Abstract
Objective
The objective of the current investigation was to examine associations between symptomatic COVID-19 history, neurocognitive function, and psychiatric symptoms using cognitive task performance, functional brain imaging, and a prospective population survey.
Methods
Study 1 was a laboratory study conducted between 3 May 2022 and 16 Nov 2022 involving 120 fully vaccinated community dwelling adults between 18 and 84 years of age (Mage = 31.96 (SD = 20.71), 63.3% female). In this cross-sectional study we examined the association between symptomatic COVID-19 infection history and performance on three computer tasks assessing cognitive function (Flanker interference, delay discounting and simple reaction time) and measured oxygen saturation within the prefrontal cortex using functional near infrared spectroscopy (fNIRS). Study 2 was a 2-wave population survey undertaken between 28 September 2021 and 21 March 2022, examining the prospective relationship between symptomatic COVID-19 and self-reported symptoms of cognitive dysfunction, depressive symptoms, anxiety symptoms, and agitation at 6-month follow up. The sample (N = 2,002, Mage = 37.0, SD = 10.4; 60.8% female) was collected using a quota process to ensure equal numbers of vaccinated and unvaccinated individuals. Structural equation modelling with latent variables was performed on the population-level data, evaluating the fit of the proposed mediational model of symptomatic COVID-19 to psychiatric symptoms through cognitive dysfunction.
Results
Findings from Study 1 revealed significant effects of symptomatic COVID-19 history on Flanker interference and delay discounting. Effects on flanker performance were significantly stronger among older adult women (effect: 9.603, SE = 4.452, t = 2.157, p = .033), and were accompanied by task-related changes cerebral oxygenation at the right superior frontal gyrus (F (1, 143.1) = 4.729, p = .031). Additionally, those with a symptomatic COVID-19 infection history showed evidence of amplified delay discounting (coefficient = 0.4554, SE = 0.2208, t = 2.0629, p = .041). In Study 2, baseline symptomatic COVID-19 history was associated with self-reported cognitive dysfunction and a latent variable reflecting psychiatric symptoms of anxiety, depression and agitation at follow-up. Mediational analyses revealed evidence of cognitive mediation of clinically significant psychiatric outcomes: depression (indirect effect = 0.077, SE = 0.026, p = .003) and generalized anxiety (indirect effect = 0.060, SE = 0.021, p = .004).
Conclusions
Converging findings from laboratory and population survey data support the conclusion that symptomatic COVID-19 infection is associated with task-related, functional imaging and self-reported indices of cognitive dysfunction as well as psychiatric symptoms. In some cases, these findings appear to be more amplified among women than men, and among older women than younger.
Highlights
-
•
Examined associations among COVID-19, cognitive function, and psychiatric symptoms.
-
•
Symptomatic COVID-19 was associated with several indicators of cognitive dysfunction.
-
•
Symptomatic COVID-19 was also associated with greater levels of psychiatric symptoms.
-
•
COVID-19 may influence cognitive and psychiatric symptoms in a mediated fashion.
1. Introduction
Like other viruses (de Araújo et al., 2016; Berger and Houff, 2008; Goenka et al., 2014; Kim et al., 2017; Levine et al., 2023), SARS-CoV-2 has the potential to adversely impact the human brain at molecular, cellular, and network levels (Mavrikaki et al., 2022; Nauen et al., 2021; Solomon, 2021). Indeed studies have shown that symptomatic COVID-19 is associated with poorer performance on cognitive tasks (Becker et al., 2021; Hampshire et al., 2021; Jaywant et al., 2021), more self-reported symptoms of cognitive dysfunction (Hall et al., 2022b), and prospective changes in structural brain parameters (Douaud et al., 2022). Moreover, older adults experiencing severe infection may be at increased risk for accelerated cognitive decline and new onset dementia (Liu et al., 2022). The phenomenon of post COVID-19 syndrome (PCS) affects approximately 1 in 3 who are infected, and cognitive symptoms are a prominent part of the PCS profile (Ceban et al., 2022; Hanson et al., 2022).
Several plausible mechanisms linking COVID-19 to brain injury have been identified including vascular damage, thrombosis, megakaryocyte invasion and cytokine storm (Boldrini et al., 2021; Nauen et al., 2021; Solomon, 2021). In parallel, several studies have examined the psychiatric sequelae associated with COVID-19 (for a review, see Zawilska and Kuczyńska, 2022). Early large-scale investigations based on hospitalized COVID-19 patients found that severe COVID-19 was associated with a variety of new onset symptoms, including clinically significant psychiatric disorders involving agitation, anxiety and depressive symptoms (Huang et al., 2021; Taquet et al., 2021); population-based estimates of increases in psychiatric symptomology mirror these findings (Hall et al., 2021). However, it remains unclear whether the brain mediates the impact of COVID-19 on the emergence of psychiatric symptoms, and although the findings appear to implicate decision-making and executive control networks, the specific cognitive processes involved as mediators remain unclear. Likewise, among the available neuroimaging modalities, only functional near infrared spectroscopy quantifies neural activation based on oxygen saturation, and no studies of fNIRS and COVID-19 have been published to date, with the sole exception of a small pilot study (Ho et al., 2021).
In this investigation, we examine the neuropsychiatric aspects of symptomatic COVID-19 in a laboratory study using cognitive tasks reflecting decision making, executive function and information processing, and employing fNIRS imaging to quantify task-related functional activation. Given that the occurrence of cognitive dysfunction (“brain fog”) is more likely if respiratory systems are impacted at the onset of infection (Asadi-Pooya et al., 2022) and brain hypoxia is part of the proposed mediational mechanism, brain imaging modalities that quantify regional oxygen saturation as part of the functional activation signal are of particular interest. Functional near-infrared spectroscopy is one such technique (Ayaz et al., 2022; Ferrari and Quaresima, 2012), and is well-suited to study the brain health impacts of COVID-19. In Study 2, we use population level data and latent variable modelling to explore whether cognitive dysfunction mediates the pathway connecting symptomatic COVID-19 to psychiatric outcomes. The prospective nature of the survey data allowed us to examine processes unfolding over time, a critical facet of unpacking mediational mechanisms.
With respect to the latter, the proposed cognitive mediational pathway linking severe COVID-19 to psychiatric symptomology is not without alternative possibilities. For example, perceived life threat and stress introduced by severe COVID-19 symptoms could cause new onset psychiatric symptoms (Pfeifer et al., 2021), with or without direct impact on cognitive function. Likewise, among hospitalized patients, intubation could impact performance on cognitive tests without a downstream impact on psychiatric symptoms. A formal mediational analysis of prospective data is required in order to examine mechanisms in a compelling manner, ideally in a sample that includes cases of symptomatic COVID-19 with and without hospitalization history. Accordingly, the current investigation 1) probed the cross-sectional relationship between symptomatic COVID-19 history and cognitive performance in the laboratory using computerized cognitive testing paired with functional brain imaging (fNIRS; Study 1), and 2) examined the prospective relationship between COVID-19 severity and psychiatric symptoms, testing for statistical evidence of mediation through indicators of cognitive dysfunction at the population level (Study 2). Given that prior studies have identified female sex and age group as risk factors for PCS (Asadi-Pooya et al., 2022; Evans et al., 2021; Hanson et al., 2022; Sigfrid et al., 2021; Torjesen, 2021; Yong, 2021), we examined these factors as moderators of effects where feasible to do so.
2. Study 1
2.1. Participants and procedure
In Study 1, a sample of 120 fully vaccinated, community dwelling adults (63.3%, n = 76 female) were recruited from a university campus and surrounding community. Age ranged from 18 to 84, with a mean age of 31.96 (SD = 20.71) years. In terms of education, 28.3% had a high school degree, 30% had technical/trade school or some university education, 25.8% had a university degree, and 15% had a post-graduate degree. Of the full sample, 52 (43.3%) reported having a positive SARS-CoV-2 infection history, the majority of whom (71.15%) reported having experienced an infection between 1 and 6 months prior. Consistent with university policy, all participants had received 2 doses of mRNA or Astra Zeneca at the time of participation; these criteria were considered ”up-to-date” in terms of vaccination status. Participants were recruited using advertisements around campus and via drawing from an older adult participant pool, in order to ensure a large age range represented within the sample. The protocol involved a 60-min laboratory session, which included computer-based cognitive tasks presented using the Inquisit software package measuring executive function (Flanker task), decision making (Delay discounting task), and simple processing speed (a simple reaction time measure was completed on two occasions and averaged together). Task-related brain activations within the medial and lateral prefrontal cortex during the Flanker were recorded using a mobile fNIRS brain imaging system, as described in detail below.
2.2. Measures
COVID-19 history. COVID-19 history was assessed using a sequence of questions starting with infection history and proceeding to severity assessment for those who reported a positive infection history. The first question asked, “What best describes YOUR experience with [SARS-CoV-2] infection?”; each participant gave a response where 1 = “I have NOT been infected”, 2 = “I have been infected,” and 3 = “not stated”. Those who indicated a positive infection history were asked, “How do you know that you HAVE BEEN infected with [SARS-CoV-2]?” and provided responses where 1 = “had symptoms but did not get tested,” 2 = “had symptoms and tested positive”, and 3 = “had no symptoms but tested positive”. Finally, these same participants were asked, “How severe was your [SARS-CoV-2] illness?”; responses were given on a 5-point response scale where 1 = “not at all severe” (asymptomatic), 2 = “slightly severe”, 3 = “moderately severe”, 4 = “very severe”, 5 = “extremely severe”. This variable was recoded such that 0 = negative history of COVID-19 (n = 68; 56.7%), 1 = positive history of asymptomatic COVID-19 (n = 10; 8.3%), and 2 = positive history of symptomatic COVID-19 (n = 42; 35%).
Flanker task. The Flanker task is a measure of the behavioral inhibition facet of executive function (Eriksen and Eriksen, 1974). In each trial, an array of 7 letters appeared in the center of the computer screen, with participants responding to the center letter (target stimulus) in a series of flanked letters (non-target stimuli) in blocks of congruent noise (i.e., HHHHHHH) or incongruent noise (i.e., CCCHCCC; derived from Lowe et al., 2018). The task consisted of 5 blocks of 50 trials, including 1 practice block; the order of blocks was fixed starting with congruent and alternating with incongruent. For each trial, the stimulus was presented for 500 ms and responses were given using a keyboard key, with both accuracy and reaction time recorded automatically. Flanker interference scores were calculated as reaction times on correct trials, with shorter reaction times representing better performance. Interference scores were calculated based on the difference in performance between the average of congruent and incongruent trials. Flanker performance values corresponding with a below chance level of accuracy were removed (both the accuracy indicator and corresponding reaction time value) given that this suggested lack of observance or understanding of the task instructions (n = 4). Accuracy corrected reaction times were converted to interference scores (incongruent-congruent) with higher values indicating more interference, or relatively “weaker” performance on the task. No transformations were necessary given that skewness statistics and kurtosis were both within acceptable limits on the interference variable.
Delay discounting task. Delay discounting (DD) is a decision-making task that examines the willingness of individuals to make short- versus far-sighed decisions that are personally consequential; performance on DD tasks is linked to value processing and the operation of the orbitofrontal cortex (OFC). Participants completed a validated 5-item delay discounting task where they identified their preferred monetary option between a fixed amount ($500) and a larger amount ($1000) at varying temporal dispersion (sooner vs. later; i.e., “Would you rather have $500 now, or $1000 in 4 h; 1 day; 3 weeks; 2 years?”) (Koffarnus and Bickel, 2014). An indifference point, denoted by k, was calculated for each choice, with higher values indicating a preference for a smaller immediate reward in lieu of a larger later reward. The k values were averaged across the four trials yielding an average k value for each participant, with higher k values representing steeper discounting of future rewards. DD k scores were significantly skewed and subjected to a log10 transformation to improve normality.
Simple reaction time. As a measure of information processing speed, participants performed a visual reaction time task, which began with a central fixation cross (+), which was followed by a target visual stimulus (a red circle) presented at variable time intervals between 2 and 8 s. Participants were asked to respond as quickly as possible to the presentation of the target stimulus via keyboard press. Mean latency was recorded from the onset of the stimulus to the time of response. The task was repeated twice, once before the above two tasks, and once following, with 20 trials on each occasion. The overall reaction time score was the average value of the two administrations. The average reaction time metric was significantly skewed, and therefore was subjected to a log10 transformation to improve normality.
Psychiatric Symptoms. Psychiatric symptoms were assessed using the Centers for Epidemiological Studies Depression 10-item scale (CESD-10; Andresen et al., 1994), the Generalized Anxiety Disorder Scale 7-item measure (GAD-7; Spitzer et al., 2006)), and a custom developed, 3-item agitation symptom measure assessed using the following items: “I felt agitated”, “I lashed out at other people in a way that was not like me”, and “I was much more irritable than usual”. Responses were provided to each stem on a 4-point scale, where 1 = “Rarely or none of the time (Less than 1 day)”, 2 = “Some or a little of the time (1–2 days)”, 3 = “Occasionally or a moderate amount of time (3–4 days)”, and 4 = “All of the time (5–7 days)”. Internal consistency reliabilities for the CESD-10, GAD-7 and agitation items were all acceptable (Cronbach's alpha = .802, .864 and 0.704, respectively). These measures were significantly intercorrelated with each other (all p's < 0.001; r's > 0.500), and visual inspection of a scree plot clearly indicated the underlying presence of a single factor representing 73.32% of the variance, with factor loadings of .860 for CESD-10, 0.878 for GAD-7, and 0.830 for agitation items. As such, for the sake of parsimony, scores on each of these psychiatric symptoms measures were subjected to z-score transformations and then averaged together to yield a psychiatric symptoms score index, with higher scores indicating higher levels of psychiatric symptoms.
Demographic Moderators and Covariates. Age, sex and ethnicity were self-reported using an online survey completed prior to arrival in the laboratory. Age was recoded such that 1 = young adult (18–24 years; n = 76), and 2 = older (25 or older; n = 44) given that early young adulthood appears to be the threshold past which sex differences in PCS are most evident (Hanson et al., 2022). Sex was treated as a binomial variable based on assigned sex at birth (male: n = 44; female: n = 76). Ethnicity was recoded such that 1 indicated white (n = 41) and 2 indicated non-white (n = 79). Age and sex were used as moderators, while ethnicity was used as a covariate, given the relatively low n in each of the 8 individual non-white categories, particularly in Study 1. Efforts were made to equate treatment of all moderators and covariates across Study 1 and Study 2.
Time since infection. Time since infection was assessed using the following item, “When did your MOST RECENT COVID-19 infection begin?” Responses were coded in 1-month increments, with 1 as the lowest value (shortest time since infection) and 6 as the highest value (longest time since infection). Those who had not been infected were coded as 7.
2.3. fNIRS signal acquisition and analysis
Cognitive task performance. Main effects and multiple moderation effects were explored using the PROCESS macro for SPSS, using non-white ethnicity and time since infection as covariates, age group and sex as moderators, and positive COVID-19 infection status as the focal predictor. Tests of the highest order interaction involving age and sex (or both) were described in each case.
Functional Near-infrared Spectroscopy (fNIRS). fNIRS is a noninvasive brain-monitoring technology that relies on optical techniques to detect changes of cortical hemodynamic responses to human perceptual, cognitive, and motor functioning with diverse field and clinical applications (Ayaz et al., 2022). For this study, a continuous-wave wearable fNIRS system Model 203c (fNIR Devices, LLC, Potomac, MD, USA) was used to record prefrontal hemodynamics. The positioning of the light emitting diode (LED) light sources and photo detectors within an ultra-thin flat sensor pad yielded a total of 16 optodes (measurement areas) with 10Hz sampling; this montage was designed to monitor dorsal and anterior frontal cortical areas underlying the forehead (Ayaz et al., 2012). Optodes in the sensor band are positioned in a rectangular grid 2 × 8 format. The bottom row of sensors with 8 optodes, closest to the eyes were saturated due to eye-tracker near-infrared light interference (see methods description in Hall et al., 2022a); as a result, only the top row (8 optodes) were used for the present analysis. Anatomical landmarks were used for sensor placement as described in (Ayaz et al., 2011). COBI Studio software was used for data acquisition and visualization (Ayaz et al., 2011). Light intensity at two near-infrared wavelengths of 730 and 850 nm was recorded. All data was filtered and processed offline after recording. Data was passed through a finite impulse response hamming filter of order 100 and cutoff frequency 0.1 Hz. Data of each participant were checked for any potential saturation (when light intensity at the detector was higher than the analog-to-digital converter limit) and motion artifact contamination by means of a coefficient of variation based statistical filter known as sliding window motion artifact rejection (SMAR) (Ayaz et al., 2010). Time synchronized blocks were processed with the Modified Beer-Lambert Law to calculate oxygenation-hemoglobin (Hbo) concentrations for each optode with local baseline and average oxygenated-hemoglobin is extracted for each block of task condition. Binning was used in order to quantify increases from local baseline (2s) to task-related activation epoch (8s). For statistical analysis involving fNIRS data, linear mixed models with repeated measures were used in NCSS Software version 21.0.5 (NCSS, LLC, Kaysville, Utah, USA). Subject was included as a random factor. Between and within fixed factors for the model were task condition (congruent/incongruent) and COVID-19 infection history (0 = negative history, 1 = positive history) as well as Sex and Age as covariates.
3. Results
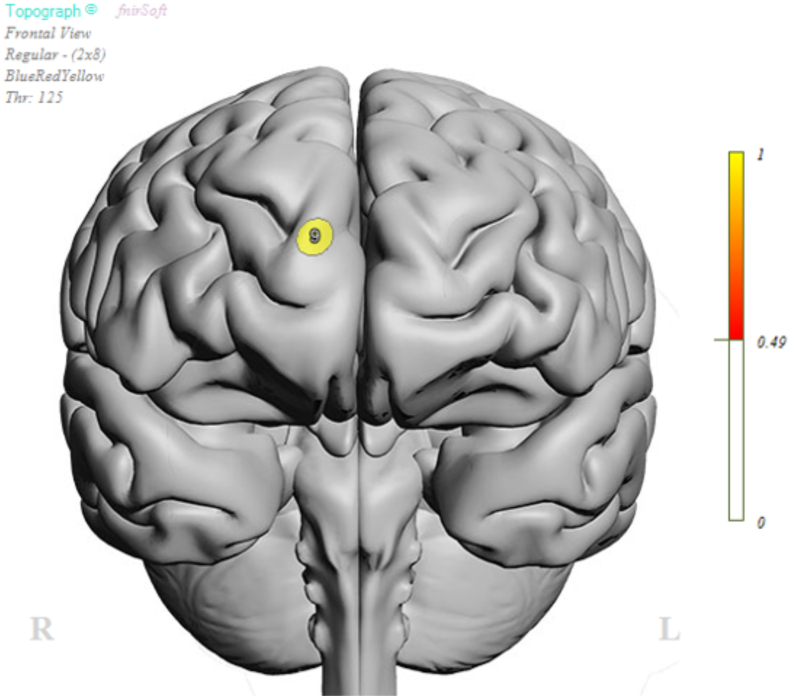
A significant main effect of symptomatic COVID-19 infection was evident in relation to Flanker interference scores (coefficient: −22.862, SE = 10.096, t = 2.265, p = .026). This main effect was qualified by a significant joint moderation effect of age and sex (ΔR2 = 0.057, F(1,108) = 3.416, p = .036), such that symptomatic COVID-19 infection history was associated with significantly greater Flanker interference scores among relatively older adult females (effect: 9.603, SE = 4.452, t = 2.157, p = .033). Functional imaging findings revealed a significant interaction between Flanker condition (Congruent/Incongruent) and COVID-19 infection history at the right superior frontal gyrus (Optode 9; F (1, 143.1) = 4.729, p = .031; Fig. 1; Table 1). There was also a significant interaction of sex and symptomatic COVID-19 history at the same channel, indicating a significantly stronger Hbo effect for females than for males (F1,143.1 = 6.352, p = .013). Age did not yield a significant interaction with Flanker condition, symptomatic COVID-19 history or sex.
Fig. 1.
Oxygenated hemoglobin (Hbo) concentration location of Optode 9.
Table 1.
Means and SE by symptomatic infection history and sex for Hbo concentration at Optode 9.
| Mean | SE | 95% CI (lower) | 95% CI (upper) | |
|---|---|---|---|---|
| COVID-19 history * Flanker Condition | ||||
| Negative, Congruent | 0.1897 | 0.1310 | −0.0706 | 0.4500 |
| Negative, Incongruent | 0.2576 | 0.1291 | 0.0011 | 0.5140 |
| Positive, Congruent | 0.3428 | 0.1445 | 0.0538 | 0.6317 |
| Positive, Incongruent | −0.0613 | 0.1417 | −0.3447 | 0.2221 |
| COVID-19 history * Sex | ||||
| Negative, Male | 0.5012 | 0.1753 | 0.1485 | 0.8539 |
| Negative, Female | −0.0539 | 0.1224 | −0.2997 | 0.1919 |
| Positive, Male | 0.2189 | 0.1826 | −0.1509 | 0.5886 |
| Positive, Female | 0.0626 | 0.1528 | −0.2473 | 0.3726 |
Analyses also revealed a significant main effect of symptomatic COVID-19 history on log10 DD task performance (k value), such that those with a positive symptomatic COVID-19 history evidenced significantly greater delay discounting than their non-infected counterparts (coefficient = 0.455, SE = 0.221, t = 2.063, p = .041). This main effect was subject to joint moderation effects of age and sex (ΔR2 = 0.050, F (2,112) = 3.143, p = .047), such that symptomatic COVID-19 history predicted significantly lower delay discounting effects among older adult females as compared to younger females and all males (effect: 0.209, SE = 0.096, t = −2.168, p = .032).
Finally, there were no significant main effects of symptomatic COVID-19 history on simple reaction time (coefficient: 0.019, SE = 0.041, t = 0.454, p = .651), and no moderation effects involving age (ΔR2 = 0.003, F(1,112) = 0.327, p = .569), sex (ΔR2 = 0.0173, F(1,112) = 2.140, p = .146), or their joint combination (ΔR2 = 0.022, F(2,112) = 1.330, p = .269). Likewise, no significant main effects of symptomatic COVID-19 history were evident on the psychiatric symptoms index (coefficient = 0.0135, SE = 0.058, t = 0.233, p = .816), and no moderation effects involving age (ΔR2 = 0.008, F(1,112) = 953, p = .331), sex (ΔR2 = 0.003, F(1,112) = 0.361, p = .549), or their joint combination (ΔR2 = 0.012, F(2,112) = 0.723, p = .488) were evident.
4. Discussion
Study 1 findings revealed a significant association between symptomatic COVID-19 and several indices of cognitive dysfunction among fully vaccinated adults. These included increased Flanker interference and reduced oxygenated hemoglobin within the right superior frontal gyrus, an effect that appeared to be more prominent in women than men, and manifested more in high demand cognitive processing (i.e., incongruent Flanker trials) more so than low demand processing. These findings, in particular the sex moderation effects, are consistent with several other investigations showing that women may be more susceptible to the cognitive effects of PCS (Asadi-Pooya et al., 2022; Evans et al., 2021; Hanson et al., 2022; Sigfrid et al., 2021; Torjesen, 2021; Yong, 2021).
The superior frontal gyrus has been shown to be responsive to Flanker task performance previously and is impacted by neurobehavioral conditions like attention deficit disorder (Melara et al., 2018; Suzuki et al., 2017, 2018; Kawai et al., 2012). Our results further support the idea that COVID-19 infection impacted higher executive functioning at a neurobehavioral level. Furthermore, results indicate additional interactions of task condition with COVID infection status and sex. Although the influence of sex on executive function are still underexplored, there is emerging evidence from human functional neuroimaging studies, showing sex differences in executive functions and that males and females engage different strategies to engage with task demands (Gaillard et al., 2021 for a review). Our results confirm that COVID-19 infection influences on inhibition also differ in males and females as they engage different strategies for the task. Further research is needed to explore the function of neural mechanisms in inhibition and other cognitive domains.
Positive symptomatic COVID-19 history was also associated with amplified delay discounting. This finding is consistent with a prior analyses of baseline survey data from the CCES population survey, wherein infection history and severity was cross sectionally associated with amplified DD in a dose-response manner (Hall et al., 2022b). These findings are meaningful to the extent that DD task performance is linked to the orbitofrontal cortex, a hypothesized primary site for SARS-CoV-2 neuroinvasion and/or localized neuroinflammation.
It is notable that the above associations were evident even in a fully vaccinated sample of male and female adults (2 doses of mRNA vaccine), during the first 3 waves of the pandemic. Although it is unclear how strongly more recent variants of SARS-CoV-2 impact the brain, it appears that for the Omicron and Delta variants, 2 mRNA dose vaccinations may not have been sufficient to offset cognitive impacts of infection completely. The use of a cross sectional dataset limits our inferential abilities, however, given that there is evidence that cognitive abilities may influence susceptibility to infection, vis-à-vis mitigation behavior performance (Hudson et al., 2022). For this reason, only a prospective study can more conclusively examine the potential for symptomatic COVID-19 to impact the brain.
Null effects were observed on a simple reaction time task, and also did not appear to extend to an index of psychiatric symptoms in this sample. The null effects of symptomatic COVID-19 history on the latter were not entirely unexpected given the fully vaccinated status of all of the participants. Study 2 provides an opportunity to test the effect of COVID-19 infection history on psychiatric symptoms in a larger sample, and one that includes an equal proportion of vaccinated and unvaccinated individuals. If it is the case that full vaccination blunts some of the effects of COVID-19 infection on psychiatric symptoms, there should be evidence of moderator effects of vaccination status in Study 2.
5. Study 2
5.1. Methods
Study 2 was a prospective population survey of 2002 adults followed over a 6-month period. Infection status was measured at baseline and follow-up.
5.2. Participants
Study 2 participants were respondents in Waves 1 and 2 of the Canadian COVID-19 Experiences Survey (CCES), part of the broader Canadian COVID-19 Experiences Project (Hall et al., 2022a). Wave 1 of the survey took place from September 28 to October 21, 2021; Wave 2 took place 6 months later, from March 3 to March 21, 2022. The sample was a representative national sample of Canadians consisting of 2002 participants aged between 18 and 56 years (Mage = 37.0, SD = 10.4; 60.8% female; Table 2); after removing those with invalid response patterns, a final sample of 1958 remained in Wave 1. Stratified sampling was undertaken to ensure a balance of vaccinated and non-vaccinated individuals at a 1:1 ratio. At Wave 1, 50.2% of participants received two vaccine doses (i.e., up-to-date or “fully vaccinated”), 43.3% had received no doses (“vaccine hesitant”) and 6.5% received one vaccine dose. A total of 182 (9.54%) of participants at Wave 1 reported having a positive SARS-CoV-2 infection history. At Wave 2, 1145 participants from Wave 1 were successfully recontacted and 674 new respondents were replenished. A total of 465 of participants at Wave 2 reported having a prior infection. Among the latter, the average time since infection was 114 days; the value was 110 days for those with an asymptomatic or minimally symptomatic infection, 128 days for those with a slightly severe infection, and 149 days for a moderate or higher symptom severity. The majority of those reporting a positive infection history (87.1%) indicated that it was their only SARS-CoV-2 infection. Changes in severe infection prevalence from Wave 1 to Wave 2 tracked emergence of the Omicron variant. In Wave 2, 75.91% of infections occurred between February 1, 2022 and November 30, 2022.
Table 2.
Study 2 sample characteristics.
| Variable | N | Percentage/Mean (SD) |
|---|---|---|
| Gender | ||
| Male | 310 | 39.79 |
| Female | 469 | 60.21 |
| Age group | ||
| 18-24 | 106 | 13.61 |
| 25-39 | 285 | 36.59 |
| 40-54 | 388 | 49.81 |
| Income | ||
| Low | 120 | 15.4 |
| Moderate | 173 | 22.21 |
| High | 410 | 52.63 |
| No answer | 76 | 9.76 |
| Education | ||
| Low | 167 | 21.44 |
| Moderate | 287 | 36.84 |
| High | 316 | 40.56 |
| No answer | 9 | 1.16 |
| Ethnicity | ||
| White | 563 | 72.27 |
| Non-white | 196 | 25.16 |
| not stated | 20 | 2.57 |
| Region | ||
| Alberta | 72 | 9.24 |
| BC | 91 | 11.68 |
| MB + SK | 44 | 5.65 |
| Maritimes | 51 | 6.55 |
| Ontario | 318 | 40.82 |
| QC-En | 57 | 7.32 |
| QC-Fr | 146 | 18.74 |
| Severity | ||
| Not infected | 708 | 95.68 |
| Infected: Not at all severe | 10 | 1.35 |
| Infected: Slightly severe | 10 | 1.35 |
| Infected: Moderately/Very/Extremely severe | 12 | 1.62 |
| Vaccination status | ||
| Hesitant | 242 | 31.07 |
| Non-hesitant (fully vaccinated/single dose) | 537 | 68.93 |
| Executive Function | 791 | 1.60 (0.62) |
| Attention | 787 | 1.57 (0.69) |
| Anxiety | 792 | 1.75 (0.84) |
| Agitation | 787 | 1.57 (0.69) |
| Depression | 793 | 1.93 (0.65) |
Note: Data are wave 1 respondents recontacted at wave 2 and did not get infected between waves.
5.3. Procedure
All measures were completed online using the Leger Opinion panel, a high-quality national web panel of Canadian adults; responses were subsequently weighted in order to achieve population representativeness. To this end, respondents were first divided into two groups: fully vaccinated and vaccine-hesitant. Within each group, respondents were further subdivided into 8 gender × age subgroups and 7 geographic region/language subgroups; for a grand total of 30 subgroups. Population totals from the 2016 Census were then combined to the CCEP disposition codes to obtain benchmark/calibration figures (e.g., estimated number of fully vaccinated 18–25 years old residing in the province of Ontario) for each subgroup. Separately for each of the 2 groups, a raking procedure was then applied to calibrate the weights based on gender × age and geographic region/language. Further details can be found in Boudreau et al. (2023).
All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. This study received ethical clearance from the institutional ethics review board at the University of Waterloo.
5.4. Measures
Symptomatic COVID-19 history. Infection history and COVID-19 symptom severity were assessed using the following question: “What best describes YOUR experience with [SARS-CoV-2] infection?”; each participant gave a response where 1 = “I have NOT been infected”, 2 = “I have been infected,” and 3 = “not stated”. Those who indicated a positive infection history were asked, “How do you know that you HAVE BEEN infected with [SARS-CoV-2]?” and provided responses where 1 = “had symptoms but did not get tested,” 2 = “had symptoms and tested positive”, and 3 = “had no symptoms but tested positive”. Finally, these same participants were asked, “How severe was your [SARS-CoV-2] illness?”; responses were given on a 5-point response scale where 1 = “not at all severe,” 2 = “slightly severe”, 3 = “moderately severe”, 4 = “very severe”, 5 = “extremely severe”. Responses to these three questions were combined to form a COVID-19 infection index where 0 = “not infected”, 1 = “asymptomatic infection” and 2 = “symptomatic infection”.
Anxiety symptoms. Respondents were evaluated using the Generalized Anxiety Disorder 7-Item Scale (GAD-7; Spitzer et al., 2006). Seven symptoms are included in the scale, including: “Feeling nervous, anxious, or on the edge”, “Not being able to stop or control worrying”, “Worrying too much about different things”, “Trouble relaxing”, “Being so restless that it’s hard to stand still”, “Becoming easily annoyed or irritable”, and “Feeling afraid as if something awful might happen”. Respondents evaluated their experiences with these symptoms at two separate time periods: in the two weeks that preceded the time of survey completion; and, for those who had experienced infection with COVID-19, the two weeks that followed their infection. Respondents reported the frequency of these feelings using the 4-point scale: “Not at all”, “Several days”, “More than half the days”, and “Nearly every day”. Cronbach's alphas indicated strong internal consistency reliability (α = 0.947).
Depressive symptoms. Symptoms of depression were evaluated using the Center for Epidemiological Studies Depression Scale (CESD-10) (Andresen et al., 1994). The following 10 items were used: “In the past week … I was bothered by things that usually don't bother me”, “I had trouble keeping my mind on what I was doing”, “I felt depressed”, “I felt everything I did was an effort”, “I felt hopeful about the future”, “I felt fearful”, “My sleep was restless”, “I was happy” (reverse scored), “I was lonely”, “I could not ‘get going’”. Participants indicated symptom frequency using a 4-point scale: 1 = “Rarely or none of the time (Less than 1 day)”, 2 = “Some or a little of the time (1–2 days)”, 3 = “Occasionally or a moderate amount of time (3–4 days)”, and 4 = “All of the time (5–7 days)”. Cronbach's alpha indicated strong internal consistency reliability (α = 0.923).
Agitation symptoms. The same set of 3 agitation items from Study 1 were used to assess agitation symptoms. Cronbach's alpha indicated good internal consistency reliability (α = 0.895).
CognitiveDysfunction. Self-reported symptoms of executive dysfunction were measured using an abbreviated version of the BDEFS (Barkley, 2011), and three custom developed items assessing attentional lapses, both described in detail elsewhere (Hall et al., 2022a). The latter attention lapse items were as follows: “How often do you have dizzy spells not experienced before”, “how often do you have more trouble concentrating than usual”, and “how often do you think slower than usual”. Responses to all items were provided using a 1 to 4 response scale where, 1="Never or rarely", 2="Sometimes", 3="Often", 4="Very often." Reliability indices for BDEFS and attention lapse scale were moderate (α = 0.796) and strong (α = 0.873) respectively.
5.5. Statistical analysis
Study 2 employed the online Canadian COVID-19 Experiences Survey (N = 2002 at inception) data from Wave 1 adult respondents aged 18 to 54 who were recontacted at Wave 2 and did not get infected between waves (n = 800); the prospective mediation analysis examined the exposure of COVID-19 severity at Wave 1 on the latent outcome of psychiatric symptoms at Wave 2, mediated by Wave 2 indicators of cognitive dysfunction. The model was further tested cross-sectionally using Wave 2 respondents (n = 1783). Additional analyses that replace the latent outcome of psychiatric symptoms with the clinical cutoff scores for diagnosable depression and generalized anxiety respectively in the above model were conducted, both prospectively and cross-sectionally using the same datasets as above. All models controlled for vaccination status, sex, age, ethnicity, and geographic regions. These hypothesized mediation models were tested through path analysis using structural equation modelling, implemented using MPlus 8.4 statistical software.
To model the complex survey data, parameters were estimated by maximizing a weighted loglikelihood function, taking into account the stratification built into the sampling design. Model fit was estimated for continuous outcomes through standard indices, including Root Mean Square Error of Approximation (RMSEA), Standardized Root Mean Square Residual (SRMR), Comparative Fit Index (CFI), and Tucker-Lewis Index (TLI). Indirect effects of the proposed paths, which represent the mediational effect, were estimated through the above path models. Statistical significances were computed at the 95% confidence level.
6. Results
Symptoms of anxiety were relatively common, with 28% of respondents meeting the GAD-7 clinical cutoff threshold at baseline, and 17% meeting the threshold at follow-up. Similarly, 41% met the CESD-10 clinical cutoff threshold at baseline. Symptoms of executive dysfunction (Mean = 6.35, SD = 2.41), agitation (Mean = 4.83, SD = 2.15), and attentional lapses (Mean = 4.69, SD = 2.05) were moderate. Study variable intercorrelations are presented in Table 3.
Table 3.
Correlation matrix for Study 2 variables.
| EF | ATT | ANX | AGIT | DEP | Vaccine | Asymptomatic | Symptomatic | Female | Age 18-24 | Age 25-39 | Eth-NW | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EF | 1 | |||||||||||
| ATT | 0.524 (0.026) | 1 | ||||||||||
| ANX | 0.402 (0.030) | 0.597 (0.023) | 1 | |||||||||
| AGIT | 0.423 (0.029) | 0.525 (0.026) | 0.739 (0.016) | 1 | ||||||||
| DEP | 0.387 (0.030) | 0.606 (0.023) | 0.794 (0.013) | 0.689 (0.019) | 1 | |||||||
| Vaccine | 0.078 (0.035) | 0.127 (0.035) | 0.088 (0.035) | 0.070 (0.035) | 0.086 (0.035) | 1 | ||||||
| Asymptomatic | −0.005 (0.035) | −0.064 (0.036) | −0.009 (0.035) | 0.010 (0.035) | −0.002 (0.035) | −0.022 (0.035) | 1 | |||||
| Symptomatic | 0.060 (0.035) | 0.019 (0.035) | 0.032 (0.035) | −0.009 (0.035) | −0.018 (0.035) | −0.046 (0.035) | −0.019 (0.035) | 1 | ||||
| Female | −0.023 (0.035) | 0.086 (0.035) | 0.125 (0.035) | 0.050 (0.035) | 0.117 (0.035) | −0.060 (0.035) | 0.023 (0.035) | −0.058 (0.035) | 1 | |||
| Age 18–24 | 0.007 (0.036) | 0.122 (0.035) | 0.085 (0.035) | 0.040 (0.036) | 0.147 (0.035) | 0.127 (0.035) | 0.056 (0.035) | −0.045 (0.035) | 0.139 (0.035) | 1 | ||
| Age 25–39 | 0.025 (0.035) | −0.007 (0.036) | −0.009 (0.035) | 0.029 (0.035) | −0.029 (0.035) | −0.041 (0.035) | 0.057 (0.035) | −0.003 (0.035) | 0.060 (0.035) | −0.291 (0.032) | 1 | |
| Eth-NW | 0.085 (0.035) | 0.040 (0.035) | −0.049 (0.035) | 0.001 (0.035) | −0.047 (0.035) | 0.050 (0.035) | 0.039 (0.035) | −0.047 (0.035) | 0.046 (0.035) | 0.159 (0.034) | 0.073 (0.035) | 1 |
| Eth-A | −0.017 (0.036) | 0.056 (0.036) | 0.074 (0.036) | 0.047 (0.036) | 0.087 (0.036) | 0.021 (0.035) | −0.018 (0.035) | −0.028 (0.035) | −0.049 (0.035) | 0.032 (0.035) | −0.052 (0.035) | −0.092 (0.035) |
Note: Data are wave 1 respondents recontacted at wave 2 who did not get infected between waves.
Abbreviations: EF = Executive function, ANX = Anxiety (GAD-7), AGIT = Agitation, DEP = Depression (CESD-10), Vaccine = Vaccination status, Eth-NW = Non-white ethnicity, Eth-A = Ethnicity not stated, AB = Alberta, BC = British Columbia, MB = Manitoba, SK = Saskatchewan, ON = Ontario, QC-EN = Quebec (English).
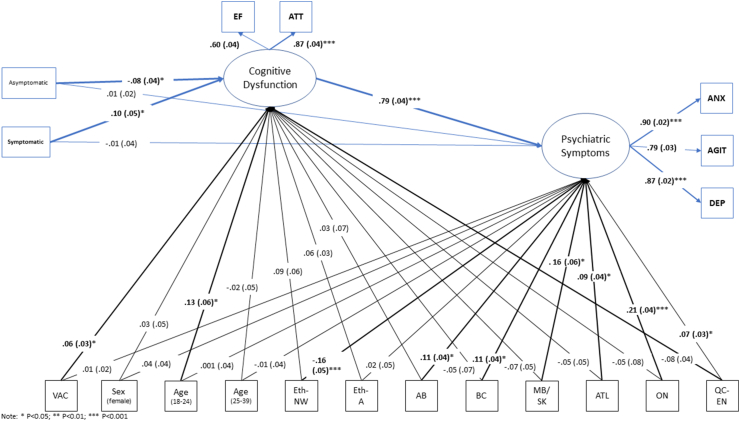
The path models fit well in both prospective (RMSEA = 0.023; SRMR = 0.024; CFI = 0.984; TLI = 0.972) and cross-sectional (RMSEA = 0.025; SRMR = 0.020; CFI = 0.981; TLI = 0.967) analyses. The measurement models indicated reasonable measurement properties, with all estimated factor loadings being positive and statistically significant, and of at least moderate magnitude (Fig. 2). Following adjustment for confounders, there was a significant association between symptomatic COVID-19 infections and BDEFS scores. Specifically, using all variables as continuous, there was a significant indirect effect of symptomatic COVID-19 infection on psychiatric symptoms, mediated through BDEFS scores (prospective indirect effect = 0.434, SE = 0.210, p = .039; cross-sectional indirect effect = 0.214, SE = 0.067, p = .001).
Fig. 2.
Structural equation model testing mediation of COVID-19 effects on psychiatric symptoms through cognitive dysfunction; EF = BDEFS scores; ATT = attentional index scores; ANX = GAD-7 scores; AGIT = agitation scores; DEP = CESD-10 depression scores. Model fit was adequate in both prospective (shown) and cross-sectional versions (prospective: RMSEA = 0.023; SRMR = 0.024; CFI = 0.984; TLI = 0.972; cross-sectional: RMSEA = 0.025; SRMR = 0.020; CFI = 0.981; TLI = 0.967).
A model examining the effects of severe symptoms on clinically significant symptomatology indicated similar findings. Of those with moderate-to-severe COVID-19, 53.25% experienced clinically significant depression compared to 41.54% of those with no infection, 34.89% with asymptomatic infection, and 45% of mild COVID-19. In cross-sectional analyses using clinical cutoff scores for each measure of affect, there were significant mediational effects of symptomatic COVID-19 infections for diagnosable depression (indirect effect = 0.077, SE = 0.026, p = .003) and generalized anxiety (indirect effect = 0.060, SE = 0.021, p = .004) through the latent cognitive dysfunction variable. Using prospective analyses involving both waves of data, the mediational effect was significant for moderate severity COVID-19 symptoms on diagnosable depression scores (indirect effect = 0.207, SE = 0.102, p = .042); a marginal mediational effect was found for diagnosable anxiety scores (indirect effect = 0.130, SE = 0.087, p = .134).
Examination of moderation effects by vaccination status showed that those unvaccinated at Wave 1 (who remained unvaccinated at Wave 2) were marginally more likely to experience psychiatric symptoms at follow-up following symptomatic COVID-19 (est = 0.589, SE = 0.321, p = .066) infection between waves. The same was not true among those unvaccinated individuals who experienced an asymptomatic COVID-19 infection (est. = 0.018, SE = 0.351, p = .958).
7. Discussion
Study 2 examined the prospective relationship between COVID-19 and psychiatric symptoms, as mediated by cognitive function in a large population representative sample, with an even distribution of vaccinated and unvaccinated individuals. Using latent variable modelling to assess psychiatric symptomology and cognitive dysfunction, we observed that cognitive dysfunction was a significant mediator of the relationship between COVID-19 and psychiatric symptoms. These findings augment prior reports of COVID-19 effects on psychiatric function by providing a test of statistical mediation through cognitive dysfunction, and via the use of a three indicator latent variable to represent psychiatric symptoms.
The use of converging evidence from a population survey and a laboratory study involving cognitive testing and functional neuroimaging is a strength of the current study. Convergence of findings from two studies using conceptually related, but different methodologies strengthens our conclusions about the mediational role of cognitive dysfunction, in accordance with the Bradford Hill principle that consistency of findings from studies employing different methodologies strengthens the likelihood of that effect (Cochran and Chambers, 1965; Hill, 2015).
There are several limitations. First, although the findings were consistent with mediation effects involving cognitive dysfunction, these mediational models did not account for all of the effects of COVID-19 severity on psychiatric outcomes. It remains possible, for instance, that mediational pathways through stress and perceived life threat also account for some of the relationship between COVID-19 symptom severity and psychiatric outcomes. Second, the measurement of COVID-19 infection status and COVID-19 symptom severity were both via self-report, which has the potential to inflate associations between this variable and self-reported psychiatric outcomes through common method variance. However when constrained to only those participants who reported a positive PCR test finding, the results of the path model in Study 2 did not differ significantly. Finally, a longer follow-up interval with multiple assessments of outcomes may provide a more complete picture of mediational processes as they play out over time.
8. Conclusion
In conclusion, evidence from a laboratory study and an interlinked population survey support the hypothesis that COVID-19 is associated with both cognitive dysfunction and psychiatric symptoms. Cognitive effects were evident at the level of self-reported symptoms, task performance differences, and task-related cerebral oxygenation levels within the prefrontal cortex. The specific pattern of findings suggests that delay discounting and executive functions may be most affected, and that cognitive symptoms and psychiatric symptoms may be mechanistically interlinked over time among those with symptomatic COVID-19 history. Additionally, our findings in relation to Flanker task performance were consistent with prior literature that older adult females are the most likely to be impacted by PCS symptoms (Hanson et al., 2022). The mechanisms by which symptomatic COVID-19 affects the brain as a function of biological sex characteristics is an important topic for future study. This being said, associations between COVID-19 history and both primary outcomes were evident at the population level even after controlling for sex and other demographic factors. Finally, the attenuating effect of vaccination on the association between symptomatic COVID-19 and psychiatric symptoms warrants further exploration in subsequent studies. Although the effect was statistically marginal in the current sample, if replicable in other studies and larger datasets, policy and practice implications would be evident with respect to vaccination efforts even as mortality from COVID-19 declines.
Author contributions
PH, GF, and SH conceived the study, planned and oversaw the statistical analyses, and wrote the final draft. GM planned and completed all statistical analyses and contributed to the writing of the final draft. HA, AH, MNS, AQ, TA, JL, and CB contributed to the planning of the study and writing of the final draft.
Funding
Funding for this study was provided by a grant from the Canadian Institutes of Health Research (GA3-177733) to P. Hall (PI), G. Fong (co-PI) and S. Hitchman (co-I).
Research ethics statement
This study protocol was reviewed by and received approval from the University of Waterloo Office of Research Ethics Committee.
Declaration of competing interest
The authors declare no conflicts of interest. fNIR Devices,LLC manufactures the optical brain imaging instrument and licensed IP and know-how from Drexel University. HA was involved in the technology development and thus offered a minor share in the startup firmfNIR Devices, LLC. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Contributor Information
Peter A. Hall, Email: pahall@uwaterloo.ca.
Hasan Ayaz, Email: ha45@drexel.edu.
Gang Meng, Email: gmeng@uwaterloo.ca.
Anna Hudson, Email: a3hudson@uwaterloo.ca.
Mohammad N. Sakib, Email: mn2sakib@uwaterloo.ca.
Anne C.K. Quah, Email: ackquah@uwaterloo.ca.
Thomas K. Agar, Email: tkagar@uwaterloo.ca.
Jessica A. Lee, Email: c259lee@uwaterloo.ca.
Christian Boudreau, Email: cboudreau@uwaterloo.ca.
Geoffrey T. Fong, Email: geoffrey.fong@uwaterloo.ca.
Data availability
Data will be made available on request.
References
- Andresen E.M., Malmgren J.A., Carter W.B., Patrick D.L. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am. J. Prev. Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- de Araújo T.V.B., Rodrigues L.C., de Alencar Ximenes R.A., de Barros Miranda-Filho D., Montarroyos U.R., de Melo A.P.L., et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect. Dis. 2016;16(12):1356–1363. doi: 10.1016/S1473-3099(16)30318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi‐Pooya A.A., Akbari A., Emami A., Lotfi M., Rostamihosseinkhani M., Nemati H., et al. Long COVID syndrome‐associated brain fog. J. Med. Virol. 2022;94(3):979–984. doi: 10.1002/jmv.27404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz H., Baker W.B., Blaney G., Boas D.A., Bortfeld H., Brady K., Brake J., Brigadoi S., Buckley E.M., Carp S.A., Cooper R.J., Cowdrick K.R., Culver J.P., Dan I., Dehghani H., Devor A., Durduran T., Eggebrecht A.T., Emberson L.L.…Zhou W. Optical imaging and spectroscopy for the study of the human brain: status report. Neurophotonics. 2022;9(S2) doi: 10.1117/1.NPh.9.S2.S24001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz H., Izzetoglu M., Shewokis P.A., Onaral B. 2010. Sliding-window Motion Artifact Rejection for Functional Near-Infrared Spectroscopy Conf Proc IEEE Eng Med Biol Soc. Buenos Aires, Argentina. [DOI] [PubMed] [Google Scholar]
- Ayaz H., Shewokis P.A., Bunce S., Izzetoglu K., Willems B., Onaral B. Optical brain monitoring for operator training and mental workload assessment. Neuroimage. 2012;59(1):36–47. doi: 10.1016/j.neuroimage.2011.06.023. [DOI] [PubMed] [Google Scholar]
- Ayaz H., Shewokis P.A., Curtin A., Izzetoglu M., Izzetoglu K., Onaral B. Using MazeSuite and functional near infrared spectroscopy to study learning in spatial navigation. JoVE. 2011;56:e3443. doi: 10.3791/3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley R. Guilford Press; New York: 2011. Barkley Deficits in Executive Functioning Scale (BDEFS) ([Google Scholar] [Google Scholar]
- Becker J.H., Lin J.J., Doernberg M., Stone K., Navis A., Festa J.R., Wisnivesky J.P. Assessment of cognitive function in patients after COVID-19 infection. JAMA Netw. Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.30645. e2130645-e2130645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J.R., Houff S. Neurological complications of herpes simplex virus type 2 infection. Arch. Neurol. 2008;65(5):596–600. doi: 10.1001/archneur.65.5.596. [DOI] [PubMed] [Google Scholar]
- Boldrini M., Canoll P.D., Klein R.S. How COVID-19 affects the brain. JAMA Psychiatr. 2021;78(6):682–683. doi: 10.1001/jamapsychiatry.2021.0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau C., Quah A.C., Agar T., Meng G., Hitchman S.C., Hall P.A., Fong G.T. 2023. Survey Design and Methods in the Canadian COVID-19 Experiences Survey. Manuscript in Progress. [Google Scholar]
- Ceban F., Ling S., Lui L.M., Lee Y., Gill H., Teopiz K.M., et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: a systematic review and meta-analysis. Brain Behav. Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran W.G., Chambers S.P. The planning of observational studies of human populations. J. Roy. Stat. Soc. 1965;128(2):234–266. doi: 10.2307/2344179. [DOI] [Google Scholar]
- Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., McCarthy P., Lange F., Andersson J.L.R., Griffanti L., Duff E., Jbabdi S., Taschler B., Keating P., Winkler A.M., Collins R., Matthews P.M., Allen N., Miller K.L., Nichols T.E., Smith S.M. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604(7907):697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen B.A., Eriksen C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974;16(1):143–149. doi: 10.3758/BF03203267. [DOI] [Google Scholar]
- Evans R.A., McAuley H., Harrison E.M., Shikotra A., Singapuri A., Sereno M., et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir. Med. 2021;9(11):1275–1287. doi: 10.1016/S2213-2600(21)00383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari M., Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage. 2012;63(2):921–935. doi: 10.1016/j.neuroimage.2012.03.049. [DOI] [PubMed] [Google Scholar]
- Gaillard A., Fehring D.J., Rossell S.L. Sex differences in executive control: a systematic review of functional neuroimaging studies. Eur. J. Neurosci. 2021;53(8):2592–2611. doi: 10.1111/ejn.15107. [DOI] [PubMed] [Google Scholar]
- Goenka A., Michael B.D., Ledger E., Hart I.J., Absoud M., Chow G., et al. Neurological manifestations of influenza infection in children and adults: results of a National British Surveillance Study. Clin. Infect. Dis. 2014;58(6):775–784. doi: 10.1093/cid/cit922. [DOI] [PubMed] [Google Scholar]
- Hall P.A., Fong G.T., Hitchman S.C., Quah A.C.K., Agar T., Meng G., Ayaz H., Dore B.P., Sakib M.N., Hudson A., Boudreau C. Brain and behavior in health communication: the Canadian COVID-19 Experiences Project. Brain. Behave. Immun. Health. 2022;22 doi: 10.1016/j.bbih.2022.100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P.A., Meng G., Hudson A., Sakib M.N., Hitchman S.C., MacKillop J., Bickel W.K., Fong G.T. Cognitive function following SARS-CoV-2 infection in a population-representative Canadian sample. Brain Behav. Immun. Health. 2022;21 doi: 10.1016/j.bbih.2022.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P.A., Sheeran P., Fong G.T., Cheah C.S.L., Oremus M., Liu-Ambrose T., Sakib M.N., Butt Z.A., Ayaz H., Jandu N., Morita P.P. Biobehavioral aspects of the COVID-19 pandemic: a review. Psychosom. Med. 2021;83(4):309–321. doi: 10.1097/psy.0000000000000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Trender W., Chamberlain S.R., Jolly A.E., Grant J.E., Patrick F., Mazibuko N., Williams S.C.R., Barnby J.M., Hellyer P., Mehta M.A. Cognitive deficits in people who have recovered from COVID-19. EClinic. Med. 2021;39 doi: 10.1016/j.eclinm.2021.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson S.W., Abbafati C., Aerts J.G., Al-Aly Z., Ashbaugh C., Ballouz T., et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. 2022;328(16):1604–1615. doi: 10.1001/jama.2022.18931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A.B. The environment and disease: association or causation? J. R. Soc. Med. 2015;108(1):32–37. doi: 10.1177/0141076814562718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho R.C., Sharma V.K., Tan B.Y., Ng A.Y., Lui Y.S., Husain S.F., et al. Comparison of brain activation patterns during olfactory stimuli between recovered COVID-19 patients and healthy controls: a functional near-infrared spectroscopy (fNIRS) study. Brain Sci. 2021;11(8):968. doi: 10.3390/brainsci11080968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Y., Li C., Peng L., Li Y., Xie W., Cui D., Shang L., Fan G., Xu J., Wang G., Wang Y., Zhong J., Wang C., Wang J., Zhang D., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/s0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A., Hall P.A., Hitchman S.C., Meng G., Fong G.T. Cognitive predictors of COVID-19 mitigation behaviors in vaccinated and unvaccinated general population members. Vaccine. 2022 doi: 10.1016/j.vaccine.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaywant A., Vanderlind W.M., Alexopoulos G.S., Fridman C.B., Perlis R.H., Gunning F.M. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology. 2021;46(13):2235–2240. doi: 10.1038/s41386-021-00978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai N., Kubo-Kawai N., Kubo K., Terazawa T., Masataka N. Distinct aging effects for two types of inhibition in older adults: a near-infrared spectroscopy study on the Simon task and the flanker task. Neuroreport. 2012;23(14):819–824. doi: 10.1097/WNR.0b013e3283578032. [DOI] [PubMed] [Google Scholar]
- Kim J.E., Heo J.H., Kim H.O., Song S.H., Park S.S., Park T.H., et al. Neurological complications during treatment of Middle East respiratory syndrome. J. Clin. Neurol. 2017;13(3):227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus M.N., Bickel W.K. A 5-trial adjusting delay discounting task: accurate discount rates in less than one minute. Exp. Clin. Psychopharmacol. 2014;22(3):222–228. doi: 10.1037/a0035973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine K., Leonard H.L., Blauwendraat C., Iwaki H., Johnson N., Bandres-Ciga S., et al. Virus exposure and neurodegenerative disease risk across national biobanks. Neuron. 2023;111:1–8. doi: 10.1016/j.neuron.2022.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.H., Chen Y., Wang Q.H., Wang L.R., Jiang L., Yang Y., Chen X., Li Y., Cen Y., Xu C., Zhu J., Li W., Wang Y.R., Zhang L.L., Liu J., Xu Z.Q., Wang Y.J. One-year trajectory of cognitive changes in older survivors of COVID-19 in wuhan, China: a longitudinal cohort study. JAMA Neurol. 2022;79(5):509–517. doi: 10.1001/jamaneurol.2022.0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C.J., Staines W.R., Manocchio F., Hall P.A. The neurocognitive mechanisms underlying food cravings and snack food consumption. A combined continuous theta burst stimulation (cTBS) and EEG study. Neuroimage. 2018;177:45–58. doi: 10.1016/j.neuroimage.2018.05.013. [DOI] [PubMed] [Google Scholar]
- Melara R.D., Singh S., Hien D.A. Neural and behavioral correlates of attentional inhibition training and perceptual discrimination training in a visual flanker task. Front. Hum. Neurosci. 2018;12:191. doi: 10.3389/fnhum.2018.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrikaki M., Lee J.D., Solomon I.H., Slack F.J. Severe COVID-19 is associated with molecular signatures of aging in the human brain. Nature Aging. 2022:1–8. doi: 10.1038/s43587-022-00321-w. [DOI] [PubMed] [Google Scholar]
- Nauen D.W., Hooper J.E., Stewart C.M., Solomon I.H. Assessing brain capillaries in coronavirus disease 2019. JAMA Neurol. 2021;78(6):760–762. doi: 10.1001/jamaneurol.2021.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer L.S., Heyers K., Ocklenburg S., Wolf O.T. Stress research during the COVID-19 pandemic and beyond. Neurosci. Biobehav. Rev. 2021;131:581–596. doi: 10.1016/j.neubiorev.2021.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigfrid L., Drake T.M., Pauley E., Jesudason E.C., Olliaro P., Lim W.S., et al. Long Covid in adults discharged from UK hospitals after Covid-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Region. Health Euro. 2021;8 doi: 10.1016/j.lanepe.2021.100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon T. Neurological infection with SARS-CoV-2 - the story so far. Nat. Rev. Neurol. 2021;17(2):65–66. doi: 10.1038/s41582-020-00453-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R.L., Kroenke K., Williams J.B., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Okumura Y., Kita Y., Oi Y., Yamashita Y., Goto T., Inagaki M. Excessive hemodynamic activity in the superior frontal cortex during the flanker task in children with attention deficit hyperactivity disorder. Neuroreport. 2017;28(13):828–832. doi: 10.1097/WNR.0000000000000834. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Okumura Y., Kita Y., Oi Y., Shinoda H., Inagaki M. The relationship between the superior frontal cortex and alpha oscillation in a flanker task: simultaneous recording of electroencephalogram (EEG) and near infrared spectroscopy (NIRS) Neurosci. Res. 2018;131:30–35. doi: 10.1016/j.neures.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatr. 2021;8(2):130–140. doi: 10.1016/s2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torjesen I. 2021. Covid-19: Middle Aged Women Face Greater Risk of Debilitating Long Term Symptoms. [DOI] [PubMed] [Google Scholar]
- Yong S.J. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis. 2021;53(10):737–754. doi: 10.1080/23744235.2021.1924397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawilska J.B., Kuczyńska K. Psychiatric and neurological complications of long COVID. J. Psychiatr. Res. 2022 doi: 10.1016/j.jpsychires.2022.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.