Abstract
Background:
Critical illness often leads to persistent functional impairment among older ICU survivors. Identification of high-risk survivors prior to discharge from their ICU hospitalization can facilitate targeting for restorative interventions after discharge, potentially improving the likelihood of functional recovery. Our objective was to develop and validate a prediction model for persistent functional impairment among older adults in the year after an ICU hospitalization.
Methods:
The analytic sample included community-living participants enrolled in the National Health and Aging Trends Study 2011 cohort who survived an ICU hospitalization through December 2017 and had a follow-up interview within 1 year. Persistent functional impairment was defined as failure to recover to the pre-ICU level of function within 12 months of discharge from an ICU hospitalization. We used Bayesian model averaging to identify the final predictors from a comprehensive set of 17 factors. Discrimination and calibration were assessed using area-under-the-curve (AUC) and calibration plots.
Results:
The development cohort included 456 ICU admissions (2,654,685 survey-weighted admissions) and the validation cohort included 227 ICU admissions (1,350,082 survey-weighted admissions). In the development cohort, the median age was 81.0 years (interquartile range [IQR] 76.0, 86.0) and 231 (50.7%) participants were women; demographic characteristics were comparable in the validation cohort. The rates of persistent functional impairment were 49.3% (development) and 50.2% (validation). The final model included age, pre-ICU disability, probable dementia, frailty, prior hospitalizations, vision impairment, depressive symptoms, and hospital length of stay. The model demonstrated good discrimination (AUC 71%, 95% confidence interval [CI] 0.66–0.76) and good calibration. When applied to the validation cohort, the model demonstrated comparable discrimination (AUC 72%, 95% CI 0.66–0.78) and good calibration.
Conclusions:
Application of the model prior to discharge from an ICU hospitalization may identify older adults at highest risk of persistent functional impairment in the subsequent year, thereby facilitating targeted interventions and follow-up.
INTRODUCTION
For the millions of patients who survive a critical illness every year, persistent impairment in physical function, cognitive function, and/or mental health is common; impairment in one or more of these domains is referred to as the “post-ICU syndrome (PICS).”1 Identifying risk factors for impairment in each of these domains has been an area of intense study over the past decade; yet, we are still unable to predict which patients will sustain impairments from which they do not recover.2 Ideally, the patients at highest risk of long-term impairments after critical illness would be identified early in the course of their recovery, so that they could be targeted for restorative interventions, close follow-up, and novel programs such as post-ICU clinics. In recognition of this urgent need, an international consensus conference was convened on the prediction and identification of long-term impairments after critical illness.3 Informed by a systematic review, the group concluded that existing tools were insufficient to predict long-term impairments in the PICS domains, and that the development and validation of prediction models should be a focus of future research.2
The need to identify patients at highest risk of persistent post-ICU functional impairment is particularly pressing among older adults aged 65 and older. In 2015, approximately 1.9 million older adults were admitted to ICUs in the U.S.4 This number is only expected to increase as the population ages; the U.S. population of older adults will nearly double between 2018 (52 million) and 2060 (95 million).5 Older adults are more likely to present with preexisting factors, such as frailty,6 cognitive impairment,7 and/or multimorbidity,8 that confer increased risk for adverse outcomes. One of these outcomes, persistent functional impairment (i.e. persistently increased disability in functional activities), is a pervasive problem among older ICU survivors.9–11 The consequences of functional impairment and disability are well-established and include increased mortality,12 nursing home placement, and greater use of home services,13 placing increased burdens on patients, caregivers, and society.
In light of the well-established associations between preexisting factors and post-ICU functional outcomes,6, 7, 11, 14 we sought to determine whether data about these factors and the ICU hospitalization could together be leveraged to predict which older ICU survivors are at greatest risk of persistent functional impairment in the year after discharge from an ICU hospitalization. By applying such a model prior to hospital discharge, providers could target these patients for close follow-up and interventions to improve their chance of functional recovery over the subsequent months. Our objective was to develop and validate a model to predict the risk of persistent functional impairment in the year after discharge from an ICU hospitalization among older ICU survivors.
METHODS
Our manuscript follows the guidelines outlined in the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD) statement (Supplemental Checklist S1).15
Source of data and participants
The National Health and Aging Trends Study (NHATS) is a nationally representative study of Medicare beneficiaries age ≥65 who completed annual, in-person interviews beginning in 2011.16, 17 The study drew a sample of older adults from the Medicare enrollment file living in the contiguous United States on September 30, 2010, with oversampling by age and for Black, non-Hispanic persons. Analytic weights in NHATS adjust for differential nonresponse and produce national prevalence estimates.18 Of the initial cohort in 2011 (n=8245), the response rate was 70.9%. The Johns Hopkins Institutional Review Board approved the protocol, and all participants provided informed consent. The Yale Institutional Review Board approved the protocol for secondary analysis of the data (IRB protocol #1607018022).
NHATS personnel administer annual interviews in the participant’s home. When the participant cannot respond, a proxy who is familiar with the participant’s routine (usually a family member) is interviewed. When a study participant is confirmed as deceased, NHATS personnel complete a last month of life interview with a proxy.16
Outcome
During the annual NHATS interviews, detailed data are collected on 7 functional activities: dressing, getting cleaned up, toileting, eating, getting out of bed, getting around inside, and going outside.17 For participants who died during the follow-up period, the same functional data were collected during the standardized last month of life interview. For each activity, functional disability was operationalized as requiring assistance from another person or unable to perform the activity. The functional measure comprised these 7 activities (range, 0–7). In cases where the functional measure was partially missing, we used multiple imputation (Supplemental Methods S1 and Supplemental Table S1).19
The outcome of persistent post-ICU functional impairment was defined as failure to return to the pre-ICU functional status within 12 months of discharge from the ICU hospitalization. If the participant did not complete the follow-up NHATS interview due to being too sick (and without a proxy), physically or mentally unable to participate, or newly in a nursing home (where a facilities questionnaire is completed, but no individual disability data are gathered), they were assigned the outcome. In the current analysis, 35 observations met these criteria (n=16, n=3, and n=16, respectively).
Identification of ICU admissions and variables
ICU admissions from 2011–2017 were identified through critical care revenue codes using linked fee-for-service Medicare claims data. We included codes for general, specialty, and coronary care units, while excluding psychiatric or intermediate critical care.20 To identify the need for mechanical ventilation, we included all observations with ICD-9 code 96.7x and ICD-10 codes 5A1935Z, 5A1945Z, and 5A1955Z.21
Predictors
A comprehensive list of potential predictors was chosen based on the literature.11, 14, 22, 23 These variables included age, sex, race and ethnicity, education, Medicaid status, body-mass index, depressive symptoms,24 Fried frailty,25 hearing impairment, vision impairment, social isolation,26 chronic conditions, any pre-ICU disability, probable dementia, number of hospitalizations in 6 months prior to ICU admission, mechanical ventilation, and hospital length of stay. Operational details are provided in Supplemental Table S3 and in the footnotes of Table 1. Data were gathered about each of the potential predictors during the annual interviews and from Medicare claims data.
Table 1.
Characteristics of Older ICU Survivors in the Development and Validation Cohorts
| Characteristica | Development cohort | Validation cohort |
|---|---|---|
| Number of observationsb | 456 | 227 |
| Weighted n | 2,654,685 | 1,350,082 |
| median (Q1, Q3) or n(%) | ||
| Age (years) | 81.0 (76.0, 86.0) | 81.0 (75.0, 87.0) |
| Female sex | 231 (50.7) | 118 (52.0) |
| Non-Hispanic white race/ethnicity | 322 (71.7) | 156 (68.7) |
| Medicaidc | 92 (20.2) | 47 (20.7) |
| Less than a high school education | 130 (29.0) | 68 (30.0) |
| Body-mass index (kg/m2) | 26.6 (23.4, 30.3) | 26.6 (23.5, 30.4) |
| Fried frailty (range, 0–5)d | 2.0 (1.0, 3.0) | 1.0 (1.0, 3.0) |
| Chronic conditions (range, 0–9)e | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) |
| Probable dementiaf | 70 (15.4) | 31 (13.7) |
| Disabled in any ADL or mobility activity (of 7 activities) | 160 (35.2) | 68 (30.0) |
| Social isolationg | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) |
| Hearing impairmenth | 150 (33.2) | 64 (28.3) |
| Vision impairmenti | 47 (10.5) | 29 (13.0) |
| Depressive symptoms (range, 0–6)j | 1.0 (0.0, 2.0) | 1.0 (0.0, 2.0) |
| Hospitalizations within the 6 months prior to admission | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) |
| None | 306 (67.1) | 157 (69.2) |
| 1 | 96 (21.1) | 48 (21.1) |
| 2 or more | 54 (11.8) | 22 (9.7) |
| Hospital length of stay (days) | 6.0 (3.0, 9.0) | 6.0 (3.0, 9.0) |
| Mechanical ventilationk | 45 (9.9) | 18 (7.9) |
| Discharge destination | ||
| Home | 269 (59.0) | 140 (61.7) |
| Skilled nursing facility (SNF) | 132 (28.9) | 56 (24.7) |
| Other | 55 (12.1) | 31 (13.7) |
Abbreviations: ICU, intensive care unit; Q1, first quartile, Q3, third quartile; ADL, activities of daily living
Participant characteristics presented in the Table are unweighted. There was minimal missingness in the NHATS variables, as follows: in the development cohort (out of 456), race 7, education 7, frailty 18 with partial missingness in the 5-item measure (7 with 3 missing, 6 with 2 missing, 5 with 1 missing), depression 8, hearing 4, vision 8, BMI 14, social isolation 1, pre-ICU disability 1; in the validation cohort (out of 227), frailty 7 with partial missingness in the 5-item measure (4 with 3 missing, 2 with 2 missing, 1 with 1 missing), depression 5, hearing 1, vision 3, BMI 6.
The 683 ICU admissions were contributed by 576 participants; 486 participants contributed 1 admission, 75 participants contributed 2 admissions, 13 participants contributed 3 admissions, and 2 participants contributed 4 admissions.
Dual status indicator from Medicare administrative data, at any time during the previous 12 months of the ICU admission.
The 5 Fried frailty criteria are weight loss, exhaustion, muscle weakness, slow gait speed, and low physical activity
Of a possible 9: hypertension, myocardial infarction, heart disease, stroke, diabetes mellitus, arthritis, osteoporosis, chronic lung disease, and cancer (other than minor skin cancers)
Probable dementia was defined as an existing diagnosis of dementia or Alzheimer’s disease, a score ≥2 on the Eight-item Informant Interview to Differentiate Aging and Dementia (AD8),37 or a score ≤1.5 standard deviations below the mean in ≥2 of 4 cognitive domains (memory, orientation, executive function, and retrieval of information).41
Defined as a score of ≥4 (range, 0–6) on a previously validated measure of social isolation42
Defined as participants not hearing well enough to carry on a conversation in a quiet room or with a radio/television playing, or using a hearing aid (or deaf).
Defined as participants (with use of corrective lenses, when applicable) having difficulty reading newspaper print, recognizing a person across the street, or seeing a television across the room.43
According to the score on the Patient Health Questionnaire-2.
Assembly of the analytic sample
Assembly of the analytic sample is outlined in Figure 1. We selected the first ICU admission per annual interval (n=1,500), excluding those who were missing all 7 items from their pre-ICU functional assessment (n=404), cases where participants were admitted from a nursing home (NH), in a NH at the time of the annual interview, or who had spent >100 days in NH care over the prior year (n=99), and those with maximum pre-ICU disability (n=33) to eliminate floor effects. As this study is focused on the functional recovery of ICU survivors, observations in which the participant died in the hospital (n=97) or was discharged to hospice (n=53) were not eligible for the analysis. Interviews obtained >1 year after discharge from the index ICU hospitalization (n=71) were excluded, as were observations missing the follow-up interview (n=60, most commonly due to participant refusal [n=37] or proxy refusal [n=10]). The analytic sample included 683 ICU admissions from 576 participants. After survey-weighting, the 683 observations represented 4,004,767 ICU admissions among the 2011 Medicare beneficiary cohort through the end of 2017, with follow-up interviews through the end of 2018.
Figure 1. Assembly of the development and validation cohorts from the 2011 NHATS cohort.
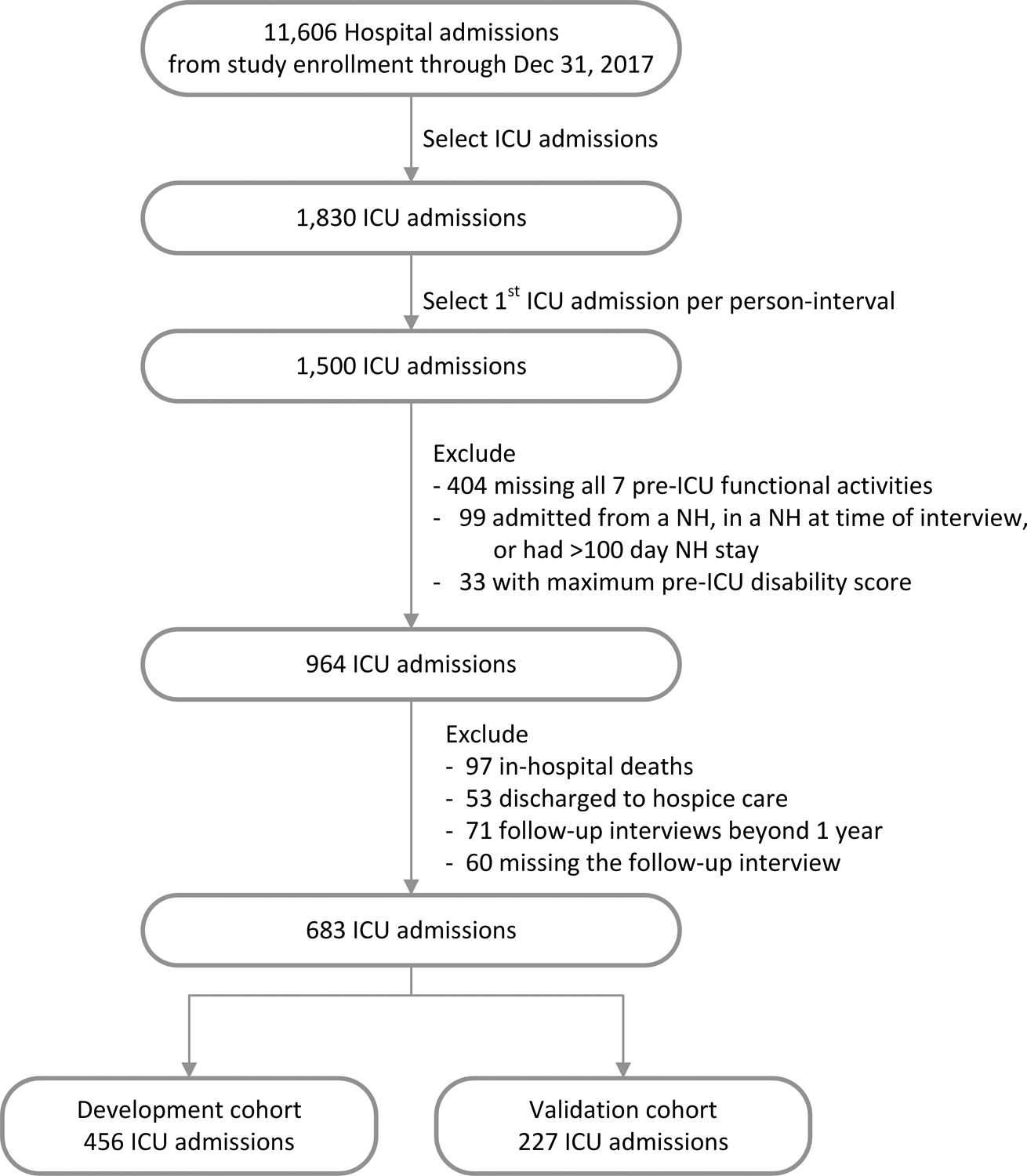
From the 2011 NHATS longitudinal cohort, fee-for-service ICU admissions from study enrollment through the end of 2017 were identified. The first ICU admission per interview interval was selected and exclusions applied as outlined in the methods. Of the 60 observations missing the follow-up interview, the reasons for missingness were participant refusal (n=37), proxy refusal (n=10), unable to locate (n=6), outside of primary sampling unit (PSU; n=2), deceased and without a proxy (n=2), language barrier (n=1), ineligible (n=1), and facility refusal (n=1). The analytic sample included 683 ICU admissions, representing 4,004,767 survey-weighted ICU admissions. This sample was divided into a development cohort (2/3 of the cohort, n=456, representing 2,654,685 survey-weighted ICU admissions) and a validation cohort (1/3 of the cohort, n=227, representing 1,350,082 survey-weighted ICU admissions).
Abbreviations: NHATS, National Health and Aging Trends Study; ICU; intensive care unit
Statistical analysis
The sample was divided into a development cohort (2/3 of the sample, n=456 ICU admissions from 399 participants, representing 2,654,685 survey-weighted ICU admissions) and a validation cohort (1/3 of the sample, n=227 ICU admissions from 212 participants, representing 1,350,082 survey-weighted ICU admissions). For each cohort, we generated descriptive statistics for the outcome. The small amount of missing predictor data (<5%, detailed in Supplemental Table S2 and the footnotes of Table 1) was multiply imputed with a fully conditional specification under an assumption of missing-at-random. In the development cohort, we employed Bayesian model averaging (BMA), which considers all combinations of all variables in its calculation of a posterior effect probability for each given variable.27 We retained only those variables exhibiting an average posterior effect probability > 50% across the imputations; additional statistical detail is provided in the statistical supplement. The count and continuous predictors selected in BMA were subsequently examined for linearity and used in a multivariable model that was fit to the development cohort. To obtain nationally representative estimates, the model was fit to the development cohort using the NHATS survey design parameters. After verifying acceptable discrimination (C-statistic) and calibration (plots) in the development cohort, those coefficients were directly applied to the validation cohort, where discrimination and calibration were also evaluated. Model selection was performed using the R package ‘BMA’,28 with details presented in Supplemental Methods S2. The final model, which used NHATS survey parameters and generalized estimating equations with an exchangeable correlation structure, was fit using SAS-callable SUDAAN Release 11.29 Otherwise, analyses were conducted using SAS Version 9.4 with SAS/STAT 14.3. Cary, NC: SAS Institute Inc; 2014.19
Integrated predictiveness curve
To help clinicians advise patients about meaningful levels of risk for the outcome, we created an integrated predictiveness curve based on the predicted probabilities in the development cohort.30 The curve specifies the observed rate of the outcome and three levels of risk: an “intervention” level that triggers an intervention to decrease the patient’s risk of the outcome, a “warning” level that triggers a discussion about the patient’s risk of the outcome, and “usual care.” Clinical judgment and deciles of risk were used to choose cutoffs between the three categories. The average rate of the outcome in the development cohort (49.3%) was chosen as the cutoff between usual care and the “warning” range, and the 7th decile of risk (corresponding to a 65% predicted risk of the outcome) was chosen as the trigger for an intervention.
RESULTS
Participants in the development and validation cohorts
Descriptive characteristics of the development and validation cohorts are presented in Table 1. The two cohorts were similar with regard to key demographic, geriatric, and hospital variables.
Model development and specification
The primary outcome of persistent post-ICU functional impairment occurred in 225 (49.3%) observations in the development cohort and 114 (50.2%) observations in the validation cohort. The Bayesian model averaging (BMA) procedure selected eight predictors: age, pre-ICU disability, probable dementia, Fried frailty count, number of hospitalizations in the 6 months prior to ICU admission, vision impairment, depressive symptoms, and hospital length of stay. Full regression results for the risk prediction model are presented in Supplemental Table S4.
Model performance
Model performance was good, with an area under the curve (AUC) of 71% (95% CI 0.66–0.76) and consistently good calibration as demonstrated in the calibration plots (Figure 2, Panel A). When the model was directly applied to the validation cohort, discrimination remained good (AUC = 0.72, 95% CI 0.66–0.78), as did calibration (Figure 2, Panel B).
Figure 2. Calibration plot of the model in the development and validation cohorts.
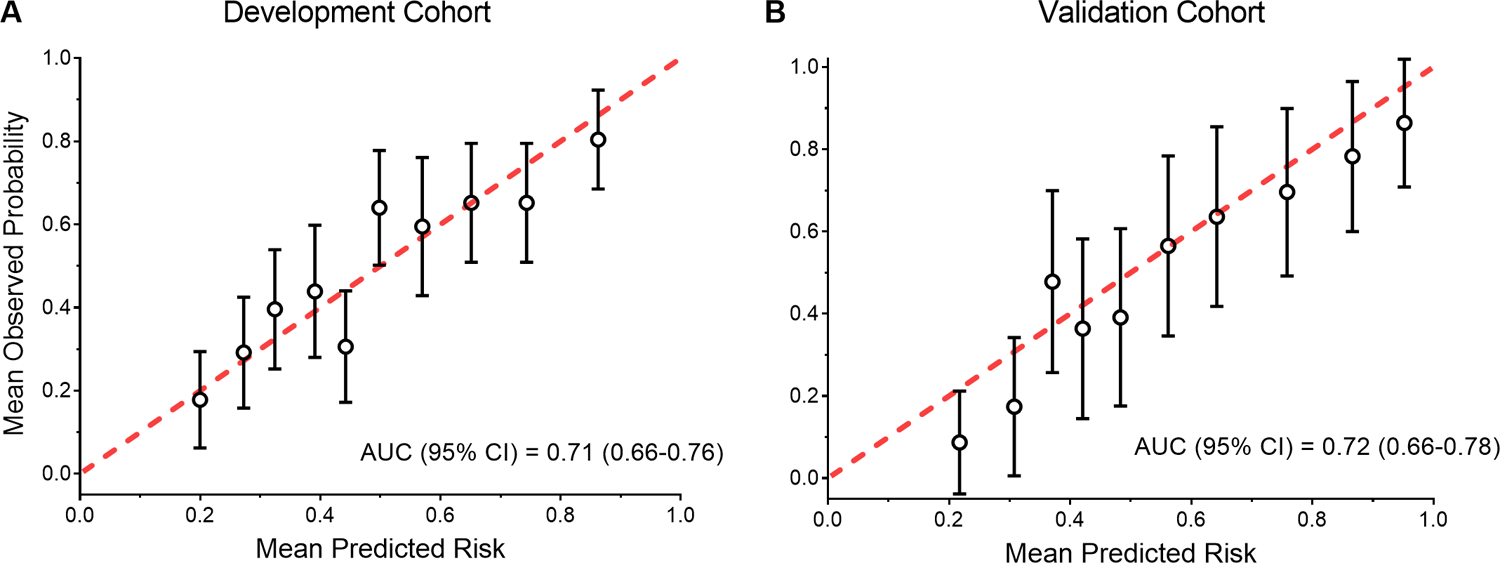
Panel A: Calibration plot in the development cohort (N=456).
Panel B: Calibration plot in the validation cohort (N=227).
For each cohort, the observed and predicted probabilities of the outcome were calculated for deciles of risk. The error bars represent 95% confidence intervals.
Abbreviation: AUC, area under the curve
Integrated predictiveness curve
The integrated predictiveness curve is presented in Figure 3. The observed rate (49.3%) of persistent post-ICU functional impairment in the development cohort is indicated by an arrow. The area between the observed rate of 49.3% and the intervention threshold of 65% (7th decile) was designated as a “warning” range of predicted risk. The equation for estimating the predicted probability of the outcome for a specific patient using the model is provided in Supplemental Methods S3.
Figure 3. Integrated predictiveness curve.
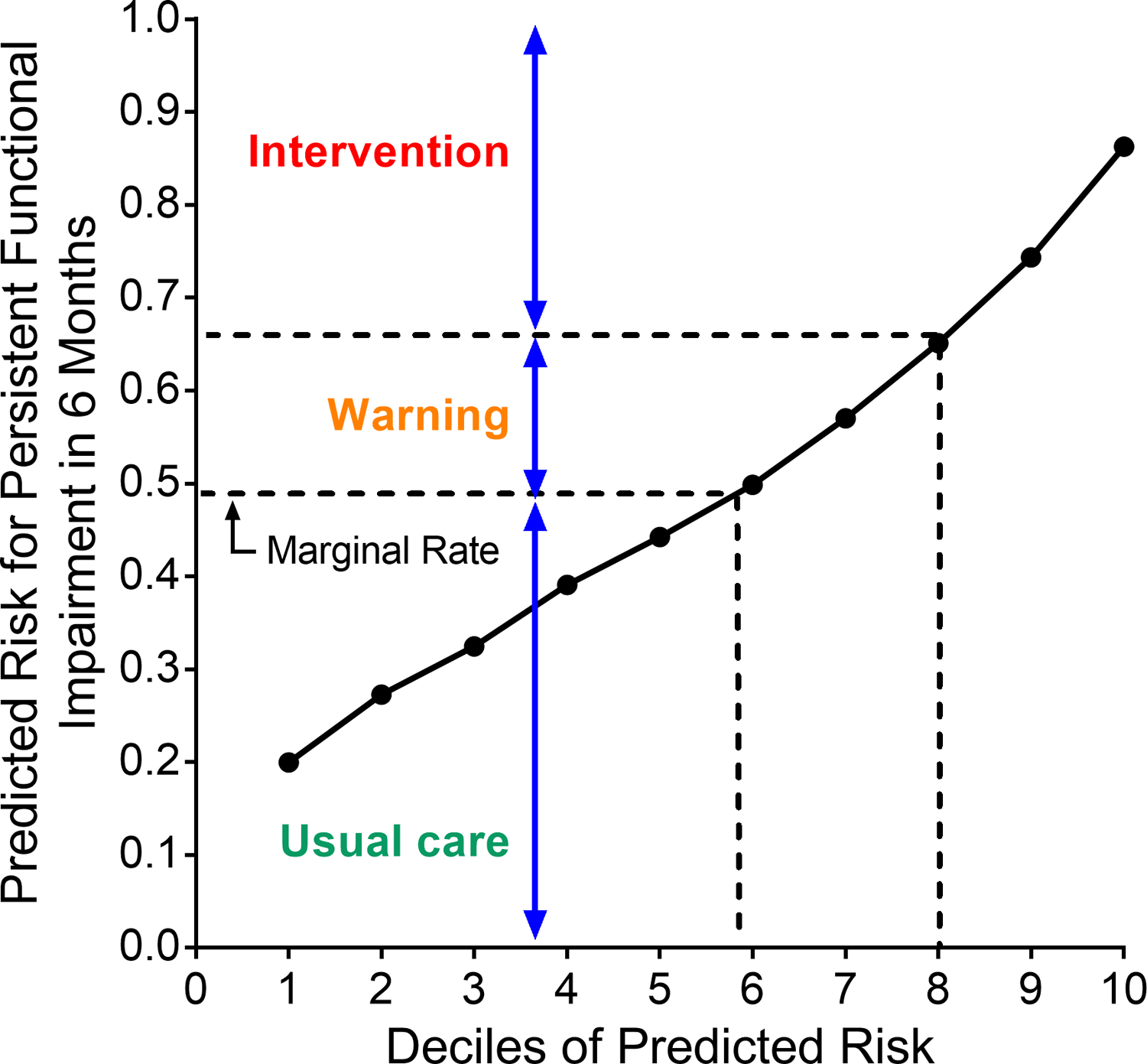
The curve is based on the predicted probabilities in the development cohort, with the marginal rate of the observed outcome (49.3%) denoted by the arrow. Using clinical judgment, the range of predicted risk between 49.3% and 65% (the 7th decile) was chosen as the “warning” range, in which the provider would warn the patient about their risk for persistent post-ICU functional impairment, while values greater than 65% predicted risk denoted the “intervention” range.
DISCUSSION
Among older adults, persistent functional impairment after an ICU hospitalization is common and may have deleterious consequences, including loss of independence, a highly valued health outcome.31 Yet, clinicians have not had tools to predict which older adults are at greatest risk of persistent functional impairment after critical illness. Our study begins to address these challenges by developing and validating a risk prediction model for persistent post-ICU functional impairment in a nationally representative population of older ICU survivors. In doing so, our work helps to fill a key knowledge gap identified by a recent international consensus conference: the need for models to predict long-term impairments after critical illness in the three domains of the post-ICU syndrome: function, cognition, and mental health..3 Our model begins to address this need for the growing population of older ICU survivors in the domain of long-term function.
To facilitate model application among ICU survivors early in the course of their recovery (i.e., shortly before discharge from the index ICU hospitalization), we considered pre-ICU factors previously associated with post-discharge outcomes as well as factors from the ICU hospitalization. The identified predictors have good face validity and can be feasibly assessed in ICU survivors prior to hospital discharge; indeed, prior research has demonstrated the feasibility of assessing Fried frailty in older ICU survivors prior to discharge from an ICU hospitalization.32 Of the other factors in the model, age, prior hospitalizations, and hospital length of stay can be drawn from the EMR, and any disability (yes/no), depressive symptoms, and vision impairment may be ascertained by a few questions. Probable dementia may be assessed in one of three ways: through the EMR (via a prior diagnosis), or by performing a brief assessment with the patient or proxy; this approach, although more extensive than the other predictors, ensures that nearly all patients can be assessed.
Our study builds on prior work demonstrating that assessments performed close to hospital discharge can help stratify ICU survivors into groups that are likely to be functionally dependent over the subsequent year33 as well as recent work in younger, mechanically ventilated patients.34 Among older ICU survivors, hospital discharge is a critical timepoint at which essential services such as rehabilitation, support services, and referral to resources such as post-ICU clinics can be arranged. Although physical and/or occupational therapy provide invaluable assessments that can inform discharge location and the need for post-hospital rehabilitation services, our prediction model builds on these assessments by targeting the patients most in need of long-term follow-up and reassessment. For high-risk patients, establishing care in a Post-ICU and/or Geriatrics clinic can help ensure long-term follow-up and functional reassessment beyond the time spent in short-term rehabilitation. Post-ICU clinics typically include assessments of physical function, cognitive function, and mental health, the ability to refer patients for subsequent rehabilitation evaluation and treatment, and emotional support services (such as support groups). The inability to target patients at high risk of post-ICU impairments for interventions such as post-ICU clinics has been identified as a barrier to improving the quality of life for ICU survivors;35 our model addresses this barrier. Moreover, prior research has shown that hospital referral for home rehabilitation after critical illness does not ensure that services are actually received by the patient.36 Identifying older ICU survivors who are most in need of long-term follow-up, functional reassessment and rehabilitation after critical illness should increase the likelihood of achieving sustained functional recovery.
To illustrate application of the model in practice, let us consider three hypothetical older adults (aged 70, 75, and 80 years) who have survived an ICU admission and are about to be discharged from the hospitalization, after a total length of stay of 1 week. The 70-year-old patient, who had one hospitalization two months ago, meets criteria for pre-frailty (due to low physical activity and slow gait speed), is dependent in 2 mobility activities at baseline, and states that he has trouble reading the newspaper (vision impairment). He does not have any depressive symptoms nor signs of dementia. Physical therapy determines that he can be discharged home with home rehabilitation services. The primary team applies the prediction model and sees that his predicted risk of persistent functional impairment over the next year is 70%, placing the patient into the “intervention” category. The team then establishes care for the patient in the local Post-ICU clinic, arranges for close follow-up with a geriatrician, ensures that a patient care navigator calls the patient weekly until he has been seen in the Post-ICU and Geriatrics clinics (with the patient care navigator ensuring that home rehabilitation services have commenced). Two weeks later, he is seen in the Post-ICU clinic, where he undergoes assessments of cognitive function, mental health, and a repeat assessment of physical function so that his long-term progress can be tracked. The cognitive screen raises concern for mild cognitive impairment, so the Post-ICU provider alerts the geriatrician, with whom the patient has a visit the following week. During his geriatrics visit, he undergoes a complete cognitive assessment and receives a referral for a vision evaluation. The patient also joins the Post-ICU support group, where he learns from other older ICU survivors about the recovery process; speaking with them improves his self-efficacy14 in returning to his baseline level of physical activity.
The 75-year-old patient, who has probable dementia (based on the Eight-item Informant Interview to Differentiate Aging and Dementia [AD8]37 questions answered by her daughter, who lives with her) and mild depressive symptoms, also had one hospitalization two months ago. She is dependent in 1 mobility activity (getting around outside) but is not frail and not vision impaired. The model indicates that her predicted risk of the outcome is 53% (the “warning” range), so the team counsels the patient and her daughter about the risk of her potentially not recovering in the months after discharge. She is referred for outpatient physical and occupational therapy, and the daughter is given information about how to contact the post-ICU clinic and a geriatrician if she feels that her mother is not recovering in the early weeks after discharge. The 80-year-old patient is not dependent in any functional activities at baseline, though he is pre-frail due to low physical activity. He does not have vision impairment, depressive symptoms, or dementia. His predicted risk of the outcome is 38%, placing him into the “usual care” category, so no extra counseling or interventions are administered at the time of discharge. On his discharge paperwork, he is advised to schedule follow-up with his primary care physician after discharge, the current standard of care.
A major strength of this study is the use of a nationally representative cohort for development and validation of the model. A related strength is the high response rate and oversampling of minority populations in the National Health and Aging Trends Study, which increases the generalizability of our findings. Additionally, our study leverages prospective measurements of baseline (pre-ICU) disability, frailty, cognitive status, and other important factors that have previously been shown to be associated with post-ICU disability. Another strength of this study is its central focus on functional outcomes, which are among the outcomes that matter most to ICU survivors38 and older adults.31 Finally, this study carefully adhered to best practices for prediction modeling as recommended in the TRIPOD statement15 and in a recent statement collectively promoted by a group of journal editors.39
This study has several limitations. Although the model underwent rigorous internal validation, it was not externally validated. Model discrimination was good but not excellent; however, this is the first model for the prediction of persistent functional impairment among older ICU survivors, represents a substantial improvement over not having any models to risk-stratify this population, and is a foundation upon which future work can build. We were unable to capture certain elements of the ICU stay that are poorly reported in administrative data, such as delirium, which may have further improved performance of the model.40 Future studies should attempt to test whether some of these factors improve model performance while maintaining feasibility of use by clinicians. Additionally, many of the geriatric factor assessments were collected prior to ICU admission, rather than between ICU admission and hospital discharge. However, these traits are unlikely to improve in an older adult during an ICU admission; if anything, these factors may increase in severity. Moreover, these factors have been previously shown to be strongly associated with post-ICU functional outcomes and are therefore important to include as potential predictors in model development.6,11, 14 Finally, our NHATS dataset could only be linked to fee-for-service Medicare admissions; therefore, this study does not include ICU admissions under managed Medicare.
For older ICU survivors, persistent functional impairment has devastating consequences for patients, families, and the health system at large. By identifying high-risk patients early (prior to discharge from the index ICU hospitalization), clinicians can target patients for restorative interventions and close follow-up, thereby improving the likelihood of functional recovery in the subsequent months. The model addresses this gap for the domain of physical function among older ICU survivors. Future studies should build on this work by developing risk prediction models for the other functional domains affected by critical illness, such as cognitive function.
Supplementary Material
Key Points
In this nationally representative study of older adults who survived a critical illness, approximately half of older ICU survivors experienced persistent functional impairment in the year after discharge.
We developed and internally validated a prediction model to identify older adults at risk of persistent functional impairment in the year after discharge from an ICU hospitalization.
The predictors in the model can all be gathered during the ICU hospitalization to facilitate application of the model prior to discharge, thereby allowing for early risk stratification. The model includes age, pre-ICU disability, probable dementia, frailty, prior hospitalizations, vision impairment, depressive symptoms, and hospital length of stay.
Why Does This Paper Matter?
Application of the model prior to discharge from an ICU hospitalization may identify older adults at highest risk of persistent functional impairment in the subsequent year, thereby facilitating targeted interventions and follow-up.
ACKNOWLEDGEMENTS
Sponsor’s Role:
The organizations funding this study had no role in the design or conduct of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
Funding information:
Dr. Ferrante is supported by a Paul B. Beeson Emerging Leaders in Aging Research Career Development Award from the National Institute on Aging (K76AG057023). Drs. Ferrante, Gill, and Murphy are supported by the Yale Claude D. Pepper Older Americans Independence Center (P30AG021342).
Footnotes
Meeting presentations: An early version of this work was presented at the 2019 American Thoracic Society meeting and the 2019 American Geriatrics Society meeting.
Conflicts of interest: The authors report no conflicts of interest.
ONLINE SUPPLEMENT
Additional supporting methods and tables can be found in the online supplement at the end of this article.
REFERENCES
- [1].Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit Care Med. 2012;40: 502–509. [DOI] [PubMed] [Google Scholar]
- [2].Haines KJ, Hibbert E, McPeake J, et al. Prediction Models for Physical, Cognitive, and Mental Health Impairments After Critical Illness: A Systematic Review and Critical Appraisal. Crit Care Med. 2020;48: 1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mikkelsen ME, Still M, Anderson BJ, et al. Society of Critical Care Medicine’s International Consensus Conference on Prediction and Identification of Long-Term Impairments After Critical Illness. Crit Care Med. 2020;48: 1670–1679. [DOI] [PubMed] [Google Scholar]
- [4].Weissman GE, Kerlin MP, Yuan YH, et al. Population Trends in Intensive Care Unit Admissions in the United States Among Medicare Beneficiaries, 2006–2015. Ann Intern Med. 2019;170: 670–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Administration on Aging, U.S. Department of Health and Human Services. 2018 Profile of Older Americans. Published April 2018.
- [6].Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. The Association of Frailty With Post-ICU Disability, Nursing Home Admission, and Mortality A Longitudinal Study. Chest. 2018;153: 1378–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ferrante LE, Murphy TE, Gahbauer EA, Leo-Summers LS, Pisani MA, Gill TM. Pre-Intensive Care Unit Cognitive Status, Subsequent Disability, and New Nursing Home Admission among Critically III Older Adults. Ann Am Thorac Soc. 2018;15: 622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sjoding MW, Prescott HC, Wunsch H, Iwashyna TJ, Cooke CR. Longitudinal Changes in ICU Admissions Among Elderly Patients in the United States. Crit Care Med. 2016;44: 1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term Cognitive Impairment and Functional Disability Among Survivors of Severe Sepsis. JAMA. 2010;304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Barnato AE, Albert SM, Angus DC, Lave JR, Degenholtz HB. Disability among Elderly Survivors of Mechanical Ventilation. Am J Respir Crit Care Med. 2011;183: 1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Functional Trajectories Among Older Persons Before and After Critical Illness. JAMA Intern Med. 2015;175: 523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gill TM, Robison JT, Tinetti ME. Difficulty and dependence: Two components of the disability continuum among community-living older persons. Ann Intern Med. 1998;128: 96–+. [DOI] [PubMed] [Google Scholar]
- [13].Coughlin TA, McBride TD, Perozek M, Liu KB. HOME CARE FOR THE DISABLED ELDERLY - PREDICTORS AND EXPECTED COSTS. Health Serv Res. 1992;27: 453–479. [PMC free article] [PubMed] [Google Scholar]
- [14].Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Factors associated with functional recovery among older intensive care unit survivors. Am J Respir Crit Care Med. 2016;194: 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): The TRIPOD Statement. Ann Intern Med. 2015;162: 55–U103. [DOI] [PubMed] [Google Scholar]
- [16].Freedman VA, Kasper JD. Cohort Profile: The National Health and Aging Trends Study (NHATS). Int J Epidemiol. 2019;48: 1044–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Freedman VA, Kasper JD, Cornman JC, et al. Validation of New Measures of Disability and Functioning in the National Health and Aging Trends Study. J Geront A Biol Sci. 2011;66: 1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].J M, Freedman VA, Spillman BC, Kasper JD. National Health and Aging Trends Study Development of Round 1 Survey Weights. Baltimore, Maryland: Johns Hopkins University School of Public Health, 2012. [Google Scholar]
- [19].SAS Institute Inc. 2013. Base SAS ® 9.4 Procedures Guide. Cary, N.C.: SAS Institute, Inc. [Google Scholar]
- [20].Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long-term Acute Care Hospital Utilization After Critical Illness. JAMA. 2010;303: 2253–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].2016 General Equivalence Mappings (GEMs) - Diagnosis Codes and Guide.
- [22].Ferrante LE, Murphy TE, Leo-Summers LS, Gahbauer EA, Pisani MA, Gill TM. The combined effects of frailty and cognitive impairment on post-ICU disability among older ICU survivors. Am J Respir Crit Care Med. 2019;200: 107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hamilton M, Tomlinson G, Chu L, et al. Determinants of Depressive Symptoms at 1 Year Following ICU Discharge in Survivors of >= 7 Days of Mechanical Ventilation Results From the RECOVER Program, a Secondary Analysis of a Prospective Multicenter Cohort Study. Chest. 2019;156: 466–476. [DOI] [PubMed] [Google Scholar]
- [24].Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5: 179–193. [DOI] [PubMed] [Google Scholar]
- [25].Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in Older Adults: A Nationally Representative Profile in the United States. J Gerontol A Biol Sci. 2015;70: 1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Falvey JR, Cohen AB, O’Leary JR, Leo-Summers L, Murphy TE, Ferrante LE. Association of Social Isolation With Disability Burden and 1-Year Mortality Among Older Adults With Critical Illness. JAMA Intern Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Murphy TE, Tsang SW, Leo-Summers LS, et al. Bayesian Model Averaging for Selection of a Risk Prediction Model for Death within Thirty Days of Discharge: The SILVER-AMI Study. Int J Stat Med Res. 2019;8: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Raftery AE, Hoeting JA, Volinsky CT, Painter I, Yeung KY. R package “BMA,” version 3.18.14. 2019.
- [29].SUDAAN Language Manual. Research Triangle Park, NC: Research Triangle Institute, 2012. [Google Scholar]
- [30].Pepe MS, Feng Z, Huang Y, et al. Integrating the predictiveness of a marker with its performance as a classifier. Am J Epidemiol. 2008;167: 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fried TR, Tinetti ME, Iannone L, O’Leary JR, Towle V, Van Ness PH. Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med. 2011;171: 1854–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Baldwin MR, Reid MC, Westlake AA, et al. The feasibility of measuring frailty to predict disability and mortality in older medical intensive care unit survivors. J Crit Care. 2014;29: 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Herridge MS, Chu LM, Matte A, et al. The RECOVER Program: Disability. Risk Groups and 1-Year Outcome after 7 or More Days of Mechanical Ventilation. Am J Respir Crit Care Med. 2016;194: 831–844. [DOI] [PubMed] [Google Scholar]
- [34].Higgins AM, Neto AS, Bailey M, et al. Predictors of death and new disability after critical illness: a multicentre prospective cohort study. Intensive Care Med. 2021;47: 772–781. [DOI] [PubMed] [Google Scholar]
- [35].Daniels LM, Johnson AB, Cornelius PJ, et al. Improving Quality of Life in Patients at Risk for Post-Intensive Care Syndrome. Mayo Clin Proc Innov Qual Outcomes. 2018;2: 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Falvey JR, Murphy TE, Gill TM, Stevens-Lapsley JE, Ferrante LE. Home Health Rehabilitation Utilization Among Medicare Beneficiaries Following Critical Illness. J Am Geriatr Soc. 2020; 68(7): 1512–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Galvin JE, Roe CM, Powlishta KK, et al. The AD8 - A brief informant interview to detect dementia. Neurology. 2005;65: 559–564. [DOI] [PubMed] [Google Scholar]
- [38].Auriemma CL, Harhay MO, Haines KJ, Barg FK, Halpern SD, Lyon SM. WHAT MATTERS TO PATIENTS AND THEIR FAMILIES DURING AND AFTER CRITICAL ILLNESS: A QUALITATIVE STUDY. Am J Crit Care. 2021;30: 11–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Leisman DE, Harhay MO, Lederer DJ, et al. Development and Reporting of Prediction Models: Guidance for Authors From Editors of Respiratory, Sleep, and Critical Care Journals. Crit Care Med. 2020;48: 623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Brummel NE, Jackson JC, Pandharipande PP, et al. Delirium in the ICU and Subsequent Long-Term Disability Among Survivors of Mechanical Ventilation. Crit Care Med. 2014;42: 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kasper JD, Freedman VA, Spillman BC. Classification of Persons by Dementia Status in the National Health and Aging Trends Study. Technical Paper #5. Baltimore: Johns Hopkins University School of Public Health, 2013. [Google Scholar]
- [42].Pohl JS, Cochrane BB, Schepp KG, Woods NF. Measuring Social Isolation in the National Health and Aging Trends Study. Res Gerontol Nurs. 2017;10: 277–287. [DOI] [PubMed] [Google Scholar]
- [43].Gell NM, Wallace RB, LaCroix AZ, Mroz TM, Patel KV. Mobility Device Use in Older Adults and Incidence of Falls and Worry About Falling: Findings from the 2011–2012 National Health and Aging Trends Study. J Am Geriatr Soc. 2015;63: 853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kerlin MP, Weissman GE, Wonneberger KA, et al. Validation of Administrative Definitions of Invasive Mechanical Ventilation across 30 Intensive Care Units. Am J Respir Crit Care Med. 2016;194: 1548–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


