Abstract
People with diabetes and chronic kidney disease (CKD) are at high risk for kidney failure, atherosclerotic cardiovascular disease, heart failure, and premature mortality. Recent clinical trials support new approaches to treat diabetes and CKD. The 2022 American Diabetes Association (ADA) Standards of Medical Care in Diabetes and the Kidney Disease: Improving Global Outcomes (KDIGO) 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease each provide evidence-based recommendations for management. A joint group of ADA and KDIGO representatives reviewed and developed a series of consensus statements to guide clinical care from the ADA and KDIGO guidelines. The published guidelines are aligned in the areas of CKD screening and diagnosis, glycemia monitoring, lifestyle therapies, treatment goals, and pharmacologic management. Recommendations include comprehensive care in which pharmacotherapy that is proven to improve kidney and cardiovascular outcomes is layered on a foundation of healthy lifestyle. Consensus statements provide specific guidance on use of renin-angiotensin system inhibitors, metformin, sodium–glucose cotransporter 2 inhibitors, glucagon-like peptide 1 receptor agonists, and a nonsteroidal mineralocorticoid receptor antagonist. These areas of consensus provide clear direction for implementation of care to improve clinical outcomes of people with diabetes and CKD.
Introduction
Clinicians and patients refer to clinical practice guidelines to synthesize data and provide expert direction on diagnosis and treatment. Guidelines must be evidence-based, systematic, transparent, and explicit to offer credibility and impact implementation. They must also allow adaptation to local circumstances and provide mechanisms for updates over time.
A rapidly expanding number of clinical trials are advancing clinical care in the field of diabetes and chronic kidney disease (CKD). The American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO) each follow structured processes to assess these data and develop rigorous, evidence-based guidelines for adults with diabetes and CKD (1,2). Areas of consensus between the two guidelines therefore represent independent agreement on high priority areas of care.
The goal of this consensus report was to identify and highlight shared recommendations from the ADA 2022 Standards of Medical Care in Diabetes (hereafter called Standards of Care) and KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease (1,2). A joint writing group of ADA and KDIGO representatives convened to compare and contrast ADA and KDIGO recommendations. A series of virtual meetings were held from March 2021 through February 2022 to define scope, review published guidelines and supportive evidence, and jointly write and revise the consensus report. Meetings were cochaired by an ADA representative (G.B.) and a KDIGO representative (I.H.d.B.) and supported by both ADA and KDIGO staff.
Consensus statements were drafted when recommendations from each organization were aligned and supported by high-quality evidence from randomized clinical trials (ada/kdigo consensus statements). These statements do not specify a level of evidence, which can be found in the individual ADA and KDIGO documents. However, all consensus statements were endorsed by both the ADA and KDIGO and represent broad agreement on evidence-based management of adults with diabetes and CKD.
ADA/KDIGO Consensus Statements
All patients with type 1 diabetes (T1D) or type 2 diabetes (T2D) and CKD should be treated with a comprehensive plan, outlined and agreed by health care professionals and the patient together, to optimize nutrition, exercise, smoking cessation, and weight, upon which are layered evidence-based pharmacologic therapies aimed at preserving organ function and other therapies selected to attain intermediate targets for glycemia, blood pressure (BP), and lipids.
An ACE inhibitor (ACEi) or angiotensin II receptor blocker (ARB) is recommended for patients with T1D or T2D who have hypertension and albuminuria, titrated to the maximum antihypertensive or highest tolerated dose.
A statin is recommended for all patients with T1D or T2D and CKD, moderate intensity for primary prevention of atherosclerotic cardiovascular disease (ASCVD) or high intensity for patients with known ASCVD and some patients with multiple ASCVD risk factors.
Metformin is recommended for patients with T2D, CKD, and estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2; the dose should be reduced to 1,000 mg daily in patients with eGFR 30–44 mL/min/1.73 m2 and in some patients with eGFR 45–59 mL/min/1.73 m2 who are at high risk of lactic acidosis.
A sodium–glucose cotransporter 2 inhibitor (SGLT2i) with proven kidney or cardiovascular benefit is recommended for patients with T2D, CKD, and eGFR ≥20 mL/min/1.73 m2. Once initiated, the SGLT2i can be continued at lower levels of eGFR.
A glucagon-like peptide 1 (GLP-1) receptor agonist with proven cardiovascular benefit is recommended for patients with T2D and CKD who do not meet their individualized glycemic target with metformin and/or an SGLT2i or who are unable to use these drugs.
A nonsteroidal mineralocorticoid receptor antagonist (ns-MRA) with proven kidney and cardiovascular benefit is recommended for patients with T2D, eGFR ≥25 mL/min/1.73 m2, normal serum potassium concentration, and albuminuria (albumin-to-creatinine ratio [ACR] ≥30 mg/g) despite maximum tolerated dose of renin-angiotensin system (RAS) inhibitor.
Background
CKD occurring among people with diabetes is common, morbid, and costly. The International Diabetes Federation estimates that 537 million people were living with diabetes in 2021, with an expected increase to 784 million by the year 2045 (3). The prevalence of CKD among people with diabetes is >25%, and it has been estimated that 40% of people with diabetes develop CKD during their lifetime (4). As the prevalence of diabetes has increased, the prevalence of CKD attributable to diabetes has grown proportionally (4).
Diabetes is the most common cause of kidney failure requiring kidney transplantation or dialysis worldwide (5). In the U.S., diabetes fueled a marked increase in the prevalence of kidney failure over the last 30 years and now accounts for half of all new cases of kidney failure (6). Moreover, CKD markedly amplifies risks of ASCVD, heart failure (HF), cardiovascular death, and all-cause mortality among people with diabetes (7,8).
In the U.S., one of every five adults with diabetes is not aware of their diagnosis (9). Awareness of CKD is even lower, with 9 of 10 individuals unaware of having underlying CKD, including 2 of 5 with severe CKD (6,10). In addition, both diabetes and CKD disproportionately affect racial and ethnic minorities and older adults. Insufficient screening, diagnosis, and awareness impair efforts to implement treatment and improve outcomes and exacerbate racial, socioeconomic, and ethnic disparities. Furthermore, recent population-based data uncovering disparities in access to glucose-lowering agents with proven kidney and cardiovascular benefits further highlight the need for interventions that ensure more equitable access to and use of these pharmacotherapies across racial and ethnic minorities (11).
In the U.S., the total estimated cost of diagnosed diabetes in 2017 was $327 billion, including $237 billion in direct medical costs and $90 billion in reduced productivity (12). The estimated global direct health expenditure on diabetes in 2019 was $760 billion (13). CKD, with and without kidney failure, is a major driver of the cost of diabetes care. Costs of CKD, stroke, and heart disease are additive (14,15).
Screening and Diagnosis
CKD is defined as persistent eGFR <60 mL/min/1.73 m2, albuminuria (ACR ≥30 mg/g), or other markers of kidney damage, such as hematuria or structure abnormalities. Importantly, these measurements can vary within individuals over time, and persistence for at least 3 months is therefore required for diagnosis (16).
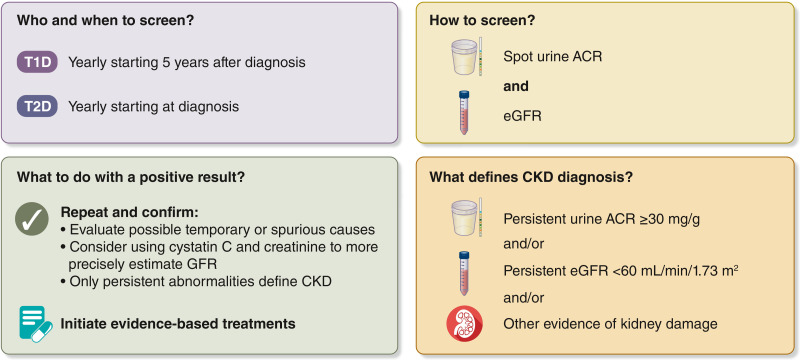
For most people, CKD is not identified as a result of symptoms; CKD is often diagnosed through routine screening. Both the ADA and KDIGO recommend annual screening of patients with diabetes for CKD (17,18) (Fig. 1). CKD screening should start at diagnosis of T2D because evidence of CKD is often already apparent at this time. For T1D, screening is recommended commencing 5 years after diagnosis, prior to which CKD is uncommon. Screening is underutilized, particularly for albuminuria. In typical practice in the U.S., less than half of patients with T2D are screened for albuminuria in a given year (19).
Figure 1.
CKD screening and diagnosis for people living with diabetes. Screening includes measurement of both urine albumin and eGFR. Abnormalities should be confirmed. Persistent abnormalities in either urine ACR or eGFR (or both) diagnose CKD and should lead to immediate initiation of evidence-based treatments. ACR, albumin-to-creatinine ratio; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; T1D, type 1 diabetes; T2D, type 2 diabetes.
Clinical laboratories routinely report eGFR calculated from serum creatinine and demographic data (20–22). The American Society of Nephrology and National Kidney Foundation advocate using the 2021 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, which was generated without inclusion of a term for race and calculates eGFR without regard to race, to estimate glomerular filtration rate (GFR) from creatinine, age, and sex (20). Another CKD-EPI equation that additionally incorporates serum cystatin C increases precision and reduces racial and ethnic bias, offering additional value in screening and for confirmation of low eGFR in appropriate cases (23–25).
Calculation of the ACR in single-voided “spot” urine samples is most convenient to measure albuminuria. Early morning urine specimens are ideal, although samples collected any time of day may be used. ACR has marked variability; therefore, a confirmatory urine sample within 3–6 months is recommended (26,27).
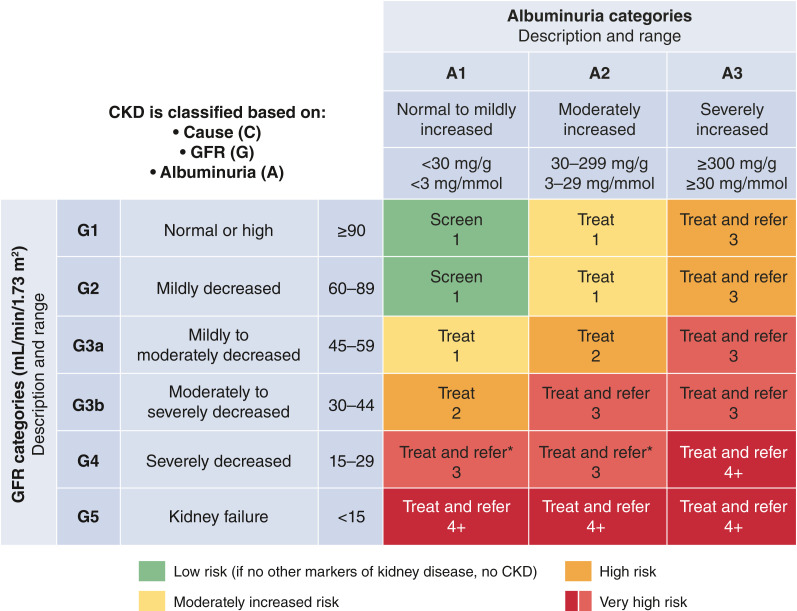
KDIGO has codified a CKD classification scheme based on eGFR and albuminuria that is endorsed by the ADA (26). In cohort studies, risks of progressive CKD, cardiovascular events, and mortality all increase with categories of increasing albuminuria or decreasing eGFR. Moreover, CKD stage and corresponding risk category can guide frequency of laboratory monitoring, treatment, and referral to nephrology care (Fig. 2).
Figure 2.
Risk of CKD progression, frequency of visits, and referral to nephrology according to GFR and albuminuria. The numbers in the boxes are a guide to the frequency of screening or monitoring (number of times per year). Green reflects no evidence of CKD by eGFR or albuminuria, with screening indicated once per year. For monitoring of prevalent CKD, suggested monitoring varies from once per year (yellow) to four times or more per year (i.e., every 1–3 months, [deep red]) according to risks of CKD progression and CKD complications. These are general parameters only, based on expert opinion, and underlying comorbid conditions and disease state must be taken into account, as well as the likelihood of impacting a change in management for any individual patient. CKD, chronic kidney disease; GFR, glomerular filtration rate.
A cause of CKD other than diabetes should be considered in the presence of other systemic diseases that cause CKD, when retinopathy is not present (particularly in T1D), or with CKD signs not common to diabetes (e.g., glomerular hematuria, large and abrupt changes in eGFR or albuminuria, or abnormal serology tests). In the absence of such “red flags,” CKD is usually attributed to diabetes and treated accordingly. Ongoing research seeks to define CKD subtypes with more granularity and link novel subtypes to precision treatments (28,29).
Comprehensive Care
Goals of Comprehensive Care
Multimorbidity is common in patients with diabetes and CKD, who are at high risk of CKD progression, cardiovascular events, and premature mortality. Therefore, both the ADA (1) and KDIGO (2) emphasize the importance of comprehensive, holistic, patient-centered medical care to improve overall patient outcomes.
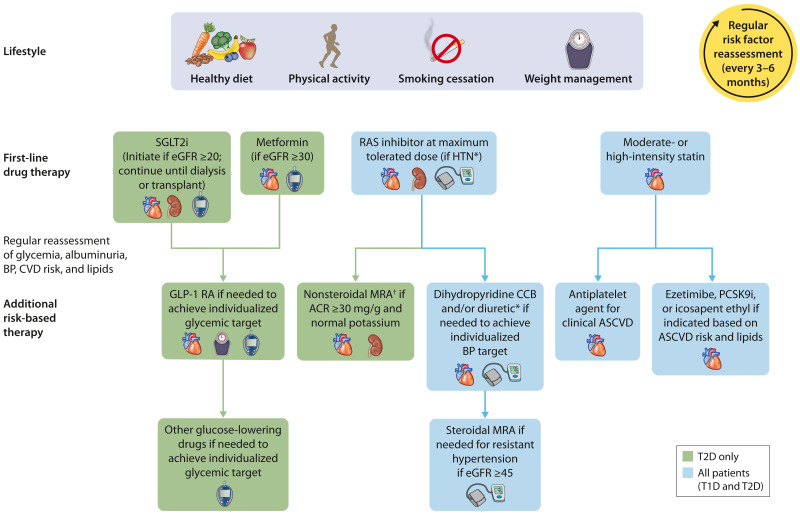
The goals of comprehensive care are to treat the patient as a “whole” person and incorporate coordinated multidisciplinary treatment, structured education to promote self-management, shared-decision making, and primary and secondary prevention of diabetes-related complications, including CKD, ASCVD, and HF (2). This approach requires treatment directed to optimize lifestyle, pharmacological therapy aimed at preserving organ function, and additional therapies aimed at improving intermediate risk factors such as glycemia, BP, and lipids (Fig. 3).
Figure 3.
Holistic approach for improving outcomes in patients with diabetes and CKD. Icons presented indicate the following benefits: BP cuff, BP lowering; glucometer, glucose lowering; heart, cardioprotection; kidney, kidney protection; scale, weight management. eGFR is presented in units of mL/min/1.73 m2. *ACEi or ARB (at maximal tolerated doses) should be first-line therapy for hypertension when albuminuria is present. Otherwise, dihydropyridine calcium channel blocker or diuretic can also be considered; all three classes are often needed to attain BP targets. †Finerenone is currently the only ns-MRA with proven clinical kidney and cardiovascular benefits. ACEi, angiotensin-converting enzyme inhibitor; ACR, albumin-to-creatinine ratio; ARB, angiotensin II receptor blocker; ASCVD, atherosclerotic cardiovascular disease; BP, blood pressure; CCB, calcium channel blocker; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; GLP-1 RA, GLP-1 receptor agonist; HTN, hypertension; MRA, mineralocorticoid receptor antagonist; ns-MRA, nonsteroidal mineralocorticoid receptor antagonist; PCSK9i, proprotein convertase subtilisin/kexin type 9 inhibitor; RAS, renin-angiotensin system; SGLT2i, sodium–glucose cotransporter 2 inhibitor; T1D, type 1 diabetes; T2D, type 2 diabetes.
With multiple interventions ubiquitously needed to optimize the care of people with diabetes and CKD, it is crucial to avoid therapeutic inertia (30). Most patients with diabetes and CKD have high residual risks of CKD progression and cardiovascular disease despite treatment, and increasing options are available for risk mitigation. Patients may need to be seen frequently to identify and implement multiple therapies, some of which may interact. For example, RAS inhibitors, SGLT2i, and the ns-MRA finerenone all cause initial hemodynamic reductions in GFR. When indicated, such medications may need to be added and adjusted sequentially, with frequent assessments to institute and optimize care in a timely manner. Empowering patients and facilitating multidisciplinary care can help institute and titrate multiple treatments expeditiously.
Consensus Statement
All patients with T1D or T2D and CKD should be treated with a comprehensive plan, outlined and agreed by health care professionals and the patient together, to optimize nutrition, exercise, smoking cessation, and weight, upon which are layered evidence-based pharmacologic therapies aimed at preserving organ function and other therapies selected to attain intermediate targets for glycemia, BP, and lipids.
Education, Self-care, and Patient Empowerment
The ADA and KDIGO guidelines both advocate for patients to take an active role in managing their diabetes and kidney disease and to have a voice in decisions that affect their well-being (2,31). Education for patients and an integrated approach to treatment is an effective approach for both patients and clinicians.
Patients know themselves better than anyone else, and although health care professionals have the medical background, when a patient and health care professional become partners in developing a shared-decision treatment plan the lives of the patients will improve. In addition, the time required by the health care professional in managing the patients care will be reduced. Patient priorities often do not align with health care professional priorities. Ideally, health care professionals will question patients about their priorities and together they will establish an agreed upon care program (32).
Ways in which patients can work with their health care professionals to manage their diabetes and CKD include asking questions; becoming educated about diet, physical activity, smoking cessation, glycemic control, and medications; talking to peers and support groups in the diabetes and CKD community; becoming familiar with technology that is available to track progress; and understanding test results in preparation for health care appointments (33).
Multidisciplinary Team Care
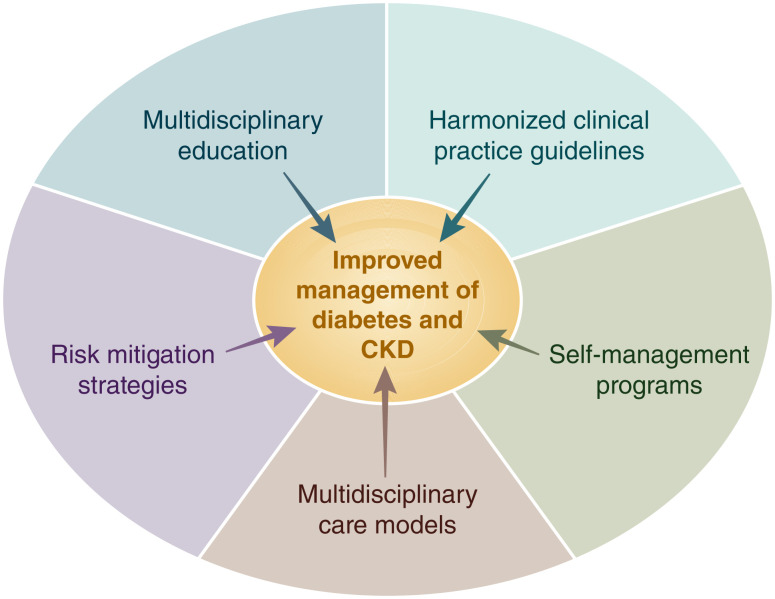
Diabetes and CKD management is ideal when the health care system model of care includes a multidisciplinary team to assist patients including the patient, physician (or other care provider), and other health care professionals (2,34). Both the ADA and KDIGO guidelines emphasize the importance of a team-based integrated approach that engages diabetes care and education specialists, physicians, nurse practitioners, physician assistants, nurses, dietitians, exercise specialists, pharmacists, dentists, podiatrists, and/or mental health professionals in the care of the patient, with multidisciplinary care models representing a key strategy to overcome barriers to effective management of patients with diabetes and CKD (Fig. 4).
Figure 4.
Overcoming barriers to management of CKD in patients with diabetes. Barriers such as low CKD awareness, high complexity of care, difficulties with adhering to increasingly complex treatment regimens, and low recognition and application of guideline-directed management all contribute to suboptimal management of patients with diabetes and CKD. Proposed strategies that may contribute to improved management of patients with diabetes and CKD include implementation of multidisciplinary models of care, structured risk mitigation strategies and education, multidisciplinary educational initiatives, harmonization of clinical practice guidelines, and provision of self-management programs for patients with diabetes and CKD.
Health care systems should include team-based care for patients and focus on both short- and long-term treatment plans. Lifestyle interventions for the patient must be included in determining an overall plan of care to ensure individual preferences are addressed and goals are established by all team members, especially the patient.
Behavioral evaluation should be considered in the initial assessment for all patients with diabetes. In addition, it should be considered in patients who are unable to meet goals in order to determine potential psychosocial barriers to treatment and self-management.
Lifestyle
Both the ADA and KDIGO guidelines underscore the integral role of medical nutritional therapy, including adequate access to nutritional management from a specialty-trained registered dietitian nutritionist (RD/RDN), for optimal diabetes management (Supplementary Table 1). The ADA and KDIGO guidelines both recommend individualized and balanced diets that are high in vegetables, fruits, and whole grains but are low in refined carbohydrates and sugar-sweetened beverages (1,2). Both guidelines also recommend a low-sodium diet (KDIGO <2,000 mg/day, ADA 1,500 to <2,300 mg/day), largely to control BP and reduce cardiovascular risk.
The ADA and KDIGO guidelines also recommend targeting a dietary protein intake of 0.8 g/kg/day, the same intake recommended by the World Health Organization for the general population. Higher protein intakes confer theoretical risk of enhancing kidney function decline (35). KDIGO performed a systematic review of randomized trials and found no conclusive evidence that restriction of dietary protein to levels <0.8 g/kg/day improves kidney or other health outcomes among people with diabetes and CKD (2). While the ADA and KDIGO are aligned in this regard, the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) has somewhat different recommendations, including restricting dietary protein to 0.55–0.60 g/kg/day (or lower with keto acid analog supplementation) for metabolically stable CKD patients without diabetes and to 0.6–0.8 g/kg/day for patients with diabetes and CKD (36). All recommendations call for higher levels of protein intake for patients with kidney failure treated with maintenance dialysis, who are often catabolic or malnourished (e.g., 1.0–1.2 g/kg/day).
The ADA and KDIGO guidelines also advise moderate to intense/vigorous physical activity with a cumulative duration of ≥150 min/week and avoidance of sedentary activity (1,2). In overweight or obese patients with diabetes, ADA and KDIGO show overall agreement with respect to achieving and maintaining healthy weight through diet, physical activity, and behavioral therapy (Supplementary Table 1). Though specific evidence is low, smoking cessation is also strongly advised.
Treatment Targets and Pharmacotherapy
Glycemic Control
Metrics and Frequency
Both the ADA and KDIGO recommend twice-yearly glycemic assessment using glycated hemoglobin (HbA1c) among stable patients with T2D who are meeting treatment goals and quarterly assessment among those who are intensively managed, whose therapy has changed, or whose treatment goals are not met (Supplementary Table 1). While both ADA and KDIGO focus on HbA1c as the primary tool for assessing long-term glycemic control, both guidelines acknowledge limitations in its accuracy and precision as an indirect metric of glycemic status, particularly in advanced CKD (i.e., CKD stages G4 and G5 without kidney replacement therapy [KRT]) and kidney failure treated by dialysis, and the inability of HbA1c to adequately capture glycemic variability and hypoglycemic events. Consequently, both guidelines emphasize the concurrent use of 1) HbA1c as a metric upon which therapeutic targets are defined based on randomized controlled trial (RCT) data, 2) continuous glucose monitoring (CGM) to assess effectiveness and safety of treatment among patients at risk for hypoglycemia or to assess overall glycemia when HbA1c is inaccurate, and 3) self-monitoring of blood glucose as a tool to guide medication adjustment, particularly in patients treated with insulin (37).
Individualized Targets
Both the ADA and KDIGO emphasize use of individualized glycemic targets that take into consideration key patient characteristics that may modify risks and benefits of intensive glycemic control (Supplementary Table 1). Based on RCT data, KDIGO recommends an individualized HbA1c target of <6.5% to <8.0% for patients with diabetes and CKD, with targets in this range having been associated with improvements in survival, cardiovascular outcomes, and microvascular end points, as well as lower risk of CKD progression. The ADA recommends a starting HbA1c target of <7% to reduce microvascular complications in most nonpregnant adult patients with T1D and T2D without hypoglycemia risk, although with higher goals (i.e., <8%) acceptable for patients with limited life expectancy and in whom the harms of treatment may outweigh the benefits.
CGM and Diabetes Technology
Diabetes technology refers to the hardware, devices, and software that patients with diabetes use to manage their chronic disease and encompasses 1) insulin administered with syringe, pen, or pump; 2) blood glucose monitoring with meter or CGM; and 3) hybrid devices that monitor glucose and deliver insulin. The ADA and KDIGO guidelines highlight the important role of CGM technology in improving diabetes management as a tool to identify and correct glycemic derangements, prevent hypoglycemia, direct medication management, and guide medical nutritional therapy and physical activity, as well as its rapid evolution in affordability and accuracy (2,37) (Supplementary Table 1). Furthermore, ADA and KDIGO underscore that CGM may provide an advantage in glycemic control assessment among patients with T1D, as well as patients with T2D using glucose-lowering therapies associated with hypoglycemia. Other technologies supported by the ADA include sensor-augmented pumps that suspend insulin when glucose is low or predicted to become low, as well as automated insulin delivery systems that increase and decrease insulin delivery based on sensor-derived glucose levels and trends.
BP Management
BP management is universally accepted as a critical goal for prevention of CKD progression, ASCVD, and HF. The ADA includes BP recommendations in each annual Standards of Care and published a position statement on diabetes and hypertension in 2017 (38). BP control was highlighted as a key component of comprehensive care in the KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease and KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease and addressed in more detail in the KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease (39).
The ADA and KDIGO BP recommendations share many similarities, including a focus on proper BP measurement techniques, individualization of BP targets, and preferred drugs for treatment. Considerations for individualization of BP targets include both anticipated benefits (e.g., higher absolute benefit for patients with higher underlying cardiovascular or kidney disease risk) and potential risks (e.g., ability to tolerate pharmacotherapy without experiencing adverse effects).
For patients with diabetes, hypertension, and high cardiovascular risk (i.e., 10-year ASCVD risk ≥15%), the ADA advises a BP target of <130/80 mmHg if this target can be safely attained. For patients with diabetes, hypertension, and low cardiovascular risk (defined as those with 10-year ASCVD risk <15%), the ADA recommends a BP target of <140/90 mmHg (grade A recommendation) (40). The KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease recommends a target systolic BP of <120 mmHg with assessment via standardized guideline-recommended office measurement in CKD patients (grade 2B recommendation), based largely on a single, high-quality RCT that was conducted exclusively in people without diabetes (39). However, the KDIGO Blood Pressure Work Group outlined certain caveats with respect to safety considerations and/or limited evidence for this threshold in certain populations, including those with diabetes and CKD. All of these thresholds are proposed as starting places for individualization of targets (41).
With respect to preferred antihypertensive pharmacotherapies, there is consensus that an RAS inhibitor, i.e., an ACEi or ARB, should be initiated in patients with concomitant diabetes, hypertension, and albuminuria, with titration to the highest tolerated approved dose. This recommendation is based on RCTs where findings demonstrated decreased risk of CKD progression, for which patients with albuminuria are at elevated risk, with a maximally dosed RAS inhibitor compared with placebo or an active antihypertensive drug comparator (42–44). In a recent study in almost three million patients, investigators found that both classes performed similarly; however, the ARB was better tolerated (45). Dihydropyridine calcium channel blockers and thiazide-like diuretics are also recommended for patients with hypertension who do not have albuminuria, for whom cardiovascular events and mortality are more common than kidney failure. Multiple drugs are often required to control BP, and an RAS inhibitor, dihydropyridine calcium channel blockers, and diuretics can be combined to attain individualized BP targets (Fig. 3).
Consensus Statement
An ACEi or ARB is recommended for patients with T1D or T2D who have hypertension and albuminuria, titrated to the maximum antihypertensive or highest tolerated dose.
Lipid Management
Statin therapy is a cornerstone of therapy for the primary and secondary prevention of ASCVD among people with diabetes and CKD. The 2013 KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease recommended statin initiation for most adults with diabetes and CKD who are not treated with dialysis (46,47). Specifically, this included 1) adults ≥50 years old with CKD and eGFR ≥60 mL/min/1.73 m2 (grade 1B recommendation) and 2) adults aged 18–49 years with CKD with diabetes, known coronary heart disease, prior ischemic stroke, or estimated 10-year incidence of coronary heart disease death or nonfatal myocardial infarction >10% (grade 2A recommendation). These recommendations are based largely on results of the Study of Heart and Renal Protection (SHARP) trial of CKD (48). Additional evidence from subsequent trials was incorporated into recommendations in the 2022 ADA Standards of Care, which are endorsed by this consensus statement.
For primary prevention of ASCVD, the ADA recommends a moderate-intensity statin for all adults with diabetes aged 40–75 years, those aged 20–39 years with additional ASCVD risk factors (such as CKD), and, with individualized decision-making, those aged >75 years (who are not well represented in completed trials). An exception may be patients with kidney failure treated with dialysis for whom primary prevention of ASCVD events with a statin has been generally ineffective (47,49,50). High-intensity statin is recommended for secondary prevention for all patients with known ASCVD. For some patients, intensification of statin therapy (for primary prevention), addition of ezetimibe, or addition of a PCSK-9 inhibitor is recommend based on ASCVD risk and attained LDL cholesterol concentrations. For patients with high triglyceride or low HDL levels, intensification of lifestyle intervention, optimization of glycemic control, and then consideration of icosapent ethyl are advised (51) (Supplementary Table 1).
Consensus Statement
A statin is recommended for all patients with T1D or T2D and CKD, moderate intensity for primary prevention of ASCVD or high intensity for patients with known ASCVD and some patients with multiple ASCVD risk factors.
Glucose-Lowering Agents in T2D and CKD
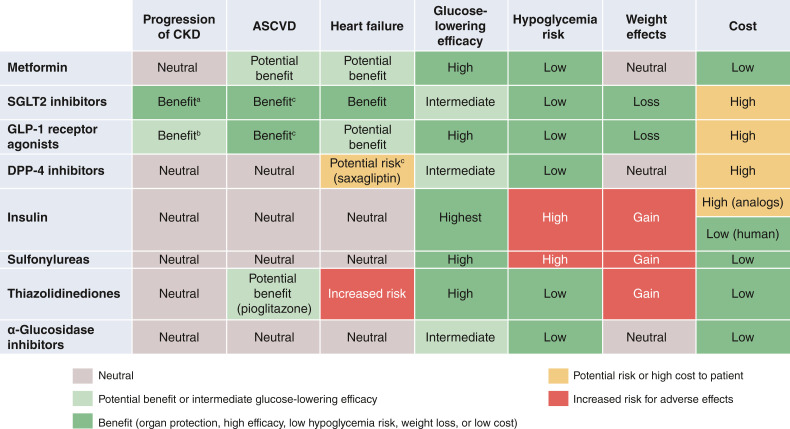
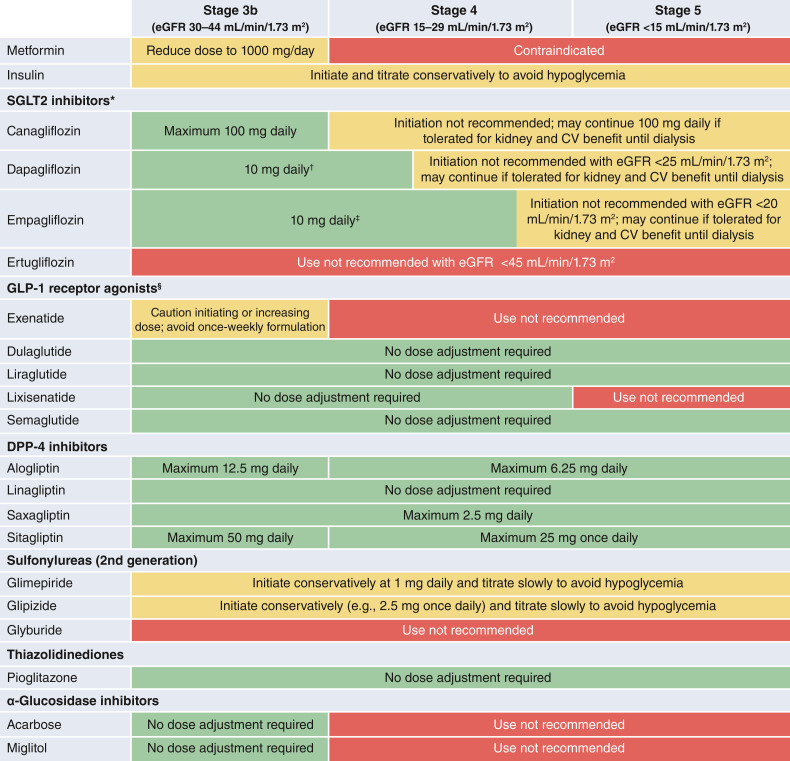
The ADA 2022 Standards Care and the KDIGO 2022 guideline recommend early initiation of metformin plus an SGLT2 inhibitor in most patients with T2D and CKD (2,17) (Table 1). Additional glucose-lowering agents can then be added as needed to meet individualized glycemic targets based on patient-specific considerations (2,17) (Table 2). Prescription of glucose-lowering medications may be limited by eGFR (Table 3). Appropriate dose adjustment based on eGFR is important for medications that increase risk of side effects with low eGFR or undergo elimination through the kidney (Table 4). When needed, careful use and titration of insulin and sulfonylurea agents is recommended to avoid hypoglycemia.
Table 1.
| Medication class | ADA 2022 Standards of Medical Care in Diabetes | KDIGO 2022 Guideline for Diabetes Management in Chronic Kidney Disease |
|---|---|---|
| Metformin | • 9.4a First-line therapy depends on comorbidities, patient-centered treatment factors, and management needs and generally includes metformin and comprehensive lifestyle modification (A). | • Recommendation 4.1.1: We recommend treating patients with T2D, CKD, and an eGFR ≥30 mL/min per 1.73 m2 with metformin (1B). |
| • Practice Point 4.1.3: Adjust the dose of metformin when the eGFR is <45 mL/min per 1.73 m2, and for some patients when the eGFR is 45–59 mL/min per 1.73 m2. | ||
| SGLT2i | • Consider use of SGLT2i for organ protection independent of baseline HbA1c, individualized HbA1c target, or metformin use. | • Recommendation 1.3.1: We recommend treating patients with T2D, CKD, and an eGFR ≥20 mL/min per 1.73 m2 with an SGLT2i (1A). |
| • 10.42 Among patients with T2D who have established ASCVD or established kidney disease, an SGLT2i or GLP-1 receptor agonist with demonstrated cardiovascular disease benefit is recommended as part of the comprehensive cardiovascular risk reduction and/or glucose-lowering regimens (A). | ||
| • 10.42a In patients with T2D and established ASCVD, multiple ASCVD risk factors, or diabetic kidney disease, an SGLT2i with demonstrated cardiovascular benefit is recommended to reduce the risk of MACE and/or HF hospitalization (A). | ||
| • 11.3a For patients with T2D and diabetic kidney disease, use of an SGLT2i in patients with an eGFR ≥20 mL/min/1.73 m2 and urinary albumin ≥200 mg/g creatinine is recommended to reduce CKD progression and cardiovascular events (A).* | ||
| • 11.3b For patients with T2D and diabetic kidney disease, use of an SGLT2i is recommended to reduce CKD progression and cardiovascular events in patients with an eGFR ≥20 mL/min/1.73 m2 and urine albumin ranging from normal to 200 mg/g creatinine (B). | ||
| GLP-1 receptor agonists | • 10.42 Among patients with T2D who have established ASCVD or established kidney disease, an SGLT2i or GLP-1 receptor agonist with demonstrated cardiovascular disease benefit is recommended as part of the comprehensive cardiovascular risk reduction and/or glucose-lowering regimens (A). | • Recommendation 4.2.1: In patients with T2D and CKD who have not achieved individualized glycemic targets despite use of metformin and SGLT2i treatment, or who are unable to use those medications, we recommend a long-acting GLP-1 receptor agonist (1B). |
The ADA issues an A level of evidence for clear or supportive evidence from well-conducted, generalizable randomized control trials that are adequately powered and a B level of evidence for supportive evidence from well-conducted cohort or case-control studies. KDIGO uses the GRADE framework, with 1A indicating a strong recommendation based on high-quality evidence and 1B indicating a strong recommendation based on moderate-quality evidence.
ADA recommendations 11.3a and 11.3b include updates made in September 2022 through ADA’s living Standards of Care guideline update process.
Table 2.
Benefit supported by primary and secondary outcome data.
Benefit supported by secondary outcome data.
Benefit or risk is agent specific.
ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; DPP-4, dipeptidyl peptidase 4; GLP-1, glucagon-like peptide 1; SGLT2, sodium–glucose cotransporter 2.
Table 3.
Key monitoring and risk mitigation strategies for preferred glucose-lowering agents
| Medication | Consideration | Monitoring and/or risk mitigation strategies |
|---|---|---|
| Metformin | Metformin-associated lactic acidosis | • Monitor eGFR with increasing frequency as eGFR falls to <60 mL/min/1.73 m2 |
| • Adjust metformin dose as appropriate per eGFR (see Table 4) | ||
| • Consider dose reduction in the presence of conditions that predispose patients to hypoperfusion and hypoxemia for eGFR 45–59 mL/min/1.73 m2 | ||
| • Discontinue for eGFR <30 mL/min/1.73 m2 | ||
| • Institute a sick day protocol | ||
| B12 malabsorption | • Monitor patients for vitamin B12 deficiency when treated with metformin for >4 years | |
| SGLT2i | Genital mycotic infections | • Counsel on genital hygiene |
| Volume depletion | • Monitor for hypovolemia and consider proactive dose reduction of diuretics in patients at high risk | |
| • Hold SGLT2i during illness | ||
| Diabetic ketoacidosis | • Educate about signs/symptoms to facilitate early recognition | |
| • Monitor blood or urine ketones in the case of very high risk | ||
| • Institute a sick day protocol | ||
| • Maintain at least low-dose insulin in insulin-requiring individuals | ||
| Hypoglycemia | • Adjust background glucose-lowering agents (e.g., insulin or sulfonylureas) as appropriate | |
| GLP-1 receptor agonists | Nausea/vomiting/diarrhea | • Educate on tolerability and symptom recognition |
| • Start at lowest recommended dose and titrate slowly | ||
| Hypoglycemia | • Adjust background glucose-lowering agents (e.g., insulin or sulfonylureas) as appropriate |
eGFR, estimated glomerular filtration rate; GLP-1, glucagon-like peptide 1; SGLT2i, sodium–glucose cotransporter 2 inhibitor.
Table 4.
Dose adjustments for eGFR <45 mL/min/1.73 m2 (information presented reflects the package inserts rather than guidance from this consensus report)
Glucose-lowering efficacy is reduced with SGLT2i as eGFR declines, but kidney and cardiovascular benefits are preserved.
Dapagliflozin is approved for use at 10 mg once daily with an eGFR of 25 to <45 mL/min/1.73 m2.
Initiation not recommended with eGFR <30 mL/min/1.73 m2 for glycemic control or <20 mL/min/1.73 m2 for HF. Higher dose can be used but is not effective for glucose lowering and does not offer further clinical benefit in this range of eGFR.
Dulaglutide, liraglutide, and injectable semaglutide have demonstrated evidence of cardiovascular benefit in large cardiovascular outcome trials.
CV, cardiovascular; DPP-4, dipeptidyl peptidase 4; GFR, estimated glomerular filtration rate; GLP-1, glucagon-like peptide 1; SGLT2, sodium–glucose cotransporter 2.
Metformin
Metformin is recommended for use in most patients with T2D and CKD who have eGFR ≥30 mL/min/1.73 m2, although careful patient selection and downward dose adjustment based on eGFR is recommended. Metformin has been proven to be a safe, effective, and affordable foundation for glycemic control in T2D. Metformin is excreted unchanged in urine, with the label including a boxed warning for increased risk of lactic acidosis in patients with CKD due to impaired metformin excretion (52). Evidence, however, suggests the overall risk for metformin-associated lactic acidosis is low (53), and the U.S. Food and Drug Administration has revised the U.S. label to reflect its safety in most patients with eGFR ≥30 mL/min/1.73 m2 (52). In facilitating safe use, eGFR should be monitored at least annually in patients with CKD, with the recommended frequency of monitoring increased to every 3–6 months once eGFR falls <60 mL/min/1.73 m2 (2) (Fig. 1). It is recommended that the dose of metformin be reduced to 1,000 mg daily in patients with eGFR between 30 and 44 mL/min/1.73 m2, and a reduction should also be considered in patients with eGFR of 45–59 mL/min/1.73 m2 if they have a comorbidity that places them at increased risk of lactic acidosis due to hypoperfusion and hypoxemia (2). Most episodes of metformin-associated lactic acidosis occur concurrent with other acute illness, often when acute kidney injury (AKI) contributes to reduced metformin clearance. Therefore, sick day protocols that specify holding metformin doses during acute illness may help reduce the risk of metformin-associated lactic acidosis.
Consensus Statement
Metformin is recommended for patients with T2D, CKD, and eGFR ≥30 mL/min/1.73 m2; the dose should be reduced to 1,000 mg daily for patients with eGFR 30–44 mL/min/1.73 m2 and for some patients with eGFR 45–59 mL/min/1.73 m2 who are at high risk of lactic acidosis.
SGLT2i
SGLT2i are recommended in most patients with T2D and CKD with eGFR ≥20 mL/min/1.73 m2 independent of HbA1c or the need for additional glucose lowering (2,17). This recommendation is based on strong evidence that SGLT2i reduce CKD progression, HF, and ASCVD risk in patients with T2D and CKD. These benefits are independent of glycemia, and an SGLT2i should be used for patients with T2D and CKD even if glycemic targets are already attained. While an SGLT2i will usually be added to lifestyle and metformin therapy, SGLT2i treatment without metformin may be reasonable for patients with eGFR too low for safe prescription of metformin, who do not tolerate metformin, or who do not need metformin to achieve glycemic targets.
To date, two clinical trials with primary kidney disease outcomes using canagliflozin and dapagliflozin (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation [CREDENCE] and Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease [DAPA-CKD]) demonstrated significant benefit for composite outcomes including end points of substantial eGFR decline, kidney failure, and mortality (54,55). These trials enrolled participants with albuminuria (ACR ≥300 mg/g and ≥200 mg/g, respectively); therefore, current evidence is strongest in this population, as emphasized by ADA recommendations (17) (Table 1). Evidence from combined major SGLT2i trials, however, suggests that kidney and cardiovascular benefits are consistent irrespective of baseline albuminuria (56), including in patients with normal albumin excretion, as reflected in the KDIGO recommendation and consensus statement supporting SGLT2i use in most patients with T2D and CKD (2).
The lower limit of eGFR for which initiation of SGLT2i is recommended has changed over time as new data have rapidly become available. The KDIGO 2022 guideline recommended initiation of an SGLT2i for patients with T2D and CKD who have eGFR ≥20 mL/min/1.73 m2 (a change from ≥30 mL/min/1.73 m2 in the 2020 guideline), and the ADA has also updated this threshold to ≥20 mL/min/1.73 m2 in its living Standards of Care (from ≥25 mL/min/1.73 m2 in the initial issue of the 2022 Standards of Care). These changes are driven largely by findings of new trials, including the DAPA-CKD trial (which provided clear evidence of efficacy and safety for dapagliflozin in patients with eGFR ≥25 mL/min/1.73 m2 and ACR ≥200 mg/g) and the Empagliflozin Outcome Trial in Patients With Chronic Heart Failure (EMPEROR) trials (which provided clear evidence of efficacy and safety for empagliflozin among patients with eGFR ≥20 mL/min/1.73 m2 and HF) (54,57,58). Additional support comes from subgroup analyses of participants in the CREDENCE and DAPA-CKD trials with baseline eGFR <30 mL/min/1.73 m2 (59,60). Based on these results, direct evidence supporting initiation of an SGLT2i for patients with T2D and eGFR 20–29 mL/min/1.73 m2 is strongest for patients with concomitant albuminuria or HF, though the efficacy and safety of SGLT2i are generally consistent among trial participants with or without these conditions (56,61,62). Moreover, SGLT2i have been observed to have consistent efficacy and safety across studied ranges of eGFR (56). Therefore, an SGLT2i can be initiated for most patients with T2D, CKD, and eGFR ≥20 mL/min/1.73 m2. Further data are anticipated from the EMPA-KIDNEY trial (EMPA-KIDNEY: The Study of Heart and Kidney Protection with Empagliflozin [clinical trial reg. no. NCT03594110, ClinicalTrials.gov]), where entry criteria was expanded to include nonalbuminuric CKD with an eGFR initiation threshold ≥20 mL/min/1.73 m2, among >6,600 participants with or without T2D. Like CREDENCE and DAPA-CKD, EMPA-KIDNEY was stopped early for clear positive efficacy (63); corresponding expansion of the indications for use of an SGLT2i in CKD may be further supported based on these findings.
SGLT2i initiation is associated with a reversible decline in eGFR, but this generally does not require drug discontinuation. In fact, SGLT2i use appears to protect patients from AKI (56). Notably, protocols for both CREDENCE and DAPA-CKD specified continuation of study drug when eGFR fell below initiation thresholds. Therefore, it is reasonable to continue therapy if the eGFR falls below the initiation thresholds unless the patient is not tolerating treatment or KRT is initiated (2).
Hypovolemia and hypoglycemia may occur with SGLT2i, but absolute risks are low, especially at low eGFR. Therefore, adjustment of background therapies is generally not required when initiating an SGLT2i, but it may be prudent in some patients, and follow-up to reassess volume status and glycemia is important (64). Euglycemic ketoacidosis with minimal to no elevation in blood glucose may occur in patients taking SGLT2i. Patients with T2D requiring insulin are at particular risk. To mitigate risk, it is important to maintain at least low-dose insulin and consider pausing SGLT2i treatment during periods of acute illness or stressors. Blood or urine ketone monitoring may be used for ketosis detection. Patients with signs, symptoms, or biochemical evidence of ketoacidosis should discontinue SGLT2i therapy and seek immediate medical attention. Genital mycotic infections are a known complication of SGLT2i. A meta-analysis of clinical trials reported that genital mycotic infections occurred in 6% of participants assigned to an SGLT2i, compared with 1% of those assigned to placebo (65). The risk is higher for women than men. Daily hygienic measures may lessen this risk, and most genital mycotic infections are easily treated, but severe cases of Fournier gangrene have been reported. Additional research is needed to determine the role of SGLT2i in improving kidney outcomes in patients with T1D, among whom diabetic ketoacidosis is more common, and posttransplant, in which case immunosuppression may modify infection risks (66).
Consensus Statement
An SGLT2i with proven kidney or cardiovascular benefit is recommended for patients with T2D, CKD, and eGFR ≥20 mL/min/1.73 m2. Once initiated, the SGLT2i can be continued at lower levels of eGFR.
Use of Additional Glucose-Lowering Agents
For patients with T2D and CKD requiring additional glucose-lowering agents, selection should be made in consideration of patient- and medication-specific considerations (Table 2). Addition of a long-acting GLP-1 receptor agonist is preferred as per KDIGO for patients not achieving individualized glycemic targets despite use of metformin and/or SGLT2i therapy or for individuals unable to take these medications (2). Similarly, the ADA gives strong support to use of GLP-1 receptor agonists in patients with T2D and CKD or ASCVD in consideration of their primary cardiovascular and secondary kidney benefits in large cardiovascular outcomes trials (17). Notably, GLP-1 receptor agonists retain glycemic efficacy and safety even in advanced CKD stages.
GLP-1 Receptor Agonists
GLP-1 receptor agonists reduce albuminuria and slow eGFR decline, as evidenced by secondary outcomes assessed in the cardiovascular outcomes trials and a clinical trial for glycemic efficacy and safety in patients with T2D and eGFR 15–59 mL/min/1.73 m2 (2). In cardiovascular outcomes trials, GLP-1 receptor agonists reduced risk of major adverse cardiovascular events (MACE) in patients with T2D (67–70). Notably, the MACE risk reduction with liraglutide was significantly greater for those with eGFR <60 mL/min/1.73 m2 than for those with eGFR ≥60 mL/min/1.73 m2 (69). Although most participants in the cardiovascular outcomes trials of GLP-1 receptor agonists had established cardiovascular disease, the MACE reduction was similar between those with and without previous cardiovascular or kidney disease (71).
Although there has not been a completed kidney disease outcome trial for GLP-1 receptor agonists, the cardiovascular outcomes trials have included participants with eGFR as low as 15 mL/min/1.73 m2. The GLP-1 receptor agonists with favorable CKD outcomes include lixisenatide, exenatide (once weekly), liraglutide, semaglutide, albiglutide, dulaglutide, and efpeglenatide (67,68,70,72–76). In a meta-analysis of eight cardiovascular outcomes trials, GLP-1 receptor agonists significantly reduced risk for a composite kidney disease outcome (macroalbuminuria, eGFR decline, progression to kidney failure, or death from kidney disease) compared with placebo, largely driven by reduction in albuminuria (71). In a glycemic efficacy and safety trial in patients with moderate-to-severe CKD (CKD stages G3 and G4), dulaglutide was compared with insulin glargine as basal therapy (71,77). Dulaglutide produced similar glycemic control but resulted in significantly slower GFR decline. There is an ongoing clinical trial for a GLP-1 receptor agonist in T2D and CKD to evaluate whether semaglutide will prevent ≥50% eGFR decline, kidney failure, or death due to kidney or cardiovascular causes (clinical trial reg. no. NCT03819153, ClinicalTrials.gov).
Nausea, vomiting, and diarrhea are the most common side effects of GLP-1 receptor agonists. These symptoms occur in 15–20% of patients with moderate-to-severe CKD (CKD stages G3 and G4) but usually are tolerable with dose titration and abate over several weeks to months (77). Injection site reactions are rare (<1%), and semaglutide is now available in an oral formulation. Heart rate typically increases by ∼5 bpm but has not been associated with higher BP or other adverse events. GLP-1 receptor agonist treatment is not recommended in patients at risk for thyroid C-cell tumors (e.g., multiple endocrine neoplasia), pancreatic cancer, or pancreatitis based on theoretical risks from preclinical models (1).
GLP-1 receptor agonists that have shown cardiovascular and CKD benefits (liraglutide, semaglutide, albiglutide [not currently available], and dulaglutide) are preferred agents. GLP-1 receptor agonists do not cause hypoglycemia per se but, when used with insulin or insulin secretagogues, doses of these drugs may be reduced to avoid hypoglycemia. However, in moderate-to-severe CKD (CKD stages G3 and G4), rates of hypoglycemia are reduced by one-half even with concurrent insulin therapy (77).
Consensus Statement
GLP-1 receptor agonist with proven cardiovascular benefit is recommended for patients with T2D and CKD who do not meet their individualized glycemic target with metformin and/or an SGLT2i or who are unable to use these drugs.
Glycemic Management in Advanced CKD (eGFR <30 mL/min/1.73 m2 With or Without KRT)
Glycemic management is particularly challenging for patients with eGFR <30 mL/min/1.73 m2, including those treated with dialysis, because of restrictions on drug use (Table 4) and lack of high-quality RCTs in this population.
For T1D, insulin remains the only approved therapy. Doses are titrated to achieve individualized glycemic goals but may need to be decreased in comparison with earlier stages of CKD due to reduced insulin clearance and other changes in metabolism with advanced CKD (78).
In T2D, advanced CKD is a risk factor for hypoglycemia (29,79) and, when possible, drugs that control glycemia without increasing risk of hypoglycemia are preferred. Metformin is contraindicated with eGFR<30 mL/min/1.73 m2 and with dialysis treatment. SGLT2i can be initiated with eGFR 20–29 mL/min/1.73 m2 and continued at lower eGFR if previously initiated and well tolerated. However, SGLT2i have minimal effects on glycemia in this range of eGFR and are of use mainly for kidney and cardiovascular benefits not mediated through glycemia.
GLP-1 receptor agonists have been studied with eGFR as low as 15 mL/min/1.73 m2 and retain glucose-lowering potency across the range of eGFR and among dialysis patients. GLP-1 receptor agonists reduced ASCVD events and albuminuria in large RCTs and, thus, are theoretically appealing for people with T2D and CKD but have not been prospectively tested for cardiovascular efficacy or safety in this population. However, findings of a meta-analysis of the cardiovascular outcomes trials showed that ASCVD risk was reduced at least as much among individuals with eGFR <60 mL/min/1.73 m2 compared with those with higher eGFR (71). GLP-1 receptor agonists induce weight loss and can cause nausea and vomiting, so caution is warranted among patients with or at risk for malnutrition. Notably, in people with T2D and advanced CKD who have obesity exceeding BMI limits required for kidney transplant listing, GLP-1 receptor agonists can be used to aid with weight loss that may facilitate qualification for transplant.
Selected dipeptidyl peptidase 4 inhibitors can be used with eGFR <30 mL/min/1.73 m2 and in dialysis (Table 4) and provide a safe and effective option for treatment of patients who are not treated with GLP-1 receptor agonists. Thiazolidinediones improve insulin sensitivity, a common abnormality in advanced CKD, and retain antihyperglycemic effects in this population. Fluid retention and HF are concerns with low eGFR and require careful monitoring. Insulin and short-acting sulfonylureas are often necessary to control glucose when medications with less propensity to cause hypoglycemia are contraindicated, not tolerated, unavailable, or insufficient.
Glycemic Management for Patients With a Kidney Transplant
Patients with a kidney transplant have been excluded from most clinical trials of glucose-lowering therapy. Therefore, data must be extrapolated from general populations with diabetes, with consideration of differences in diabetes pathophysiology (i.e., posttransplant diabetes) and unique aspects of treatment (such as immunosuppressive medications). High-quality trial data are needed for this population.
For T2D and posttransplant diabetes, it is reasonable to treat kidney transplant recipients with metformin according to eGFR, as for the broader population with T2D, because risks of metformin are related to kidney function (80–84). SGLT2i are promising drugs for kidney transplant recipients because they reduce intraglomerular pressure, which may be elevated in single functional kidneys, and may improve graft outcomes through this and other mechanisms. However, these benefits have not been confirmed in clinical trials, and there is a theoretical concern that infection risks (i.e., genital mycotic infections, urinary tract infections, Fournier gangrene) may be increased due to immunosuppression. Therefore, more data are needed prior to making recommendations for or against treatment with SGLT2i for kidney transplant recipients. Kidney transplantation and its treatments do not substantially modify the known risks and benefits of other glucose-lowering medications, other than restrictions associated with eGFR.
Renin-Angiotensin-Aldosterone System Inhibition
ACE Inhibitors and ARBs
RAS inhibition with ACEi or ARBs has been standard of care in patients with T1D and T2D and CKD for decades. ACEi or ARBs are the preferred first-line agent for BP treatment among patients with diabetes, hypertension, and ACR ≥300 mg/g because of their proven benefits for prevention of CKD progression. In the setting of lower levels of albuminuria (30–299 mg/g), ACEi or ARB therapy has been demonstrated to reduce progression to more advanced albuminuria (≥300 mg/g) and cardiovascular events but not progression to kidney failure. Therefore, both KDIGO and the ADA recommend an ACEi or ARB for treatment of hypertension among people with T1D or T2D who have hypertension and ACR ≥30 mg/g (1,2).
Rarely, patients with albuminuria have normal BP, and in this situation, evidence for treatment with RAS inhibition is less strong. Although short-term studies demonstrated added benefit of the combination of ACEi and ARBs in albuminuria reduction, long-term studies showed no benefit and more adverse events, particularly hyperkalemia and AKI, and thus avoidance of this combination is recommended.
ns-MRAs
The steroidal mineralocorticoid receptor antagonist spironolactone is effective for management of resistant hypertension and treatment of primary hyperaldosteronism, in the setting of normal eGFR. Additionally, spironolactone reduces mortality in patients with HF with reduced ejection fraction. However, spironolactone causes hyperkalemia, particularly with reduced kidney function (i.e., eGFR <45 mL/min/1.73 m2). There are no long-term kidney outcome studies with spironolactone, and only one study in heart failure with reduced ejection fraction with a mean follow-up of 2 years that showed benefit.
A novel class of ns-MRAs, including esaxerenone and finerenone, has recently been investigated among people with T2D and CKD, added to RAS inhibition. Esaxerenone lowered BP and albuminuria with limited changes in potassium, but long-term studies with clinical end points are lacking (85). Finerenone was investigated in two complementary phase 3 studies of patients with T2D, kidney disease (defined primarily as ACR ≥30 mg/g), and potassium <4.8 mmol/L and is the only ns-MRA approved in the world for slowing CKD progression and reducing cardiovascular events. In Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD), both the primary kidney end point of progression of kidney disease (40% decline in eGFR or kidney failure) and the prespecified secondary composite cardiovascular end point (MACE or hospitalization for HF) were reduced with finerenone compared with placebo. Serum potassium was monitored regularly, and 2.6% of participants stopped treatment because of hyperkalemia with finerenone compared with 0.9% on placebo (86). In Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD), the primary composite cardiovascular end point (MACE or hospitalization for HF) was reduced with finerenone compared with placebo, with estimates of effect for kidney outcomes and hyperkalemia similar to those seen in FIDELIO-DKD (87).
Findings from the FIDELITY (Finerenone in Chronic Kidney Disease and Type 2 Diabetes: Combined FIDELIO-DKD and FIGARO-DKD Trial Programme Analysis) individual patient, prespecified combined analysis of both trials (13,191 total participants) demonstrated significant reductions of 18% for the composite cardiovascular outcome; 23% for a composite outcome of doubling of creatinine, kidney failure, or renal death; and 20% for dialysis initiation with a 22% reduction in HF hospitalizations (88). While <10% of participants were treated with an SGLT2i or a GLP-1 receptor agonist, results of subgroup analyses suggested that benefits of finerenone were similar with and without concomitant SGLT2i or GLP-1 receptor agonist treatment. Moreover, the risk of hyperkalemia was significantly reduced by the presence of an SGLT2i (89).
In summary, FIDELIO-DKD and FIGARO-DKD demonstrated cardiovascular and kidney benefits for finerenone among people with T2D who were treated with standard of care (including an RAS inhibitor at maximally tolerated doses and good control of glycemia and BP) who were at high residual risk, based largely on albuminuria (ACR ≥30 mg/g). These effects appear to be additive, based on preclinical studies, to those of SGLT2i and GLP-1 receptor agonists, though further clinical research on these combinations is needed. Therefore, it is reasonable to add finerenone to the treatment regimen of patients with T2D who have any level of persistent albuminuria despite current standard of care treatment with glucose-lowering and antihypertensive medications (Fig. 3).
Finerenone can be initiated with eGFR ≥25 mL/min/1.73 m2 (as per trial eligibility) and serum potassium 4.8 mmol/L (per trial eligibility criteria) or ≤5.0 mmol/L (as per U.S. Food and Drug Administration label). As per trial protocols, finerenone should be started at a dose of 20 mg daily for eGFR >60 mL/min/1.73 m2 and 10 mg for eGFR 25–60 mL/min/1.73 m2 and uptitrated to 20 mg daily if possible. Potassium should be followed 4 weeks after dose change and regularly during treatment. With potassium <4.8 mmol/L, dose can be uptitrated to 20 mg and continued with potassium ≤5.5 mmol/L. If potassium increases to >5.5 mmol/L, finerenone should be withheld and can be restarted at 10 mg daily when potassium is ≤5.0 mmol/L. Finerenone can be continued with eGFR <25 mL/min/1.73 m2 as long as potassium is acceptable and the drug is otherwise tolerated.
Consensus Statement
An ns-MRA with proven kidney and cardiovascular benefit is recommended for patients with T2D, eGFR ≥25 mL/min/1.73 m2, normal serum potassium concentration, and albuminuria (ACR ≥30 mg/g) despite maximum tolerated dose of RAS inhibitor.
Conclusions
The 2022 ADA Standards of Care and KDIGO 2022 guideline are aligned on issues of CKD screening and diagnosis, glycemia monitoring, lifestyle therapies, treatment goals, and pharmacologic management (1,2). Both recommend comprehensive care in which pharmacotherapy that is proven to improve clinical kidney and cardiovascular outcomes is layered upon a foundation of healthy lifestyle approaches. This consensus approach to management is based on high-quality evidence. Randomized clinical trial data are most abundant for drug therapies, and other professional societies have also made similar recommendations for use of these agents.
Implementation of proven therapies is paramount to improving health outcomes. There is a critical need for patients with diabetes and CKD to be treated in accord with the most up-to-date recommendations. The ADA and KDIGO, individually and now in combination, offer clear guidance on applying and prioritizing interventions. High cost, limited workforce, and other resource constraints in health care systems will limit implementation of some recommendations among individuals and populations, and efforts to improve accessibility are essential to maximizing benefit and minimizing disparities.
Investigation remains active in the fields of diabetes, CKD, and cardiovascular disease, and additional data on existing and novel approaches are anticipated. Clinical practice guidelines will continue to evolve. When possible, consensus approaches to diagnosis and management will help interpret new data in context and translate discoveries to improved outcomes for patients.
Article Information
Duality of Interest. I.H.d.B.’s employer receives research support from Dexcom, and he has received honoraria from the ADA. He is a consultant to or advisory board member of AstraZeneca, Bayer, Boehringer Ingelheim, Cyclerion Therapeutics, George Clinical, Goldfinch Bio, and Ironwood Pharmaceuticals. He is also deputy editor for the Clinical Journal of the American Society of Nephrology. K.K.’s institution has received research grants from Boehringer Ingelheim, AstraZeneca, Novartis, Novo Nordisk, Sanofi, Lilly, and Merck Sharp & Dohme, and he is a consultant to Novo Nordisk, AstraZeneca, Sanofi, Servier, Merck Sharp & Dohme, Novartis, Abbott, Amgen, Bayer, Lilly, Roche, Berlin-Chemie AG/Menarini Group, and Boehringer Ingelheim. T.S.’s employer receives research support from Transplant House, and she has received honoraria from AstraZeneca. K.R.T. has received research grants from Goldfinch Bio, Bayer, and Travere Therapeutics. She is a consultant to or advisory board member of Eli Lilly, AstraZeneca, Boehringer Ingelheim, Gilead Sciences, Goldfinch Bio, Novo Nordisk, Bayer, and Travere Therapeutics. J.J.N. is an advisory board member for Novo Nordisk and Sanofi and is on Dexcom’s speakers bureau. C.M.R. has received a research grant from Dexcom and honoraria from AstraZeneca. She has also received funding from Fresenius Medical Care and ReCor Medical. S.E.R.’s employer receives research grants from Bayer, and she is a consultant to or advisory board member of Bayer, Relypsa, and Reata Pharmaceuticals. She is the president-elect of the National Kidney Foundation. P.R. has received research support from Novo Nordisk, AstraZeneca, Bayer, Gilead Sciences, Boehringer Ingelheim, Vifor Pharma, Mundipharma, Sanofi, Astellas Pharma, and Merck Sharp & Dohme. G.B. is a consultant to or advisory board member of Merck, Bayer, KBP Biosciences, Ionis Pharmaceuticals, Alnylam Pharmaceuticals, AstraZeneca, Quantum Genomics, Horizon Therapeutics, Novo Nordisk, DiaMedica Therapuetics, and inRegen. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. I.H.d.B. and G.B. were co-chairs for the consensus report writing group. J.J.N., C.M.R., and S.E.R. were the writing group members for the ADA. K.K., T.S., K.R.T., and P.R. were the writing group members for the KDIGO. All authors were responsible for drafting the report and revising it critically for important intellectual content. All authors approved the version to be published.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.20272404.
This article is featured in a podcast available at diabetesjournals.org/journals/pages/diabetes-core-update-podcasts.
This article is being simultaneously published in Diabetes Care and Kidney International. The articles are identical except for stylistic changes in keeping with each journal’s style. Either of these versions may be used in citing this article.
A consensus report of a particular topic contains a comprehensive examination and is authored by an expert panel (i.e., consensus panel) and represents the panel’s collective analysis, evaluation, and opinion. The need for a consensus report arises when clinicians, scientists, regulators, and/or policy makers desire guidance and/or clarity on a medical or scientific issue related to diabetes for which the evidence is contradictory, emerging, or incomplete. Consensus reports may also highlight gaps in evidence and propose areas of future research to address these gaps. A consensus report is not an American Diabetes Association (ADA) position but represents expert opinion only and is produced under the auspices of the ADA by invited experts. A consensus report may be developed after an ADA Clinical Conference or Research Symposium.
References
- 1. American Diabetes Association . Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S1–S264 [DOI] [PubMed] [Google Scholar]
- 2. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group . KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022;102(4S):S1–S123 [DOI] [PubMed] [Google Scholar]
- 3. International Diabetes Federation . Diabetes facts & figures. Accessed 1 October 2020. Available from https://idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html
- 4. Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA 2016;316:602–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levin A, Tonelli M, Bonventre J, et al.; ISN Global Kidney Health Summit participants . Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 2017;390:1888–1917 [DOI] [PubMed] [Google Scholar]
- 6. United States Renal Data System . 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2020 [Google Scholar]
- 7. Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 2013;24:302–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fox CS, Matsushita K, Woodward M, et al.; Chronic Kidney Disease Prognosis Consortium . Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 2012;380:1662–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020. Atlanta, GA, Centers for Disease Control and Prevention, 2020 [Google Scholar]
- 10. Chu CD, McCulloch CE, Banerjee T, et al.; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team . CKD awareness among US adults by future risk of kidney failure. Am J Kidney Dis 2020;76:174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eberly LA, Yang L, Eneanya ND, et al. Association of race/ethnicity, gender, and socioeconomic status with sodium-glucose cotransporter 2 inhibitor use among patients with diabetes in the US. JAMA Netw Open 2021;4:e216139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marathe PH, Gao HX, Close KL. American Diabetes Association Standards of Medical Care in Diabetes 2017. J Diabetes 2017;9:320–324 [DOI] [PubMed] [Google Scholar]
- 13. Williams R, Karuranga S, Malanda B, et al. Global and regional estimates and projections of diabetes-related health expenditure: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2020;162:108072. [DOI] [PubMed] [Google Scholar]
- 14. Chen HY, Kuo S, Su PF, Wu JS, Ou HT. Health care costs associated with macrovascular, microvascular, and metabolic complications of type 2 diabetes across time: estimates from a population-based cohort of more than 0.8 million individuals with up to 15 years of follow-up. Diabetes Care 2020;43:1732–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wan EYF, Chin WY, Yu EYT, et al. The impact of cardiovascular disease and chronic kidney disease on life expectancy and direct medical cost in a 10-year diabetes cohort study. Diabetes Care 2020;43:1750–1758 [DOI] [PubMed] [Google Scholar]
- 16. Stevens PE; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members . Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013;158:825–830 [DOI] [PubMed] [Google Scholar]
- 17. Draznin B, Aroda VR, Bakris G, et al.; American Diabetes Association Professional Practice Committee . 11. Chronic kidney disease and risk management: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S175–S184 [DOI] [PubMed] [Google Scholar]
- 18. Shlipak MG, Tummalapalli SL, Boulware LE, et al.; Conference Participants . The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2021;99:34–47 [DOI] [PubMed] [Google Scholar]
- 19. Stempniewicz N, Vassalotti JA, Cuddeback JK, et al. Chronic kidney disease testing among primary care patients with type 2 diabetes across 24 U.S. health care organizations. Diabetes Care 2021;44:2000–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis 2022;79:268–288.e1 [DOI] [PubMed] [Google Scholar]
- 21. Hatlen G, Romundstad S, Hallan SI. The accuracy of predicting cardiovascular death based on one compared to several albuminuria values. Kidney Int 2014;85:1421–1428 [DOI] [PubMed] [Google Scholar]
- 22. Inker LA, Eneanya ND, Coresh J, et al.; Chronic Kidney Disease Epidemiology Collaboration . New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 2021;385:1737–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA 2019;322:1294–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hsu CY, Yang W, Parikh RV, et al.; CRIC Study Investigators . Race, genetic ancestry, and estimating kidney function in CKD. N Engl J Med 2021;385:1750–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013;3(Suppl):1–150 [DOI] [PubMed] [Google Scholar]
- 27. KDOQI . KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis 2007;49(Suppl. 2):S12–S154 [DOI] [PubMed] [Google Scholar]
- 28. Townsend RR, Guarnieri P, Argyropoulos C, et al.; TRIDENT Study Investigators . Rationale and design of the Transformative Research in Diabetic Nephropathy (TRIDENT) Study. Kidney Int 2020;97:10–13 [DOI] [PubMed] [Google Scholar]
- 29. de Boer IH, Alpers CE, Azeloglu EU, et al.; Kidney Precision Medicine Project . Rationale and design of the Kidney Precision Medicine Project. Kidney Int 2021;99:498–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Draznin B, Aroda VR, Bakris G, et al.; American Diabetes Association Professional Practice Committee; American Diabetes Association Professional Practice Committee . 5. Facilitating behavior change and well-being to improve health outcomes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S60–S82 [DOI] [PubMed] [Google Scholar]
- 32. Tuttle KR, Knight R, Appelbaum PS, et al.; Kidney Precision Medicine Project . Integrating patient priorities with science by community engagement in the Kidney Precision Medicine Project. Clin J Am Soc Nephrol 2021;16:660–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sadusky T, Hurst C. The patient voice in health care decision making: the perspective of people living with diabetes and CKD. Clin J Am Soc Nephrol 2021;16:991–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Draznin B, Aroda VR, Bakris G, et al.; American Diabetes Association Professional Practice Committee; American Diabetes Association Professional Practice Committee . 4. Comprehensive medical evaluation and assessment of comorbidities: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S46–S59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Joint WHO/FAO/UNU Expert Consultation . Protein and Amino Acid Requirements in Human Nutrition. Geneva, World Health Org., 2007. (Tech. Rep. Ser., no. 935) [PubMed] [Google Scholar]
- 36. Ikizler TA, Burrowes JD, Byham-Gray LD, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis 2020;76(Suppl. 1):S1–S107 [DOI] [PubMed] [Google Scholar]
- 37. Draznin B, Aroda VR, Bakris G, et al.; American Diabetes Association Professional Practice Committee . 7. Diabetes technology: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S97–S112 [DOI] [PubMed] [Google Scholar]
- 38. de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017;40:1273–1284 [DOI] [PubMed] [Google Scholar]
- 39. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group . KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int 2021;99(3S):S1–S87 [DOI] [PubMed] [Google Scholar]
- 40. American Diabetes Association Professional Practice Committee . 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S144–S174 [DOI] [PubMed] [Google Scholar]
- 41. de Boer IH, Bakris G, Cannon CP. Individualizing blood pressure targets for people with diabetes and hypertension: comparing the ADA and the ACC/AHA recommendations. JAMA 2018;319:1319–1320 [DOI] [PubMed] [Google Scholar]
- 42. Brenner BM, Cooper ME, de Zeeuw D, et al.; RENAAL Study Investigators . Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–869 [DOI] [PubMed] [Google Scholar]
- 43. Keane WF, Brenner BM, de Zeeuw D, et al.; RENAAL Study Investigators . The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int 2003;63:1499–1507 [DOI] [PubMed] [Google Scholar]
- 44. Lewis EJ, Hunsicker LG, Bain RP; The Collaborative Study Group . The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 1993;329:1456–1462 [DOI] [PubMed] [Google Scholar]
- 45. Chen R, Suchard MA, Krumholz HM, et al. Comparative first-line effectiveness and safety of ACE (angiotensin-converting enzyme) inhibitors and angiotensin receptor blockers: s multinational cohort study. Hypertension 2021;78:591–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wanner C; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members . KDIGO clinical practice guideline for lipid management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int 2014;85:1303–1309 [DOI] [PubMed] [Google Scholar]
- 47. Sharp Collaborative Group . Study of Heart and Renal Protection (SHARP): randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. Am Heart J 2010;160:785–794.e10 [DOI] [PubMed] [Google Scholar]
- 48. Baigent C, Landray MJ, Reith C, et al.; SHARP Investigators . The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011;377:2181–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fellström BC, Jardine AG, Schmieder RE, et al.; AURORA Study Group . Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009;360:1395–1407 [DOI] [PubMed] [Google Scholar]
- 50. Wanner C, Krane V, März W, et al.; Deutsche Diabetes-Dialyse-Studie (4D) Study Group . Randomized controlled trial on the efficacy and safety of atorvastatin in patients with type 2 diabetes on hemodialysis (4D study): demographic and baseline characteristics. Kidney Blood Press Res 2004;27:259–266 [DOI] [PubMed] [Google Scholar]
- 51. American Diabetes Association . Introduction: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S1–S2 [DOI] [PubMed] [Google Scholar]
- 52. Glucophage [package insert] . Princeton, NJ, Bristol-Myers Squibb Company, 2018. Accessed 31 March 2022. Available from https://packageinserts.bms.com/pi/pi_glucophage.pdf
- 53. Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 2014;312:2668–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al.; DAPA-CKD Trial Committees and Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446 [DOI] [PubMed] [Google Scholar]
- 55. Perkovic V, Jardine MJ, Neal B, et al.; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 56. Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2019;7:845–854 [DOI] [PubMed] [Google Scholar]
- 57. Anker SD, Butler J, Filippatos G, et al.; EMPEROR-Preserved Trial Investigators . Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–1461 [DOI] [PubMed] [Google Scholar]
- 58. Packer M, Anker SD, Butler J, et al.; EMPEROR-Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424 [DOI] [PubMed] [Google Scholar]
- 59. Bakris G, Oshima M, Mahaffey KW, et al. Effects of canagliflozin in patients with baseline eGFR <30 ml/min per 1.73 m2: subgroup analysis of the randomized CREDENCE trial. Clin J Am Soc Nephrol 2020;15:1705–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chertow GM, Vart P, Jongs N, et al.; DAPA-CKD Trial Committees and Investigators . Effects of dapagliflozin in stage 4 chronic kidney disease. J Am Soc Nephrol 2021;32:2352–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Razuk V, Chiarito M, Cao D, et al. SGLT-2 inhibitors and cardiovascular outcomes in patients with and without a history of heart failure: a systematic review and meta-analysis. Eur Heart J Cardiovasc Pharmacother 2022;8:557–567 [DOI] [PubMed] [Google Scholar]
- 62. Staplin N, Roddick AJ, Emberson J, et al. Net effects of sodium-glucose co-transporter-2 inhibition in different patient groups: a meta-analysis of large placebo-controlled randomized trials. EClinicalMedicine 2021;41:101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Boehringer Ingelheim . Jardiance (empagliflozin) Phase III EMPA-KIDNEY trial will stop early due to clear positive efficacy in people with chronic kidney disease, 2022. Accessed 16 March 2022. Available from https://www.boehringer-ingelheim.com/human-health/metabolic-diseases/early-stop-chronic-kidney-disease-trial-efficacy
- 64. Zoungas S, de Boer IH. SGLT2 inhibitors in diabetic kidney disease. Clin J Am Soc Nephrol 2021;16:631–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shi FH, Li H, Shen L, et al. Appraisal of non-cardiovascular safety for sodium-glucose co-transporter 2 inhibitors: a systematic review and meta-analysis of placebo-controlled randomized clinical trials. Front Pharmacol 2019;10:1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tuttle KR, Brosius FC 3rd, Cavender MA, et al. SGLT2 inhibition for CKD and cardiovascular disease in type 2 diabetes: report of a scientific workshop sponsored by the National Kidney Foundation. Diabetes 2021;70:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gerstein HC, Colhoun HM, Dagenais GR, et al.; REWIND Investigators . Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394:121–130 [DOI] [PubMed] [Google Scholar]
- 68. Hernandez AF, Green JB, Janmohamed S, et al.; Harmony Outcomes committees and investigators . Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519–1529 [DOI] [PubMed] [Google Scholar]
- 69. Mann JFE, Fonseca V, Mosenzon O, et al. Effects of liraglutide versus placebo on cardiovascular events in patients with type 2 diabetes mellitus and chronic kidney disease. Circulation 2018;138:2908–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Marso SP, Bain SC, Consoli A, et al.; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 71. Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 2021;9:653–662 [DOI] [PubMed] [Google Scholar]
- 72. Bethel MA, Mentz RJ, Merrill P, et al. Microvascular and cardiovascular outcomes according to renal function in patients treated with once-weekly exenatide: insights from the EXSCEL trial. Diabetes Care 2020;43:446–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gerstein HC, Sattar N, Rosenstock J, et al.; AMPLITUDE-O Trial Investigators . Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med 2021;385:896–907 [DOI] [PubMed] [Google Scholar]
- 74. Holman RR, Bethel MA, Mentz RJ, et al.; EXSCEL Study Group . Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mann JFE, Ørsted DD, Brown-Frandsen K, et al.; LEADER Steering Committee and Investigators . Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 2017;377:839–848 [DOI] [PubMed] [Google Scholar]
- 76. Pfeffer MA, Claggett B, Diaz R, et al.; ELIXA Investigators . Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–2257 [DOI] [PubMed] [Google Scholar]
- 77. Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol 2018;6:605–617 [DOI] [PubMed] [Google Scholar]
- 78. Hahr AJ, Molitch ME. Optimizing insulin therapy in patients with type 1 and type 2 diabetes mellitus: optimal dosing and timing in the outpatient setting. Dis Mon 2010;56:148–162 [DOI] [PubMed] [Google Scholar]
- 79. Pathak RD, Schroeder EB, Seaquist ER, et al.; SUPREME-DM Study Group . Severe hypoglycemia requiring medical intervention in a large cohort of adults with diabetes receiving care in U.S. integrated health care delivery systems: 2005–2011. Diabetes Care 2016;39:363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Attallah N, Yassine L. Use of empagliflozin in recipients of kidney transplant: a report of 8 cases. Transplant Proc 2019;51:3275–3280 [DOI] [PubMed] [Google Scholar]
- 81. Cehic MG, Muir CA, Greenfield JR, et al. Efficacy and safety of empagliflozin in the management of diabetes mellitus in heart transplant recipients. Transplant Direct 2019;5:e450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Halden TAS, Kvitne KE, Midtvedt K, et al. Efficacy and safety of empagliflozin in renal transplant recipients with posttransplant diabetes mellitus. Diabetes Care 2019;42:1067–1074 [DOI] [PubMed] [Google Scholar]
- 83. Mahling M, Schork A, Nadalin S, Fritsche A, Heyne N, Guthoff M. Sodium-glucose cotransporter 2 (SGLT2) inhibition in kidney transplant recipients with diabetes mellitus. Kidney Blood Press Res 2019;44:984–992 [DOI] [PubMed] [Google Scholar]
- 84. Schwaiger E, Burghart L, Signorini L, et al. Empagliflozin in posttransplantation diabetes mellitus: a prospective, interventional pilot study on glucose metabolism, fluid volume, and patient safety. Am J Transplant 2019;19:907–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ito S, Kashihara N, Shikata K, et al. Esaxerenone (CS-3150) in patients with type 2 diabetes and microalbuminuria (ESAX-DN): phase 3 randomized controlled clinical trial. Clin J Am Soc Nephrol 2020;15:1715–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bakris GL, Agarwal R, Anker SD, et al.; FIDELIO-DKD Investigators . Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020;383:2219–2229 [DOI] [PubMed] [Google Scholar]
- 87. Pitt B, Filippatos G, Agarwal R, et al.; FIGARO-DKD Investigators . Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021;385:2252–2263 [DOI] [PubMed] [Google Scholar]
- 88. Agarwal R, Filippatos G, Pitt B, et al.; FIDELIO-DKD and FIGARO-DKD investigators . Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J 2022;43:474–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Agarwal R, Joseph A, Anker SD, et al.; FIDELIO-DKD Investigators . Hyperkalemia risk with finerenone: results from the FIDELIO-DKD trial. J Am Soc Nephrol 2022;33:225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]