Abstract
The impact of egg consumption, a major source of dietary cholesterol, on the risk of atherosclerotic cardiovascular diseases (ASCVD) is controversial. Venous thromboembolism (VTE) is a CVD which shares common risk factors and mechanistic pathways with ASCVD. However, there is no data on the relationship between egg or cholesterol intake and VTE risk. Therefore, we evaluated the prospective associations of egg and cholesterol intakes with VTE risk and whether the apoE4 phenotype, which influences cholesterol metabolism, could modify the associations. Data involving 1852 men aged 42–61 years at baseline without a history of VTE or CHD in the population-based Kuopio Ischaemic Heart Disease Risk Factor Study were analysed. Dietary intakes were assessed with 4-d food records. Incident VTE events were identified by record linkage to hospital discharge registries. Hazard ratios (95 % CI) for incident VTE were estimated using Cox regression. During a median follow-up of 28·8 years, 132 VTE events occurred. Comparing the top (> 38 g/d) v. bottom (< 20 g/d) tertiles of egg consumption, the hazard ratio (95 % CI) for VTE was 0·99 (0·64, 1·53) in analysis adjusted for several established risk factors and other dietary factors. There was also no evidence of an association between cholesterol intake and VTE risk. Imputed results were consistent with the observed results. The apoE4 phenotype did not modify the associations. In middle-aged and older Finnish men, egg or cholesterol intakes were not associated with future VTE risk. Other large-scale prospective studies are needed to confirm or refute these findings.
Key words: Egg consumption, Dietary cholesterol, Venous thromboembolism, ApoE4, risk factor, Cohort study
Atherosclerotic cardiovascular disease (ASCVD) (arterial thrombotic disease), which includes CHD and cerebrovascular disease (ischemic stroke)(1), is the major manifestation of CVD; CVD is the leading cause of morbidity and mortality globally(1) and also associated with substantial costs to healthcare systems. Major risk factors for ASCVD include age, sex, blood cholesterol (lipids), blood pressure, diabetes and smoking status(2). Venous thromboembolism (VTE) (comprising deep vein thrombosis and pulmonary embolism) is the third leading vascular disease after CHD and stroke(3); it is also associated with significant morbidity and economic costs and is a preventable cause of death(4,5). Emerging evidence suggests that ASCVD and VTE are closely related via shared risk factors such as age, obesity and cigarette smoking(6,7) and pathophysiological pathways such as coagulation, platelet activation and dyslipidaemia(8). The association between serum cholesterol and CHD risk has been documented as very strong(9). It has been reported that dyslipidemia may not only be associated with arterial thrombotic disease, but with VTE as well(8). Given that previous reports have shown that statins (lipid-lowering drugs) are associated with decreased risk of VTE(10–13), there might be a potential role for lipids in the pathophysiology of VTE. In line with this plausibility, a number of observational studies have demonstrated associations between serum cholesterol parameters and VTE risk(14,15).
Major advances have been made in the prevention of ASCVD through risk factor modification via physical activity, healthy dietary patters and lipid lowering. Though several factors explain a considerable proportion of VTE cases, the causes are still unknown in a substantial number of VTE cases(16). Like ASCVD, VTE constitutes a major public health burden and there is a need to identify risk factors that could aid in the development of preventive strategies. There is evidence to suggest that the adoption of healthy lifestyles such as engaging in habitual physical activity and consuming a healthy diet could help prevent VTE(17,18). Conversely, unhealthy lifestyles such as prolonged sedentary behaviours increase the risk of developing VTE(19,20). Unlike ASCVD, the primary prevention of VTE through lifestyle modification has been largely ignored in guideline recommendations(21).
Though dietary cholesterol does not make an appreciable contribution to serum cholesterol concentrations in most people(22), associations between cholesterol intake and risk of ASCVD have been demonstrated in some studies(23). The impact of egg consumption, a major source of dietary cholesterol (about 200 mg per medium-sized egg), on the risk of ASCVD and other cardiometabolic conditions such as type 2 diabetes is controversial. Meta-analyses of prospective cohort studies suggest little adverse cardiovascular effects with egg intake up to 1 egg per d(24) but have rather observed an inverse association with risk of stroke(25) and hypertension(26). In studies conducted in the USA, egg intake has been associated with higher risk of type 2 diabetes, but such an association has not been observed in studies conducted in Europe or in Asia(27). However, no study has previously assessed if a prospective association exists between egg or cholesterol intakes and future VTE risk. Given the nature of the overall existing evidence, we hypothesised that egg and cholesterol intakes will not be associated with the risk of VTE. In this context, our primary objective was to evaluate the prospective associations of egg and cholesterol intakes with the risk of VTE using a population-based prospective cohort of 1852 middle-aged Finnish men without VTE or CHD at baseline. Given that the influence of dietary cholesterol on serum LDL-cholesterol concentrations is more pronounced among individuals with apoE allele 4 (apoE4) in the general population(28), a subsidiary analysis assessed if the apoE4 phenotype could modify the association between egg or cholesterol intakes and VTE risk.
Materials and methods
Study design and participants
Reporting of the study conforms to broad EQUATOR guidelines(29) and was conducted according to STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines for reporting observational studies in epidemiology (online Supplementary Material 1). The study protocol and design were approved by the Research Ethics Committee of the University of Kuopio. Each study participant provided written informed consent. All study procedures adhered to the Declaration of Helsinki. Study participants included in this analysis were part of the Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD), a population-based prospective study designed to investigate risk factors for ASCVD and other related outcomes. Participants included in the KIHD comprised a representative sample of men living in the city of Kuopio and its surrounding rural communities in eastern Finland. Details of the study design and recruitment methods have been described in previous reports(30–33). Briefly, participants were men aged 42, 48, 54, or 60 years during baseline examinations performed between March 1984 and December 1989. During recruitment, a total of 3433 men were potentially eligible and of these, 3235 were found to be eligible for inclusion into study. Of this number, 2682 volunteered to participate and 553 did not respond to the invitation or declined to give informed consent. From the analyses, we excluded (i) men with a history of CHD (n 677) as they are likely to have changed their diet as part of lifestyle modification or upon advice from a physician and (ii) those with missing data on dietary intakes and confounders (n 153). The current analysis included 1852 men with no previous history of VTE or CHD and complete information on egg consumption, dietary cholesterol intake, relevant covariates and first VTE events (online Supplementary Material 2).
Measurement of covariates and outcome ascertainment
The collection of blood samples, physical measurements, assessment of lifestyle characteristics, medical history and dietary intakes, and measurement of blood biomarkers have been described in detail in previous reports(34–36). For blood sample collection, participants fasted overnight and abstained from drinking alcohol for at least 3 d and from smoking for at least 12 h before blood samples were taken between 08.00 and 10.00. Smoking, alcohol consumption and medical history were assessed by self-administered questionnaires(34). The apoE4 phenotype was determined from plasma using isoelectric focusing and immunoblotting techniques. Subjects who had the phenotype 3/4 or 4/4 were included in the apoE4 group. The consumption of foods was assessed with the use of a 4-d-guided food record, during three weekdays and one weekend day using household measures. A picture book of common foods and dishes was used to help in the estimation of portion sizes. Instructions were provided and completed food records were checked by a nutritionist together with the participant, to ensure accuracy. Nutrient intakes were estimated with the use of NUTRICA version 2.5 software (Social Insurance Institution, Finland), and these were energy-adjusted with the use of the residual method(37). The egg consumption variable represented total egg consumption (g) per d and included the intake of eggs in mixed dishes and recipes. We included all first incident VTE events that occurred from study entry to 31 December 2018. All VTE events required positive imaging tests for their diagnoses and were identified by computer linkage to the National Hospital Discharge Registry data. Each event was validated by two physicians who were blinded to the exposures following detailed cross-checking of medical documents. The ICD 10 codes (I26, I80 and I82) were used to code and classify each VTE case.
Statistical analysis
Baseline characteristics were presented as means and standard deviation or median (interquartile range) for continuous variables and percentages for categorical variables. To assess the cross-sectional associations of egg consumption with various risk markers, Pearson’s correlation coefficients were estimated using linear regression models adjusted for age. We also assessed univariable relationships between egg consumption and baseline characteristics using ANOVA (for continuous variables) and χ2 tests (for categorical variables). Hazard ratios with 95 % CI for incident VTE were estimated using Cox proportional hazard models after confirmation of no major departure from the proportionality of hazards assumptions using Schoenfeld residuals(38). The adjustment for confounders were based on three models: (model 1) age and total energy intake; (model 2) model 1 plus systolic blood pressure, BMI, serum TAG, smoking status, alcohol consumption, leisure-time physical activity, socio-economic status, total energy intake, serum albumin, intake of fruits, berries and vegetables, intake of processed and unprocessed red meat, and history of cancer; and (model 3) a potential mediator-adjusted model comprising history of type 2 diabetes, serum total cholesterol, serum TAG and serum high-sensitivity C-reactive protein concentrations. The confounders selected for models 1–2 were based on their previously established roles as risk factors for VTE, evidence from previous research, previously published associations with VTE in the KIHD study(39–41), or their potential as confounders based on known associations with VTE outcomes and observed associations with egg consumption using the available data(42). Tests of interaction were used to formally assess if the risk of VTE associated with egg consumption and dietary cholesterol intake was modified by the apoE4 phenotype. We conducted multiple imputation by chained equations to handle potential selection bias originating from missingness. The imputation model included all model covariates as well as VTE outcome status. Given the computational time required, ten imputations were computed. Cox regression analyses were run across the ten imputed datasets, and the pooled estimates were reported. All statistical analyses were conducted using Stata version MP 16 (Stata Corp).
Results
Baseline characteristics
Table 1 shows baseline characteristics of study participants and cross-sectional correlates of egg consumption. The mean (standard deviation) age, egg consumption and dietary cholesterol intake of the 1852 men at baseline were 52 (5) years, 33 (26) g/d and 404 (107) mg/d, respectively. Egg consumption was weakly and inversely correlated with age, blood pressure and TAG. There were moderate to strong positive correlations with total energy intake and dietary cholesterol. At baseline, men with higher egg intake were less likely to smoke, have lower concentrations of serum TAG and high-sensitivity C-reactive protein, and had higher intakes of energy, dietary cholesterol and processed and unprocessed red meat (Table 2). Furthermore, men with lower egg intake were more likely to consume alcohol.
Table 1.
Baseline participant characteristics and correlates of egg consumption (n 1852)
| Mean or % | sd | Pearson’s correlation r | 95 % CI† | Percentage difference in values of egg consumption per 1 sd higher or compared with reference category of correlate‡ | 95 % CI | |
|---|---|---|---|---|---|---|
| Egg consumption (g/d) | 33 | 26 | – | – | ||
| Questionnaire/prevalent conditions | ||||||
| Age at survey (years) | 52 | 5 | –0·06 | –0·10, −0·01* | –1·44 % | –2·62, −0·27* |
| Alcohol consumption (g/week) | ||||||
| Median | 32·1 | –0·02 | –0·07, 0·03 | –0·58 % | –1·8, 0·66 | |
| IQR | 6·6–89·8 | |||||
| Socio-economic status | 7·99 | 4·22 | 0·04 | –0·01, 0·08 | 0·94 % | –0·27, 2·14 |
| History of type 2 diabetes | ||||||
| No | 1801 | 97·3 | – | ref | ||
| Yes | 51 | 2·8 | – | –4·30 % | –11·48, 2·88 | |
| Smoking status | ||||||
| Other | 1306 | 70·5 | – | ref | ||
| Current | 546 | 29·5 | – | –3·28 % | –5·86,–0·71* | |
| History of cancer | ||||||
| No | 1823 | 98·4 | – | ref | ||
| Yes | 29 | 1·6 | – | –1·76 % | –11·23, 7·70 | |
| Physical measurements | ||||||
| BMI (kg/m2) | 26·7 | 3·5 | –0·01 | –0·05, 0·04 | –0·20 % | –1·37, 0·98 |
| SBP (mmHg) | 134 | 17 | –0·04 | –0·08, 0·01 | –0·93 % | –2·12, 0·25 |
| DBP (mmHg) | 89 | 11 | –0·05 | –0·09, −0·00* | –1·19 % | –2·37, −0·02* |
| Physical activity (kJ/d) | ||||||
| Median | 1199 | –0·00 | –0·05, 0·04 | –0·10 % | –1·29, 1·08 | |
| IQR | 642–1968 | |||||
| Blood-based markers | ||||||
| Albumin (g/l) | 42·4 | 3·6 | –0·01 | –0·06, 0·03 | –0·33 % | –1·51, 0·85 |
| Total cholesterol (mmol/l) | 5·86 | 1·03 | –0·01 | –0·05, 0·04 | –0·16 % | –1·34, 1·02 |
| HDL-C (mmol/l) | 1·31 | 0·29 | 0·03 | –0·01, 0·08 | 0·81 % | –0·37, 1·98 |
| TAG (mmol/l) | ||||||
| Median | 1·08 | –0·06 | –0·10, −0·01* | –1·45 % | –2·63, −0·28* | |
| IQR | 0·78–1·52 | |||||
| High-sensitivity CRP (mg/l) | ||||||
| Median | 1·19 | –0·03 | –0·08, 0·01 | –0·89 % | –2·06, 0·29 | |
| IQR | 0·66–2·20 | |||||
| Dietary intakes | ||||||
| Total energy intake (kJ/d) | 9935 | 2575 | 0·24 | 0·20, 0·29*** | 6·38 % | 5·23, 7·54*** |
| Cholesterol (mg/d) | 404 | 107 | 0·73 | 0·71, 0·75*** | 18·75 % | 17·95, 19·56*** |
| Processed and unprocessed red meat (g/d) | 146 | 79 | 0·02 | –0·02, 0·07 | 0·56 % | –0·65, 1·77 |
| Fruits, berries and vegetables (g/d) | 258 | 157 | 0·04 | –0·01, 0·09 | 1·03 % | –0·14, 2·21 |
IQR, interquartile range; SBP, systolic blood pressure; DBP, diastolic blood pressure; CRP, C-reactive protein.
Pearson’s correlation coefficients between egg consumption and the row variables.
Percentage change in values of egg consumption per 1 sd increase in the row variable (or for categorical variables, the percentage difference in mean values of egg consumption for the category v. the reference); asterisks indicate the level of statistical significance: *P < 0·05; ***P < 0·001.
Table 2.
Baseline participant characteristics according to egg consumption (Mean values and standard deviations; numbers and percentage; median values and interquartile range)
| Egg consumption tertiles | |||||||
|---|---|---|---|---|---|---|---|
| T1 (20 g/d) | T2 (20–38 g/d) | T3 (> 38 g/d) | |||||
| Mean or % | sd | Mean or % | sd | Mean or % | sd | P | |
| Questionnaire/prevalent conditions | |||||||
| Age at survey (years) | 53 | 5 | 52 | 5 | 52 | 5 | 0·19 |
| Alcohol consumption (g/week) | |||||||
| Median | 36·6 | 29·9 | 32·0 | 0·036 | |||
| IQR | 7·6–105·3 | 6·5–81·3 | 6·4–86·5 | ||||
| Socio-economic status | 8·05 | 4·24 | 7·74 | 4·30 | 8·18 | 4·09 | 0·18 |
| History of type 2 diabetes | |||||||
| No | 622 | 96·3 | 605 | 97·9 | 574 | 97·6 | 0·17 |
| Yes | 24 | 3·7 | 13 | 2·1 | 14 | 2·4 | |
| Smoking status | |||||||
| Other | 414 | 64·1 | 450 | 72·8 | 442 | 75·2 | < 0·01 |
| Current | 232 | 35·9 | 168 | 27·2 | 146 | 24·8 | |
| History of cancer | |||||||
| No | 636 | 98·5 | 608 | 98·4 | 579 | 98·5 | 0·99 |
| Yes | 10 | 1·5 | 10 | 1·6 | 9 | 1·5 | |
| Physical measurements | |||||||
| BMI (kg/m2) | 26·8 | 3·6 | 26·7 | 3·4 | 26·7 | 3·3 | 0·86 |
| SBP (mmHg) | 135 | 17 | 135 | 17 | 133 | 16 | 0·084 |
| DBP (mmHg) | 90 | 11 | 89 | 11 | 88 | 10 | 0·015 |
| Physical activity (kJ/d) | |||||||
| Median | 1151 | 1239 | 1220 | 0·17 | |||
| IQR | 631–1958 | 679–1940 | 606–2014 | ||||
| Blood-based markers | |||||||
| Albumin (g/l) | 42·3 | 3·8 | 42·5 | 3·70 | 42·3 | 3·1 | 0·39 |
| Total cholesterol (mmol/l) | 5·89 | 1·07 | 5·81 | 1·04 | 5·88 | 0·99 | 0·35 |
| HDL-C (mmol/l) | 1·30 | 0·29 | 1·29 | 0·28 | 1·33 | 0·30 | 0·11 |
| TAG (mmol/l) | |||||||
| Median | 1·15 | 1·07 | 1·03 | 0·016 | |||
| IQR | 0·80–1·59 | 0·80–1·53 | 0·75–1·41 | ||||
| High-sensitivity CRP (mg/l) | |||||||
| Median | 1·24 | 1·16 | 1·15 | 0·018 | |||
| IQR | 0·67–1·59 | 0·65–2·16 | 0·64–2·07 | ||||
| Dietary intakes | |||||||
| Total energy intake (kJ/d) | 9204 | 2503 | 9894 | 2432 | 10 782 | 2548 | < 0·001 |
| Cholesterol (mg/d) | 343 | 73 | 385 | 73 | 492 | 112 | < 0·001 |
| Processed and unprocessed red meat (g/d) | 143 | 82 | 145 | 77 | 149 | 76 | 0·35 |
| Fruits, berries and vegetables (g/d) | 245 | 167 | 259 | 148 | 270 | 153 | 0·022 |
T, tertile; IQR, interquartile range; SBP, systolic blood pressure; DBP, diastolic blood pressure; CRP, C-reactive protein.
Associations of egg and cholesterol intake with venous thromboembolism risk
A total of 132 VTE events were recorded during a median (interquartile range) follow-up of 28·8 (19·6, 31·2) years. In analysis adjusted for age and total energy intake, the hazard ratio (95 % CI) for VTE comparing the top v. bottom tertiles of egg consumption was 1·01 (0·66, 1·54), which remained non-significant 0·99 (0·64, 1·53) on further adjustment for systolic blood pressure, BMI, TAG, smoking status, alcohol consumption, physical activity, socio-economic status, serum albumin, intake of fruits, berries and vegetables, intake of processed and unprocessed red meat, and history of cancer (Fig. 1). There was no evidence of an association in the model that adjusted for potential mediators (Fig. 1). The results were similar for the association between dietary cholesterol intake and VTE risk (Fig. 2). The null associations persisted when both exposures were modelled as continuous variables (Fig. 1 and 2). Data were imputed for 2005 participants, and the imputed results were consistent with those obtained using observed values (online Supplementary Materials 3–4).
Fig. 1.
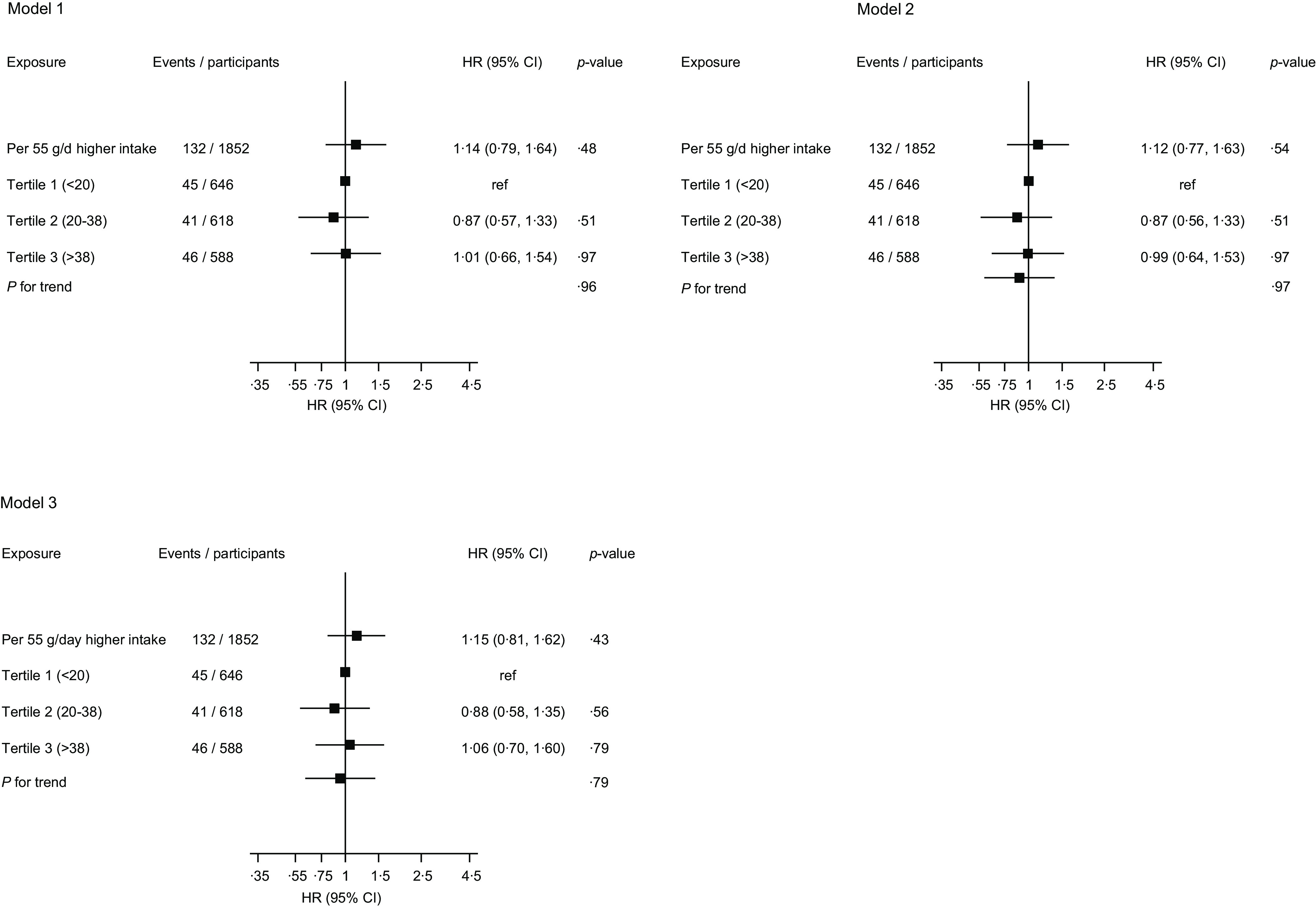
Association between egg consumption and risk of venous thromboembolism. HR, hazard ratio; ref, reference. Model 1: adjusted for age and total energy intake. Model 2: model 1 plus total energy intake, systolic blood pressure, BMI, serum TAG, smoking status, alcohol consumption, physical activity, socio-economic status, serum albumin, intake of fruits, berries and vegetables, intake of processed and unprocessed red meat, and history of cancer. Model 3: history of type 2 diabetes, serum total cholesterol, serum TAG and serum high-sensitivity C-reactive protein.
Fig. 2.
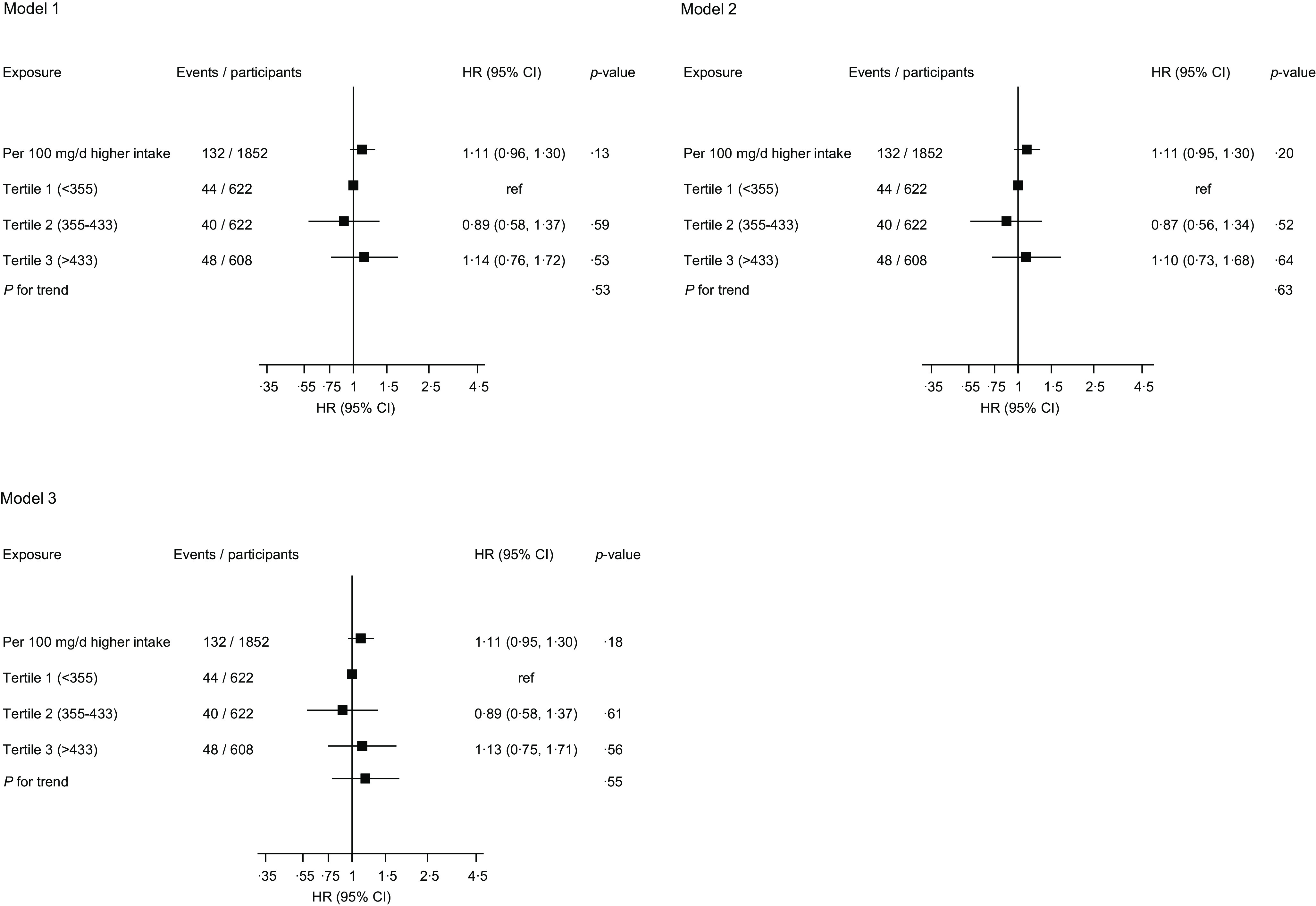
Association between dietary cholesterol intake and risk of venous thromboembolism. HR, hazard ratio; ref, reference. Model 1: adjusted for age and total energy intake. Model 2: model 1 plus total energy intake, systolic blood pressure, BMI, serum TAG, smoking status, alcohol consumption, physical activity, socio-economic status, serum albumin, intake of fruits, berries and vegetables, intake of processed and unprocessed red meat, and history of cancer. Model 3: history of type 2 diabetes, serum total cholesterol, serum TAG and serum high-sensitivity C-reactive protein
Following exclusions of men with pre-existing CHD and missing data on dietary intakes and relevant confounders, there were 988 men with available data on apoE phenotype: 335 were carriers of the apoE4 phenotype (n of VTE events = 17) and 653 were non-carriers (n of VTE events = 54). For each 55 g/d (1 egg) higher egg intake, the hazard ratio (95 % CI) for VTE (based on model 2) were 1·11 (0·33, 3·72) and 1·17 (0·63, 2·16) for apoE4 carriers and non-carriers, respectively (P-value for interaction = 0·94). For each 100 mg/d higher cholesterol intake, the hazard ratio (95 % CI) for VTE (based on model 2) were 1·10 (0·69, 1·76) and 1·17 (0·91, 1·49) for apoE4 carriers and non-carriers, respectively (P-value for interaction = 0·83).
Discussion
In this general population-based cohort of middle-aged and older Finnish men without a history of VTE or CHD, there were generally weak correlations of egg consumption with several VTE risk markers. In analysis adjusted for several established and emerging risk factors, egg consumption or dietary cholesterol intake was not associated with VTE risk, not even in participants with the apoE4 phenotype. The imputed results were similar to the observed results.
Given that this is the first reported evaluation of the prospective associations of egg and cholesterol intakes with VTE risk, the current findings cannot be discussed in comparison with previous studies. Other large-scale studies will be needed to refute or confirm these findings. However, given our previous findings in the KIHD cohort of no evidence of associations between egg or cholesterol intakes and carotid atherosclerosis or risk of CHD(43) or stroke(36), not even among the carriers of the apoE4, and an inverse association with type 2 diabetes(44), the null findings with VTE risk are not that unexpected. There is a possibility that the absence of evidence of an association may represent the true relationship between egg consumption, a major source of dietary cholesterol, and VTE risk. Serum cholesterol is well established to be strongly associated with the risk of ASCVD. For several years, dietary cholesterol was implicated to increase serum cholesterol levels, leading to an increased risk of ASCVD(45). As a result, major guideline bodies such as the American Heart Association made recommendations of limiting dietary cholesterol intake to 300 mg/d in healthy individuals and restricting egg consumption to not more than three whole eggs per week(46). However, there is convincing evidence that dietary cholesterol only makes modest contributions to serum cholesterol concentrations in general populations(22). There is also evidence suggesting that increased dietary cholesterol intake decreases the synthesis of endogenous de novo cholesterol, to maintain cholesterol homoeostasis(47). An extensive review of observational and experimental research also does not provide conclusive evidence that dietary cholesterol is involved in the development of ASCVD(45). This has led to the removal of recommendations restricting dietary cholesterol intake to 300 mg/d in the 2015–2020 Dietary Guidelines for Americans(48). It has been reported that the observed association between dietary cholesterol and ASCVD could be driven by SFA which are high in foods containing dietary cholesterol(45). SFA, especially when replacing PUFA in diet, are well known to increase the levels of LDL-cholesterol, which are associated with an increased risk of ASCVD(49).
Though there is a wealth of evidence supporting a close relationship between ASCVD and VTE, definite conclusions have not been drawn as evidence on their shared risk factors and mechanisms has not been consistent. With regard to shared risk factors, some studies have demonstrated associations between traditional ASCVD risk factors and VTE risk(7,50), whereas others have not(51,52). While some studies have reported that ASCVD is an underlying condition and precedes the development of VTE(53), other studies have shown that ASCVD does not precede VTE development(54,55) or VTE rather precedes ASCVD(56). Historically, ASCVD and VTE have been viewed as distinct pathophysiological entities, as a result of the obvious anatomical differences and their distinct clinical presentations(57). Consistent with this is the inability of our study to demonstrate an association in apoE4 carriers, given that the subjects with the apoE4 phenotype have more pronounced elevations in LDL-cholesterol levels due to dietary cholesterol intake(28) and higher risk of ASCVD(58,59). Other potential reasons for the null results include the low event rate and underestimation of the true strength of the association due to regression dilution bias, given the use of only one dietary assessment at the baseline and the long follow-up duration. Due to the low incidence rates of VTE and hence few events in the first few years of follow-up, we were unable to conduct sufficiently powered targeted analyses to ascertain if the observed null associations could be due to potential regression dilution bias. However, our use of multiple imputation methods (based on 2005 participants) showed that the results of our complete-case analyses were not biased. Taking the overall evidence together and contrary to what was previously thought, it appears that dietary cholesterol intakes (including egg consumption) may not be associated with an increased risk of venous and arterial thromboembolic conditions, which are associated with substantial morbidity, premature mortality and high economic costs. Eggs are nutrient-dense food items, rich in several micronutrients including vitamins and minerals, and contain high-quality protein with minimal SFA (1·56 g/egg)(45). Hence, it is appropriate to include moderate egg consumption as part of a healthy eating pattern.
We have conducted the first evaluation of the temporal relationships of egg and cholesterol intakes with VTE risk. Other strengths include the population-based prospective cohort design and the ability to assess if the apoE4 phenotype modified the associations. This was especially relevant given that the apoE4 phenotype is very common among the Finnish population; it has been reported that about one-third of the Finnish population possess ≥ 1 E4 allele(60). There were limitations which deserve consideration and include as follows: (i) dietary intake was only assessed at baseline, which did not consider the possible dietary changes during the long follow-up; (ii) possible random errors in food recording that could attenuate the true associations; (iii) lack of specific data on nutrient intake from a specific method of preparation; however, all intake of nutrients including egg consumption represented total consumption and these included intake in mixed dishes and recipes; (iv) inability to generalise the findings to women and other populations; (v) inability to adjust for examination year as a number of participants (n 395) did not have the specific year of examination assigned to them; however, it is unlikely this would have impacted on our results given that the examination years spanned a relatively short period from 1984 to 1989; (vi) the relatively low event rate; (vii) lack of specific data on deep vein thrombosis and pulmonary embolism; and (viii) potential for biases due to the observational design. Given the limitations and being the first evaluation of its kind, caution is needed in interpreting the findings; they should be regarded mainly as hypothesis generating.
In summary, egg or cholesterol intake was not associated with future VTE risk in middle-aged and older men. Furthermore, apoE4 phenotype did not modify the associations. Other large-scale prospective studies conducted in other populations are needed to confirm or refute these findings.
Acknowledgements
The authors thank the staff of the Kuopio Research Institute of Exercise Medicine and the Research Institute of Public Health and University of Eastern Finland, Kuopio, Finland, for the data collection in the study.
Prof. Laukkanen acknowledges support from The Finnish Foundation for Cardiovascular Research, Helsinki, Finland.
Authors’ responsibilities were as follows: S. K. K.: study design, data analysis and interpretation, drafting manuscript, and revising manuscript content and approving final version of manuscript; J. A. L.: study design and conduct, responsibility for the patients and data collection, and revising manuscript content and approving final version of manuscript; J. K. V.: study design and conduct, responsibility for the patients and data collection, and revising manuscript content and approving final version of manuscript. All authors approved the final version of the manuscript.
The authors declare no conflicts of interest.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0007114522000988.
click here to view supplementary material
References
- 1. Barquera S, Pedroza-Tobias A, Medina C, et al. (2015) Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res 46, 328–338. [DOI] [PubMed] [Google Scholar]
- 2. Wood D (2001) Established and emerging cardiovascular risk factors. Am Heart J 141, S49–57. [DOI] [PubMed] [Google Scholar]
- 3. Di Nisio M, van Es N & Buller HR (2016) Deep vein thrombosis and pulmonary embolism. Lancet 388, 3060–3073. [DOI] [PubMed] [Google Scholar]
- 4. Douketis JD, Gu CS, Schulman S, et al. (2007) The risk for fatal pulmonary embolism after discontinuing anticoagulant therapy for venous thromboembolism. Ann Intern Med 147, 766–774. [DOI] [PubMed] [Google Scholar]
- 5. Cohen AT, Agnelli G, Anderson FA, et al. (2007) Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost 98, 756–764. [DOI] [PubMed] [Google Scholar]
- 6. Glynn RJ & Rosner B (2005) Comparison of risk factors for the competing risks of coronary heart disease, stroke, and venous thromboembolism. Am J Epidemiol 162, 975–982. [DOI] [PubMed] [Google Scholar]
- 7. Ageno W, Becattini C, Brighton T, et al. (2008) Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation 117, 93–102. [DOI] [PubMed] [Google Scholar]
- 8. Ray JG (2003) Dyslipidemia, statins, and venous thromboembolism: a potential risk factor and a potential treatment. Curr Opin Pulm Med 9, 378–384. [DOI] [PubMed] [Google Scholar]
- 9. Ference BA, Ginsberg HN, Graham I, et al. (2017) Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 38, 2459–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kunutsor SK, Seidu S & Khunti K (2017) Statins and secondary prevention of venous thromboembolism: pooled analysis of published observational cohort studies. Eur Heart J 38, 1608–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kunutsor SK, Whitehouse MR, Blom AW, et al. (2017) Statins and venous thromboembolism: do they represent a viable therapeutic agent? Expert Rev Cardiovasc Ther 15, 629–637. [DOI] [PubMed] [Google Scholar]
- 12. Kunutsor SK, Seidu S & Khunti K (2017) Statins and primary prevention of venous thromboembolism: a systematic review and meta-analysis. Lancet Haematol 4, e83–e93. [DOI] [PubMed] [Google Scholar]
- 13. Zaccardi F, Kunutsor SK, Seidu S, et al. (2018) Is the lower risk of venous thromboembolism with statins related to low-density-lipoprotein reduction? A network meta-analysis and meta-regression of randomised controlled trials. Atherosclerosis 271, 223–231. [DOI] [PubMed] [Google Scholar]
- 14. Delluc A, Malecot JM, Kerspern H, et al. (2012) Lipid parameters, lipid lowering drugs and the risk of venous thromboembolism. Atherosclerosis 220, 184–188. [DOI] [PubMed] [Google Scholar]
- 15. Ray JG & Rosendaal FR (2001) The role of dyslipidemia and statins in venous thromboembolism. Curr Control Trials Cardiovasc Med 2, 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosendaal FR (1999) Risk factors for venous thrombotic disease. Thromb Haemost 82, 610–619. [PubMed] [Google Scholar]
- 17. Kunutsor SK, Makikallio TH, Seidu S, et al. (2020) Physical activity and risk of venous thromboembolism: systematic review and meta-analysis of prospective cohort studies. Eur J Epidemiol 35, 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kunutsor SK (2021) Can a healthy dietary pattern alone prevent venous thromboembolism in the general population? Nutr Metab Cardiovasc Dis 31, 2839–2841. [DOI] [PubMed] [Google Scholar]
- 19. Kunutsor SK, Dey RS & Laukkanen JA (2022) Television viewing and venous thrombo-embolism: a systematic review and meta-analysis. Eur J Prev Cardiol. doi: 10.1093/eurjpc/zwab220. [DOI] [PubMed] [Google Scholar]
- 20. Kunutsor SK & Laukkanen JA (2021) TV viewing and venous thromboembolism: risk or red herring? J Thromb Haemost 19, 2635–2637. [DOI] [PubMed] [Google Scholar]
- 21. Arnett DK, Blumenthal RS, Albert MA, et al. (2019) 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 140, e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hopkins PN (1992) Effects of dietary cholesterol on serum cholesterol: a meta-analysis and review. Am J Clin Nutr 55, 1060–1070. [DOI] [PubMed] [Google Scholar]
- 23. Zhong VW, Van Horn L, Cornelis MC, et al. (2019) Associations of dietary cholesterol or egg consumption with incident cardiovascular disease and mortality. JAMA 321, 1081–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Godos J, Micek A, Brzostek T, et al. (2021) Egg consumption and cardiovascular risk: a dose-response meta-analysis of prospective cohort studies. Eur J Nutr 60, 1833–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang H, Cao Y, Yang X, et al. (2020) Egg consumption and stroke risk: a systematic review and dose-response meta-analysis of prospective studies. Front Nutr 7, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Y & Zhang DZ (2018) Red meat, poultry, and egg consumption with the risk of hypertension: a meta-analysis of prospective cohort studies. J Hum Hypertens 32, 507–517. [DOI] [PubMed] [Google Scholar]
- 27. Tamez M, Virtanen JK & Lajous M (2016) Egg consumption and risk of incident type 2 diabetes: a dose-response meta-analysis of prospective cohort studies. Br J Nutr 115, 2212–2218. [DOI] [PubMed] [Google Scholar]
- 28. Bennet AM, Di Angelantonio E, Ye Z, et al. (2007) Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA 298, 1300–1311. [DOI] [PubMed] [Google Scholar]
- 29. Simera I, Moher D, Hoey J, et al. (2010) A catalogue of reporting guidelines for health research. Eur J Clin Invest 40, 35–53. [DOI] [PubMed] [Google Scholar]
- 30. Kunutsor SK, Kurl S, Zaccardi F, et al. (2016) Baseline and long-term fibrinogen levels and risk of sudden cardiac death: a new prospective study and meta-analysis. Atherosclerosis 245, 171–180. [DOI] [PubMed] [Google Scholar]
- 31. Kunutsor SK, Whitehouse MR, Blom AW, et al. (2017) Low serum magnesium levels are associated with increased risk of fractures: a long-term prospective cohort study. Eur J Epidemiol 32, 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Laukkanen T, Kunutsor SK, Zaccardi F, et al. (2018) Acute effects of sauna bathing on cardiovascular function. J Hum Hypertens 32, 129–138. [DOI] [PubMed] [Google Scholar]
- 33. Kunutsor SK, Khan H, Nyyssonen K, et al. (2016) Lipoprotein(a) and risk of sudden cardiac death in middle-aged Finnish men: a new prospective cohort study. Int J Cardiol 220, 718–725. [DOI] [PubMed] [Google Scholar]
- 34. Salonen JT, Nyyssonen K, Korpela H, et al. (1992) High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation 86, 803–811. [DOI] [PubMed] [Google Scholar]
- 35. Kunutsor SK, Khan H & Laukkanen JA (2016) γ-Glutamyltransferase and risk of sudden cardiac death in middle-aged Finnish men: a new prospective cohort study. J Am Heart Assoc 5, e002858. [DOI] [PMC free article] [PubMed]
- 36. Abdollahi AM, Virtanen HEK, Voutilainen S, et al. (2019) Egg consumption, cholesterol intake, and risk of incident stroke in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr 110, 169–176. [DOI] [PubMed] [Google Scholar]
- 37. Willett W (2013) Implications of Total Energy Intake for Epidemiologic Analyses. In: Nutritional Epidemiology. New York: Oxford University Press. [Google Scholar]
- 38. Therneau TM & Grambsch PM (2000) Modeling Survival Data: Extending the Cox Model. New York: Springer.
- 39. Kunutsor SK, Makikallio TH, Araujo CGS, et al. (2019) Cardiorespiratory fitness is not associated with risk of venous thromboembolism: a cohort study. Scand Cardiovasc J 53, 255–258. [DOI] [PubMed] [Google Scholar]
- 40. Kunutsor SK, Dey RS & Laukkanen JA (2021) Circulating serum copper is associated with atherosclerotic cardiovascular disease, but not venous thromboembolism: a prospective cohort study. Pulse 9, 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kunutsor SK & Laukkanen JA (2021) Circulating serum magnesium and the risk of venous thromboembolism in men: a long-term prospective cohort study. Pulse 8, 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Groenwold RH, Klungel OH, Grobbee DE, et al. (2011) Selection of confounding variables should not be based on observed associations with exposure. Eur J Epidemiol 26, 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Virtanen JK, Mursu J, Virtanen HE, et al. (2016) Associations of egg and cholesterol intakes with carotid intima-media thickness and risk of incident coronary artery disease according to apolipoprotein E phenotype in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr 103, 895–901. [DOI] [PubMed] [Google Scholar]
- 44. Virtanen JK, Mursu J, Tuomainen TP, et al. (2015) Egg consumption and risk of incident type 2 diabetes in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr 101, 1088–1096. [DOI] [PubMed] [Google Scholar]
- 45. Soliman GA (2018) Dietary cholesterol and the lack of evidence in cardiovascular disease. Nutrients 10, 780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. American Heart Association (1968) Diet and Heart Disease. Dallas, TX: American Heart Association. [Google Scholar]
- 47. Hu YW, Zheng L & Wang Q (2010) Regulation of cholesterol homeostasis by liver X receptors. Clin Chim Acta 411, 617–625. [DOI] [PubMed] [Google Scholar]
- 48. U.S. Department of Health and Human Services & U.S. Department of Agriculture (2015) 2015–2020 Dietary Guidelines for Americans. 8th Edition. https://health.gov/our-work/food-nutrition/previous-dietary-guidelines/2015 (accessed January 2022).
- 49. Maki KC, Eren F, Cassens ME, et al. (2018) n-6 polyunsaturated fatty acids and cardiometabolic health: current evidence, controversies, and research gaps. Adv Nutr 9, 688–700. [DOI] [PMC free article] [PubMed]
- 50. Gregson J, Kaptoge S, Bolton T, et al. (2019) Cardiovascular risk factors associated with venous thromboembolism. JAMA Cardiol 4, 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wattanakit K, Lutsey PL, Bell EJ, et al. (2012) Association between cardiovascular disease risk factors and occurrence of venous thromboembolism. A time-dependent analysis. Thromb Haemost 108, 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mahmoodi BK, Cushman M, Anne Naess I, et al. (2017) Association of traditional cardiovascular risk factors with venous thromboembolism: an individual participant data meta-analysis of prospective studies. Circulation 135, 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Prandoni P, Bilora F, Marchiori A, et al. (2003) An association between atherosclerosis and venous thrombosis. N Engl J Med 348, 1435–1441. [DOI] [PubMed] [Google Scholar]
- 54. Reich LM, Folsom AR, Key NS, et al. (2006) Prospective study of subclinical atherosclerosis as a risk factor for venous thromboembolism. J Thromb Haemostasis 4, 1909–1913. [DOI] [PubMed] [Google Scholar]
- 55. van der Hagen PB, Folsom AR, Jenny NS, et al. (2006) Subclinical atherosclerosis and the risk of future venous thrombosis in the Cardiovascular Health Study. J Thromb Haemostasis 4, 1903–1908. [DOI] [PubMed] [Google Scholar]
- 56. Prandoni P, Ghirarduzzi A, Prins MH, et al. (2006) Venous thromboembolism and the risk of subsequent symptomatic atherosclerosis. J Thrombosis Haemostasis: JTH 4, 1891–1896. [DOI] [PubMed] [Google Scholar]
- 57. Prandoni P (2007) Venous thromboembolism and atherosclerosis: is there a link? J Thromb Haemost 5, 270–275. [DOI] [PubMed] [Google Scholar]
- 58. Khan TA, Shah T, Prieto D, et al. (2013) Apolipoprotein E genotype, cardiovascular biomarkers and risk of stroke: systematic review and meta-analysis of 14 015 stroke cases and pooled analysis of primary biomarker data from up to 60 883 individuals. Int J Epidemiol 42, 475–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kumar A, Kumar P, Prasad M, et al. (2016) Association between Apolipoprotein epsilon4 gene polymorphism and risk of ischemic stroke: a meta-analysis. Ann Neurosci 23, 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ehnholm C, Lukka M, Kuusi T, et al. (1986) Apolipoprotein E polymorphism in the Finnish population: gene frequencies and relation to lipoprotein concentrations. J Lipid Res 27, 227–235. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0007114522000988.
click here to view supplementary material


