Abstract
Background
The aim of this study was to evaluate the safety and effectiveness of robot‐assisted thymectomy (RAT) in large anterior mediastinal tumors (AMTs) (size ≥6 cm) compared with video‐assisted thymectomy (VAT) and open surgery.
Methods
A total of 132 patients with large AMTs who underwent surgical resection from January 2016 to June 2022 were included in this study. A total of 61 patients underwent RAT, 36 patients underwent VAT and 35 patients underwent open surgery. Perioperative outcomes were compared.
Results
There were no significant differences in tumor size (p = 0.141), or pathological types (p = 0.903). Compared with the open group, the RAT and VAT groups were associated with a shorter operation time (115.00 vs. 160.00, p = 0.012; 122.50 vs. 160.00, p = 0.071), and less blood loss (50.00 vs. 200.00, p < 0.001; 50.00 vs. 200.00, p < 0.001), respectively. The rate of conversion in the RAT group was similar to that in the VAT group (6.56% vs. 13.89%, p = 0.229). Concomitant resection was less frequently performed in the VAT group than in the RAT and open groups (5.56% vs. 31.15%, p = 0.040; 5.56% vs. 31.43%, p = 0.006). VAT patients had a lower drainage volume (365.00 vs. 700.00 and 910.00 mL, p < 0.001), shorter duration of chest tube (2.00 vs. 3.00 and 4.00, p < 0.001), and shorter hospital stay (5.00 vs. 6.00 and 7.00, p < 0.001) than the RAT and open groups. There was no 30‐day mortality in any group. No difference was seen in R0 resection rates (p = 0.846). The postoperative complication rates were similar among the three groups (p = 0.309). Total in‐hospital costs (66493.90 vs. 33581.05 and 42876.40, p < 0.001) were significantly higher in the RAT group.
Conclusions
RAT is safe and effective for the resection of large AMTs compared to VAT and open surgery. Vascular resection in RAT is technically feasible. A long‐term follow‐up is required.
Keywords: anterior mediastinal tumor, robot‐assisted thymectomy, video‐assisted thymectomy
The robot‐assisted thymectomy (RAT) can be performed using various approaches. The sub‐xiphoid approach is routinely selected for centered and advanced large AMTs in our center; it can provide excellent visualization for exposing the proximal and distal ends of the innominate vein, which is convenient for resection of the invaded innominate vein, even for partial resection of the superior vena cava (SVC).
INTRODUCTION
Complete surgical resection is a standard and effective treatment for anterior mediastinal tumors (AMTs). 1 The classic surgical approaches are sternotomy, and anterolateral or posterolateral thoracotomy. 2 Both sternotomy and thoracotomy can provide excellent surgical visualization and satisfying manipulation space. However, open surgery has considerable morbidity, such as postoperative pain, blood loss, and incisional wound infection. 3 Over the past few decades, video‐assisted thymectomy (VAT) has become the preferable approach for AMTs because of its better perioperative outcomes and oncological results versus open surgery. 4 Robot‐assisted thymectomy (RAT) has also been an alternative approach for AMT because of the advantages of 3D‐magnified visualization and highly stable and mobile operating arms, which provide comparable surgical outcomes. 5
A large AMT may narrow the retrosternal space and invade adjacent structures such as the lung, pericardium, phrenic nerve, and vessels. It requires the surgeon to be very experienced and have superb skills to avoid incomplete resection and tumor rupture. In the published literature, mini‐invasive approaches including RAT and VAT used for the resection of large AMTs are rare. Very few studies have compared the perioperative outcomes among RAT, VAT, and open surgery. 6 In this study, we aimed to identify the safety and effectiveness of RAT for the resection of large AMTs (tumor size ≥6 cm) in comparison to VAT and open surgery.
METHODS
Patients
A total of 132 patients with AMTs 6 cm or larger that underwent surgical resection at Daping Hospital from January 2016 to June 2022 were reviewed. A total of 61 patients underwent robot‐assisted thymectomy (RAT group), 36 patients underwent video‐assisted thymectomy (VAT group), and 35 patients underwent open surgery (open group). Enhanced chest computed tomography was used to assess tumor size, location, and potential invasion of adjacent structures. Patients with myasthenia gravis (MG) were assessed for symptoms. This retrospective study was approved by the Ethics Committee of Army Medical Center of PLA (Daping Hospital, Chongqing City, China) on August 24, 2022 (IRB: 2022 to 256).
Patient demographics, presence of MG, tumor size, histology, adjuvant treatment, and the intraoperative (operation time, estimated blood loss, conversion, R0 resection, concomitant resection, and laterality), and postoperative outcomes (total drainage volume, duration of chest tube, hospital stay, complications, total in‐hospital costs, and 30 days mortality were collected and analyzed).
Surgical technique
Mini‐invasive surgery was performed under general anesthesia with single‐lumen bronchial intubation, whereas open surgery was performed with double‐lumen bronchial intubation.
Patients with MG underwent extended thymectomy with tumor included, and patients without MG underwent classical thymectomy with tumor included.
Robot‐assisted surgery
The Da Vanci Si system with four arms (Intuitive Surgical) was used for the operation.
In the transthoracic approach patients were placed in the 30° lateral position, the 3‐port method was commonly used and one assistant port wound was made if necessary. A 12 mm camera port was placed at the mid‐axillary line in the 5th intercostal space, and carbon dioxide (CO2) was insufflated into the pleural cavity at a pressure of 8–10 mm Hg. Two 8‐mm working ports were placed in the 3rd and 4th intercostal spaces at the anterior axillary and mid‐clavicular lines. The assistant port was usually placed in the 4th intercostal space at the mid‐axillary line. Electrocautery and bipolar forceps (Maryland) were commonly used.
In the sub‐xiphoid approach patients were placed in the dorsal elevated position, the 3‐port method was commonly used and one assistant port wound was made if necessary. A 12‐mm camera port was placed below the xiphoid process, by using finger and sponge forceps from the port, the retrosternal space was enlarged blindly. CO2 was insufflated into the retrosternal space at a pressure of 8–10 mm Hg, and two 8‐mm working ports were placed below the bilateral costal arches at the mid‐clavicular line. The assistant port was usually placed in the 4th intercostal space at the mid‐axillary line (right or left). Electrocautery and bipolar forceps (Maryland) were also used. To enlarge the manipulation space, bilateral mediastinal pleura were usually dissected, and the lower part of sternum could be slightly elevated by the camera arm. The procedure consisted of a complete tumor resection including the whole thymus, mediastinal fat and adjacent structures that had been invaded, following a non‐squeeze concept to avoid tumor rupture. An endoscopic stapler was used in concomitant resection of the invaded lung, innominate vein and superior vena cava (SVC) through the assistant port. The specimen was removed in an endobag through a working port, and the incision was enlarged if necessary. (Figure 1).
FIGURE 1.
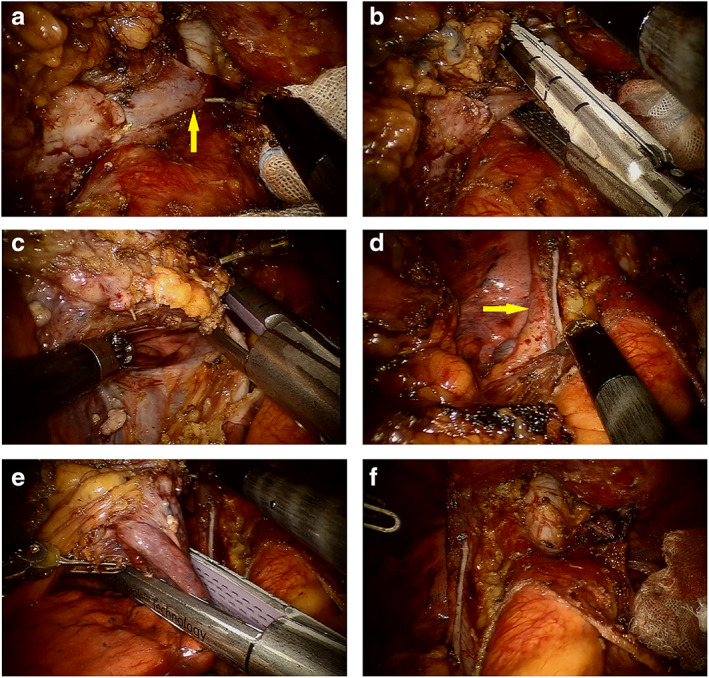
Robot‐assisted thymectomy via sub‐xiphoid approach for a large and advanced tumor combined resection of the innominate vein, partial superior vena cava (SVC) and lung. (a) The distal end of the innominate vein was exposed. (b) The distal end of the innominate vein was cut off with an endoscopic stapler. (c) Partial resection of the SVC with a stapler. (d) Sufficient diameter of the SVC. (e) Removal of the invaded lung. (f) Surgical field after complete resection of the tumor and invaded vital structures.
Video‐assisted surgery
In VAT, the patient's position and incision design were similar to those in RAT. A harmonic scalpel replaced the bipolar forceps and electrocautery and aspirator were used.
Open surgery
The operation was performed using thoracotomy (3rd or 4th intercostal incision), sternotomy or a hemi‐Clamshell incision. The non‐squeeze policy was followed in tumor resection.
Statistical analysis
Statistical analysis was performed using SPSS version 25.0. The continuous data are described as the median and interquartile range (IQR) or the mean with standard deviation (SD). A Kruskal‐Wallis test or ANOVA was performed for comparisons among the three groups. The Wilcoxon rank‐sum test or LSD test (homogeneity of variance) was performed for comparisons between any two groups. Categorical variables are displayed as patient counts and percentages, and were compared using the χ2 or Fisher's exact test. Statistical significance was defined as a p value <0.05.
RESULTS
Patient and tumor characteristics
A total of 132 patients were included in this study. There were no significant differences among the three groups in age, sex, BMI, tumor size, presence of MG, pathological type, or adjuvant treatment. Patient and tumor characteristics are summarized in Table 1.
TABLE 1.
Clinical pathological characteristics of the 132 patients with anterior mediastinal tumor
| RAT group (n = 61) | VAT group (n = 36) | Open group (n = 35) | p‐value | |
|---|---|---|---|---|
| Age (years), mean ± SD | 46.10 ± 14.10 | 47.60 ± 14.60 | 47.70 ± 16.30 | 0.468 |
| Gender, no. (%) | 0.447 | |||
| Male | 40 (65.57) | 19 (52.78) | 22 (62.86) | |
| Female | 21 (34.43) | 17 (47.22) | 13 (37.14) | |
| BMI (kg/m2), mean ± SD | 23.01 ± 2.92 | 22.98 ± 3.35 | 22.79 ± 4.18 | 0.384 |
| Tumor size (cm), median (IQR) | 8.00 (7.00, 9.90) | 7.35 (6.58, 8.50) | 8.00 (6.80, 10.50) | 0.141 |
| Myasthenia gravis, no. (%) | 0.366 | |||
| Yes | 5 (8.20) | 2 (5.56) | 5 (14.29) | |
| No | 56 (91.80) | 34 (94.44) | 30 (85.71) | |
| Pathological types, no. (%) | 0.903 | |||
| Thymic epithelial tumor | 45 (73.77) | 24 (66.67) | 27 (77.14) | |
| A | 3 | 4 | 1 | |
| AB | 14 | 7 | 8 | |
| B1 | 5 | 6 | 5 | |
| B2 | 14 | 6 | 4 | |
| B3 | 4 | 1 | 5 | |
| C | 5 | 0 | 4 | |
| Mature teratoma | 8 (13.11) | 6 (16.66) | 4 (11.43) | |
| Other types | 8 (13.11) | 6 (16.66) | 4 (11.43) | |
| Adjuvant treatment, no. (%) | 0.245 | |||
| Yes | 1 (1.64) | 0 (0) | 2 (5.71) | |
| No | 60 (98.36) | 36 (100) | 33 (94.29) |
Abbreviations: BMI, body mass index; IQR, interquartile range; RAT, robot‐assisted thymectomy; VAT, video‐assisted thymectomy.
Intraoperative outcomes
The median operation time in the open group was significantly longer than that in the RAT group (160.00 vs. 115.00, p = 0.012), but similar to that in the VAT group (160.00 vs. 122.50, p = 0.071); however, there was no significant difference between the RAT group and the VAT group (p = 1.000). The median estimated blood loss in both the RAT group (50.00, p < 0.001) and the VAT group (50.00, p < 0.001) was significantly less than that in the open group (200.00), and there was no significant difference between the RAT group and the VAT group (p = 0.881). The concomitant resection rates in both the RAT group (31.15%, p = 0.040) and the open group (31.43%, p = 0.006) were significantly higher than those in the VAT group (5.56%), but there was no significant difference between the RAT group and the open group (p = 0.977). There were no significant differences among the three groups in the R0 resection rate (p = 0.846). The conversion rate (p = 0.229) and mini‐invasive laterality (p = 0.742) in the RAT group were similar to those in the VAT group (Table 2).
TABLE 2.
Surgical characteristics stratified by operative approach
| RAT group (n = 61) | VAT group (n = 36) | Open group (n = 35) | p‐value | |
|---|---|---|---|---|
| Operation time (min) | 115.00 | 122.50 | 160.00 | 0.012 |
| Median (IQR) | (92.50, 145.00) | (86.25, 170.00) | 115.00, 190.00) | |
| RAT vs. VAT | 1.000 | |||
| RAT vs. open | 0.012 | |||
| VAT vs. open | 0.071 | |||
| Estimated blood loss (ml) | 50.00 | 50.00 | 200.00 | 0.000 |
| Median (IQR) | (25.00, 100.00) | (30.00, 187.50) | (100.00, 300.00) | |
| RAT vs. VAT | 0.881 | |||
| RAT vs. open | 0.000 | |||
| VAT vs. open | 0.000 | |||
| Conversion, no. (%) | 4 (6.56) | 5 (13.89) | NA | 0.229 |
| R0 resection, no. (%) | 58 (95.08) | 34 (94.44) | 35 (100.00) | 0.846 |
| Concomitant resection, no. (%) | 19 (31.15) | 2 (5.56) | 11 (31.43) | 0.009 |
| Lung | 13 | 2 | 5 | |
| Pericardium | 14 | 0 | 8 | |
| Innominate vein | 8 | 0 | 1 | |
| Phrenic nerve | 3 | 0 | 2 | |
| RAT vs. VAT | 0.040 | |||
| RAT vs. open | 0.977 | |||
| VAT vs. open | 0.006 | |||
| Open approach, no. (%) | ||||
| Sternotomy | NA | NA | 20 (57.14) | |
| Thoracotomy | NA | NA | 15 (42.86) | |
| Mini‐invasive laterality, no. (%) | 0.742 | |||
| Right side | 34 (55.74) | 18 (50.00) | NA | |
| Left side | 20 (32.79) | 12 (33.33) | NA | |
| Subxyphoid | 7 (11.47) | 6 (16.67) | NA |
Abbreviations: IQR, interquartile range; RAT, robot‐assisted thymectomy; VAT, video‐assisted thymectomy.
Postoperative outcomes
There was no 30‐day mortality. There were no significant differences in postoperative complications among the three groups. The median drainage volume in the VAT group was significantly less than that in the RAT group (365.00 vs. 700.00, p = 0.003) and open group (365.00 vs. 910.00, p < 0.001); however, there was no significant difference between the RAT group and open group (p = 0.726). The duration of chest tube in both the RAT group (3.00, p = 0.004) and the open group (4.00, p < 0.001) was significantly longer than that in the VAT group (2.00), and there was no significant difference between the RAT group and the open group (p = 0.651). The hospital stay in the open group was significantly longer than that in the VAT group (7.00 vs. 5.00, p = 0.002), but similar to that in the RAT group (7.00 vs. 6.00, p = 0.187). However, there was no significant difference between the RAT group and the VAT group (p = 0.157). The total in‐hospital costs in the RAT group were significantly higher than those in the VAT group (66,493.90 vs. 33,581.05, p < 0.001) and open group (66,493.90 vs. 42,876.40, p < 0.001), whereas there was no significant difference between the VAT group and open group (p = 0.414) (Table 3).
TABLE 3.
Postoperative outcomes stratified by operative approach
| RAT group (n = 61) | VAT group (n = 36) | Open group (n = 35) | p‐value | |
|---|---|---|---|---|
| Total drainage volume | 700.00 | 365.00 | 910.00 | 0.000 |
| (mL), median (IQR) | (405.00, 1250.00) | (172.50, 705.00) | (460.00, 1200.00) | |
| RAT vs. VAT | 0.003 | |||
| RAT vs. open | 0.726 | |||
| VAT vs. open | 0.000 | |||
| Duration of chest tube | 3.00 | 2.00 | 4.00 | 0.000 |
| (Days), median (IQR) | (3.00, 5.00) | (2.00, 3.75) | (3.00, 6.00) | |
| RAT vs. VAT | 0.004 | |||
| RAT vs. open | 0.651 | |||
| VAT vs. open | 0.000 | |||
| Hospital stay | 6.00 | 5.00 | 7.00 | 0.003 |
| (Days), median (IQR) | (5.00, 8.50) | (4.00, 7.00) | (6.00, 9.00) | |
| RAT vs. VAT | 0.157 | |||
| RAT vs. open | 0.187 | |||
| VAT vs. open | 0.002 | |||
| Postoperative complication, no. (%) | 7 (11.48) | 3 (8.33) | 7 (20.00) | 0.309 |
| Pulmonary infection | 2 | 1 | 4 | |
| Myasthenia gravis crisis | 4 | 0 | 0 | |
| Pleural effusion | 0 | 1 | 1 | |
| Air leak | 1 | 1 | 0 | |
| Arrhythmia | 0 | 0 | 1 | |
| Hydropneumothorax | 0 | 0 | 1 | |
| Total in‐hospital costs | 66,493.90 | 33,581.05 | 42,876.40 | 0.000 |
| Median (IQR) | (57,488.65, 86,880.05) | (26,202.05, 49,312.43) | (32,531.20, 52,623.70) | |
| RAT vs. VAT | 0.000 | |||
| RAT vs. open | 0.000 | |||
| VAT vs. open | 0.414 | |||
| 30 days mortality, no. (%) | 0 | 0 | 0 |
Abbreviations: IQR, interquartile range; RAT, robot‐assisted thymectomy; VAT, video‐assisted thymectomy.
DISCUSSION
Primary tumors of the anterior mediastinum account for 50% of all mediastinum masses, and the most frequent histological types are thymoma, teratoma, lymphoma, and thyroid tumors, 7 which is consistent with the results shown in our study. Standard treatment of AMTs requires complete surgical resection and necessary adjuvant therapy. 8 , 9 The limited retrosternal space and adjacent vital structures make resection challenging for surgeons, especially for large tumors. The traditional open approach for AMTs can provide excellent exposure of the surgical field, which is convenient for combined resection and reconstruction of invaded vital structures, satisfying safety and oncological principles. In recent decades, VAT and RAT have been adopted more often because of notable advantages, such as shorter operation time, 10 , 11 less estimated blood loss, 12 , 13 shorter duration of chest tube and hospital stay, 14 , 15 lower rate of postoperative complications, and comparable oncological results. 16 , 17 Tumor size has frequently been used to determine the surgical approach in patients with AMTs. Several studies have demonstrated that RAT or VAT for large AMTs can achieve comparable perioperative outcomes versus open surgery. Kneuertz et al. 6 reported that RAT can be performed safely and effectively in a radical fashion for large thymomas (median size 6 cm). Weng et al. 18 demonstrated that VAT is a safe and effective approach for large thymomas (≥5 cm) with comparable surgical outcomes and oncological results. In our own center, tumor size is not the determining factor in the choice of approach, and quite a few patients with larger and more advanced AMTs underwent RAT.
To our knowledge, this is the first study to compare these three approaches for the treatment of large AMTs (≥6 cm). Our data showed a reduced operative time in RAT versus open surgery (p = 0.012), which is comparable to VAT (p = 0.071). Considering the robot setup and docking time included in the calculation of operative time, RAT may have the potential to be significantly faster than VAT. As previous studies have demonstrated, 6 , 19 we also confirmed that open surgery is associated with much more blood loss (p < 0.001). Complete resection is the gold standard for solid tumors, and the R0 resection rate has been known to be a major surgical prognostic factor correlated with the risk of tumor recurrence. 20 In our study, the R0 resection rates were equivalent between the three groups and more than 90% in any group, which is comparable with previous results in smaller AMTs based on published data. 21 In mini‐invasive approaches, both the RAT group and the VAT group had equivalent conversion rates and similar surgical route selections. In our earlier study, 22 we confirmed that RAT can be performed using various approaches. The sub‐xiphoid approach is routinely selected for centered and advanced large AMTs in our center, because it can provide excellent visualization for exposing the proximal and distal ends of the innominate vein, which is convenient for resection of the invaded innominate vein, even for partial resection of the SVC.
Technically, the advantages of robotic operating systems have expanded the indications for RAT. Because of its magnified 3D vision, flexible endo‐wristed instruments, and stable operating system, RAT can be performed safely for large and advanced AMTs requiring combined resection of vital structures, such as the lung, pericardium, phrenic nerve, and innominate vein. In the RAT group, 19 cases (31.15%) of concomitant resection were successfully performed, and the concomitant resection rate in the RAT group was much higher than that in the VAT group (5.56%), and similar to that in the open group (31.43%). In particular, in the RAT group, resection of the innominate vein was performed in 8 cases, including 1 case combined with partial resection of the SVC. Several studies have shown promising perioperative outcomes in RAT for large AMTs requiring concomitant resection. Kneuertz et al. 6 reported that 50% of patients who underwent RAT for large thymomas had undergone concomitant resection of adjacent structures, but in their study, no patient underwent major vascular dissection because of selection. In a report by Na et al., 23 29 patients (17.3%) underwent concomitant resection of adjacent organs, including innominate vein resection in six patients. If the proximal end of the innominate vein is invaded by the tumor, partial resection of the SVC seems technically feasible in RAT. However, a sufficient vascular diameter must be ensured.
In the present study, compared to both RAT and open surgery, VAT had a lower drainage volume and shorter duration of chest tube, which we consider to be caused by a lower rate of concomitant resection in the VAT group. The RAT had a hospital stay comparable to that of open surgery, whereas the VAT was slightly shorter than that of open surgery. All three groups had equivalent rates of postoperative complications, which was consistent with previous reports. 6 , 22 There was no 30‐day mortality.
The much higher costs in RAT have been criticized for a long time. The costs for the Da Vinci robot include the initial costs of the Da Vinci system and the extra costs related to disposable materials. Our data showed that the costs in the RAT group were nearly 40%~45% higher than those in the other two groups. Some scholars believe that robot‐assisted surgery allows for a faster recovery, which can potentially reduce the total costs. 24 The costs of robot‐assisted surgery are still controversial.
Limitations
There are several limitations in this study. The first, it is single‐center cohort with a relatively small study size, which limits the statistical power. In addition, the retrospective nature of the study may lead to selection bias, because propensity score matching was not used because of the small sample size in each group. Moreover, we have no record of some important postoperative outcomes, such as pain scores and quality of life. Our study also lacked long‐term follow‐up data to demonstrate oncological efficacy.
CONCLUSION
In conclusion, RAT is safe and effective for the resection of large AMTs compared to VAT and open surgery. RAT is associated with a higher rate of concomitant resection versus VAT, and vascular resection in RAT is technically feasible. A long‐term follow‐up is required in the future.
AUTHOR CONTRIBUTIONS
Conception and design: Yi‐Dan Lin, Bin Jiang, and Long‐Fei Zhu. Administrative support: Yi‐Dan Lin. Provision of study materials or patients: Bin Jiang, Qun‐You Tan, and Bo Deng. Collection and assembly of data: Bin Jiang, Long‐Fei Zhu, and Long‐Yong Mei. Data analysis and interpretation: Bin Jiang and Long‐Fei Zhu. Manuscript writing: all authors. Final approval of manuscript: all authors.
CONFLICT OF INTEREST
The authors report no conflicts of interests.
Jiang B, Tan Q‐Y, Deng B, Mei L‐Y, Lin Y‐D, Zhu L‐F. Robot‐assisted thymectomy in large anterior mediastinal tumors: A comparative study with video‐assisted thymectomy and open surgery. Thorac Cancer. 2023;14(3):267–273. 10.1111/1759-7714.14744
Contributor Information
Yi‐Dan Lin, Email: linyidan.academy@foxmail.com.
Long‐Fei Zhu, Email: zhu_longfei@aliyun.com.
REFERENCES
- 1. Bacha EA, Chapelier AR, Macchiarini P, Fadel E, Dartevelle PG. Surgery for invasive primary mediastinal tumors. Ann Thorac Surg. 1998;66:234–9. [DOI] [PubMed] [Google Scholar]
- 2. Imielski B, Kurihara C, Manerikar A, Chaudhary S, Kosterski S, Odell D, et al. Comparative effectiveness and cost‐efficiency of surgical approaches for thymectomy. Surgery. 2020;168:737–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hess NR, Sarkaria IS, Pennathur A, Levy RM, Christie NA, Luketich JD. Minimally invasive versus open thymectomy: a systematic review of surgical techniques, patient demographics, and perioperative outcomes. Ann Cardiothorac Surg. 2016;5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Friedant AJ, Handorf EA, Su S, Scott WJ. Minimally invasive versus open thymectomy for thymic malignancies: systematic review and meta‐analysis. J Thorac Oncol. 2016;11:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shen C, Li J, Li J, Che G. Robot‐assisted thoracic surgery versus video‐assisted thoracic surgery for treatment of patients with thymoma: a systematic review and meta‐analysis. Thorac Cancer. 2022;13:151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kneuertz PJ, Kamel MK, Stiles BM, Lee BE, Rahouma M, Nasar A, et al. Robotic Thymectomy is feasible for large Thymomas: a propensity‐matched comparison. Ann Thorac Surg. 2017;104:1673–8. [DOI] [PubMed] [Google Scholar]
- 7. Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest. 2005;128:2893–909. [DOI] [PubMed] [Google Scholar]
- 8. Parker D, Holford CP, Begent RH, Newlands ES, Rustin GJ, Makey AR, et al. Effective treatment for malignant mediastinal teratoma. Thorax. 1983;38:897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Modh A, Rimner A, Allen PK, Greenfield B, Marom EM, Rice D, et al. Treatment modalities and outcomes in patients with advanced invasive Thymoma or Thymic carcinoma: a retrospective multicenter study. Am J Clin Oncol. 2016;39:120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kimura T, Inoue M, Kadota Y, Shiono H, Shintani Y, Nakagiri T, et al. The oncological feasibility and limitations of video‐assisted thoracoscopic thymectomy for early‐stage thymomas. Eur J Cardiothorac Surg. 2013;44:e214–8. [DOI] [PubMed] [Google Scholar]
- 11. Cakar F, Werner P, Augustin F, Schmid T, Wolf‐Magele A, Sieb M, et al. A comparison of outcomes after robotic open extended thymectomy for myasthenia gravis. Eur J Cardiothorac Surg. 2007;31:501–4. [DOI] [PubMed] [Google Scholar]
- 12. Xie A, Tjahjono R, Phan K, Yan TD. Video‐assisted thoracoscopic surgery versus open thymectomy for thymoma: a systematic review. Ann Cardiothorac Surg. 2015;4:495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ye B, Li W, Ge XX, Feng J, Ji CY, Cheng M, et al. Surgical treatment of early‐stage thymomas: robot‐assisted thoracoscopic surgery versus transsternal thymectomy. Surg Endosc. 2014;28:122–6. [DOI] [PubMed] [Google Scholar]
- 14. Pennathur A, Qureshi I, Schuchert MJ, Dhupar R, Ferson PF, Gooding WE, et al. Comparison of surgical techniques for early‐stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg. 2011;141:694–701. [DOI] [PubMed] [Google Scholar]
- 15. Seong YW, Kang CH, Choi JW, et al. Early clinical outcomes of robot‐assisted surgery for anterior mediastinal mass: its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur J Cardiothorac Surg. 2014;45:e68–73. [DOI] [PubMed] [Google Scholar]
- 16. Zahid I, Sharif S, Routledge T, Scarci M. Video‐assisted thoracoscopic surgery or transsternal thymectomy in the treatment of myasthenia gravis. Interact Cardiovasc Thorac Surg. 2011;12:40–6. [DOI] [PubMed] [Google Scholar]
- 17. Kang CH, Na KJ, Park S, Park IK, Kim YT. Long‐term outcomes of robotic thymectomy in patients with thymic epithelial tumors. Ann Thorac Surg. 2021;112:430–5. [DOI] [PubMed] [Google Scholar]
- 18. Weng W, Li X, Meng S, Liu X, Peng P, Wang Z, et al. Video‐assisted thoracoscopic thymectomy is feasible for large thymomas: a propensity‐matched comparison. Interact Cardiovasc Thorac Surg. 2020;30:565–72. [DOI] [PubMed] [Google Scholar]
- 19. Azenha LF, Deckarm R, Minervini F, Dorn P, Lutz J, Kocher GJ. Robotic vs. transsternal thymectomy: a single center experience over 10 years. J Clin Med. 2021;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weis CA, Yao X, Deng Y, Detterbeck FC, Marino M, Nicholson AG, et al. The impact of thymoma histotype on prognosis in a worldwide database. J Thorac Oncol. 2015;10:367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burt BM, Yao X, Shrager J, Antonicelli A, Padda S, Reiss J, et al. Determinants of complete resection of Thymoma by minimally invasive and open thymectomy: analysis of an international registry. J Thorac Oncol. 2017;12:129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang B, Kang P, Tao S, et al. Short‐term efficacy of robot‐assisted surgery versus video‐assisted thoracoscopic surgery for anterior mediastinal mass. J Amry Med Univ. 2019;41(16):1578–82. [Google Scholar]
- 23. Na KJ, Kang CH. Robotic thymectomy for advanced thymic epithelial tumor: indications and technical aspects. J Thorac Dis. 2020;12:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marulli G, Comacchio GM, Schiavon M, Rebusso A, Mammana M, Zampieri D, et al. Comparing robotic and trans‐sternal thymectomy for early‐stage thymoma: a propensity score‐matching study. Eur J Cardiothorac Surg. 2018;54:579–84. [DOI] [PubMed] [Google Scholar]


