Abstract
Supraventricular tachycardia (SVT) with ventriculoatrial (VA) block can represent a diagnostic challenge. We present a case of SVT where His-His interval shortening was repeatedly observed during episodes of VA block. This novel observation is more diagnostically suggestive of atrioventricular nodal re-entrant tachycardia, as opposed to orthodromic re-entry using a nodofascicular or nodoventricular pathway where a constant His-His is recorded during episodes of VA block. (Level of Difficulty: Intermediate.)
Key Words: AVNRT, His, nodoventricular or nodofascicular, supraventricular tachycardia, ventriculoatrial block
Abbreviations and Acronyms: AH, atrio-His; AV, atrioventricular; AVNRT, atrioventricular nodal re-entrant tachycardia; HA, His-atrial; HH, His-His; HV, His-ventricular; LBBB, left bundle branch block; NF, nodofascicular; NV, nodoventricular; ORT, orthodromic re-entry; RBBB, right bundle branch block; SVT, supraventricular tachycardia; VA, ventriculoatrial
Central Illustration
Supraventricular tachycardias (SVTs) can represent diagnostic challenges even for experienced electrophysiologists. This is particularly the case when atrioventricular (AV) or, less commonly, ventriculoatrial (VA) block occurs. Specifically, distinguishing AV nodal re-entrant tachycardia (AVNRT) from orthodromic re-entry (ORT) using a concealed nodofascicular (NF) or nodoventricular (NV) pathway can be especially difficult. Recent data have highlighted the diagnostic utility that His-His (HH) interval prolongation may have before VA block in supporting a diagnosis of AVRNT. However, to the best of our knowledge, HH interval shortening has not been described in a similar setting.
Learning Objectives
-
•
To be able to make the differential diagnosis of SVT with VA block by observing changes in HH intervals.
-
•
To understand the role of HH interval changes consistent with multiple nodal pathways supporting AVNRT.
History of Presentation
A 54-year-old man presented with vagal- and adenosine-sensitive recurrent SVT. Electrocardiograms demonstrated sinus rhythm without evidence of pre-excitation and narrow-complex tachycardia between 190 and 220 beats/min with a pseudo R′ in lead V1.
Differential Diagnosis
Although the history is supportive of particular SVTs over others, an electrophysiology study is required for definitive diagnosis.
Investigations
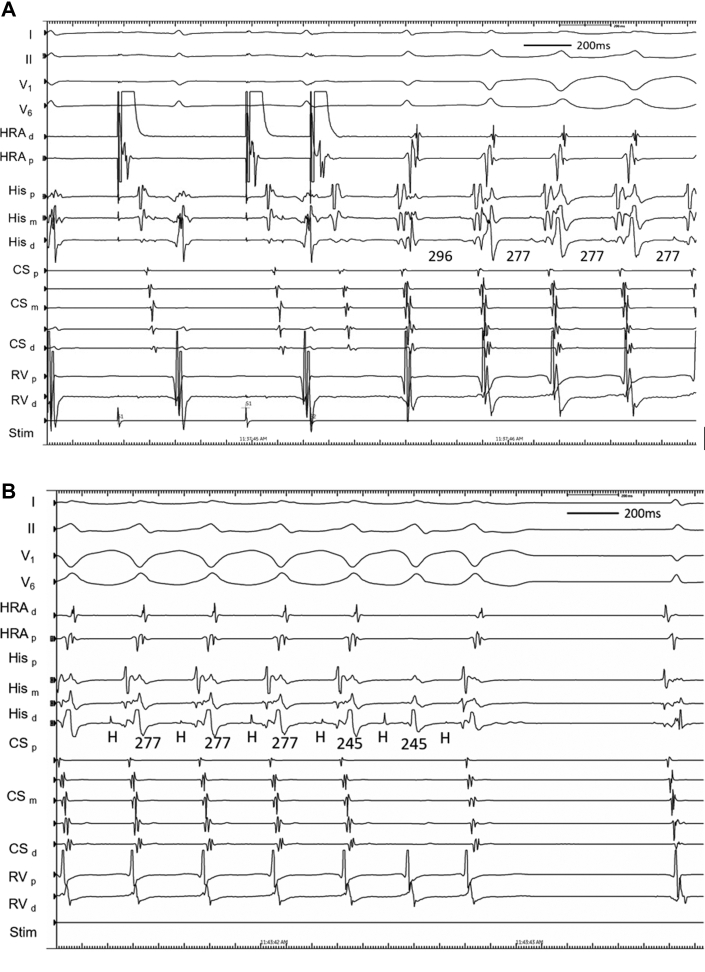
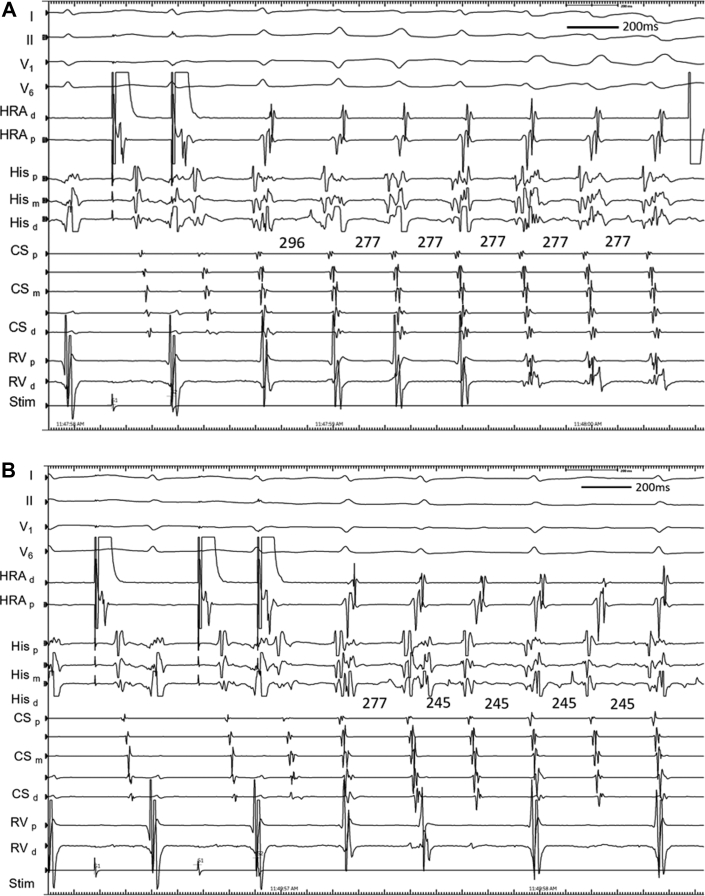
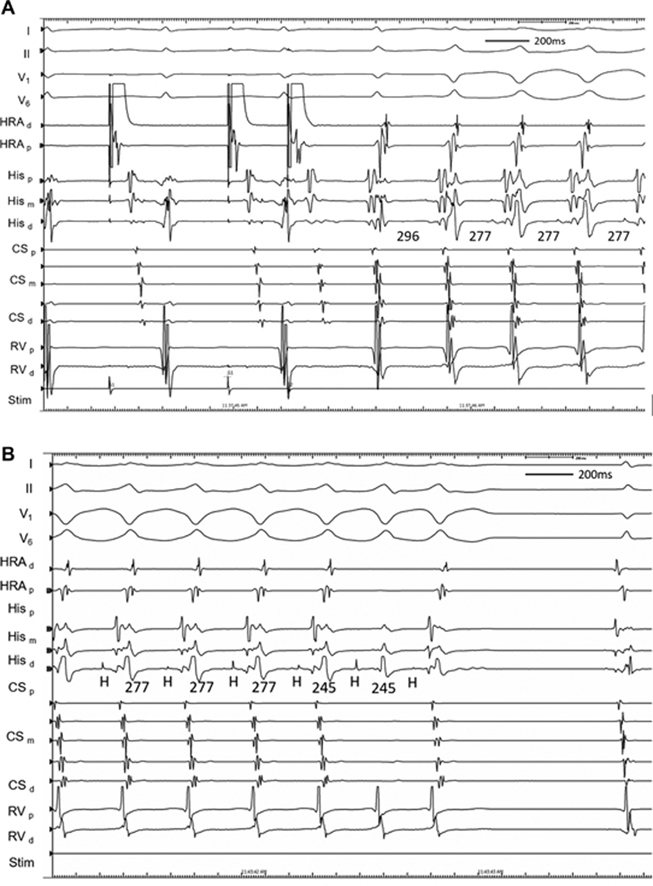
At electrophysiology study, the patient was in sinus rhythm with atrio-His (AH) and His-ventricular (HV) intervals of 67 and 56 milliseconds, respectively. VA conduction was concentric and decremental, with the earliest atrial activation at the right superoparaseptum. There was a reproducible AH jump and typical nodal echoes observed with single atrial extrastimuli from the high right atrium, consistent with dual AV nodal physiology. The atrial effective refractory period was 270 milliseconds. Para-Hisian pacing was consistent with a nodal response. With isoproterenol, tachycardia was induced after critical AH prolongation during programmed atrial extrastimulation. After an initial narrow-complex beat, transition to a left bundle branch block (LBBB) configuration tachycardia occurred, with a cycle length of 277 milliseconds, a short VA time of 10 milliseconds, and concentric retrograde atrial activation earliest at the right superoparaseptum (Figure 1A). During LBBB tachycardia, supra-Hisian VA block occurred spontaneously (Figure 1B). Although His-atrial (HA) and His-His (HH) intervals were constant during 1:1 VA conduction, the HH interval shortened by 32 milliseconds (from 277 to 245 milliseconds) for the cycle preceding and straddling the occurrence of VA block. Further episodes of tachycardia initiated similarly, with a narrow-complex QRS interval before transitioning to a right bundle branch block (RBBB) configuration (Figure 2A). Notably, VA or HA intervals and retrograde atrial activation sequences were also comparable to LBBB configuration and narrow-complex tachycardias. Subsequently, a tachycardia configuration was observed to transition among narrow, LBBB, and RBBB configurations without variation in cycle length or VA or HA intervals; these were associated with numerous episodes of 2:1 AV block without change in HA or HH intervals (Figure 2B). Despite reproducible tachycardia initiation, episodes were not sufficiently sustained to allow for ventricular overdrive pacing, or atrial or ventricular extrastimuli, to be reliably delivered. Nevertheless, a diagnosis of typical, slow-fast AVNRT was made on the basis of multiple supportive features. VA block is inconsistent with atrial tachycardia or ORT using an AV pathway. AV block excludes ORT using an NV pathway, and it renders ORT using an NF pathway very unlikely. Furthermore, a lack of VA change during RBBB or LBBB also strongly argues against NF or NV ORT. Junctional tachycardia remains possible and was not excluded by atrial pacing maneuvers. Bundle branch re-entry is excluded by the transition from LBBB or RBBB to a normal QRS complex during tachycardia. Upper septal fascicular ventricular tachycardia is also excluded by the site of successful ablation. Finally, intra-His re-entry is theoretically possible but would seem less likely in the absence of a split His.
Figure 1.
Initial Tachycardia Induction With Subsequent Ventriculoatrial Block
(A) Induction of tachycardia after critical atrial-His interval prolongation during programmed atrial extrastimulation. The initial beat of tachycardia had a narrow QRS complex before transition to a left bundle branch block configuration. (B) During tachycardia with a left bundle branch block configuration, ventriculoatrial block occurred spontaneously before termination of tachycardia was observed. CS = coronary sinus; d = distal; HRA = high right atrium; m = mid; p = proximal; RV = right ventricle.
Figure 2.
Repeat Tachycardia Induction With Varying QRS Configuration and Atrioventricular Block
(A) Repeat induction of tachycardia with initially narrow QRS complex and subsequently a right bundle branch block configuration. (B) Repeat induction of tachycardia followed by atrioventricular block. Abbreviations as in Figure 1.
Management
A traditional electroanatomic approach to slow pathway modification was undertaken with slow junctional beats during radiofrequency ablation. Following this, an AH jump and single typical echoes were observed with atrial extrastimuli, but the previous tachycardia that was repeatedly inducible was no longer inducible despite isoproterenol administration.
Discussion
SVTs with AV or, less commonly, VA block occur occasionally and can represent a diagnostic challenge, particularly in the latter scenario. In this case, HH variation during VA block was observed and provided useful diagnostic information. In NF or NV ORT, HH intervals should remain constant with VA block because the re-entry circuit is independent of any upper common exit. In contrast, HH intervals can vary in AVNRT and suggest the involvement of multiple pathways.1, 2, 3, 4 Although HH prolongation has been described before VA block in AVNRT, a decrease in HH interval has not been previously reported.
A recent series of SVTs with VA block elegantly characterized the diagnostic utility of several features for differentiation of NF or NV ORT from AVNRT.1 One of these features was HH prolongation during VA block, where ≥10 milliseconds (37 ± 28 milliseconds) was observed in most (79%) AVNRT cases, in contrast to a lack of change in the majority of NF or NV ORT cases. Several other previous reports also noted a similar phenomenon of HH prolongation during VA block in AVNRT, and it was ascribed to the presence of multiple nodal pathways.2, 3, 4
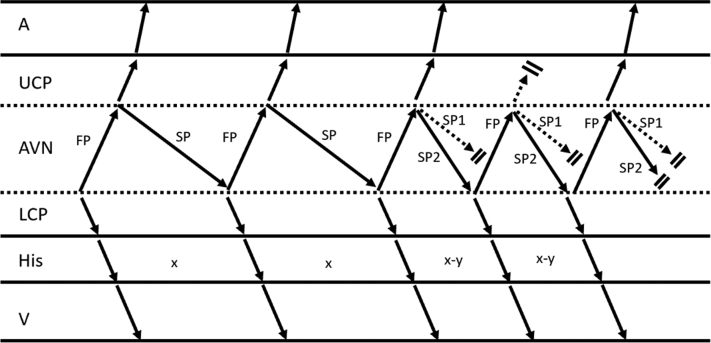
In our case, we uniquely observed a decrease in the HH interval of 32 milliseconds associated with VA block. The most likely explanation for this observation is the presence of multiple pathways, as has been previously postulated for patients with AVNRT and HH prolongation. For example, conduction could proceed down another anterograde pathway with faster conduction, manifesting as a decrease in HH interval and subsequent AH shortening that leads to VA block (Figure 3). Despite a lack of multiple discontinuities during programmed atrial extrastimulation, this remains the most likely explanation. Other structural possibilities are also plausible, including a faster retrograde pathway (although this should result in VA block before HH shortening) or a bystander NF-NV pathway. Furthermore, functional explanations that may also allow for a shorter HH interval are theoretically possible, including a change in upper turnaround point of the nodal pathways resulting in a decrease in circuit size and upper common pathway block, changes in conduction velocity of a single pathway, or shortening of the refractory period of the antegrade or retrograde nodal pathways, given the previous suggestion that changes in refractoriness determined cycle length alternans in AVNRT.5 In contrast, in addition to a slower nodal pathway, an alternative explanation for the analogous HH prolongation documented by other investigators is the association of VA block with conduction delay or block below the upper turnaround point of the re-entry circuit. Although 1 mechanism for VA block contemplated in earlier reports was an additional pathway with a lack of an atrial exit, this would be unlikely in our case because of resumption of VA conduction in the subsequent cycle with persistently shorter HH interval, and the site of VA block may instead be in an upper common pathway.6 Interestingly, faster tachycardias were subsequently initiated in our patient. In some induced episodes, the first cycle was of similar cycle length to previous tachycardias (277 milliseconds) before stabilizing into a shorter cycle length (245 milliseconds) similar to that observed with VA block. We believe that the induction of these tachycardias also provides further evidence for the existence of a third pathway. Slow pathway modification was successful in eliminating all inducible tachycardias, although this site of successful ablation alone does not distinguish AVNRT from NF or NV ORT because the latter can also be ablated from this region.
Figure 3.
Ladder Diagram Depicting a His-His Decrement During Atrioventricular Nodal Re-Entrant Tachycardia, as Shown in Figure 1B
This finding is postulated to result from conduction over another antegrade slow pathway with faster conduction and associated atrio-His shortening resulting in an upper common pathway block. Termination of tachycardia occurs in the next beat with block in both antegrade slow pathways. A = atrium; AVN = atrioventricular node; FP = fast pathway; His = bundle of His; LCP = lower common pathway; SP = slow pathway; UCP = upper common pathway; V = ventricle.
Conclusions
We describe a consistently shorter HH interval associated with VA block in a patient with SVT. We conclude that the most likely explanation for this observation is AVNRT, and a third nodal pathway similar to that postulated when HH interval prolongation has occurred, although other possibilities are discussed. In addition to advancing our mechanistic understanding of SVTs, this observation may prove helpful diagnostically when attempting to differentiate AVNRT from NF or NV ORT where the former is more likely to have multiple pathways and tachycardias present, but this observation needs further confirmation.1
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Nagashima K., Kaneko Y., Maruyama M., et al. Novel diagnostic observations of nodoventricular/nodofascicular pathway-related orthodromic reciprocating tachycardia differentiating from atrioventricular nodal re-entrant tachycardia. J Am Coll Cardiol EP. 2020;6(14):1797–1807. doi: 10.1016/j.jacep.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Ino T., Tadera T., Miyamoto S., et al. Ventriculoatrial block during atrioventricular nodal reentrant tachycardia utilizing multiple retrograde pathways. J Cardiovasc Electrophysiol. 1998;9(11):1206–1213. doi: 10.1111/j.1540-8167.1998.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 3.Otomo K., Okamura H., Noda T., et al. Unique electrophysiologic characteristics of atrioventricular nodal reentrant tachycardia with different ventriculoatrial block patterns: effects of slow pathway ablation and insights into the location of the reentrant circuit. Heart Rhythm. 2006;3(5):544–554. doi: 10.1016/j.hrthm.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Morihisa K., Yamabe H., Uemura T., et al. Analysis of atrioventricular nodal reentrant tachycardia with variable ventriculoatrial block: characteristics of the upper common pathway. Pacing Clin Electrophysiol. 2009;32(4):484–493. doi: 10.1111/j.1540-8159.2009.02309.x. [DOI] [PubMed] [Google Scholar]
- 5.Ross D.L., Dassen W.R., Vanagt E.J., Brugada P., Bär F.W., Wellens H.J. Cycle length alternation in circus movement tachycardia using an atrioventricular accessory pathway. A study of the role of the atrioventricular node using a computer model of tachycardia. Circulation. 1982;65(5):862–868. doi: 10.1161/01.cir.65.5.862. [DOI] [PubMed] [Google Scholar]
- 6.Munawar D.A., Arstall M., Lypourlis D. The existence of upper common pathway: evidence from concomitant atrioventricular nodal reentrant tachycardia and atrial fibrillation. HeartRhythm Case Rep. 2021;7(1):21–25. doi: 10.1016/j.hrcr.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]