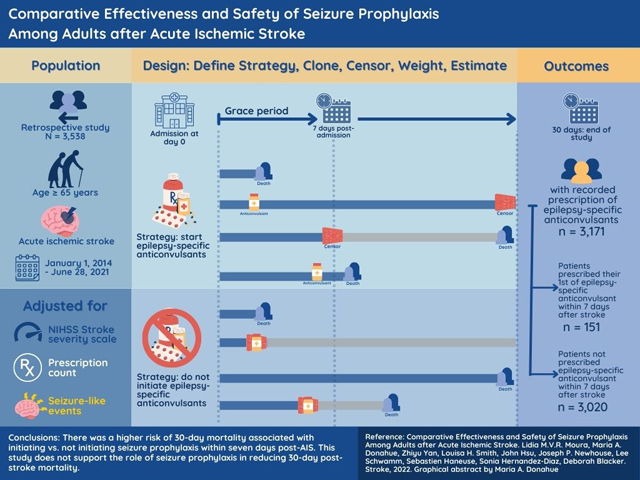
Abstract
Background:
Older adults occasionally receive seizure prophylaxis in an acute ischemic stroke (AIS) setting, despite safety concerns. There are no trial data available about the net impact of early seizure prophylaxis on post-AIS survival.
Methods:
Using a stroke registry (American Heart Association’s Get With The Guidelines) individually linked to electronic health records (EHR), we examined the effect of initiating seizure prophylaxis (i.e., epilepsy-specific ASDs) within seven days of an AIS admission versus not initiating in patients ≥65 years admitted for a new, non-severe AIS (NIH-Stroke Severity Score ≤ 20) between 2014–2021 with no recorded use of epilepsy-specific ASDs in the previous three months. We addressed confounding by using inverse-probability weights. We performed standardization accounting for pertinent clinical and healthcare factors (e.g., NIH Stroke Severity scale, prescription counts, seizure-like events).
Results:
The study sample included 151 patients who received ASDs and 3,020 who did not. The crude 30-day mortality risks were 219 deaths per 1000 patients among epilepsy-specific ASDs initiators and 120 deaths per 1000 among non-initiators. After standardization, the estimated mortality was 251 (95% CI, 190–307) deaths per 1000 among initiators and 120 (95% CI, 86 to 144) deaths per 1000 among non-initiators, corresponding to a risk difference (RD) of 131 (95% CI, 65– 200) excess deaths per 1000 patients. In the prespecified subgroup analyses, the RD was 52 (CI, 11–72) among patients with minor AIS and 138 (CI, 52–222) among moderate-to-severe AIS patients. Similarly, the RDs were 86 (95% CI 18–118) and 157 (95% CI 57–219) among patients aged 65–74 years and ≥75 years, respectively.
Conclusions:
There was a higher risk of 30-day mortality associated with initiating vs. not initiating seizure prophylaxis within seven days post-AIS. This study does not support the role of seizure prophylaxis in reducing 30-day post-stroke mortality.
Keywords: Acute ischemic stroke, antiseizure drug, seizure prophylaxis
Graphical Abstract
INTRODUCTION
Acute ischemic stroke (AIS) is a common cause of older adults’ short-term mortality and long-term disability.1, 2 For those ≥65 years, stroke is the second leading cause of hospitalization and carries a post-stroke 30-day mortality risk of 9–24%.3 Seizures are common and challenging-to-predict stroke complications. Incidence of post-stroke seizure risk varies widely and often measures different outcomes, e.g., from a one-year incidence of 5–7% in community-based studies to a one-week incidence of 10–50% in patients with continuous electroencephalography (cEEG).4
Over recent decades, cEEG utilization has doubled.5 cEEG shows epileptic abnormalities resembling seizures in 46–60% of patients in the acute symptomatic phase.6 Because those patterns are associated with greater post-stroke seizure risk, and healthcare providers debate whether these epileptic abnormalities should be treated,7 as observational studies suggest that antiseizure drug (ASD) treatment may cause net harm.8 Others indicate that the inability to demonstrate benefit is due to confounding by indication (e.g., failing to adequately adjust for traits that impact the probability of clinically significant seizures, treatment initiation, and death) since those at higher risk are more likely to receive seizure prophylaxis.9 Nonetheless, cEEG utilization has increased prophylaxis with levetiracetam and other ASDs.4, 10
Despite the increasing concerns of the prevalence of use, there remains limited real-world information about the effectiveness and safety of seizure prophylaxis among older adults in the United States.11–13 ASDs may lead to life-threatening adverse effects (e.g., falls, infections, somnolence).14, 15 Older adults on polytherapy are more sensitive to drug toxicity,16, 17 as are those with acute brain insults such as AIS. Since older adults are typically excluded from phase III and IV clinical trials,18 the effect of seizure prophylaxis remains underexplored in this population.16, 17
We used observational data to evaluate the effect of seizure prophylaxis initiation within seven days post-AIS on 30-day mortality among patients ≥65 years.
METHODS
Study Design
We used a target trial approach to emulate a hypothetical pragmatic randomized clinical trial.19, 20 Specifying the ideal study to answer the research question forces a rigorous conceptualization of the study design components and the assumptions necessary to answer the question using observational data.19 The target trial to answer the question of interest would randomly assign eligible patients at the time of AIS admission to one of two treatment strategies: (1) initiate seizure prophylaxis (ASD hereafter refers to epilepsy-specific anti-seizure drugs) within seven days post-AIS or b) do not initiate within the same seven-day period. The outcome is mortality, evaluated in a follow-up period of 30 days following treatment initiation. The following sections describe the observational study to emulate this target trial (Table 1).
Table 1.
Description of a target trial and the corresponding observational study
| TARGET TRIAL SPECIFICATION | EMULATION (OBSERVATIONAL STUDY) |
|---|---|
| Eligibility criteria | |
| Admission for cerebrovascular accident between 1/2014 and 6/2021 at Massachusetts General Hospital | Same |
| Age ≥ 65 | Same |
| Confirmed Acute Ischemic Stroke (AIS) | Same |
| No previous history of AIS in the last 12 months | No recorded diagnosis of AIS in the last 12 months. |
| No use of ASD* in the last three months. | No recorded prescription of ASD in the last three months. |
| Treatment strategies | |
| Treatment arm: Initiate seizure prophylaxis (ASD) within seven days of AIS admission. Control arm: Do not initiate seizure prophylaxis (ASD) within seven days of AIS admission. |
Same |
| Treatment assignment | |
| Open label, randomized treatment assignment | Emulated randomization by balancing confounders using IPTW for treatment selection |
| Outcomes | |
| Time to death from the day of AIS admission | Same. Time to death (as recorded in EHR and/or GWTG registry) from the day AIS admission. |
| Follow-up | |
| Starts at randomization (at admission) and ends at death, or end of the 30-day observation period in the study, whichever occurs first. | Starts at AIS admission and ends at death, or 30 days of follow-up, whichever occurs first. |
| Causal contrast | |
| Intention-to-treat effect. | Observational analog of intention-to-treat effect. |
| Statistical analysis | |
| Intention-to-treat effect analysis of time to death, accounting for censoring. | Same, additionally accounting for baseline confounding. |
Abbreviations: ASD, antiseizure drugs; AIS, Acute Ischemic Stroke; GWTG, Get-with-The-Guidelines Stroke Registry; EHR, electronic health record; IPTW, Inverse Probability of Treatment Weights.
ASDs: Acetazolamide, Acetazolamide XR, Brivaracetam, Cannabidiol, Eslicarbazepine, Ethosuximide, Felbamate, Lacosamide, Lamotrigine, Lamotrigine ER, Levetiracetam, Levetiracetam ER, Methsuximide, Perampanel, Phenobarbital, Phenytoin, Retigabine, Ezogabine, Rufinamide, Tiagabine, Vigabatrin.
Setting & Data Sources
We used a comprehensive registry, the American Heart Association’s Get With The Guidelines (GWTG)-Stroke Registry (see Supplementary Text), to identify eligible patients.21 We then linked the data to patients’ electronic health records (EHR) from the Mass General Brigham Healthcare System (MGB) to obtain demographic, clinical, and healthcare utilization data (e.g., inpatient diagnoses, procedures, outpatient and inpatient drug administration).22
This study was approved by the Institutional Review Board of Massachusetts General Hospital, and informed consent was waived. The data that support the findings of this study are available from the corresponding author (LMVRM) upon reasonable request.
Study Population
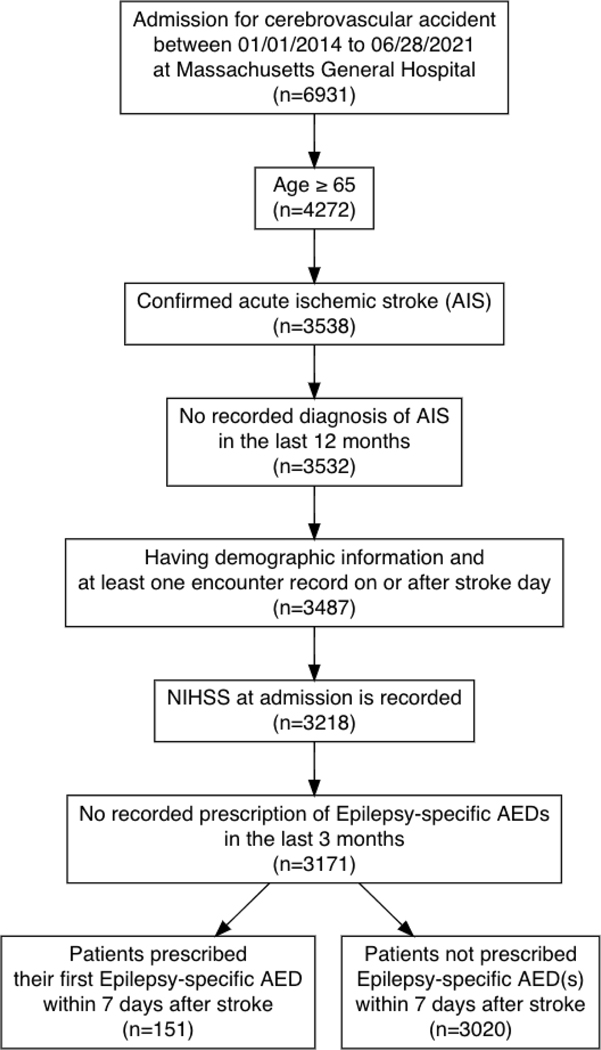
From January 1, 2014, to June 28, 2021, we identified 3,538 patients ≥ 65 years who had specifically AIS,21, 23 and had no recorded diagnosis of prior AIS in the last 12 months. We excluded 45 patients without the minimum information in the EHR to determine eligibility, e.g., NIHSS not recorded at admission. This enhanced the selection of AIS patients admitted at MGH on the day of the AIS because those with missing NIHSS values were typically transferred from another hospital one or more days after the AIS. We also excluded patients with severe NIHSS admitted for new non-severe AIS (NIHSS ≤ 20), and patients with one or more recorded prescriptions of ASDs within the three-month period before admission. The final eligible sample was 3,171 (Figure 1).
Figure 1. Selection of eligible patients with new acute ischemic stroke (AIS) ≥65 years, 2014–2021.
Describes the sampling process that resulted in a sample of 3,171 subjects, including patients ≥ 65 years at the time of new acute ischemic stroke admission, with available data in the EHR system and who had not received ASDs in the three months before admission.
Treatment Strategies
We obtained information on ASD use from inpatient and outpatient pharmacy data. We classified ASDs as those prescribed for seizure prophylaxis (i.e., not used for other indications like pain management or anxiety. Table S1). We defined the following treatment strategies: a) initiate seizure prophylaxis within seven days of admission, or b) no seizure prophylaxis during these seven days.
Emulated Randomization & Covariates
In the target trial, balanced baseline characteristics would be attained through randomization. In the emulation, we ascertained information on clinical and sociodemographic characteristics, assessed differences in their distribution between treated and non-treated groups, and standardized for relevant confounders in the analysis.
We examined a comprehensive list of clinical (e.g., stroke severity, seizures, and seizure-like events, comorbidities, code status) and healthcare utilization variables (inpatient visits, outpatient visits, procedures [electroencephalogram, brain imaging]). Supplemental Text details the operational definition of each measure of interest.
As our measure of stroke severity at baseline, we chose the NIHSS,24, 25 a summary measure that has been strongly associated with seizure risk, seizure prophylaxis, and mortality. NIHSS was reliably assessed, measured, and documented upon hospital admission (study time zero), making it an ideal baseline measure for use in the weights for treatment initiation. We also considered baseline comorbidities and prescription drug utilization before the AIS using data from 90 days before admission. We obtained several sociodemographic measures from the MGB database (i.e., age, sex, race, and ethnicity).26
As time-varying characteristics, during the seven-day window, we used a comprehensive list of clinical and healthcare utilization variables, including inpatient and outpatient visits and procedures related to AIS management and cumulative in-hospital prescription count, which we divided into four categories; no prescription recorded, one to four drugs, five to nine drugs, and more than nine drugs (excluding ASDs).19, 27–30
Follow-up & Outcome – 30-day Mortality
Patients were followed from AIS admission for 30 days or until death (Figure S1). We extracted the death date from the EHR Demographics data file (Death Master File). MGB updates death data monthly from the Social Security Administration. Thus, deaths were captured even if the patient was transferred into a nursing home or another non-MGB facility (i.e., no losses to follow-up).
Statistical Analysis
We first described the characteristics of the eligible sample.31 We obtained a naïve crude 30-day mortality estimate for ASD initiators from treatment during the first-week post-AIS and non-initiators from AIS admission.32, 33
To evaluate the effect of ASD initiation in the first seven days post-AIS on 30-day mortality, we estimated mortality probabilities using model-based predictions of the conditional survival for each day under each treatment strategy.34, 35 We provide details of the statistical approach, missing data, and pre-planned stratified analysis in the Supplemental Text and we separately created IPT weights with some variables collected at baseline (i.e., NIHSS, prescription count at baseline, and seizure-like events at baseline) to show the balance (i.e., all SMDs <0.2 after applying IPT weights), please see Supplemental Table S2.
RESULTS
Study Population Characteristics
Among AIS patients ≥65 years, 3,171 were eligible for our emulated trial. Of those, 151 received seizure prophylaxis within seven days post-AIS, and 3,020 did not. Table 2 describes patient characteristics among initiators versus non-initiators. The most frequently administered ASD was levetiracetam at 84%, followed by phenytoin at 6% (Table S3). Additionally, in Figure S2 we provide a breakdown of when the medications of interest were started by post-AIS days within the seven days exposure window (from day zero to day six). In the observational data, 64 patients (42%) received one of the ASDs of interest within the first 24 hours post-AIS admission. Cumulatively, 133 patients (88%) received one of the ASDs of interest within the first 72 hours post-AIS admission. In Figure S3, we demonstrate the counts of deaths over the same period to illustrate the issue of immortal time bias, which we have addressed using the proposed methods. Further, in Figure S3, we show that prophylaxis has been the primary use within the study cohort. 67% of the patients initiated on ASDs were discontinued within 24h; 85% of the patients continued within the first seven days post-AIS but greater than 90% were discontinued within 30 days.
Table 2.
Characteristics of patients by ASD exposure
| ASD initiator (N=151) | ASD non-initiator (N=3,020) | SMD | |
|---|---|---|---|
| Socio-Demographic Characteristics (recorded at admission) | |||
| Age, mean (SD) | 77.30 (8.49) | 78.05 (8.43) | 0.089 |
| Female (%) | 71 (47.0) | 1540 (51.0) | 0.080 |
| Non-white | 22 (15.4) | 472 (16.3) | 0.026 |
| Hispanic (%) | 1 (0.7) | 42 (1.5) | 0.073 |
| Primary insurance Medicare or other government (vs private) (%) | 120 (79.5) | 2441 (80.9) | 0.035 |
| Baseline Medication Use (recorded during the 90 days before admission) | |||
| Prescription count, Mean (SD) | 19.86 (37.09) | 7.90 (30.49) | 0.352 |
| Categories of medication use (%) | |||
| No prescription recorded** | 55 (36.4) | 2161 (71.6) | |
| 1–4 drugs | 18 (11.9) | 333 (11.0) | |
| 5–9 drugs | 13 (8.6) | 141 (4.7) | |
| >9 drugs | 65 (43.0) | 385 (12.7) | |
| Baseline Clinical Characteristics (recorded during 12 months before admission) | |||
| Charlson comorbidity score, mean (SD) | 2.27 (1.97) | 1.15 (1.75) | 0.604 |
| Alzheimer’s Disease and Related Dementias | 10 (6.6) | 104 (3.4) | 0.146 |
| Baseline Health-Resource Utilization (recorded during 12 months before admission), % | |||
| Fall-related injury | 22 (14.6) | 325 (10.8) | 0.115 |
| Seizure-like events | 51 (33.8) | 160 (5.3) | 0.770 |
| EEG | 13 (8.6) | 23 (0.8) | 0.378 |
| Acute Ischemic Stroke Severity (recorded at admission), % | |||
| NIHSS (mean (SD)) | 11.95 (8.91) | 7.59 (7.80) | 0.521 |
| Mild (0–4) | 39 (25.8) | 1536 (50.9) | |
| Moderate (5–15) | 54 (35.8) | 920 (30.5) | |
| Moderate to severe (16–20) | 23 (15.2) | 287 (9.5) | |
| Severe (>20) | 35 (23.2) | 277 (9.2) | |
| In-hospital Measures of Stroke Severity and Complications (recorded during first day of admission)*** (%) | |||
| Observed large vessel occlusion | 34 (39.5) | 594 (34.4) | 0.107 |
| In-hospital prescription count | 15.22 (12.84) | 9.53 (11.83) | 0.461 |
| IV injection of tissue plasminogen activator (tPA) | 8 (5.3) | 220 (7.3) | 0.082 |
| Endovascular thrombectomy (EVT) | 2 (1.3) | 67 (2.2) | 0.068 |
| Computed tomography (CT/CAT) Scan | 82 (54.3) | 1854 (61.4) | 0.144 |
| Magnetic resonance imaging (MRI) of the brain | 42 (27.8) | 1502 (49.7) | 0.462 |
| Comfort Measures Only (%) | 0.367 | ||
| Day 0 or 1 | 8 (5.3) | 134 (4.4) | |
| Day 2 or after | 34 (22.5) | 289 (9.6) | |
| Not on CMO | 109 (72.2) | 2597 (86.0) | |
Abbreviations: ASD, antiseizure drugs; SD, standard deviation; SMD, standardized mean difference; EEG, electroencephalogram.
No prescription recorded: the prescription information was a) missing from the MGB structured health system data warehouse, b) the patient was not taking any prescription drug, c) the patient was taking prescription drugs given elsewhere (e.g., over the counter, prescribed and recorded in another healthcare system), d) other unknown reason.
For simplicity, we present just the values obtained during the first day of admission, but we include time-varying values of those measures in the model for treatment initiation (updated daily).
Outcome – Mortality
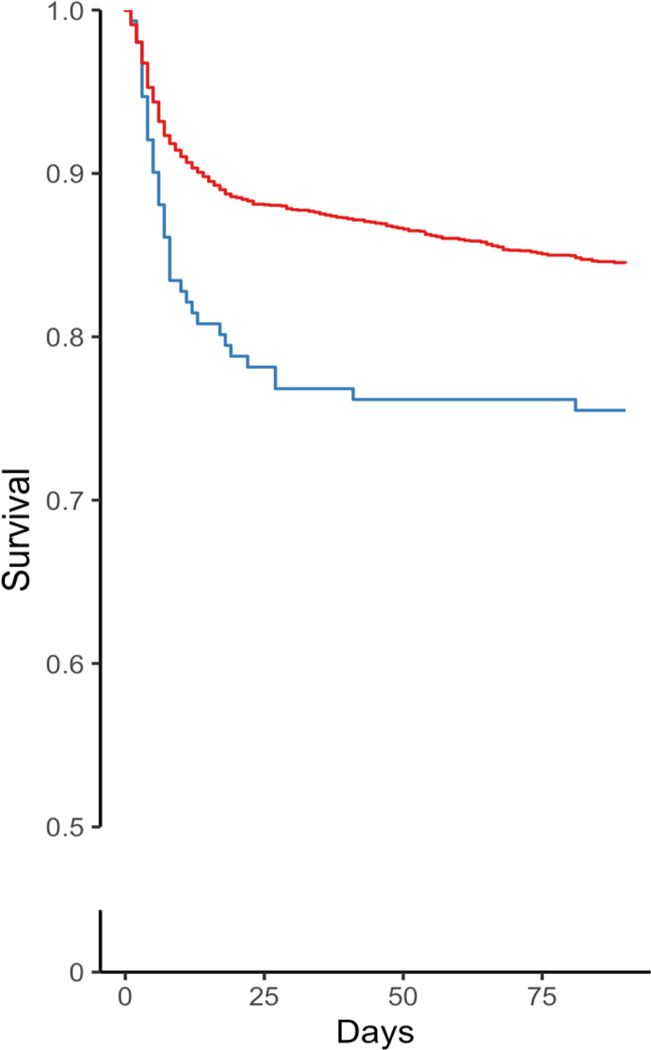
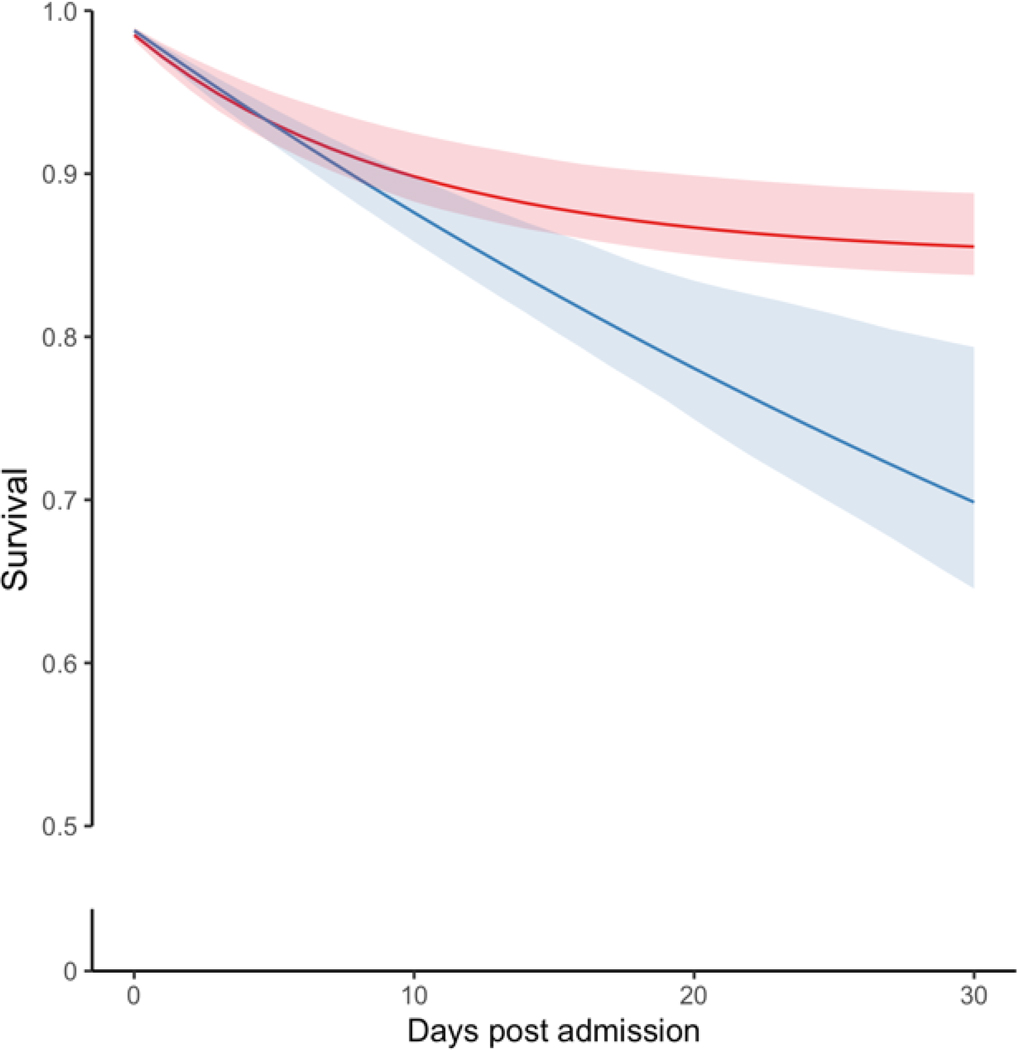
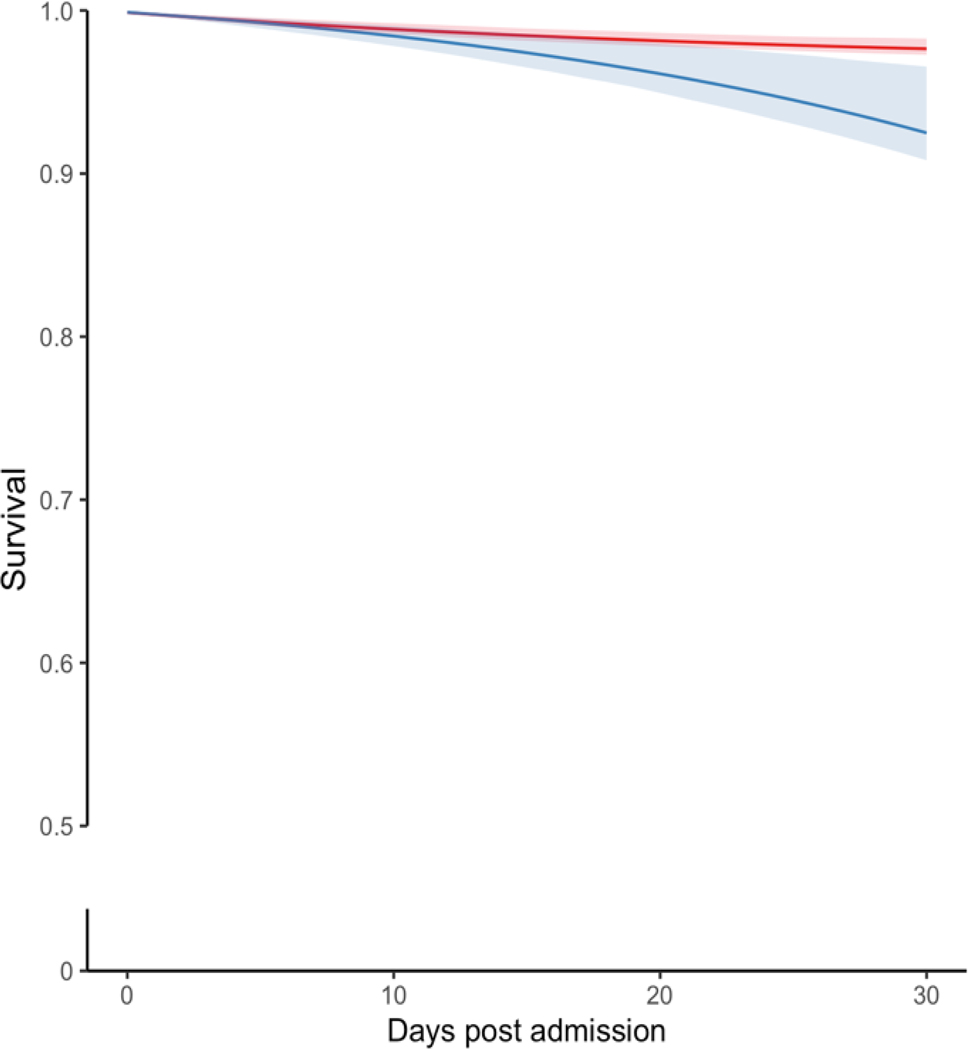
Figure 2 provides the crude Kaplan-Meier and standardized survival curves for all 3,171 eligible patients. The crude 30-day mortality risks were 219 deaths per 1000 patients among ASD initiators within seven days (Figure 2A, Table S4) and 120 deaths per 1000 among non-initiators. Since we had no missing data with respect to death, we provided crude curves with 90 days in the X-axis. The apparent difference in crude excess mortality in patients with seizure prophylaxis was predominantly seen during the first 30 days (Figure 2A). The standardized differences could increase beyond 30 days (Figures 2B and 3), but with a decreasing degree of certainty over time (i.e., larger confidence interval) because a model was run each day with fewer subjects and covariates in the data. We showed the most conservative analysis and produce standardized curves setting the follow-up to 30 days post-AIS.
Figure 2. Crude and standardized survival curves by seizure prophylaxis initiation strategy during the first 30 days post-stroke admission.
a Blue: ASD initiated within seven days post-AIS admission. a Red: ASD not initiated within seven days post-AIS. b Blue: Strategy for ASD initiation within seven days post-AIS admission. b Red: Strategy for no initiation of ASD within seven days post-AIS admission. Shaded areas: 95% confidence intervals constructed using bootstrap with 500 replications.
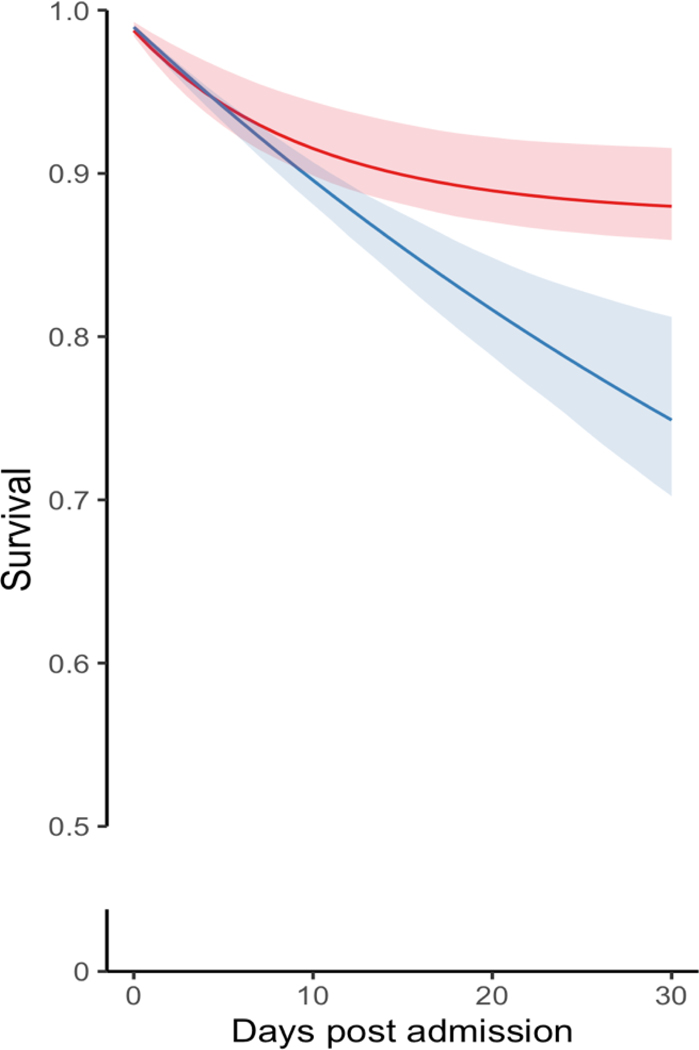
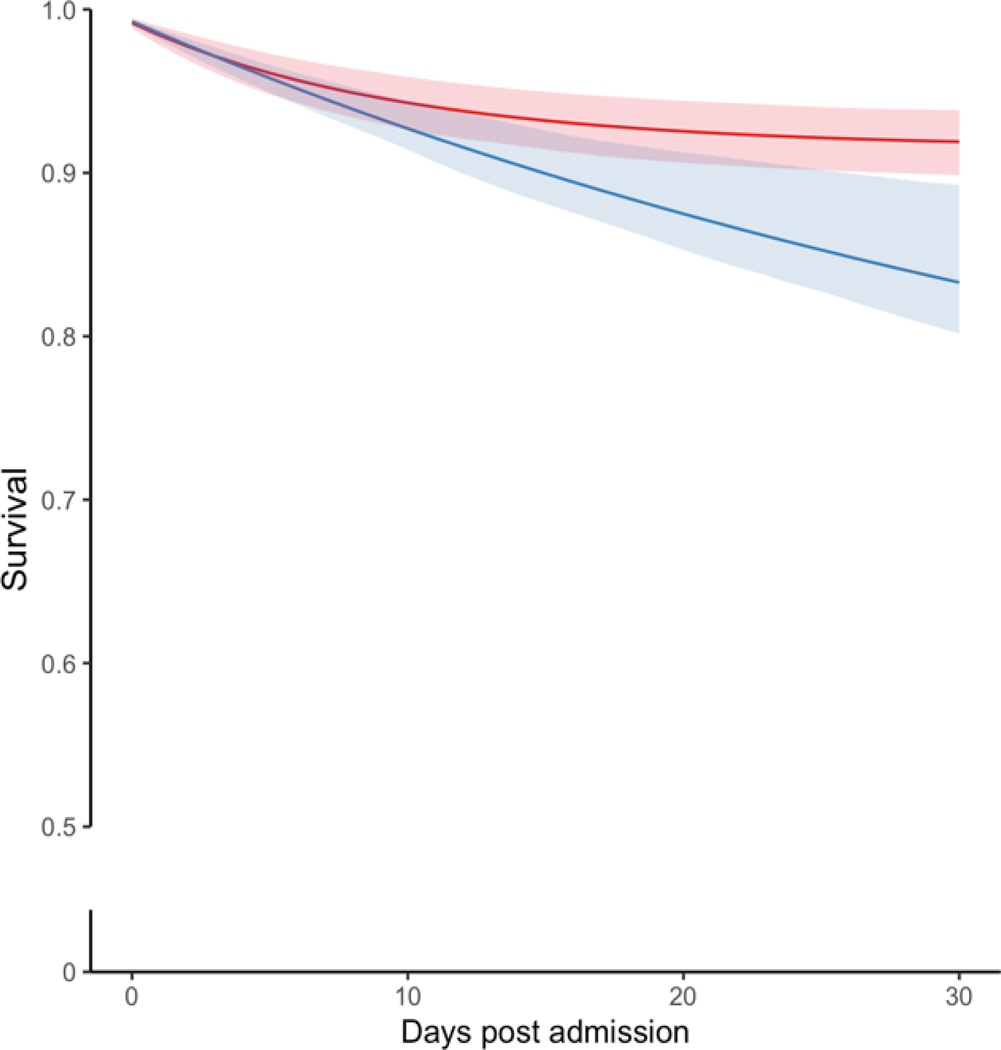
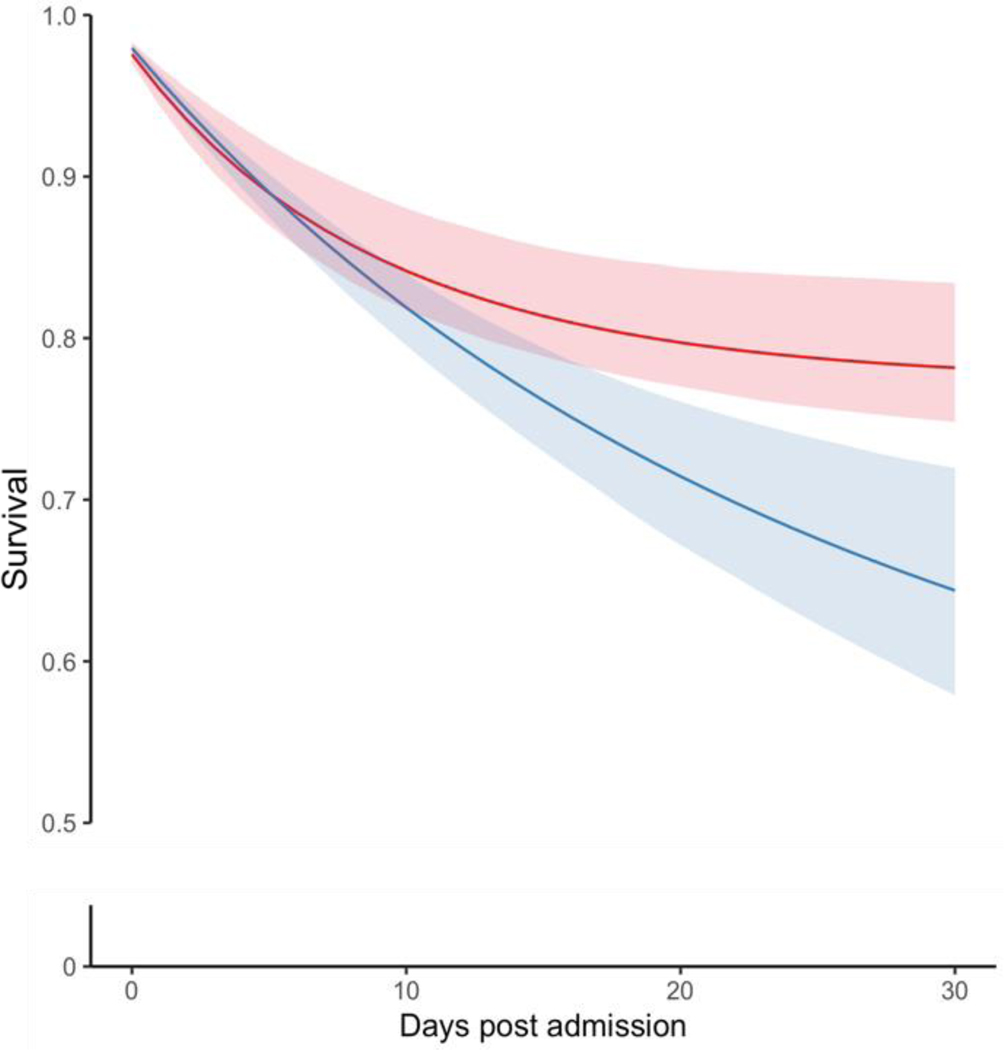
Figure 3. Standardized Survival Curves by ASD Initiation Strategy Across Categories of Age and Stroke Severity.
Blue: Strategy for ASD initiation within seven days post-AIS admission. Red: Strategy for no initiation of ASD within seven days post-AIS admission. Shaded areas: 95% confidence intervals constructed using bootstrap with 500 replications.
The standardized 30-day mortality was 230 (95% CI, 210–254) deaths/1000 patients who initiated ASDs and 121 (95% CI, 116–127) per 1000 non-initiators, yielding a risk difference of 109 (95% CI, 91–132) deaths/1000 patients. When further corrected for confounding (Figure 2B, Table S4), standardized 30-day mortality was 251 (95% CI, 190–307) deaths/1000 patients who initiated ESAs and 120 (95% CI, 86–145) per 1000 non-initiators, yielding a risk difference of 131 (95% CI, 65–200) deaths/1000 patients. Inspection of the curves suggests greater mortality rates for the initiate-seizure prophylaxis strategy than no-initiation, especially later after admission.
Among AIS patients 65–74 years and ≥74 years, the RDs were 86 (95% CI 18–118) and 157 (95% CI 57–219)/1000 patients, respectively (Figure 3 A–B). Among patients with mild and moderate-to-severe AIS, the 30-day mortality RD was 52 (95% CI, 11 to 72) deaths/1000 and 138 (95% CI, 52–222), respectively (Figure 3 C–D). The Tables S5 and S6 present the main standardized estimates stratified by age group and AIS severity. Table S7 presents Model Parameters for Estimating Epilepsy-specific ASD Initiation Weights.
Table S8 displays this study’s compliance with reporting recommendations. The Supplemental Text provides the Statistical Code used to conduct the analysis.
DISCUSSION
In this study, using rich information on predictors of seizure prophylaxis and mortality among AIS patients ≥65 years, we observed a crude higher risk of 30-day mortality associated with initiating seizure prophylaxis within seven days post-AIS compared with not initiating. Although residual confounding by indication remains a concern, our findings suggest that any net-benefit is likely small (as illustrated in the standardized survival curves). Stated differently, this manuscript does not support a role for short-term seizure prophylaxis in reducing post-stroke mortality.
ASDs are occasionally used for primary seizure prophylaxis, even though the American Geriatrics Society’s Beers Criteria explicitly states that ASDs should be “avoid[ed] unless safer alternatives are not available; avoid antiepileptics except for seizure and mood disorders”.36 However, there have been no well-designed randomized clinical trials with sufficient sample size to address the safety and effectiveness of ASDs during the acute stage of AIS among older adults.37–41 Specifically, there is some understanding of seizure prophylaxis after specific stroke types: spontaneous intracerebral hemorrhage,37, 42 intracerebral hemorrhage,43 subarachnoid hemorrhage,44 cryptogenic stroke,45 and least assessed, ischemic stroke.40
This study was motivated by the limitations of existing guidelines regarding which type of patients could benefit from seizure prophylaxis within the early symptomatic stroke recovery period and which type of patients could experience adverse effects from this treatment.14, 46 Seizures and seizure-like events are considered AIS symptoms (i.e., symptomatic seizures), and prophylaxis may be unnecessary unless they recur after the acute AIS recovery period is over (i.e., post-AIS epilepsy, by definition).11, 47, 48
The most examined ASDs have been levetiracetam,37, 39, 40 valproic acid,38 and sodium valproate,41 with an urgent need to evaluate the safety and effectiveness of newer drugs such as lacosamide, carbamate, brivaracetam, vigabatrin, and eslicarbazepine.49 Evidence shows the side effects of levetiracetam are: behavioral disturbances (e.g., anxiety, anger, depression), nausea/vomiting, infections, somnolence, and fatigue that may precipitate fall-related injuries.50 Additionally, documented phenytoin side effects include ataxia, incoordination, arrhythmia, cognitive impairment, and acute skin allergic reactions.51 While ASDs might cause adverse reactions with potential long-term effects consequences, their benefit may be limited, especially when used in the very short term (e.g., 85% of the patients who were started on ASDs had stopped it in the first 7 days in this study).
Strengths
Our approach has several important strengths when compared to previous studies in the presence of staggered treatment initiation.52–55 For instance, rather than moving the start of follow-up for the ASD group to the time of treatment initiation, we aligned time-zero for exposed and reference groups, thus comparing the same periods post-AIS, which is critical because there is substantially more significant mortality in the first days.
To address confounding and improve precision, we linked multiple data sources over numerous years, incorporating granular measurements of baseline variables and time-dependent covariates up to treatment strategy assignment and statistical methods of addressing time-dependent confounding.20 Lastly, there were no losses to follow-up since we had information on mortality, even when the patient stopped using the healthcare system.
Limitations
Residual confounding.
Our crude versus standardized analysis showed that confounding was present in this setting. Residual confounding by unmeasured factors associated with prescribing ASDs could still explain some of the observed associations.
Generalizability.
Our single-center study based on a large academic institution in a region with a predominantly white, non-Hispanic, and insured population might have favored the selection of those patients with greater previous use of the healthcare system. We favored the latter in the tradeoff between generalizability and internal validity by obtaining rich baseline data from those using healthcare to control for confounding. From our results, we observed that the primary use of antiseizure medication in this study cohort was seizure prophylaxis. Determining the duration and dosage of ASD prophylaxis is not part of the scope of this study, as our data was sparse and limited the type of analysis we could perform. We will apply this methodology in a larger, linked dataset to perform sensitivity analysis and increase the study’s external validity by increasing its generalizability and representativeness.
Power.
Our sample’s overall mortality risk was low, especially in the mild stroke severity subset. Our mortality results represent the lower bounds of exposure patterns and outcome effects than other practice patterns.56 This is partly because this study took place in a certified Advanced Comprehensive Stroke Center that aims to treat patients with AIS with the highest quality of care. Lastly, even though we were able to obtain accurate death dates, examining the cause of death for each patient was out of scope for this study. The cause of death is worth investigating further in future studies.
CONCLUSIONS
This study examined the 30-day mortality risk associated with the initiation of seizure prophylaxis within seven days after an AIS in patients ≥65 years. Our findings suggest that any net-benefit is likely small and insufficient to support a role for short-term seizure prophylaxis in reducing post-stroke mortality.
Supplementary Material
ACKNOWLEDGEMENTS
This study was conducted as part of a Ph.D. thesis in Public Health Science and constituted one of the graduation requirements.
FUNDING SOURCES
This study was funded by the NIH (1R01AG073410-01)
DISCLOSURE STATEMENT
L.M.V.R.M. receives support from the Centers for Diseases Control and Prevention (U48DP006377), the National Institutes of Health (NIH-NIA 5R01AG062282-02, NIH-NIA 2P01AG032952-11, NIH- NIA 3R01AG062282-03S1), and the Epilepsy Foundation of America.
Z.Y. and M.A.D. none
J.P.N. receives funding from NIH (2P01- AG032952, T32-AG51108) and reports being a director of Aetna until May 2018 and holding equity in Aetna until November 2018.
L.H.S. is a scientific consultant regarding trial design and conduct on late window thrombolysis and a member of the steering committee for Genentech (TIMELESS NCT03785678); user interface design and usability to LifeImage; stroke systems of care to the Massachusetts Dept of Public Health; member of a Data Safety Monitoring Board for Penumbra (MIND NCT03342664); Diffusion Pharma (PHAST-TSC NCT03763929); principal investigator, multicenter trial of stroke prevention for Medtronic (Stroke AF NCT02700945); principal investigator, StrokeNet Network NINDS (New England Regional Coordinating Center U24NS107243).
J.H. receives support from the NIH (1R01AG062282-012, P01AG032952).
J.P.N. receives funding from NIH (2P01- AG032952, T32-AG51108) and reports being a director of Aetna until May 2018 and holding equity in Aetna until November 2018.
S.H. receives support from the NIH (R01HD098421, R01NS104143, P50CA244433, 1R01DK128150-01, R01DK107972) and Gates Foundation (INV-003612).
S.H.D. Receives funding from NIH (5R01HD088393-02) and reports grants to her institution from Takeda and consulting for Bayer and UCB, all outside the submitted work.
D.B. Receives support from the NIH (5P30 AG062421-03, 2P01AG036694-11, 5U01AG032984-12, 1U24NS100591-04, 1R01AG058063-04, R01AG063975-03, 5R01AG062282-04, 3R01AG062282-03S1, 5R01AG066793-02, 1U19AG062682-03, 2P01AG032952-11, 2T32MH017119-34, 3P01AG032952-12S3, 1U01AG068221-01, 1U01AG076478-01, 5R01AG048351-05).
Non-standard Abbreviations and Acronyms:
- AIS
acute ischemic stroke
- ASD
antiseizure drug
REFERENCES
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, et al. Heart Disease and Stroke Statistics-2017 Update: A Report from the American Heart Association. Circulation. 2017;135:e146–e603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Health Data Exchange 2017. Available At: Http://Ghdx.Healthdata.Org/Gbd-Results-Tool.
- 3.Katan M, Luft A. Global Burden of Stroke. Semin Neurol. 2018;38:208–211 [DOI] [PubMed] [Google Scholar]
- 4.Zafar SF, Postma EN, Biswal S, Boyle EJ, Bechek S, O’Connor K, Shenoy A, Kim J, Shafi MS, Patel AB, et al. Effect of Epileptiform Abnormality Burden on Neurologic Outcome and Antiepileptic Drug Management after Subarachnoid Hemorrhage. Clinical Neurophysiology. 2018;129:2219–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medicare Payment Advisory Commission (Medpac). March 2019 Report to the Congress: Medicare Payment Policy. Available At: Http://Medpac.Gov/Docs/Default-Source/Reports/Mar19_Medpac_Entirereport_Sec.Pdf?Sfvrsn=0. 2019 [Google Scholar]
- 6.Westover MB, Shafi MM, Bianchi MT, Moura LMVR, O’Rourke D, Rosenthal ES, Chu CJ, Donovan S, Hoch DB, Kilbride RD, et al. The Probability of Seizures During Eeg Monitoring in Critically Ill Adults. Clinical Neurophysiology. 2015;126:463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Putten MJ, Hofmeijer J. Generalized Periodic Discharges: Pathophysiology and Clinical Considerations. Epilepsy Behav. 2015;49:228–233 [DOI] [PubMed] [Google Scholar]
- 8.Scoppettuolo P, Gaspard N, Depondt C, Legros B, Ligot N, Naeije G. Epileptic Activity in Neurological Deterioration after Ischemic Stroke, a Continuous EEG Study. Clinical Neurophysiology. 2019;130:2282–2286 [DOI] [PubMed] [Google Scholar]
- 9.Fountain NB, Fugate JE. Refractory Status Epilepticus: What to Put Down: The Anesthetics or the Patient? Neurology. 2014;82:650–651 [DOI] [PubMed] [Google Scholar]
- 10.Perucca E, Berlowitz D, Birnbaum A, Cloyd JC, Garrard J, Hanlon JT, Levy RH, Pugh MJ. Pharmacological and Clinical Aspects of Antiepileptic Drug Use in the Elderly. Epilepsy Research. 2006;68 Suppl 1:S49–63 [DOI] [PubMed] [Google Scholar]
- 11.Moura LMVR, Smith JR, Yan Z, Blacker D, Schwamm LH, Newhouse JP, Hernandez-Diaz S, Hsu J. Patterns of Anticonvulsant Use and Adverse Drug Events in Older Adults. Pharmacoepidemiol Drug Safety. 2021;30:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Lott LB, Burke JF, Kerber KA, Skolarus LE, Callaghan BC. Medicare Part D Payments for Neurologist-Prescribed Drugs. Neurology. 2016;86:1491–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callaghan BC, Reynolds E, Banerjee M, Kerber KA, Skolarus LE, Magliocco B, Esper GJ, Burke JF. Out-of-Pocket Costs Are on the Rise for Commonly Prescribed Neurologic Medications. Neurology. 2019;92:e2604–e2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang JZ, Vyas MV, Saposnik G, Burneo JG. Incidence and Management of Seizures after Ischemic Stroke: Systematic Review and Meta-Analysis. Neurology. 2017;89:1220–1228 [DOI] [PubMed] [Google Scholar]
- 15.Moura LMVR Westover MB, Kwasnik D Cole AJ, Hsu J. Causal Inference as an Emerging Statistical Approach in Neurology: An Example for Epilepsy in the Elderly. Clin Epidemiol. 2017;9:9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corsonello A, Pedone C, Incalzi RA. Age-Related Pharmacokinetic and Pharmacodynamic Changes and Related Risk of Adverse Drug Reactions. Curr Med Chem. 2010;17:571–584 [DOI] [PubMed] [Google Scholar]
- 17.Hilmer SN, McLachlan AJ, Le Couteur DG. Clinical Pharmacology in the Geriatric Patient. Fundamental & Clinical Pharmacology. 2007;21:217–230 [DOI] [PubMed] [Google Scholar]
- 18.Ridda I, Lindley R, MacIntyre RC. The Challenges of Clinical Trials in the Exclusion Zone: The Case of the Frail Elderly. Australas J Ageing. 2008;27:61–66 [DOI] [PubMed] [Google Scholar]
- 19.Hernan MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. American Journal of Epidemiol. 2016;183:758–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernan MA. How to Estimate the Effect of Treatment Duration on Survival Outcomes Using Observational Data. BMJ. 2018;360:k182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ormseth CH, Sheth KN, Saver JL, Fonarow GC, Schwamm LH. The American Heart Association’s Get with the Guidelines (GWTG)-Stroke Development and Impact on Stroke Care. Stroke Vasc Neurol. 2017;2:94–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nalichowski R, Keogh D, Chueh HC, Murphy SN. Calculating the Benefits of a Research Patient Data Repository. AMIA Annu Symp Proc. 2006:1044 [PMC free article] [PubMed] [Google Scholar]
- 23.Get with the Guidelines (GWTG) Stroke. 2020;2020 [Google Scholar]
- 24.Kasner SE, Chalela JA, Luciano JM, Cucchiara BL, Raps EC, McGarvey ML, Conroy MB, Localio AR. Reliability and Validity of Estimating the NIH Stroke Scale Score from Medical Records. Stroke. 1999;30:1534–1537 [DOI] [PubMed] [Google Scholar]
- 25.Runde D.Calculated Decisions: NIH Stroke Scale/Score (NIHSS). Emerg Med Pract. 2020;22:CD6-CD7 [PubMed] [Google Scholar]
- 26.Nordahl H.Social Inequality in Chronic Disease Outcomes. Dan Med J. 2014;61:B4943. [PubMed] [Google Scholar]
- 27.Hernan MA. Methods of Public Health Research - Strengthening Causal Inference from Observational Data. New England Journal of Medicine. 2021;385:1345–1348 [DOI] [PubMed] [Google Scholar]
- 28.Hernan MA, Robins JM. Estimating Causal Effects from Epidemiological Data. Journal of Epidemiology and Community Health. 2006;60:578–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernan MA, Hernandez-Diaz S, Robins JM. A Structural Approach to Selection Bias. Epidemiology. 2004;15:615–625 [DOI] [PubMed] [Google Scholar]
- 30.VanderWeele TJ, Hernan MA, Robins JM. Causal Directed Acyclic Graphs and the Direction of Unmeasured Confounding Bias. Epidemiology. 2008;19:720–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hedges LV, Pustejovsky JE, Shadish WR. A Standardized Mean Difference Effect Size for Multiple Baseline Designs across Individuals. Res Synth Methods. 2013;4:324–341 [DOI] [PubMed] [Google Scholar]
- 32.Kloecker DE, Davies MJ, Khunti K, Zaccardi F. Uses and Limitations of the Restricted Mean Survival Time: Illustrative Examples from Cardiovascular Outcomes and Mortality Trials in Type 2 Diabetes. Annals of Internal Medicine. 2020;172:541–552 [DOI] [PubMed] [Google Scholar]
- 33.Lu Y, Tian L. Statistical Considerations for Sequential Analysis of the Restricted Mean Survival Time for Randomized Clinical Trials. Stat Biopharm Res. 2021;13:210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cole SR, Hernan MA. Constructing Inverse Probability Weights for Marginal Structural Models. American Journal of Epidemiology. 2008;168:656–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen L, Young JG, Robins JM, Hernan MA. Parametric G-Formula Implementations for Causal Survival Analyses. Biometrics. 2021;77:740–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.By the American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society 2019 Updated Ags Beers Criteria(R) for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67:674–694 [DOI] [PubMed] [Google Scholar]
- 37.Christie C, Daggubati L, Patel N, Matthews N, Lehman EB, Cockroft KM. Effect of Newer Generation Anticonvulsant Prophylaxis on Seizure Incidence after Spontaneous Intracerebral Hemorrhage. World Neurosurg. 2020;141:e461–e465 [DOI] [PubMed] [Google Scholar]
- 38.Gilad R, Boaz M, Dabby R, Sadeh M, Lampl Y. Are Post Intracerebral Hemorrhage Seizures Prevented by Anti-Epileptic Treatment? Epilepsy Res. 2011;95:227–231 [DOI] [PubMed] [Google Scholar]
- 39.Daou BJ, Maher CO, Holste K, Palmateer G, Lint C, Elenbaas J, Thompson BG, Pandey AS. Seizure Prophylaxis in Unruptured Aneurysm Repair: A Randomized Controlled Trial. J Stroke Cerebrovasc Dis. 2020;29:105171 [DOI] [PubMed] [Google Scholar]
- 40.van Tuijl JH, van Raak EP, de Krom MC, Lodder J, Aldenkamp AP. Early Treatment after Stroke for the Prevention of Late Epileptic Seizures: A Report on the Problems Performing a Randomised Placebo-Controlled Double-Blind Trial Aimed at Anti-Epileptogenesis. Seizure. 2011;20:285–291 [DOI] [PubMed] [Google Scholar]
- 41.Hu X, Fang Y, Li H, Liu W, Lin S, Fu M, Li X, Cao X, Zhang H, You C, et al. Protocol for Seizure Prophylaxis Following Intracerebral Hemorrhage Study (Spich): A Randomized, Double-Blind, Placebo-Controlled Trial of Short-Term Sodium Valproate Prophylaxis in Patients with Acute Spontaneous Supratentorial Intracerebral Hemorrhage. Int J Stroke. 2014;9:814–817 [DOI] [PubMed] [Google Scholar]
- 42.Angriman F, Tirupakuzhi Vijayaraghavan BK, Dragoi L, Lopez Soto C, Chapman M, Scales DC. Antiepileptic Drugs to Prevent Seizures after Spontaneous Intracerebral Hemorrhage. Stroke. 2019;50:1095–1099 [DOI] [PubMed] [Google Scholar]
- 43.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of Icd-9-Cm Codes for Identifying Cardiovascular and Stroke Risk Factors. Med Care. 2005;43:480–485 [DOI] [PubMed] [Google Scholar]
- 44.Feng R, Mascitelli J, Chartrain AG, Margetis K, Mocco J. Anti-Epileptic Drug (AED) Use in Subarachnoid Hemorrhage (SAH) and Intracranial Hemorrhage (ICH). Curr Pharm Des. 2017;23:6446–6453 [DOI] [PubMed] [Google Scholar]
- 45.Labovitz DL, Hauser WA. Preventing Stroke-Related Seizures: When Should Anticonvulsant Drugs Be Started? Neurology. 2003;60:365–366 [DOI] [PubMed] [Google Scholar]
- 46.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418 [DOI] [PubMed] [Google Scholar]
- 47.Jones FJS, Sanches PR, Smith JR, Zafar SF, Blacker D, Hsu J, Schwamm LH, Newhouse JP, Westover MB, Moura LMVR. Seizure Prophylaxis after Spontaneous Intracerebral Hemorrhage. JAMA Neurol. 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones FJS, Sanches PR, Smith JR, Zafar SF, Hernandez-Diaz S, Blacker D, Hsu J, Schwamm LH, Westover MB, Moura LMVR. Anticonvulsant Primary and Secondary Prophylaxis for Acute Ischemic Stroke Patients: A Decision Analysis. Stroke. 2021:2782–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klein P, Friedman A, Hameed MQ, Kaminski RM, Bar-Klein G, Klitgaard H, Koepp M, Jozwiak S, Prince DA, Rotenberg A, et al. Repurposed Molecules for Antiepileptogenesis: Missing an Opportunity to Prevent Epilepsy? Epilepsia. 2020;61:359–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuller KL, Wang YY, Cook MJ, Murphy MA, D’Souza WJ. Tolerability, Safety, and Side Effects of Levetiracetam Versus Phenytoin in Intravenous and Total Prophylactic Regimen among Craniotomy Patients: A Prospective Randomized Study. Epilepsia. 2013;54:45–57 [DOI] [PubMed] [Google Scholar]
- 51.Wilder BJ. Antiepileptic Drugs--Current Use. Can J Neurol Sci. 1996;23:S18–23 [DOI] [PubMed] [Google Scholar]
- 52.Colin O, Labreuche J, Deguil J, Mendyk AM, Deken V, Cordonnier C, Deplanque D, Leys D, Bordet R. Preadmission Use of Benzodiazepines and Stroke Outcomes: The Biostroke Prospective Cohort Study. BMJ Open. 2019;9:e022720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Porter B, Arthur A, Savva GM. How Do Potentially Inappropriate Medications and Polypharmacy Affect Mortality in Frail and Non-Frail Cognitively Impaired Older Adults? A Cohort Study. BMJ Open. 2019;9:e026171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Betensky RA, Mandel M. Recognizing the Problem of Delayed Entry in Time-to-Event Studies: Better Late Than Never for Clinical Neuroscientists. Ann Neurol. 2015;78:839–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu KY, Hartz SM, Borodovsky JT, Bierut LJ, Grucza RA. Association between Benzodiazepine Use with or without Opioid Use and All-Cause Mortality in the United States, 1999–2015. JAMA Netw Open. 2020;3:e2028557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moura LMVR, Smith JR, Yan Z, Blacker D, Schwamm LH, Newhouse JP, Hernandez-Diaz S, Hsu J. Patterns of Anticonvulsant Use and Adverse Drug Events in Older Adults Pharmacoepidemiology Drug Safety. 2020:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, Engel J Jr., Forsgren L, French JA, Glynn M, et al. Ilae Official Report: A Practical Clinical Definition of Epilepsy. Epilepsia. 2014;55:475–482 [DOI] [PubMed] [Google Scholar]
- 58.Galovic M, Ferreira-Atuesta C, Abraira L, Dohler N, Sinka L, Brigo F, Bentes C, Zelano J, Koepp MJ. Seizures and Epilepsy after Stroke: Epidemiology, Biomarkers and Management. Drugs and Aging. 2021;38:285–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doria JW, Forgacs PB. Incidence, Implications, and Management of Seizures Following Ischemic and Hemorrhagic Stroke. Current Neurology and Neuroscience Reports. 2019;19:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hall RE, Porter J, Quan H, Reeves MJ. Developing an Adapted Charlson Comorbidity Index for Ischemic Stroke Outcome Studies. BMC Health Services Research. 2019;19:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zelano J.Poststroke Epilepsy: Update and Future Directions. Therapeutic Advances in Neurological Disorders. 2016;9:424–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bryndziar T, Sedova P, Kramer NM, Mandrekar J, Mikulik R, Brown RD Jr., Klaas JP. Seizures Following Ischemic Stroke: Frequency of Occurrence and Impact on Outcome in a Long-Term Population-Based Study. Journal of Stroke and Cerebrovascular Diseases. 2016;25:150–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kogan E, Twyman K, Heap J, Milentijevic D, Lin JH, Alberts M. Assessing Stroke Severity Using Electronic Health Record Data: A Machine Learning Approach. BMC Medical Informatics and Decision Making. 2020;20:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moore KL, Patel K, Boscardin WJ, Steinman MA, Ritchie C, Schwartz JB. Medication Burden Attributable to Chronic Co-Morbid Conditions in the Very Old and Vulnerable. PloS One. 2018;13:e0196109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lamy C, Domigo V, Semah F, Arquizan C, Trystram D, Coste J, Mas JL, Patent Foramen O, Atrial Septal Aneurysm Study G. Early and Late Seizures after Cryptogenic Ischemic Stroke in Young Adults. Neurology. 2003;60:400–404 [DOI] [PubMed] [Google Scholar]
- 66.Cole SR, Hernan MA. Adjusted Survival Curves with Inverse Probability Weights. Comput Methods Programs Biomed. 2004;75:45–49 [DOI] [PubMed] [Google Scholar]
- 67.Willis AW, Schootman M, Tran R, Kung N, Evanoff BA, Perlmutter JS, Racette BA. Neurologist-Associated Reduction in Pd-Related Hospitalizations and Health Care Expenditures. Neurology. 2012;79:1774–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hernan MA, Sauer BC, Hernandez-Diaz S, Platt R, Shrier I. Specifying a Target Trial Prevents Immortal Time Bias and Other Self-Inflicted Injuries in Observational Analyses. Journal of Clinical Epidemiology. 2016;79:70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yadav K, Lewis RJ. Immortal Time Bias in Observational Studies. JAMA. 2021;325:686–687 [DOI] [PubMed] [Google Scholar]
- 70.Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, Sorensen HT, von Elm E, Langan SM, Committee RW. The Reporting of Studies Conducted Using Observational Routinely-Collected Health Data (RECORD) Statement. PLoS Med. 2015;12:e1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.