 39.7) |
45.5 (44.9, 46.2) |
| Rates of change in adiposity measures (95% CI) | ||||
| All | Normal/underweight | Overweight | Obese | |
| Body weight (kg/year) | ||||
| Tc ≤ 6 years | 0.17 (0.099, 0.24) |
0.30 (0.23, 0.38) |
0.20 (0.090, 0.31) |
−0.020 (−0.19, 0.15) |
| T > 6 years | −0.079 (−0.13, −0.028) |
−0.00047 (−0.051, 0.050) |
0.037 (−0.033, 0.11) |
−0.28 (−0.41, −0.16) |
| Fat mass (kg/year) | 0.015 (−0.010, 0.041) |
0.094 (0.064, 0.13) |
0.077 (0.037, 0.12) |
−0.15 (−0.20, −0.086) |
| Body fat percentage (percentage point/year) | 0.030 (0.011, 0.049) |
0.096 (0.065, 0.13) |
0.053 (0.022, 0.083) |
−0.075 (−0.11, 22120.041) |
Least-squared means and rates of change were obtained from linear mixed effects models. For each outcome, the model included time (linear spline for BW; linear for FM and BF%), age at baseline, race/ethnicity, site, educational attainment at baseline, baseline dietary energy intake, and time-varying menopausal status, HT use, smoking status, and physical activity as predictors. Random intercepts and time effects were included. Models were fit for all women and by baseline obesity status.
Sample sizes varied by outcome. For body weight, the sample sizes for “all”, “normal/underweight”, “overweight”, and “obese” were 1369, 502, 407, and 460, respectively. For fat mass and body fat percentage, the sample sizes for “all”, “normal/underweight”, “overweight”, and “obese” were 1344, 499, 403, and 442, respectively.
“T” stands for “time since baseline”.
Among all women, none of the phthalate metabolites were significantly associated with the changes in BW during follow-up (Fig1; Table S6 in Appendix A). In contrast, all phthalate metabolites except MCNP were associated with faster increases in FM and BF% (Fig 2; Tables S7 and S8 in Appendix A). Per doubling of phthalate metabolite concentrations, differences in the five-year change in FM ranged from 0.04 kg (95% CI: −0.05, 0.14) for MiBP to 0.12 kg (95% CI: 0.03, 0.20) for ∑HMW phthalates (Fig 2, Panel A; Table S7 in Appendix A); differences in the five-year change in BF% ranged from 0.03 ppt (95% CI: −0.03, 0.09) for MiBP to 0.09 ppt (95% CI: 0.02, 0.16) for MCPP (Fig 2, Panel B; Table S8 in Appendix A). The associations with BF% change were statistically significant for all DEHP metabolites, MBzP, MCOP, MCPP, and ∑HMW phthalates, and were borderline significant for MEP (p-value = 0.09) and MnBP (p-value = 0.08) (Table S8 in Appendix A).
Fig 1. Differences in five-year body weight change per doubling of phthalate metabolite concentrations.
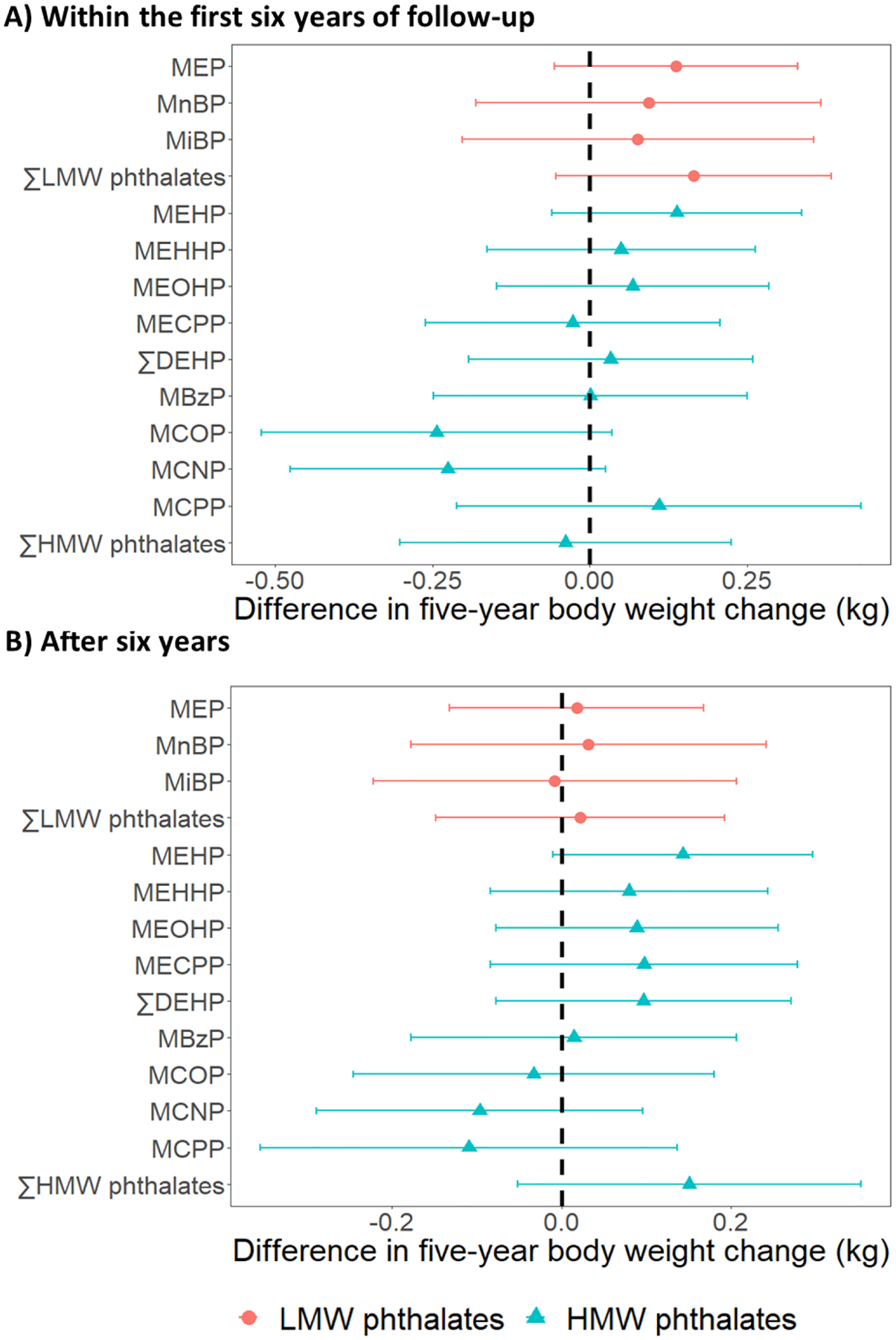
Results were based on 14380 observations from 1369 women. Differences were adjusted for age at baseline (1999/2000), race/ethnicity, the interaction between race/ethnicity and time, site, education level, daily dietary energy intake at baseline, and time-varying physical activity, smoking status, menopausal status, and use of hormone therapy. ∑LMW phthalates = molar sum of MEP, MnBP, and MiBP; ∑DEHP = molar sum of MEHP, MEHHP, MEOHP, and MECPP; ∑HMW phthalates = molar sum of MEHP, MEHHP, MEOHP, MECPP MBzP, MCOP, MCNP, and MCPP.
Fig 2. Differences in five-year changes in fat mass and body fat percentage per doubling of phthalate metabolite concentrations.
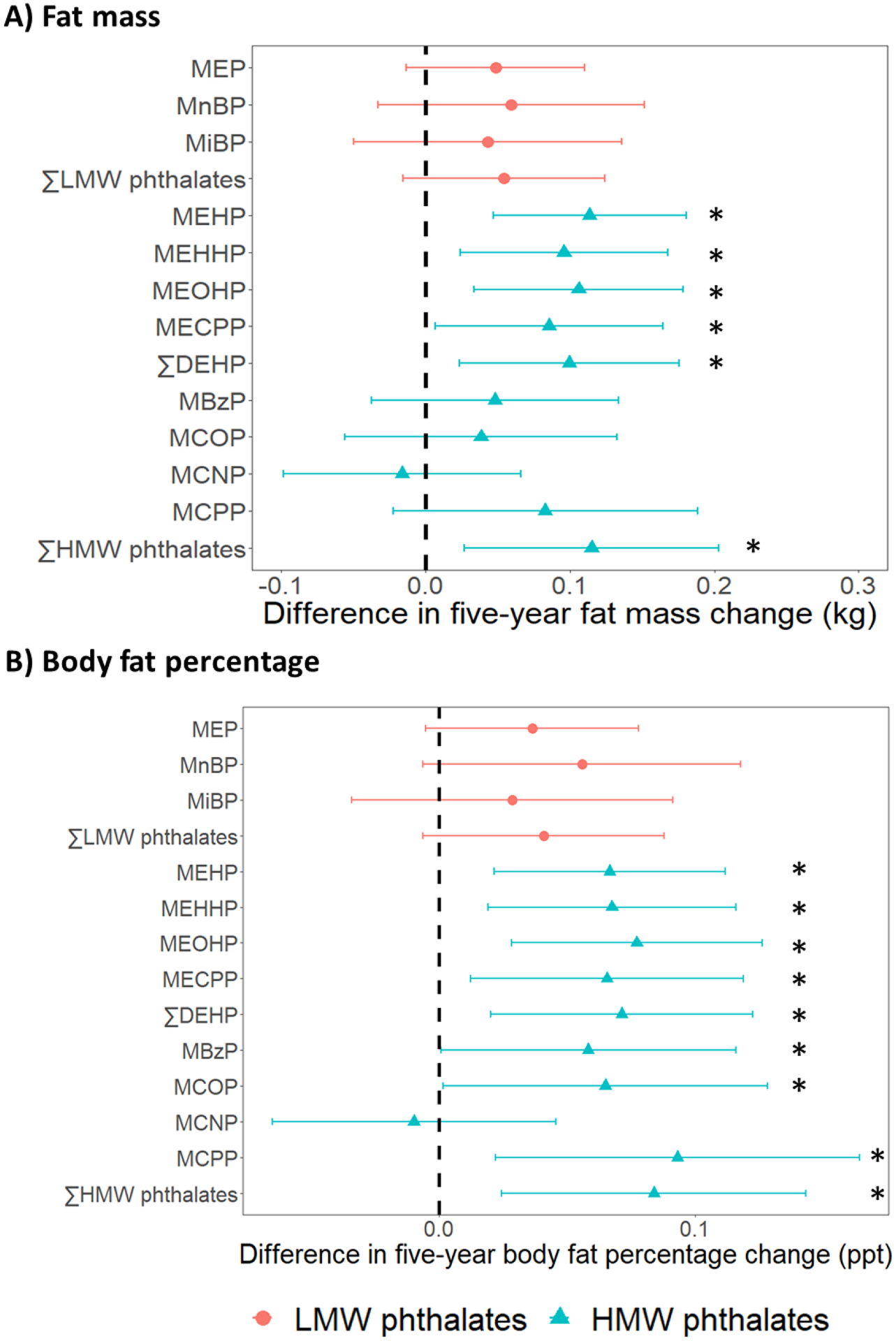
Results were based on 12746 observations from 1344 women. Differences were adjusted for age at baseline (1999/2000), race/ethnicity, site, the interaction between time and site, education level, daily dietary energy intake at baseline, and time-varying physical activity, smoking status, menopausal status, and use of hormone therapy. ∑LMW phthalates = molar sum of MEP, MnBP, and MiBP; ∑DEHP = molar sum of MEHP, MEHHP, MEOHP, and MECPP; ∑HMW phthalates = molar sum of MEHP, MEHHP, MEOHP, MECPP, MBzP, MCOP, MCNP, and MCPP. * = p-value < 0.05.
In analyses stratified by baseline obesity status, the associations between phthalate metabolites and changes in adiposity measures were strongest among women who were normal/underweight at baseline (Figs 3 and 4; Tables S6–S8 in Appendix A). In this group, all phthalate metabolites except MCNP were positively associated with the changes in all adiposity measures. Per doubling of phthalate metabolite concentrations, differences in the five-year change in BW during the period of BW increase ranged from 0.11 kg (95% CI: −0.10, 0.31) for MEHP to 0.40 kg (95% CI: 0.09, 0.70) for MCPP (Fig 3; Table S6 in Appendix A); differences in the five-year change in FM ranged from 0.08 kg (95% CI: −0.03, 0.18) for MiBP to 0.21 kg (95% CI: 0.11, 0.31) for ∑HMW phthalates (Fig 4, Panel A; Table S7 in Appendix A); and differences in the five-year change in BF% ranged from 0.06 ppt (95% CI: −0.04, 0.16) for MiBP to 0.18 ppt (95% CI: 0.09, 0.28) for MBzP (Fig 4, Panel D; Table S8 in Appendix A). In contrast, the associations between phthalate metabolites and the five-year changes in adiposity measures were appreciably smaller in magnitude and largely not statistically significant among overweight and obese women (Figs 3 and 4; Tables S6–S8 in Appendix A).
Fig 3. Differences in five-year body weight change per doubling of phthalate metabolite concentrations, by baseline obesity status.
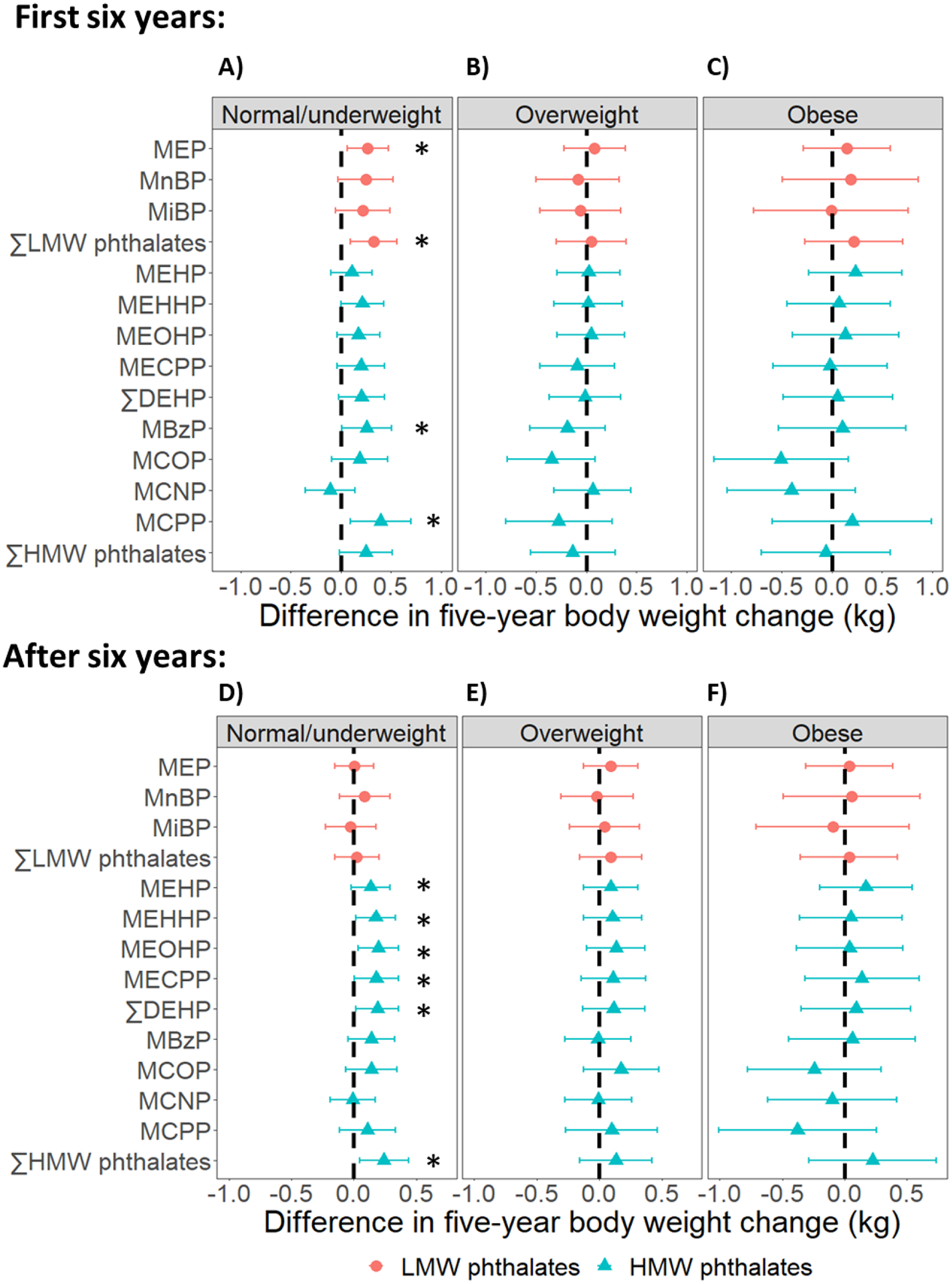
Results were based on 5279 observations from 502 women who were normal/underweight, 4313 observations from 407 women who had overweight, and 4788 observations from 460 women who had obesity. Differences were adjusted for age at baseline (1999/2000), race/ethnicity, the interaction between race/ethnicity and time, site, education level, daily dietary energy intake at baseline, and time-varying physical activity, smoking status, menopausal status, and use of hormone therapy. ∑LMW phthalates = molar sum of MEP, MnBP, and MiBP; ∑DEHP = molar sum of MEHP, MEHHP, MEOHP, and MECPP; ∑HMW phthalates = molar sum of MEHP, MEHHP, MEOHP, MECPP MBzP, MCOP, MCNP, and MCPP. * = p-value < 0.05.
Fig 4. Differences in five-year fat mass and body fat percentage changes per doubling of phthalate metabolite concentrations, by baseline obesity status.
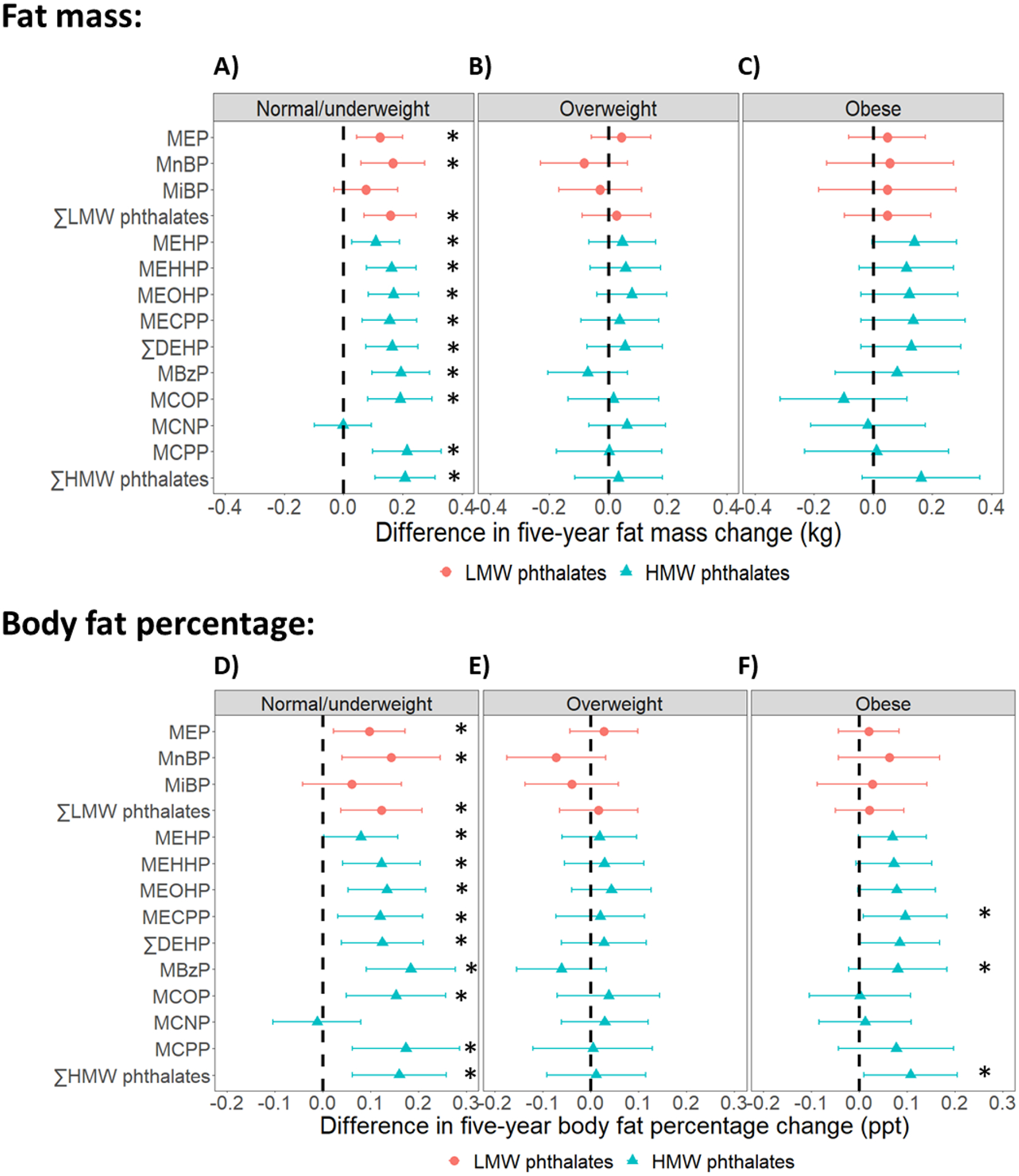
Results were based on 4750 observations from 499 women who were normal/underweight, 3942 observations from 403 women who had overweight, and 4054 observations from 442 women who had obesity. Differences were adjusted for age at baseline (1999/2000), race/ethnicity, site, the interaction between time and site, education level, daily dietary energy intake at baseline, and time-varying physical activity, smoking status, menopausal status, and use of hormone therapy. ∑LMW phthalates = molar sum of MEP, MnBP, and MiBP; ∑DEHP = molar sum of MEHP, MEHHP, MEOHP, and MECPP; ∑HMW phthalates = molar sum of MEHP, MEHHP, MEOHP, MECPP, MBzP, MCOP, MCNP, and MCPP. * = p-value < 0.05.
Fig 5 visualizes adiposity trajectories for women who were normal/underweight at baseline and exposed to different levels of MEP, ∑DEHP, and MBzP. Women at the 75th percentile of each metabolite experienced steeper increases in all adiposity measures as compared to those at the 25th percentile (Fig 5; Tables S9–S11 in Appendix A). For example, during the phase of BW gain, the additional change in BW per year for those at the 75th versus those at the 25th percentile of MEP was 0.11 kg/year (95% CI: 0.03, 0.20) (Table S9 in Appendix A). This difference was equivalent to the impact of watching approximately 3 (0.11/0.035 = 3.1) more hours of TV per day in terms of expected weight gain (Mozaffarian et al., 2011). The diverging adiposity trajectories between women at high versus low levels of exposure were also evident for the other metabolites, except MCNP (Tables S9–S11 in Appendix A).
Fig 5. Predicted adiposity trajectories at two levels of phthalate exposure among normal/underweight women.
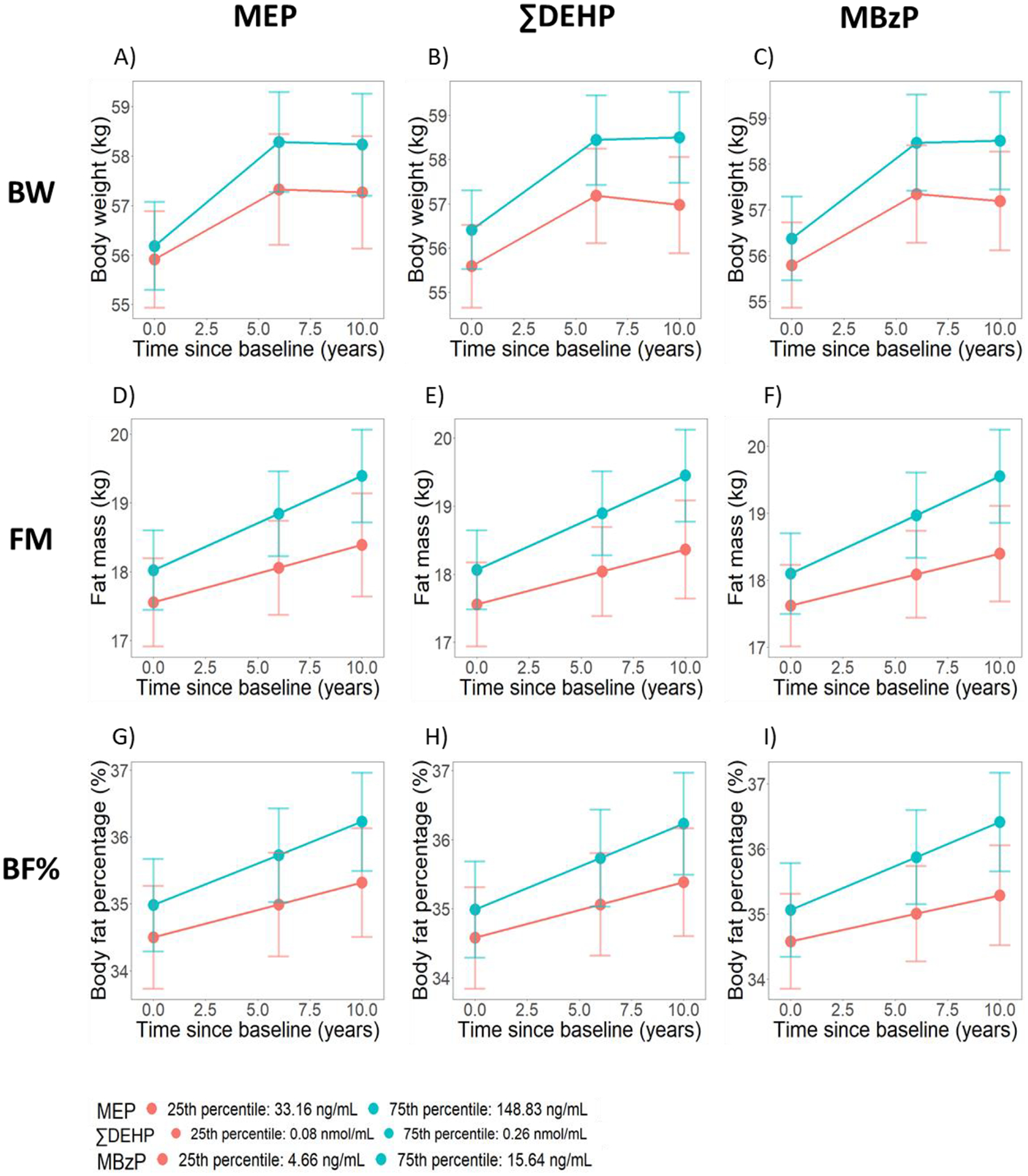
The predicted trajectories were least-squared means from the linear mixed effects model for each outcome and each phthalate metabolite among those who were normal/underweight at baseline. All models were adjusted for age at baseline (1999/2000), race/ethnicity, site, education level, daily dietary energy intake at baseline, and time-varying physical activity, smoking status, menopausal status, and use of hormone therapy. For BW, models were additionally adjusted for time by race/ethnicity interaction. For FM and BF%, models were additionally adjusted for time by site interaction.
Sensitivity analyses adjusting for dietary intake of food items or censoring data at the time of cancer or diabetes onset did not change estimates for the baseline or longitudinal associations between phthalate metabolites and all outcomes (data not shown). When metabolites from 2002/2003 were used as exposures, the associations between most metabolites and the five-year changes in adiposity measures were attenuated (Tables S12 – S14 in Appendix A). However, the degree of attenuation was smaller for women who were normal/underweight at baseline as compared to women who were overweight or obese. For normal or underweight women, positive associations in the primary analyses remained positive in the sensitivity analyses. When within-person mean phthalate metabolite concentrations were used as exposures, the associations between most metabolites and changes in adiposity measures were also attenuated, although generally to a smaller extent than when metabolites from 2002/2003 were used as exposures (Tables S15 – S17 in Appendix A).
Discussion
In this study of a diverse group of midlife women followed for almost 20 years, we found that phthalate metabolites were associated with faster increases in fat mass and body fat percentage. The associations were strongest and most persistent in women who were normal/underweight at baseline. The associations between phthalate metabolites and body weight gain were less consistent, perhaps reflecting the fact that body weight is not an accurate measure of body fat in an aging cohort (Kuk et al., 2009). Overall, this study suggests that phthalates contribute to body fat gain in mid-life women. However, our results were not replicated in sensitivity analyses with a second set of phthalate metabolites from a different time point, so our findings should be interpreted cautiously.
This study is the first piece of evidence directly linking phthalate exposure to body fat gain in women. Prior studies have linked MEP, MnBP, MiBP, DEHP metabolites, MBzP and MCPP to faster increases or slower declines in body weight or BMI, but results were highly heterogeneous both within and across studies (Díaz Santana et al., 2019; Haggerty et al., 2021; Perng et al., 2020; Philips et al., 2020; Rodríguez-Carmona et al., 2019; Song et al., 2014). Few studies reported statistically significant, positive associations between body weight changes and all metabolites, and few metabolites have been consistently associated with faster body weight gain across studies. Consequently, whether phthalate exposure leads to body fat gain and obesity is still unclear. One critical limitation in most prior studies is the use of body weight to approximate body fat. Because changes in body fat do not always result in changes in body weight, many studies may have missed or underestimated the associations between phthalate exposure and increases in adiposity. Only one prior study examined percent body fat as the outcome (van der Meer et al., 2021). While that study found positive associations between some phthalate metabolites and greater retention of body fat, its generalizability is limited because participants were all overweight/obese and underwent intense caloric restriction in order to lose weight. By examining the association of phthalates and fat mass and body fat percentage among a general population of midlife women, our findings provide stronger evidence for phthalates’ obesogenic potential than has previously been reported. Whether our findings are generalizable to women at other life stages and men should be investigated in future studies, preferably with precise measures of adiposity rather than body weight alone.
The finding that some phthalate metabolites were associated with accelerated body fat gain in midlife women has important public health implications. Virtually all individuals are exposed to phthalates daily through using personal care products (Guo and Kannan, 2013), ingesting food (Serrano et al., 2014), or inhaling indoor dust (Yang et al., 2020) contaminated with phthalates. The near 100% detection rates of most phthalate metabolites in this and many other studies (Wang et al., 2019) despite the short half-lives of phthalates testify to the widespread and ongoing nature of phthalate exposure. Although some phthalates commonly used 20 years ago, such as di-n-butyl phthalate (the parent of MnBP), DEHP, and butyl benzyl phthalate (the parent of MBzP and MnBP), have been banned in children’s toys and childcare articles since 2008 due to concerns about developmental toxicity (United States Consumer Product Safety Commission, 2019), they are still used in other applications such as food packaging and food handling contact materials (Edwards et al., 2021), and their metabolites continue to be found in recent urine samples (Centers for Disease Control and Prevention, 2019). The finding that these widely used chemicals are associated with more rapid changes in fat mass, a risk factor for numerous chronic diseases, is concerning. Although the associations between some phthalate metabolites and the five-year changes in FM and BF% may be small at the individual level, the potential population-level impact could be substantial given widespread phthalate exposure. Our findings have added to the evidence suggesting phthalates’ potential obesogenic effects. If phthalates’ obesogenic potential is confirmed in future studies, it would be important to incorporate limiting phthalate exposures as part of a comprehensive obesity prevention strategy. Measures to limit phthalate exposures may include requiring the disclosure of phthalates in consumer products or further restricting their use in products.
One remarkable finding of this study was that phthalate metabolites were associated with faster increases in body fat primarily in women who were normal/underweight at baseline. This was somewhat unexpected, as previous studies did not always show stronger associations between phthalate metabolites and body weight gain in normal/underweight women (Díaz Santana et al., 2019; Song et al., 2014). It is unclear why women who were normal/underweight at baseline in this study appeared more susceptible to phthalates’ potential obesogenic effects. Since the adiposity trajectories differed substantially by baseline obesity status, we speculate that women’s potential to gain additional body fat may modify the associations between phthalate metabolites and body fat gain. Those who are normal/underweight may be more susceptible to gain additional fat, whereas women who are already overweight or obese may have reached the upper limits of their adipose tissues’ expandability. Thus, this may result in normal/underweight women being more susceptible to phthalates’ obesogenic effects. This is consistent with the observation that levels of PPAR-γ, a nuclear receptor through which phthalates promote adipogenesis, are reduced in the fat tissues of obese individuals (Tchkonia et al., 2010).
PPAR-γ is essential for the growth and maintenance of body fat (Ferré, 2004), and many phthalate metabolites activate PPAR-γ (Bility et al., 2004; Hurst and Waxman, 2003). Phthalates may also disrupt the hypothalamic-pituitary-thyroid axis (HPT), leading to a lower basal metabolic rate and hence less energy expenditure (Lv et al., 2016), although it is unclear if this mechanism is more prominent in normal/underweight individuals. Our findings underscore the existence of individuals with different susceptibility to phthalates in the population. Identifying these individuals and understanding the mechanisms behind different susceptibility is important for tailoring public health measures to specific populations.
Another notable result of this study was that the associations between phthalate metabolites and five-year changes in adiposity measures were attenuated when metabolites from 2002/2003 were used as exposures. This may be due to random exposure measurement error, as the degree of attenuation generally increased when the intraclass correlation coefficient of a metabolite decreased. However, we note that the timing of exposure had also changed in sensitivity analyses. Given that women were three years older, and many had transitioned to post-menopause in 2002/2003, we cannot rule out that the effects of phthalates truly differ by the age or menopausal status at which women are exposed. There might exist a critical age window or life stage during which women are more sensitive to phthalates’ obesogenic effects. Unfortunately, with only two sets of phthalate metabolites measured three years apart, we were not able to pinpoint the reason for the differences between primary and sensitivity analyses. Future studies should repeatedly measure phthalates at closer intervals within different life stages of interest.
This study has many important strengths and limitations. Unlike the majority of previous studies, our analysis considered fat mass and body fat percentage, measured precisely with DXA. This allowed us to minimize outcome measurement error and provide the first piece of evidence directly linking phthalate metabolites to changes in body fat in midlife women. Also unlike previous studies, we used a prospective study design with long-term follow-up, thereby reducing concerns about reverse causation. The SWAN-MPS cohort is diverse in terms of race/ethnicity, geographic location, and socioeconomic status. This diversity increases our confidence in the generalizability of our findings. Finally, several high-molecular-weight phthalate metabolites we examined, including MCOP, MCNP, and MCPP were infrequently studied in previous investigations on phthalates and adiposity measures in adults, so our work expands our understanding of a broad set of phthalate metabolites.
Despite these notable strengths, there are some key limitations to acknowledge. In 1999/2000, participants of SWAN-MPS had lower BMI, were more physically active, less likely to smoke, and had higher levels of education than SWAN participants excluded from SWAN-MPS (Ding et al., 2022). Women with high levels of phthalate exposure before 1999/2000, who might have high BMI in 1999/2000 as a result, may have been excluded. This could have potentially created selection bias in our study, leading to potential attenuations of the associations between phthalate metabolites and changes in adiposity measures. This study utilized a single spot urine per woman to measure phthalate exposure. Because the half-lives of phthalate metabolites in the body are relatively short (Johns et al., 2015), phthalate metabolites in a single urine sample may not reflect habitual exposure. Thus, the use of spot urine samples may result in non-differential exposure measurement error, which would have attenuated the associations between phthalate metabolites and adiposity measures. Despite having limited dietary data to account for confounding by diet, we observed positive associations for low-molecular-weight phthalate metabolites, for which diet is not a major source of exposure (Koch et al., 2013; Radke et al., 2019). Thus, confounding by diet is unlikely to fully explain the positive associations between phthalate metabolites and body fat gain. Given the observational nature of this study, residual confounding by other factors is possible, including confounding by other phthalate metabolites and other environmental chemicals. Future analyses will explore multi-pollutant models to consider this limitation. While body fat distribution in addition to total body fat is an independent risk factor of cardiometabolic disease, we lacked imaging-based measures of body fat distribution. Finally, we did not adjust for multiple comparisons, so statistical significance should be interpreted cautiously.
Conclusions
In conclusion, in this longitudinal study on a diverse group of midlife women, we found that exposure to phthalates was associated with more rapid body fat gain, especially in women who were normal/underweight. These findings support the hypothesis that certain environmental chemicals may cause obesity. Limiting exposure to phthalates and potentially other synthetic chemicals may help prevent obesity and its comorbidities.
Supplementary Material
Highlights.
Phthalate metabolites were associated with more rapid increases in body fat in women
The associations were stronger in women who were normal/underweight at baseline
Phthalates’ associations with body weight increases were largely null
Funding sources
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, and U19AG063720). The study was also supported by the SWAN Repository (U01AG017719). This publication was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131.
This study was also supported by grants from the National Institute of Environmental Health Sciences (NIEHS) R01-ES026578, R01-ES026964 and P30-ES017885, and by the Center for Disease Control and Prevention (CDC)/National Institute for Occupational Safety and Health (NIOSH) grant T42-OH008455. Mia Peng was supported by the Interdisciplinary Research Training on Health and Aging Grant (T32 AG-027708).
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers:
University of Michigan, Ann Arbor - Carrie Karvonen-Gutierrez, PI 2021 - present, Siobán Harlow, PI 2011 – 2021, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA - Joel Finkelstein, PI 1999 - present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL - Howard Kravitz, PI 2009 - present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser - Ellen Gold, PI; University of California, Los Angeles - Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY - Carol Derby, PI 2011 - present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry - New Jersey Medical School, Newark - Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA - Karen Matthews, PI.
NIH Program Office:
National Institute on Aging, Bethesda, MD - Rosaly Correa-de-Araujo 2020 - present; Chhanda Dutta 2016– 2020; Winifred Rossi 2012–2016; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD - Program Officers.
Central Laboratory:
University of Michigan, Ann Arbor - Daniel McConnell (Central Ligand Assay Satellite Services).
NIA Biorepository:
Rosaly Correa-de-Araujo 2019 - Present; SWAN Repository: University of Michigan, Ann Arbor - Siobán Harlow 2013 – 2018; Dan McConnell 2011 – 2013; MaryFran Sowers 2000 – 2011.
Coordinating Center:
University of Pittsburgh, Pittsburgh, PA - Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
The study protocols of SWAN were approved by institutional review boards at each study site, and all participants provided informed consent to participate in the study at each study visit. We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Declaration of interests:
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Andersen C, Krais AM, Eriksson AC, Jakobsson J, Löndahl J, Nielsen J, Lindh CH, Pagels J, Gudmundsson A, Wierzbicka A, 2018. Inhalation and Dermal Uptake of Particle and Gas-Phase Phthalates—A Human Exposure Study. Environ. Sci. Technol 52, 12792–12800. 10.1021/acs.est.8b03761 [DOI] [PubMed] [Google Scholar]
- Bai PY, Wittert GA, Taylor AW, Martin SA, Milne RW, Shi Z, 2015. The association of socio-demographic status, lifestyle factors and dietary patterns with total urinary phthalates in Australian men. PLoS ONE 10, e0122140. 10.1371/journal.pone.0122140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL, 2005. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ. Health Perspect 113, 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bility MT, Thompson JT, McKee RH, David RM, Butala JH, Vanden Heuvel JP, Peters JM, 2004. Activation of mouse and human peroxisome proliferator-activated receptors (PPARs) by phthalate monoesters. Toxicol. Sci 82, 170–182. 10.1093/toxsci/kfh253 [DOI] [PubMed] [Google Scholar]
- Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L, 1986. A data-based approach to diet questionnaire design and testing. American Journal of Epidemiology 124, 453–469. 10.1093/oxfordjournals.aje.a114416 [DOI] [PubMed] [Google Scholar]
- Buckley JP, Kim H, Wong E, Rebholz CM, 2019. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013–2014. Environ Int 131, 105057. 10.1016/j.envint.2019.105057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2019. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, January 2019. URL https://www.cdc.gov/exposurereport/index.html (accessed 3.26.20).
- Colacino JA, Harris TR, Schecter A, 2010. Dietary intake is associated with phthalate body burden in a nationally representative sample. Environ. Health Perspect 118, 998–1003. 10.1289/ehp.0901712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz Santana MV, Hankinson SE, Bigelow C, Sturgeon SR, Zoeller RT, Tinker L, Manson JAE, Calafat AM, Meliker JR, Reeves KW, 2019. Urinary concentrations of phthalate biomarkers and weight change among postmenopausal women: a prospective cohort study. Environ Health 18, 20. 10.1186/s12940-019-0458-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Karvonen-Gutierrez CA, Mukherjee B, Calafat AM, Harlow SD, Park SK, 2022. Per- and Polyfluoroalkyl Substances and Incident Hypertension in Multi-Racial/Ethnic Women: The Study of Women’s Health Across the Nation. Hypertension 79, 1876–1886. 10.1161/HYPERTENSIONAHA.121.18809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards L, McCray NL, VanNoy BN, Yau A, Geller RJ, Adamkiewicz G, Zota AR, 2021. Phthalate and novel plasticizer concentrations in food items from U.S. fast food chains: a preliminary analysis. J Expo Sci Environ Epidemiol 1–8. 10.1038/s41370-021-00392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige JN, Gelman L, Rossi D, Zoete V, Métivier R, Tudor C, Anghel SI, Grosdidier A, Lathion C, Engelborghs Y, Michielin O, Wahli W, Desvergne B, 2007. The Endocrine Disruptor Monoethyl-hexyl-phthalate Is a Selective Peroxisome Proliferator-activated Receptor γ Modulator That Promotes Adipogenesis*. Journal of Biological Chemistry 282, 19152–19166. 10.1074/jbc.M702724200 [DOI] [PubMed] [Google Scholar]
- Ferré P, 2004. The Biology of Peroxisome Proliferator-Activated Receptors: Relationship With Lipid Metabolism and Insulin Sensitivity. Diabetes 53, S43–S50. 10.2337/diabetes.53.2007.S43 [DOI] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT, 2015. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev 36, E1–E150. 10.1210/er.2015-1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greendale GA, Sternfeld B, Huang M, Han W, Karvonen-Gutierrez C, Ruppert K, Cauley JA, Finkelstein JS, Jiang S-F, Karlamangla AS, 2019. Changes in body composition and weight during the menopause transition. JCI Insight 4. 10.1172/jci.insight.124865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grün F, Blumberg B, 2006. Environmental Obesogens: Organotins and Endocrine Disruption via Nuclear Receptor Signaling. Endocrinology 147, s50–s55. 10.1210/en.2005-1129 [DOI] [PubMed] [Google Scholar]
- Guo Y, Kannan K, 2013. A Survey of Phthalates and Parabens in Personal Care Products from the United States and Its Implications for Human Exposure. Environ. Sci. Technol 47, 14442–14449. 10.1021/es4042034 [DOI] [PubMed] [Google Scholar]
- Haggerty DK, Flaws JA, Li Z, Strakovsky RS, 2021. Phthalate exposures and one-year change in body mass index across the menopausal transition. Environmental Research 194, 110598. 10.1016/j.envres.2020.110598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CM, 2020. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018 8. [PubMed]
- Heindel JJ, Blumberg B, 2019. Environmental Obesogens: Mechanisms and Controversies. Annu Rev Pharmacol Toxicol 59, 89–106. 10.1146/annurev-pharmtox-010818-021304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, vom Saal FS, Blumberg B, Bovolin P, Calamandrei G, Ceresini G, Cohn BA, Fabbri E, Gioiosa L, Kassotis C, Legler J, La Merrill M, Rizzir L, Machtinger R, Mantovani A, Mendez MA, Montanini L, Molteni L, Nagel SC, Parmigiani S, Panzica G, Paterlini S, Pomatto V, Ruzzin J, Sartor G, Schug TT, Street ME, Suvorov A, Volpi R, Zoeller RT, Palanza P, 2015. Parma consensus statement on metabolic disruptors. Environmental Health 14, 54. 10.1186/s12940-015-0042-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst CH, Waxman DJ, 2003. Activation of PPAR and PPAR by Environmental Phthalate Monoesters. Toxicological Sciences 74, 297–308. 10.1093/toxsci/kfg145 [DOI] [PubMed] [Google Scholar]
- Johns LE, Cooper GS, Galizia A, Meeker JD, 2015. Exposure assessment issues in epidemiology studies of phthalates. Environment International 85, 27–39. 10.1016/j.envint.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joslin Diabetes Center, 2016. Asian BMI Calculator [WWW Document]. Joslin Diabetes Center Asian American Diabetes Initiative. URL https://aadi.joslin.org/en/am-i-at-risk/asian-bmi-calculator (accessed 2.9.21). [Google Scholar]
- Jung SK, Choi W, Kim SY, Hong S, Jeon HL, Joo Y, Lee C, Choi K, Kim S, Lee K-J, Yoo J, 2022. Profile of Environmental Chemicals in the Korean Population—Results of the Korean National Environmental Health Survey (KoNEHS) Cycle 3, 2015–2017. Int J Environ Res Public Health 19, 626. 10.3390/ijerph19020626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Lorber M, Christensen KLY, Pälmke C, Koslitz S, Brüning T, 2013. Identifying sources of phthalate exposure with human biomonitoring: results of a 48h fasting study with urine collection and personal activity patterns. Int J Hyg Environ Health 216, 672–681. 10.1016/j.ijheh.2012.12.002 [DOI] [PubMed] [Google Scholar]
- Koniecki D, Wang R, Moody RP, Zhu J, 2011. Phthalates in cosmetic and personal care products: concentrations and possible dermal exposure. Environ. Res 111, 329–336. 10.1016/j.envres.2011.01.013 [DOI] [PubMed] [Google Scholar]
- Kuk JL, Saunders TJ, Davidson LE, Ross R, 2009. Age-related changes in total and regional fat distribution. Ageing Research Reviews 8, 339–348. 10.1016/j.arr.2009.06.001 [DOI] [PubMed] [Google Scholar]
- Lv Z, Cheng J, Huang S, Zhang Y, Wu S, Qiu Y, Geng Y, Zhang Q, Huang G, Ma Q, Xie X, Zhou S, Wu T, Ke Y, 2016. DEHP induces obesity and hypothyroidism through both central and peripheral pathways in C3H/He mice: DEHP-Induced Obesity and Hypothyroidism. Obesity 24, 368–378. 10.1002/oby.21359 [DOI] [PubMed] [Google Scholar]
- Majeed KA, Ur Rehman H, Yousaf MS, Zaneb H, Rabbani I, Tahir SK, Rashid MA, 2017. Sub-chronic exposure to low concentration of dibutyl phthalate affects anthropometric parameters and markers of obesity in rats. Environ Sci Pollut Res Int 24, 25462–25467. 10.1007/s11356-017-9952-y [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB, 2011. Changes in Diet and Lifestyle and Long-Term Weight Gain in Women and Men. N Engl J Med 364, 2392–2404. 10.1056/NEJMoa1014296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council, 2008. Phthalate Exposure Assessment in Humans, Phthalates and Cumulative Risk Assessment: The Tasks Ahead. National Academies Press; (US: ). [PubMed] [Google Scholar]
- O’Brien KM, Upson K, Cook NR, Weinberg CR, 2016. Environmental Chemicals in Urine and Blood: Improving Methods for Creatinine and Lipid Adjustment. Environ. Health Perspect 124, 220–227. 10.1289/ehp.1509693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng W, Kasper NM, Watkins DJ, Sanchez BN, Meeker JD, Cantoral A, Solano-González M, Tellez-Rojo MM, Peterson K, 2020. Exposure to Endocrine-Disrupting Chemicals During Pregnancy Is Associated with Weight Change Through 1 Year Postpartum Among Women in the Early-Life Exposure in Mexico to Environmental Toxicants Project. J Womens Health (Larchmt) 29, 1419–1426. 10.1089/jwh.2019.8078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips EM, Jaddoe VWV, Deierlein A, Asimakopoulos AG, Kannan K, Steegers EAP, Trasande L, 2020. Exposures to phthalates and bisphenols in pregnancy and postpartum weight gain in a population-based longitudinal birth cohort. Environment International 144, 106002. 10.1016/j.envint.2020.106002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke EG, Galizia A, Thayer KA, Cooper GS, 2019. Phthalate exposure and metabolic effects: a systematic review of the human epidemiological evidence. Environ Int 132, 104768. 10.1016/j.envint.2019.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Carmona Y, Cantoral A, Trejo-Valdivia B, Téllez-Rojo MM, Svensson K, Peterson KE, Meeker JD, Schnaas L, Solano M, Watkins DJ, 2019. Phthalate exposure during pregnancy and long-term weight gain in women. Environ. Res 169, 26–32. 10.1016/j.envres.2018.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D, 2007. Obesity in women: a life cycle of medical risk. Int J Obes (Lond) 31 Suppl 2, S3–7; discussion S31–32. 10.1038/sj.ijo.0803729 [DOI] [PubMed] [Google Scholar]
- Saravanabhavan G, Guay M, Langlois É, Giroux S, Murray J, Haines D, 2013. Biomonitoring of phthalate metabolites in the Canadian population through the Canadian Health Measures Survey (2007–2009). Int J Hyg Environ Health 216, 652–661. 10.1016/j.ijheh.2012.12.009 [DOI] [PubMed] [Google Scholar]
- Schmidt J-S, Schaedlich K, Fiandanese N, Pocar P, Fischer B, 2012. Effects of di(2-ethylhexyl) phthalate (DEHP) on female fertility and adipogenesis in C3H/N mice. Environ. Health Perspect 120, 1123–1129. 10.1289/ehp.1104016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, Redman LM, Leibel RL, 2017. Obesity Pathogenesis: An Endocrine Society Scientific Statement. Endocrine Reviews 38, 267–296. 10.1210/er.2017-00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S, 2014. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health 13, 43. 10.1186/1476-069X-13-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AR, Kogut KR, Parra K, Bradman A, Holland N, Harley KG, 2021. Dietary intake and household exposures as predictors of urinary concentrations of high molecular weight phthalates and bisphenol A in a cohort of adolescents. J Expo Sci Environ Epidemiol 1–11. 10.1038/s41370-021-00305-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Hauser R, Hu FB, Franke AA, Liu S, Sun Q, 2014. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. Int J Obes (Lond) 38, 1532–1537. 10.1038/ijo.2014.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternfeld B, Ainsworth BE, Quesenberry CP, 1999. Physical Activity Patterns in a Diverse Population of Women. Preventive Medicine 28, 313–323. 10.1006/pmed.1998.0470 [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Morbeck DE, von Zglinicki T, van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL, 2010. Fat tissue, aging, and cellular senescence. Aging Cell 9, 667–684. 10.1111/j.1474-9726.2010.00608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Sathyanarayana S, Jo Messito M, S. Gross R, Attina TM, Mendelsohn AL, 2013. Phthalates and the diets of US children and adolescents. Environmental Research 126, 84–90. 10.1016/j.envres.2013.07.007 [DOI] [PubMed] [Google Scholar]
- United States Consumer Product Safety Commission, 2019. Phthalates Business Guidance & Small Entity Compliance Guide [WWW Document]. U.S. Consumer Product Safety Commission. URL http://www.cpsc.gov/Business--Manufacturing/Business-Education/Business-Guidance/Phthalates-Information (accessed 11.8.21). [Google Scholar]
- van der Meer TP, Thio CHL, van Faassen M, van Beek AP, Snieder H, van Berkum FNR, Kema IP, Makris KC, Wolffenbuttel BHR, van Vliet-Ostaptchouk JV, 2021. Endocrine disrupting chemicals during diet-induced weight loss - A post-hoc analysis of the LOWER study. Environ Res 192, 110262. 10.1016/j.envres.2020.110262 [DOI] [PubMed] [Google Scholar]
- Varshavsky JR, Morello-Frosch R, Woodruff TJ, Zota AR, 2018. Dietary sources of cumulative phthalates exposure among the U.S. general population in NHANES 2005–2014. Environment International 115, 417–429. 10.1016/j.envint.2018.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhu H, Kannan K, 2019. A Review of Biomonitoring of Phthalate Exposures. Toxics 7, 21. 10.3390/toxics7020021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner GR, Flaws JA, 2018. Bisphenol A and Phthalates: How Environmental Chemicals Are Reshaping Toxicology. Toxicological Sciences 166, 246–249. 10.1093/toxsci/kfy232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittassek M, Wiesmüller GA, Koch HM, Eckard R, Dobler L, Müller J, Angerer J, Schlüter C, 2007. Internal phthalate exposure over the last two decades--a retrospective human biomonitoring study. Int J Hyg Environ Health 210, 319–333. 10.1016/j.ijheh.2007.01.037 [DOI] [PubMed] [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, Wetmur J, Calafat AM, 2008. Prenatal Phenol and Phthalate Exposures and Birth Outcomes. Environ Health Perspect 116, 1092–1097. 10.1289/ehp.11007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Harris SA, Jantunen LM, Kvasnicka J, Nguyen LV, Diamond ML, 2020. Phthalates: Relationships between Air, Dust, Electronic Devices, and Hands with Implications for Exposure. Environ. Sci. Technol 54, 8186–8197. 10.1021/acs.est.0c00229 [DOI] [PubMed] [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ, 2014. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ. Health Perspect 122, 235–241. 10.1289/ehp.1306681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Phillips CA, Mitro SD, 2016. Recent Fast Food Consumption and Bisphenol A and Phthalates Exposures among the U.S. Population in NHANES, 2003–2010. Environ. Health Perspect 124, 1521–1528. 10.1289/ehp.1510803 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


