Abstract
Background:
Older adults with cancer are at increased risk of treatment-related toxicities and excess mortality. We evaluated whether a patient-reported geriatric assessment (GA) based frailty index can identify those at risk of adverse outcomes.
Methods:
Older adults (≥60y) enrolled in a single-institutional prospective registry underwent patient-reported GA at initial evaluation in our medical oncology clinic. Using deficit accumulation method, we constructed a 44-item frailty index (CARE-FI), categorizing patients as robust, pre-frail and frail. The primary outcome was overall survival (OS). Secondary outcomes included a) functional decline at three months post-therapy b) incident grade ≥3 treatment-related toxicities at six-month post-treatment. We used multivariate Cox and logistic regression models respectively to study the impact of frailty on primary and secondary outcomes.
Results:
We identified 589 older adults with a median age of 69y; 55% males and 73% Whites. Overall, 168 (29%) were pre-frail and 230 (39%) frail. Being frail (vs robust) was associated with worse overall survival (Hazards Ratio, HR 1.83, 95% Confidence Interval, CI 1.34–2.49, P<.001) after adjusting for age, sex, race/ethnicity, cancer type, cancer stage and line of therapy. Similarly, frailty was associated with increased risk of functional decline (OR 3.01; 95% CI 1.33–6.81; P= .008) and grade ≥3 non-hematologic toxicities (OR 3.65; 95% CI 1.54–8.69; P=.003) but not hematologic toxicities (OR 1.01; 95% CI 0.46–2.22; P=.97).
Conclusions:
Our frailty index using a patient-reported GA is a robust predictor of survival, functional decline and treatment related toxicity among older adults with GI malignancies.
Keywords: Geriatric Assessment, Frailty Index, Cancer, Older Adults
Introduction:
Cancer is a disease of aging; over half of all new cancer cases and 70% of all cancer-related deaths occur among adults 65 years or older.1 Older adults with cancer continue to have suboptimal outcomes including excess treatment-related toxicities as well as inferior survival as compared to their younger counterparts.2 This vulnerability of older adults to adverse outcomes is inadequately explained by chronologic age and clinician-assessed performance status.2 Therefore, novel tools to capture aging-associated vulnerabilities are urgently needed.
Frailty is a clinically recognizable state of increased vulnerability resulting from aging-associated decline in reserve and function across multiple physiologic systems.3 Frailty reflects a reduced ability of an older adult to recover or restore homeostasis from acute stressors, putting them at risk of adverse health events including falls, incident disability, hospitalization and mortality.4 Whereas, there is no universally accepted standard for operationalizing frailty, two most commonly cited approach exist in the literature. In 2001, Fried defined frailty syndrome as meeting three out of five phenotypic criteria indicating compromised energetics: low grip strength, low energy, slowed waking speed, low physical activity, and/or unintentional weight loss.3 In the same year, Rockwood et al proposed that frailty should be viewed as the proportion of accumulated deficits (symptoms, signs, functional impairments and laboratory abnormalities).5 These two approaches represent the most commonly utilized operational definitions of frailty in the oncology literature as well as other fields to date.6
Geriatric assessment (GA) is a multidimensional evaluation of the overall health status of an older adult. Recently, it has been shown that GA is helpful for evaluation of older adults with cancer and can help predict those at risk of excess toxicities, guide treatment selection and targeted geriatric interventions.7–10 Information from GA can be used to construct a frailty index using the deficit accumulation principle as proposed by Rockwood et al.11 In addition to providing an operational definition of frailty, such an approach can highlight potential deficits unique to an older adult that can be used to guide appropriate interventions.
Despite the emerging role of frailty among older adults with cancer, a frailty index is not used as part of routine oncologic care and none is available specifically for patients with GI malignancies. We have previously described the feasibility of integrating a patient-reported GA in a medical oncology clinic at our institution. The objective of the current study was to evaluate the ability of a frailty index constructed from our patient-reported GA to predict survival, functional decline, and treatment-related toxicities among older adults with GI malignancies.
Materials and Methods
Study Population:
We used participants enrolled in the University of Alabama at Birmingham (UAB) Cancer and Aging Resilience Evaluation (CARE) study – a prospective registry enrolling older adults (≥60y) undergoing cancer treatment at UAB Hospitals and Clinics.12,13 We chose 60y of age as the criterion for enrollment in this registry given recognition of the uncertainty of the appropriate chronologic age cutoff and given the high prevalence of GA impairments and frailty among 60–65 year-olds.14 For the current report, we included patients completing GA at the time of initial consultation to the UAB medical oncology clinic between 9/2017 and 10/2019. The Institutional Review Board of UAB (IRB-300000092) approved the study protocol prior to the conduct of this study and all participants gave written informed consent. The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines, the principles originating from the Declaration of Helsinki, and institutional regulations.
Baseline Geriatric Assessment:
All participants enrolled in the CARE registry undergo a baseline patient-reported GA as previously described.12 Briefly, this GA includes evaluation of multiple domains including functional status, nutrition, cognition, mental health status, social support, comorbidity/polypharmacy and health-related quality of life, consistent with recommendations of the International Society of Geriatric Oncology and National Comprehensive Cancer Network guidelines.10,15 The CARE GA is completely patient-reported and was developed to overcome barriers to implementing the GA in routine oncology clinics.16 The specific instruments to evaluate each domain are described in detail in Table S6 in the Supplement.
CARE Frailty Index (CARE-FI):
We constructed a frailty index using the principle of deficit accumulation as described by Rockwood et al5, and following the standard procedures outlined by Searle et al.11 Each patient was scored based on 44 deficit items using data from the CARE survey (Appendix S1, Supplement), and CARE-FI was calculated as the proportion of deficits for each patient (range 0–1). We categorized patients as robust (0–0.2), pre-frail (0.2–0.35) and frail (>0.35), as previously described.11 We required participants to have non-missing data for at least 30 items to compute a valid frailty score. An index constructed with ≥30 items has been previously shown to predict adverse outcomes and survival among older adults.17–19
Study Outcomes:
The primary outcome of interest included overall survival. Secondary outcomes of interest included severe (grade ≥3) chemotherapy-associated hematologic and non-hematologic toxicities and functional decline, defined below.
Primary Study Outcome
Overall Survival (OS):
We obtained information regarding vital status by linking the study cohort to Accurint database20, which uses death information from Social Security Administration records, obituaries and state death records; we supplemented mortality data with manual review of UAB Electronic Health Records. The date of GA evaluation was defined as the index date for survival time, and information on vital status was updated until 12/1/2020.
Secondary Outcomes
Functional Decline:
Of all patients enrolled in the CARE registry, those who continued to receive systemic therapy at UAB were invited to participate in a follow-up survey at 90 ±15 days from the baseline assessment. The choice of 3 months was largely based on evidence that functional decline occurs early among older adults receiving systemic anti-cancer therapy and to ensure consistency with other studies that have used this time point.21–23 The follow-up survey included a repeat GA including an assessment of activities of daily living (ADL) and instrumental activities of daily living (IADL) captured and scored using the Older Americans Resources and Services Program (OARS) questionnaire. Using these data, we defined functional decline as a one-point decline (or increase in dependence) in ADL or IADL score from baseline to follow up surveys consistent with prior literature.24
Grade ≥3 Hematologic and non-Hematologic Toxicity:
For a subset of patients meeting the above criteria and receiving systemic cytotoxic chemotherapy at UAB, we extracted information on incident grade ≥3 toxicity by retrospectively reviewing electronic health records. Each patient was followed for toxicity evaluation until 6 months from treatment initiation, treatment discontinuation or death, whichever occurred earlier. The choice of 6 month time point for toxicity evaluation was to ensure uniformity in therapy duration between those patients receiving adjuvant systemic therapy for early stage disease (usually 6 months) and those who receiving palliative chemotherapy for advanced stage disease (usually indefinitely). Data extraction was completed by a trained physician investigator (CH) under the direct supervision of a board-certified medical oncologist (GRW) and graded using common terminology criteria for adverse events (CTCAE) version 5.0.
Additional Covariates:
We abstracted information on demographic and clinical characteristics including age at time of GA evaluation, sex, self-reported race/ethnicity, cancer type, cancer stage, and line of therapy from the electronic health records.
Statistical Analysis:
We compared baseline characteristics between the frailty categories using distribution-appropriate bivariate statistical tests, namely analysis of variance/Kruskal Wallis test for continuous variables and chi-square test/Fisher exact test for categorical variables. The median follow-up of the entire cohort was calculated using reverse Kaplan Meier Method.25 We used Kaplan Meier methods to compute survival function and log-rank test of trend to compare survival distributions across the three ordered categories (robust, pre-frail and frail). We used Cox proportional hazards regression model to measure the association between frailty categories and overall survival adjusting for potential confounders including age, sex, race/ethnicity, cancer type, cancer stage, and line of therapy. We assessed proportional hazards assumption for all covariates using a Kolmogorov-type supremum test computed on 1000 simulated patients.26 Lastly, a sensitivity analysis was conducted to study the impact of CARE-FI score (as a continuous variable in 0.1 unit increments) on survival adjusting for the above confounders. The functional form of CARE-FI score (i.e linear vs non-linear) was assessed by plotting Martingale and Deviance residuals against the absolute frailty score and testing for alternative non-linear specifications using fractional polynomials.27
With regards to our secondary outcomes, we compared the impact of frailty categories on functional decline and grade ≥3 treatment-related toxicities using logistic regression models adjusting for potential confounders. Separate models were constructed for grade ≥3 hematologic and non-hematologic toxicities. All hypothesis testing was two sided and the level of significance was set at 0.05. We used STATA, version 16 (StataCorp LLC, College Station, TX, USA) and SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) for all statistical analysis.
Results
Of the 765 consecutive patients with GI malignancies presenting for an initial consultation at UAB at age ≥60 during the study period, 631 (82%) were enrolled in the CARE registry and underwent GA. Of these, 589 had valid non-missing data for frailty evaluation and were included in the current study (FigureS1, Supplement). Participants were similar in baseline characteristics to the non-participants (Table S1, Supplement). The median time from diagnosis to baseline GA was 31 days (IQR 16–69 days). The overall median age at enrollment was 69 (IQR 64–74y); 55% were males and 73% non-Hispanic white. Common cancer types included colorectal cancer (30%) and pancreatic cancer (27%); most presented with advanced stage disease (stage III 28%; IV 46%) (Table 1).
Table 1:
Comparison of Baseline Demographic and Clinical Characteristics of the Study Population by Frailty Status
| Variable | Robust | Pre-Frail | Frail | P value |
|---|---|---|---|---|
|
| ||||
| N | 189 | 168 | 232 | |
|
| ||||
| Age, median (IQR) | 68 (64–74) | 69 (64–74) | 70 (65–75) | .37 |
|
| ||||
| Age category | .47 | |||
| - 60–65 | 66 (34.9%) | 50 (30.1%) | 62 (27%) | |
| -66–70 | 45(23.8%) | 40 (24.1%) | 55 (23.9%) | |
| - >70y | 78 (41.3%) | 76 (45.8%) | 113 (49.1%) | |
|
| ||||
| Sex | .76 | |||
| - Male | 104 (55%) | 88 (52.4%) | 130 (56%) | |
| - Female | 85 (45%) | 80 (47.6%) | 102 (44%) | |
|
| ||||
| Race | .01 | |||
| - White/Caucasian | 151 (80.3%) | 123 (74.6%) | 151 (65.7%) | |
| - Black/African-American | 32 (17%) | 38 (23%) | 76 (33.0%) | |
| - Hispanic | 15 (2.7%) | 4 (2.4%) | 3 (1.3%) | |
| - Unknown | 1 | 2 | 0 | |
|
| ||||
| Cancer Stage | .04 | |||
| - Stage I | 15 (7.9%) | 13 (7.8%) | 23 (10%) | |
| - Stage II | 32 (16.9%) | 34 (20.5%) | 38 (16.5%) | |
| - Stage III | 68 (36%) | 44 (26.5%) | 50 (21.7%) | |
| - Stage IV | 74 (39.2%) | 75 (45.2%) | 120 (52%) | |
| - Unknown | 0 | 2 | 1 | |
|
| ||||
| Cancer Stage | .04 | |||
| - Colorectal | 66 (34.9%) | 55 (32.7%) | 55 (23.7%) | |
| - Pancreatic | 45 (23.8%) | 33 (19.6%) | 78 (33.6%) | |
| - Hepatobiliary | 32 (16.9%) | 33 (19.6%) | 39 (16.8%) | |
| - Gastroesophageal | 20(10.6%) | 21 (12.5%) | 19 (8.2%) | |
| - Other GI cancersa | 26 (13.8%) | 26 (15.5%) | 41 (17.7%0 | |
|
| ||||
| Line of Therapy | .01 | |||
| - 1st line | 165 (91.7%) | 137 (87.3%) | 178 (80.9%) | |
| - 2nd line and beyond | 15 (8.3%) | 20 (12.7%) | 42 (19.1%) | |
Other GI includes Anal cancer (13), Appendiceal Cancer (7), Gastrointestinal stromal tumor (17), Neuroendocrine carcinoma (51), and GI not otherwise specified (5)
Baseline Frailty Assessment:
The median CARE-FI score in the entire cohort was 0.28 (IQR 0.16–0.44). Overall, 189 patients (32%) were characterized as robust, 168 (28%) as pre-frail and 230 (39.3%) as frail. Patients who were frail as compared to robust or pre-frail individuals were more likely to be non-Hispanic Black (33% vs 17% vs 23% respectively; P <.001), with stage IV disease (52% vs 40% vs 45% respectively; P=.05), diagnosed with pancreatic cancer (34% vs 24% vs 20% respectively; P=.04) and receiving 2nd or latter lines of therapy (34% vs 27% vs 22% respectively; P=.02) (Table 1)
Impact of CARE-FI on Overall Survival:
Over a median follow-up of 31 months (range 0.3–48.6), 256 (43%) patients had died. The median survival of the entire cohort was not reached, and the 1y and 2y overall survival rates were 70.8% (95% confidence interval [CI] 66.9–74.3%) and 58.55% (95% CI 54.3–62.5%). There were significant differences in 2y overall survival between robust, pre-frail and frail patients (67% vs 63% vs 48%; log rank test of trend P <.001) (Figure 1).
Figure 1:
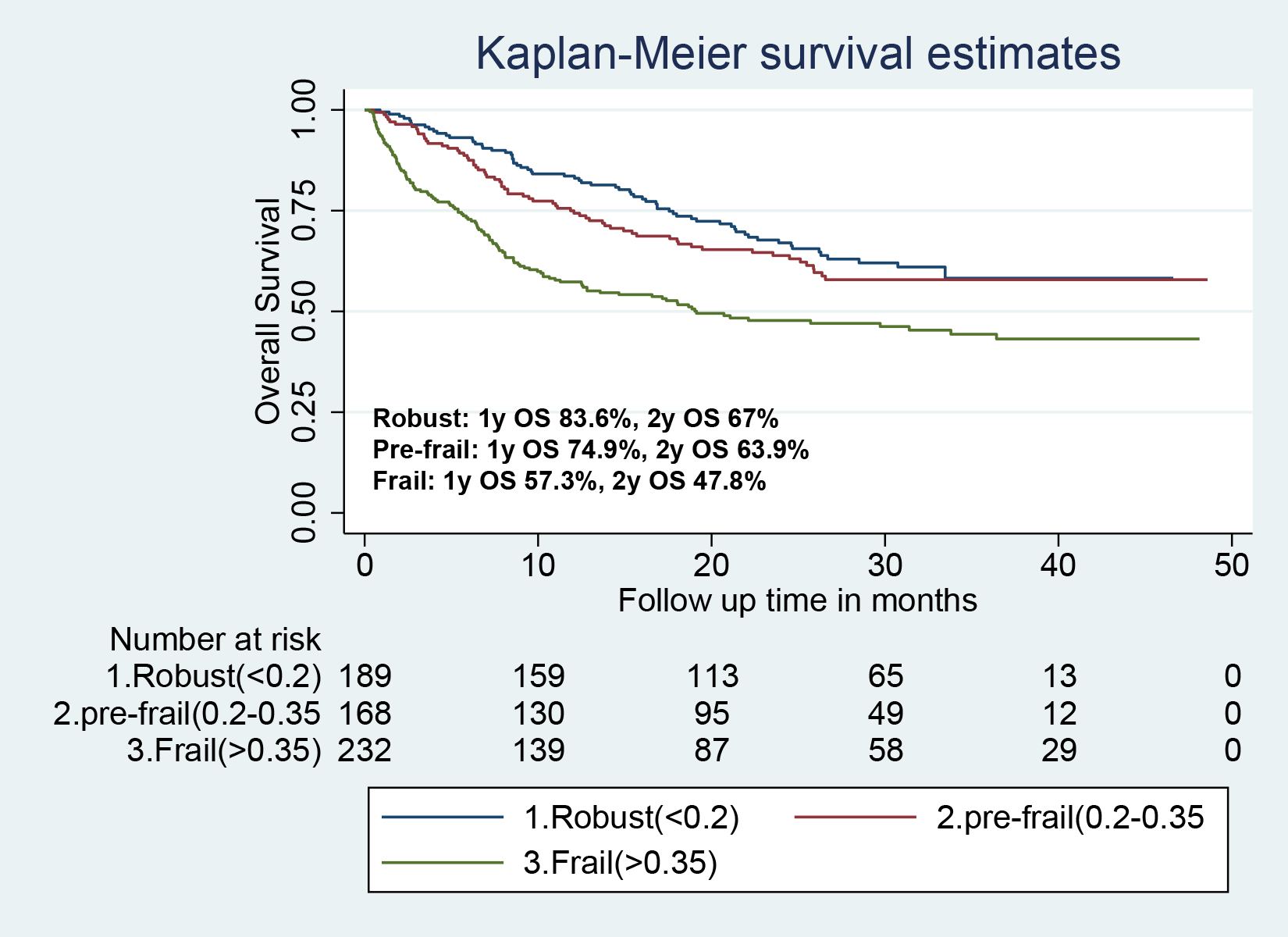
Comparison of survival distributions among patients who were robust, pre-frail or frail at baseline. The log rank test of trend was statistically significant with a p value <.001.
In a multivariable Cox regression, as compared to robust patients, frailty was associated with significantly worse OS (HR 1.83; 95% CI 1.34–2.49; P< .001) after adjusting for age, sex, race/ethnicity, cancer stage, cancer type, and line of therapy (Table 2). In a sensitivity analysis, each 0.1 unit increase in frailty score was associated with a 12% increased hazards of all-cause mortality (95% CI 1.05–1.20; P=.001) (Table S2, Supplement). An evaluation of Martingale and Deviance residual plots suggested an almost linear relationship between frailty score and survival (Table S3, Supplement), whereas alternative specifications of frailty score using first- and second-order fractional polynomials did not significantly improve the model fit (data not shown).
Table 2:
Cox Regression Model showing the association of baseline frailty status on overall survival among older adults with GI malignancies.
| Variable | Hazards Ratio | 95% CI | P value |
|---|---|---|---|
|
| |||
| Frailty Category | |||
| - Robust | Ref | - | |
| - Pre-frail | 1.16 | 0.81–1.65 | .42 |
| - Frail | 1.83 | 1.34–2.49 | <.001 |
|
| |||
| Age category | |||
| - 60–65 y | Ref | - | |
| -66–70 y | 0.85 | 0.59–1.22 | .39 |
| - >70 y | R1.06 | 0.79–1.43 | .70 |
|
| |||
| Sex | .28 | ||
| - Female | Ref | - | |
| - Male | 1.15 | 0.89–1.50 | |
|
| |||
| Race | |||
| - White/Caucasian | Ref | - | |
| - Othersa | 0.73 | 0.54–1.00 | .05 |
|
| |||
| Cancer Stage | |||
| - Stage I-II | Ref | - | |
| - Stage III-IV | 1.38 | 1.00–1.89 | .05 |
|
| |||
| Cancer Type | |||
| - Colorectal | Ref | - | |
| - Pancreatic | 2.03 | 1.45–2.86 | <.001 |
| - Other GI cancersb | 1.12 | 0.80–1.56 | .51 |
|
| |||
| Line of Therapy | |||
| - 1st line | Ref | ||
| - 2nd line and beyond | 1.36 | 0.95–1.94 | .09 |
GI, gastrointestinal; CI, confidence interval
Other race includes Black (146) and Hispanics (12).
Other GI includes Hepatobiliary (104), Gastroesophageal (60), Anal cancer (13), Appendiceal Cancer (7), Gastrointestinal stromal tumor (17), Neuroendocrine carcinoma (51), and GI not otherwise specified (5)
Impact of CARE-FI on Functional Decline:
Overall, 182 patients underwent follow-up GA evaluation including repeat functional assessment at a median of 112 days (IQR 91–147 days) from baseline evaluation. Common reasons for lack of follow up data included no systemic therapy received (24%), chose not to follow up at UAB (22%), death or hospice care prior to follow up (10%) and missed/lost to follow up (10%). Patients with or without follow up data were similar in terms of baseline demographic/clinical characteristics or frailty status with the exception of line of therapy (Table S7, Supplement). Of those with available follow up data, 38% experienced functional decline, whereas 39.8% and 22.2% had stable and improved functional status respectively. Patients who were pre-frail or frail at baseline were more likely to experience functional decline than those who were robust (43.3% vs 46.4% vs 24.2% respectively; P .02). In a logistic regression model, adjusted for age, sex, race/ethnicity, cancer type, cancer stage and line of therapy, being prefrail (OR 3.24; 95% CI 1.47–8.52; P= .005) or frail (OR 3.76; 95% CI 1.36–10.39; P= .01) was associated with an increased risk of functional decline as compared to those who were robust at baseline (Table 3)
Table 3:
Logistic Regression Model showing the association of baseline frailty status on Functional Decline among adults with GI malignancies*
| Variable | Odds Ratio | 95% CI | P value |
|---|---|---|---|
|
| |||
| Frailty Category | |||
| - Robust | Ref | - | |
| - Pre-frail | 2.99 | 1.31–6.84 | .009 |
| - Frail | 3.01 | 1.33–6.81 | .008 |
|
| |||
| Age category | |||
| - 60–65 | Ref | - | |
| -66–70 | 1.31 | 0.54–3.16 | .54 |
| - >70y | 0.98 | 0.46–2.11 | .96 |
|
| |||
| Sex | .70 | ||
| - Female | Ref | - | |
| - Male | 1.14 | 0.59–2.22 | |
|
| |||
| Race | |||
| - White/Caucasian | Ref | - | |
| - Othersa | 1.01 | 0.48–2.13 | .98 |
|
| |||
| Cancer Stage | - | ||
| - I/II | Ref | - | |
| - III/IV | 0.69 | 0.33–1.46 | .34 |
|
| |||
| Cancer Type | |||
| - Colorectal | Ref | - | |
| - Pancreatic | 1.65 | 0.67–4.04 | .27 |
| - Other GI cancersb | 1.06 | 0.48–2.34 | .88 |
|
| |||
| Line of Therapy | |||
| - 1st line | Ref | ||
| - 2nd line and beyond | 0.41 | 0.08–2.13 | .28 |
GI, gastrointestinal; CI, confidence interval
Other race includes Black (146) and Hispanics (12).
Other GI includes Hepatobiliary (104), Gastroesophageal (60), Anal cancer (13), Appendiceal Cancer (7), Gastrointestinal stromal tumor (17), Neuroendocrine carcinoma (51), and GI not otherwise specified (5)
Functional decline
Impact of CARE-FI on Grade ≥3 toxicities:
Of the 168 patients with available toxicity data, 70 patients (42%) experienced of grade ≥3 hematologic toxicity and 45 patients (26.8%) experienced grade ≥3 non-hematologic toxicity during the follow up period. On bivariate analysis, those who were frail (vs robust or pre-frail) were more likely to experience non-hematologic toxicity (44% vs 19.7% vs 19.2%; P=.005) but not hematologic toxicity (42% vs 42.6% vs 40.9%; P= .98). In a logistic regression model, being frail (vs robust) was associated with a trend towards increased risk of all-cause grade ≥3 toxicities (OR 2.21; 95% CI 1.00–4.88; P=.05) after adjusting for age, sex, race/ethnicity, cancer type, cancer stage and line of therapy (Table S4, Supplement). Frailty was associated with an increased risk of grade ≥3 non-hematologic toxicity (OR 3.11; 95% CI 1.227.93; P= .02) but not grade ≥3 hematologic toxicity (OR 0.89; 95% CI 0.39–2.06; P=.37) after adjusting for relevant confounders. (Table S5–S6, Supplement).
Discussion
In this study, we describe a patient-reported GA-based frailty index that can be used to identify older adults with GI malignancies at risk of adverse outcomes including chemotherapy-related toxicities, functional decline, and excess mortality. Our study adds to the growing evidence on the use of GA to uncover aging-related vulnerability in this population to tailor treatment approaches and plan interventions.
Our study is consistent with similar observations in the recent literature showing the impact of frailty in predicting adverse outcomes among older adults with cancer. Prior to our study, Guerard et al developed a 36-item Carolina Frailty Index, developed on the principle of deficit accumulation, among 546 older adults with predominantly breast cancer. As compared to robust patients, frail patients were at a 2-fold increased risk of all-cause mortality.19 Similarly, Cohen et al showed that a 51-item deficit accumulation frailty index from a GA can be used to predict grade ≥3 toxicity, chemotherapy discontinuation and unplanned hospitalization among 500 older adults ≥65y with cancer The latter was also used by Gillmore et al in a secondary analysis of a nationwide cluster randomized trial showing that frail patients were more likely to have more and higher quality conversations about aging-related concerns with their oncologists.28 However, both frailty indices require objective assessments to be conducted by clinic staff (Blessed Orientation Memory Concentration test and Timed up and Go tests) and therefore are not fully patient-reported. Additionally, these studies included few patients with GI cancers were (8%,27% and 22% respectively) and a Frailty Index focused on GI cancers was not available.29
In contrast to the above studies, the CARE-FI is entirely based on a patient-reported GA and does not require objective assessments from clinic staff. A recent American Society of Clinical Oncology (ASCO) survey showed that less than 20% of providers routinely performed a GA in clinical care with lack of time and lack of support staff reported as the two most common barriers.16 Another study reported that only 5% of community oncology clinics have access to a geriatrician to facilitate traditional comprehensive geriatric assessments.32 Our patient reported GA was developed to specifically address these limitations and represents a practical tool for incorporation in routine oncology practice. We have previously reported on the feasibility of integrating our patient-reported GA in a GI oncology setting with a median time to completion of 10 min.12 Therefore, the CARE-FI has a potential for broader and widespread implementation into routine care, and thus warrants specific validation in oncology.
In addition to predicting treatment-related toxicities and overall survival, the CARE-FI was also associated with functional decline. To our knowledge, this is the first study to evaluate the association of a cancer specific Frailty Index with functional decline. Prior studies have shown that functional independence is of utmost importance to older adults with cancer and identification of patients at risk of functional decline can provide opportunities for treatment modification and guided interventions.33,34 Given rising interest in and evidence for cancer rehabilitation, the CARE-FI may help identify those at increased risk of functional decline and that could benefit most from targeted rehabilitation strategies.35,36
Our study has several limitations. Patients were recruited from a single site in the US within the Deep South. It is conceivable that our findings may not be readily applicable to other malignancies or populations. Furthermore, our study population was heterogeneous including a mixture of GI malignancies across different stages necessitating varying type and duration of systemic therapies. As such, our results indicate the average impact of frailty across all GI cancer types and stages, however it is possible that effect sizes may be different for each subgroup of patients. We cannot rule out the possibility of selection bias, where more fit patients and those reporting good health may be more likely to complete the CARE GA as opposed to those in poor health. It is important to note however, that 82% of all newly diagnosed gastrointestinal malignancies presenting to the GI oncology clinic were included in the CARE registry during the study time period and no differences in observable baseline characteristics was noted between those who did or did not participate in the study. Follow up data on functional status was only available on a small proportion of patients raising a possibility of selection bias between those with and without repeat assessments. However, we did not see statistically significant differences between the two groups by demographic or clinical characteristics and frailty status, with the exception of line of therapy. Our measurement of treatment related toxicity was based on review of clinical data captured in electronic health data during routine cancer therapy with no pre-defined standard follow up time points. It is possible that future integration of electronic or remote patient reported outcome assessments could improve mitigate this loss of follow up information and more accurately capture symptom burden among patients. We did not have complete data on clinician reported performance status and the incremental value of CARE-FI beyond clinical reported performance status could not be assessed. We had limited clinical information regarding prognostic variables such as the type and intensity of chemotherapy, detailed prognostic data such as tumor grade, lymph node involvement, and tumor markers that may adversely affect survival in our cohort. Our follow-up time was relatively short and more mature survival data are needed. Lastly, we did not compare the predictive performance of CARE-FI with other established frailty indices utilizing clinical assessments, It is possible that the frailty classification may vary between a purely patient-reported FI vs partially or fully clinician assessed FI, as patients tend to over-estimate their cognitive or functional capacity,37,38 potentially leading to an under-estimation of frailty using patient-reported frailty indices which may impact their downstream clinical care. Future studies are needed to compare the predictive performance and degree of misclassification between patient reported and objectively assessed frailty indices.
In summary, our study shows that the CARE-FI constructed from a completely patient-reported GA can be used to identify older adults with GI malignancies at increased risk of severe toxicities, functional decline, and worse all-cause mortality. Future work is needed to independently validate our findings in a geographically diverse cohorts of older adults with cancer and explore how these results can improve cancer treatment decision-making.
Supplementary Material
1. Figure S1: Flow Chart showing Cohort selection
2. Table S1: Comparison of baseline demographic and clinical characteristics between participants who underwent GA assessment versus those who did not
3. Table S2: Cox Regression Model showing the impact of CARE Frailty Index as a continuous variable on overall survival
4. Figure S2: Relationship between absolute CARE Frailty Index scores and Martingale/Deviance Residuals from Cox regression model
5. Table S3 Logistic Regression Model showing the association of baseline frailty status on grade ≥3 chemotherapy related toxicity
6. Table S4: Logistic Regression Model showing the association of baseline frailty status on grade ≥3 non hematologic toxicity
7. Table S5: Logistic Regression Model showing the association of baseline frailty status on grade ≥3 hematologic toxicity
8. Appendix S1: Construction of CARE Frailty Index
9. Table S6: Overview of Geriatric Assessment Domains in the CARE registry
10. Table S7: Comparison of baseline demographic/clinical characteristics as well as frailty status between those with and without 3 month functional status assessment
Key Points:
Despite emerging evidence on the utility of geriatric assessment in the evaluation of older adults with cancer, routine implementation in busy oncology practices remain challenging.
We have previously developed a patient-reported Geriatric Assessment (GA) tool that has been successfully incorporated medical oncology clinic at our institution.
Here, we show that a deficit-accumulation Frailty Index based on our patient reported GA is strongly associated with adverse outcomes relevant to older adults with cancer including treatment related toxicities, functional decline and overall survival.
Why does this paper matter?
Frailty Index derived from our patient-reported GA tool can provide clinically meaningful information for prognostication as well as selection of candidates for treatment modifications and focused interventions.
Funding:
Supported in part by the Walter B. Frommeyer Fellowship in Investigative Medicine at the University of Alabama at Birmingham and the National Cancer Institute of the National Institutes of Health (K08CA234225). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Sponsor’s Role: The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Conflicts of Interest: Smith Giri: Honoraria: CareVive, OncLive; Research Funding: Carevive Systems, Pack Health, Sanofi. Olumide Gbolahan: Consulting or Advisory Role: Exelixis, QED therapeutics, AstraZeneca Speakers Bureau: MJH Life Sciences Research Funding: AstraZeneca. Mohd Khushman: Consulting or Advisory Role: Taiho, Bayer; Speakers’ Bureau: Pfizer, AstraZeneca, MJH life sciences; Research Funding: AstraZeneca, BMS, Erasca, Arcus; Shareholder: Moderna, Regeneron, Cardiff oncology and Blueprint Medicine. None of the other authors have anything relevant to disclose.
Presented in an abstract form at the American Society of Clinical Oncology 2021 Annual Meeting.
Data Availability Statement:
The data underlying this article cannot be shared publicly to protect the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
References:
- 1.National Cancer Institute Surveillance Epidemiology and End Results Program. Cancer Stat Facts: Cancer of Any Site. https://seer.cancer.gov/statfacts/html/all.html. Published 2021. Accessed May 18, 2021.
- 2.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology Series A, Biological sciences and medical sciences. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–263. [DOI] [PubMed] [Google Scholar]
- 5.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. The journals of gerontology Series A, Biological sciences and medical sciences. 2007;62(7):722–727. [DOI] [PubMed] [Google Scholar]
- 6.Ethun CG, Bilen MA, Jani AB, Maithel SK, Ogan K, Master VA. Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA: a cancer journal for clinicians. 2017;67(5):362–377. [DOI] [PubMed] [Google Scholar]
- 7.Hurria A, Mohile S, Gajra A, et al. Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(20):2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li D, Sun CL, Kim H, et al. Geriatric Assessment-Driven Intervention (GAIN) on Chemotherapy-Related Toxic Effects in Older Adults With Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohile SG, Mohamed MR, Xu H, et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. Lancet. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC geriatrics. 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams GR, Kenzik KM, Parman M, et al. Integrating geriatric assessment into routine gastrointestinal (GI) consultation: The Cancer and Aging Resilience Evaluation (CARE). J Geriatr Oncol. 2020;11(2):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mir N, MacLennan P, Al-Obaidi M, et al. Patient-reported cognitive complaints in older adults with gastrointestinal malignancies at diagnosis- Results from the Cancer & Aging Resilience Evaluation (CARE) study. Journal of geriatric oncology. 2020;11(6):982–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giri S, Al-Obaidi M, Weaver A, et al. Association Between Chronologic Age and Geriatric Assessment-Identified Impairments: Findings From the CARE Registry. Journal of the National Comprehensive Cancer Network : JNCCN. 2021:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dotan E, Walter LC, Browner IS, et al. NCCN Guidelines(R) Insights: Older Adult Oncology, Version 1.2021. Journal of the National Comprehensive Cancer Network : JNCCN. 2021;19(9):1006–1019. [DOI] [PubMed] [Google Scholar]
- 16.Dale W, Williams GR, A RM, et al. How Is Geriatric Assessment Used in Clinical Practice for Older Adults With Cancer? A Survey of Cancer Providers by the American Society of Clinical Oncology. JCO Oncol Pract. 2021;17(6):336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53(12):2184–2189. [DOI] [PubMed] [Google Scholar]
- 18.Cohen HJ, Smith D, Sun CL, et al. Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer. 2016;122(24):3865–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerard EJ, Deal AM, Chang Y, et al. Frailty Index Developed From a Cancer-Specific Geriatric Assessment and the Association With Mortality Among Older Adults With Cancer. J Natl Compr Canc Netw. 2017;15(7):894–902. [DOI] [PubMed] [Google Scholar]
- 20.Accurint L http://www.accurint.com. Accessed October 30, 2019.
- 21.Alibhai SM, Breunis H, Timilshina N, et al. Impact of androgen-deprivation therapy on physical function and quality of life in men with nonmetastatic prostate cancer. J Clin Oncol. 2010;28(34):5038–5045. [DOI] [PubMed] [Google Scholar]
- 22.Kenis C, Decoster L, Bastin J, et al. Functional decline in older patients with cancer receiving chemotherapy: A multicenter prospective study. J Geriatr Oncol. 2017;8(3):196–205. [DOI] [PubMed] [Google Scholar]
- 23.Puts MTE, Monette J, Girre V, et al. Changes in functional status in older newly-diagnosed cancer patients during cancer treatment: A six-month follow-up period. Results of a prospective pilot study. Journal of Geriatric Oncology. 2011;2(2):112–120. [Google Scholar]
- 24.Owusu C, Margevicius S, Schluchter M, Koroukian SM, Schmitz KH, Berger NA. Vulnerable elders survey and socioeconomic status predict functional decline and death among older women with newly diagnosed nonmetastatic breast cancer. Cancer. 2016;122(16):2579–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–346. [DOI] [PubMed] [Google Scholar]
- 26.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80(3):557–572. [Google Scholar]
- 27.Royston P Model selection for univariable fractional polynomials. Stata J. 2017;17(3):619–629. [PMC free article] [PubMed] [Google Scholar]
- 28.Gilmore N, Xu H, Kehoe L, et al. Evaluating the association of frailty with communication about aging-related concerns between older patients with advanced cancer and their oncologists. Cancer. 2022;128(5):1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen HJ, Smith D, Sun C-L, et al. Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer. 2016;122(24):3865–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams GR, Deal AM, Sanoff HK, et al. Frailty and health-related quality of life in older women with breast cancer. Support Care Cancer. 2019;27(7):2693–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams GR, Dunham L, Chang Y, et al. Geriatric Assessment Predicts Hospitalization Frequency and Long-Term Care Use in Older Adult Cancer Survivors. Journal of oncology practice / American Society of Clinical Oncology. 2019:JOP1800368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams GR, Weaver KE, Lesser GJ, et al. Capacity to Provide Geriatric Specialty Care for Older Adults in Community Oncology Practices. The oncologist. 2020;25(12):1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. The New England journal of medicine. 2002;346(14):1061–1066. [DOI] [PubMed] [Google Scholar]
- 34.Soto-Perez-de-Celis E, Li D, Su CY, et al. Patient-defined goals and preferences among older adults with cancer starting chemotherapy J Clin Oncol 36, 2018. (suppl 15; abstr 10009). 2018. [Google Scholar]
- 35.Pergolotti M, Deal AM, Williams GR, et al. Older Adults with Cancer: A Randomized Controlled Trial of Occupational and Physical Therapy. Journal of the American Geriatrics Society. 2019;67(5):953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pergolotti M, Lyons KD, Williams GR. Moving beyond symptom management towards cancer rehabilitation for older adults: Answering the 5W’s. Journal of geriatric oncology. 2018;9(6):543–549. [DOI] [PubMed] [Google Scholar]
- 37.Spitzer S, Weber D. Reporting biases in self-assessed physical and cognitive health status of older Europeans. PLoS One. 2019;14(10):e0223526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ando M, Ando Y, Hasegawa Y, et al. Prognostic value of performance status assessed by patients themselves, nurses, and oncologists in advanced non-small cell lung cancer. British journal of cancer. 2001;85(11):1634–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1. Figure S1: Flow Chart showing Cohort selection
2. Table S1: Comparison of baseline demographic and clinical characteristics between participants who underwent GA assessment versus those who did not
3. Table S2: Cox Regression Model showing the impact of CARE Frailty Index as a continuous variable on overall survival
4. Figure S2: Relationship between absolute CARE Frailty Index scores and Martingale/Deviance Residuals from Cox regression model
5. Table S3 Logistic Regression Model showing the association of baseline frailty status on grade ≥3 chemotherapy related toxicity
6. Table S4: Logistic Regression Model showing the association of baseline frailty status on grade ≥3 non hematologic toxicity
7. Table S5: Logistic Regression Model showing the association of baseline frailty status on grade ≥3 hematologic toxicity
8. Appendix S1: Construction of CARE Frailty Index
9. Table S6: Overview of Geriatric Assessment Domains in the CARE registry
10. Table S7: Comparison of baseline demographic/clinical characteristics as well as frailty status between those with and without 3 month functional status assessment
Data Availability Statement
The data underlying this article cannot be shared publicly to protect the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.


