Abstract
The radial sensory nerve can be injured during many common procedures, including intravenous cannulation, first extensor compartment release, and radial-sided wrist surgery. Injury to the nerve may result in neuroma formation that can lead to chronic and debilitating pain. Nonsurgical treatments and surgical interventions, including excision of the neuroma and burying the nerve into local muscle, are frequently ineffective. Here, we present a technique for treating recalcitrant neuromas of the radial sensory nerve with targeted muscle reinnervation to a redundant motor nerve branch of the extensor carpi radialis brevis.
Key words: Pain, Peripheral nerve, Targeted muscle reinnervation, Upper extremity
Injury to a peripheral nerve may result in the development of a neuroma if the proximal axons regenerate in a disorganized or incomplete fashion. Symptomatic neuromas can present with patients complaining of pain, paresthesia, numbness, cold intolerance, or complex regional pain syndrome in the affected nerve distribution.1,2 Nonsurgical management includes pain medication, radiofrequency ablation, neuromodulation, or desensitization, though these interventions are frequently ineffective at controlling symptoms.3 Surgical treatment of symptomatic neuromas includes excision of the neuroma and typically another adjunctive strategy to mitigate the risk of neuroma recurrence. Adjunctive strategies may be “passive” or “active.” Passive strategies focus on relocating the nerve end away from the surgical field. Examples include excision with traction neurectomy, excision with implantation into muscle or other tissue, nerve cap, or a relocating nerve graft. Active surgical strategies focus on promoting physiologic regeneration to distal targets. Examples include excision and reconstruction to the distal nerve end, end-to-side neurorrhaphy, regenerative peripheral nerve interface, or targeted muscle reinnervation (TMR).1
Targeted muscle reinnervation is the transfer of the distal end of an injured peripheral nerve to an adjacent expendable motor nerve, originally described as a technique to provide enhanced control of myoelectric prosthetics for upper extremity amputees.4, 5, 6 Multiple studies have since noted that TMR has an impact on decreasing rates of painful neuroma formation and phantom limb pain in amputees.7, 8, 9, 10, 11 The transected peripheral nerve is provided with a distal nerve end to establish axonal continuity, thought to decrease disorganized axonal sprouting and subsequent neuroma formation. This also establishes an interface with a denervated motor end plate, providing continuity to efferent and afferent pathways. Importantly, TMR has been reported to be superior to traditional, passive methods of neuroma resection and implantation and represents a changing paradigm in the surgical management of neuromas.3
For patients with symptomatic, refractory terminal neuromas, TMR provides terminal nerve stabilization if the distal nerve cannot be reconstructed. For patients with neuromas in continuity, neuroma resection and nerve reconstruction may be attempted as a first-line surgical option, and TMR may be indicated for patients who either fail nerve reconstruction or who have gaps that are too long to effectively be reconstructed after neuroma resection.
Neuromas of the radial sensory nerve (RSN) may present with debilitating pain and other symptoms in the distal dorsal radial hand and forearm. Besides trauma, RSN neuromas have been reported to occur after attempted intravenous cannulation of the distal cephalic vein, de Quervain release, and Kirschner wire fixation of distal radius fractures, as well as carpal surgery.12, 13, 14 Radial sensory nerve neuromas are particularly difficult to treat. Along with duration of pain, the number of surgeries, social factors, and digital neuromas, neuromas of the RSN are associated with recurrent pain after surgery.15 One study reported the rate of secondary surgery for RSN neuromas to be 20%, which is higher than that of other upper extremity neuromas.16
Theoretically, the RSN can be transferred to any motor branch within reach to complete a successful TMR. In this paper, we present our surgical technique for the treatment of RSN neuromas with TMR to the expendable distal motor nerve to the extensor carpi radialis brevis (ECRB).
Materials and Methods
Patient evaluation
When evaluating for RSN neuroma, a detailed history focused on previous traumatic injury or surgery near the RSN will aid in determining whether a neuroma is the likely cause of symptoms. Physical examination findings will frequently demonstrate a positive Tinel sign overlying the course of the RSN, typically focused over the point of injury, but also proximally at the brachioradialis (BR) and extensor carpi radialis longus (ECRL) interval where the RSN emerges. The degree of sensory deficit (ie, complete anesthesia vs diminished sensation) in the distribution of the RSN—the dorsoradial wrist and hand—can help determine whether the patient has a complete or partial nerve injury and will help guide surgical treatment options. Finally, motor examination, with a particular focus on wrist extension, will help determine whether the ECRB is a suitable target for TMR.
Prior to treating a neuroma with TMR, the patient must be carefully evaluated with a preoperative diagnostic nerve block. A suitable candidate for TMR will have nearly complete relief of symptoms transiently after a nerve block of the affected nerve. The duration of pain is important to consider as well when evaluating a patient, as patients with long-standing pain tend to do worse after TMR, likely secondary to reorganization of the somatosensory cortex and centralization of pain after neuroma formation.1
Statement of informed consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Surgical anatomy
The radial nerve bifurcates into the RSN and posterior interosseous nerve proximal to the leading edge of the supinator muscle. The RSN runs deep to the BR muscle and pierces the forearm fascia between the tendons of the BR and ECRL about 9 cm proximal to the radial styloid. It then divides into multiple branches across the dorsal radial hand in a variable pattern. The RSN and its branches are deep to the superficial veins, but superficial to the extensor pollicis longus tendon and the tendons of the first extensor compartment.
Motor branches to the ECRB may originate from the radial nerve or the posterior interosseous nerve.17 As with most forearm muscles, the ECRB has multiple innervation points, usually 3 or 4.18 The nerves can be found in the proximal forearm in an interval between the BR and ECRL muscles, which is the interval used for a radial tunnel surgical approach.
We chose ECRB for several reasons. The motor nerves lie immediately adjacent to the RSN in a standard radial tunnel exposure. Its function to extend the wrist is redundant. If the entire ECRB muscle is denervated, the patient may experience radial wrist deviation on wrist extension or overall weakness with wrist extension; however, the ECRB is typically segmentally innervated by at least 2–4 motor branches, and native innervation is preserved if the most distal motor branch is selected as the target for the TMR.16
To find the RSN and motor branches to the ECRB in the proximal forearm, it is helpful to mark the skin incision before surgery with the patient fully awake (Fig. 1). To mark the interval between the BR and ECRL, the patient should be asked to flex at their elbow against resistance with the forearm in neutral rotation, delineating the BR. During the dissection, the posterior cutaneous nerve of the forearm is encountered in the subcutaneous plane at the interval between the ECRL and BR. Additionally, the forearm fascia is typically lighter and thicker over the ECRL muscle, and darker and thinner over the BR. The fascia should be incised directly at this interval. The interval between the muscles can be easily bluntly dissected, which leads directly to a fat stripe containing the RSN, the posterior interosseous nerve, and the motor branches to the ECRB.
Figure 1.
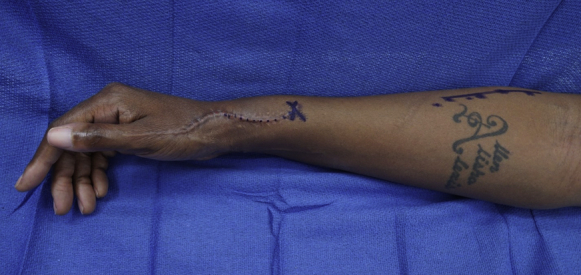
Skin markings of the proximal and distal incisions. The distal incision over the pathologic RSN is generally through the prior surgical site. The proximal incision is at the interval between the BR and ECRB muscles.
Surgical technique
The procedure is performed with the patient in the supine position and their upper extremity on a hand table. Based on the patient’s comorbidities and preference, it can be done under general anesthesia or under a regional block with sedation.
For the patient presented in this report, a distal incision was made over the RSN to explore for a neuroma, 9 cm proximal to the radial styloid and between the BR and ECRL tendons (Fig. 1). The BR and ECRL tendons were identified, and the RSN was seen exiting from the fascia between these tendons (Fig. 2). The nerve was dissected from proximal to distal to release scar adhesions from prior procedures, to locate the neuroma, and to gain length for the nerve transfer. The RSN can be left in situ and trimmed back to healthy fascicles after it is transferred to the proximal incision.
Figure 2.
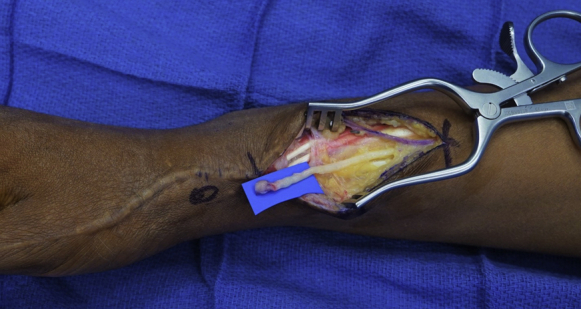
Radial sensory nerve with a neuroma. The prior incision was extended proximally to identify the nerve in an unscarred surgical field.
The proximal forearm incision is centered over the interval between the BR and ECRL. The posterior cutaneous nerve of the forearm, located just superficial to the forearm fascia running along the interval between the ECRL and BR, should be identified and preserved. The forearm fascia is incised in the interval between the ECRL and BR. Both the posterior cutaneous nerve of the forearm and the juxtaposition of the lighter fascia over the ECRL and the darker fascia over the BR are helpful markers of the interval between the ECRL and BR. Blunt dissection is used to develop the interval between the ECRL and BR until a fat stripe is located, where the RSN can be found. A self-retaining retractor is placed. The RSN is dissected proximally to the proper radial nerve, at which point the ECRB motor nerve can be identified branching from the proper radial nerve. Posterior interosseous nerve exploration is not required. The ECRB motor nerve is then dissected to the ECRB muscle. Generally, there are multiple branches from the ECRB motor nerve that segmentally innervate the muscle (Fig. 3). The proximal branches are preserved, and the most distal branch identified is used for the TMR nerve transfer.
Figure 3.
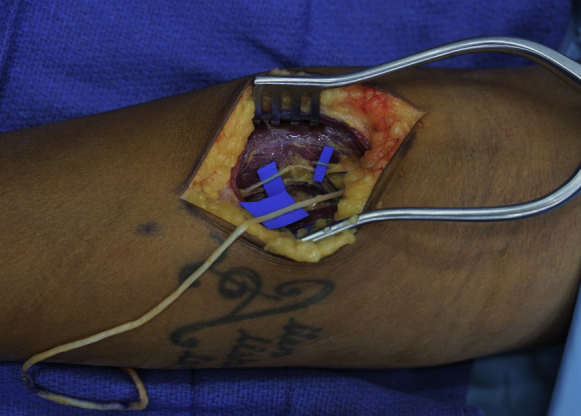
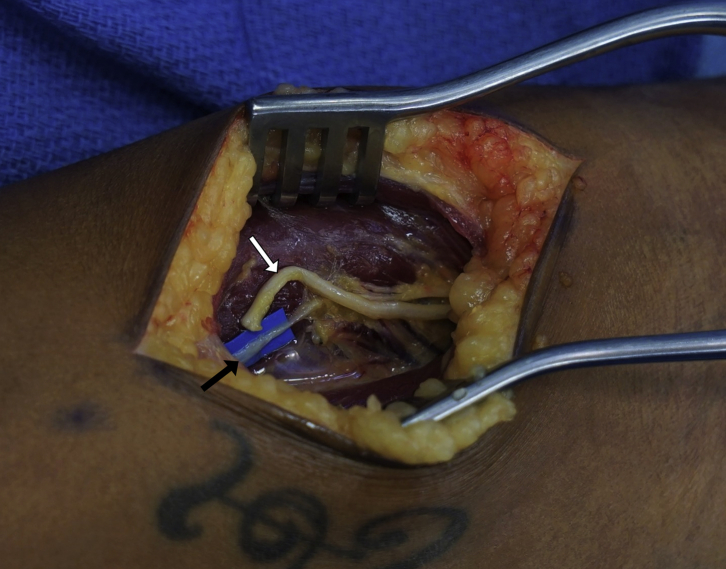
Multiple motor nerves to ECRB are highlighted with blue backgrounds. Note that the most distal motor nerve is used, and the proximal motor nerves are spared. The RSN has been withdrawn from the distal incision into the proximal exposure.
Once the RSN is cleared of its distal soft tissue attachments, it may be easily withdrawn into the proximal incision (Fig. 3). The RSN should now be sharply trimmed back to healthy fascicles and to a point where the caliber is a better match for the ECRB motor nerve (Fig. 4). The distal ECRB motor branch is sharply divided, and an end-to-end antegrade TMR nerve transfer is performed from the RSN to the ECRB distal motor nerve. The coaptation should be tension free (Fig. 5). A size mismatch between the nerves is expected and is the norm for TMR.
Figure 4.
The RSN (white arrow) has been transposed and cut back to the appropriate length. A distal motor nerve to ECRB (black arrow) has been selected as the target. Note the proximal motor nerves left in continuity.
Figure 5.
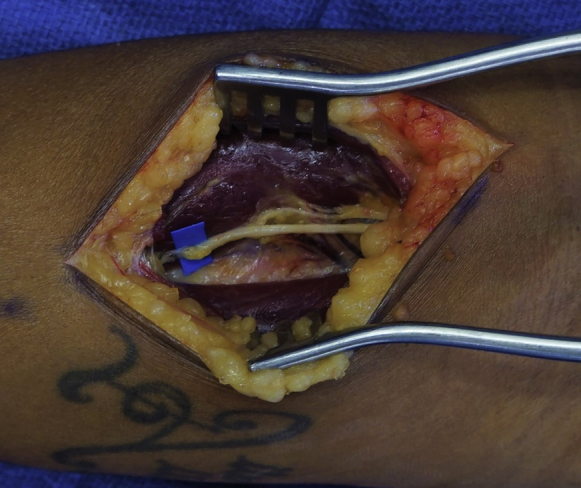
The selected motor nerve to ECRB has been cut and the RSN has been coapted to the distal end of the motor nerve.
Liposomal bupivacaine may be infiltrated adjacent to the nerves and subcutaneously to control postoperative pain, particularly in patients with chronic pain.
Postoperative management
Generally, this is an outpatient procedure unless the patient has medical comorbidities necessitating observation after anesthesia. The patient is discharged in a soft dressing that they remove on postoperative day 3. Gabapentin or pregabalin are prescribed as an adjunct for pain control for 1 month. Patients are instructed to manually desensitize their affected extremity after surgery and are referred to hand therapy as needed for desensitization, range of motion, and/or strengthening.
Pearls and pitfalls
Prior to surgery, every patient receives a diagnostic block of the sensory branch of the radial nerve that serves to confirm the RSN as the dominant cause of pain. This is also useful to prepare the patients for loss of sensation in this distribution if their nerve is still in continuity. Especially if the patient’s pain is chronic, they are educated that the procedure is likely to improve their pain but not eliminate it completely.
There is an expected size mismatch between the RSN and the distal motor branch to the ECRB. The motor branch of the ECRB can be dissected further into the muscle, where it gains more connective tissue, and therefore caliber. The increased caliber aids in the coaptation with the RSN.
Discussion
Including the patient presented in this report, we have performed the described procedure in 6 extremities for refractory symptomatic radial sensory neuromas. Follow-up has ranged from 5 months to 20 months. Patients’ preoperative visual analog scale pain scores ranged from 7 to 10 and their postoperative scores ranged from 0 to 4. All patients retained 5 out of 5 wrist extension, despite using the distal ECRB motor nerve as a target. Two procedures were performed under nerve block and the other 4 under general anesthesia.
Reported outcomes on the surgical treatment of RSN neuromas are limited. One study reported patient satisfaction after surgical intervention on 18 RSN neuromas to be 33%, and another study on 54 RSN neuromas reported that revision surgery for persistent pain was required in 20% of the cases and that only 68% of patients described themselves as improved after surgery.16,19 Techniques employed in these studies were excision alone, excision with implantation, excision and repair, and neurolysis alone, with or without nerve wrapping. Targeted muscle reinnervation for the treatment and prevention of RSN neuromas has been described for upper extremity amputees, but for TMR in nonamputees the literature is limited.7, 8, 9, 10, 11
Additional patients with longer follow-ups are required to better understand the effectiveness of this technique or compare it to other surgical treatments of RSN neuromas; however, this technique shows promising results of improved symptoms with unaffected wrist extension. We considered it a safe and potentially impactful additional option in the treatment of refractory, symptomatic RSN neuromas.
Footnotes
Declaration of interests: No benefits in any form have been received or will be received related directly or indirectly to the subject of this article.
References
- 1.Eberlin K.R., Ducic I. Surgical algorithm for neuroma management: a changing treatment paradigm. Plast Reconstr Surg Glob Open. 2018;6(10) doi: 10.1097/GOX.0000000000001952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ives G.C., Kung T.A., Nghiem B.T., et al. Current state of the surgical treatment of terminal neuromas. Neurosurgery. 2018;83(3):354–364. doi: 10.1093/neuros/nyx500. [DOI] [PubMed] [Google Scholar]
- 3.Chappell A.G., Jordan S.W., Dumanian G.A. Targeted muscle reinnervation for treatment of neuropathic pain. Clin Plast Surg. 2020;47(2):285–293. doi: 10.1016/j.cps.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Cheesborough J.E., Souza J.M., Dumanian G.A., Bueno R.A. Targeted muscle reinnervation in the initial management of traumatic upper extremity amputation injury. Hand (N Y) 2014;9(2):253–257. doi: 10.1007/s11552-014-9602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gart M.S., Souza J.M., Dumanian G.A. Targeted muscle reinnervation in the upper extremity amputee: a technical roadmap. J Hand Surg Am. 2015;40(9):1877–1888. doi: 10.1016/j.jhsa.2015.06.119. [DOI] [PubMed] [Google Scholar]
- 6.Cheesborough J.E., Smith L.H., Kuiken T.A., Dumanian G.A. Targeted muscle reinnervation and advanced prosthetic arms. Semin Plast Surg. 2015;29(1):62–72. doi: 10.1055/s-0035-1544166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valerio I.L., Dumanian G.A., Jordan S.W., et al. Preemptive treatment of phantom and residual limb pain with targeted muscle reinnervation at the time of major limb amputation. J Am Coll Surg. 2019;228(3):217–226. doi: 10.1016/j.jamcollsurg.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Dumanian G.A., Potter B.K., Mioton L.M., et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial. Ann Surg. 2019;270(2):238–246. doi: 10.1097/SLA.0000000000003088. [DOI] [PubMed] [Google Scholar]
- 9.Mioton L.M., Dumanian G.A., Shah N., et al. Targeted muscle reinnervation improves residual limb pain, phantom limb pain, and limb function: a prospective study of 33 major limb amputees. Clin Orthop Relat Res. 2020;478(9):2161–2167. doi: 10.1097/CORR.0000000000001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang B.L., Harbour P., Mondshine J., Kleiber G.M. Targeted muscle reinnervation to expendable motor nerves for the treatment of refractory symptomatic neuromas in nonamputees. Plast Reconstr Surg Glob Open. 2021;9(2) doi: 10.1097/GOX.0000000000003436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Brien A.L., Jordan S.W., West J.M., Mioton L.M., Dumanian G.A., Valerio I.L. Targeted muscle reinnervation at the time of upper-extremity amputation for the treatment of pain severity and symptoms. J Hand Surg Am. 2021;46(1):72.e1–72.e10. doi: 10.1016/j.jhsa.2020.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Stahl S., Kaufman T., Ben-David B. Neuroma of the superficial branch of the radial nerve after intravenous cannulation. Anesth Analg. 1996;83(1):180–182. doi: 10.1097/00000539-199607000-00032. [DOI] [PubMed] [Google Scholar]
- 13.Louis D.S., Greene T.L., Noellert R.C. Complications of carpal tunnel surgery. J Neurosurg. 1985;62(3):352–356. doi: 10.3171/jns.1985.62.3.0352. [DOI] [PubMed] [Google Scholar]
- 14.Singh S., Trikha P., Twyman R. Superficial radial nerve damage due to Kirschner wiring of the radius. Injury. 2005;36(2):330–332. doi: 10.1016/j.injury.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Stokvis A., Coert J.H., van Neck J.W. Insufficient pain relief after surgical neuroma treatment: prognostic factors and central sensitisation. J Plast Reconstr Aesthet Surg. 2010;63(9):1538–1543. doi: 10.1016/j.bjps.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb R.W., Westenberg R.F., Chen N.C., Coert J.H., Eberlin K.R. Long-term outcomes after surgical treatment of radial sensory nerve neuromas: patient-reported outcomes and rate of secondary surgery. Plast Reconstr Surg. 2021;147(1):101–111. doi: 10.1097/PRS.0000000000007437. [DOI] [PubMed] [Google Scholar]
- 17.Branovacki G., Hanson M., Cash R., Gonzalez M. The innervation pattern of the radial nerve at the elbow and in the forearm. J Hand Surg Br. 1998;23(2):167–169. doi: 10.1016/s0266-7681(98)80166-6. [DOI] [PubMed] [Google Scholar]
- 18.Abrams R.A., Ziets R.J., Lieber R.L., Botte M.J. Anatomy of the radial nerve motor branches in the forearm. J Hand Surg Am. 1997;22(2):232–237. doi: 10.1016/S0363-5023(97)80157-8. [DOI] [PubMed] [Google Scholar]
- 19.Stokvis A., van der Avoort D.J.C., van Neck J.W., Hovius S.E.R., Coert J.H. Surgical management of neuroma pain: a prospective follow-up study. Pain. 2010;151(3):862–869. doi: 10.1016/j.pain.2010.09.032. [DOI] [PubMed] [Google Scholar]