Abstract
PURPOSE
To identify and quantify the barriers and facilitators to the use of clinical decision support systems (CDSSs) by primary care professionals (PCPs).
METHODS
A mixed-methods systematic review was conducted using a sequential synthesis design. PubMed/MEDLINE, PsycInfo, Embase, CINAHL, and the Cochrane library were searched in July 2021. Studies that evaluated CDSSs providing recommendations to PCPs and intended for use during a consultation were included. We excluded CDSSs used only by patients, described as concepts or prototypes, used with simulated cases, and decision supports not considered as CDSSs. A framework synthesis was performed according to the HOT-fit framework (Human, Organizational, Technology, Net Benefits), then a quantitative synthesis evaluated the impact of the HOT-fit categories on CDSS use.
RESULTS
A total of 48 studies evaluating 45 CDSSs were included, and 186 main barriers or facilitators were identified. Qualitatively, barriers and facilitators were classified as human (eg, perceived usefulness), organizational (eg, disruption of usual workflow), and technological (eg, CDSS user-friendliness), with explanatory elements. The greatest barrier to using CDSSs was an increased workload. Quantitatively, the human and organizational factors had negative impacts on CDSS use, whereas the technological factor had a neutral impact and the net benefits dimension a positive impact.
CONCLUSIONS
Our findings emphasize the need for CDSS developers to better address human and organizational issues, in addition to technological challenges. We inferred core CDSS features covering these 3 factors, expected to improve their usability in primary care.
Key words: primary health care; information technology; medical informatics; quality of health care; decision support systems, clinical
INTRODUCTION
Achieving best practice in primary care is a challenge because primary care professionals (PCPs) face a variety of health care issues and cannot always identify and access all the relevant information within the timeframe of the consultation.1,2 Clinical decision support systems (CDSSs) are software designed to be a direct aid to clinical decision making, in which an inference engine matches the features of an individual patient to a computerized clinical knowledge base or a machine learning algorithm and then presents patient-specific assessments or recommendations to the clinician or the patient for a decision.3,4 Clinical decision support systems are intended to improve the quality, safety, and efficiency of care.5-7 In primary care, they have not yet proven effectiveness on clinical outcomes, such as morbidity or mortality.8,9 However, according to a large recent meta-analysis of controlled trials in any settings, CDSSs increase the proportion of patients receiving the desired element of care by 5.8% overall, with a trend toward a worse outcome in the outpatient setting.10
Qualitative evaluations are needed to obtain a comprehensive understanding of the barriers and facilitators to CDSS use, which are key to their implementation success.11,12 For this purpose, several systematic reviews focused on specific types of CDSS (knowledge-based CDSSs,13 clinical reminders14), specific processes of care (drug prescription,15,16 diagnosis17), or specific health issues (antibiotics prescription,18 HIV management19), without any restriction to their context of use. However, health information systems implementation or evaluation models such as the HOT-fit framework (Human, Organization, Technology, Net Benefits20) and others21-25 emphasize the influence of context-specific factors in the use of health information systems. This is even more important for the primary care setting owing to the unique combination of the diversity and complexity of health issues managed (often multiple in the same consultation), its patient-centered care approach, and its particular decision-making context,26 which may generate specific needs for decision support systems.
The objective of the present systematic review was therefore to identify and quantify the barriers and facilitators to the use of CDSSs by PCPs. From a qualitative synthesis based on the HOT-fit framework, we derived a quantitative synthesis assessing the mean impacts of the human, organizational, technological factors, and the net benefits dimension on CDSS use by PCPs.
METHODS
The present study was a mixed-methods systematic review that followed the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) reporting guideline. The study protocol was registered on PROSPERO on July 14, 2020 (CRD42020185199).
Search Strategy
The search strategy was built in cooperation with medical librarians. We searched PubMed/MEDLINE, PsycInfo, Embase, CINAHL, and the Cochrane library for relevant studies. We tracked citations from included records to identify additional relevant references. The search was performed on July 5, 2021 without date limitation. The complete search strategy for each database is available in Supplemental Appendix 1.
Eligibility Criteria
We included all qualitative, quantitative, and mixed methods studies and only original articles for which the primary or secondary objectives were to identify barriers and facilitators to the use of CDSSs in primary care. For studies involving various professions or specialties, we only considered those that had at least 50% PCPs in the study sample. We included CDSSs that provided recommendations to PCPs (and possibly to patients) and were intended for use during the consultation.
We excluded publication types that were posters, dissertations or theses, conference proceedings, commentaries, letters, or editorials. We excluded the following decision supports that were not considered as being CDSSs: drug-drug interaction alert systems, risk assessment tools that provided assessments but not recommendations, and clinical decision supports without inference engine. We excluded the following CDSSs: decision aids only used by patients, CDSSs described as concepts or prototypes, and CDSSs evaluated with simulated clinical scenarios.
Selection Process
The selection process was performed using Covidence software (Veritas Innovation Ltd).27 After automatic removal of duplicates, 2 authors (P-Y.M. and C.R.) independently screened titles and abstracts and excluded irrelevant records. They independently screened potentially relevant articles in full text while documenting reasons for exclusion. The concordance ( ) was 0.62 for title and abstract screening and 0.72 for full text screening. The disagreements were resolved by seeking consensus between the 2 authors.
) was 0.62 for title and abstract screening and 0.72 for full text screening. The disagreements were resolved by seeking consensus between the 2 authors.
Quality Appraisal
The quality of the included studies was independently appraised by 2 authors (P-Y.M. and C.R.) using the Covidence software. We applied the QuADS tool that has been designed to appraise the methodological and reporting quality of qualitative, quantitative, and mixed-methods studies in systematic reviews, based on 13 common criteria.28 Each criterion is assessed according to the 4 following proposals: no mention at all, very slightly, moderately, complete. The concordance ( ) was 0.39. The disagreements were resolved by seeking consensus between the 2 authors.
) was 0.39. The disagreements were resolved by seeking consensus between the 2 authors.
Data Extraction
We used a structured data collection form to extract CDSS features (Supplemental Table 1) and methodological features of the included studies. The extraction process was performed independently by 2 authors (P-Y.M. and C.R.); disagreements were resolved by seeking consensus between them. We contacted the main authors of the included studies to obtain data on CDSS features not reported in the published article.
The HOT-fit Framework
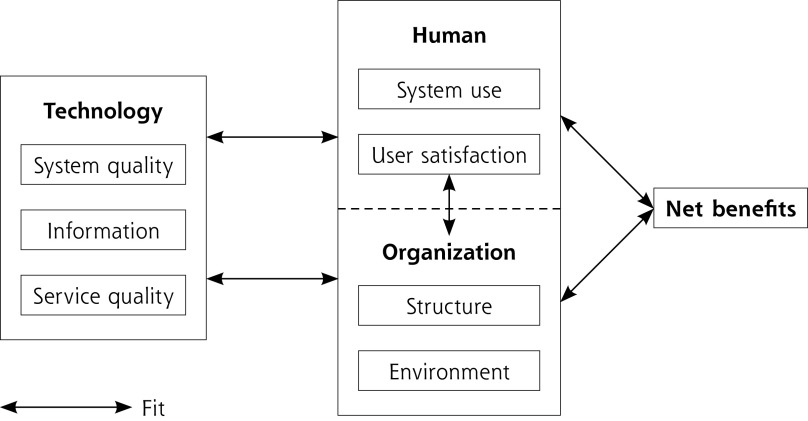
The HOT-fit framework describes the interdependent human, organizational, and technological factors related to health information system adoption. These 3 factors are described through 7 dimensions: system use and user satisfaction related to the human factor; environment and structure related to the organizational factor; system, information and service quality related to the technological factor. The framework is complemented by an additional dimension, net benefits, which captured the positive and negative effects of CDSS recommendations on PCPs (Figure 1).20 Each HOT-fit dimension includes several evaluation measures. The HOT-fit framework was chosen as it assesses barriers and facilitators to the use of health information systems from a pragmatic, user-centered approach.
Figure 1:
HOT-fit framework (derived from Yusof et al29).
HOT-fit = human, organization, technology, net benefits.
Note: The HOT-fit framework describes the interdependent human, organizational, and technological factors related to health information system adoption. A fit between the human, organizational, technological factors and the net benefit dimension is required for the adoption of these systems.
Data Synthesis
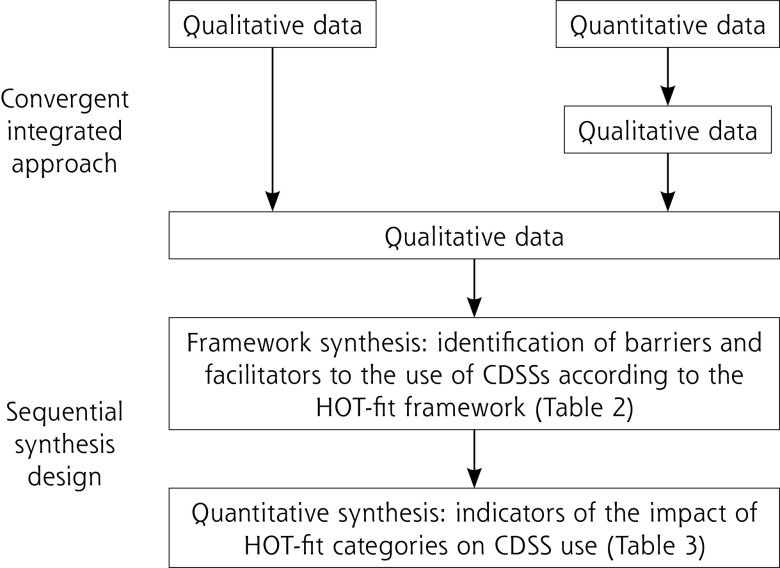
The review followed a sequential synthesis design, using a framework synthesis to inform a quantitative synthesis (Figure 2).30-32 First, a convergent integrated approach built up a common set of qualitative data from the included qualitative and quantitative data. It involved data transformation through a narrative interpretation of quantitative data.31 The resulting framework synthesis was based on the HOT-fit framework. Second, we performed a quantitative synthesis by calculating the difference between barriers and facilitators categorized according to the HOT-fit framework.
Figure 2.
Mixed-methods synthesis design.
CDSS = clinical decision support system; HOT-fit = human, organization, technology, net benefits.
The framework synthesis was performed using NVivo (QSR International), released in March 2020.33 Barriers and facilitators to the use of CDSSs were coded inductively as concepts and then classified into the appropriate HOT-fit evaluation measures.32 This was done independently by 2 authors (P-Y.M. and E.G.), who reviewed together every coded citation and reached consensus on the concepts and the associated HOT-fit evaluation measures. We extended the HOT-fit framework to include a few concepts we were unable to classify in existing evaluation measures. Due to the various level of detail in the description of the barriers and facilitators to CDSS use in the included studies, certain codes were considered as explanatory elements of higher concepts that we named main barriers and facilitators.
From the framework synthesis results, we first quantified the individual impacts of the 3 HOT-fit factors (human, organization, technology) and the net benefits dimension on the use of each CDSS. In practice, facilitators to CDSS use were quantified as +1 and barriers as −1. For example, if a given CDSS had X barriers and Y facilitators classified in the human factor, this factor’s impact on the use of this CDSS was calculated as Y-X. For each CDSS, barriers and facilitators were considered only once, regardless of the number of their occurrences in the included studies. Then, we calculated the mean impact of each HOT-fit factor and the net benefits dimension on the use of the whole sample of CDSSs included, with a confidence interval (CI).
RESULTS
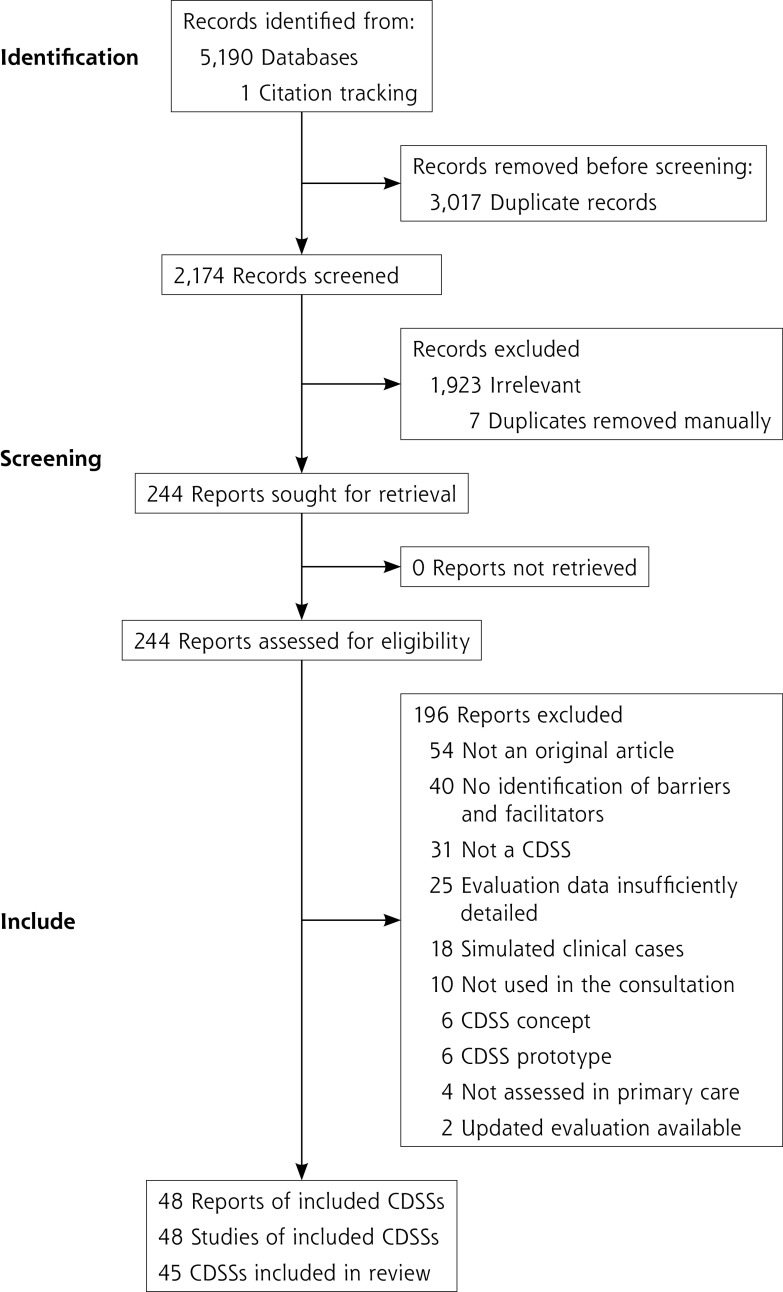
A total of 48 studies assessing 45 CDSSs were included in the review (Figure 3).34-81 Of these, 29 were mixed-methods studies, 15 were qualitative studies, and 4 were quantitative studies. Included studies were published from 1998 to 2021 (median year: 2016). Three CDSSs (EBMeDS, NHGDoc, and PRIMA-EDS) were assessed in 2 studies.
Figure 3.
PRISMA flow chart.
CDSS = clinical decision support system; PRISMA = Preferred Reporting Items for Systematic Review and Meta-Analysis.
Description of the CDSSs
Clinical decision support systems were mainly developed (n = 18) and used (n = 17) in the United States. The main users were primary care physicians (n = 37) and nurses (n = 22). Clinical decision support systems were used for preventive care (n = 27), treatment (n = 21), management of chronic disease(s) (n = 13), and/or diagnosis (n = 7). All CDSSs were knowledge based; none integrated a machine learning algorithm. Updates to the knowledge database were made in 11/33 CDSSs for which this information was available. The strength of evidence of CDSS recommendations was provided in 4/19 studies for which the information was available. Twenty-three CDSSs provided a direct link to the source of recommendations. Thirteen CDSSs provided educational materials to help patients with shared decision making. Primary care professionals were trained to the use of 36 CDSSs. Most CDSSs were partially or fully integrated in the electronic health record (EHR) (n = 28). The main features of the included CDSS are described in Table 1. Detailed features are presented in Supplemental Appendix 2.
Table 1.
Main Features of the 45 Identified CDSSs
| CDSS n = 45 in 48 Studies | Name | Country of Use | Care Procedures | Targeted Health Issues |
|---|---|---|---|---|
| North America | ||||
| Alagiakrishnan et al,36 2016 | SMART-CDS | Canada | Prevention (iatrogenesis) | Adaption of medication to renal function from the patient’s EHR |
| Ash et al38 2011 | USA | Diagnosis, Therapeutics (prescribing, vaccination) | Drug-drug, drug-condition, and drug-allergy interaction checking, patient care plan dashboard with reminders, nearly 3,000 condition specific point-and-click templates for documentation | |
| Curry et al42 2011 | Decision Support Server | Canada | Prevention (disease, test ordering) | Prescription of diagnostic imaging |
| Dixon et al43 2013 | USA | Prevention (disease, test ordering), Management of chronic disease(s) | Diabetes mellitus type II, hypertension, coronary artery disease | |
| Doerr et al44 2014 | My Family | USA | Prevention (disease, test ordering) | Cancer risk management |
| Edelman et al45 2014 | The Pregnancy and Health Profile (PHP) | USA | Prevention (disease, test ordering) | Prenatal genetic screening |
| Feldstein et al46 2013 | Patient Panel-Support Tool (PST) | USA | Prevention (iatrogenesis, disease, test ordering), Management of chronic disease | Graphically displays ‘‘care gaps’’ (eg, for screening, medication use, monitoring, risk-factor control, vaccination) |
| Guenter et al47 2019 | McMaster Pain Assistant (MPA) | Canada | Diagnosis, Therapeutics (prescribing, vaccination) | Neuropathic pain |
| Jenssen et al51 2016 | USA | Prevention (disease, test ordering) Therapeutics (prescribing, vaccination) |
Smoking cessation | |
| Kempe et al52 2017 | Immunization information systems | USA | Prevention (disease, test ordering) | Vaccination |
| Lam Shin Cheung et al54 2020 | eAMS | Canada | Therapeutics (prescribing, vaccination), Management of chronic disease(s) | Asthma |
| Lemke et al55 2020 | GWA | USA | Prevention (disease, test ordering) | Genetic risk assessment |
| Litvin et al. (2012) | ABX-TRIP CDSS | USA | Prevention (disease, test ordering), Diagnosis, Therapeutics (prescribing) | Acute respiratory infections |
| Litvin et al56 2016 | USA | Prevention (disease, test ordering) | Identification and management of chronic kidney disease | |
| Minian et al62 2021 | Canada | Prevention (disease, test ordering) | Alcohol cessation | |
| Montini et al63 2013 | USA | Prevention (disease, test ordering) Therapeutics (prescribing, vaccination) |
Tobacco cessation | |
| Price et al67 2017 | Canada | Prevention (iatrogenesis) | Potentially inappropriate prescriptions in the elderly | |
| Richardson et al68 2019 | USA | Therapeutics (prescribing, vaccination) | Sore throat, upper respiratory tract infections | |
| Rubin et al72 2006 | USA | Therapeutics (prescrbiing, vaccination), Diagnosis | Acute respiratory tract infections | |
| Trafton et al76 2010 | ATHENA-OT | USA | Therapeutics (prescribing, vaccination) | Opioid therapy for chronic, noncancer pain |
| Trinkley et al77 2021 | USA | Therapeutics (prescribing, vaccination) | Heart failure | |
| Williams et al79 2016 | USA | Prevention (disease, test ordering) | Pediatric cardiovascular risk | |
| Zheng et al81 2005 | CRS | USA | Prevention (iatrogenesis, disease, test ordering), Therapeutics (prescribing, vaccination), Management of chronic disease(s) | Diabetes mellitus type II, hyperlipidemia, steroid-induced osteoporosis, influenza, pneumonia, breast cancer, cervical cancer |
| Europe | ||||
| af Klercker et al35 1998 | Sweden | Diagnosis | Ear, nose, throat diseases | |
| Arts et al37 2018 | The Netherlands | Management of chronic disease(s) | Diabetes mellitus type II, atrial fibrillation, hypertension, medication prescriptions relating to care of older adults | |
| Bindels et al41 2003 | GRIF Automated Feedback System | The Netherlands | Prevention (disease, test ordering) | Comments on the appropriateness of diagnostic tests ordered by general practitioners |
| Helldén et al48 2015 | The renal button | Sweden | Prevention (iatrogenesis) | Adaption of medication to renal function from the patient’s EHR |
| Heselmans et al49 2020 and Koskela et al53 2016 | EBMeDS | Belgium, Estonia, Finland, Italy | Prevention (iatrogenesis, disease, test ordering), Therapeutics (prescribing, vaccination), Management of chronic disease | > 1,000 NICE-accredited international guidelines |
| Lugtenberg et al58,59 2015 (2 articles) | NHGDoc | The Netherlands | Prevention (disease, test ordering, iatrogenesis), Therapeutics (prescribing, vaccination), Management of chronic disease(s) | Diabetes mellitus type II, cardiovascular risk management, asthma/COPD, thyroid disorders, viral hepatitis and other liver diseases, atrial fibrillation, subfertility |
| Pannebakker et al64 2019 | England | Prevention (disease, test ordering) | Pigmented skin lesions | |
| Rieckert et al68,69 2018, 2019 | PRIMA-EDS | Germany, Austria, Italy, England | Prevention (iatrogenesis) | Polypharmacy in older and chronically ill people |
| Rousseau et al71 2003 | England | Therapeutics (prescribing, vaccination), Management of chronic disease(s) | Asthma and angina in adults | |
| Toth-Pal et al75 2008 | Evibase | Sweden | Management of chronic disease(s) | Congestive heart failure |
| Australia | ||||
| Abimbola et al34 2019 | Health Tracker | Australia | Prevention (disease, test ordering) | Cardiovascular risk management |
| Bandong et al39 2019 | My Whiplash Navigator |
Australia | Therapeutics (prescribing, vaccination) | Whiplash-associated disorders |
| Peiris et al65 2014 | Australia | Diagnosis, Therapeutics (prescribing, vaccination) | Back pain management | |
| Wan et al78 2012 | Australia | Management of chronic disease(s) | Diabetes mellitus type 2 | |
| Wilson et al80 2007 | EMPOWER | Australia | Prevention (disease, test ordering), Therapeutics (prescribing, vaccination) | Cardiovascular risk management, hypertension |
| South America | ||||
| Maia et al60 2016 | Brazil | Management of chronic disease(s) | Diabetes mellitus type II | |
| Marcolino et al61 2021 | Brazil | Prevention (disease, test ordering), Management of chronic diseases | Diabetes mellitus type II, hypertension, cardiovascular risk treatment | |
| Silveira et al73 2019 | TeleHAS | Brazil | Prevention (disease, test ordering), Therapeutics | Cardiovascular risk management, hypertension |
| Africa | ||||
| Bessat et al40 2019 | REC | Burkina Faso | Diagnosis, Therapeutics (prescribing, vaccination) | Follow-up and treatment of children under the age of 5 years in developing countries |
| Jensen et al50 (2019) | eIMCI | South Africa | Prevention (disease, test ordering), Therapeutics (prescribing, vaccination) | Management of childhood illness |
| Sukums et al74 2015 | QUALMAT | Burkina Faso, Ghana, Tanzania | Therapeutics (prescribing, vaccination) | Antenatal and intrapartum care |
| Asia | ||||
| Praveen et al66 2014 | India, Indonesia, Thailand | Management of chronic disease(s) | Cardiovascular risk management | |
CDSS = clinical decision support system; COPD = chronic obstructive pulmonary disease; EHR = electronic health record; NICE = National Institute for Health and Care Excellence.
Quality Assessment
The rationale for the choice of the data collection tool was not mentioned at all in 18 studies and very slightly in 6 studies. The format and content of the data collection tool was estimated as completely appropriate to meet the stated research objectives in 27 studies, and moderately appropriate in 19 studies. The appraisal of the 13 quality criteria of the QuADS tool is presented in Supplemental Table 2. The methodological features of the included studies are presented in Supplemental Table 3.
Framework Synthesis
In total, 186 main barriers and facilitators and 69 explanatory elements were identified. All CDSSs reported technological barriers or facilitators, but 4 CDSSs did not assess the human factor, 4 did not assess the organizational factor, and 3 did not assess the net benefits dimension. Among the 186 main barriers and facilitators, 43 were classified as human factors, 49 as organizational factors, 70 as technological factors, and 24 in the net benefits dimension. The full list is presented in Supplemental Appendix 3, and barriers and facilitators (or their pairs) identified in at least 7 CDSSs are described in Tables 2 and 3 with their explanatory elements. The mean number of main barriers and facilitators identified per CDSS was 28.9; this ranged from 8 to 86 (Supplemental Table 4). We complemented the HOT-fit framework by adding an evaluation measure called “hardware” in the dimension “structure” of the factor “organization” to describe barriers and facilitators related to hardware issues in PCPs facilities.
Table 2.
Main Facilitators Reported in More Than 7 CDSSs, and Their Explanatory Elements, Classified According to the HOT-Fit Framework
| HOT-Fit Framework | Main Facilitatorsa (No. CDSSs Concerned) | Explanatory Elements | ||
|---|---|---|---|---|
| Factors and Dimensions (No. CDSSs concerned) | Evaluation Measures | |||
| Human (n = 41) User satisfaction (n = 31) |
Perceived usefulness Training Software satisfaction Motivation to use Overall satisfaction |
Perceived usefulness of the CDSS (n = 23) Training before use is appreciated (n = 10) PCPs would continue to use the CDSS (n = 9) Patients’ perceived usefulness of the CDSS increases PCPs motivation to use it (n = 7) CDSSs increase PCPs satisfaction (n = 7) |
||
| Organization (n = 41) Structure (n = 39) |
Clinical process | Natural integration of the CDSS in the clinical workflow (n = 13) | ||
| Autonomy | Producing reports of quality measures through collected data increases the value from the CDSS’s use in clinical practice (n = 7) | Expansion of skill set and roles in assisting physicians and patients in meeting care needs | ||
| Teamwork | Other professionals ease physician’s increased workload with the CDSS (n = 6) | |||
| Technology (n = 45) System quality (n = 45) |
Ease of use | The CDSS is user-friendly (ergonomic) (n = 30) CDSS recommendations are easy to understand (n = 9) |
||
| Usefulness of system features and functions | Reminders (n = 8) | |||
| Ease of learning | Easy to use after a short learning period (n = 9) | |||
| Information quality (n = 40) | Usefulness | Information provided is useful for the targeted process of care (n = 13) Educational materials for patients are valuable (n = 7) |
General agreement with the validity of recommendations | |
| Format | Pleasing visual layout [n = 12] | |||
| Relevance | Recommendations are relevant (n = 11) | |||
| Reliability | Recommendations are reliable (n = 9) | |||
| Service quality (n = 11) | Technical support | Satisfaction with the CDSS service support (n = 7) | CDSS technical staff availability | |
| Net benefits (n = 42) | Effectiveness | Potential to improve the quality of care (n = 23) | Brings preventive care to the forefront | |
| Helps to systematize assessment of every patient | ||||
| Facilitates patient care management | ||||
| CDSS helps PCPs to improve guideline adherence (n = 11) | ||||
| Efficiency | Using CDSS saves time (n = 22) | Shortening documentation time | ||
| Giving a quick patient evaluation from relevant data in patients’ EHRs | ||||
| Decision-making quality | CDSS facilitates decision making (n = 22) | CDSS is facilitating decision making about referral | ||
| Communication | CDSS helps focus on patient education (n = 18) CDSS eases patient-PCP communication (n = 13) |
CDSS helps increase patient engagement | ||
| Clinical practice | CDSS is a way to update PCP’s knowledge (n = 17) CDSS leads to better teamwork in primary care (n = 7) CDSS increases PCPs’ self-confidence (n = 7) |
|||
| Error reduction | CDSS helps PCPs to identify unrecognized information needs (n = 17) | |||
CDSS = clinical decision support system; EHR = electronic health record; HOT-fit = human, organization, technology, net benefits; PCP = primary care professional.
Main facilitators are ranked by the number of CDSSs concerned.
Table 3.
Main Barriers Reported in at Least 7 CDSSs, and Their Explanatory Elements, Classified According to the HOT-Fit Framework
| HOT-Fit Framework | Main Barriersa (No. CDSSs Concerned) | Explanatory Elements | |
|---|---|---|---|
| Factors and Dimensions (No. CDSSs Concerned) | Evaluation Measures | ||
| Human (n = 41) System use (n = 39) |
Resistance or reluctance |
Conflicts between CDSS recommendations and PCP expertise or beliefs (n = 18) |
CDSS recommendations do not reflect the complexity of the situation |
| Report acceptance | Alert fatigue (n = 13) Information overload (n = 8) |
Lack of a concise synthesis of the CDSS recommendation | |
| Training | Training before use is needed (n = 11) | The training session to the CDSS is inadequate or too short | |
| Attitude | PCPs don’t need help with the targeted health issue (n = 8) | ||
| Lack of engagement from PCPs (inertia of previous practice) (n = 8) | |||
| Knowledge and expertise | Lack of computer skills (n = 7) | ||
| Motivation to use | Ask for financial compensation to use the CDSS (n = 7) | ||
| Organization (n = 41) | Clinical process | Using CDSS disrupts usual workflow (n = 25) | |
| Structure (n = 39) | Teamwork | Need of more teamwork with other PCPs to help physicians with CDSS’s increased workload (n = 13) | Physicians fear more the CDSS workload than assistants or nurses |
| Hardware | Lack or computers or tablets (n = 7) | ||
| Environment (n = 18) | Inter-organizational relationship | Difficulty to use CDSSs for patients comanaged by other specialists (n = 11) | Information is sometimes missing or not integrated from external sources |
| Technology (n = 45) System quality (n = 45) |
Ease of use | The CDSS is not user-friendly (n = 21) | Need to switch windows in the EHR while using CDSSs |
| Location of CDSS recommendations should be changed | |||
| Need to switch windows between the EHR and the CDSS | |||
| Turnaround time | CDSS slowness (n = 16) | CDSS’s slowness impairs the interaction with the patient and increases the consultation time | |
| Usefulness of system features and functions | CDSS not fully integrated in the EHR (n = 14) | A CDSS not fully integrated in the EHR is time consuming and disrupts workflow | |
| The most current information collected in the EHR is sometimes not updated in the CDSS | |||
| Database contents | The CDSS should target more health issues (n = 11) | ||
| Questioning validity of CDSS’s knowledge database (n = 7) | Concerns about the CDSS’s independence from pharmaceutical industry | ||
| Flexibility | Need of customization options (n = 8) | ||
| Information quality (n = 40) | Format | Format of recommendations (length, structure, font colors) (n = 13) | |
| Reliability | Doubtful reliability of the recommendations (n = 12) | The reliability of the recommendations depends on the quality and completeness of the information collected | |
| Relevance | Recommendations are not relevant (n = 11) | Conflicts between patient complaints and unrelated CDSS recommendations General recommendations are often irrelevant |
|
| Usefulness | Recommendations are not helpful (n = 8) | ||
| Net benefits (n = 42) | Efficiency | Increased workload during the consultation (n = 33) | Lack of time to use the CDSS during the consultation Structured data collection takes too much time Duplication of data collection Coping strategies: increased consultation time, need of additional time to use the CDSS outside the consultation, scheduling follow-up consultations |
| Negative effect on patient-PCP communication (n = 7) | |||
CDSS = clinical decision support system; EHR = electronic health record; HOT-Fit = human, organization, technology, net benefits; PCP = primary care provider.
Main barriers are ranked by the number of CDSSs concerned.
From the human perspective, PCPs valued CDSSs for which they were trained and perceived to be useful. Conversely, they did not appreciate CDSS that provided recommendations conflicting with their beliefs or expertise. They frequently experienced alert fatigue or information overload.
From the organizational perspective, PCPs appreciated that the CDSS was well integrated into the clinical workflow and physicians appreciated that other professionals eased their workload. Conversely, PCPs expressed difficulties in using CDSSs with patients managed by other specialists because of disagreements between CDSS recommendations and specialists’ prescriptions.
From the technological perspective, PCPs appreciated fully integrated and easy-to-use CDSSs, providing reminders for PCPs and educational materials to patients, and relevant and reliable recommendations. Conversely, they disliked CDSSs that were slow, or targeting only a few health issues. They sometimes questioned the reliability of the recommendations, which they attributed to the quality and completeness of the information collected. PCPs frequently requested CDSS customization features.
From the net benefits perspective, PCPs felt that using CDSSs increased workload during the consultation for 33 of the 45 included CDSSs. Despite this major barrier, PCPs largely agreed on the benefits of CDSSs in terms of their potential to improve quality of care, particularly for preventive care. Primary care clinicians also felt that CDSSs improved the automatic identification of all useful information that they did not systematically recognize at the moment the clinical decision is made, as well as shared medical decision making through the provision of educational material to patients and their adherence to guidelines.
Quantitative Synthesis
Organizational and human HOT-fit factors had an overall negative impact on CDSS use by PCPs. The technological factor had a neutral overall impact, and the net benefits dimension an overall positive impact on CDSS use (Table 4). Individual impacts of the 3 HOT-fit factors and the net benefits dimension on the use of each CDSS are presented in Supplemental Table 5.
Table 4.
Mean Impacts of HOT-Fit Categories on CDSS Use by PCPs
| Human | Organization | Technology | Net Benefits | |
|---|---|---|---|---|
| Mean impact of HOT-fit categories on CDSS use | Slightly negative | Slightly negative | Neutral | Positive |
| Mean difference between barriers and facilitators (95% CI) | −1.5 (−2.2 to −0.8) | −1.9 (−2.6 to −1.1) | −0.5 (−1.5 to 0.5) | + 3.1 (2.2 to 3.9) |
CDSS = clinical decision support system; HOT-fit = human, organization, technology, net benefits; PCP = primary care professional.
Note: In the human factor, there was 1.5 additional barriers per CDSS than there were facilitators. In the net benefits dimension, there was 3.1 additional facilitators per CDSS than there were barriers.
DISCUSSION
All barriers and facilitators to CDSS use by PCPs were distributed across the 3 HOT-fit factors and the net benefits dimension, with a predominance of the technological factor, that was the only factor explored in all studies. However, the overall impact of the technological factor on the use of CDSSs by PCPs was neutral, which indicates a balance between technological barriers and facilitators, while the human and organizational factors had overall negative impacts and the net benefit dimension an overall positive impact. The net benefits reported by PCPs support the potential effectiveness of CDSSs in improving quality and safety of care. However, they seem unable to improve care efficiency since they are believed to increase PCP workload.
Comparison With Other Studies
This review is the first to identify and quantify barriers and facilitators to the use of CDSSs specifically in the primary care setting. In 2017, Kilsdonk et al conducted a systematic review and gap analysis of barriers and facilitators to the use of knowledge-based CDSSs in any setting (including hospitals) according to the HOT-fit framework, including CDSS evaluations based on simulated clinical scenarios.13 The quantitative gap analysis revealed the predominance of technological and human factors, and a knowledge gap regarding the organizational factor and the net benefits dimension. However, no conclusion could be drawn on the relative impact of these factors on CDSS use. In the present review, the organizational factor was not less frequently identified than the human factor, and the quantitative synthesis assessed for the first time the relative impact of the HOT-fit factors on CDSS use. In 2013, Moxey et al analyzed both health care providers’ general views on, and use of, CDSSs including computerized guidelines and risk assessment tools, in various settings.15 These systematic reviews identified barriers and facilitators mostly concerning time consumption, workflow, integration in the EHR, user friendliness, and relevance of the recommendations. Moxey et al conducted a subgroup analysis that identified the lack of CDSS integration into the EHR and patient negative opinion as barriers specific to the ambulatory care setting.15 Poor CDSS integration was confirmed as a main technological barrier in our review. The diversity of EHRs developed for primary care may explain the persistence of this barrier over time.82 Patient negative opinion was less frequently reported in our review, presumably because of the increasing acceptance over time of the use of health information systems during consultations.83
Other barriers and facilitators identified in the present review are original as compared with previous reviews.13,15 First, teamwork needs for and benefits of using CDSSs were frequently reported. The importance of teamwork could not be identified previously since it is just emerging in primary care in many health care systems, contrarily to the hospital setting.84,85 Second, PCPs expressed difficulties in using CDSSs with patients co-managed by specialists, due to discrepancies between specialist and CDSS recommendations or to outdated patient information in the EHR. Third, PCPs expected CDSSs covering a large array of conditions in agreement with the diversity of the health issues they manage. Fourth, the contribution of CDSSs to reporting on quality measures was valued by PCPs, in a context of evaluation programs implemented in primary care.
Strengths and Weaknesses
The scope of this review was limited to strictly defined CDSSs providing recommendations to PCPs based on individual patient characteristics, according to a medical decision-making perspective. Two previous systematic reviews13,15 also included decision supports providing individualized assessments (risks) or general recommendations (not personalized to the patient), which presumably increased the heterogeneity of the barriers and facilitators identified. They also have included decision supports used in any care setting (including hospitals), and those evaluated using simulated clinical scenarios. In the present review, only CDSSs used in clinical settings and in the specific context of primary care were included, which further strengthens the external validity of the results. It may, however, not be fully representative of the various CDSSs developed for primary care, as some of them were probably not studied regarding barriers and facilitators to their use.
Frequency-based indicators of the impact of HOT-fit categories on CDSS use were useful for comparison purposes; they were, however, limited by the heterogeneity of CDSS barriers and facilitators and by the difficulty to weight them individually according to their perceived importance by PCPs. In addition, PCPs reported some barriers such as conflicts between CDSS recommendations and their own expertise or lack of computer skills, while the first barrier is more likely related to the use of guidelines86 and the second to the uptake of EHRs.87,88 The classification of barrier and facilitators in the evaluation measures was sometimes subjective because of similarities between, or lack of clear definition of some HOT-fit evaluation measures, as already reported by other authors.13 For instance, the HOT-fit evaluation measure “clinical process” in the dimension “structure” of the organizational factor is close to the evaluation measure “clinical practice” of the net benefits dimension.
Implications
The present systematic review highlights barriers and facilitators to the use of CDSSs related to its feasibility (eg, increased workload), acceptability (eg, conflicts with PCPs expertise or beliefs), meaningfulness (eg, relevance of recommendations), and effectiveness (net benefits dimension). These different forms of evidence refer to the feasibility, appropriateness, meaningfulness, and effectiveness (FAME) evidence-based model,89 which is useful to understand complex interventions such as implementing CDSSs. Based on these findings, we inferred an operational list of 11 intrinsic and contextual CDSS features expected to make them more feasible, acceptable, meaningful, and effective in primary care (Table 5). They are spread across the 3 interdependent human, organizational, and technological factors. Among intrinsic features, the expectation of decision support for preventive care is consistent with the great importance of prevention in primary care practice. The expectation of a large array of conditions covered by CDSSs is explained by the preference of PCPs for a single comprehensive system rather than several CDSSs with limited clinical coverage displaying recommendations in separate windows, each requiring a specific training. Information overload refers to PCPs facing more information than they have the time or cognitive ability to process.90,91 Clinical decision support systems aim at both rationalizing patient management while avoiding overwhelming PCPs with information, which can be achieved by CDSSs providing concise recommendations and prioritizing the most appropriate interventions recommended for each patient.92 In addition, the feature of providing patients with educational material supports shared decision making within a patient-centered approach. Among contextual features, developing CDSSs in close collaboration with PCPs according to a bottom-up approach is needed to improve their perceived usefulness and user-friendliness and the relevance of their recommendations. Providing the rationale for selecting the sources of the CDSS knowledge base is expected to increase CDSS reliability. This seems critical, even more for future non–knowledge-based CDSSs, as health care professionals are exposed to automation bias, which consists of over-relying on automated advice.93 Teamwork for data collection and use of CDSSs may ease physician workload and make EHR patient data more complete and reliable. Several of these intrinsic and contextual features may allow the leading barrier, increased workload, to be overcome. Since the findings of the present review show that human and organizational factors are the most impeding to CDSS use, we recommend that CDSS developers investigate the human and organizational requirements in the early stages of CDSS development and that evaluation studies of CDSS use systematically evaluate these factors. In addition, quantitative studies are particularly needed to assess the weight of the main barriers and facilitators identified through the present framework synthesis.
Table 5.
Expected Features of a CDSS for Primary Care
| Intrinsic features |
| Including preventive care |
| Covering a large array of conditions |
| Providing reminders personalized to the patient |
| Minimizing information overload and alert fatigue |
| Providing educational materials to patients |
| Integrated in the EHR, with the fewest possible duplicate data entries |
| Fast processing |
| Contextual features |
| Developed in close collaboration with PCPs |
| Providing the rationale for the selection of sources of its knowledge base |
| With teamwork for data collection and use of the CDSS |
| With systematic training for its use |
CDSS = clinical decision support system; EHR = electronic health record; PCPs = primary care professionals.
CONCLUSION
Although benefits reported by PCPs support the potential effectiveness of CDSS use in improving quality and safety of care, they also highlight its lack of efficiency due to increased workload. Our findings emphasize the need for CDSS developers to better address human and organizational issues in addition to technological stakes. Covering theses 3 factors, we inferred core intrinsic and contextual CDSS features expected to improve their usability in primary care.
Supplementary Material
Acknowledgments
We would like to thank Mathieu Fauvernier and Maxime Bonjour (Service de Biostatistiques des Hospices Civils de Lyon, France; UMR5558, Laboratoire de Biométrie et Biologie Évolutive, Équipe Biostatistique-Santé, Villeurbanne, France) for their comments on the data synthesis method. We would like to thank Colin Sidre and Bastien Blanchon (BIU Santé Médecine, Paris, France) for help with the search strategy. We also thank Philip Robinson (DRS, Hospices Civils de Lyon) for help in manuscript preparation.
Footnotes
Conflicts of interest: authors report none.
Funding support: The study funder was a public health institution (Agence Régionale de Santé Auvergne-Rhône-Alpes) through a grant attributed to Pierre-Yves Meunier.
Disclaimer: The study funder did not interfere in the conduct of this study. All authors confirm the independence of researchers from funders. All authors had full access to all of the data (including tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Trial registration: Protocol registered on PROSPERO (CRD42020185199) (no amendments to information provided in the protocol).
REFERENCES
- 1.World Health Organisation . Technical Series on Primary Health Care: Quality in Primary Health Care. World Health Organisation; 2018. Accessed Oct 5, 2021. https://www.who.int/docs/default-source/primary-health-care-conference/quality.pdf [Google Scholar]
- 2.van der Keylen P, Tomandl J, Wollmann K, et al. The online health information needs of family physicians: systematic review of qualitative and quantitative studies. J Med Internet Res. 2020; 22(12): e18816. 10.2196/18816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sim I, Gorman P, Greenes RA, et al. Clinical decision support systems for the practice of evidence-based medicine. J Am Med Inform Assoc. 2001; 8(6): 527-534. 10.1136/jamia.2001.0080527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutton RT, Pincock D, Baumgart DC, Sadowski DC, Fedorak RN, Kroeker KI.. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med. 2020; 3(1): 17. 10.1038/s41746-020-0221-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organisation . WHO Guideline : Recommendations on Digital Interventions for Health System Strenghtening. World Health Organisation; 2019. Accessed Oct 5, 2021. https://apps.who.int/iris/handle/10665/331883 [PubMed] [Google Scholar]
- 6.Osheroff JA, Teich JM, Middleton B, Steen EB, Wright A, Detmer DE.. A roadmap for national action on clinical decision support. J Am Med Inform Assoc. 2007; 14(2): 141-145. 10.1197/jamia.M2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tcheng JE, National Academy of Medicine , eds. Optimizing Strategies for Clinical Decision Support: Summary of a Meeting Series. National Academy of Medicine; 2017. [PubMed] [Google Scholar]
- 8.Heselmans A, Van de Velde S, Donceel P, Aertgeerts B, Ramaekers D.. Effectiveness of electronic guideline-based implementation systems in ambulatory care settings - a systematic review. Implement Sci. 2009; 4(1): 82. 10.1186/1748-5908-4-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Souza NM, Sebaldt RJ, Mackay JA, et al. ; CCDSS Systematic Review Team . Computerized clinical decision support systems for primary preventive care: a decision-maker-researcher partnership systematic review of effects on process of care and patient outcomes. Implement Sci. 2011; 6(1): 87. 10.1186/1748-5908-6-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwan JL, Lo L, Ferguson J, et al. Computerised clinical decision support systems and absolute improvements in care: meta-analysis of controlled clinical trials. BMJ. 2020; 370: m3216. 10.1136/bmj.m3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lobach DF. The road to effective clinical decision support: are we there yet? BMJ. 2013; 346: f1616. 10.1136/bmj.f1616 [DOI] [PubMed] [Google Scholar]
- 12.Sarkar U, Samal L.. How effective are clinical decision support systems? BMJ. 2020; 370: m3499. 10.1136/bmj.m3499 [DOI] [PubMed] [Google Scholar]
- 13.Kilsdonk E, Peute LW, Jaspers MWM.. Factors influencing implementation success of guideline-based clinical decision support systems: A systematic review and gaps analysis. Int J Med Inform. 2017; 98: 56-64. 10.1016/j.ijmedinf.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 14.Saleem JJ, Patterson ES, Militello L, Render ML, Orshansky G, Asch SM.. Exploring barriers and facilitators to the use of computerized clinical reminders. J Am Med Inform Assoc. 2005; 12(4): 438-447. 10.1197/jamia.M1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moxey A, Robertson J, Newby D, Hains I, Williamson M, Pearson SA.. Computerized clinical decision support for prescribing: provision does not guarantee uptake. J Am Med Inform Assoc. 2010; 17(1): 25-33. 10.1197/jamia.M3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westerbeek L, Ploegmakers KJ, de Bruijn GJ, et al. Barriers and facilitators influencing medication-related CDSS acceptance according to clinicians: a systematic review. Int J Med Inform. 2021; 152: 104506. 10.1016/j.ijmedinf.2021.104506 [DOI] [PubMed] [Google Scholar]
- 17.Nurek M, Kostopoulou O, Delaney BC, Esmail A.. Reducing diagnostic errors in primary care. A systematic meta-review of computerized diagnostic decision support systems by the LINNEAUS collaboration on patient safety in primary care. Eur J Gen Pract. 2015; 21(Suppl): 8-13. 10.3109/13814788.2015.1043123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laka M, Milazzo A, Merlin T.. Factors that impact the adoption of clinical decision support systems (CDSS) for antibiotic management. Int J Environ Res Public Health. 2021; 18(4): 1901. 10.3390/ijerph18041901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oluoch T, Santas X, Kwaro D, et al. The effect of electronic medical record-based clinical decision support on HIV care in resource-constrained settings: a systematic review. Int J Med Inform. 2012; 81(10): e83-e92. 10.1016/j.ijmedinf.2012.07.010 [DOI] [PubMed] [Google Scholar]
- 20.Yusof MM, Kuljis J, Papazafeiropoulou A, Stergioulas LK.. An evaluation framework for Health Information Systems: human, organization and technology-fit factors (HOT-fit). Int J Med Inform. 2008; 77(6): 386-398. 10.1016/j.ijmedinf.2007.08.011 [DOI] [PubMed] [Google Scholar]
- 21.Delone William H., McLean Ephraim R.. The DeLone and McLean model of information systems success: a ten-year update. J Manage Inf Syst. 2003; 19(4): 9-30. 10.1080/07421222.2003.11045748 [DOI] [Google Scholar]
- 22.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC.. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009; 4: 50. 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sittig DF, Singh H.. A new sociotechnical model for studying health information technology in complex adaptive healthcare systems. Qual Saf Health Care. 2010; 19(Suppl 3): i68-i74. 10.1136/qshc.2010.042085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venkatesh V, Thong J, Xu X.. Unified theory of acceptance and use of technology: a synthesis and the road ahead. J Assoc Inf Syst. 2016; 17(5). 10.17705/1jais.00428 [DOI] [Google Scholar]
- 25.Greenhalgh T, Wherton J, Papoutsi C, et al. Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J Med Internet Res. 2017; 19(11): e367. 10.2196/jmir.8775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen J, Gay B, Crebolder H, et al. The european definition of general practice/family medicine. Published 2011. Accessed Feb 20, 2022. https://www.woncaeurope.org/file/520e8ed3-30b4-4a74-bc35-87286d3de5c7/Definition%203rd%20ed%202011%20with%20revised%20wonca%20tree.pdf
- 27.Veritas Health Innovation . Covidence systematic review software. www.covidence.org
- 28.Harrison R, Jones B, Gardner P, Lawton R.. Quality assessment with diverse studies (QuADS): an appraisal tool for methodological and reporting quality in systematic reviews of mixed- or multi-method studies. BMC Health Serv Res. 2021; 21(1): 144. 10.1186/s12913-021-06122-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yusof MM. A case study evaluation of a Critical Care Information System adoption using the socio-technical and fit approach. Int J Med Inform. 2015; 84(7): 486-499. 10.1016/j.ijmedinf.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 30.Hong QN, Pluye P, Bujold M, Wassef M.. Convergent and sequential synthesis designs: implications for conducting and reporting systematic reviews of qualitative and quantitative evidence. Syst Rev. 2017; 6(1): 61. 10.1186/s13643-017-0454-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lizarondo L, Stern C, Carrier J, et al. Chapter 8: mixed methods systematic reviews. In: Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis. JBI; 2020. 10.46658/JBIMES-20-09 [DOI] [PubMed] [Google Scholar]
- 32.Dixon-Woods M. Using framework-based synthesis for conducting reviews of qualitative studies. BMC Med. 2011; 9(1): 39. 10.1186/1741-7015-9-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.QSR International Pty Ltd. NVivo. Published 2021. https://www.qsrinternational.com/nvivo-qualitative-data-analysis-software/home
- 34.Abimbola S, Patel B, Peiris D, et al. The NASSS framework for ex post theorisation of technology-supported change in healthcare: worked example of the TORPEDO programme. BMC Med. 2019; 17(1): 233. 10.1186/s12916-019-1463-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.af Klercker T, Zetraeus S.. Dilemmas in introducing World Wide Web-based information technology in primary care: a focus group study. Fam Pract. 1998; 15(3): 205-210. 10.1093/fampra/15.3.205 [DOI] [PubMed] [Google Scholar]
- 36.Alagiakrishnan K, Wilson P, Sadowski C, et al. Physicians’use of computerized clinical decision supports to improve medication management in the elderly; the Seniors Medication Alert and Review Technology intervention. Clin Interv Aging. 2016; (11): 73-81. 10.2147/CIA.S94126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arts DL, Medlock SK, van Weert HCPM, Wyatt JC, Abu-Hanna A.. Acceptance and barriers pertaining to a general practice decision support system for multiple clinical conditions: a mixed methods evaluation. PLoS One. 2018; 13(4): e0193187. 10.1371/journal.pone.0193187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ash JS, Sittig DF, Wright A, et al. Clinical decision support in small community practice settings: a case study. J Am Med Inform Assoc. 2011; 18(6): 879-882. 10.1136/amiajnl-2010-000013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bandong AN, Mackey M, Leaver A, et al. an interactive website for whiplash management (My Whiplash Navigator): process evaluation of design and implementation. JMIR Form Res. 2019; 3(3): e12216. 10.2196/12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bessat C, Zonon NA, D’Acremont V.. Large-scale implementation of electronic Integrated Management of Childhood Illness (eIMCI) at the primary care level in Burkina Faso: a qualitative study on health worker perception of its medical content, usability and impact on antibiotic prescription and resistance. BMC Public Health. 2019; 19(1): 449. 10.1186/s12889-019-6692-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bindels R, Hasman A, Derickx M, Van Wersch JW, Winkens RA.. User satisfaction with a real-time automated feedback system for general practitioners: a quantitative and qualitative study. Int J Qual Health Care. 2003; 15(6): 501-508. 10.1093/intqhc/mzg076 [DOI] [PubMed] [Google Scholar]
- 42.Curry L, Reed MH.. Electronic decision support for diagnostic imaging in a primary care setting. J Am Med Inform Assoc. 2011; 18(3): 267-270. 10.1136/amiajnl-2011-000049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dixon BE, Simonaitis L, Goldberg HS, et al. A pilot study of distributed knowledge management and clinical decision support in the cloud. Artif Intell Med. 2013; 59(1): 45-53. 10.1016/j.artmed.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 44.Doerr M, Edelman E, Gabitzsch E, Eng C, Teng K.. Formative evaluation of clinician experience with integrating family history-based clinical decision support into clinical practice. J Pers Med. 2014; 4(2): 115-136. 10.3390/jpm4020115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edelman EA, Lin BK, Doksum T, et al. Evaluation of a novel electronic genetic screening and clinical decision support tool in prenatal clinical settings. Matern Child Health J. 2014; 18(5): 1233-1245. 10.1007/s10995-013-1358-y [DOI] [PubMed] [Google Scholar]
- 46.Feldstein AC, Schneider JL, Unitan R, et al. Health care worker perspectives inform optimization of patient panel-support tools: a qualitative study. Popul Health Manag. 2013; 16(2): 107-119. 10.1089/pop.2012.0065 [DOI] [PubMed] [Google Scholar]
- 47.Guenter D, Abouzahra M, Schabort I, et al. Design process and utilization of a novel clinical decision support system for neuropathic pain in primary care: mixed methods observational study. JMIR Med Inform. 2019; 7(3): e14141. 10.2196/14141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Helldén A, Al-Aieshy F, Bastholm-Rahmner P, et al. Development of a computerised decisions support system for renal risk drugs targeting primary healthcare. BMJ Open. 2015; 5(7): e006775. 10.1136/bmjopen-2014-006775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heselmans A, Delvaux N, Laenen A, et al. Computerized clinical decision support system for diabetes in primary care does not improve quality of care: a cluster-randomized controlled trial. Implement Sci. 2020; 15(1): 5. 10.1186/s13012-019-0955-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jensen C, McKerrow NH, Wills G.. Acceptability and uptake of an electronic decision-making tool to support the implementation of IMCI in primary healthcare facilities in KwaZulu-Natal, South Africa. Paediatr Int Child Health. 2019; 40(4): 215-226. 10.1080/20469047.2019.1697573 [DOI] [PubMed] [Google Scholar]
- 51.Jenssen BP, Bryant-Stephens T, Leone FT, Grundmeier RW, Fiks AG.. Clinical decision support tool for parental tobacco treatment in primary care. Pediatrics. 2016; 137(5): e20154185-e20154185. 10.1542/peds.2015-4185 [DOI] [PubMed] [Google Scholar]
- 52.Kempe A, Hurley LP, Cardemil CV, et al. Use of immunization information systems in primary care. Am J Prev Med. 2017; 52(2): 173-182. 10.1016/j.amepre.2016.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koskela T, Sandström S, Mäkinen J, Liira H.. User perspectives on an electronic decision-support tool performing comprehensive medication reviews - a focus group study with physicians and nurses. BMC Med Inform Decis Mak. 2016; 16: 6. 10.1186/s12911-016-0245-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lam Shin Cheung J, Paolucci N, Price C, Sykes J, Gupta S; Canadian Respiratory Research Network . A system uptake analysis and GUIDES checklist evaluation of the Electronic Asthma Management System: a point-of-care computerized clinical decision support system. J Am Med Inform Assoc. 2020; 27(5): 726-737. 10.1093/jamia/ocaa019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lemke AA, Thompson J, Hulick PJ, et al. Primary care physician experiences utilizing a family health history tool with electronic health record-integrated clinical decision support: an implementation process assessment. J Community Genet. 2020; 11(3): 339-350. 10.1007/s12687-020-00454-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Litvin CB, Hyer JM, Ornstein SM.. Use of clinical decision support to improve primary care identification and management of chronic kidney disease (CKD). J Am Board Fam Med. 2016; 29(5): 604-612. 10.3122/jabfm.2016.05.160020 [DOI] [PubMed] [Google Scholar]
- 57.Litvin CB, Ornstein SM, Wessell AM, Nemeth LS, Nietert PJ.. Adoption of a clinical decision support system to promote judicious use of antibiotics for acute respiratory infections in primary care. Int J Med Inform. 2012; 81(8): 521-526. 10.1016/j.ijmedinf.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 58.Lugtenberg M, Weenink JW, van der Weijden T, Westert GP, Kool RB.. Implementation of multiple-domain covering computerized decision support systems in primary care: a focus group study on perceived barriers. BMC Med Inform Decis Mak. 2015; 15: 82. 10.1186/s12911-015-0205-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lugtenberg M, Pasveer D, van der Weijden T, Westert GP, Kool RB.. Exposure to and experiences with a computerized decision support intervention in primary care: results from a process evaluation. BMC Fam Pract. 2015; 16(1): 141. 10.1186/s12875-015-0364-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maia JX, de Sousa LAP, Marcolino MS, et al. The impact of a clinical decision support system in diabetes primary care patients in a developing country. Diabetes Technol Ther. 2016; 18(4): 258-263. 10.1089/dia.2015.0253 [DOI] [PubMed] [Google Scholar]
- 61.Marcolino MS, Oliveira JAQ, Cimini CCR, et al. Development and implementation of a decision support system to improve control of hypertension and diabetes in a resource-constrained area in Brazil: mixed methods study. J Med Internet Res. 2021; 23(1): e18872. 10.2196/18872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Minian N, Noormohamed A, Lingam M, et al. Integrating a brief alcohol intervention with tobacco addiction treatment in primary care: qualitative study of health care practitioner perceptions. Addict Sci Clin Pract. 2021; 16(1): 17. 10.1186/s13722-021-00225-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montini T, Schenkel AB, Shelley DR.. Feasibility of a computerized clinical decision support system for treating tobacco use in dental clinics. J Dent Educ. 2013; 77(4): 458-462. 10.1002/j.0022-0337.2013.77.4.tb05491.x [DOI] [PubMed] [Google Scholar]
- 64.Pannebakker MM, Mills K, Johnson M, Emery JD, Walter FM.. Understanding implementation and usefulness of electronic clinical decision support (eCDS) for melanoma in English primary care: a qualitative investigation. BJGP Open. 2019; 3(1): X101635. 10.3399/bjgpopen18X101635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peiris D, Williams C, Holbrook R, et al. A web-based clinical decision support tool for primary health care management of back pain: development and mixed methods evaluation. JMIR Res Protoc. 2014; 3(2): e17. 10.2196/resprot.3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Praveen D, Patel A, Raghu A, et al. SMARTHealth India: development and field evaluation of a mobile clinical decision support system for cardiovascular diseases in rural India. JMIR Mhealth Uhealth. 2014; 2(4): e54. 10.2196/mhealth.3568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Price M, Davies I, Rusk R, Lesperance M, Weber J.. Applying STOPP guidelines in primary care through electronic medical record decision support: randomized control trial highlighting the importance of data quality. JMIR Med Inform. 2017; 5(2): e15. 10.2196/medinform.6226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Richardson S, Feldstein D, McGinn T, et al. Live usability testing of two complex clinical decision support tools: observational study. JMIR Hum Factors. 2019; 6(2): e12471. 10.2196/12471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rieckert A, Sommerauer C, Krumeich A, Sönnichsen A.. Reduction of inappropriate medication in older populations by electronic decision support (the PRIMA-eDS study): a qualitative study of practical implementation in primary care. BMC Fam Pract. 2018; 19(1): 110. 10.1186/s12875-018-0789-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rieckert A, Teichmann AL, Drewelow E, et al. Reduction of inappropriate medication in older populations by electronic decision support (the PRIMA-eDS project): a survey of general practitioners’ experiences. J Am Med Inform Assoc. 2019; 26(11): 1323-1332. 10.1093/jamia/ocz104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rousseau N, McColl E, Newton J, Grimshaw J, Eccles M.. Practice based, longitudinal, qualitative interview study of computerised evidence based guidelines in primary care. BMJ. 2003; 326(7384): 314-314. 10.1136/bmj.326.7384.314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rubin MA, Bateman K, Donnelly S, et al. Use of a personal digital assistant for managing antibiotic prescribing for outpatient respiratory tract infections in rural communities. J Am Med Inform Assoc. 2006; 13(6): 627-634. 10.1197/jamia.M2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silveira DV, Marcolino MS, Machado EL, et al. Development and evaluation of a mobile decision support system for hypertension management in the primary care setting in Brazil: mixed-methods field study on usability, feasibility, and utility. JMIR Mhealth Uhealth. 2019; 7(3): e9869-e9869. 10.2196/mhealth.9869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sukums F, Mensah N, Mpembeni R, et al. Promising adoption of an electronic clinical decision support system for antenatal and intrapartum care in rural primary healthcare facilities in sub-Saharan Africa: the QUALMAT experience. Int J Med Inform. 2015; 84(9): 647-657. 10.1016/j.ijmedinf.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 75.Toth-Pal E, Wårdh I, Strender LE, Nilsson G.. Implementing a clinical decision-support system in practice: a qualitative analysis of influencing attitudes and characteristics among general practitioners. Inform Health Soc Care. 2008; 33(1): 39-54. 10.1080/17538150801956754 [DOI] [PubMed] [Google Scholar]
- 76.Trafton J, Martins S, Michel M, et al. Evaluation of the acceptability and usability of a decision support system to encourage safe and effective use of opioid therapy for chronic, noncancer pain by primary care providers. Pain Med. 2010; 11(4): 575-585. 10.1111/j.1526-4637.2010.00818.x [DOI] [PubMed] [Google Scholar]
- 77.Trinkley KE, Kroehl ME, Kahn MG, et al. Applying clinical decision support design best practices with the practical robust implementation and sustainability model versus reliance on commercially available clinical decision support tools: randomized controlled trial. JMIR Med Inform. 2021; 9(3): e24359. 10.2196/24359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wan Q, Makeham M, Zwar NA, Petche S.. Qualitative evaluation of a diabetes electronic decision support tool: views of users. BMC Med Inform Decis Mak. 2012; 12(1): 61. 10.1186/1472-6947-12-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Williams PA, Furberg RD, Bagwell JE, LaBresh KA.. Usability testing and adaptation of the pediatric cardiovascular risk reduction clinical decision support tool. JMIR Hum Factors. 2016; 3(1): e17. 10.2196/humanfactors.5440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilson A, Duszynski A, Turnbull D, Beilby J.. Investigating patients’ and general practitioners’ views of computerised decision support software for the assessment and management of cardiovascular risk. Inform Prim Care. 2007; 15(1): 33-44. 10.14236/jhi.v15i1.642 [DOI] [PubMed] [Google Scholar]
- 81.Zheng K, Padman R, Johnson MP, Diamond HS.. Understanding technology adoption in clinical care: clinician adoption behavior of a point-of-care reminder system. Int J Med Inform. 2005; 74(7-8): 535-543. 10.1016/j.ijmedinf.2005.03.007 [DOI] [PubMed] [Google Scholar]
- 82.Turbow S, Hollberg JR, Ali MK.. Electronic health record interoperability: how did we get here and how do we move forward? JAMA Health Forum. 2021; 2(3): e210253. 10.1001/jamahealthforum.2021.0253 [DOI] [PubMed] [Google Scholar]
- 83.Luchenski SA, Reed JE, Marston C, Papoutsi C, Majeed A, Bell D.. Patient and public views on electronic health records and their uses in the United kingdom: cross-sectional survey. J Med Internet Res. 2013; 15(8): e160. 10.2196/jmir.2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zijl ALV, Vermeeren B, Koster F, Steijn B.. Interprofessional teamwork in primary care: the effect of functional heterogeneity on performance and the role of leadership. J Interprof Care. 2021; 35(1): 10-20. 10.1080/13561820.2020.1715357 [DOI] [PubMed] [Google Scholar]
- 85.Harris M, Advocat J, Crabtree B, et al. Interprofessional teamwork innovations for primary health care practices and practitioners: evidence from a comparison of reform in three countries. J Multidiscip Healthc. 2016(9): 35-46. 10.2147/JMDH.S97371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999; 282(15): 1458-1465. 10.1001/jama.282.15.1458 [DOI] [PubMed] [Google Scholar]
- 87.Ancker JS, Kern LM, Edwards A, et al. ; HITEC Investigators . How is the electronic health record being used? Use of EHR data to assess physician-level variability in technology use. J Am Med Inform Assoc. 2014; 21(6): 1001-1008. 10.1136/amiajnl-2013-002627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boonstra A, Broekhuis M.. Barriers to the acceptance of electronic medical records by physicians from systematic review to taxonomy and interventions. BMC Health Serv Res. 2010; 10(1): 231. 10.1186/1472-6963-10-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pearson A, Wiechula R, Court A, Lockwood C.. The JBI model of evidence-based healthcare. Int J Evid Based Healthc. 2005; 3(8): 207-215. 10.1111/j.1479-6988.2005.00026.x [DOI] [PubMed] [Google Scholar]
- 90.Schick AG, Gordon LA, Haka S.. Information overload: a temporal approach. Account Organ Soc. 1990; 15(3): 199-220. 10.1016/0361-3682(90)90005-F [DOI] [Google Scholar]
- 91.Eppler MJ, Mengis J.. The concept of information overload - a review of literature from organization science, accounting, marketing, MIS, and related disciplines (2004). Info Soc. 2004(20)5: 1-20. 10.1007/978-3-8349-9772-2_15 [DOI] [Google Scholar]
- 92.Taksler GB, Hu B, DeGrandis F Jr, et al. Effect of individualized preventive care recommendations vs usual care on patient interest and use of recommendations: a pilot randomized clinical trial. JAMA Netw Open. 2021; 4(11): e2131455. 10.1001/jamanetworkopen.2021.31455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goddard K, Roudsari A, Wyatt JC.. Automation bias: empirical results assessing influencing factors. Int J Med Inform. 2014; 83(5): 368-375. 10.1016/j.ijmedinf.2014.01.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.