Abstract
Background:
The Quality of Communication (QOC) Questionnaire has been widely used to assess foundational aspects of patient-clinician communication about end-of-life (EOL) care. However, this instrument has never before been fielded with primary care patients who have cognitive impairment and their caregivers, a population with unique communication needs.
Design:
We report on baseline data from a completed pilot study and ongoing efficacy trial of advance care planning involving dyads of primary care patients ages 80 and older with cognitive impairment and their family care partners. Two QOC subscales assessed ratings of general communication and EOL care communication from 0 (“worst”) to 10 (“best”). Due to challenges piloting the EOL subscale, we integrated skip logic to improve cognitive accessibility and measurement precision. Participants were first asked whether EOL communication occurred (yes/no); those responding affirmatively were subsequently asked to rate communication. We report experiences with EOL subscale adaptations from our ongoing trial (NCT04593472).
Results:
Using the original instrument in our pilot (13 dyads), mean patient and family general communication ratings were similar (9.65 and 9.60, respectively), but EOL ratings diverged (4.23 and 5.88, respectively), and “Don’t Know” comprised 5% of patient and 32% of family responses. Interviewers reported patient and family participants expressed confusion when asked to rate EOL communication behaviors that had not occurred. Using the adapted instrument in our efficacy trial (43 dyads), EOL communication behaviors were most often reported as not having occurred (76% of patient and 73% of family responses across all items). Mean patient and family EOL subscale ratings were similar (2.23 and 2.26) and responses of “Don’t Know” were minimal (<1%).
Conclusion:
The original QOC EOL subscale involves rating conversations that rarely occur in primary care but are important for older adults with cognitive impairment. Subscale adaptations may reduce confusion and response uncertainty and improve measurement accuracy.
Keywords: communication, end-of-life, primary care, dementia, caregiver
Introduction
Communication is the process by which patients and providers establish a therapeutic relationship, engage in information exchange, and deliberate on a clinical course of action.1 High quality communication is especially critical in the context of serious illness and end-of-life (EOL) care due to the frequency of high-stakes decisions, and because it is through ongoing communication that patient preferences are elicited and understood as a step toward achieving goal-concordant care.2, 3 The measurement of high-quality communication about EOL care is especially challenging and important in the context of cognitive impairment and dementia due to the necessity of involving knowledgeable informants, who commonly assume a role in surrogate decision-making.4
The Quality of Communication (QOC) Questionnaire is a validated 13-item instrument that assesses the quality of foundational aspects of communication about EOL care.5–7 The questionnaire was developed with the input of patients, families, and clinicians, and has been widely used in interventional research8–13 due to established psychometric properties,5 demonstrated responsiveness to change,9–12 and the capacity to assess perspectives of both patients and knowledgeable informants.11, 13, 14 Based on these strengths, the QOC instrument was selected as the primary outcome for an ongoing NIA-funded trial testing the effects of a multicomponent advance care planning intervention in primary care among older adults with cognitive impairment (NCT04593472). However, the instrument has not previously been fielded to older adults with cognitive impairment and involved family in primary care.
Studies fielding the QOC instrument commonly report on two subscales assessing general communication skills (6 items) and communication about EOL (7 items). Average ratings of general communication are consistently higher than ratings of communication about EOL care,5, 11, 12 reflecting pervasive structural, systemic, and cultural challenges that inhibit proactive conversations about EOL care, even in the context of serious illness such as in hospice and palliative care settings. In a recent study that interviewed family decision-makers of persons with advanced dementia residing in nursing homes, the mean EOL subscale rating was 2.0 of 10.0 points (higher scores indicating better communication), reflecting notable omissions in foundational aspects of EOL communication.14
Because our trial examines the effects of an advance care planning intervention, we selected the EOL subscale of the QOC Questionnaire as the primary endpoint, as it reflects our goal of increasing the frequency and comprehensiveness of proactive primary care conversations about future care at EOL. Primary care is a vital setting for initiating conversations about EOL among older adults living with cognitive impairment and dementia. It is the most common setting of initial dementia diagnosis and ongoing medical management, typically involves frequent interactions in long-term trusted relationships, and older patients commonly expect and desire their primary care clinician to initiate conversations about their wishes for future medical care.15–17 Evaluating opportunities for improving these EOL conversations is important, but this population’s unique communication needs may impact the utility of existing EOL communication measures.
This report describes our experiences piloting the originally worded QOC Questionnaire to older adults who demonstrate signs of cognitive impairment and their involved family in the context of primary care. We summarize modifications made to EOL subscale question-wording to address challenges observed during our pilot, and we report on experiences fielding the adapted instrument in the ongoing efficacy trial.
Methods
Setting and Participants
Eligibility criteria were constructed to identify a population with mild to severe cognitive impairment at high risk of mortality. For both the pilot and efficacy trial, participants must be English-speaking, age 80 or older, and respond incorrectly to at least two items on a validated six-item telephone screening instrument for cognitive impairment.18 Participants must also attend primary care visits with a family member or unpaid friend who agrees to participate in the trial. Both phases follow a recruitment protocol used in prior studies in primary care involving the same target population.19–21
Instrument
The QOC Questionnaire5 measures the quality of EOL communication from the perspective of patients and family (Supplementary Table S1). The instrument measures communication in two subscales: a 6-item “general communication skills” subscale, which reflects how the primary care team generally supports and communicates with patients and families and a 7-item “communication about end-of-life care” subscale, which reflects communication of the primary care team about dying and care at EOL.
Respondents are prompted to rate individual items from 0 (“worst”) to 10 (“best”) based on interactions to date, without reference to a specific recall period. Prior studies fielding the QOC have recorded “Don’t Know” when respondents report they are unsure what rating to give, or “Doctor Didn’t Do” when reporting that the communication had never occurred when speaking with their doctor. In the initial QOC validation study, Engelberg and colleagues established a precedent of replacing nonnumeric responses of “Don’t Know” with the sample median for the item. They recoded responses of “Doctor Didn’t Do” with a value of 0, the rationale being that all items encompass important aspects of communication, and the failure of the care provider to address an item therefore warrants a low score.5
For this study, interviews were conducted over the telephone or through a REDCap survey. When fielding the original QOC instrument in the pilot study (13 dyads), responses of “Doctor Didn’t Do” or “Don’t Know” were provided in the preamble but not restated unless requested, in accordance with administration instructions. Interviewers reported some participants appeared confused by how to rate EOL subscale items. Staff reported that participants appeared to have trouble reconciling how to respond when asked to rate EOL communication that had not occurred and that some participants stated that EOL communication had not occurred, but insisted on providing a numeric rating. Family participants appeared unsure how to respond when asked to rate EOL communication that they had not witnessed but that could have occurred when not present.
Based on interviewer input and audio review of recorded participant exit interviews, in our efficacy trial we adapted the QOC instrument to introduce skip logic for each EOL subscale item, with approval from our data safety monitoring board (Supplementary Table S1). We first ascertained whether the communication had occurred (yes/no). Responses of “no” were treated as “Doctor Didn’t Do”. Participants responding affirmatively were asked to numerically rate the communication as in the original instrument; responses of “Don’t Know” were also recorded. Interviewers were instructed to clarify as needed that the questions pertained to any member of the primary care team and that items were intended to assess communication that were observed in their presence.
Analyses
We present descriptive statistics characterizing 13 dyads who participated in the pilot phase and the first 43 dyads enrolled in the efficacy trial. We then report response frequencies and patient and family mean ratings for each item and subscale for the original QOC Questionnaire in the pilot phase, as well as observations of research staff who fielded the instrument to participants. Finally, we present patient and family response frequencies and mean ratings for each item and subscale for the adapted QOC Questionnaire in our efficacy trial. In alignment with prior studies that have fielded the QOC, all mean ratings that are presented include responses of “Doctor Didn’t Do” and “Don’t Know” which have been recoded using Engelberg’s non-numeric substitution rules.
Results
In the pilot and efficacy studies, respectively, patient participants were on average 84 and 86 years old, female (62% and 44%), and mostly white (92% and 74%; Supplementary Table S1). Family participants in the pilot and efficacy studies were respectively on average 66 and 67 years old, female (69% and 77%), mostly white (84% and 79%), and most often adult children (46% and 49%) or spouses (38% and 42%).
Pilot Results
In the pilot study, mean ratings for the general communication subscale were similar for patient and family participants (9.65 and 9.60 of 10, respectively; Supplementary Table S1). Responses of “Don’t Know” were infrequent (3% of patients and 5% of family responses, respectively) and no patient or family participant responded “Doctor Did Not Do” to general communication items.
For the EOL subscale, nonnumeric ratings were more common: less than half of EOL subscale responses were numerically rated by patients (46%) and family (48%). Patient and family participants reported “Don’t Know” for 5% and 32% of responses, respectively, and “Doctor Didn’t Do” for 48% and 20% of responses, respectively. After using the established procedures to recode nonnumeric responses of “Don’t Know” to the item median response and “Did Not Do” to 0, the EOL subscale mean ratings were low and diverged for patients and family (4.23 vs 5.88 of 10, respectively; Figure 1a).
Figure 1:
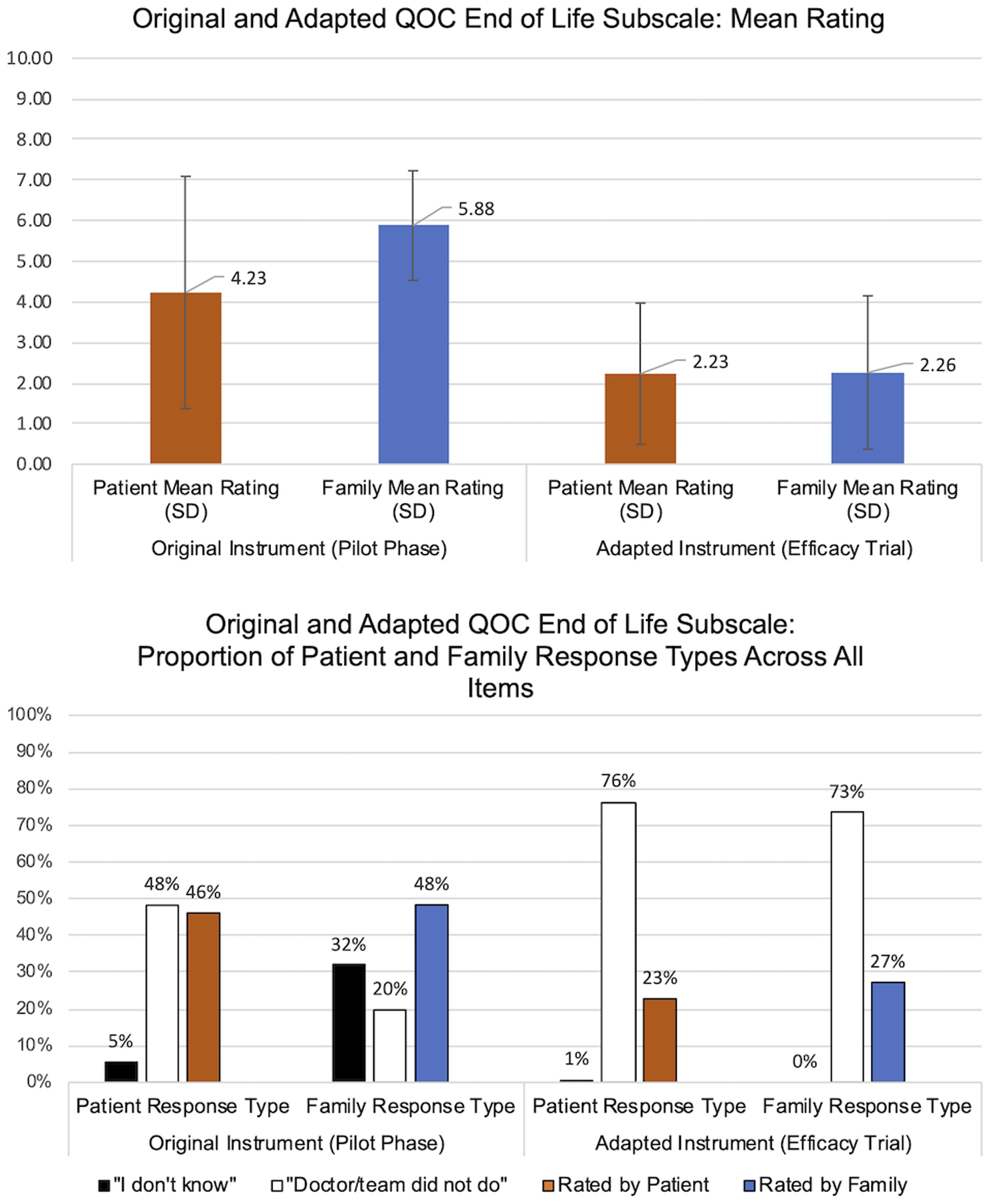
a) Mean ratings reported by patients living with cognitive impairment and their family members for the 7-item End-of-Life (EOL) subscale of Quality of Communication (QOC) instrument in the pilot (using the original instrument; patient n=13, family n=13) and in the efficacy trial (patient n=37, family n=43); a rating of 0 corresponds to worst and 10 to best. In the pilot, the original EOL subscale was used and in the efficacy trial, an adapted EOL subscale was fielded that first ascertained whether an item had occurred and then asked for a rating from those responding affirmatively. b) Distribution of aggregate responses to the 7-item End-of-Life (EOL) subscale of Quality of Communication (QOC) instrument in the pilot and in the efficacy trial. Each item represents a different aspect of EOL communication. Participants are asked to rate their doctor on each item from 0 (worst) to 10 (best); responses of “don’t know” or that the doctor or primary care team “did not do” that item are recorded if offered by the respondent.
Fielding Adapted QOC Questionnaire in the Ongoing Efficacy Trial
Using the adapted EOL subscale in the efficacy trial, responses to the newly introduced yes/no questions varied by item but responses of patient and family participants were generally aligned (Supplementary Table S1). “Don’t Know” comprised <1% of patients and family responses across all items (Figure 1b). EOL communication behaviors were frequently reported to have not occurred (76% and 73% of patient and family responses across all EOL items, respectively). After numeric substitution (treating responses of “no” as “Did Not Do”), EOL subscale mean ratings were low and comparable for patients and family (2.23 and 2.26 of 10, respectively).5
Discussion
The QOC Questionnaire has been used to assess quality of EOL communication in a range of populations and care settings but has not previously been fielded to older adults with cognitive impairment and involved family in the primary care context. The QOC EOL subscale asks respondents to rate the quality of conversations that rarely occur in primary care.22, 23 Accordingly, comprehending the originally worded questions in this context requires abstraction and cognitive flexibility–skills which decline with age, especially in the presence of cognitive decline.24 Family respondents may have difficulty judging response options and confidently selecting an answer if they are not consistently present during patient and provider interactions -- for example, among those who jointly share responsibilities or only assist intermittently as needed. The sensitive nature of EOL topics may compound reporting challenges.25, 26
When fielding the original QOC EOL subscale to older adults with cognitive impairment and their family in our pilot, respondents appeared uncertain of how to rate conversations that had not occurred. “Don’t Know” comprised a nontrivial percentage of patient and family responses in the pilot study, and mean EOL subscale ratings were markedly lower for patients than family. After introducing skip logic to first assess whether each EOL subscale communication behavior had occurred, a reduction in “Don’t Know” responses and increased concordance between patient and family EOL subscale mean ratings were observed in our efficacy trial.
Answering survey questions requires a complex series of cognitive processes of comprehension, retrieval, judgment, and selection.25 If the cognitive load of a question is high due to complexity in any of these processes, a respondent is more likely to engage in satisficing or shortcut strategies such as answering “Don’t Know”.25, 26 Our adaptations to reduce cognitive burden decreased shortcut responses, and the observed increased concordance of EOL subscale mean ratings for patients and family suggests an improvement in measurement reliability. This is imperative for accurate intervention effect estimation in our study.
When responding to the adapted EOL subscale, patients and family frequently reported EOL communication had not occurred; factoring in these responses, mean ratings of EOL communication were low. These findings are comparable to findings from other studies that have fielded the QOC Questionnaire to persons with serious illness in the nursing home and hospice contexts,5, 9, 14 and align with the broader evidence identifying gaps in communication about EOL among persons living with cognitive impairment and dementia, and in primary care.22, 23, 27 Quality communication that is aligned with individual values, needs, and preferences is strongly associated with patient satisfaction, well-being, treatment adherence, and health outcomes.28, 29 Our findings provide evidence that population-specific adaptations can reduce cognitive burden and response uncertainty and improve measurement accuracy when assessing quality of communication about EOL care among older adults with cognitive impairment and involved family.
Supplementary Material
Supplementary Table S1: Original and Adapted Quality of Communication Instruments, Sample Characteristics, and Responses from the Pilot and Efficacy Trials.
Key Points:
The Quality of Communication (QOC) Questionnaire assesses foundational aspects of patient-clinician communication about end-of-life (EOL) care but has not been used among older adults with cognitive impairment in the primary care context.
When fielding the original instrument in our pilot work, patient and family ratings on the subscale assessing communication about end-of-life care diverged and a notable proportion of responses were “Don’t Know.”
Adapting questions to integrate skip-logic in the end-of-life subscale in our ongoing trial of an advanced care planning intervention (NCT04593472), ratings of “Don’t Know” were negligible, and patient and family ratings were similar.
Why Does This Matter?
The availability of appropriate measures are necessary in evaluating the success of interventions to improve communication about end-of-life care among older adults who are living with cognitive impairment, a topic which is crucial but infrequently addressed in primary care.
Acknowledgements:
Funding Sources:
This work was supported by grants R01AG058671 and T32AG066576 from the National Institute on Aging.
Sponsor’s role:
No sponsor had any role in study design, data analysis and interpretation, or manuscript preparation.
Footnotes
Conflicts of interest: The authors have no conflict of interest to disclose.
Contributor Information
Jenni S. Reiff, Department of Health Policy and Management, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD.
John G. Cagle, School of Social Work, University of Maryland, Baltimore, Baltimore, MD.
Talan Zhang, Center on Aging and Health, Division of Geriatric Medicine and Gerontology, Johns Hopkins University, Baltimore, MD.
David L. Roth, Center on Aging and Health, Division of Geriatric Medicine and Gerontology, Johns Hopkins University, Baltimore, MD.
Jennifer L. Wolff, Department of Health Policy and Management and Johns Hopkins Bloomberg School of Public Health, Baltimore, MD.
References
- 1.Ong L, de Haes J, Hoos A, Lammes F. Doctor-patient communication: a review of the literature. Social science & medicine. Apr 1995;40(7):903–18. doi:027795369400155M [pii] [DOI] [PubMed] [Google Scholar]
- 2.Sanders JJ, Curtis JR, Tulsky JA. Achieving Goal-Concordant Care: A Conceptual Model and Approach to Measuring Serious Illness Communication and Its Impact. Journal of palliative medicine. Mar 2018;21(S2):S17–S27. doi: 10.1089/jpm.2017.0459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sudore RL, Lum HD, You JJ, et al. Defining Advance Care Planning for Adults: A Consensus Definition from a Multidisciplinary Delphi Panel. Journal of pain and symptom management. Jan 03 2017;doi: 10.1016/j.jpainsymman.2016.12.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried TR, Zenoni M, Iannone L, O’Leary J, Fenton BT. Engagement in Advance Care Planning and Surrogates’ Knowledge of Patients’ Treatment Goals. Journal of the American Geriatrics Society. Mar 20 2017;doi: 10.1111/jgs.14858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engelberg R, Downey L, Curtis JR. Psychometric characteristics of a quality of communication questionnaire assessing communication about end-of-life care. Validation Studies. Journal of palliative medicine. Oct 2006;9(5):1086–98. doi: 10.1089/jpm.2006.9.1086 [DOI] [PubMed] [Google Scholar]
- 6.Wenrich MD, Curtis JR, Shannon SE, Carline JD, Ambrozy DM, Ramsey PG. Communicating with dying patients within the spectrum of medical care from terminal diagnosis to death. Archives of internal medicine. Mar 26 2001;161(6):868–74. doi: 10.1001/archinte.161.6.868 [DOI] [PubMed] [Google Scholar]
- 7.Curtis JR, Engelberg RA, Nielsen EL, Au DH, Patrick DL. Patient-physician communication about end-of-life care for patients with severe COPD. The European respiratory journal. Aug 2004;24(2):200–5. doi: 10.1183/09031936.04.00010104 [DOI] [PubMed] [Google Scholar]
- 8.Curtis JR, Back AL, Ford DW, et al. Effect of communication skills training for residents and nurse practitioners on quality of communication with patients with serious illness: a randomized trial. JAMA : the journal of the American Medical Association. Dec 4 2013;310(21):2271–81. doi: 10.1001/jama.2013.282081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Au DH, Udris EM, Engelberg RA, et al. A randomized trial to improve communication about end-of-life care among patients with COPD. Randomized Controlled Trial Chest Mar 2012;141(3):726–35. doi: 10.1378/chest.11-0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox CE, Lewis CL, Hanson LC, et al. Development and pilot testing of a decision aid for surrogates of patients with prolonged mechanical ventilation. Research Support, N.I.H., Extramural. Critical care medicine. Aug 2012;40(8):2327–34. doi: 10.1097/CCM.0b013e3182536a63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson LC, Zimmerman S, Song MK, et al. Effect of the Goals of Care Intervention for Advanced Dementia: A Randomized Clinical Trial. JAMA internal medicine. Nov 28 2016;doi: 10.1001/jamainternmed.2016.7031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houben CHM, Spruit MA, Luyten H, et al. Cluster-randomised trial of a nurse-led advance care planning session in patients with COPD and their loved ones. Thorax Apr 2019;74(4):328–336. doi: 10.1136/thoraxjnl-2018-211943 [DOI] [PubMed] [Google Scholar]
- 13.White DB, Angus DC, Shields AM, et al. A Randomized Trial of a Family-Support Intervention in Intensive Care Units. The New England journal of medicine. May 23 2018;doi: 10.1056/NEJMoa1802637 [DOI] [PubMed] [Google Scholar]
- 14.Toles M, Song MK, Lin FC, Hanson LC. Perceptions of Family Decision-makers of Nursing Home Residents With Advanced Dementia Regarding the Quality of Communication Around End-of-Life Care. Journal of the American Medical Directors Association. Oct 2018;19(10):879–883. doi: 10.1016/j.jamda.2018.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geldmacher DS, Kerwin DR. Practical Diagnosis and Management of Dementia Due to Alzheimer’s Disease in the Primary Care Setting: An Evidence-Based Approach. Review. The primary care companion to CNS disorders. 2013;15(4)doi: 10.4088/PCC.12r01474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang M, Chang CH, Carmichael D, Oh ES, Bynum JP. Who Is Providing the Predominant Care for Older Adults With Dementia? Journal of the American Medical Directors Association. Sep 1 2016;17(9):802–6. doi: 10.1016/j.jamda.2016.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lakin JR, Block SD, Billings JA, et al. Improving Communication About Serious Illness in Primary Care: A Review. JAMA internal medicine. Jul 11 2016;doi: 10.1001/jamainternmed.2016.3212 [DOI] [PubMed] [Google Scholar]
- 18.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Medical care Sep 2002;40(9):771–81. doi: 10.1097/01.MLR.0000024610.33213.C8 [DOI] [PubMed] [Google Scholar]
- 19.Vick J, Amjad H, Smith KC, et al. “Let him speak:” A descriptive qualitative study of the roles and behaviors of family companions in primary care visits among older adults with cognitive impairment. International journal of geriatric psychiatry. 2018;33(1):e103–e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolff JL, Roter DL, Boyd CM, et al. Patient-Family Agenda Setting for Primary Care Patients With Cognitive Impairment: The SAME Page Trial. Journal of general internal medicine. 2018;33(9):1478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolff JL, Aufill J, Echavarria D, et al. Sharing in Care: Engaging care partners in the care and communication of breast cancer patients. Breast Cancer Research and Treatment. 2019;177(1):127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glaudemans JJ, Moll van Charante EP, Willems DL. Advance care planning in primary care, only for severely ill patients? A structured review. Review. Family practice. Feb 2015;32(1):16–26. doi: 10.1093/fampra/cmu074 [DOI] [PubMed] [Google Scholar]
- 23.Keary S, Moorman SM. Patient-Physician End-of-Life Discussions in the Routine Care of Medicare Beneficiaries. Journal of aging and health. Sep 2015;27(6):983–1002. doi: 10.1177/0898264315569458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson HJ, Brunsdon VEA, Bradford EEF. The developmental trajectories of executive function from adolescence to old age. Sci Rep Jan 14 2021;11(1):1382. doi: 10.1038/s41598-020-80866-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts C Response Styles in Surveys: Understanding their Causes and Mitigating their Impact on Data Quality. In: Steele M, ed. The SAGE Handbook of Survey Methodology. SAGE; 2017:chap 36. The SAGE Handbook of Suvey Methodology. [Google Scholar]
- 26.Krosnick JA. Response strategies for coping with the cognitive demands of attitude measures in surveys. Appl Cognitive Psychology. 1991;5:213–236. [Google Scholar]
- 27.Harrison KL, Adrion ER, Ritchie CS, Sudore RL, Smith AK. Low Completion and Disparities in Advance Care Planning Activities Among Older Medicare Beneficiaries. JAMA internal medicine. Oct 31 2016;doi: 10.1001/jamainternmed.2016.6751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogardus ST, Bradley EH, Williams CS, Maciejewski PK, van Doorn C, Inouye SK. Goals for the care of frail older adults: do caregivers and clinicians agree? The American journal of medicine. Feb 1 2001;110(2):97–102. doi:S0002–9343(00)00668–9 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Bogardus ST, Bradley EH, Williams CS, Maciejewski PK, Gallo WT, Inouye SK. Achieving goals in geriatric assessment: role of caregiver agreement and adherence to recommendations. Journal of the American Geriatrics Society. Jan 2004;52(1):99–105. doi:52017 [pii] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Original and Adapted Quality of Communication Instruments, Sample Characteristics, and Responses from the Pilot and Efficacy Trials.


