Abstract
Ganglioneuromas are benign neuroblastic tumors seen most in pediatric population. The most common locations are mediastinal, retroperitoneal and adrenal regions. Ganglioneuromas rarely occur in presacral space. We present one such case of an incidentally diagnosed presacral ganglioneuroma in an asymptomatic 71-year-old male who initially presented with hematuria.
Keywords: Presacral, Ganglioneuroma, Hematuria
Introduction
Ganglioneuromas (GN) are rare benign neuroblastic tumors typically seen in young patients in the posterior mediastinal, retroperitoneal and adrenal regions [1,2]. The presacral region is a rare location for ganglioneuromas, where they are often discovered incidentally [3]. We present a case of a 71-year-old male presenting with lower urinary tract symptoms who was incidentally found to have a presacral GN.
Case report
A 71-year-old-male with no significant past medical history presented with short term history of hematuria and mild low back pain. The patient was neurologically intact. Contrast-enhanced abdominopelvic CT and pelvic MRI with and without intravenous gadolinium were performed and incidentally revealed a sacral lesion with a large exophytic presacral mass.
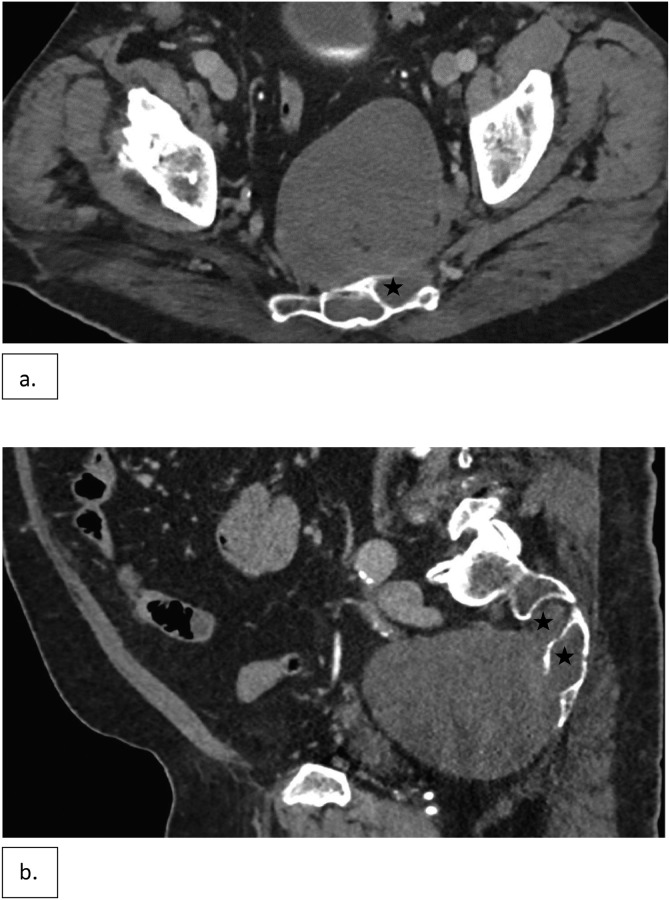
Initial abdominopelvic CT (Fig. 1) demonstrated an expansile lesion involving the S2-S4 region of the sacrum with a large well circumscribed exophytic presacral soft tissue component without internal matrix. The portion of the lesion in the sacrum was associated with indolent expansion of the bone with expansion involving the spinal canal and the left S2-S4 sacral foramina (Fig. 1). MRI of the pelvis was performed on a 1.5 Tesla magnet (Siemens MRI-MAGNETOM Aera) with axial and sagittal T1, T2 fat suppressed and post-gadolinium T1-weighted fat suppressed image acquisitions (Fig. 2). The combined osseous and extraosseous components measured approximately 11 × 10 × 10.8 cm (oblique anteroposterior X transverse X oblique craniocaudal). The mass demonstrated predominantly low to intermediate signal intensity on T1, but also contained scattered foci of linear and nodular T1 hyperintensity, that were shown to represent fat on correlative T2-weighted images with fat suppression. The mass was predominantly T2 hyperintense but showed scattered areas of intermediate and low T2 signal intensity. The bulk of the mass showed minimal enhancement with scattered areas of hazy enhancement. The mass showed minimal activity on 2-deoxy-2 -F18-fluoro-D-glucose (FDG) PET CT with a SUV max of 3.0 (Fig. 3).
Fig. 1.
Axial (a) and sagittal (b) CT images of the pelvis show a lytic expansile lesion in the sacrum with a large presacral soft tissue mass that is in anatomic continuity with the left S2-S4 neural foramina (stars).
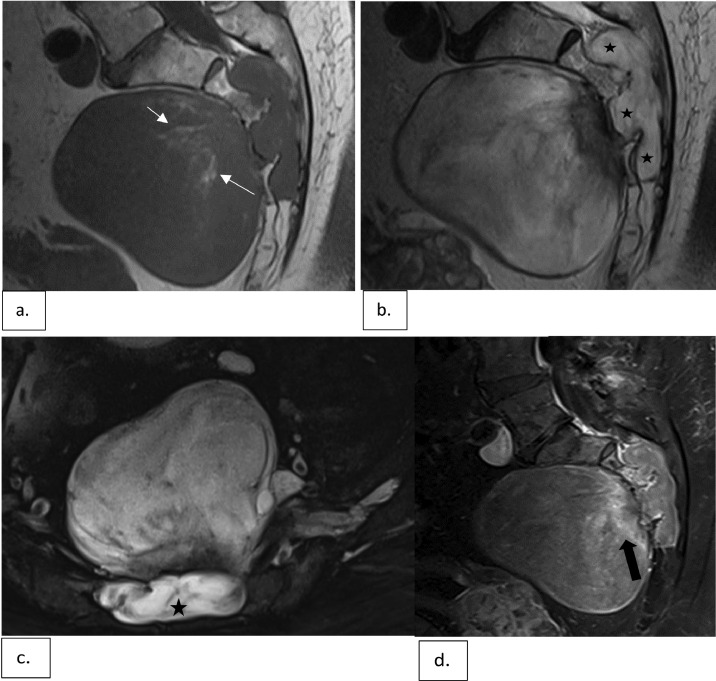
Fig. 2.
Sagittal T1-weighted (a), sagittal T2-weighted (b) and axial fat-suppressed T2-weighted (c) images demonstrate a lesion involving S2-S4 segments with a large associated presacral soft tissue mass. The lesion is associated with expansion of the spinal canal and left S2-4 neural foramina (star). The mass is predominantly hypointense on T1 and hyperintense on T2 with subtle hazy and linear internal foci of fat signal intensity (short white arrows in a). Sagittal gadolinium enhanced T1-weighted images with fat suppression (d) show minimal internal enhancement with a few patchy areas of enhancement near the sacrum (black arrow).
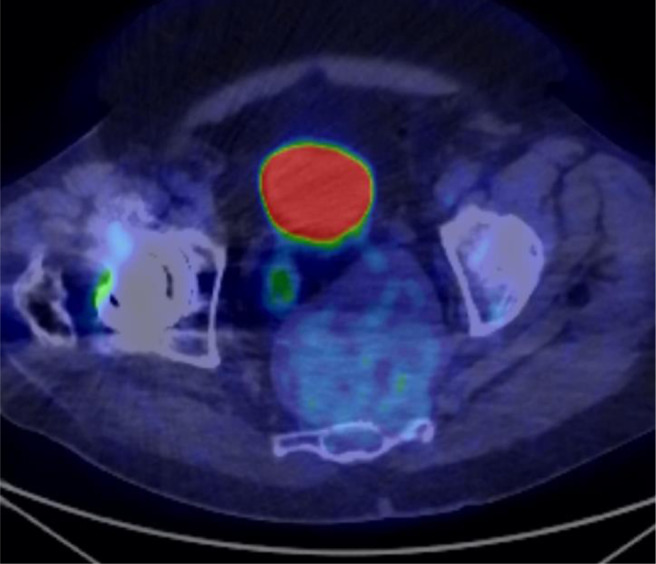
Fig. 3.
Axial fused image from F18-FDG PET-CT show minimal FDG uptake in the mass with a SUV max of 3.0.
The differential diagnosis for this mass involving the sacrum with a large presacral soft tissue mass primarily included chordoma and neurogenic tumors. In the neurogenic category, benign and malignant neurogenic tumors were considered including schwannoma and malignant peripheral nerve sheath tumor. The pattern of indolent expansion of the spinal canal and neural foramina rather than aggressive lytic destruction of the bone favored the diagnosis of a benign neurogenic tumor over chordoma. Of interest, the presence of internal fat, along with a significant component of fluid-like signal intensity (T2 hyperintense with minimal enhancement) suggestive of a myxoid component also raised the possibility of a myxoid liposarcoma. However, the anatomic location and associated involvement of the sacrum would be highly unusual for this diagnosis. The presence of internal fat and retroperitoneal location could also raise the possibility of a low-grade liposarcoma (atypical lipomatous tumor), but this tumor would also not typically show involvement of the sacrum and are less common in the presacral region of the retroperitoneum.
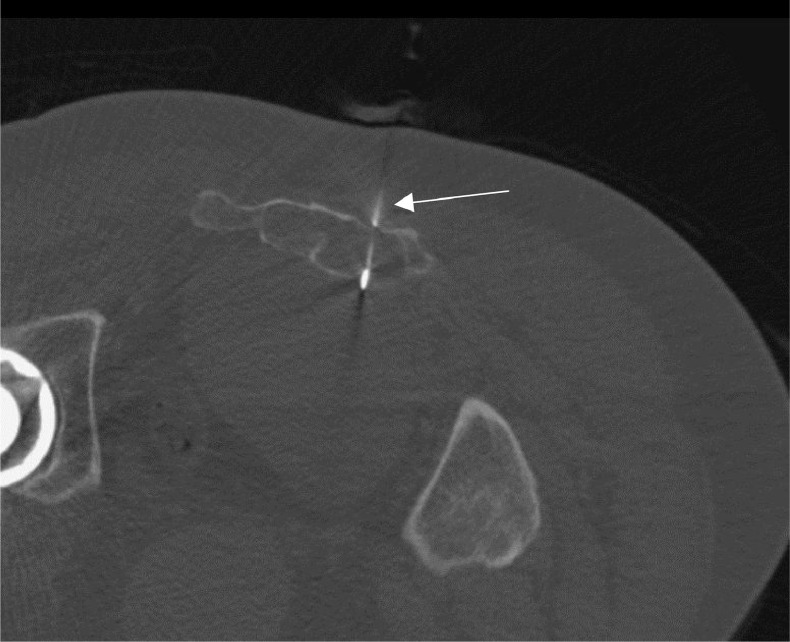
A CT-guided percutaneous transsacral biopsy (Fig. 4) was performed for histologic diagnosis. The procedure was performed utilizing sterile technique and moderate sedation. Given the diagnostic possibility of chordoma, the biopsy approach was coordinated with the surgical team and sterile tattoo ink was injected at the needle entry site to facilitate biopsy tract identification at the time of surgical resection.
Fig. 4.
Axial CT-guided biopsy of sacral mass with patient in a prone position show the biopsy needle (white arrow) traversing the sacral canal just to the left of midline.
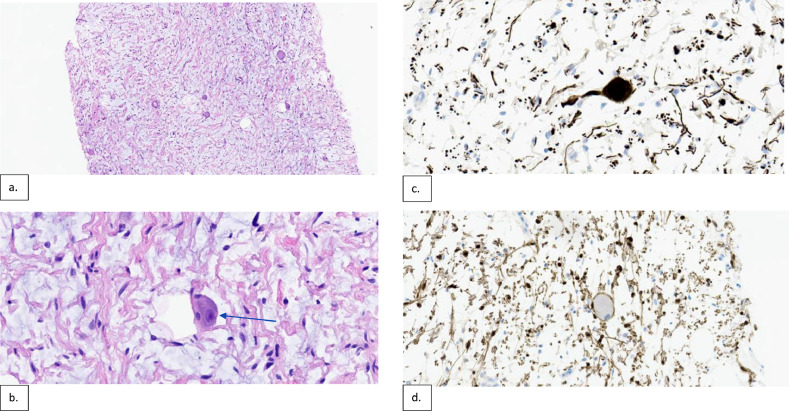
Histologic evaluation revealed tissue composed of schwannian stroma with scattered large ganglion cells on H&E staining (Fig. 5). A strong positive reaction was identified in the small nerve axons within the stroma and ganglion cells with a neurofilament stain. The schwannian stroma and sustentacular cells surrounding the ganglion cell were positive for S100. These findings were consistent with a ganglioneuroma. Due to the patient’s age, comorbidities and lack of significant symptomatology, a decision was made to forego surgical resection. The underlying cause for hematuria was benign prostatic hyperplasia.
Fig. 5.
Mid-power and high-power H&E stains (a & b respectively) demonstrate histopathology of the sacral mass with schwannian stroma composed of Schwann cells with elongated nuclei and tapered or pointed ends, with a collagenous and variably edematous background. There are also scattered large ganglion cells (blue arrow in b) with abundant amphophilic cytoplasm and distinct round nuclei with prominent nucleoli. The neurofilament stain shows strong positive reaction in the small nerve axons within the stroma and ganglion cells (c). The schwannian stroma and sustentacular cells surrounding the ganglion cell were positive for S100 (d).
Discussion
Ganglioneuromas are mature benign tumors that comprise less than 1% of soft tissue neoplasms. They occur most commonly in the pediatric population, but have a reported mean age ranging from the first to fifth decades of life [4,18]. The presacral space is bordered posteriorly by the sacrum and coccyx and contains neuronal (sacral plexus), mesenchymal (adjacent pelvic organs), vascular and connective tissues [5]. In adults, a variety of benign and malignant masses can occur in this location according to the tissue of origin [5,6]. Of interest, it is not a common location for ganglioneuromas, which are usually located in the retroperitoneum and posterior mediastinum [7]. Space occupying masses in the presacral region present with variable symptomatology, often with nonspecific symptoms of lower abdominal/pelvic, flank or back pain. When endocrinologically active, GN can present with symptoms such as diarrhea or hypertension [4,5]. However, the majority are indolent and rarely undergo malignant transformation [7]. Presacral masses can be discovered incidentally during the work up of nonspecific lower abdominal or pelvic symptoms, as in our case, where lower urinary tract symptoms were the precipitating event.
The CT and MR imaging characteristics of GN are well documented with GN typically presenting as well-defined, oval shaped mass with intermediate attenuation on CT, predominantly T2 hyperintense and T1 iso- to hypointense on MRI and heterogeneous enhancement with 40%-60% lesions demonstrating internal calcifications [3,8]. The size of these lesions varies from a few millimeters to a few centimeters with few reported cases of lesions approaching or greater than 10 cm [3,8,9]. Given the rarity of presacral GN and overlapping imaging features with the relatively more common etiologies in this location such as chordoma and schwannoma, a histologic diagnosis is required [10]. A definitive histologic diagnosis is necessary to devise an optimal treatment plan. A malignant lesion, such as chordoma, would require entirely different management including wide surgical resection and/or radiation [11,12]. The mainstay of treatment of GN has been surgical resection, through a retroperitoneal approach, which allows easier access and localization of tumors in the presacral region [13,19]. No adjuvant therapy is required or proven beneficial in the treatment of GN [14,15]. Radiologic surveillance following surgical resection is indicated since cases of recurrent tumor have been reported [16,17]; however, the incidence remains unknown to the best of our knowledge. Surgical resection is not warranted in asymptomatic cases as reported in the study by Lee et al. [1]. Patients’ quality of life and surgical risk calculation should be considered before consideration of surgical resection.
Conclusion
Ganglioneuromas are benign tumors originating from the sympathetic chain and may be discovered incidentally on imaging. Given the rarity of presacral GN and overlapping imaging features with other benign or malignant presacral lesions, a biopsy is recommended for histologic diagnosis and can be safely and accurately performed with CT-guidance. The decision to treat with surgical resection or observation is dependent on clinical symptomatology, age, co-morbidities of the patient and not strictly dependent on the size of the lesion. Asymptomatic lesions, even if large, can be safely observed negating the need to risk surgical morbidity.
Patient consent
Written informed consent was obtained from patient's representative.
Footnotes
Competing Interests: None.
References
- 1.Lee D., Choe W.J., Lim S.D. Ganglioneuroma of the Sacrum. Korean J Spine. 2017;14(3):106–108. doi: 10.14245/kjs.2017.14.3.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozluoglu L.N., Yilmaz I., Cagici C.A., Bal N., Erdogan B. Ganglioneuroma of the internal auditory canal: a case report. Audiol Neurootol. 2007;12(3):160–164. doi: 10.1159/000099018. [DOI] [PubMed] [Google Scholar]
- 3.Vardas K., Manganas D., Papadimitriou G., Vougas V., Bakalis A., Chantziara M., et al. Presacral ganglioneuroma: diagnostic considerations and therapeutic strategy. Case Rep Oncol. 2013;6(3):561–568. doi: 10.1159/000356707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fliedner S.M.J., Winkelmann P.E.R., Wesley R., Vonthein R., Lehnert H. Ganglioneuromas across age groups: systematic review of individual patient data. Clin Endocrinol (Oxf) 2021;94(1):12–23. doi: 10.1111/cen.14297. [DOI] [PubMed] [Google Scholar]
- 5.Hain K.S., Pickhardt P.J., Lubner M.G., Menias C.O., Bhalla S. Presacral masses: multimodality imaging of a multidisciplinary space. Radiographics. 2013;33(4):1145–1167. doi: 10.1148/rg.334115171. [DOI] [PubMed] [Google Scholar]
- 6.Hassan I., Wietfeldt E.D. Presacral tumors: diagnosis and management. Clin Colon Rectal Surg. 2009;22(2):84–93. doi: 10.1055/s-0029-1223839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kołodziejek A., Pronobis K., Derlatka P., Grabowska-Derlatka K., Grabowska-Derlatka L. Presacral ganglioneuroma in an adult with 6-year follow-up without surgical treatment. Radiol Case Rep. 2020;15(10):1983–1987. doi: 10.1016/j.radcr.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AlShammari S., Alsalouli M.M., Alkabli A.M., Abanumay F.M., AlAli M.N., Al-Sakkaf H., et al. Large asymptomatic retroperitoneal ganglioneuroma displacing major abdominal organs and vessels in an adult. Am J Case Rep. 2021;22 doi: 10.12659/AJCR.931725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamatam N., Jr., Rayappan E., Smile S., Vivekanandan R. Large ganglioneuroma presenting as presacral mass. BJR Case Rep. 2016;2(4):20150361. doi: 10.1259/bjrcr.20150361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou F., Dai M., Zhang B., Nie T. Misdiagnosis of a giant intrapelvic schwannoma: A case report. Oncol Lett. 2013;6(6):1646–1648. doi: 10.3892/ol.2013.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keykhosravi E., Rezaee H., Tavallaii A., Tavassoli A., Maftouh M., Aminzadeh B. A giant sacrococcygeal chordoma: a case report. Brain Tumor Res Treat. 2022;10(1):29–33. doi: 10.14791/btrt.2022.10.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phang Z.H., Saw X.Y., Nor N.F.B.M., Ahmad Z.B., Ibrahim S.B. Rare case of neglected large sacral chordoma in a young female treated by wide en bloc resection and sacrectomy. BMC Cancer. 2018;18(1):1112. doi: 10.1186/s12885-018-5012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi C., Li F., Wang Y., Pei L., Wang T. Retroperitoneoscopic resection of retroperitoneal nonadrenal ganglioneuromas: our technique and clinical outcomes. Int Braz J Urol. 2018;44(6):1166–1173. doi: 10.1590/S1677-5538.IBJU.2017.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Decarolis B., Simon T., Krug B., Leuschner I., Vokuhl C., Kaatsch P., et al. Treatment and outcome of ganglioneuroma and ganglioneuroblastoma intermixed. BMC Cancer. 2016;16:542. doi: 10.1186/s12885-016-2513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marmor E., Fourney D.R., Rhines L.D., Skibber J.M., Fuller G.N., Gokaslan Z.L. Sacrococcygeal ganglioneuroma. J Spinal Disord Tech. 2002;15(3):265–268. doi: 10.1097/00024720-200206000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Geoerger B., Hero B., Harms D., Grebe J., Scheidhauer K., Berthold F. Metabolic activity and clinical features of primary ganglioneuromas. Cancer. 2001;91(10):1905–1913. doi: 10.1002/1097-0142(20010515)91:10<1905::aid-cncr1213>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Okita Y., Narita Y., Yoshida A., Miyakita Y., Ohno M., Saio M., et al. The late recurrence of ganglioneuroma 21 years after initial presentation with neuroblastoma. Pediatr Hematol Oncol. 2012;29(7):647–651. doi: 10.3109/08880018.2012.721871. [DOI] [PubMed] [Google Scholar]
- 18.Abbou W., Guerrouj I., Tiabi M., Derouich Y., Bennani A., Benhaddou H., et al. Presacral ganglioneuroma in an 8-year-old child: case report, and literature review. Radiol Case Rep. 2022;17(6):1866–1869. doi: 10.1016/j.radcr.2022.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouzid A., Belhadj A., Saidani A., Bokal Z., Khefacha F., Chebbi F. Unusual retrorectal ganglioneuroma: a case report of laparoscopic assisted approach. Pan Afr Med J. 2021;38:241. doi: 10.11604/pamj.2021.38.241.27028. [DOI] [PMC free article] [PubMed] [Google Scholar]