Abstract
Larvae of the sea urchin, Strongylocentrotus purpuratus, have pigmented migratory cells implicated in immune defense and gut patterning. The transcription factor SpGcm activates the expression of many pigment cell-specific genes, including those involved in pigment biosynthesis (SpPks1 and SpFmo3) and immune related genes (e.g. SpMif5). Despite the importance of this cell type in sea urchins, pigmented cells are absent in larvae of the sea star, Patiria miniata. In this study, we tested the premises that sea stars lack genes to synthesize echinochrome pigment, that the genes are present but are not expressed in the larvae, or rather that the homologous gene expression does not contribute to echinochrome synthesis. Our results show that orthologs of sea urchin pigment cell-specific genes (PmPks1, PmFmo3-1 and PmMifL1-2) are present in the sea star genome and expressed in the larvae. Although no cell lineage homologous to migratory sea urchin pigment cells is present, dynamic gene activation accomplishes a similar spatial and temporal expression profile. The mechanisms regulating the expression of these genes, though, is highly divergent. In sea stars; PmGcm lacks the central role in pigment gene expression since it is not expressed in PmPks1 and PmFmo3-1-positive cells, and knockdown of Gcm does not abrogate pigment gene expression. Pigment genes are instead expressed in the coelomic mesoderm early in development before later being expressed in the ectoderm. These findings were supported by in situ RNA hybridization and comparative scRNA-seq analyses. We conclude that simply the co-expression of Pks1 and Fmo3 orthologs in cells of the sea star is not sufficient to underlie the emergence of the larval pigment cell in the sea urchin.
Introduction
Biological pigmentation is an evolutionary Swiss army knife. Pigments are found everywhere in the natural world, serving diverse functions in a countless number of organisms. Functions of pigment include energy synthesis, camouflage, communication, sexual selection, warning, and imitation. An additional function of biological pigment is in providing defense and innate immunity. Such is the case in sea urchins, including the purple sea urchin Strongylocentrotus purpuratus. Sea urchin larvae and adults produce a suite of naphthoquinone pigments called echinochromes and spinochromes [1]-[4]. These organic molecules consist of two fused six carbon rings and extensive systems of conjugated pi bonds with ketones, alcohols, and aliphatic groups as common ring substituents [3]. In the adult sea urchin, echinochromes and spinochromes are produced in the spines and test, as well as in pigmented coelomocytes (named red spherule cells) which survey the coelomic cavity [5],[6]. Extracted pigments from any of these sources have been shown to impede bacterial growth in culture, and adults of the same species with distinct spine pigmentation based on gene manipulations show distinct microbial colonization, providing strong corroborative evidence for an antimicrobial pigment function [5],[7],[8], [74]. Furthermore, red spherule cells store echinochrome A in cytoplasmic granules, which are released upon contact with microbes or tissue damage [9]. Albino adult sea urchins produced through genetic manipulation have been reported to have reduced pathogenic resistance relative to wild type adults, suggesting a significant increase in fitness provided by naphthoquinone pigments [4].
As a phylum, echinoderms display notable developmental diversity (Supplementary Fig. S1). Two major morphological features are found only in the larvae of certain echinoderm taxa, making them useful targets for exploring evolutionary change; the larval presence of a skeleton and of pigment. Larval skeletogenesis has been the subject of a sizable body of research [e.g. 65]. Sea urchins, sea cucumbers and brittle stars are known to synthesize skeletons as larvae, while crinoids and sea stars do not. Considerably less attention has been paid to the other morphological novelty appearing through the course of echinoderm evolution, the advent of pigment cells in larvae.
Echinoid larvae are alone within the echinoderm phylum in possessing pigment cells. In sea urchins, pigment cell precursors, a subset of the non-skeletogenic mesoderm, are specified after the 7th cleavage stage [10],[11]. At that stage in development, the adjacent large micromeres (which give rise to the skeletogenic primary mesenchyme cells) present the Delta ligand, activating the Notch cascade in cells receiving the signal [12],[13]. The transcription factor glial cells missing (SpGcm in S. purpuratus) is a direct target of this Notch signaling, and acts as an essential transcription factor for pigment cell fate [11],[14]-[16]. SpGcm is first expressed symmetrically in the non-skeletogenic mesoderm, though by the mesenchyme blastula stage its expression is restricted to the aboral region of the vegetal plate [11],[17]. SpGcm activates the expression of many pigment cell-specific genes, including polyketide synthase 1 (SpPks1), flavin-dependent monooxygenase 3 (SpFmo3) and macrophage migration inhibitory factor 5 (SpMif5), all of which remain present in the pigment cell lineage throughout development [14],[16],[18]-[20]. Once gastrulation commences, cells of the aboral non-skeletogenic mesoderm undergo epithelial-to-mesenchymal transition and migrate into the blastocoel. By the larval stage, pigment cells acquire pigment and reside within or just beneath the aboral ectoderm [21]-[23]. Pigment cells function as immunocytes in the larval sea urchin [24] and echinochrome A, the major naphthoquinone pigment in pigment cells, is an antimicrobial agent [9],[14],[25]. When pathogenic bacteria are introduced to the larval gut or injected directly into the blastocoel, pigment cells migrate from the ectoderm to the site of infection, where they directly interact with immune cell populations [26],[27].
Previous studies have identified an extensive suite of genes specifically expressed in pigment cells and include SpPks1 and SpFmo3, which are responsible for larval pigment production [4],[14],[28]. The polyketide synthase family consists of large, modular enzymes most commonly found in plants, bacteria and fungi, and are known to catalyze the synthesis of diverse products [29],[30]. Pks proteins have multiple enzymatically active modules for product formation that are thought to function as an assembly line [14],[31]. Flavin-dependent monooxygenase proteins are generally involved in the oxidation of a wide variety of xenobiotic substrates [32] and given the enzymatic activities of Pks and Fmo proteins, it has been hypothesized that SpPks1 constructs a polyketide template which may then be modified by various Fmo enzymes to produce naphthoquinone pigment products [4].
Pigment cells also express lineage-specific factors not involved in pigment biosynthesis, including SpMif5. Members of the macrophage migration inhibitory factor family (like Pks and Fmo genes) are ancient and have been identified in bacteria, plants and animals [33]. Genes in this family encode inflammatory cytokines that possess enzymatic tautomerase activity [34]. In adult sea urchins, exposure to pathogens causes an increase in the expression of certain Mif genes, suggesting a conserved role in immunity [35].
Despite the importance of pigment cells in the sea urchin, sea star larvae lack both pigment and a cell lineage homologous to pigment cells (Supplementary Fig. S2A&B). To explain this divergence, we hypothesized that the sea star did not have, or did not express, the enzymes necessary for pigmentation. However, our results provide evidence of a distinct and dynamic gene regulatory network of orthologous pigment genes in the sea star larva that appear to lack the activity of echinochrome biosynthesis.
Results
Sea star embryos possess orthologs of sea urchin pigment cell-specific genes
The absence of pigment in sea star embryos could be attributed to the genomic absence of the necessary genes for pigment. To investigate this possibility, we searched for orthologs of sea urchin pigment cell-specific genes in the genome of the sea star P. miniata (73). Sea urchins possess only two genes in the Pks family, SpPks1 and SpPks2, the latter of which is expressed in skeletogenic cells and is required for spicule formation [36],[37]. Due to the small size of the Pks gene family in sea urchins, a protein BLAST was used to identify a potential ortholog of SpPks1 in P. miniata using the Echinobase BLAST suite [38]-[40]. We conclude that the best aligned sequence named PmPloyksL_3 on Echinobase and here renamed PmPks1 (PMI_000680), is the SpPks1 ortholog (see STable 4, 5 for gene IDs). Assuming a complete PmPks1 amino acid sequence, it shares 61% amino acid similarity with SpPks1. SpPks1 and PmPks1 share 9 out of 10 predicted protein domains, with SpPks1 containing an additional predicted alcohol dehydrogenase domain not observed in PmPks1 (Supplementary Fig. S3A).
The Fmo family in sea urchins has many paralogs. Previous work has identified 15 sea urchin Fmo proteins, 4 of which are evolutionarily closely related and specifically expressed in pigment cells (SpFmo3, SpFmo5-1, SpFmo2-2, and SpFmo2) [16]. To identify a potential sea star ortholog to SpFmo3, amino acid sequences were obtained for all annotated sea urchin and sea star Fmo proteins and a maximum likelihood tree was constructed (Supplementary Fig. S4). Three sea star Fmo sequences were located within the group of sea urchin pigment cell-specific Fmo genes (bracketed in red in Supplementary Fig. S4): PmFmo3-1 (PMI_000684), PmFmo3 (PMI_000684), and PmFmo3-3 (PMI_024504). PmFmo3-1 (red arrow) shares the closest alignment with SpFmo3, with 62% amino acid similarity. We conclude it to be the SpFmo3 ortholog.
Sea urchin pigment cell-specific factors extend beyond those implicated in pigment biosynthesis, including SpMif5. A protein BLAST was sufficient to identify an SpMif5 ortholog, PmMifL1-2, having the greatest similarity to SpMif5 (35% amino acid similarity) (Supplementary Fig. 3C). The presence of these genes suggests that the sea star genome does not lack orthologs of sea urchin pigment producing genes.
PmPks1, PmFmo3-1 and PmMifL1-2 are co-expressed in the same cells
We tested the expression and localization of these gene products by quantitative reverse transcriptase PCR (qRT-PCR) and fluorescent in situ hybridization (FISH), respectively. Surprisingly, despite the absence of pigment, the orthologs of sea urchin pigment cell-specific genes are indeed expressed during sea star development (Supplementary Fig. S5). PmPks1 transcripts appear at the early gastrula stage (30h post fertilization) and remain present through the late larval stage. Expression drastically peaks at the late gastrula stage (72h post fertilization). PmFmo3-1 and PmMifL1-2 expression follows a nearly identical pattern as PmPks1, with slight variations at the early larval stage (96h post fertilization). This profile is very similar to that documented by qPCR in the sea urchin [41].
Double FISH revealed a striking conservation in the spatial and temporal expression patterns of pigment cell specific-genes between sea urchin and sea star embryos. In S. purpuratus, pigment cell precursors (expressing SpPks1, SpFmo3 and SpMif5) are located in the mesodermal vegetal plate. During gastrulation, these cells migrate from the tip of the archenteron and differentiate into functional pigment cells. In plutei, most pigment cells are either embedded in or just basal to the ectoderm [21]-[23]. In P. miniata, the genes encoding PmPks1, PmFmo3-1 and PmMifL1-2 are co-expressed throughout development (Fig. 1 & 2). These genes are initially expressed in cells in the presumptive mesoderm during the early gastrula stage. As gastrulation proceeds, their transcripts remain detectable in the mesodermal cells of the archenteron.
Figure 1. PmPks1 and PmFmo3-1 are coexpressed during sea star development.
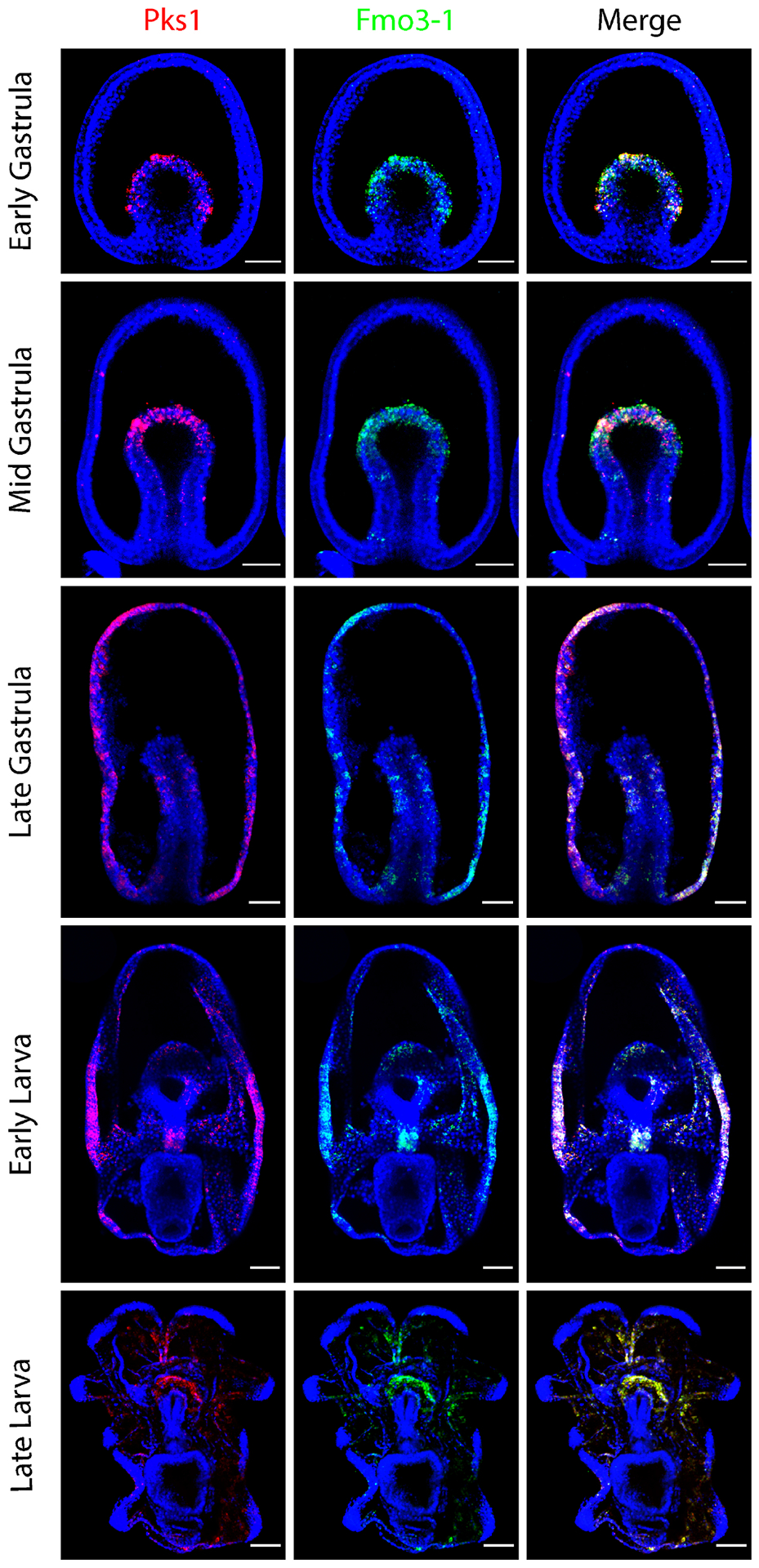
Confocal images show the expression pattern of PmPks1 and PmFmo3-1 using double FISH. Both genes are expressed in the same mesodermal cells located in the archenteron during gastrulation. Between the mid gastrula and late gastrula stages, PmPks1 and PmFmo3-1 expression shifts from mesodermal cells to cells scattered throughout the ectoderm. Some cells in the foregut also express both genes by the late gastrula stage. During the larval stages, ectodermal expression remains consistent while foregut expression is lost by the late larval stage. Nuclei are shown in blue with DAPI. Scale bars are 50 μm.
Figure 2. PmPks1 is coexpressed with PmMifL1-2.
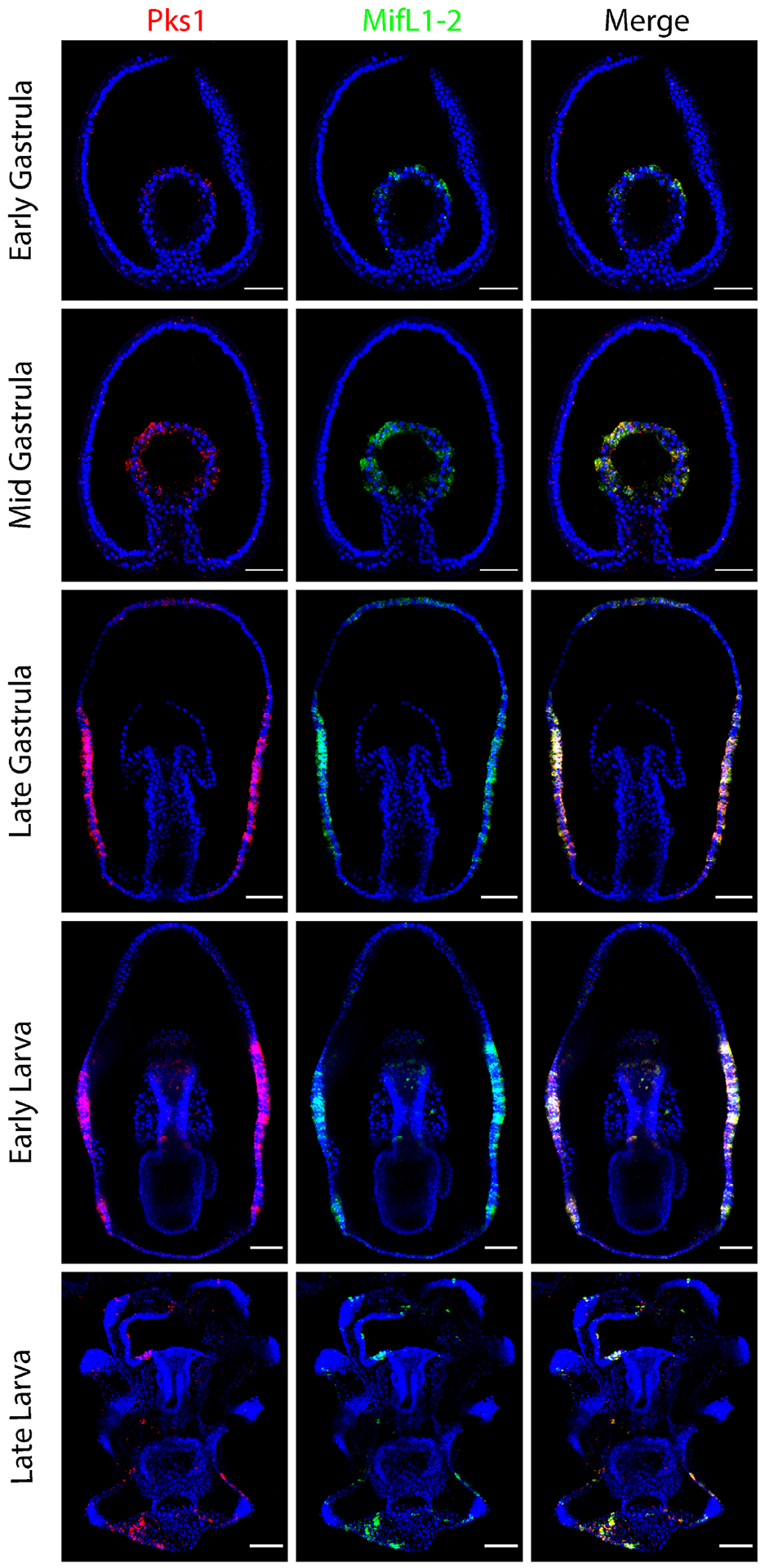
Confocal images show the expression pattern of PmPks1 and PmMifL1-2 using double FISH. PmMifL1-2 is expressed in the same cells as PmPks1 at all developmental stages. PmPks1 and PmMifL1-2 expression is restricted to mesodermal cells through the mid gastrula stage. After this stage, expression of these two genes is shifted to cells mainly found in the ectoderm. Nuclei are shown in blue with DAPI. Scale bars are 50 μm.
While the pigment cell precursors ingress into the blastocoel during gastrulation in sea urchins, in the sea star, PmPks1, PmFmo3-1 and PmMifL1-2 expression dynamically transitions from the mesoderm to the ectoderm between the mid- and late-gastrula stages (Fig. 1 & 2). After this point, expression in scattered ectodermal cells remains present through the late larval stage of development. While ectodermal expression after mid gastrula stage is always observed, endodermal expression of PmPks1 is highly variable between embryos and is not always apparent. At the late gastrula stage, three distinct patterns of PmPks1 (and thus PmFmo3-1 and PmMifL1-2) expression are observed: 1) presumptive foregut expression (Fig. 1 and Supplementary Fig. S6); 2) broad endodermal expression (Fig. 4 & 6 and Supplementary Fig. S7); and 3) no endodermal expression (Fig. 2 & 3). Variable expression of PmPks1 is also observed in the foregut at the early larval stage (present in Fig. 1, 2 & 6 and Supplementary Fig. S6 & S7; absent in Fig. 3 & 4). In late larvae, no endodermal expression of PmPks1 is detected. This constant ectodermal and variable endodermal expression profile in the larval stages of development is partially conserved between sea urchin and sea stars; when pathogenic bacteria are present in the gut, pigment cells migrate to the gut to combat the infection [26],[27]. The key difference between sea urchin and sea star larvae lies in the presence of a stable lineage of cells, the pigment cells in the sea urchin versus dynamic changes in gene expression between various lineages in the sea star. During gastrulation, sea urchin pigment cells migrate to the ectoderm whereas in the sea star PmPks1, PmFmo3-1 and PmMifL1-2 are activated de novo in ectodermal cells.
Figure 4. PmPks1 expression colocalizes with PmSix3 expression at early but not later stages of development.
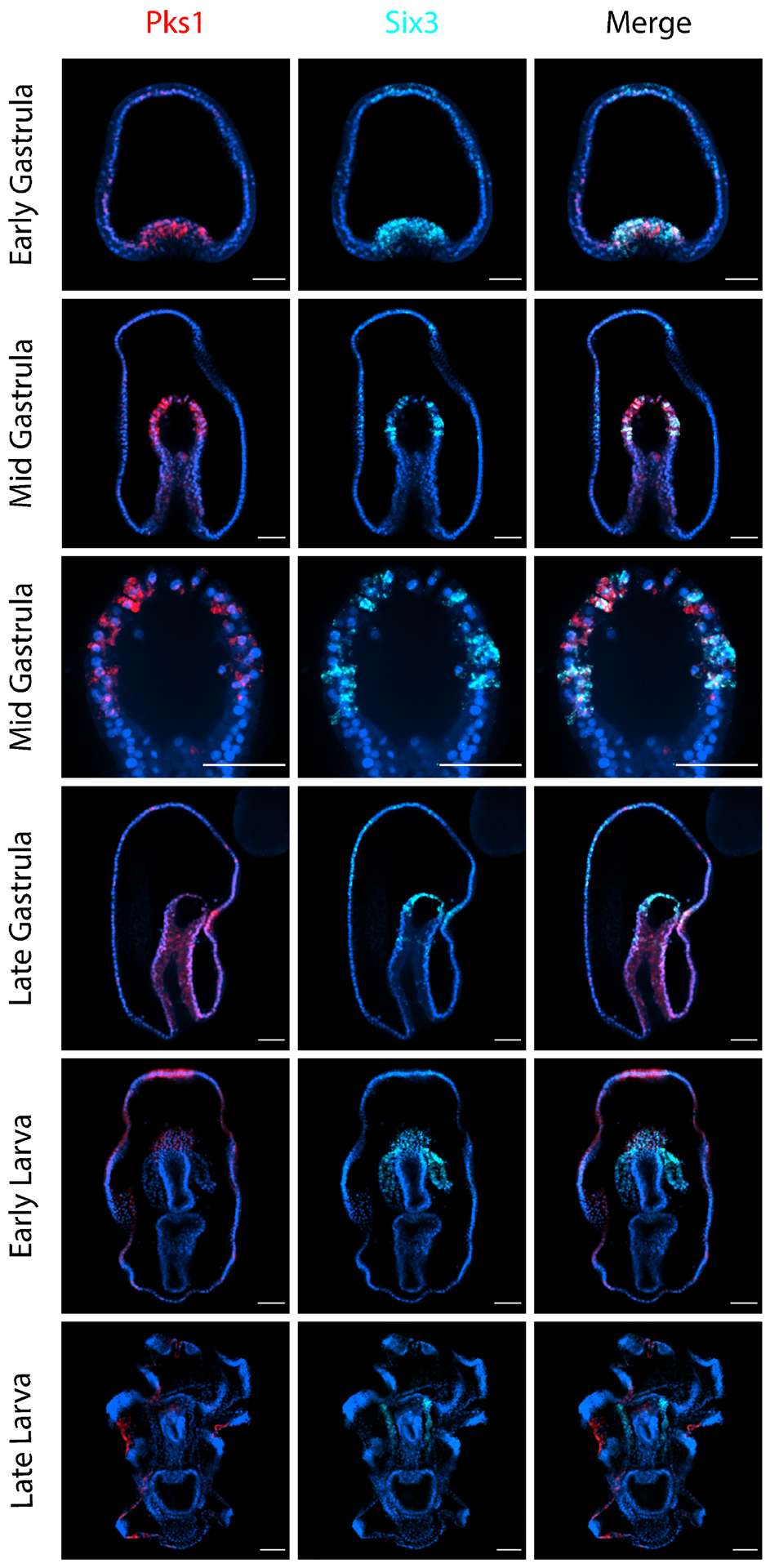
Confocal images show the expression pattern of PmPks1 and PmSix3 using double FISH. At the early gastrula stage, PmSix3 is expressed in the invaginating vegetal pole and the anterior domain of the embryo. Anterior expression of PmSix3 is present until the late larval stage. PmPks1 is also expressed in the vegetal pole during the early gastrula stage, however not the anterior region. By the mid gastrula stage, PmPks1 and PmSix3 transcripts are detected in the same cells in the archenteron. Following this stage, colocalization of PmSix3 and PmPks1 is lost, as PmSix3 expression becomes restricted to the coelomic pouches and PmPks1 expression shifts to the ectoderm. Nuclei are shown in blue with DAPI. Scale bars are 50 μm.
Figure 6. PmGataC and PmPks1 are coexpressed in the ectoderm of larvae.
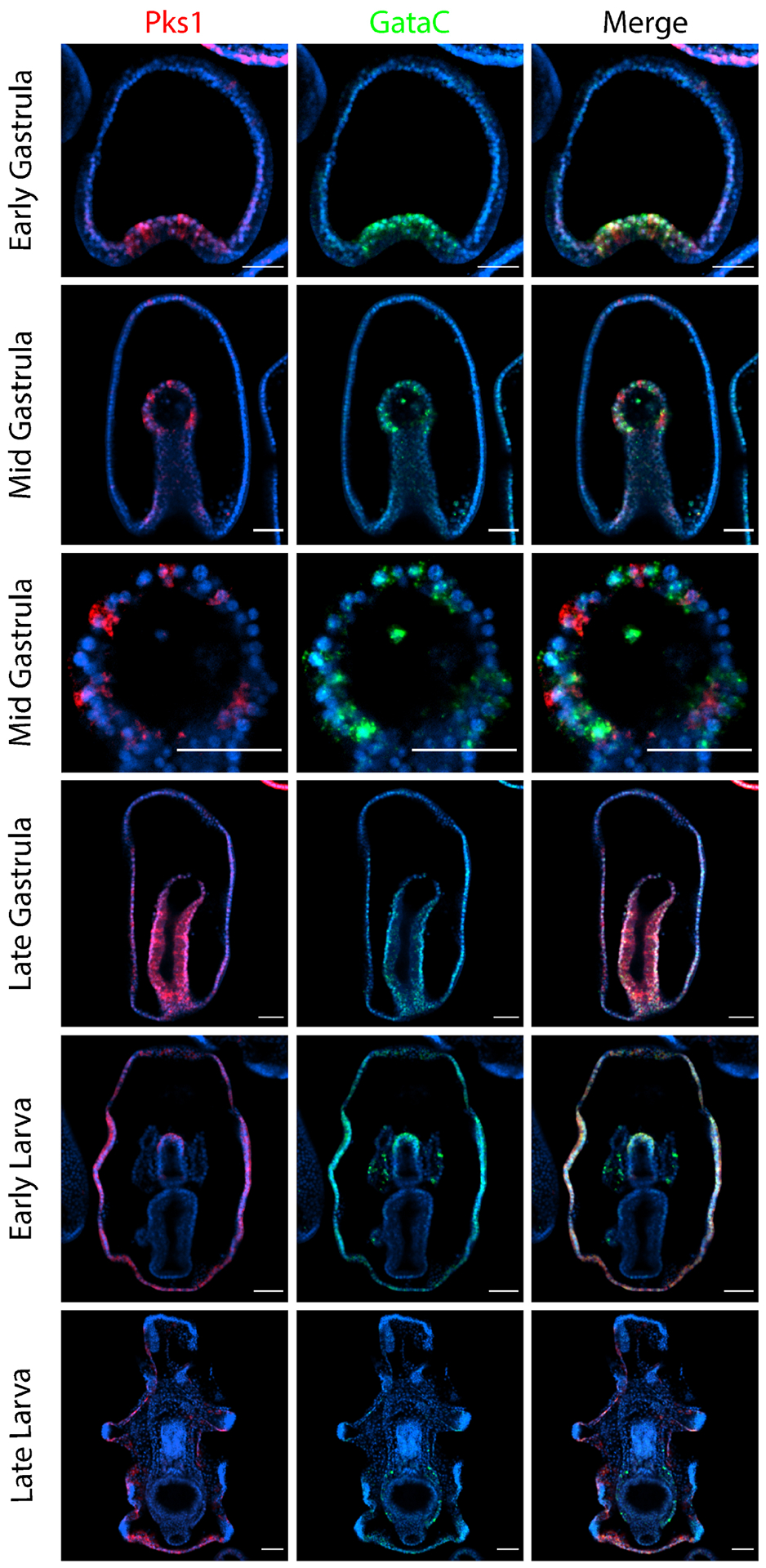
Confocal images show the expression pattern of PmPks1 and PmGataC using double FISH. PmGataC and PmPks1 are both expressed in the vegetal domain of early gastrula stage embryos. However, by the mid gastrula stage, PmPks1 and PmGataC are expressed in distinct groups of cells in the archenteron. Starting at the late gastrula stage and continuing through the late larval stage, PmPks1 and PmGataC transcripts colocalize in the same cells in the ectoderm. PmGataC is also expressed in the coelomic pouches and the posterior enterocoel. Nuclei are shown in blue with DAPI. Scale bars are 50 μm.
Figure 3. PmPks1 and PmGcm are expressed in distinct cells throughout development.
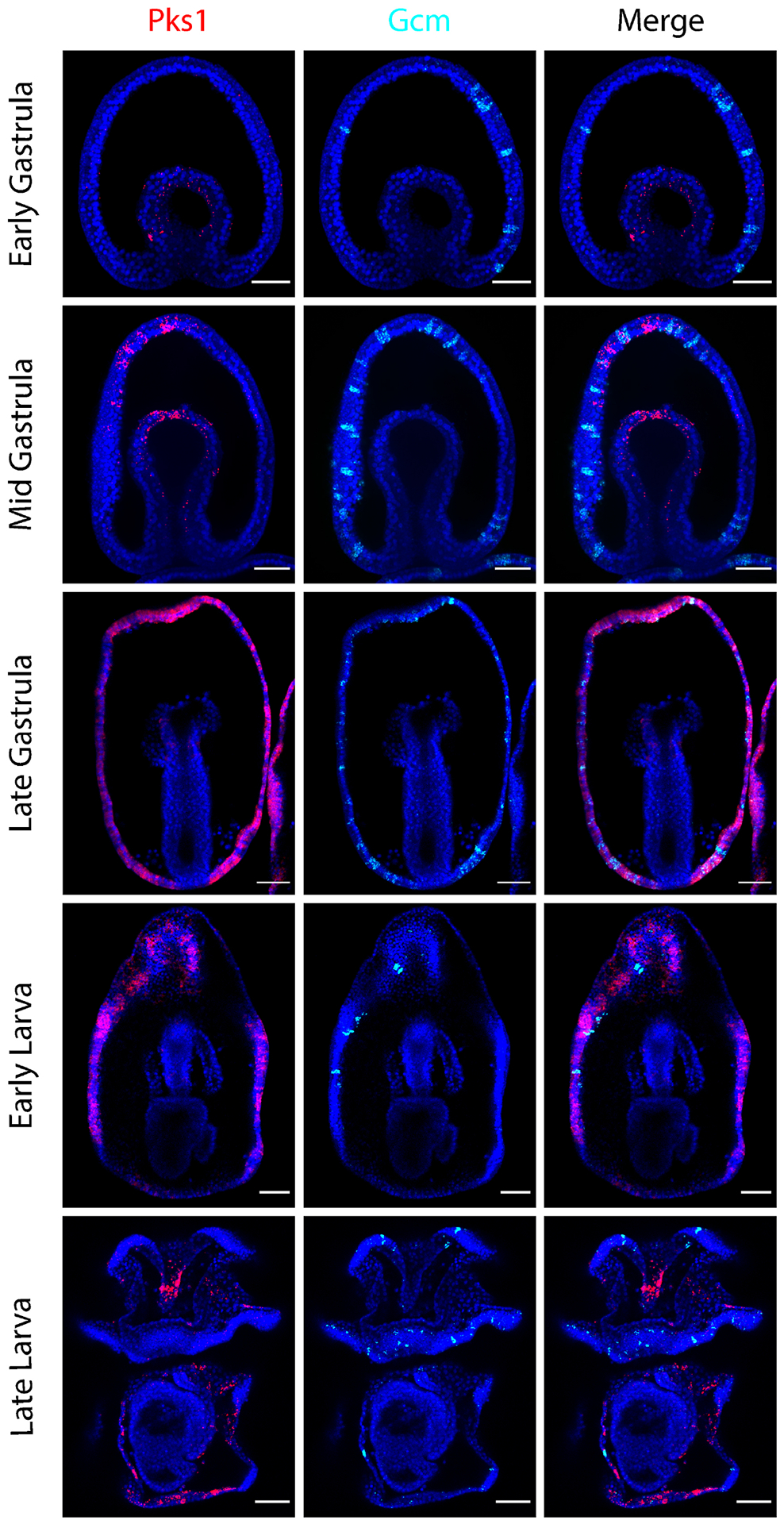
Confocal images show the expression pattern of PmPks1 and PmGcm using double FISH. As previously reported [47],[48], PmGcm is expressed by cells in the ectoderm throughout development. By the late larval stage, PmGcm-expressing cells appear mainly in the ciliary bands. At early stages of development, PmGcm is expressed in the ectoderm while PmPks1 is expressed in the mesoderm. Following the late gastrula stage transition in which PmPks1 expression appears in the ectoderm, PmPks1 and PmGcm still do not coexpress. Nuclei are shown in blue with DAPI. Scale bars are 50 μm.
The regulation of pigment genes is divergent between sea urchins and sea stars
In S. purpuratus, SpGcm is an essential transcription factor for pigment cell specification and differentiation. After the 7th cleavage, skeletogenic precursors activate SpGcm in the adjacent endomesoderm via Delta/Notch signaling [10],[11]. By the mesenchyme blastula stage, SpGcm is restricted to the aboral non-skeletogenic mesoderm by Nodal signaling in the oral non-skeletogenic mesoderm and repression by the endodermal gene regulatory network [17],[42],[43]. After the Delta/Notch input, SpGcm expression is maintained by self-activation and drives the expression of pigment cell differentiation genes, including SpPks1, SpFmo3 and SpMif5 [16],[44],[45]. SpGcm is necessary to specify cells to follow a pigment cell fate, as perturbation of SpGcm has been shown to cause a sharp reduction in differentiated pigment cells [4],[11],[16],[28]. Additionally, ectopic expression of SpGcm in skeletogenic precursors rewires these cells to follow a pigment cell fate [46].
Previous studies in P. miniata have demonstrated divergence in PmGcm localization during embryogenesis from sea urchins. PmGcm expression is absent in the mesoderm and is instead found in cells scattered throughout the ectoderm beginning at the blastula stage [47],[48]. Double FISH probing for PmGcm and PmPks1 was performed to test their coexpression (Fig. 3). As previously reported, PmGcm is expressed in ectodermal cells throughout development [47],[48]. In the larval stages, PmGcm-expressing cells are located in the ciliary bands, the structures which allow for larval motility and aid in feeding [49]. During the early phases of gastrulation, PmPks1 and PmGcm are expressed in distinct germ layers (mesoderm and ectoderm, respectively). Following the transition from mesodermal to ectodermal PmPks1 expression in the later phases of gastrulation, PmPks1 and PmGcm continue to be expressed in mostly nonoverlapping cell populations. Additionally, PmPks1 transcripts appear in far more cells than PmGcm transcripts. Knowing that Gcm is an activator of Pks in the sea urchin pigmented cells, we tested whether Gcm could control Pks expression in the few cells were these two genes overlap in the sea star embryos. To this aim, we knocked down PmGcm expression by injecting oocytes with a morpholino antisense oligonucleotide and found that the expression of PmPks1 was not abrogated as may be expected for expression in distinct cells. Pks mRNA actually increased in the absence of Gcm, while PmFmo3-1 and PmMifL1-2 mRNA levels showed no change (Supplementary Fig. S11). Given these observations, we conclude that PmGcm does not regulate pigment genes, and could instead be acting indirectly, between cells, as a repressor of Pks in sea star, whereas in sea urchins it is a central activator of Pks1 and other genes in the pigmentation pathway (14, 44). Thus, the regulatory paradigm governing these pigment cell effector genes has diverged significantly between sea urchins and sea stars.
Conserved regulatory exclusion from the blastocoelar cell lineage
Sea urchin and sea star larvae possess a population of transparent mesodermal mesenchymal cells that migrate through the blastocoel and function as immunocytes [26],[27],[50],[51]. In S. purpuratus, blastocoelar cells originate from the oral non-skeletogenic mesoderm and expresses the transcription factor SpErg [17]. In P. miniata, the blastocoelar lineage also expresses PmErg, first in the vegetal plate at the blastula stage and then in mesenchymal cells after migration from the archenteron during gastrulation [52],[53].
Do the PmPks1+ mesodermal cells in the sea star also express Erg? Double FISH was performed to test this premise (Supplementary Fig. S6) and results show that throughout development, PmPks1 and PmErg are not expressed in the same cells. Unlike in sea urchins though, PmPks1 and PmErg-expressing cell populations are not spatially segregated, but are rather intermixed in early gastrula stage. Overall though, the exclusion of Pks transcripts from blastocoelar precursors marked by Erg during gastrulation is conserved between sea urchins and sea stars.
Mesodermal PmPks1-expressing cells are coelomic pouch precursors
Six3 and Pax6 are components of a gene regulatory network implicated in coelomogenesis in both S. purpuratus and P. miniata [52],[56]. Additionally, Six3 has been shown to possess pleiotropic functions in sea urchin and sea star embryos, also serving as an anterior determinant [48],[57].
To investigate the possibility that PmPks1, PmFmo3-1 and PmMifL1-2 are expressed in the coelomic mesoderm during gastrulation, double FISH was performed for PmSix3/PmPax6 and PmPks1 (Fig. 4 & Supplementary Fig. S7). As previously reported [48],[52], PmSix3 transcripts are detected in the anterior ectoderm and broadly in mesodermal cells during the early gastrula stage (Fig. 4). By the mid gastrula stage, PmSix3 expression is restricted to a subset of mesodermal cells. During the transition to the early larval phase, PmSix3 expression fades from the anterior ectoderm yet remains consistently expressed in the coelomic mesoderm throughout development. PmPks1 expression colocalizes with PmSix3 expression at the early stages of development, most notably at the mid gastrula stage. Whereas PmSix3 transcripts are ubiquitous in the early gastrula mesoderm, PmPks1 transcripts are found only in certain cells. By the mid gastrula stage, though, both genes are clearly expressed in the same cells in the archenteron. This colocalization is lost at the end of gastrulation in the transition to larval stages as the coelomic pouches begin to form (Fig. 4). A similar pattern of strong mesodermal colocalization between PmPks1 and PmPax6 until the mid-gastrula stage is also observed (Supplementary Fig. S7). Due to the observed colocalization of PmPks1 transcripts with PmSix3 and PmPax6 transcripts at the mid gastrula stage, we conclude that PmPks1, PmFmo3-1 and PmMifL1-2 are expressed in the coelomic mesoderm until the end of gastrulation, but their expression is lost in the true coelom of the larval stages.
Requirement of Notch input for mesodermal Pks expression is conserved
Delta/Notch signaling directly activates SpGcm expression in pigment cell precursors of S. purpuratus [11]. Once present, SpGcm upregulates SpPks1, SpFmo3 and SpMif5 expression [16],[44]. If Delta/Notch signaling is inhibited by early treatment of embryos with the γ-secretase inhibitor DAPT, pigment cells fail to form [58],[59]. Thus, Notch indirectly activates the expression of pigment cell effector genes in sea urchin embryos.
Previous reports have demonstrated the critical role of Delta/Notch signaling in specifying the coelomic mesoderm in P. miniata [52]. Embryos treated with DAPT displayed an expansion of blastocoelar cell precursors expressing PmErg and a reduction in coelomic pouch precursors expressing PmSix3 and PmPax6 in the archenteron. To determine if Notch has a conserved role in activating mesodermal Pks expression in sea stars as it does in sea urchins, Notch activity was inhibited using DAPT as previously described [52]. The results (Fig. 5) demonstrate that Notch is required to specify the coelomic mesoderm and to activate PmPks1 expression. While the DMSO-carrier-treated control embryos display interwoven cells expressing either PmPks1 or PmErg (blastocoelar cell precursor marker), DAPT-treated embryos contain almost exclusively PmErg-expressing cells. Thus, Delta/Notch signaling remains the most upstream factor leading to mesodermal Pks expression in both sea urchin and sea star embryos.
Figure 5. Notch signaling is required to establish mesodermal PmPks1 expression.
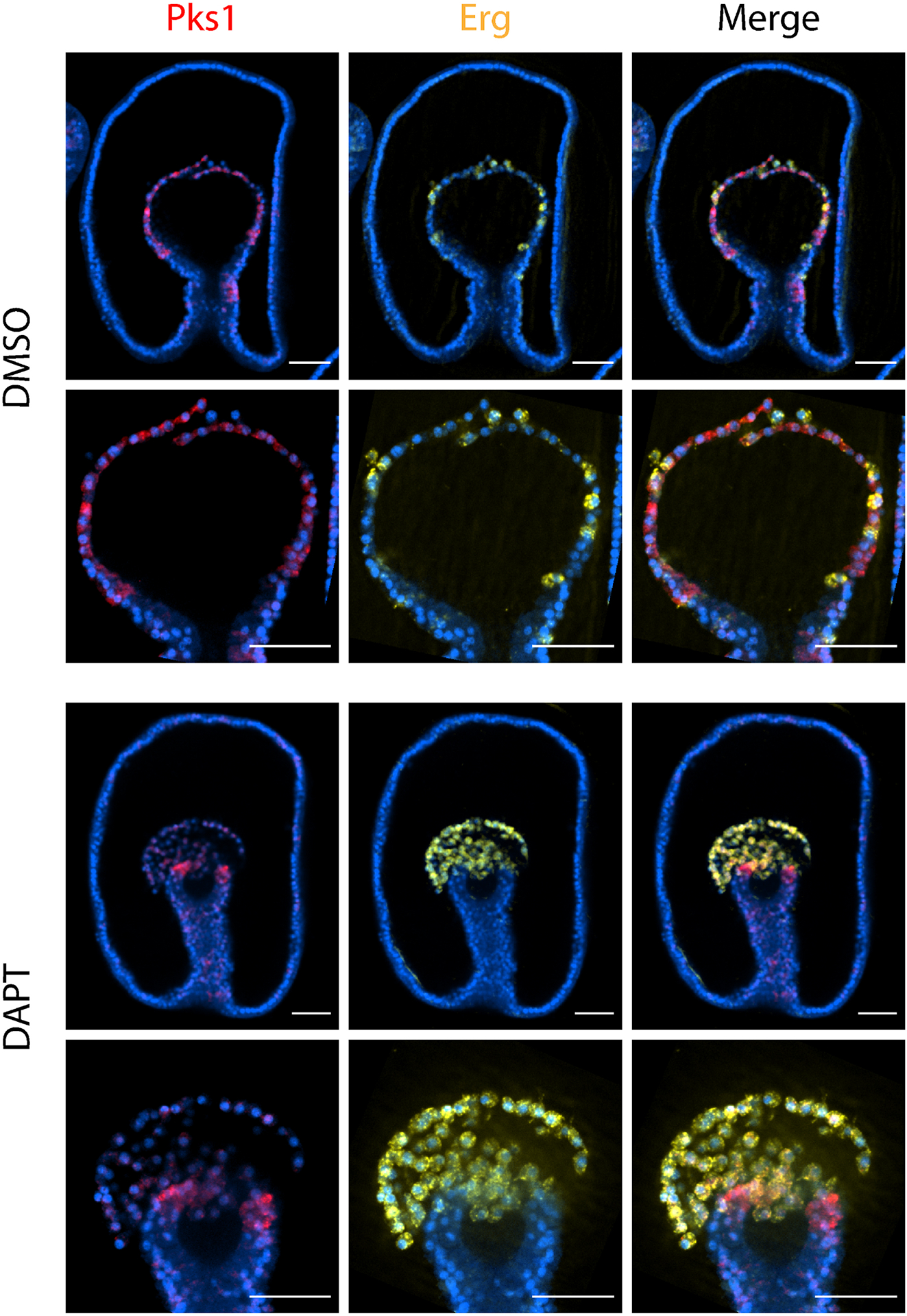
Confocal images show the expression pattern of PmPks1 and PmErg using double FISH at the mid gastrula stage. Delta/Notch signaling was inhibited by treating embryos with 32 μM DAPT at the 2 cell stage. Control embryos received an equivalent volume of DMSO. In control embryos, cells express either PmPks1 or PmErg in the archenteron. When Notch is inhibited, PmPks1 expressing cells are lost and the vast majority of mesodermal cells express PmErg. Nuclei are shown in blue with DAPI. Scale bars are 50 μm.
The GATA-binding factor is expressed in PmPks1+ cells of the larva
To identify potential transcriptional mechanisms that may regulate PmPks1, PmFmo3-1 and PmMifL1-2, MussaGL pairwise alignment software was applied on the genomic sequences 10kb upstream of these genes. As shown in Supplementary Fig. S8, multiple instances of a shared sequence are present in the region upstream of each gene. TFBind software was then used to determine whether this sequence contained potential transcription factor binding sites [60]. Hits were returned for the transcription factors GATA1, GATA2 and GATA3, which have known roles in hematopoietic regulation in mammals [61]. P. miniata has two genes encoding products in the GATA-binding protein family, PmGataC (also referred to as PmGata1/2/3) and PmGataE (also referred to as PmGata4/5/6). PmGataE is required for endoderm specification, while PmGataC is expressed in the mesoderm through gastrulation [47],[53]. In S. purpuratus embryos, SpGataC is expressed in the oral mesoderm (blastocoelar cell precursors) while it is repressed by SpGcm in the aboral mesoderm (pigment cell precursors) [62].
Since PmGataC is known to be expressed in the sea star mesoderm and is the closest ortholog of GATA1, GATA2 and GATA3, its expression pattern was analyzed in relation to PmPks1 expression using double FISH (Fig. 6). As previously reported, PmGataC transcripts are broadly present in the mesoderm in the early stages of gastrulation [47],[53]. By the mid gastrula stage, PmGataC expression is restricted to certain cells in the archenteron. PmPks1 transcripts are located in cells adjacent to those expressing PmGataC. This pattern is much like the one observed for PmErg and PmPks1 in Supplementary Fig. S6. Due to this similarity and the known expression of SpGataC in sea urchin blastocoelar cell precursors [62], it is likely that PmGataC is expressed in the sea star blastocoelar cell precursors, though this was not assessed directly. Once in the late gastrula stage, though, PmGataC expression is lost from the blastocoelar cell lineage. Curiously, PmGataC transcripts colocalize with PmPks1 transcripts in the ectoderm, most readily apparent at the early larval stage. PmGataC is also expressed in the coelomic pouches and the germline-containing posterior enterocoel in the larval stages. In light of the predicted PmGataC binding sites and the observed expression pattern, PmGataC may activate PmPks1, PmFmo3-1 and PmMifL1-2 in the ectoderm at later developmental stages.
Comparative analysis of pigment cell-specific factors using scRNA-seq
To further test the expression of pigment cell transcript localization with a discovery mode, we utilized single cell RNA sequencing (scRNA-seq) datasets representing early development for S. purpuratus and P. miniata [16],[63]. We first analyzed transcript distribution across clusters, focusing on the gastrula stage as we could detect the expression of most genes of interest (Fig. 7A&B). In sea stars, PmPks1, PmFmo3-1 and PmMifL1-2 transcripts are detected across many clusters (Fig. 7A), akin to the observed patterns in FISH images. PmGcm is enriched in cluster 9, likely representing the positive cells seen by in situ hybridization (Fig. 3). PmSix3 is enriched in the mesodermal cluster 3 while also being expressed in ectodermal clusters, consistent with the FISH staining. Contrary to the broad distribution of pigment cell-specific transcripts appearing across clusters in sea stars, specific clusters (2 and 13) express these genes in sea urchins (Fig. 7B).
Figure 7. Comparative scRNA-seq analysis of sea star and sea urchin gastrulae.
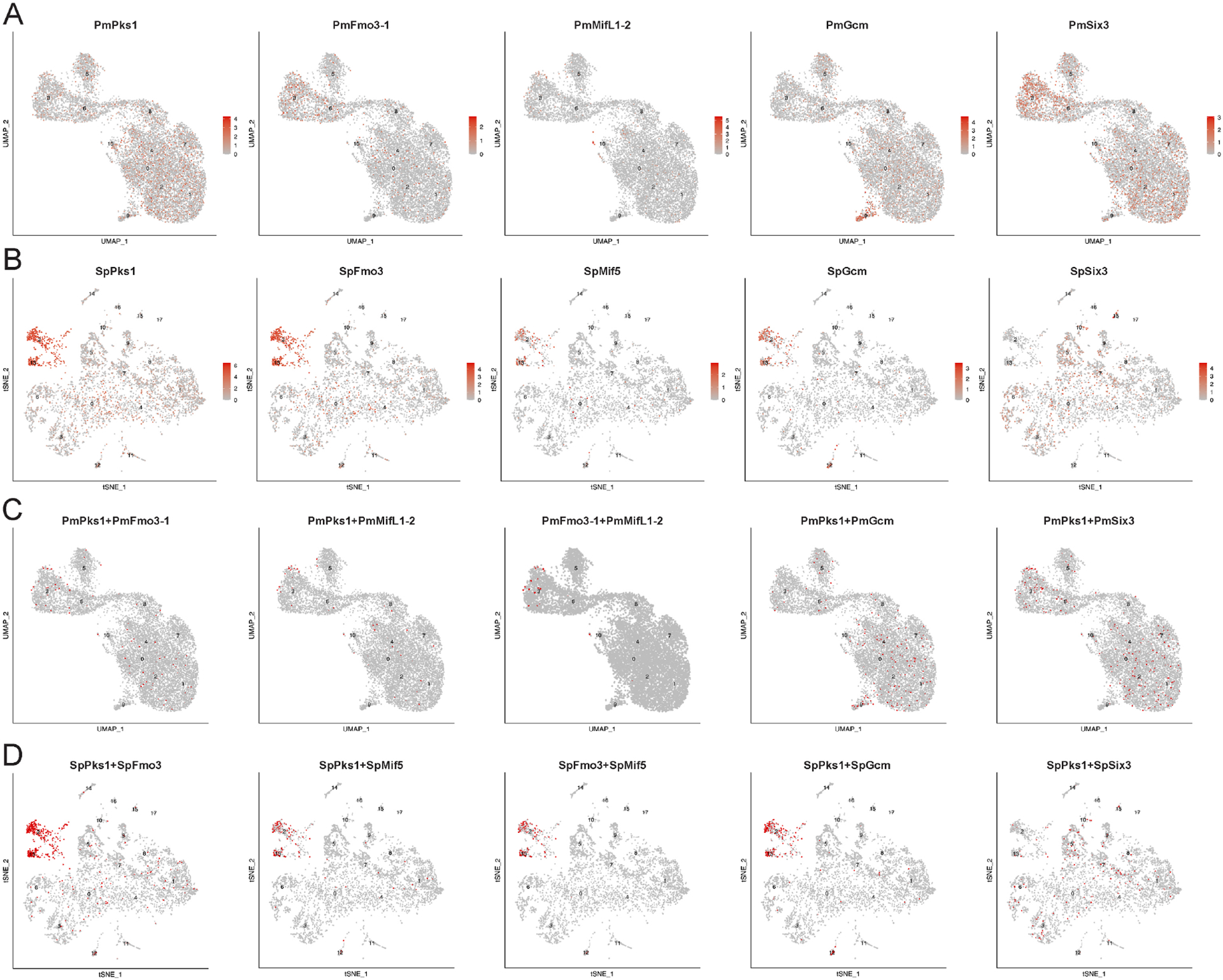
Cluster annotations are shown in Supplementary Table S1 for sea star data [63] and Supplementary Table S2 for sea urchin data [16]. A-B. Feature plots for selected transcripts for sea star (A) and sea urchin (B) at gastrular stages. C-D. Coexpression analysis highlights cells double positive for indicated transcripts in red for sea star (C) and sea urchin (D).
We then utilized co-expression analysis to test whether the patterns observed in the double FISH images were consistent using an orthogonal approach. We utilized pairwise comparison for PmPks1, PmFmo3-1 and PmMifL1-2 to mitigate transcript dropout, finding that these transcripts are indeed frequently co-expressed (Fig. 7C). Given that PmPks1 was observed to become broadly expressed in the ectoderm immediately following the mid gastrula stages (into ectodermal cells both having and lacking PmGcm expression), it appears likely that the scRNA-seq data likely captured this transition. Importantly, out of 1143 cells detected in the mesodermal cluster 3, only 0.9% were positive for both PmPks1 and PmGcm, further supporting the notion that PmGcm does not drive mesodermal activation of PmPks1 expression as it does in sea urchins (Supplementary Fig. S9). Additionally, we observed differences between the expression of pigment cell-transcripts and PmSix3/SpSix3. In sea stars, we observed the co-expression of PmPks1 and PmSix3 in cluster 3 (Fig. 7C), supporting our hypothesis that PmPks1 is expressed in the coelomic mesoderm. However, SpSix3 was not found to be expressed in the clusters enriched for pigment cell-specific genes (Fig. 7B&D). These findings support the paradigm of two separate lineages for pigment cells and coelomic mesoderm cells in sea urchin versus a single integrated lineage in gastrulae of sea stars.
A unique molecular signature is present in sea star larvae
Currently, SpPks1 and SpFmo3 are the only known enzymes implicated in larval sea urchin pigment biosynthesis [4],[14]. Given the finding that P. miniata co-express orthologs of these genes (PmPks1 and PmFmo3-1) throughout development (Fig. 1), the generation of molecules structurally related to sea urchin pigments may occur in sea stars. To investigate the hypothesis that sea star larvae produce pigment molecules that are simply not sufficiently concentrated to detect in live larvae, chemical extraction followed by mass spectroscopy was performed on both sea urchin and sea star larvae. Previous reports have shown that both sea urchin larvae and adults produce a variety of naphthoquinone pigments, including echinochrome A and a multitude of spinochrome molecules [1]-[4].
We found that as previously reported [1], both echinochrome and spinochrome molecules are present in larval S. purpuratus (Supplementary Table S3). Echinochrome A is the most abundant pigment molecule present by a considerable margin. Spinochromes E, B, 282 and C were also detected. No known naphthoquinone molecule was identified in the sea star larvae extract despite the appearance of a light orange pigment (Supplementary Fig. S10). The most abundant peak appeared at 253.1431 m/z, just roughly 0.9 m/z units from the mass of spinochrome E. While no molecular formula could be assigned to this peak, its affinity for the extraction solvent and molecular mass suggest that it may result from a molecule in the naphthoquinone family.
Discussion
Ancestral coupling of pigment genes
Larval pigment cells in echinoderms are an evolutionary novelty found only in echinoids and have important functions in immunity and development [26],[27],[64]. Such roles are accomplished due to the expression of a set of pigment cell-specific genes. However, the activities of orthologs to these genes in pigment cell-lacking echinoderm larvae have until now yet to be identified. Here, orthologs of SpPks1, SpFmo3 and SpMif5 were identified in sea stars, and spatial and temporal gene expression profiles throughout development were assessed. Strikingly, many characteristic features of these genes observed in sea urchin embryos are also found in sea star development. The orthologs, PmPks1, PmFmo3-1 and PmMifL1-2, are stably co-expressed from the early gastrula stage through the late larval stage. In sea urchins, these genes are likewise consistently expressed throughout development in a pigment cell lineage [14],[16],[18],[19]. Both sea urchins and sea stars initiate the expression of these genes in the mesoderm. As gastrulation proceeds, transcripts remain present in a lineage of migratory cells in sea urchins whereas they are dynamically transitioned to a new population of cells in sea stars. Though cell migration and dynamic changes in expression are mechanistically different processes, they both functionally result in pigment cell-specific gene transcripts in or near the ectoderm in larval stages. The co-expression of these genes in both sea urchins and sea stars suggests an ancestral regulatory paradigm to activate each gene within the same cells. This simplifies the emergence of larval pigment cells, as ancestral echinoids already possessed a regulatory kernel containing genes necessary for pigment cell function, such as pigment production.
Though the characterization of these pigment cell-specific genes in sea stars provides insights into the emergence of sea urchin pigment cells, it also introduces new questions. First, what are the products of these enzyme-encoding genes in sea star larvae? SpPks1 and SpFmo3 are required for sea urchin larval pigment biosynthesis, though no such molecules are readily apparent in sea star larvae [4],[14],[28]. As shown in Supplementary Fig. S10, sea star larvae may produce a naphthoquinone molecule. Whether the most abundant peak observed, just 0.9 m/z from spinochrome E, is actually a molecule related to sea urchin pigments remains unclear. If indeed it is, PmPks1 and PmFmo3-1 would present the most likely candidates for its production. In future experiments, perturbation of both PmPks1 and PmFmo3-1 followed by chemical extraction and LC-MS could address this possibility. If PmPks1 and PmFmo3-1 are found not to synthesize a naphthoquinone molecule, it is likely that differences in protein coding sequences are responsible for pigment in sea urchins and the lack thereof in sea stars. We find it striking that in the coelomocytes of the sea star, Pks and Fmo 3 mRNA is readily detectable, yet pigment in the coelomocytes is rarely seen. Since the Pks protein family is known to create diverse polyketide products across different species, these enzymes are the most likely candidates to explain the restriction of naphthoquinone pigment synthesis to only sea urchins and other echinoids [31]. Specific amino acid changes, like the alcohol dehydrogenase domain present in SpPks1 yet absent from PmPks1, may underlie the abilities of the two enzymes to create distinct polyketide products (Supplementary Fig. S3A). Moreover, what is the broader functionality of PmPks1, PmFmo3-1 and PmMifL1-2? In sea urchin larvae, the immune role of pigment cells is mediated by many cell-type specific genes, including those that synthesize the antimicrobial pigment [7],[27]. Whether an immunological function for these genes also exists in sea star larvae remains an open question.
Regulatory divergence despite downstream similarity
Despite the similarities in the expression patterns of pigment-cell specific genes between sea urchins and sea stars, the architecture governing gene activation has significantly diverged. The developmental gene regulatory networks of both sea urchins and sea stars are shown in Fig. 8. The two lineages demonstrate a shared segregation of pigment-cell specific gene expression from blastocoelar cell precursors (expressing Erg) early in development. This, along with a common Notch input, make up the known few regulatory conservations between sea urchins and sea stars. Mesodermal expression of Gcm appears to be the focal point for pigment cell evolution. In sea urchins, SpGcm is required for specification of pigment cells and activation of pigment cell-specific genes [14],[16]. Previous observations that PmGcm is expressed in the ectoderm suggested the lack of pigment cells in sea stars could be attributed to the absence of pigment cell gene network activity in the mesoderm [47]. However, this is not the case, as orthologs of pigment cell-specific genes are indeed expressed in the mesoderm early in development independently of PmGcm. Additionally, PmGcm is not activated by Notch as it is in sea urchins [47]. Therefore, the evolutionary transitions in sea urchins which allowed Notch to activate SpGcm may have allowed for mesodermal SpGcm expression, which in turn could drive expression of the preformed regulatory network consisting of SpPks1, SpFmo3 and SpMif5. Future comparative analyses of the regulatory elements of these genes are needed to evaluate this hypothesis.
Figure 8. Comparative gene regulatory networks of sea urchins and sea stars.
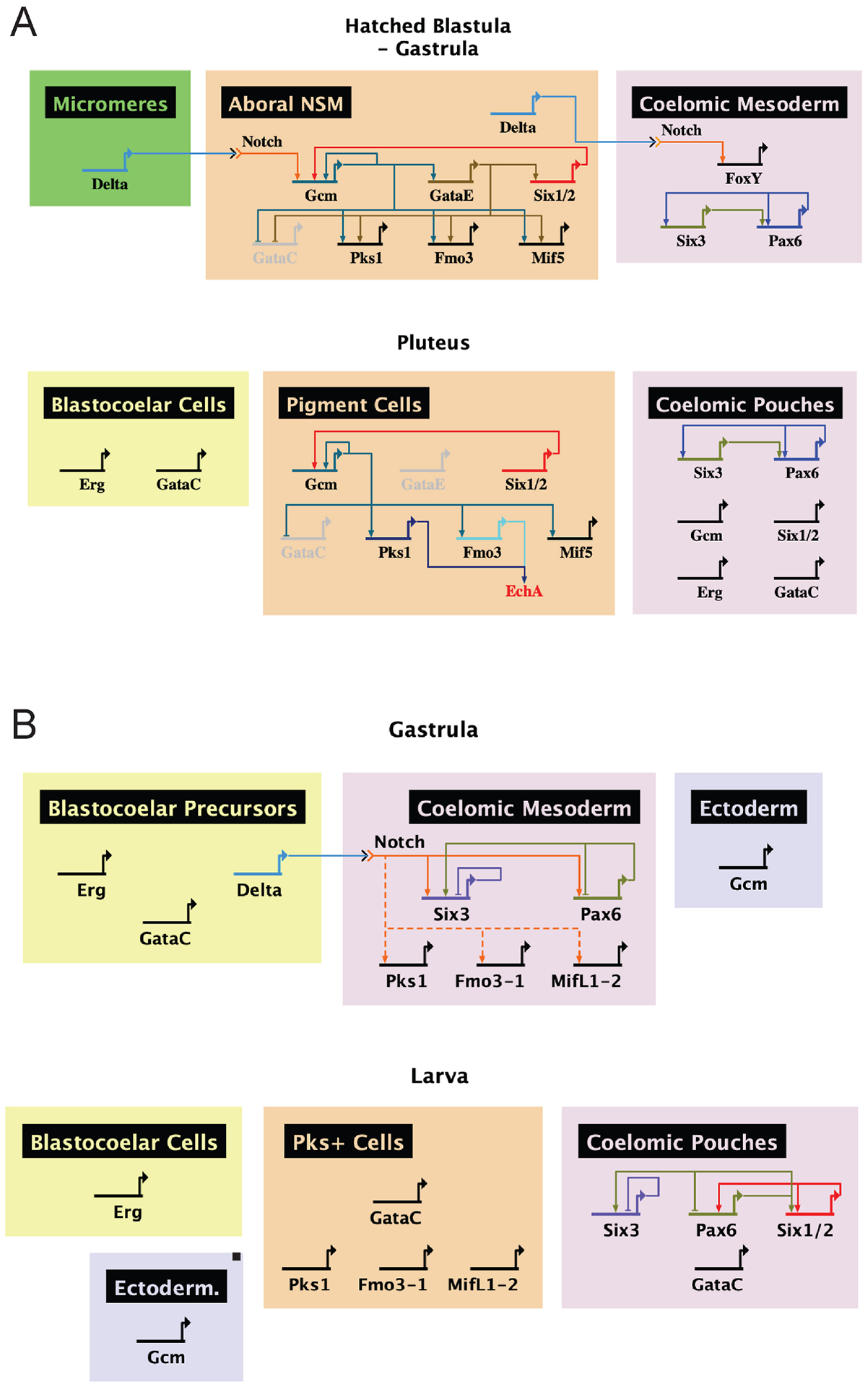
Networks were constructed using BioTapestry software. A. The sea urchin has a stable lineage of pigment cells throughout development specified by Notch. These cells are marked by SpGcm, which activates the expression of SpPks1, SpFmo3 and SpMif5. SpPks1 and SpFmo3 synthesize the pigment Echinochrome A. Relationships between genes are described elsewhere [14],[17],[18],[45],[56],[58],[62],[72]. B. The sea star lacks a lineage of cells expressing PmPks1, PmFmo3-1 and PmMifL1-2. The mesodermal expression of these genes during gastrulation is conserved, as is the requirement of Notch, though they do not depend on PmGcm for expression. Additional work is required to determine if Notch activation is direct or proceeds through an intermediate as it does in sea urchins. Later in development, PmPks1, PmFmo3-1 and PmMifL1-2 are expressed in the ectoderm with PmGataC. Relationships between genes are derived from this work and elsewhere [52].
Pigment cell lineage versus dynamic gene expression
While the early mesodermal expression of pigment cell-specific genes is conserved between sea urchins and sea stars, substantial differences emerge towards the end of gastrulation. Sea urchin pigment cells migrate whereas mesodermal cells of sea stars lose PmPks1, PmFmo3-1 and PmMifL1-2 transcripts that are later activated their expression in the ectoderm. In sea urchins, SpGcm represses SpGataC, as the two genes mark the aboral and oral mesodermal regions in the vegetal plate, respectively [17]. Until larval stages, SpGcm sustains the transcription of pigment cell-specific genes in a stable cell lineage [14],[16]. In the sea star, though, PmGcm does not drive PmPks1, PmFmo3-1 and PmMifL1-2. These genes are first expressed in the coelomic mesodermal cells distinct from those expressing PmGataC. Interestingly, PmPks1, PmFmo3-1 and PmMifL1-2 expression then shifts to the ectoderm, colocalizing with PmGataC. The observed colocalization further demonstrates the divergent function of Gcm, as SpGcm represses SpGataC in SpPks1, SpFmo3 and SpMif5 expressing cells. The transition to ectodermal expression of PmGataC coincides with that of PmPks1, PmFmo3-1 and PmMifL1-2, though more substantial evidence is required to assess whether PmGataC is responsible for the dynamic shift in PmPks1, PmFmo3-1 and PmMifL1-2 expression.
Association between sea star coelomic mesoderm and sea urchin mesodermal cell types
The results presented in this manuscript demonstrate that pigment cell-specific genes are integrated into the mesoderm gene regulatory network prior to larval stages in pigment cell-lacking sea stars. Specifically, our results showed that this GRN is excluded from the mesoderm that will give rise to the blastocoelar cells. We propose that a pigment cell-specific GRN is active in the other types of mesodermal cells, specifically in the precursors of muscles and coelomic pouches. This finding parallels what has been discovered regarding the evolution of a larval skeleton, a structure formed by another mesodermal cell population observed in sea urchins yet absent from sea stars. The transcription factor Alx1 is required for skeletogenic primary mesenchyme cell specification in sea urchins and sea cucumbers, and likely brittle stars too [65]-[67]. In sea stars, PmAlx1 is expressed in the coelomic mesoderm notwithstanding the absence of a skeletogenic lineage, much like PmPks1, PmFmo3-1 and PmMifL1-2 shown here [67]. Moreover, we found that the main players of the sea urchin pigment cell GRN are expressed by subsets of ectodermal cells in the larval stages, suggesting a novel function for these genes in the sea star that diverged from the sea urchin larva.
Together, these results highlight that genes involved in the pigment cell function in sea urchins are dynamically expressed during the sea star embryonic development and can be key to understand the appearance of mesodermal pigment and skeletogenic cell lineages in sea urchin development.
Conclusion
In summary, this work has uncovered key elements of evolutionary transition between sea urchin and sea star developmental processes underpinning the appearance of larval pigment cells. Despite conserved expression patterns of downstream pigment cell-specific genes, their regulation has diverged significantly. Given the unexpected similarities, a small number of developmental transitions were likely capable of giving rise to a novel cell type in sea urchin larvae, perhaps in addition to key changes in enzymatic activities. A more detailed examination of regulatory mechanisms that make the Pks1+/pigment cell type will help reveal specific changes allowing for the emergence of a new and critical cell lineage in this animal taxa.
Materials and Methods
Phylogenetic and Genomic Analysis
Phylogenetic analysis was done using MEGA X[68]. Amino acid sequences were obtained from NCBI and echinobase.org [38]-[40], [73]. The maximum likelihood method was used for phylogenetic reconstruction, with a bootstrap value of 500. Initial trees for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the JTT model, and then selecting the topology with superior log likelihood value. 70 amino acid sequences were analyzed, with gene names and NCBI/Echinobase accession numbers located on the tree. For MussaGL analysis, P. miniata V2.0 Scaffolds from Echinobase were used. The sequencing data generated here have been made publicly available at Gene Expression Omnibus [https://www.ncbi.nlm.nih.gov/geo/] (GSE155427).
Animals and Culture
Adult Patiria miniata and Strongylocentrotus purpuratus were obtained from info@scbiomarine.com and peterhalmay@gmail.com, respectively, off the California coast and kept in artificial seawater at 16°C. Fertilization and embryo culture was performed as previously described [69],[70]. Sea star gametes were obtained by surgical removal and dissection of gonads from adult sea stars. Sea star oocytes were matured by treatment with 1 μM 1-methyladenine (Fisher Scientific) in filtered seawater from the Marine Biological Laboratory (MBL) for 45 minutes. Sea urchin gametes were obtained by shaking adult sea urchins or by intracoelomic injection of 0.5M KCl. Mature sea urchin and sea star eggs were incubated at room temperature for 10 minutes with a 1:1000 dilution of sperm in filtered sea water for fertilization. Fertilized eggs were decanted and washed with filtered sea water at least 3 times to remove excess sperm, and then transferred to a 6 well plate or a large beaker with electric stirrer for development. Embryos were cultured at 16°C in filtered seawater. If late stage larvae were desired, larvae were fed algae approximately 4 days after fertilization.
RNA Isolation and qRT-PCR
RNA was isolated from embryos and larvae using the RNeasy Micro Kit (Qiagen) and cDNA was produced using the Maxima First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). Quantitative reverse transcriptase PCR (qRT-PCR) was performed with cDNA and the primer sequences in Supplementary Table S4 using the Maxima SYBR Green/ROX qPCR Master Mix (Thermo Fisher Scientific). Triplicates were performed for each reaction to account for technical variability. Cycle threshold (Ct) values were averaged within triplicates and normalized to ubiquitin Ct values.
RNA Fluorescent in situ Hybridization (FISH)
FISH was performed as previously described [71]. Primers for probe generation were selected using the Primer3 web application. Primer sequences are located in Supplementary Table S5. Selected primers were used to amplify embryonic sea star cDNA by polymerase chain reaction (PCR) using Platinum Taq polymerase (Invitrogen). Amplified sequences were cloned into pGEM-T Easy Vector (Promega) and transformed in E. coli XL-1 blue competent cells, grown on Luria Broth agar plates. DNA was linearized by M13 primer PCR of transformed plasmids. Labeled probes were transcribed from linearized DNA using digoxigenin-11-UTP or fluorescein-12-UTP (Promega), or transcribed using unlabelled NTPs and labelled with dinitrophenol (Mirus) following kit instructions. Embryos and larvae were fixed in 4% paraformaldehyde in filtered sea water overnight at 4°C. Fixed samples were washed in MOPS buffer prior to hybridization. Alternatively, fixed samples could be dehydrated in 70% ethanol and stored at −20°C for later use. Fixed samples were incubated with a 1:2000 dilution of labeled probe(s) in a 70% formamide hybridization buffer for 5 to 10 days at 60°C. Following hybridization, signal was developed with fluorophore-conjugated tyramide (1:400 reagent diluents, Perkin Elmer) using maleic acid buffer to wash between steps. Nuclei were stained using a 1:10000 dilution of 4′,6-diamidino-2-phenylindole (DAPI, Fisher Scientific). Samples were imaged using the Olympus SpinSR10 Spinning Disk Confocal Super Resolution Microscope (Olympus), the Zeiss LSM 800 Confocal Laser Scanning Microscope (Zeiss) or the Nikon Eclipse Ti2 Microscope (Nikon).
Delta/Notch Perturbation
Notch signaling was inhibited in sea star embryos by treatment with the γ-secretase inhibitor N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl Ester (DAPT). 32 μM DAPT in DMSO or an equivalent volume of DMSO alone was added to cultures at the 2 cell-stage as described previously [52].
Perturbations using morpholino antisense oligonucleotides
Translation-blocking antisense morpholino (MO) against Pm Gcm (5’-TACCGGCCACTTGCTGATCCAT-3’) was synthesized by Gene-Tools; and used at a concentration of 1 mM. MO was injected with 10,000 MW fluorescent dextran (injection solution). Immature oocytes were injected, cultured overnight at 16 degrees before being matured and fertilized. For each condition, 30 of these injected embryos were used for qPCR analysis at 48hpf.
scRNA-seq Analysis
Single cell RNA-seq datasets are described for Sp[16] and Pm[63]. Feature plots and coexpression plots were obtained using the R package Seurat.
Pigment Extraction
Pigment extraction was performed as previously described [4]. Sea urchin and sea star embryos were cultured until larval stages. Larvae were dissolved in 1 mL of aqueous 6M HCl for an hour. Samples were centrifuged at maximum speed for 1 minute and supernatants were transferred to new tubes. Diethyl ether was added to supernatants in a 1:1 ratio. Following a brief vortex and another 1 minute centrifuge spin at maximum speed, the diethyl ether layer was moved to a new tube and partitioned with 200 μL of aqueous 5M NaCl. The diethyl ether layer was moved to a new tube, and the partitioning process was repeated 2 additional times. The final isolated diethyl ether layer was evaporated until dry. Dry samples were resuspended in methanol and analyzed by liquid chromatography-mass spectrometry.
LC-MS Analysis
Pigment analyses were performed as previously described [4] using an HPLC system (1260 series, Agilent Technologies) coupled to a 6530 Accurate-Mass Q-TOF (Agilent Technologies) operated in negative electrospray ionization (ESI-) mode. Vials containing samples in methanol were kept at −20°C prior to LC-MS analysis. Reversed phase column Waters XTerra MS C18, 3.5 μm 2.1 × 50 mm column was used at 40°C with a sample volume injected of 8 μL and flow rate of 0.3 μL/min. The HPLC mobile phases consist of: A = 0.1% formic acid in water, B = acetonitrile. The linear gradient elution used the following time program: 0 min 5% B, linear to 95% B at 9.5 min, hold at 95% for 2 min, back to 5% B at 14 min, and equilibrate for 8 min. The injection volume was 8 μL. The ESI source conditions were gas temperature 300 C, drying gas 11 L/min, nebulizer 35 psig, VCap voltage 3500 V, fragmentor 175 V, and skimmer 65 V. The instrument was tuned using an Agilent calibration tuning mix for mass calibration of the Q-TOF instrument. The reference solution provided reference masses m/z 112.9856 and m/z 1033.9881 for ESI- were used to correct small mass drift during acquisition. Data were collected in both centroid and profile formats and data analysis used Agilent MassHunter Qualitative Analysis (v. B.06.00).
Supplementary Material
Highlights:
Larvae of sea stars lack the pigment seen in larvae of echinoids
Sea star larvae have the known genes necessary to make pigment
The gene regulatory network for pigment is distinct in the ancestral sea star
Gcm not an essential activator of the pigmentation pathway in the sea star
We conclude that multiple distinct changes had to occur for pigment in echinoids
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The authors declare no competing interests.
Data Availability
scRNA-seq data are available for S. purpuratus [16] and P. miniata [63].
References
- 1.Griffiths M A study of the synthesis of naphthaquinone pigments by the larvae of two species of sea urchins and their reciprocal hybrids. Dev. Biol 11, 433–447 (1965). [DOI] [PubMed] [Google Scholar]
- 2.McClendon JF Echinochrome, a red substance in sea urchins. J. Biol. Chem, 435–441 (1912). [Google Scholar]
- 3.Thomson RH Naturally Occuring Quinones. Second edn, 257–275 (Academic Press, 1971). [Google Scholar]
- 4.Wessel GM, Kiyomoto M, Shen TL & Yajima M Genetic manipulation of the pigment pathway in a sea urchin reveals distinct lineage commitment prior to metamorphosis in the bilateral to radial body plan transition. Sci. Rep 10, 1973; 10.1038/s41598-020-58584-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brasseur L et al. The Roles of Spinochromes in Four Shallow Water Tropical Sea Urchins and Their Potential as Bioactive Pharmacological Agents. Mar. Drugs 15, 179; 10.3390/md15060179 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heatfield BM & Travis DF Ultrastructural studies of regenerating spines of the sea urchin Strongylocentrotus purpuratus. II. Cell types with spherules. J. Morphol 145, 51–71 (1975). [DOI] [PubMed] [Google Scholar]
- 7.Gerardi P, Lassegues M & Canicatti C Cellular distribution of sea urchin antibacterial activity. Biol. Cell 70, 153–157 (1990). [Google Scholar]
- 8.Smith LC et al. Echinoderm immunity. Adv. Exp. Med. Biol 708, 260–301 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Coates CJ, McCulloch C, Betts J & Whalley T Echinochrome A Release by Red Spherule Cells Is an Iron-Withholding Strategy of Sea Urchin Innate Immunity. J. Innate Immun 10, 119–130 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cameron RA, Fraser SE, Britten RJ & Davidson EH Macromere cell fates during sea urchin development. Development 113, 1085–1091 (1991). [DOI] [PubMed] [Google Scholar]
- 11.Ransick A & Davidson EH cis-regulatory processing of Notch signaling input to the sea urchin glial cells missing gene during mesoderm specification. Dev. Biol 297, 587–602 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Sherwood DR & McClay DR LvNotch signaling mediates secondary mesenchyme specification in the sea urchin embryo. Development 126, 1703–1713 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Sweet HC, Hodor PG & Ettensohn CA The role of micromere signaling in Notch activation and mesoderm specification during sea urchin embryogenesis. Development 126, 5255–5265 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Calestani C, Rast JP & Davidson EH Isolation of pigment cell specific genes in the sea urchin embryo by differential macroarray screening. Development 130, 4587–4596 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Croce JC & McClay DR Dynamics of Delta/Notch signaling on endomesoderm segregation in the sea urchin embryo. Development 137, 83–91 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perillo M et al. Regulation of dynamic pigment cell states at single-cell resolution. eLife 9, e60388; 10.7554/eLife.60388 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Materna SC, Ransick A, Li E & Davidson EH Diversification of oral and aboral mesodermal regulatory states in pregastrular sea urchin embryos. Dev. Biol 375, 92–104 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson EH et al. A genomic regulatory network for development. Science 295, 1669–1678 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Ransick A, Rast JP, Minokawa T, Calestani C & Davidson EH New early zygotic regulators expressed in endomesoderm of sea urchin embryos discovered by differential array hybridization. Dev. Biol 246, 132–147 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Foster S, Oulhen N & Wessel G A single cell RNA sequencing resource for early sea urchin development. Development 147, dev191528; doi: 10.1242/dev.191528 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibson AW & Burke RD The origin of pigment cells in embryos of the sea urchin Strongylocentrotus purpuratus. Dev. Biol 107, 414–419 (1985). [DOI] [PubMed] [Google Scholar]
- 22.Gustafson T & Wolpert L Cellular movement and contact in sea urchin morphogenesis. Biol. Rev. Camb. Philos. Soc 42, 442–498 (1967). [DOI] [PubMed] [Google Scholar]
- 23.Kominami T, Takata H & Takaichi M Behavior of pigment cells in gastrula-stage embryos of Hemicentrotus pulcherrimus and Scaphechinus mirabilis. Dev. Growth. Differ 43, 699–707 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Buckley KM & Rast JP An Organismal Model for Gene Regulatory Networks in the Gut-Associated Immune Response. Front. Immunol 8, 1297; 10.3389/fimmu.2017.01297 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perry G & Epel D Ca2+−stimulated production of H2O2 from naphthoquinone oxidation in Arbacia eggs. Exp. Cell Res 134, 65–72 (1981). [DOI] [PubMed] [Google Scholar]
- 26.Fleming TJ, Schrankel CS, Vyas H, Rosenblatt HD & Hamdoun A CRISPR/Cas9 mutagenesis reveals a role for ABCB1 in gut immune responses to Vibrio diazotrophicus in sea urchin larvae. J. Exp. Biol 224, jeb.232272; 10.1242/jeb.232272 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho ECH et al. Perturbation of gut bacteria induces a coordinated cellular immune response in the purple sea urchin larva. Immunol. Cell Biol 94, 861–874 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oulhen N & Wessel GM Albinism as a visual, in vivo guide for CRISPR/Cas9 functionality in the sea urchin embryo. Mol. Reprod. Dev 83, 1046–1047 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schröder J et al. Plant polyketide synthases: a chalcone synthase-type enzyme which performs a condensation reaction with methylmalonyl-CoA in the biosynthesis of C-methylated chalcones. Biochem. 37, 8417–8425 (1998). [DOI] [PubMed] [Google Scholar]
- 30.Wang B, Guo F, Huang C & Zhao H Unraveling the iterative type I polyketide synthases hidden in Streptomyces. Proc. Natl. Acad. Sci. U.S.A 117, 8449–8454 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nivina A, Yuet KP, Hsu J & Khosla C Evolution and Diversity of Assembly-Line Polyketide Synthases. Chem. Rev 119, 12524–12547 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Başaran R & Can Eke B Flavin Containing Monooxygenases and Metabolism of Xenobiotics. Turk. J. Pharm. Sci 14, 90–94 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang WS et al. Macrophage migration inhibitory factor (MIF) family in arthropods: Cloning and expression analysis of two MIF and one D-dopachrome tautomerase (DDT) homologues in mud crabs, Scylla paramamosain. Fish Shellfish Immunol. 50, 142–149 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Lubetsky JB et al. The tautomerase active site of macrophage migration inhibitory factor is a potential target for discovery of novel anti-inflammatory agents. J. Biol. Chem 277, 24976–24982 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Romero A, Novoa B & Figueras A Cell mediated immune response of the Mediterranean sea urchin Paracentrotus lividus after PAMPs stimulation. Dev. Comp. Immunol 62, 29–38 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Castoe TA, Stephens T, Noonan BP & Calestani C A novel group of type I polyketide synthases (PKS) in animals and the complex phylogenomics of PKSs. Gene 392, 47–58 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Hojo M et al. Unexpected link between polyketide synthase and calcium carbonate biomineralization. Zool. Lett 1, 3; 10.1186/s40851-014-0001-0 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cameron RA, Samanta M, Yuan A, He D & Davidson E SpBase: the sea urchin genome database and web site. Nucleic Acids Res. 37, D750–D754; 10.1093/nar/gkn887 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kudtarkar P & Cameron RA Echinobase: an expanding resource for echinoderm genomic information. Database 2017, bax074; 10.1093/database/bax074 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cary GA, Cameron RA & Hinman VF EchinoBase: Tools for Echinoderm Genome Analyses in Eukaryotic Genomic Databases: Methods and Protocols (ed. Kollmar M) 349–369 (Springer New York, 2018). [DOI] [PubMed] [Google Scholar]
- 41.Ageenko NV, Kiselev KV & Odintsova NA Expression of Pigment Cell-Specific Genes in the Ontogenesis of the Sea Urchin Strongylocentrotus intermedius. Evid. Based Complement Alternat. Med 2011, 730356; 10.1155/2011/730356 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peter IS & Davidson EH The endoderm gene regulatory network in sea urchin embryos up to mid-blastula stage. Dev. Biol 340, 188–199 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peter IS & Davidson EH A gene regulatory network controlling the embryonic specification of endoderm. Nature 474, 635–639 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calestani C & Rogers DJ Cis-regulatory analysis of the sea urchin pigment cell gene polyketide synthase. Dev. Biol 340, 249–255 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Ransick A & Davidson EH Cis-regulatory logic driving glial cells missing: self-sustaining circuitry in later embryogenesis. Dev. Biol 364, 259–267 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Damle SS & Davidson EH Synthetic in vivo validation of gene network circuitry. Proc. Natl. Acad. Sci. U.S.A 109, 1548–1553 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinman VF & Davidson EH Evolutionary plasticity of developmental gene regulatory network architecture. Proc. Natl. Acad. Sci. U.S.A 104, 19404–19409 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yankura KA, Koechlein CS, Cryan AF, Cheatle A & Hinman VF Gene regulatory network for neurogenesis in a sea star embryo connects broad neural specification and localized patterning. Proc. Natl. Acad. Sci. U.S.A 110, 8591–8596 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strathmann RR The feeding behavior of planktotrophic echinoderm larvae: Mechanisms, regulation, and rates of suspensionfeeding. J. Exp. Mar. Biol. Ecol 6, 109–160 (1971). [Google Scholar]
- 50.Furukawa R, Takahashi Y, Nakajima Y, Dan-Sohkawa M & Kaneko H Defense system by mesenchyme cells in bipinnaria larvae of the starfish, Asterina pectinifera. Dev. Comp. Immunol 33, 205–215 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Metchnikoff E Lectures on the Comparative Pathology of Inflammation: Delivered at the Pasteur Institute in 1891. xii–218 (Kegan Paul, Trench, Rtubner & Co., Ltd., 1893). [Google Scholar]
- 52.Cary GA et al. Systematic comparison of sea urchin and sea star developmental gene regulatory networks explains how novelty is incorporated in early development. Nat. Commun 11, 6235; 10.1038/s41467-020-20023-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCauley BS, Akyar E, Saad HR & Hinman VF Dose-dependent nuclear β-catenin response segregates endomesoderm along the sea star primary axis. Development 142, 207–217 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duboc V, Röttinger E, Lapraz F, Besnardeau L & Lepage T Left-Right Asymmetry in the Sea Urchin Embryo Is Regulated by Nodal Signaling on the Right Side. Dev. Cell 9, 147–158 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Wessel GM, Fresques T, Kiyomoto M, Yajima M & Zazueta V Origin and development of the germ line in sea stars. Genesis 52, 367–377 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martik ML & McClay DR Deployment of a retinal determination gene network drives directed cell migration in the sea urchin embryo. eLife 4, e08827; 10.7554/eLife.08827 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei Z, Yaguchi J, Yaguchi S, Angerer RC & Angerer LM The sea urchin animal pole domain is a Six3-dependent neurogenic patterning center. Development 136, 1179–1189 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Materna SC & Davidson EH A comprehensive analysis of Delta signaling in pre-gastrular sea urchin embryos. Dev. Biol 364, 77–87 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foster S, Teo YV, Neretti N, Oulhen N & Wessel GM Single cell RNA-seq in the sea urchin embryo show marked cell-type specificity in the Delta/Notch pathway. Mol. Reprod. Dev 86, 931–934 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsunoda T & Takagi T Estimating transcription factor bindability on DNA. Bioinformatics 15, 622–630 (1999). [DOI] [PubMed] [Google Scholar]
- 61.Gao J, Chen Y-H & Peterson LC GATA family transcriptional factors: emerging suspects in hematologic disorders. Exp. Hematol. Oncol 4, 28; 10.1186/s40164-015-0024-z (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Solek CM et al. An ancient role for Gata-1/2/3 and Scl transcription factor homologs in the development of immunocytes. Dev. Biol 382, 280–292 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Foster S, Oulhen N, Fresques T, Zaki H & Wessel G Vasa and Nanos are regulated differently during induction primordial germ cells. (submitted). [Google Scholar]
- 64.Shipp LE, Hill RZ, Moy GW, Gökırmak T & Hamdoun A ABCC5 is required for cAMP-mediated hindgut invagination in sea urchin embryos. Development 142, 3537–3548 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ettensohn CA, Illies MR, Oliveri P & De Jong DL Alx1, a member of the Cart1/Alx3/Alx4 subfamily of Paired-class homeodomain proteins, is an essential component of the gene network controlling skeletogenic fate specification in the sea urchin embryo. Development 130, 2917–2928 (2003). [DOI] [PubMed] [Google Scholar]
- 66.Koga H et al. Experimental Approach Reveals the Role of alx1 in the Evolution of the Echinoderm Larval Skeleton. PLOS ONE 11, e0149067; 10.1371/journal.pone.0149067 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCauley BS, Wright EP, Exner C, Kitazawa C & Hinman VF Development of an embryonic skeletogenic mesenchyme lineage in a sea cucumber reveals the trajectory of change for the evolution of novel structures in echinoderms. Evodevo 3, 17; 10.1186/2041-9139-3-17 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar S, Stecher G, Li M, Knyaz C & Tamura K MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol 35, 1547–1549 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Foltz KR, Adams NL & Runft LL Echinoderm eggs and embryos: procurement and culture. Methods Cell Biol. 74, 39–74 (2004). [DOI] [PubMed] [Google Scholar]
- 70.Wessel GM, Reich AM & Klatsky PC Use of sea stars to study basic reproductive processes. Syst. Biol. Reprod. Med 56, 236–245 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perillo M, Paganos P, Spurrell M, Arnone MI & Wessel GM Methodology for Whole Mount and Fluorescent RNA In Situ Hybridization in Echinoderms: Single, Double, and Beyond. Methods Mol. Biol 2219, 195–216 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee PY & Davidson EH Expression of Spgatae, the Strongylocentrotus purpuratus ortholog of vertebrate GATA4/5/6 factors. Gene Expr. Patterns 5, 161–165 (2004). [DOI] [PubMed] [Google Scholar]
- 73.Arshinoff BI, Cary GA, Karimi K, Foley S, Agalakov S, Delgado F, Lotay VS, Ku CJ, Pells TJ, Beatman TR, Kim E, Cameron RA, Vize PD, Telmer Cheryl A., Croce JC, Ettensohn CA, & Hinman VF (2021). Echinobase: leveraging an extant model organism database to build a knowledgebase supporting research on the genomics and biology of echinoderms. Nucleic Acids Research, 50(D1), D970–D979. 10.1093/nar/gkab1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wessel GM, Kiyomoto M, Reitzel A, Carrier T (2022). Pigmentation biosynthesis influences the microbiome in sea urchins. Proceedings of the Royal Society B doi.org/ 10.1098/rspb.2022.1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
scRNA-seq data are available for S. purpuratus [16] and P. miniata [63].


