Summary
Background
Primary SARS-CoV-2 vaccination has been shown to wane with time and provide lower protection from disease with new viral variants, prompting the WHO to recommend the administration of booster doses. We determined the safety and immunogenicity of homologous or heterologous boosters with ChAdOx1 nCoV-19 (COVISHIELD™) or BBV152 (COVAXIN®), the two vaccines used widely for primary immunization in India, in participants who had already received two primary doses of these vaccines.
Methods
Participants primed with two doses each of COVISHIELD™ or COVAXIN® 12–36 weeks previously, were randomised to receive either COVISHIELD™ or COVAXIN® booster in a 1:1 ratio. The primary outcome was day 28 post-booster anti-spike IgG seropositivity and secondary outcomes were anti-spike IgG levels and assessment of safety and reactogenicity. The results of 90 days intention-to-treat analysis are presented. This trial is registered with ISRCTN (CTRI/2021/08/035648).
Findings
In the COVISHIELD™ primed group with 200 participants, the seropositivity 28 days post booster in the heterologous COVAXIN® arm was 99% and non-inferior to the homologous COVISHIELD™ arm, which was also 99% (difference 0%; 95% CI: −2.8% to 2.7%). The geometric mean concentration (GMC) of anti-spike antibodies following heterologous COVAXIN® boost on day 28 was 36,190.78 AU/mL (95% CI: 30,526.64–42,905.88) while the GMC following homologous COVISHIELD™ boost was 97,445.09 AU/mL (82,626.97–114,920.7). In the COVAXIN® primed group with 204 participants, the seropositivity 28 days post booster in the heterologous COVISHIELD™ arm was 100% and non inferior to the homologous COVAXIN® arm which was 96% (difference 4%, 95% CI: 0.2%–7.8%). The GMC following heterologous COVISHIELD™ boost was 241,681.6 AU/mL (95% CI: 201,380.2–290,048.3) compared to homologous COVAXIN® boost, which was 48,473.94 AU/mL (95% CI: 38,529.56–60,984.95). The day 28 geometric mean ratio (GMR) of the anti-spike IgG between the heterologous and homologous boosted arms was 0.42 (95% CI: 0.34–0.52) in the COVISHIELD™ primed group and 5.11 (95% CI: 3.83–6.81) in the COVAXIN® primed group. There were no related serious adverse events reported in any group.
Interpretation
Homologous and heterologous boosting with COVISHIELD™ or COVAXIN® in COVISHIELD™ or COVAXIN® primed individuals are immunogenic and safe. A heterologous boost with COVISHIELD™ after COVAXIN® prime offers the best immune response among the four combinations evaluated.
Funding
Azim Premji Foundation and Bill and Melinda Gates Foundation.
Keywords: COVID-19, Vaccine trial, Homologous booster, Heterologous booster, ChAdOx1 nCov-19, COVISHIELD, BBV152, Covaxin, Immunogenicity, Safety
Research in context.
Evidence before this study
We searched standard databases, to identify pertinent literature until June 4, 2021, using the key words “vaccination”, “heterologous”, “homologous”, “booster”, “immunogenicity”, “SARS-CoV-2”, and “COVID-19”. The multiple waves of the pandemic resulted in infections, cases and deaths, affected healthcare systems and impacted society. Waning of neutralizing antibodies and reduction in protection from infection due to variants from the primary doses of the Covid-19 vaccines prompted researchers in several countries to study the effect of booster doses with both homologous and heterologous schedules. The preliminary safety and reactogenicity results from the Com-COV study published on 12th May 2021 comparing four prime-boost permutations of the ChAdOx1 nCoV-19 (ChAd) COVID-19 vaccine (Vaxzevria, AstraZeneca) and BNT162b2 (BNT) COVID-19 vaccine (Comirnaty, Pfizer-BioNTech) vaccines showed that both heterologous vaccine schedules induced greater systemic reactogenicity following the boost dose than their homologous counterparts. In the Com-COV immunogenicity study, heterologous schedules of BNT and ChAd produced higher SARS-CoV-2 anti-spike IgG concentrations of both heterologous schedules were higher than that of a licensed vaccine schedule (ChAd/ChAd).
Added value of this study
COVISHIELD and COVAXIN are the two vaccines used in the majority of the Indian population. The four permutations for a booster dose evaluated in this trial are primary immunization with COVISHIELD™ followed by a homologous booster or a heterologous COVAXIN® booster and primary immunization with COVAXIN® followed by a homologous booster or a heterologous COVISHIELD™ booster. Based on study results, homologous and heterologous boosting with COVISHIELD™ or COVAXIN® in COVISHIELD™ or COVAXIN® primed individuals are immunogenic and safe. For both binding and functional antibodies, in participants primed with COVISHIELD™, homologous boosting with COVISHIELD™ offers better immune responses than heterologous boosting with COVAXIN®; and in those primed with COVAXIN®, heterologous boost with COVISHIELD™ offers the better immune response. A heterologous boost with COVISHIELD™ after a COVAXIN® primary schedule offers the best immune response among the approaches evaluated in this study.
Implications of all the available evidence
Even though homologous and heterologous boosting with COVISHIELD™ or COVAXIN® in COVISHIELD™ or COVAXIN® primed individuals are immunogenic and safe, boosting with COVISHIELD™ produce better binding and functional antibodies irrespective of whether the primary vaccination series used COVISHIELD™ or COVAXIN®.
Introduction
Antibodies and clinical protection have been shown to decrease after two primary doses of mRNA vaccines against SARS-CoV-2.1,2 Similar results have been seen with other vaccine products and therefore, the World Health Organisation (WHO) revised its recommendations in 2022 to include a booster dose.3 The vaccine to be used for boosting remains a question for countries with access to a limited number of vaccines, especially with uncertain performance data against variants of concern (VOC) such as the B.1.617.2 (Delta) variant, first reported in India in December 20204 and the B.1.1.529 (Omicron) variant, first reported in November 2021 in South Africa, which have had a considerable impact worldwide.5 Limited sequencing data are available from India prior to December 2021, but the available sequences show no evidence of Omicron circulation until end-November 2021. From mid-late December 2021, Omicron sequences are reported nationally, and from 20th December, through a separate community based surveillance in Vellore, Omicron circulation was identified and increased rapidly in January 2022.
The waning immunity offered by primary vaccination against variants, especially among the elderly and the clinically vulnerable,6 has prompted the study of boosting with either homologous or heterologous vaccines in many countries.7 The preliminary safety and reactogenicity results from the UK Com-COV study comparing all four prime-boost permutations of the ChAdOx1 nCoV-19 (ChAd) COVID-19 vaccine (Vaxzevria, AstraZeneca) and BNT162b2 (BNT) COVID-19 vaccine (Comirnaty, Pfizer-BioNTech) vaccines showed that both heterologous vaccine schedules induced greater systemic reactogenicity following the boost dose than their homologous counterparts.8 The study also demonstrated higher immunogenicity with the ChAdOx1 nCoV-19/BNT162b2 combination as compared to homologous doses of ChAdOx1 nCoV-19.9 A study from Sweden also reported that the effectiveness of heterologous ChAdOx1 nCoV-19/BNT162b2 and ChAdOx1 nCoV-19/mRNA-1273 (Moderna) prime-boost vaccination was significantly higher than using homologous ChAdOx1 nCoV-19 boosts.10 Further, inactivated adjuvanted vaccines have been less effective than mRNA or vectored vaccines when used as homologous boosters as shown with the CoronaVac® vaccine in the RHH-001 study or heterologous boosters as with the VLA2001(Valneva) vaccine in the UK COV-BOOST trial.11,12
In India, two vaccines were widely used for primary immunisation viz., the ChAdOx1 nCoV-19 vaccine, an adenoviral vectored vaccine encoding the SARS-CoV-2 Spike (S) glycoprotein, and BBV152, a whole virion inactivated vaccine adjuvanted with Alhydroxiquim-II, marketed in India as COVISHIELD™ and COVAXIN® respectively. The utilization of vaccines as on June 2, 2022 was 80.6% for COVISHIELD™ and 16.8% for COVAXIN®.13 The existing governmental policy is for a third ‘precautionary’ dose of a homologous vaccine 9 months after the second dose.14 To address lacunae in data regarding the two Indian WHO-approved products, we undertook a study to assess the safety and immunogenicity of homologous or heterologous booster vaccinations with COVISHIELD™ and COVAXIN® in individuals who had completed their primary immunisation at least 12–36 weeks previously. The interim results for safety at 90 days and immunogenicity are presented here.
Method
Study design
The trial was a participant and observer-blinded randomised homologous/heterologous boost vaccine non-inferiority phase 4 clinical trial. The trial has two study groups based on the primary vaccine administered, each with its own randomisation. The study was an investigator-initiated trial conducted at the Christian Medical College (CMC), Vellore, India, and approved by the Drugs Controller General of India (Ref no. BIO/CT04/FF/2021/27076) and the Institutional Review Board and Ethics Committee.
Participants
All participants were screened after written informed consent. Adults above 18 years who received two primary homologous doses of COVISHIELD™ or COVAXIN® and had completed 12–36 weeks after their second dose were eligible to participate. The primary vaccine status was based on their records in the COWIN portal, a governmental platform on which every dose of vaccine is required to be recorded. The COVISHIELD™ primed group and the COVAXIN® primed group were stratified and randomised to receive either a COVISHIELD™ or COVAXIN® booster dose in a 1:1 ratio. Over 80% of the adults in the study area had received COVISHIELD™ and the rest had received COVAXIN®.15 Hence COVISHIELD™ primed participants were recruited in a month between 30th August 2021 and 30th September 2021, but an extended period between 7th September 2021 and 19th January 2022 was needed for the COVAXIN® primed group. Complete exclusion and inclusion criteria are listed in Supplementary Table S1.
Randomisation and blinding
Randomisation used an Interactive Web Response System (IWRS) with permuted random blocks, and participants were randomly assigned to the homologous or heterologous arms with equal probability. The study statistician generated the sequence and controlled access to the randomisation code.
Trained staff enrolled the participants and assigned them to the trial groups. Pharmacists and vaccine administrators, who were aware of the treatment assignment, maintained confidentiality, loaded the vaccines in a separate cubicle, and administered them in the vaccination room. Study staff, blinded to the randomisation, were responsible for the safety evaluation of the participants following vaccination and for data analysis.
Procedures
The trial duration was 6 months with visits at baseline, day 28 ± 2 and day 180 ± 14 (Supplementary Table S2). At baseline and on day 28, 19 mL of blood was collected for safety and immunogenicity assessment, and on day 180, 9 mL of blood was collected for immunogenicity assessment. An additional 18 ml of blood was collected during each visit in a subset of 40% randomly selected participants for detailed evaluation, including cell-mediated immune responses. Blood parameters such as complete blood counts, liver function tests, blood sugar and creatinine were assessed for safety at baseline and on day 28, and for immunogenicity on all the visits. COVAXIN® and COVISHIELD™ vaccines were used with 0.5 mL of either vaccine injected into the deltoid muscle.
Participants were also followed-up telephonically every fortnight for safety monitoring. Data from the case report forms (CRF) were entered and managed on electronic data capture forms developed on REDCap®, hosted at CMC Vellore.16,17
Outcomes
Safety assessment
Daily monitoring for solicited local and systemic adverse events was by telephonic follow-up during the first 7 days and thereafter every fortnight for 6 months. The safety endpoints were the incidence of related adverse events, serious adverse events following vaccination and any clinically significant change in the physical examination parameters and laboratory values. RT-PCR was done for those reporting fevers and influenza-like symptoms during follow-up.
Immunogenicity assessment
The primary immunogenicity endpoint was seropositivity on day 28 post booster. Secondary end points were the geometric mean concentration ratio (GMR) of anti-SARS-CoV-2 spike IgG between the two boosted arms in each vaccine primed group on day 28, as measured using the Meso Scale Discovery (MSD) platform, GMR at day 180, and the incidence of virologically confirmed RT-PCR cases of COVID-19 between arms after the booster dose. Breakthrough infection with COVID-19 was defined as any fever with influenza-like illness and a positive RT-PCR.
Commercial assays were used to measure antibodies.
-
1)
LIAISON® SARS-CoV-2 TrimericS IgG assay18: The LIAISON® SARS-CoV-2 TrimericS IgG assay, an indirect chemiluminescence immunoassay (CLIA), was used for the quantitative determination of anti-trimeric spike protein-specific IgG antibodies to SARS-CoV-2 in human serum or plasma samples. A result above 33.8 BAU/mL indicates the presence of IgG antibodies.
-
2)
MSD assay19: The MSD multiplexed chemiluminescence assay was used for the simultaneous detection of a broader repertoire of IgG antibodies against SARS-CoV-2 spike (S), spike receptor-binding domain (RBD), spike N terminal domain (NTD), nucleocapsid antigen and SARS-CoV-1 spike using the V-PLEX SARS-CoV-2 Panel 1 Kit, according to the manufacturer's protocol. All samples were tested at an initial dilution of 1: 5000. Any samples which were above or below the range of detection were retested in lower (1:1000) or higher dilutions (up to 1 in 50:000).
Surrogate neutralisation/ACE2 binding inhibition assay
The angiotensin-converting enzyme 2 (ACE2) binding inhibition assay using the MSD V-PLEX SARS-CoV-2 Panel 13 and 24 (ACE2) kits were used according to the manufacturer's protocol at a dilution of 1:50, to detect antibodies that block the binding of ACE2 to the RBD of the Wuhan strain of SARS-CoV-2, and spike antigens from variants including the Wuhan, B.1.1.7 (Alpha), B.1.351(Beta), B.1.617.1 (Kappa), B.1.617.2 (Delta), P.1 (Gamma), P.2 (Zeta), and B.1.1.529 (Omicron) variants. Results are presented as percentage inhibition.
Statistical analysis
The sample size was based on the assumption that both homologous and heterologous booster combinations in COVISHIELD™ and COVAXIN® primed groups would provide 95% seropositivity 28 days after the booster dose. With a 0.05 significance level, 90% power and non-inferiority margin of 0.1, 82 individuals were required in each group. The number was inflated to 100 to account for any loss to follow-up. The primary analysis presented here is the intention to treat (ITT) analysis.
The seropositivity, defined as IgG level ≥33.8 BAU/mL on the LIAISON® SARS-CoV-2 TrimericS IgG assay, on day 28 between the heterologous and homologous booster schedules is the primary outcome with a non-inferiority margin of 0.1. The number who achieved seroconversion on day 28 is presented for each arm. Seroconversion is defined as an IgG level of <33.8 BAU/mL at baseline and ≥33.8 BAU/mL on day 28. The anti-spike IgG at baseline and on day 28 are also reported as GMCs with 95% CI based on the results from the MSD assay. The day 28 anti-spike IgG GMR with 95% confidence interval (CI) between the COVISHIELD™ boosted, and COVAXIN® boosted arms in the COVISHIELD™ and COVAXIN® primed groups are reported. Analysis of Covariance (ANCOVA), in which the log-transformed IgG values on day 28 were included as the outcome variable, and age, baseline log-transformed IgG levels and booster vaccine arms as covariates, were also performed. Based on the GMR, non-inferiority of the heterologous schedule was inferred if the lower limit of the two-sided 95% CI for the ratio of two geometric means (log normalised) was ≥0.67.20 The GMCs and GMRs were calculated for different subgroups. Analysis was performed using STATA version 15.1. Graphs were created using GraphPad Prism software version 9, RStudio software version 2021.09.2 and Microsoft® Excel® for Microsoft 365 MSO (Version 2205).
Safety and reactogenicity parameters were analysed for all randomised participants. Adverse events reported during the follow-up period were coded according to the Medical Dictionary for Regulatory Activities (MedDRA 24.1) and tabulated at the Preferred Term (PT) and the System Organ Class (SOC) level across vaccine groups. An independent data and safety monitoring board were responsible for reviewing the safety and immunogenicity data. This trial is registered with ISRCTN, number: CTRI/2021/08/035648.
Role of the funding source
The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Recruitment
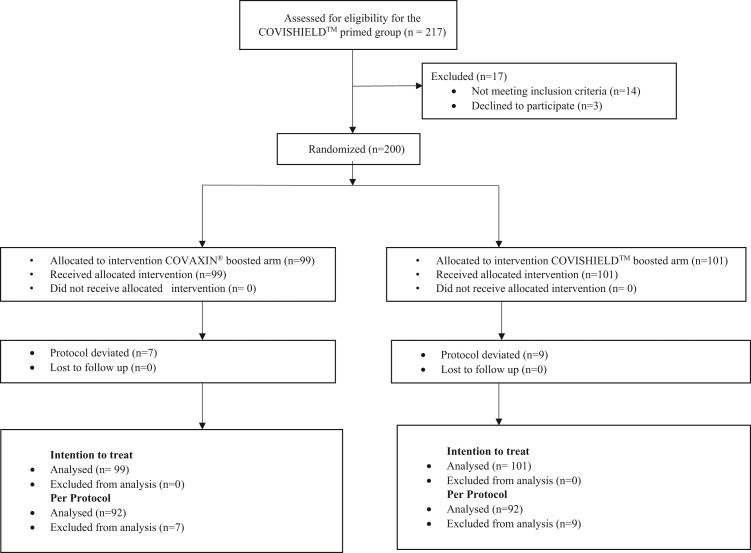
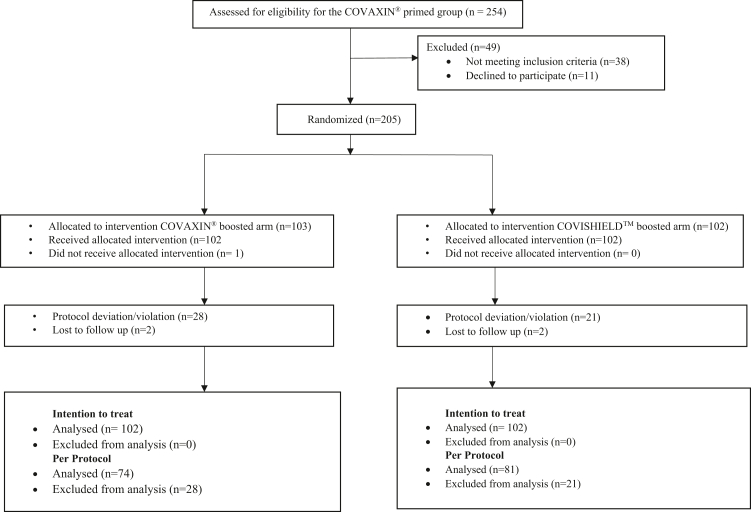
In the COVISHIELD™ primed group, 217 participants were screened, of which 14 participants did not meet the inclusion criteria, and 3 withdrew consent after randomisation. In the COVAXIN® primed group, 254 participants were screened, and 205 were randomised. In the intention to treat analyses, 200 participants were included in each study group (Fig. 1). The COVISHIELD™ primed group participants were recruited within 1 month in 2021. The COVAXIN® primed group participants were recruited over 5 months, and hence the period of recruitment overlapped with the Omicron wave in December 2021 and January 2022 in India. Therefore, 5th January 2022, an estimated 2 weeks after the onset of the Omicron wave, was used for stratified analysis for the COVAXIN® primed group depending on whether or not the day 28 visit was during the Omicron wave.
Fig. 1.
Enrolment, Randomization and Analysis in COVISHIELD™ primed group and COVAXIN® primed group.
Participants
In the COVISHIELD™ primed group, ages ranged from 21 to 93 years, and the mean age of the participants was 43.60 years (SD: 13) in the COVAXIN® boosted arm and 39.30 years (SD: 11.30) in the COVISHIELD™ boosted arm (Table 1). In the COVAXIN® boosted arm, 39.39% of the participants were women, while in the COVISHIELD™ boosted arm, 35.64% were women. The mean number of days since receiving the second primary dose of the COVISHIELD™ vaccine was 186.92 days in COVAXIN® boosted arm and 187.13 days in the other arm.
Table 1.
Baseline characteristics of study participants who are previous recipients of two doses of either COVISHIELD™ or COVAXIN® vaccines at least 12–36 weeks prior and were randomised to receive either homologous or heterologous booster vaccination.
| Category | COVISHIELD™ primed group |
COVAXIN® primed group |
||
|---|---|---|---|---|
| COVISHIELD™ (homologous) boosted arm (n = 101) | COVAXIN® (heterologous) boosted arm (n = 99) | COVAXIN® (homologous) boosted arm (n = 102)a | COVISHIELD™ (heterologous) boosted arm (n = 102)a | |
| Age in years | ||||
| Mean (SD) | 39.3 (11.3) | 43.6 (13.0) | 44.38 (13.6) | 43.23 (14.73) |
| Gender | ||||
| Male | 65 (64.36%) | 60 (60.61%) | 63 (61.76%) | 52 (50.98%) |
| Female | 36 (35.64%) | 39 (39.39%) | 39 (38.24%) | 50 (49.02%) |
| Marital status | ||||
| Single | 22 (21.78%) | 16 (16.16%) | 20 (19.61%) | 24 (23.53%) |
| Married | 79 (78.22%) | 83 (83.84%) | 82 (80.39%) | 78 (76.47%) |
| Body Mass Index | ||||
| <30 | 85 (84.16%) | 81 (81.82%) | 79 (77.45%) | 82 (80.39%) |
| ≥30 | 16 (15.84%) | 18 (18.18%) | 23 (22.55%) | 20 (19.61%) |
| With a past history of Covid-19 | 18 (17.82%) | 16 (16.16%) | 17 (16.67%) | 16 (15.69%) |
| Seronegative for Spike IgG on Day-0 (TrimericS) | 20 (19.8%) | 27 (27.27%) | 38 (38%) | 38 (38%) |
| Baseline SARS-CoV-2 Spike Baseline GMC | 19,244.55 (14,687.48–25,215.52) | 12,880.31 (9592.7–17,294.64) | 9107.33 (6005.29–13,811.74) | 7748.63 (5232.87–11,473.86) |
| Co-morbiditiesb | ||||
| Hypertension | 22 (21.78%) | 26 (26.26%) | 43 (42.16%) | 38 (37.25%) |
| Diabetes mellitus | 5 (4.95%) | 7 (7.07%) | 17 (16.67%) | 16 (15.69%) |
| Dyslipidaemia | 5 (4.95%) | 10 (10.1%) | 7 (6.86%) | 7 (6.86%) |
| Bronchial asthma | 4 (3.96%) | 4 (4.04%) | 4 (3.92%) | 2 (1.96%) |
| Thyroid dysfunction | 7 (6.93%) | 3 (3.03%) | 5 (4.9%) | 7 (6.86%) |
| Malignancy | 2 (1.98%) | 0 | 1 (0.98%) | 0 |
| Tuberculosis | 2 (1.98%) | 2 (2.02%) | 0 | 0 |
| Heart disease | 0 | 1 (1.01%) | 1 (0.98%) | 0 |
| Interval between the doses, mean (SD) | ||||
| Interval in days between the two primary doses of the vaccine | 36.34 (8.94) | 36.22 (8.84) | 43.86 (22.8) | 42.87 (22.78) |
| Interval in days between the second primary dose and the booster dose | 187.13 (14.12) | 186.92 (13.6) | 208.73 (45.6) | 206.83 (45.4) |
TrimericS IgG assays results are available for only 100 participants.
Comorbidities were self-reported by participants, with review by study team doctor.
Among those in the COVAXIN® primed group, the ages ranged from 18 to 86 years, and the mean age was 44.38 years (SD: 13.6) and 43.23 years (SD: 14.73) in the COVAXIN®, and the COVISHIELD™ boosted arms, respectively. In the COVAXIN® boosted arm, 38.24% of the participants were women, and it was 49.02% in the COVISHIELD™ boosted arm. The mean number of days since receiving the primary doses of COVAXIN® was 208.73 days in the COVAXIN® boosted arm and 206.83 days in the COVISHIELD™ boosted arm.
Antibody response measured by the LIAISON® SARS-CoV-2 TrimericS IgG assay
In the COVISHIELD™ primed group, the booster was given at a mean of 187 days post-primary immunisation. The seropositivity post heterologous COVAXIN® booster (99.0%; 95% CI: 94.5–100%) was non-inferior to homologous COVISHIELD™ booster (99.0%; 95% CI: 94.6–100%) since the difference was 0% (95% CI: −2.8% to 2.7%) and the lower limit of 95% CI was above the non-inferiority margin of 10%. Pre-boost, 47 participants (23.5%; 95% CI: 17.8–30.0%) were seronegative, with 27 receiving and 20 receiving COVISHIELD™ booster. Four weeks after the booster dose, 26 of the 27 seronegative participants (96.3%; 95% CI: 81.0–99.9%) in the COVAXIN® boosted arm and 19 out of 20 seronegative participants (95%; 95% CI: 75.1–99.9%) in the COVISHIELD™ boosted arm had seroconverted. Among the seropositive participants at baseline, a four-fold increase in the anti-spike IgG levels was noticed in 19.4% (95% CI: 11.1–30.5%) in the COVAXIN® boosted arm and 54.3% (95% CI: 42.9–65.4%) in the COVISHIELD™ boosted arm.
In the COVAXIN® primed group, the booster was administered at a mean of 207 days post-primary immunisation. The seropositivity post heterologous COVISHIELD™ booster (100%; 95% CI: 96.4–100%) was non-inferior to homologous COVAXIN® booster (96%; 95% CI: 90.1–98.9%) since the difference was 4% (95% CI: 0.2%–7.8%) and the lower limit of 95% CI was above the non-inferiority margin of 10%. Pre-boost, 76 participants (38%; 95% CI: 31.2–45.1%) were seronegative and were equally distributed in the two booster arms. By day 28, 35 of the 38 seronegative participants (92.1%; 95% CI: 78.6–98.3%) at baseline seroconverted in the COVAXIN® boosted arm, whereas all the 38 seronegative participants (100%; 95% CI: 90.7–100%) at baseline seroconverted in the COVISHIELD™ boosted arm The four-fold titre rise among the baseline seropositive participants was 24.2% (95% CI: 14.2–36.7%) in the COVAXIN® boosted arm and 80.6% (95% CI: 68.6–89.6%) in the COVISHIELD™ boosted arm. Please refer to Supplementary Tables S3 and S4 for GMCs and seroconversion based on LIAISON® assay.
Antibody response measured by the MSD assay
As shown in Table 2 and Supplementary Table S5 both vaccines were effective in boosting antibody responses by day 28, regardless of the primary immunogen. In the COVISHIELD™ primed group, anti-spike IgG GMCs on day 28 following heterologous COVAXIN® boost was 36,190.78 AU/mL (95% CI: 30,526.64–42,905.88) and following homologous COVISHIELD™ boost was 97,445.09 AU/mL (95% CI: 82,626.97–114,920.7). The day 28 GMR was 0.42 (95% CI: 0.34–0.52), with no evidence of non-inferiority of heterologous over homologous booster in COVISHIELD™ primed group as the lower limit of the CI is below the non-inferiority margin of 0.67. The fold rise from baseline to day 28 in the heterologous COVAXIN® boosted arm was 2.81 (95% CI: 2.21–3.57) and the homologous COVISHIELD™ boosted arm was 5.06 (95% CI: 3.88–6.61).
Table 2.
Immune response measured using the Meso Scale Discovery assay by booster dose vaccine allocation at baseline and 28 days post booster dose among COVISHIELD™ primed and COVAXIN® primed modified intention-to-treat population.
| COVISHIELD™ primed group |
COVAXIN® primed group |
||||
|---|---|---|---|---|---|
| COVISHIELD™ (homologous) boosted arm (n = 101) | COVAXIN® (heterologous) boosted arm (n = 99) | COVAXIN® (homologous) boosted arm (n = 100) | COVISHIELD™ (heterologous) boosted arm (n = 100) | ||
| SARS-CoV-2 Spike | |||||
| Day 0 | GMC (95% CI) | 19,244.55 (14,687.48–25,215.52) | 12,880.31 (9592.7–17,294.64) | 9107.33 (6005.29–13,811.74) | 7748.63 (5232.87–11,473.86) |
| Day 28 | GMC (95% CI) | 97,445.09 (82,626.97–114,920.7) | 36,190.78 (30,526.64–42,905.88) | 48,473.94 (38,529.56–60,984.95) | 241,681.6 (201,380.2–290,048.3) |
| GMFR | 5.06 (3.88–6.61) | 2.81 (2.21–3.57) | 5.32 (3.55–7.99) | 31.19 (20.38–47.74) | |
| Adjusted Day 28 GMRa | Ref | 0.42 (0.34–0.52) | Ref | 5.11 (3.83–6.81) | |
| SARS CoV2 S1 NTD | |||||
| Day 0 | GMC (95% CI) | 326.35 (250.33–425.45) | 212.58 (159.85–282.71) | 104.44 (65.97–165.33) | 90.06 (58.27–139.19) |
| Day 28 | GMC (95% CI) | 1826.23 (1548.36–2153.98) | 637.47 (538.02–755.31) | 617.62 (461.02–827.42) | 3051.35 (2260.68–4118.55) |
| GMFR | 5.6 (4.32–7.25) | 3 (2.32–3.88) | 5.91 (3.75–9.32) | 33.88 (21.65–53.02) | |
| Adjusted Day 28 GMRa | Ref | 0.39 (0.31–0.49) | Ref | 5 (3.37–7.43) | |
| SARS CoV2 S1 RBD | |||||
| Day 0 | GMC (95% CI) | 8694.21 (6454.71–11,710.72) | 5870.33 (4188.87–8226.74) | 3782.06 (2378.38–6014.17) | 3253.2 (2132–4964.03) |
| Day 28 | GMC (95% CI) | 56,607.76 (47,798.17–67,041.02) | 19,802.87 (16,503.48–23,761.88) | 26,709.89 (20,650.07–34,547.98) | 137,250.9 (106,357.6–177,117.6) |
| GMFR | 6.51 (4.85–8.75) | 3.37 (2.54–4.48) | 7.06 (4.54–10.99) | 42.19 (26.61–66.89) | |
| Adjusted Day 28 GMRa | Ref | 0.39 (0.31–0.49) | Ref | 5.29 (3.73–7.51) | |
| SARS-CoV-2 Nucleocapsid | |||||
| Day 0 | GMC (95% CI) | 1216.83 (827.75–1788.79) | 874.41 (595.34–1284.31) | 7677.79 (4823.95–12,219.95) | 6832.16 (4584.92–10,180.86) |
| Day 28 | GMC (95% CI) | 834.11 (484.53–1435.92) | 2068.49 (1149–3723.81) | 96,408.59 (69,817.21–133,127.9) | 31,472.68 (19,409.93–51,032.09) |
| GMFR | 0.69 (0.39–1.19) | 2.37 (1.29–4.34) | 12.56 (7.26–21.72) | 4.61 (2.79–7.62) | |
| Adjusted Day 28 GMRa | Ref | 3.01 (1.39–6.53) | Ref | 0.33 (0.19–0.58) | |
| SARS-CoV-1 Spike | |||||
| Day 0 | GMC (95% CI) | 2903.53 (2221.56–3794.84) | 1992.96 (1519.24–2614.38) | 2577.74 (1797.66–3696.32) | 2111.86 (1535.95–2903.73) |
| Day 28 | GMC (95% CI) | 14,220.13 (11,995.78–16,856.93) | 6409.59 (5459.51–7525) | 10,353.19 (8410.88–12,744.04) | 33,229.7 (27,791.6–39,731.88) |
| GMFR | 4.9 (3.78–6.35) | 3.22 (2.57–4.03) | 4.02 (2.87–5.62) | 15.73 (11.14–22.22) | |
| Adjusted Day 28 GMRa | Ref | 0.51 (0.41–0.63) | Ref | 3.32 (2.55–4.32) | |
Adjusted for age and baseline log transformed IgG using ANCOVA.
In the COVAXIN® primed group, anti-spike IgG GMCs on day 28 after a heterologous COVISHIELD™ boost was 241,681.6 AU/mL (95% CI: 201,380.2–290,048.3) and following homologous COVAXIN® boost, was 48,473.94 AU/mL (95% CI: 38,529.56–60,984.95). The day 28 GMR was 5.11 (95% CI: 3.83–6.81), confirming non-inferiority of heterologous over homologous booster in the COVAXIN® primed group with respect to the GMR as the lower limit of the CI is above the non-inferiority margin of 0.67. The fold rise from baseline to day 28 in the heterologous COVISHIELD™ boosted arm was 31.19 (95% CI: 20.38–47.74) and in the COVAXIN® boosted arm was 5.32 (95% CI: 3.55–7.99).
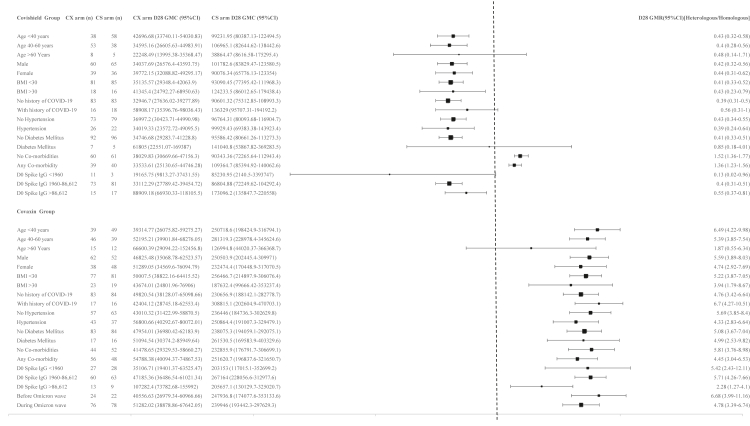
The individual anti-spike IgG levels at baseline and day 28 are shown in Supplementary Fig. S1, with stratified analyses of the anti-spike IgG in Fig. 2. The GMC, GMR and GMFR of the antibodies to the other targeted antigens, such as the RBD, NTD, nucleocapsid antigen and SARS-CoV-1 spike protein, showed similar patterns as the anti-spike IgG across groups and arms (Supplementary Fig. S1 and Supplementary Table S5).
Fig. 2.
Forest plot with the stratified immunogenicity analyses for anti-spike IgG at 28 days post booster dose between the COVISHIELD™ and COVAXIN® boosted arms in the COVISHIELD™ primed group and the COVAXIN® primed group. Age, sex, Body Mass Index (BMI), past history of COVID-19, comorbidities, and baseline IgG were stratified and analysed for both the arms in both the groups. The sample size in each arm, anti-spike IgG at 28 days (and 95% CI) post booster dose and the day 28 GMR (and 95% CI) are shown in the forest plot. The baseline spike IgG was classified into three categories: <1960, 1960–86,612, >86,612 AU/mL. 1960 AU/mL is the cut-off as per the product insert,19 86,612 AU/mL is the day 28 median GMC of the COVISHIELD™ and COVAXIN® primed groups together. CS = COVISHIELD™; CX = COVAXIN®; D28 = Day 28; GMC = Geometric Mean Concentration; GMR = Geometric Mean Ratio.
Effect of the Omicron wave
The Omicron wave occurred during the period after the COVISHIELD™ primed group had completed their booster and immunogenicity assessment, but while the COVAXIN® primed group was still being given booster vaccination and evaluated for immunogenicity. Antibodies to nucleocapsid were not increased in the COVISHIELD™ primed and COVISHIELD™ boosted participants, but in the COVAXIN® primed COVISHIELD™ boosted participants, In the COVAXIN® primed group, during the pre-Omicron period, anti-nucleocapsid IgG was 9609.15 AU/mL (4815.38–19,175.17) at baseline and 50,558.24 AU/mL (34,418.2–74,266.96) on day 28 in the COVAXIN® boosted arm; and 6924.49 AU/mL (3180.64–15,075.13) at baseline and 5656.3 AU/mL (2942.92–10,871.42) in the COVISHIELD™ boosted arm. In the COVAXIN® primed group, during the Omicron wave, anti-nucleocapsid IgG was 7152.59 AU/mL (4012.55–12,749.86) at baseline and 118,205.9 (79,247.28–176,316.8) on day 28 in the COVAXIN® boosted arm; and 6806.34 AU/mL (4251.62–10,896.14) at baseline and 51,071.09 AU/mL (29,456.04–88,547.41) in the COVISHIELD™ boosted arm. There was an increase in nucleocapsid antibodies in those for whom the day 28 sample was collected after January 5, 2022, indicating infection (Supplementary Table S6 and Supplementary Fig. S2).
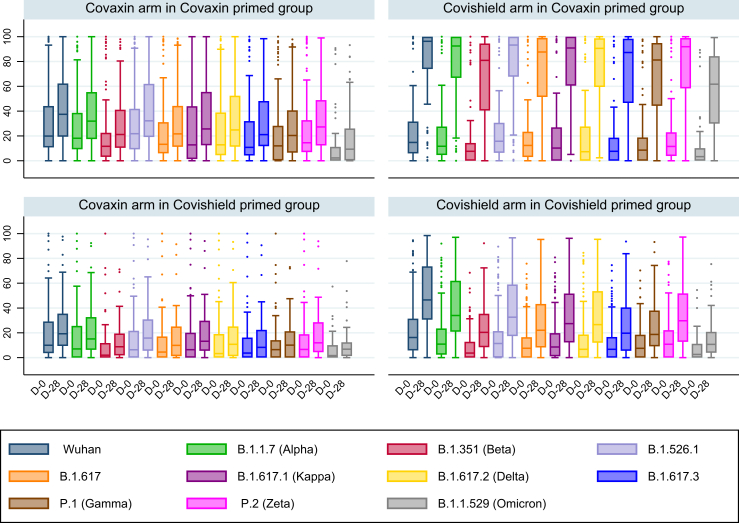
Antibody response to VOC
Surrogate neutralisation tests showing the percentage inhibition of the ACE2 receptor binding to the S antigen at baseline and on day 28 are shown in Fig. 3 and Supplementary Table S7. In both the COVISHIELD™ primed group and the COVAXIN® primed group, the percentage inhibition of the ACE2 receptor binding to the S antigen on day 28 by the variants Wuhan, B.1.1.7 (Alpha), B.1.351 (Beta), B.1.526.1, B.1.617, B.1.617.1 (Kappa), B.1.617.2 (Delta), B.1.617.3, P.1 (Gamma), P.2 (Zeta), and B.1.1.529 (Omicron) were significantly higher in the COVISHIELD™ boosted arm than in the COVAXIN® boosted arm. The best neutralising response was observed in the COVISHIELD™ boosted arm in the COVAXIN® primed group, with the median percentage inhibition of the ACE2 receptor binding to the spike antigen on day 28 being 96.27% (IQR: 73.92–100) for the Wuhan strain, 92.50% (IQR: 66.77–100) for the B.1.1.7 (Alpha) variant, 80.90% (IQR: 40.48–94.51) for the B.1.351 (Beta) variant, 90.58% (IQR: 59.47–98.73) for the B.1.617.2 (Delta) variant and 61.70% (IQR: 29.90–84.30) for the B.1.1.529 (Omicron) variant.
Fig. 3.
Box plot with the percentage of ACE2-Spike-inhibition for the wild type and variants of concern by arms in the COVISHIELD™ primed group and the COVAXIN® primed group, performed using the MSD surrogate neutralisation assay. The percentage of ACE2-Spike-inhibition on day 0 and day 28 for the wild type and each of the variants of concern are represented by different colours in the box plot by arms in the COVISHIELD™ primed group and the COVAXIN® primed group. The upper two panels represent the two arms in the COVAXIN® primed group and the lower two represent the two arms in the COVISHIELD™ primed group. D-0 = Day 0; D-28 = Day 28 of follow-up.
On stratified analysis of the COVISHIELD™ boosted arm in the COVAXIN® primed group based on day 28 blood sampling before and during the Omicron wave, the day 28 ACE2 receptor inhibition for Omicron variant was 69.45% (IQR: 24.40–79.90%) before the Omicron wave and 59.75% (IQR: 35.40–85.20%) during the Omicron wave. However, in the COVAXIN® boosted group, it was 1.45% (IQR: 0–8.95%) before the onset of the Omicron wave and 14.45% (2.3–31.35) during the Omicron wave. The relative lack of neutralizing antibody response in the COVAXIN® boosted arm, and the significant response in COVISHIELD™ boosted arm even before the Omicron wave indicate that the increase in functional antibody response in the COVISHIELD™ boosted arm was not significantly influenced by the Omicron wave (Supplementary Fig. S3).
Breakthrough infections and adverse effects
Three symptomatic and RT-PCR positive breakthrough COVID-19 infections were reported in the COVISHIELD™ primed group, all in the COVAXIN® boosted arm; and 20 in the COVAXIN® primed group, with ten in each arm (Supplementary Table S8). All the breakthrough infections in the COVISHIELD™ primed group occurred before the onset of the Omicron wave, and all in the COVAXIN® primed group occurred after the onset. None of the breakthrough infections required hospitalisation. The study was not powered for efficacy and the follow up period presented here is only for 90 days. Hence efficacy was not analysed.
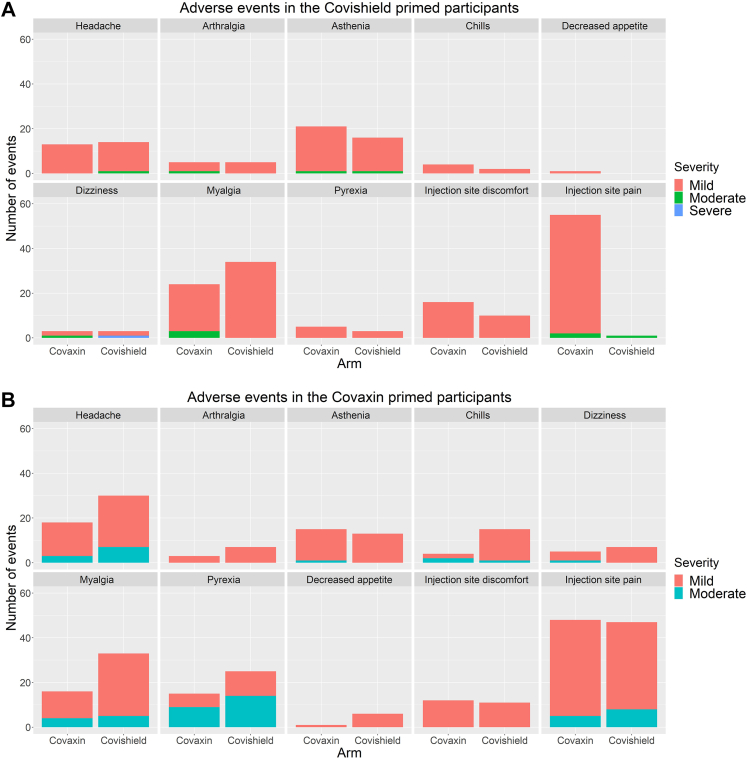
In the COVISHIELD™ primed group, the most common adverse events recorded during the first 7 days after the booster dose were injection site pain, myalgia, asthenia and headache. It was similar in both the arms. In the COVAXIN® primed group, the most common adverse events recorded during the first 7 days after the booster dose were injection site pain, myalgia, headache, and pyrexia. Conditions such as myalgia, headache and chills were significantly more frequent in those who received COVISHIELD™ as the booster. Severe adverse events were reported in 3% and 1% of those who received homologous COVISHIELD™ booster and homologous COVAXIN® booster, respectively in the first 7 days. There were no severe adverse events in the first 7 days with heterologous schedules (Fig. 4A and B; Supplementary Tables S9 and S10). During the 90 days follow-up period, four serious adverse events (SAE) were reported, two in the COVISHIELD™ primed group and two in the COVAXIN® primed group. None of the SAEs was considered related to the booster vaccination, and no deaths were reported (Supplementary Table S11). The haematological and biochemical parameters were found to be within normal limits on day 28 in both the arms in both the groups (Supplementary Tables S12 and S13).
Fig. 4.
A and B: Adverse events in the first seven days post-vaccination among the participants in the COVISHIELD™ boosted arm and COVAXIN® boosted arm in the COVISHIELD™ primed group and the COVAXIN® primed group.
Discussion
In both COVISHIELD™ and COVAXIN® primed groups, seropositivity on day 28 post booster in heterologous schedules were non-inferior to homologous schedules. Both homologous and heterologous booster vaccines produced significantly elevated GMCs 28 days post booster compared to baseline GMCs. Since there was no placebo arm, we used the absolute GMC on day 28 and the fold rise of GMC from baseline to day 28 to assess the immune responses. In the COVISHIELD™ primed group, the day 28 GMFRs were 2.81 (2.21–3.57) and 5.06 (3.88–6.61) in the COVAXIN® and COVISHIELD™ boosted arms, respectively. This was in keeping with the lower GMFR of 1.45 (95% CI: 1.31–1.60) after the heterologous inactivated VLA2001 vaccine compared to 2.58 (95% CI: 2.1–3.08) with homologous ChAdOx1 vaccine in the COV-BOOST trial.11 In the COVAXIN® primed group, the day 28 GMFRs in the COVAXIN® boost arm [5.32 (95% CI: 3.55–7.99)] was significantly lower than in the COVISHIELD™ boost arm [31.19 (95% CI: 20.38–47.74)]. In the RHH-001 trial, homologous inactivated CoronaVac® had a GMFR of 12 (11–14) compared to 77 (67–88) in those boosted with Ad26COV2-S, and 90 (77–104) with ChAdOx1 boost, both adenoviral vectored vaccines.12 The post-booster GMCs in our study followed a trend similar to the GMFRs, with the highest in the COVISHIELD™ boosted arm in the COVAXIN® primed group and the least in the COVAXIN® boosted arm in the COVAXIN® primed group. The use of mRNA, adenoviral vectored, whole-cell or sub-unit vaccines, too, have shown significantly elevated GMCs after booster doses.11 Immunogenicity assessment using antibodies to other antigenic components of the spike protein, such as the NTD and the RBD, also generated similar results. Our findings are consistent with the COV-BOOST trial in that the boosting responses are related to the individual vaccines used in the primary series and as boosters, therefore reiterating the need for generating data for each vaccine and combinations.11
Although all participants had received two doses of vaccine, a proportion of participants were seronegative before the booster administration in the COVISHIELD™ (23.5% seronegative) and COVAXIN® (38% seronegative) primed groups respectively. The GMC in the COVAXIN® primed group was considerably lower than the COVISHIELD™ primed group at baseline, but there was a difference of 20 days in the mean duration between the second dose of the primary series and the booster dose between the two groups (187 days in the COVISHIELD™ primed group vs 207 days in the COVAXIN® primed group). This difference in the IgG at approximately 6 months post-vaccination in the COVISHIELD™ primed and the COVAXIN® primed groups are consistent with the COVAT study on humoral antibody kinetics that showed higher anti-spike IgG at the end of 6 months with COVISHIELD™ compared to COVAXIN®.21 Other studies have also shown that humoral immunity wanes within 6 months of the second dose of vaccine,22, 23, 24 and longer intervals after the second dose have been associated with more breakthrough infection.25,26
ACE2 inhibition surrogate neutralisation for wild-type Wuhan and VOCs showed the lowest neutralisation for Omicron in the pre-boost baseline samples for both vaccines. Following the booster dose, all arms showed an increase in neutralisation against all evaluated viral spike proteins (Fig. 3), with the best results seen in those who received a COVISHIELD™ booster following COVAXIN® primary immunisation, and the least increase in those who received COVAXIN® booster after COVAXIN® prime. The maximal increase in Omicron neutralisation was also seen in those who received a COVISHIELD™ booster following COVAXIN® primary immunisation. The RHH-001 study showed a similar response with better neutralising antibody levels to the wild type and the Delta variants in the heterologous schedule with vectored vaccines ChAdOx1 nCoV-19 and Ad.26CoV2-S, as compared to homologous inactivated CoronaVac® vaccine.12 The COV-BOOST trial showed better neutralising antibodies to the Delta variant with homologous vectored ChAdOx1 vaccine than heterologous inactivated VLA2001 vaccine, similar to our results.11 COVAXIN®, which has the novel adjuvant Alhydroxiquim-II, has a similar pattern of lower immune responses when used in a homologous or heterologous series, as seen with other inactivated vaccines which are adjuvanted with alum.
All homologous and heterologous schedules showed acceptable reactogenicity in the first 7 days after booster vaccination (Fig. 4A and B, Supplementary Tables S9 and S10). Most adverse events in the first 7 days were mild or moderate, and none led to hospitalisation or death. In COVISHIELD™ primed individuals, there was no significant difference between the adverse events in those who received a homologous or heterologous booster. However, in COVAXIN® primed individuals, there were more vaccinees with myalgia, headache and chills, with COVISHIELD™ booster compared to COVAXIN® booster, although none of these events was severe. Our findings are consistent with other studies that reported acceptable reactogenicity after booster doses with multiple heterologous schedules.11,27 The increased reactogenicity with heterologous boosting with a vectored vaccine compared to homologous boosting with inactivated vaccines is similar to the RHH-001 study.12 The safety profile in the first seven days is consistent with the safety record of these vaccines when used in primary schedules.28
The WHO interim recommendations in December 2021 on homologous and heterologous schedules are to consider using vectored or mRNA vaccines after initial doses of inactivated vaccines depending on vaccine availability, with no recommendation for subsequent heterologous doses with inactivated vaccines.29 In India, where COVISHIELD™ or COVAXIN® form almost all COVID vaccination in adults, a precautionary third dose for adults >18 years was recommended using homologous schedules only. Our study results show that homologous boosting with COVISHIELD™ and COVAXIN® produces boosting immune responses. However, with heterologous schedules, COVISHIELD™ boost after COVAXIN® prime produces a robust immune boost, whereas COVAXIN® boost after COVISHIELD™ prime produces a weak immune boost substantiating the existing WHO recommendations. Based on our binding and functional antibody results, COVISHIELD™ would be a better booster in India irrespective of whether the primary series has been COVISHIELD™ or COVAXIN®. Further research on the longevity of the antibody responses and analysis of cellular data post heterologous or homologous boosters would be useful to determine the role of memory responses with these boosters.
There were several limitations in our study. Firstly, the study was planned with a non-inferiority design using seroconversion to calculate sample size as there was poor clarity on the role of GMCs with different assays that were being used at the time of planning the study. Over time, there was more information available on the various assays and we added a non-inferiority outcome based on GMRs and amended our protocol. Secondly, participants who had a natural infection were not excluded. Natural infection would be expected to boost the immune response to the vaccines, and participants with a history of COVID-19 infection did have a higher baseline IgG levels, and consequently the GMFR was lower than those without infection. However, the absolute values of the GMCs were similar on day 28 (Fig. 2). Thirdly, the COVISHIELD™ primed participants were recruited within a month, whereas with COVAXIN® primed participants, the recruitment took 5 months as fewer people received COVAXIN® as primary doses. The Omicron wave that started in Vellore at the end of December 2021 could have impacted the boosting in the COVAXIN® arm. Nevertheless, on stratified analysis, there was no significant difference in the day 28 GMCs between those who completed their day 28 visit before and after the Omicron wave (Fig. 2). Fourthly, in the COVISHIELD™ primed group, there were statistically significant baseline differences in the mean age and baseline spike IgG levels. However, all GMRs presented are adjusted for age and baseline IgG (Table 2 and Supplementary Table S5).
In conclusion, this interim analysis shows that homologous and heterologous boosting with COVISHIELD™ or COVAXIN® in COVISHIELD™ or COVAXIN® primed individuals are immunogenic and safe. For both binding and functional antibodies, in participants primed with COVISHIELD™, homologous boosting with COVISHIELD™ offers better immune responses than heterologous boosting with COVAXIN®; and in those primed with COVAXIN®, heterologous boost with COVISHIELD™ offers the better immune response. A heterologous boost with COVISHIELD™ after COVAXIN® prime offers the best immune response among the approaches evaluated in this study.
Contributors
G.K. conceived the trial. W.R. was the chief investigator. G.K., W.R., J.J., V.R.M., P.S., S.B., R.M. and R.R. contributed to the protocol and design of the study. R.R., O.S.N., J.V.L.X., J.S.D.C., S.V., P.R., K.P.P.A. and K.B.T. contributed to the project administration. S.B., R.M., S.I. and A.G. contributed to the investigations performed in the study. R.R., P.S. and A.A.D. designed the statistical analysis and accessed and verified the underlying data. R.R. and W.R. drafted the report. All authors reviewed and approved the final report.
Data sharing statement
De-identified data may be available upon reasonable request to the corresponding author.
Declaration of interests
The study is funded by Azim Premji Foundation the Bill and Melinda Gates Foundation. The funding is to the institution, Christian Medical College, Vellore. K.B.T. was an investigator in a study which received funding support from Bharath Biotech, manufacturer of Covaxin. The funding was given to his institute, Centre for Cellular and Molecular Biology, Hyderabad. None of the other authors have declared any competing interests.
Acknowledgements
The investigators express their gratitude for the contribution of all trial participants.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lansea.2023.100141.
Appendix A. Supplementary data
IgG levels measured at baseline and at 28 days post booster among the participants in the COVISHIELD™ and COVAXIN® boosted arms in the COVISHIELD™ primed group and COVAXIN® primed group. The graphs represent paired data, with the blue dots representing the log transformed IgG level at baseline and the red representing that on day 28 for each participant.
Anti-spike and anti-nucleocapsid IgG levels at baseline and at 28 days post booster among the participants in the COVISHIELD™ and COVAXIN® boosted arms of the COVAXIN® primed group before and during the Omicron wave in the study site. The graphs represent paired data, with the blue dots representing the log transformed anti-spike and anti-nucleocapsid IgG levels at baseline, and the red representing that on day 28 in both the arms of the COVAXIN® primed group, stratified based on onset of the Omicron wave in the study site.
Box plot with the percentage inhibition of ACE2 for the Omicron variant by arms in the COVAXIN® primed group, stratified by the onset of the Omicron wave in the study site. The percentage of ACE2-Spike-inhibition on day 0 and day 28 for the Omicron variant, stratified based on the onset of Omicron wave, in the COVAXIN® primed group. The blue boxes represent the percentage inhibition on day 0 and the red boxes represent that on day 28.
References
- 1.Tartof S.Y., Slezak J.M., Fischer H., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chemaitelly H., Tang P., Hasan M.R., et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385(24):e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Interim statement on the use of additional booster doses of emergency use listed mRNA vaccines against COVID-19 [internet] https://www.who.int/news/item/17-05-2022-interim-statement-on-the-use-of-additional-booster-doses-of-emergency-use-listed-mrna-vaccines-against-covid-19 Available from:
- 4.Mlcochova P., Kemp S.A., Dhar M.S., et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599(7883):114–119. doi: 10.1038/s41586-021-03944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karim S.S.A., Karim Q.A. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398(10317):2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrews N., Tessier E., Stowe J., et al. Duration of protection against mild and severe disease by Covid-19 vaccines. N Engl J Med. 2022;386(4):340–350. doi: 10.1056/NEJMoa2115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews N., Stowe J., Kirsebom F., et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022;28(4):831–837. doi: 10.1038/s41591-022-01699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw R.H., Stuart A., Greenland M., et al. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397(10289):2043–2046. doi: 10.1016/S0140-6736(21)01115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X., Shaw R.H., Stuart A.S.V., et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398(10303):856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordström P., Ballin M., Nordström A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic Covid-19 infection in Sweden: a nationwide cohort study. Lancet Reg Health Eur. 2021;11:100249. doi: 10.1016/j.lanepe.2021.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munro A.P.S., Janani L., Cornelius V., et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398(10318):2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clemens S.A.C., Weckx L., Clemens R., et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399(10324):521–529. doi: 10.1016/S0140-6736(22)00094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CoWIN dashboard [internet] https://dashboard.cowin.gov.in/ Available from:
- 14.MoHFW. Guidelines for COVID-19 vaccination of children between 15-18 years and precaution dose to HCWs, FLWs & 60+ population with comorbidities.
- 15.Comparison of vaccination of Covishield and Covaxin to the beneficiaries Health Unit District wise in Tamil Nadu upto 30th November 2021 [internet]. Open Gov. DataOGD Community. https://community.data.gov.in/comparison-of-vaccination-of-covishield-and-covaxin-to-the-beneficiaries-health-unit-district-wise-in-tamil-nadu-upto-30th-november-2021/ Available from:
- 16.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiaSorin's LIAISON® SARS-CoV-2 diagnostic solutions [internet]. DiaSorin. https://www.diasorin.com/en/immunodiagnostic-solutions/clinical-areas/infectious-diseases/covid-19 Available from:
- 19.Meso Scale Diagnostics, LLC. V-PLEX Covid-19 ACE2 neutralization assay product insert.
- 20.Guidelines on clinical evaluation of vaccines: regulatory expectations [internet]. WHO. 2017. https://www.who.int/publications/m/item/WHO-TRS-1004-web-annex-9 Available from:
- 21.Singh A.K., Phatak S.R., Singh R., et al. Antibody response after first and second-dose of ChAdOx1-nCOV (CovishieldTM®) and BBV-152 (CovaxinTM®) among health care workers in India: the final results of cross-sectional coronavirus vaccine-induced antibody titre (COVAT) study. Vaccine. 2021;39(44):6492–6509. doi: 10.1016/j.vaccine.2021.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogbe A., Pace M., Bittaye M., et al. Durability of ChAdOx1 nCov-19 (AZD1222) vaccination in people living with HIV - responses to SARS-CoV-2, variants of concern and circulating coronaviruses [internet] 2021. https://www.medrxiv.org/content/10.1101/2021.09.28.21264207v1 2021.09.28.21264207. Available from:
- 23.Almendro-Vázquez P., Laguna-Goya R., Ruiz-Ruigomez M., et al. Longitudinal dynamics of SARS-CoV-2-specific cellular and humoral immunity after natural infection or BNT162b2 vaccination. PLoS Pathog. 2021;17(12) doi: 10.1371/journal.ppat.1010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levin E.G., Lustig Y., Cohen C., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iacobucci G. Covid-19: protection from two doses of vaccine wanes within six months, data suggest. BMJ. 2021;374:n2113. doi: 10.1136/bmj.n2113. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg Y., Mandel M., Bar-On Y.M., et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385(24):e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sablerolles R.S.G., Rietdijk W.J.R., Goorhuis A., et al. Immunogenicity and reactogenicity of vaccine boosters after Ad26.COV2.S priming. N Engl J Med. 2022;386(10):951–963. doi: 10.1056/NEJMoa2116747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaur R.J., Dutta S., Bhardwaj P., et al. Adverse events reported from COVID-19 vaccine trials: a systematic review. Indian J Clin Biochem. 2021;36(4):427–439. doi: 10.1007/s12291-021-00968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization Interim recommendations for heterologous COVID-19 vaccine schedules: interim guidance, 16 December 2021 [internet]. World Health Organization. 2021. https://apps.who.int/iris/handle/10665/350635 Available from:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IgG levels measured at baseline and at 28 days post booster among the participants in the COVISHIELD™ and COVAXIN® boosted arms in the COVISHIELD™ primed group and COVAXIN® primed group. The graphs represent paired data, with the blue dots representing the log transformed IgG level at baseline and the red representing that on day 28 for each participant.
Anti-spike and anti-nucleocapsid IgG levels at baseline and at 28 days post booster among the participants in the COVISHIELD™ and COVAXIN® boosted arms of the COVAXIN® primed group before and during the Omicron wave in the study site. The graphs represent paired data, with the blue dots representing the log transformed anti-spike and anti-nucleocapsid IgG levels at baseline, and the red representing that on day 28 in both the arms of the COVAXIN® primed group, stratified based on onset of the Omicron wave in the study site.
Box plot with the percentage inhibition of ACE2 for the Omicron variant by arms in the COVAXIN® primed group, stratified by the onset of the Omicron wave in the study site. The percentage of ACE2-Spike-inhibition on day 0 and day 28 for the Omicron variant, stratified based on the onset of Omicron wave, in the COVAXIN® primed group. The blue boxes represent the percentage inhibition on day 0 and the red boxes represent that on day 28.