Abstract
Background:
Our objective is to describe adoption of the post-hospitalization behaviors associated with successful transition of care and related baseline characteristics.
Methods:
This study includes 550 participants in the Transition of Care Stroke Disparities Study, a prospective observational cohort derived from the Florida Stroke Registry. Participants had an ischemic stroke (2018–2021), discharged home or to rehabilitation, with mRS=0–3 (44% women, 24% Black, 48% White, 26% Hispanic, 35% foreign-born). We collected baseline socio-demographic and clinical characteristics. A structured telephone interview at 30-day post-discharge evaluated outcomes including medication adherence, medical appointment attendance, outpatient therapy, exercise, diet modification, toxic habit cessation, and a calculated composite adequate transition of care (ATOC) measure. Multivariable analyses assessed the association of baseline characteristics with 30-day behaviors.
Results:
At 30 days, medication adherence was achieved by 89%, medical appointments by 82%, outpatient therapy by 76%, exercise by 71%, diet modification by 68%, toxic habit cessation by 35%, and ATOC measure by 67%. Successful ATOC participants were more likely to be employed full-time (42% vs 31%, p=0.02), live with a spouse (60% vs 47%, p=0.01), feel close to 3 or more individuals (84% vs 71%, p<0.01), have history of dyslipidemia (45 vs 34%, p=0.02), have thrombectomy (15% vs 8%, p=0.02), but less likely to have a history of smoking (17% vs 32%, p<0.001), coronary artery disease (14% vs 21%, p=0.04), and heart failure (3% vs 11%, p<0.01). Multivariable logistic regression analyses revealed that multiple socio-economic factors and pre-stroke comorbid diseases predicted fulfillment of transition of care measures. There was no difference in outcomes during the Covid-19 pandemic (2020–2021) compared to prepandemic years (2018–2019).
Conclusions:
One in three patients did not attain adequate 30-day transition of care behaviors. Their achievement varied substantially among different measures and was influenced by multiple socioeconomic and clinical factors. Interventions aimed at facilitating transition of care from hospital after stroke are needed.
Registration:
Keywords: stroke, ischemic stroke, continuity of care, hospital to home transition, patient compliance, Cerebrovascular Disease
Graphical Abstract
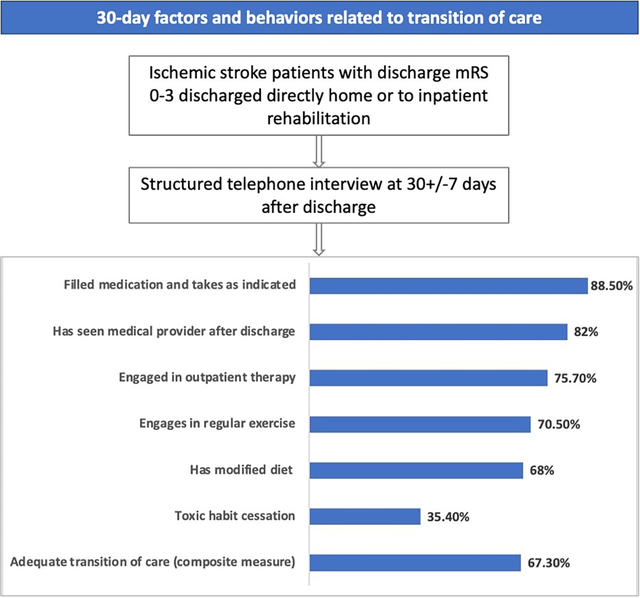
Introduction
Improving the quality of transition of stroke care from hospital to home has become critical in reducing stroke-related burden. It is evident that transition of care is suboptimal for a proportion of patients; indeed, all cause readmission after a stroke is estimated as high as 17.4% at 30 days,1 at great cost to the health care system. Moreover, 23% of all strokes are recurrent cases, representing a missed opportunity for the deployment of effective secondary stroke prevention.2 Medication adherence, medical follow up and lifestyle modifications are well-established factors associated with prevention of vascular events.3,4 Transition of care (TOC) requires “a set of actions (by the patient, caregiver, family or health care providers) designed to ensure the coordination and continuity of health care as patients transfer between different locations or different levels of care”.5
The ongoing Transitions of Care Stroke Disparities Study (TCSD-S) is an investigator-initiated, prospective, multi-center observational study funded by the NIH/NIMHD that aims to understand the drivers and disparities in factors that lead to successful transition of care after hospital discharge as well as its effects on stroke recurrence and readmission (NCT 03452813). In this prespecified intermediate analysis of TCSD-S we aimed to assess specific transition of care factors and behaviors in which stroke patients engage after hospital discharge and their association with baseline personal and stroke clinical characteristics. Understanding these factors and associated covariates may identify those at highest risk of not achieving adequate transition of care to focus resources and design interventions to address a successful transition of care after stroke. The period included in the current study provides a unique opportunity to examine whether trends in TOC health measures changed during the COVID-19 pandemic.
Methods
As TCSD-S utilizes data from American Heart Association (AHA) Get With The Guidelines-Stroke (GWTG-S), data-sharing agreements require an application process for other researchers to access data. Researchers can submit proposals at www.heart.org/qualityresearch to be considered by the GWTG-S and TCSD-S steering committees.
Cohort:
the TCSD-S population includes patients from 10 Florida Comprehensive Stroke Centers (Supplemental Table 1) that participate in the Florida Stroke Registry (FSR)6 with an ischemic stroke or intracerebral hemorrhage who are discharged to home, assisted living facility or inpatient rehabilitation. All participants signed informed consent and were enrolled prior to hospital discharge. TCSD-S is approved by the University of Miami Central Institutional Review Board. For this analysis, to identify a more homogeneous population able to engage in the factors associated with successful transition of care evaluated in this study, we included only those with a final diagnosis ischemic stroke and a discharge modified Rankin Score7 (mRS) of 0–3, enrolled between 08/2018 and 12/2021.
Baseline characteristics:
Baseline demographic and clinical characteristics, as well as medication class at discharge were obtained from the FSR which utilizes data abstracted by trained hospital abstractors and recorded in GWTG-S.8 In addition, we collected the stroke mechanism at discharge (TOAST classification),9 and through an in-person structured interview recorded factors related to social determinants of care, including language spoken at home, country of birth and years in the US (for foreign born), highest level of education, employment status before stroke, difficulty paying for basics and medical expenses, and living arrangements.
Measures reflective of effective transition of care:
We identified a priori the transition of care measures recommended for patients after stroke discharge. These measures were selected based on their known association with stroke recurrence and hospital readmission. Measures were collected through a structured telephone questionnaire by trained study site personnel at 30+/−7 days from discharge. We included: a) Medication adherence: filled medications and taken as prescribed 90–100% of the time (vs. did not fill prescription or taking <90% of the time); b) Outpatient therapy (only applicable to those that were prescribed outpatient physical, occupational or speech therapy): currently attending outpatient therapy or attended and completed therapy (vs. did not start prescribed therapy); c) Medical appointments: has seen a medical provider after discharge (vs. does not have appointment or has appointment but has not seen provider); d) Toxic habit cessation (only applicable to those that used tobacco, marihuana, or other drugs before stroke): completely stopped using tobacco, marihuana, other drugs (vs. did not stop); e) Engaging in regular exercise: regular walking on a treadmill or outside, or regular exercise other than walking (vs. not exercising or <1/week); f) Diet modification (excludes those with feeding/gastric tube or who only use prepared shakes): modified diet per recommendation after stroke (vs. no change in diet). We created a summary metric of adequate transition of care (ATOC) behavior, which was accomplished if the patients engaged in all activities and behaviors for which s/he was eligible at 30 days. We also collected the reasons for not engaging in these activities and behaviors through a structured interview.
Statistical analysis:
Demographic and clinical characteristics were summarized as means ± standard deviation or medians (interquartile ranges) for continuous variables and as frequency and percentage for categorical variables, overall and by group. A binary variable of enrolled time (pre- or during COVID-19) was used to explore the potential impact of COVID-19 on the performance. Each 30-day TOC measure was dichotomized as a binary outcome variable. For the univariable analyses, group comparisons were conducted using t test or F-test for continuous variables and chi-square test for categorical variables. For the multivariable analysis of each outcome, the baseline personal socio-demographic and clinical characteristics were simultaneously included in logistic regression models. Model discrimination was compared with receiver-operator-characteristic curves and C-statistic, whereas model goodness-of-fit was evaluated with AIC and likelihood ratio test. Following the primary analyses, interaction effects between discharge destination and the multivariable-adjusted significant factors were explored and sensitivity analyses were performed only for those discharged directly to home. For each TOC measure, all statistical tests were conducted against a 2-sided alternative hypothesis at a significance level of 0.05 given that they were planned exploratory analyses in the proposal. Data missingness was minimal (<12%) and all analyses utilized the complete case approach.
This study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Results
There were 624 participants with a final diagnosis of ischemic stroke, discharged directly home or to inpatient rehabilitation, having a discharge mRS 0–3 and data abstracted in GWTG-S. Among them, 550 participants completed the 30-day interview (Figure 1). The personal and stroke characteristics of this cohort are described in Table 1. As noted, this was a diverse cohort (44% women, 24% Black patients, 26% Hispanic patients, 35% foreign-born). Overall, the group had relatively mild stroke severity (NIHSS mean ± SD: 3.8±4.8, median (IQR): 2 (1–5)), 66% had a discharge mRS of 0–1, and 83% ambulated independently at discharge. A total of 474 (86%) were discharged directly home, while the rest went to inpatient rehabilitation.
Figure 1.
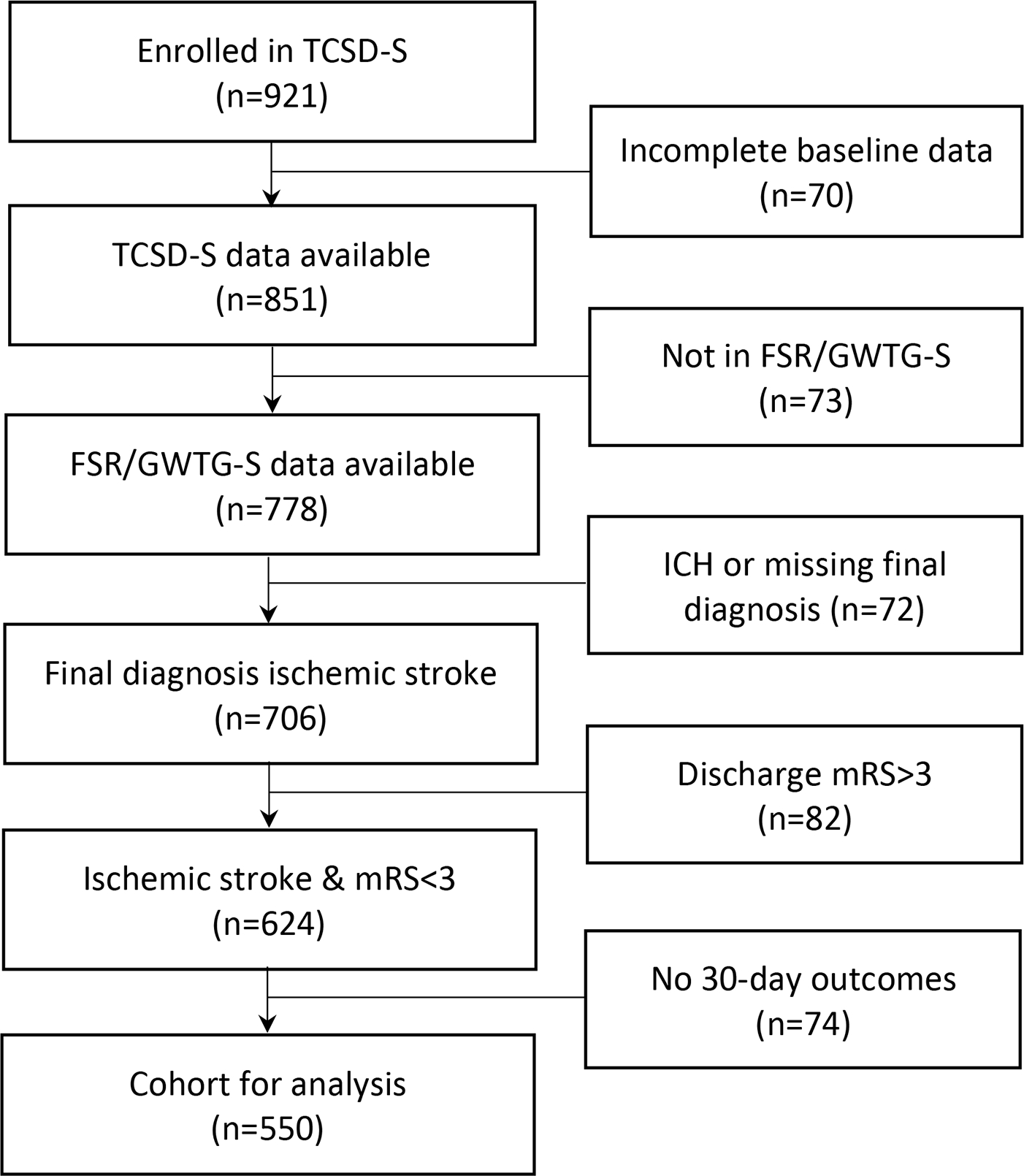
Flowchart of study cohort
Table 1.
Baseline sociodemographic and clinical stroke-related characteristics
| Characteristics | All (N=550) | Characteristics | All (N=550) |
|---|---|---|---|
| Age, years, mean (SD) | 63 (14) | Coronary artery disease/prior MI, % | 16 |
| Neighborhood, years, mean (SD) | 15 (15) | Peripheral vascular dis, % | 3.3 |
| Female, % | 43.5 | Previous Stroke/TIA, % | 20.9 |
| Race | Carotid stenosis, % | 4 | |
| Non-Hispanic White, % | 47.8 | Heart failure, % | 5.8 |
| Non-Hispanic Black, % | 23.5 | Hypertension, % | 76.5 |
| Hispanic, % | 25.8 | Diabetes mellitus, % | 31.1 |
| Non-Hispanic Other, % | 2.9 | Dyslipidemia, % | 41.5 |
| Foreign born% | 34.7 | Atrial fibrillation/flutter, % | 13.8 |
| English, % | 75.1 | Baseline Anticoagulation meds, % | 37.6 |
| Education | Baseline Antihypertensive meds, % | 40.5 | |
| Less than high school, % | 12.2 | Baseline Antilipidemic meds, % | 36.5 |
| Completed high school, % | 33.3 | Baseline Antidiabetic meds, % | 18.4 |
| Some college or more, % | 54.5 | Discharge Anticoagulation, % | 13.3 |
| Health insurance | Discharge Antidiabetic, % | 24.5 | |
| Private Insurance, % | 25.6 | Discharge Antihypertensive meds, % | 60.9 |
| Medicare, % | 44 | Discharge Cholesterol reducing meds, % | 98.5 |
| Medicaid, % | 5.3 | Discharge Antidepressant medications, % | 7.8 |
| Self/None, % | 25.1 | Stroke mechanisms | |
| Employment | Large artery atherosclerosis, % | 17.1 | |
| Full-time, % | 38 | Cardioembolic, % | 24.7 |
| Part-time, % | 11.6 | Small artery occlusion % | 22.2 |
| Retired, % | 38.5 | Other determined cause, % | 10.7 |
| Unemployed, % | 11.8 | Stroke of undetermined cause, % | 25.3 |
| Difficulty paying for basics | IV alteplase, % | 54 | |
| Hard or very hard, % | 20.5 | Thrombectomy, % | 12.4 |
| Somewhat hard, % | 15.8 | Arrival mode | |
| Not very hard, % | 63.6 | Arrival by EMS, % | 39.8 |
| Difficulty paying for medical care | Arrival self, % | 44.4 | |
| Very hard, % | 16.2 | Transfer, % | 14.5 |
| Hard, % | 12.2 | Onset-arrival time | |
| Somewhat hard, % | 15.8 | < 4.5 hours, % | 31.1 |
| Not very hard, % | 55.8 | 4.5–8.0 hours, % | 9.1 |
| Live with | > 8.0 hours, % | 40.2 | |
| Alone, % | 21.1 | Unknown, % | 19.6 |
| Spouse/partner, % | 55.8 | NIHSS score, mean (SD) | 3.8 (4.8) |
| Other, % | 10.7 | Modified Rankin Score at discharge, 0–1, % | 66 |
| Children, % | 12.4 | Independent ambulation at discharge, % | 82.9 |
| Person close to, 3 or more, % | 79.6 | Discharged directly to home, % | 86.2 |
| Smoker, % | 22.2 |
Not all participants were eligible for all measures: only 46% (n=225) were prescribed outpatient therapy and 23% (n=127) used tobacco or had other toxic habits at the time of their stroke. At 30 days, medication adherence was achieved by 89%, medical appointments by 82%, outpatient therapy by 76%, exercise by 71%, diet modification by 68%, and toxic habit cessation by 35%, leading to an overall ATOC measure achieved by 67% (Figure 2).
Figure 2.
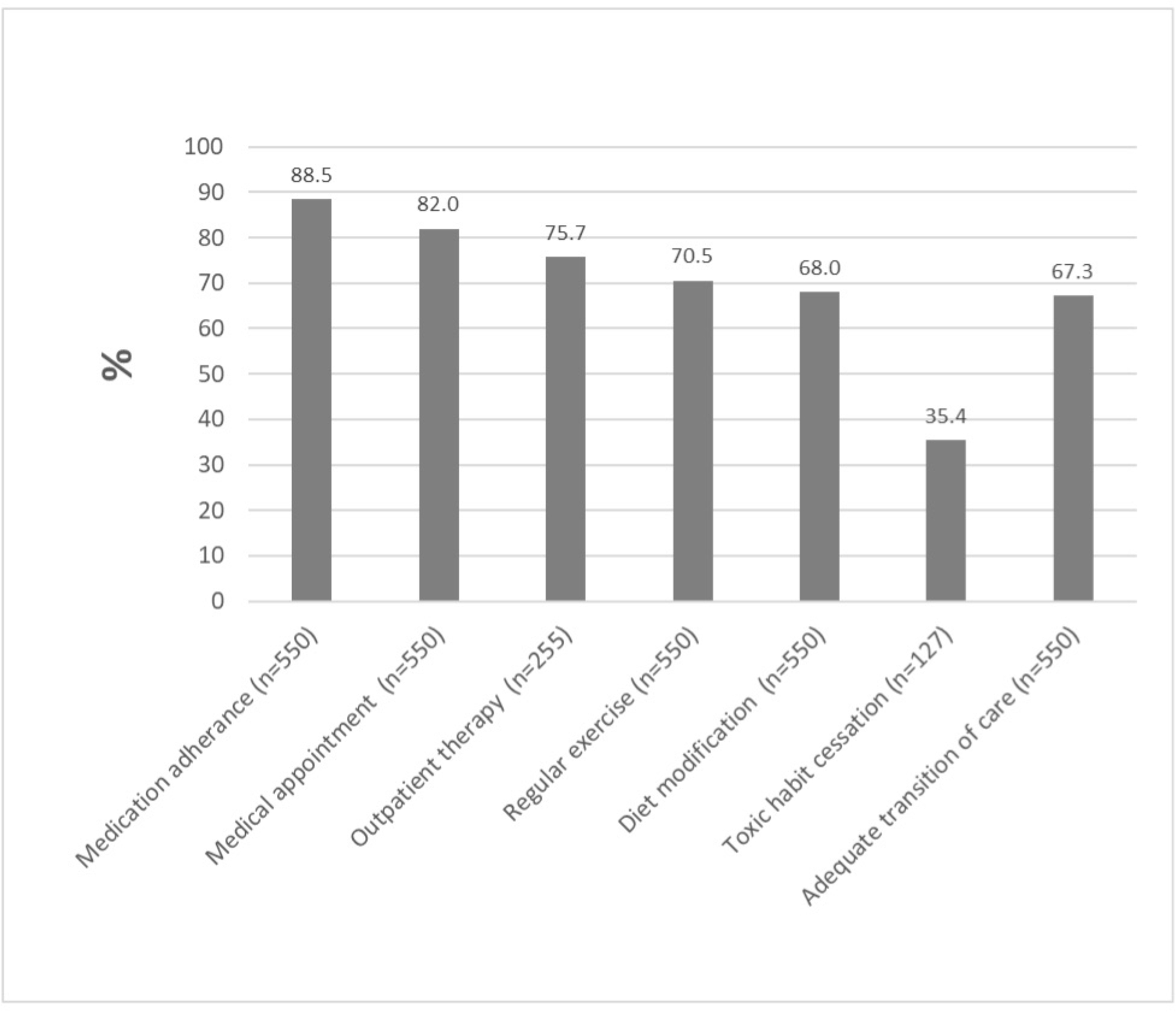
Proportion of successful 30-day transition of stroke care measures
The univariable associations between 30-day overall ATOC and baseline personal and stroke clinical characteristic are presented in Supplemental Table 2. Compared with those who failed to achieve overall ATOC, ATOC stroke patients were more likely to be employed full-time (41.6% vs 30.6%, p=0.02), live with a spouse (60% vs 47.2%, p=0.01), have 3 or more individuals they felt close to (83.8% vs71.1%, p<0.01), have a history of dyslipidemia (44.9% vs 34.4%, p=0.02), and underwent thrombectomy treatment (14.6% vs 7.8%, p=0.02), but less likely to have a history of smoking (17.3% vs 32.2%, p<0.001), coronary artery disease (13.8% vs 20.6%, p=0.04), and heart failure (3.2% vs 11.1%, p<0.01). In multivariable analyses (Table 2), the associations with ATOC remained statistically significant for those that felt close to 3 or more individuals (OR=1.86, p=0.007), living with children vs spouse (OR=0.48, p=0.012), having a history of smoking (OR=0.47, p=0.001) or heart failure (OR=0.27, p=0.001).
Table 2.
Factors associated with outcomes with a p-value <0.05 in multivariable analyses
| Outcome measures | Effects | OR | 95% CI for OR | p-Value | |
|---|---|---|---|---|---|
| ATOC | Person close to (3 or more vs <3) | 1.86 | 1.19 | 2.92 | 0.007 |
| Live with (Children vs Spouse) | 0.48 | 0.27 | 0.85 | 0.012 | |
| History of smoker (yes vs no) | 0.47 | 0.31 | 0.74 | 0.001 | |
| History of heart failure (yes vs no) | 0.27 | 0.12 | 0.59 | 0.001 | |
| Medication Adherence | Difficulty paying for medic care (somewhat hard vs not hard) | 0.37 | 0.19 | 0.72 | 0.004 |
| Difficulty paying for medic care (very hard vs not hard) | 0.46 | 0.22 | 0.96 | 0.038 | |
| Antihypertension medication at discharge (yes vs no) | 0.43 | 0.22 | 0.83 | 0.012 | |
| Thrombectomy (yes vs no) | 7.94 | 1.07 | 58.83 | 0.043 | |
| Outpatient Therapy | Health insurance (Private vs Self/None) | 3.38 | 1.45 | 7.88 | 0.005 |
| Health insurance (Medicare vs Self/None) | 4.12 | 1.89 | 8.96 | 0.0004 | |
| Discharged to (home vs rehab) | 0.14 | 0.03 | 0.59 | 0.008 | |
| Medical Appointment | Health insurance (Private vs Self/None) | 5.43 | 2.44 | 12.06 | <.0001 |
| Health insurance (Medicare vs Self/None) | 2.17 | 1.28 | 3.7 | 0.004 | |
| Live with (Children vs Spouse) | 0.36 | 0.19 | 0.67 | 0.001 | |
| Difficulty paying for basics (very hard/hard vs 3-not hard) | 0.47 | 0.27 | 0.82 | 0.008 | |
| Discharged to (home vs rehab) | 1.92 | 1.05 | 3.51 | 0.035 | |
| Diet Modification | US born (yes vs no) | 0.55 | 0.36 | 0.85 | 0.006 |
| Employment (Full-time vs Retired) | 3.73 | 2.33 | 5.99 | <.0001 | |
| Employment (Full-time Vs Unemployed) | 2.21 | 1.14 | 4.3 | 0.02 | |
| History of hypertension (yes vs no) | 1.89 | 1.19 | 3.03 | 0.007 | |
| History of diabetes (yes vs no) | 1.69 | 1.08 | 2.70 | 0.022 | |
| History of heart failure (yes vs no) | 0.43 | 0.19 | 0.94 | 0.036 | |
| Independent ambulation (yes vs no) | 2.08 | 1.20 | 3.57 | 0.008 | |
| Discharged to (home vs rehab) | 0.25 | 0.12 | 0.50 | 0.0001 | |
| Exercise | Age (per year) | 0.98 | 0.96 | 0.99 | 0.003 |
| Difficulty paying for medic care (hard vs not hard) | 0.35 | 0.19 | 0.64 | 0.0006 | |
| History of dyslipidemia (yes vs no) | 1.89 | 1.23 | 2.86 | 0.004 | |
| History of coronary artery disease/prior MI (yes vs no) | 0.55 | 0.33 | 0.92 | 0.021 | |
| Antihypertensive medication at baseline (yes vs no) | 0.63 | 0.41 | 0.98 | 0.04 | |
| Independent ambulation (yes vs no) | 1.92 | 1.16 | 3.13 | 0.01 | |
| Toxic Habit Cessation | US born (yes vs no) | 0.30 | 0.10 | 0.88 | 0.028 |
| Anticoagulation medication at baseline (yes vs no) | 0.19 | 0.07 | 0.52 | 0.001 | |
The univariable associations between 30-day medical related factors and baseline personal and stroke clinical characteristic are presented in Supplemental table 3, and the results are consistent with the multivariable regression analyses as shown in Table 2. Medication adherence was less likely among those who reported difficulty paying for medical care (OR=0.37, p=0.004 for somewhat hard vs not hard) and among those who were specifically prescribed antihypertensive medication at discharge (OR=0.43, p=0.012), and more likely among those who received thrombectomy (OR=7.94, P=0.043). Attendance at medical appointments was more likely among those with health insurance (OR=5.43, p<0.0001 for private vs none, OR=2.17, p=0.004 for Medicare vs none) and discharged home (OR=1.92, p=0.035), and less likely among those living with children (OR=0.36, p=0.001 for living with children vs spouse), and those who reported difficulty paying for basics (OR=0.47, p=0.008 for hard or very hard vs not hard).
The univariable associations between 30-day lifestyle-related TOC measures and baseline personal and stroke clinical characteristic are described in Supplemental table 4 and these associations remained statistically significant in multivariable regression analysis as shown in Table 2. Diet modification was more likely achieved by those who were foreign born (OR=1.81, p=0.006), employed full-time (OR=3.73, p<0.0001 vs retired, OR=2.21, p=0.02 vs unemployed), discharged to inpatient habilitation (OR=4.04, p=0.0001), without a history of heart failure (OR=2.33, p=0.035), with diabetes (OR=1.69, p=0.022), with hypertension (OR=1.89, p=0.007), or ambulating independently (OR=2.08, p=0.008). Regular exercise was more likely achieved by those who ambulated independently (OR=1.92, p=0.01) and without history of CAD (OR=1.82, p=0.021) or without antihypertension medication use at baseline (OR=1.58, p=0.04), but less likely achieved for those who were older (OR=0.98 per year increase, p=0.003), had difficulty paying for medical expenses (OR=0.35, p=0.0006), and did not have history of dyslipidemia (OR=0.53, p=0.004). For toxic habit cessation, the odds were greater for foreign born participants (OR=3.37, p=0.001) but smaller for those with baseline anticoagulation medication use (OR=0.19, p=0.001).
In an exploratory analysis, we compared participants enrolled before (2018–2019, n=178) and during the SARS-Cov2 public health emergency (2020–2021, n=372). We did not find a difference in the achievement of ATOC measure nor for each individual TOC measures between these two periods (Supplemental Table 5).
The reasons for not achieving the 30-day measures are shown in Table 3. The most common reason for not taking medications as prescribed was forgetting to take them. One in three not attending outpatient therapy reported issues with cost or insurance. The most common reason given for not stopping tobacco was lack of prescription of nicotine supplementation or anti-craving medications. Over 70% reported not feeling able to engage in regular physical activity, while over 50% reported not modifying their diet as they felt they were already following an ideal diet.
Table 3.
Reasons for not adopting factors/outcomes
| Outcome | N | % |
|---|---|---|
| Medication use | 44 | |
| Have not been able to get to pharmacy | 2 | 4.5 |
| Do not have the prescriptions | 4 | 9.1 |
| Too expensive or cannot afford | 6 | 13.6 |
| I feel poorly when I take them | 5 | 11.4 |
| I forget to take them | 22 | 50.0 |
| I ran out of the medication | 1 | 2.3 |
| Other or do not know | 4 | 9.1 |
| Outpatient therapy | 62 | |
| Insurance did not cover, awaiting coverage decision, or too expensive | 21 | 33.9 |
| No transportation available | 1 | 1.6 |
| Felt worse during/after therapy | 0 | 0.0 |
| Plan to start soon | 12 | 19.4 |
| Other | 28 | 45.2 |
| Medical appointment | 24 | |
| I do not have insurance | 8 | 33.3 |
| I do not have a doctor | 2 | 8.3 |
| I did not receive any follow up appointments | 8 | 33.3 |
| I missed my appointment(s) and did not reschedule | 4 | 16.7 |
| Other | 2 | 8.3 |
| Diet modification | 176 | |
| I already follow an ideal diet with low sodium, low fat, low calorie | 123 | 69.9 |
| I can’t get to the market to buy the needed ingredients | 3 | 1.7 |
| I can’t afford to change my diet as it would be more expensive | 7 | 4.0 |
| I don’t cook. I eat what the rest of the family eats or what I am given | 12 | 6.8 |
| Other | 31 | 17.6 |
| Toxic habit cessation | 82 | |
| Given sufficient information about how to stop these habits=no | 21 | 25.6 |
| Not referred to cessation clinic or support group | 53 | 64.6 |
| Not prescribed nicotine patches or medications for cravings | 59 | 72.8 |
| Exercise | 117 | |
| I feel that I cannot do any exercise due to my physical condition | 61 | 52.1 |
| I do not have the energy to exercise/too fatigued | 45 | 38.5 |
| I want to exercise but don’t have access to a park/gym | 11 | 9.4% |
The characteristics of the those with complete 30-day interview (n=550) were comparable to those not contacted at 30-days (n=74) except for country of birth, spoken language at home and discharge destination (Supplemental table 6). Compared with the patients discharged directly home, patients discharged to inpatient rehabilitation were more likely to have outpatient therapy (96% vs 71%, p<0.001) and diet modification (83% vs 66%, p=0.003), but less likely to make a follow-up medical appointment (71% vs 84%, p=0.008) (Supplemental Figure 1).
Discussion
We describe the adoption of specific behaviors after stroke hospitalization and identified substantial areas for improvement related to quality of transition of care for the population of stroke patients. Overall, about one in three patients did not achieve ideal 30-day transition of care metrics, which varied substantially across the six TOC measures. The rates of adequate TOC for three lifestyle measures (diet modification, exercise, and toxic habit cessation) were lower than those for the three medical care related measures (medication use, outpatient therapy and follow-up medical appointment). Although the factors influencing TOC performance differed by the specific measure, several social determinants of health, such as poor insurance status, difficulty paying for medical care, not being fully employed, not living with a spouse, and not having a support structure, were associated with poor transition of care measures. Our findings support the concept that transition of stroke care is affected by complex interactions of socioeconomic, environmental, and stroke-related clinical factors. Our study also highlights that even ambulatory patients discharged directly home may need additional resources to attain adequate transitions of care.
The COVID-19 pandemic has stressed stroke systems of care, including discharge planning and post-acute care.10 Indeed, some have documented a decrease in discharges to inpatient rehabilitation during the pandemic.11 To clarify if post-stroke transitions of care metrics were affected by the pandemic, we compared attainment of TOC measures in the prepandemic years with the pandemic years. Surprisingly, we did not identify a difference in the individual measures nor the composite ATOC measure. This may have been due to the selection of specific measures that did not capture pandemic-related disruptions in TOC, or to mitigation initiatives at our recruiting hospitals.
While we have not yet described the associations of these 30-day health behaviors with stroke recurrence and readmission in the TCSD Study population, successful adoption of these TOC factors has been associated with fewer readmissions and lower stroke recurrence in previous studies. For example, lower medication adherence has been associated with a greater risk of stroke,3,12 while being seen by a primary care provider after an emergency department visit has been shown to reduce recurrent emergency department visits.13
Successful transition of care is particularly challenging after stroke hospitalization. Physical disability, depression and cognitive impairment affect patients’ ability to follow instructions, participate in educational activities, and navigate the complex health care system; socioeconomic and environmental factors may considerably affect access and utilization of post-hospitalization stroke care that impact successful TOC and long-term outcomes.14,15 Interventions designed to improve these factors, including patient-centered individualized care plans, education, and physical therapy to facilitate TOC, have had limited success. In a systematic review of programs designed to improve secondary stroke prevention, a minority of studies had persistent effects on successful transition of care and stroke prevention, and these mostly involved interventions focused on reinforcing adherence to a care plan.16 More recently, a randomized trial focused on effective transition of care showed that the intervention was inconsistently implemented, with no effect on functional outcome or readmission.17,18 Some explanations proposed for this negative result include that hospitals had difficulty sustaining the intervention, there was significant loss to follow up, and outcome measures may have been insensitive in a milder stroke population.19
Nonetheless, we posit that a detailed understanding of the associations and drivers behind poor adoption of important post-hospitalization behaviors, as we have described in the current analysis, is important to inform interventions, identify high risk patients, and direct limited healthcare resources to these individuals. For example, we have identified variables related to social determinants of health, including lack of social support, not living with spouse and difficulty paying for basics and medical care, which are associated with inadequate achievement of TOC measures; these variables could be assessed at acute hospital discharge to identify patients at-risk for poor transitions of care and in need of more intense follow-up.
Moreover, we have described the self-reported barriers to adopting these health-protective behaviors, such as forgetting to take medications, perception that current diet is appropriate, and lack of prescription of tobacco cessation medications, which offer the basis to design future interventions focused on these barriers. Based on factors identified by our studies and lessons learned from pragmatic trials, one can envision interventions targeting those most at risk, supported by additional resources, that focus not only on education but also provide individualized navigation resources and behavior modification.
It is important to note that progress towards perfect achievement of each of the health-related behaviors should still be considered an improvement. For example, cutting down on smoking or incremental improvements to diet and medication adherence remain important health accomplishments after stroke. For these analyses we dichotomized the health outcomes to maximize statistical power and because we were looking at multiple outcomes. simultaneously, but acknowledge that incremental behavioral change may also be impactful. At the same time, the design of the current study may in fact over-estimate the proportion of stroke patients who are successful at making health changes after stroke because of the reliance on self-reported outcomes, selection based on the ability to consent into the current study, and the potential impact of non-random missing data at 30 days.
This study has limitations, including its modest size, the fact that it is limited to patients discharged from hospitals in Florida, evaluated shorter term follow-up at 30-days, and includes only those discharged directly home or to inpatient rehabilitation, which may limit generalizability. Nonetheless, we systematically interrogated behaviors after discharge and correlated them with baseline demographic and clinical characteristics, as well as with social determinants of health. This study focuses on demographic and clinical characteristics as predictors of transition of care health behaviors, but we lacked data on other important predictors including biomarkers of recovery (such as corticospinal tract function and lesion location), which could play a role in successful transitions of care after stroke and should be further explored in future studies. We also lacked data on the amount or intensity of rehabilitation therapy after hospital discharge. The current study includes stroke survivors discharged to home, assisted living facility or inpatient rehabilitation with a discharge mRS of 0–3. Therefore, we included patients with milder strokes, and the findings may not generalize to more severe stroke survivors. Lastly, there may a concern for type I error inflation due to multiple analyses, which should be considered in the future final analysis of the study with larger sample size.
Conclusions
In summary, this prospective study showed that one in three patients did not achieve adequate 30-day transition of care behaviors. The achievement of these behaviors varied among different TOC measures and was influenced by multiple personal, socioeconomic and clinical factors. Focused interventions aimed at facilitating TOC from hospital after stroke are urgently needed.
Supplementary Material
Source of Funding
NIH/National Institute on Minority Health and Health Disparities 1R01MD012467
Non-standard Abbreviations and Acronyms.
- ATOC
adequate transition of care
- GWTG-S
Get With The Giodelines-Stroke
- TCSD-S
Transitions of Care Stroke Disparities Study
- TOC
transition of care
Footnotes
Conflicting Interests/Disclosures
Jose G. Romano receives research support from NIH grants.
Ralph L. Sacco receives research support from NIH grants and the Florida Department of Health, and personal compensation from the AHA for serving as Editor-In-Chief of Stroke.
All other authors have no relevant conflicting interests or disclosures.
References
- 1.Zhong W, Geng N, Wang P, Li Z, Cao L. Prevalence, causes and risk factors of hospital readmissions after acute stroke and transient ischemic attack: A systematic review and meta-analysis. Neurol Sci. 2016;37:1195–1202 [DOI] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics-2021 update: A report from the american heart association. Circulation. 2021;143:e254–e743 [DOI] [PubMed] [Google Scholar]
- 3.Bailey JE, Wan JY, Tang J, Ghani MA, Cushman WC. Antihypertensive medication adherence, ambulatory visits, and risk of stroke and death. J Gen Intern Med. 2010;25:495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: The northern manhattan study. Circulation. 2012;125:2975–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman EA, Berenson RA. Lost in transition: Challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141:533–536 [DOI] [PubMed] [Google Scholar]
- 6.Sacco RL, Gardener H, Wang K, Dong C, Ciliberti-Vargas MA, Gutierrez CM, Asdaghi N, Burgin WS, Carrasquillo O, Garcia-Rivera EJ, et al. Racial-ethnic disparities in acute stroke care in the Florida-Puerto Rico collaboration to reduce stroke disparities study. J Am Heart Assoc. 2017;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607 [DOI] [PubMed] [Google Scholar]
- 8.Ormseth CH, Sheth KN, Saver JL, Fonarow GC, Schwamm LH. The american heart association’s get with the guidelines (gwtg)-stroke development and impact on stroke care. Stroke Vasc Neurol. 2017;2:94–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams HP Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, March EE. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41 [DOI] [PubMed] [Google Scholar]
- 10.Leira EC, Russman AN, Biller J, Brown DL, Bushnell CD, Caso V, Chamorro A, Creutzfeldt CJ, Cruz-Flores S, Elkind MSV, et al. Preserving stroke care during the COVID-19 pandemic: Potential issues and solutions. Neurology. 2020;95(3):124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thau L, Siegal T, Heslin ME, Rana A, Yu S, Kamen S, Chen A, Vigilante N, Gallagher S, Wegner K, et al. Decline in Rehab Transfers Among Rehab-Eligible Stroke Patients During the COVID-19 Pandemic. J Stroke Cerebrovasc Dis. 2021;30(8):105857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cummings DM, Letter AJ, Howard G, Howard VJ, Safford MM, Prince V, Muntner P. Medication adherence and stroke/tia risk in treated hypertensives: Results from the regards study. J Am Soc Hypertens. 2013;7:363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magidson PD, Huang J, Levitan EB, Westfall AO, Sheehan OC, Roth DL. Prompt outpatient care for older adults discharged from the emergency department reduces recidivism. West J Emerg Med. 2020;21:198–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broderick JP, Abir M. Transitions of care for stroke patients: Opportunities to improve outcomes. Circ Cardiovasc Qual Outcomes. 2015;8:S190–192 [DOI] [PubMed] [Google Scholar]
- 15.Bushnell CD, Olson DM, Zhao X, Pan W, Zimmer LO, Goldstein LB, et al. Secondary preventive medication persistence and adherence 1 year after stroke. Neurology. 2011;77:1182–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambert CM, Olulana O, Bailey-Davis L, Abedi V, Zand R. “Lessons learned” preventing recurrent ischemic strokes through secondary prevention programs: A systematic review. J Clin Med. 2021;10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bushnell CD, Kucharska-Newton AM, Jones SB, Psioda MA, Johnson AM, Daras LC, Halladay JR, Prvu Bettger J, Freburger JK, Gesell SB, et al. Hospital readmissions and mortality among fee-for-service medicare patients with minor stroke or transient ischemic attack: Findings from the compass cluster-randomized pragmatic trial. J Am Heart Assoc. 2021;10:e023394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan PW, Bushnell CD, Jones SB, Psioda MA, Gesell SB, D’Agostino RB Jr, Sissine ME, Coleman SW, Johnson AM, Barton-Percival BF, et al. Randomized pragmatic trial of stroke transitional care: The compass study. Circ Cardiovasc Qual Outcomes. 2020;13:e006285. [DOI] [PubMed] [Google Scholar]
- 19.Reeves MJ. Compass trial in transitional stroke care: Navigating towards true north. Circ Cardiovasc Qual Outcomes. 2020;13:e006745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


