Abstract
In schizophrenia, the age of first episode onset can reflect genetic loading and predict prognosis. Little is known about the association between the age of onset and cognition among individuals with early-stage schizophrenia. We aimed to compare the pre-treatment neurocognition profile between individuals with early-onset schizophrenia (EOS, the age of onset < 18 years), typical-onset schizophrenia (TOS, the age of onset between 18 and 39 years), and late-onset schizophrenia (LOS, the age of onset between 40 and 59 years). We included individuals with a current diagnosis of schizophrenia within 3 years and medication naive or less than 2 weeks of cumulative antipsychotic exposure and current daily antipsychotic dosage equivalent to ≤ 15 mg of olanzapine. Assessments included the MATRICS Consensus Cognitive Battery (MCCB) and the Positive and Negative Syndrome Scale (PANSS). We used linear regression to compare the difference between age-of-onset groups. We included 356 participants (67 EOS, 195 TOS, and 94 LOS). Compared with LOS, TOS was associated with lower scores in the verbal learning scores of the MCCB after adjusting for education years and the subscale scores of the PANSS (45.5 ± 12.9 vs. 40.5 ± 14.1, adjusted B = − 5.79, p = 0.001). The three groups had no difference in other cognitive domain scores. The association between the age of onset and MCCB verbal memory was U-shape (square of the age of onset, adjusted B = 0.02, p = 0.003). Patients with LOS had a better verbal learning function compared with individuals with TOS. These findings suggest that involvement of cognition assessment and rehabilitation training is necessary for patients with TOS.
Keywords: Schizophrenia, Cognition, Age of onset, Psychosis symptoms, Antipsychotics
Introduction
Schizophrenia is a serious mental disorder and the peak age of onset is at 20.5 years (Solmi et al. 2021). The younger age of onset can predict some long-term clinical outcomes, such as more negative symptoms, higher risk for relapses, and poor social/occupational function (Immonen et al. 2017). Although cognition impairment is one of the core symptoms suffering by individuals with schizophrenia (Zhang et al. 2019), little is known about the association between the age of onset and neurocognition among schizophrenia at the early phase.
A few studies have compared early-onset schizophrenia (EOS, the age of onset < 18 years), typical-onset schizophrenia (TOS, the age of onset between 18 and 39), and late-onset schizophrenia (LOS, the age of onset between 40 and 59) on cognitive function, but they had small samples, inconsistent criteria for the age of onset, and long duration of illness (Suen et al. 2019). A meta-analysis has reported that those with EOS demonstrated larger deficits in visual and auditory attention than those with LOS and performed better in digit symbol coding and vocabulary (Rajji et al. 2009). However, its major limitation was that the age of onset was missing for approximately 1/4 participants. Patients with LOS were reported to perform better in the speed of processing, spatial working memory, and verbal fluency than those whose onset of schizophrenia was before 45 years old (n = 69; Brichant-Petitjean et al. 2013). A study on > 40-year-old patients, who varied greatly on the course of illness, found that the LOS subgroup had better processing speed, abstraction, and verbal memory than the chronic EOS or TOS subgroup; however, their performance in auditory working memory was similar (Vahia et al. 2010). More studies focus on the comparison between the onset at puberty and adulthood. One study found that the age of onset (before/after 18 years old) was not associated with any between-group difference in inhibition, switching, and cognitive flexibility (Holmén et al. 2012). Among individuals with chronic schizophrenia, the age of onset after 18 years old was associated with worse processing speed and cognitive flexibility, but better verbal fluency, than EOS (Grover et al. 2019). Another study found that first-episode EOS patients performed worse on tasks of working memory, language, and motor function than first-episode TOS patients (White et al. 2006). Latest research among individuals with a psychotic disorder of fewer than 12 months’ duration reported that the age of onset < 18 years old had a lower score in sustained attention and executive functions compared with those with the age of onset between 18 and 35 years old (De la Serna et al. 2021). Differences in familiality between EOS and TOS may lead to a different performance in attention, working memory, and verbal learning (Bigdeli et al. 2020). In a group of 12–43 years old, first-episode antipsychotic-naïve schizophrenia patients, age had an inverted U-curve effect on the performance in the verbal memory task and the digit sequencing task (Fagerlund et al. 2021). This contrasts with a previous study (Tuulio-Henriksson et al. 2004), which found the earlier onset of age among adults with schizophrenia was linearly associated with lower scores in verbal learning and memory.
The comparison of LOS with EOS and TOS at the early stage of illness is few, although several studies have compared the cognition between first-episode EOS and TOS (Bigdeli et al. 2020; De la Serna et al. 2021; Fagerlund et al. 2021; White et al. 2006). The cognition function of individuals with schizophrenia may be stable over the first two years of illness (Sánchez-Torres et al. 2018) but declines in the long term. Speed processing, learning, and executive function decline among individuals with schizophrenia compared with no change in healthy controls, reported in a reprehensive birth-cohort study (from age 7 to age 38) (Meier et al. 2014). Latest longitudinal studies with matched controls reported that cognitive aging in memory, verbal knowledge (Fett et al. 2019; Zanelli et al. 2019), attention, and processing speed (Fett et al. 2019) were more rapid than general populations in the decade followings the first episode (Fett et al. 2022). Thus, it is needed to restudy the association between the age of onset and cognition in those with a short duration of illness.
Another limitation of previous research is that, to date, no study has investigated the association between the age of onset and cognitive performance measured using the MATRICS Consensus Cognitive Battery (MCCB; Nuechterlein et al. 2008). The United States National Institute of Mental Health developed this battery. With its advantages on test selection, reliability, and validity, it has become a standard tool for assessing cognitive ability in schizophrenia and related disorders (Green et al. 2014). In addition, normative data for this battery allow for multiple demographic corrections (Kern et al. 2008), especially limiting the confounding effect of age.
Cognition is important for occupational functioning and independent living among individuals with schizophrenia (Fu et al. 2017; Kharawala et al. 2022; Nuechterlein et al. 2014; Santesteban-Echarri et al. 2017). Finding associates of cognitive impairment would help deliver personalized interventions and improve their quality of life. Our study aimed to compare the pre-treatment baseline cognition performance by different onset-age groups. Also, we would explore the linear or nonlinear association between cognition with the age of onset.
Method
This cross-sectional study was conducted at five mental health hospitals, located in five cities in China: Beijing Huilongguan Hospital, Chongqing Sanmenxia Mental Health Center, Changchun Sixth Hospital, Harbin First Specialist Hospital, and Zhumadian Second People’s Hospital. The Institutional Review Board (IRB) of Beijing Huilongguan Hospital, the central IRB, approved this research and other research sites accepted this approval. Written informed consent was provided by adult participants or legal guardians of juveniles.
Participants
The inclusion criteria were: (1) age between 15 and 59 years old; (2) met a current diagnosis of schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV-TR); (3) duration of illness < 36 months; and (4) medication naive or less than 2 weeks of cumulative antipsychotic exposure and current daily antipsychotic dosage equivalent to ≤ 15 mg of olanzapine. Exclusion criteria included: (1) ≤ 6 years of education; (2) mental retardation, serious somatic or neurological illness, or history of brain injury; (3) substance dependence or abuse (except nicotine); (4) having received electroconvulsive therapy or repetitive transcranial magnetic stimulation within 6 months. Duration of illness was the months between the date of the first onset of psychotic symptoms and the interview date.
Procedures
Trained psychiatrists administered the diagnoses, interviews, and clinical assessments.
Measurement
Age of schizophrenia onset
Its definition is the age when the participant first appears psychotic symptoms (Howard et al. 2000). We collected this information through interviewing patients and their family members and reviewing their medical records. EOS was the age of onset < 18, TOS was the age of onset between 18 and 39, and LOS was the age of onset between 40 and 59 according to the international consensus (Howard et al. 2000).
Psychopathological symptoms
The Positive and Negative Syndrome Scale for Schizophrenia—30 items (PANSS; Kay et al. 1987) was used to assess symptom severity where symptoms were rated from 1 absent to 7 extreme. Subscale scores (positive symptoms, negative symptoms, and general psychopathology) were the sum of corresponding items (Xiao et al. 2015). The inter-observer reliability was greater than 0.8.
Neurocognitive function
Cognitive assessments were obtained using the MCCB. The MCCB (Nuechterlein et al. 2008; Zou et al. 2009) uses ten tests to cover seven neurocognitive domains: speed of processing, verbal learning, working memory, reasoning and problem-solving, visual learning, social cognition, and attention/vigilance. As most Chinese are not familiar with alphabetical order, the Letter-Number Span Test in the verbal working memory domain is replaced with the Digit Sequencing Test of the Wechsler Adult Intelligence Scale in the adapted Chinese MCCB (Zou et al. 2009). Raw scores were standardized to T scores, which were corrected for age and sex based on the Chinese norm.
Statistical analysis
Between-group differences in continuous variables were determined using ANOVA. Tukey–Kramer HSD correction was performed for correcting multiple comparisons problems. Pearson’s χ2 tests were used to compare sex proportions across different groups.
Multivariate analysis of variance (MANOVA) was used to test the overall between-group difference in the MCCB domains between age-of-onset groups. Multivariable linear regression was used to explore the onset-age-group difference in the corrected T score for each MCCB domain after adjusting for education years, duration of illness (months), and the subscale scores of the PANSS. The age was not included as a covariate because the high collinearity of age and the age of onset would introduce bias (van der Werf et al. 2012). Then, the inclusion of the age of schizophrenia onset and the squared age-of-onset-term in a multi-variable linear model was to explore possible nonlinear associations between the age of onset and each MCCB domain score. Multiple comparisons for scores of each MCCB domain were adjusted for Bonferroni corrections.
The software was Stata 15.0. Statistical significance was defined as a two-sided p < 0.05.
Results
Demographics and clinical characteristics
The study recruited 356 patients with first-episode schizophrenia, including EOS (n = 65), TOS (n = 195), and LOS (n = 94). Three groups had no difference in gender, education, and the positive symptoms and negative symptoms subscales scores of the PANSS (Table 1). Three group differed in the general psychopathology subscale scores of the PANSS (F = 8.90, p < 0.001) and the average scores of the LOS group were lower than that of the EOS group (Turkey HSD test, p < 0.001) and that of the TOS group (Turkey HSD test, p < 0.001).
Table 1.
Demographics and clinical characteristics
| Variables | Early-onset n = 67 |
Typical-onset n = 195 |
Late-onset n = 94 |
Statistic | P |
|---|---|---|---|---|---|
| Mean(SD) | Mean( SD) | Mean (SD) | |||
| Age, year | 17.4 (1.2) | 25.8 (5.8) | 43.1 (2.9) | F = 703.63 | < 0.001 |
| Female, n (%) | 31 (46.3%) | 91 (46.7%) | 52 (55.3%) | χ2 = 2.12 | 0.346 |
| Education, year | 10.0 (2.3) | 10.0 (3.1) | 10.4 (3.2) | F = 0.57 | 0.568 |
| Age of onset, year | 16.3 (1.0) | 24.9 (5.7) | 42.7 (2.9) | F = 788.34 | < 0.001 |
| Duration of illness, month | 15.3 (9.0) | 14.3 (10.8) | 8.3 (3.1) | F = 16.94 | < 0.001 |
| PANSS | |||||
| Positive | 23.6 (5.7) | 24.6 (4.9) | 23.6 (4.1) | F = 1.75 | 0.176 |
| Negative | 20.8 (7.5) | 19.9 (6.3) | 19.1 (6.2) | F = 1.39 | 0.250 |
| General psychopathology | 40.9 (8.2) | 39.9 (7.7) | 36.5 (6.2) | F = 8.90 | < 0.001 |
| MCCB | |||||
| Speed of processing | 42.3 (9.4) | 41.1 (10.2) | 43.7 (8.8) | F = 2.27 | 0.105 |
| Verbal learning | 44.7 (10.9) | 40.5 (14.1) | 45.5 (12.9) | F = 5.58 | 0.004 |
| Working memory | 41.7 (11.1) | 40.3 (11.8) | 44.8 (10.6) | F = 4.99 | 0.007 |
| Reasoning and problem solving | 43.4 (11.3) | 41.6 (12.5) | 44.5 (11.6) | F = 2.02 | 0.134 |
| Visual learning | 43.0 (11.1) | 39.6 (11.1) | 43.2 (10.4) | F = 4.54 | 0.011 |
| Social cognition | 46.6 (8.9) | 45.3 (10.6) | 47.6 (10.4) | F = 1.66 | 0.193 |
| Attention/vigilance | 39.2 (8.8) | 38.3 (9.7) | 40.4 (8.8) | F = 1.66 | 0.192 |
PANSS Positive and Negative Syndrome Scale for Schizophrenia—30 items; MCCB the MATRICS Consensus Cognitive Battery
The age of onset and cognition
The scores of the MCCB by three groups (EOS, TOS, and LOS) were shown in Table 1. Individuals with TOS had lower scores than those with LOS in verbal learning (40.5 ± 14.1 vs. 45.5 ± 12.9; Cohen’s d = − 0.36; Turkey HSD test, p = 0.008, Fig. 1), working memory (40.3 ± 11.8 vs. 44.8 ± 10.6, Cohen’s d = − 0.39; Turkey HSD test, p = 0.005), and visual learning (39.6 ± 11.1 vs. 43.2 ± 10.4; Cohen’s d = − 0.33; Turkey HSD test, p = 0.026). Other bi-variate tests did not find any difference in domain scores of the MCCB between the three onset-age groups (p > 0.05).
Fig. 1.
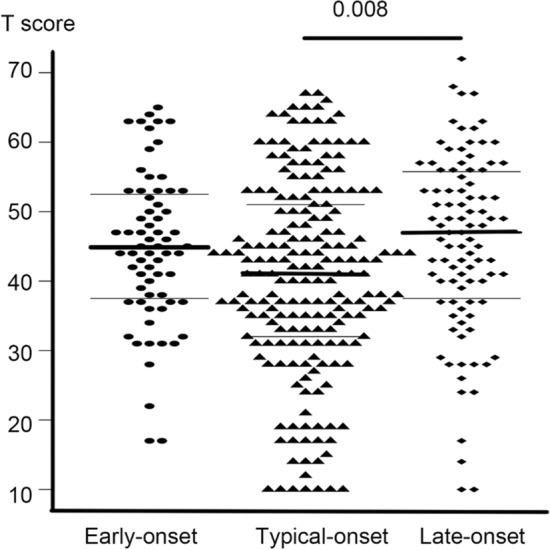
Scores for the verbal learning domain in the MATRICS Consensus Cognitive Battery by different onset-age groups
MANOVA showed that the three groups did not significantly differ overall in the seven MCCB domain scores (seven scores were dependent variables simultaneously), F(14, 692) = 1.27, exact p = 0.219. The association was still nonsignificant after adjusting for education years and duration of illness, F(14, 676) = 1.33, exact p = 0.183.
Multivariable linear regression showed that compared with LOS, the TOS only performed worse in verbal learning (B = − 5.79, p = 0.001) after adjusting for education years, duration of illness, and subscale scores of the PANSS (Table 2). The difference between bi-variate associations of EOS, TOS, and LOS in each MCCB domain was nonsignificant.
Table 2.
Multi-variable linear regression of each domain score in the MATRICS Consensus Cognitive Battery (MCCB) on different age-of-onset groups
| Variables | (1) SP |
(2) VEL |
(3) WM |
(4) RP |
(5) VIL |
(6) SC |
(7) AV |
|---|---|---|---|---|---|---|---|
| B (p) | B(p) | B(p) | B(p) | B(p) | B(p) | B(p) | |
|
Age of onset ref: Late-onset Early-onset |
− 1.60 | − 1.78 | − 2.37 | − 1.75 | − 0.36 | − 0.29 | − 1.48 |
| (0.335) | (0.431) | (0.220) | (0.392) | (0.845) | (0.868) | (0.351) | |
| Typical-onset | − 2.88 | − 5.79 | − 4.09 | − 3.32 | − 3.59 | − 1.72 | − 2.31 |
| (0.029) | (0.001) | (0.008) | (0.041) | (0.015) | (0.214) | (0.067) |
a. The sample size for the model (1) was 351, model (7) 354, and others 356. b. Adjusted for years of education, duration of illness (months), and subscale scores of the Positive and Negative Syndrome Scale. c. P values < 0.007 (≈ 0.05/7) are in boldface
SP Speed of processing; VEL Verbal learning; WM Working memory; RP Reasoning and problem solving; VIL Visual learning; SC Social cognition; AV Attention/vigilance
Then, the multi-variable linear regression showed that the association between the age of onset and MCCB’s verbal learning scores was a U curve after adjusting for education years, duration of illness, and subscale scores of the PANSS (Table 3; the age of onset, adjusted B = − 1.34, p = 0.009; the age of onset ^2, adjusted B = 0.02, p = 0.003). The significance of the associations between the age of onset and other MCCB scores did not reach a significant level after Bonferroni’s corrections.
Table 3.
Multi-variable linear regression of each domain score in the MATRICS Consensus Cognitive Battery (MCCB) on different age-of-onset groups
| Variables | (1) SP |
(2) VEL |
(3) WM |
(4) RP |
(5) VIL |
(6) SC |
(7) AV |
|---|---|---|---|---|---|---|---|
| B (p) | B(p) | B(p) | B(p) | B(p) | B(p) | B(p) | |
| Age of Onset | 0.10 | − 1.34 | 0.15 | 0.13 | − 0.81 | 0.01 | 0.12 |
| (0.070) | (0.009) | (0.014) | (0.042) | (0.056) | (0.830) | (0.020) | |
| Age of Onset ^2 | 0.02 | 0.01 | |||||
| (0.003) | (0.035) |
a. The sample size for the model (1) was 351, model (7) 354, and others 356. b. Adjusted for years of education, duration of illness (months), and subscale scores of the Positive and Negative Syndrome Scale. c. P values < 0.007 (≈ 0.05/7) are in boldface
SP Speed of processing; VEL Verbal learning; WM Working memory; RP Reasoning and problem solving; VIL Visual learning; SC Social cognition; AV Attention/vigilance
Power analysis
We computed the power for a two-sided two-sample means test using the verbal learning scores of the MCCB in the TOS group and the LOS group: mean1 [sd1] = 40.5 (14.1), mean2 [sd2] = 45.5 [12.9], α = 5%, n = 289, n2: n1 = 0.482. The power was 0.843.
Discussion
This study demonstrated that LOS was associated with small but significantly better performance in verbal learning than TOS among patients with minimal-treated, first-episode schizophrenia. Our study also showed a U-curve association between the age of onset and verbal learning.
Previous research found individuals with first-episode schizophrenia had deficiencies in all cognitive domains compared to healthy controls (Zhang et al. 2019). The cognitive profile has been regarded as a distinct dimension of clinical symptoms to picture the heterogeneity of individuals with schizophrenia. However, our study revealed that the effect of the age of onset on cognitive impairment was domain-specific rather than global. We did not find evidence for differential effects of the onset of age on majority cognition domains. The LOS was associated with better performance in verbal learning than TOS, although the effect size was relatively small. This immediate recall ability was evaluated through the Hopkins Verbal Learning Test, which used twelve words from three semantic groups. This finding was consistent with a study that detected the individuals with LOS were less impaired in verbal learning, assessed using the corrected long-delay free recall score of the California Verbal Learning Test, than those whose age of onset schizophrenia was before 40 years old (Vahia et al. 2010). However, their sample included individuals with chronic TOS and the difference in verbal learning became nonsignificant after adjusting for the duration of illness. Moreover, another study also found that the LOS group performed better in verbal fluency tests than those whose age of onset schizophrenia before 45 years old (Brichant-Petitjean et al. 2013). Compared to their study design, our study used standardized MCCB scores, making it possible for controlling the effect of age and sex when we compared the cognitive difference in different age-of-onset groups.
In addition, our study was the first to show that the association between the age of onset and performance in verbal learning was U-shape (the lowest point at 34 years old) independent of psychopathology. A study among antipsychotic-naive schizophrenia patients aged between 12 and 43 years old has detected an inverted U-curve relationship between age and verbal memory (Fagerlund et al. 2021). This difference may be because their study did not include individuals with LOS and the performance may be task-specific. Another study demonstrated that earlier onset of psychosis was associated with worse age-corrected performance on immediate recall of verbal learning, measured using the Groningen Word Learning Task, before adjusting for demographics and clinical variables (van der Werf et al. 2012). However, the majority (93.3%) of their participants had the onset of psychosis before 40 years old and they had fewer psychotic symptoms than our samples. Thus, our study can better demonstrate the cognitive profile of individuals with LOS. Less genetic loading for LOS may explain their better verbal learning abilities (Woolston et al. 2017). Also, genetic alternations during special development windows can also result in progressive brain changes (Gogtay et al. 2011; Zalesky et al. 2015). However, the poor performance in verbal learning of the TOS group than the LOS group could not be explained by aging-induced central nervous system differences (Howard et al. 2000).
Additionally, we did not detect any significant cognitive differences between the EOS and LOS groups, although EOS is likely to reflect neurodevelopmental vulnerability. The reason may be that strategic verbal memory recall ability reflects the maturation of the prefrontal cortex, which is late neurodevelopment (Yu et al. 2018). It means that the verbal learning function does not fully develop for both adolescents with or without EOS, resulting in the nonsignificant difference in cognitive impairment between individuals with EOS and those with TOS. Moreover, in our study, the speed of processing did not differ between onset-age groups. In contrast, a previous study showed that patients with LOS had better scores on the digit symbol test compared with those whose onset of schizophrenia was before 40 years old, but they did not control for the confounding effects of illness duration and psychopathology symptoms (Vahia et al. 2010).
A major strength of our study was that all participants were in the early stage of illness, thus minimalizing confounding effects of treatment history and illness phases. Additionally, we measured cognition function using a standard cognitive battery and the resultant standardized domain scores allowed us to control for the confounding effects of age and sex. Then, our samples were across the entire age-of-onset range and the study had enough sample with EOS or TOS. Our sample had a higher proportion of individuals with LOS than other studies because we over-selected individuals with LOS to enlarge the sample’s power. Then, LOS, especially for paranoia schizophrenia among middle-aged women, was not rare in China’s clinical settings. The reason may be associated with adversity in the early years, such as famine (Wang and Zhang 2017).
There were several limitations. First, recall bias was inevitable in collecting information, such as the age of schizophrenia onset. Second, the representative problem existed because completing cognitive assessment required relatively high education levels, skills of using the computer, and stable condition of illness. Finally, this was a cross-sectional study and it was hard to clarify the mechanisms underlying cognitive differences between the TOS and LOS groups. In addition, future researchers can use other techniques, such as electroencephalogram (EEG) (Sharma and Acharya 2021), to detect the effect of the onset of age on cognition.
In conclusion, the age of schizophrenia onset was associated with specific cognitive impairment among individuals with first-episode, minimally treated schizophrenia. Individuals with LOS were less impaired in verbal learning than those with TOS.
Funding
This work was supported by the National Natural Science Foundation of China [YLT, 81761128021, 81771452, 82171507]; Beijing Natural Science Foundation [YLT, 7151005]; the National Institutes of Health [LEH, R01MH112180].
Data availability
Please contact YL Tan for reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
Dr. Hong has received or is planning to receive research funding or consulting fees from Mitsubishi, Your Energy Systems LLC, Neuralstem, Taisho, Heptares, Pfizer, Sound Pharma, Luye Pharma, Takeda, and Regeneron. None was involved in the design, analysis, or outcomes of the study. Other authors declare that the research was conducted without any relationships that could be interpreted as a potential conflict of interest or financial conflict.
Consent to participate
Written informed consent was provided by adult participants or legal guardians of juveniles.
Consent for publication
All participants provided consent for publication.
Ethics approval
The Institutional Review Board (IRB) of Beijing Huilongguan Hospital, central IRB, approved this research and other research sites accept this approval.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yi Yin and Shuangshuang Li have equal contributions as first authors.
References
- Bigdeli TB, Nuechterlein KH, Sugar CA, Subotnik KL, Kubarych T, Neale MC, Asarnow RF. Evidence of shared familial factors influencing neurocognitive endophenotypes in adult- and childhood-onset schizophrenia. Psychol Med. 2020;50(10):1672–1679. doi: 10.1017/S0033291719001715. [DOI] [PubMed] [Google Scholar]
- Brichant-Petitjean C, Legauffre C, Ramoz N, Ades J, Gorwood P, Dubertret C. Memory deficits in late-onset schizophrenia. Schizophr Res. 2013;151(1–3):85–90. doi: 10.1016/j.schres.2013.08.021. [DOI] [PubMed] [Google Scholar]
- De la Serna E, Puig O, Mezquida G, Moreno-Izco L, Merchan-Naranjo J, Amoretti S, Castro-Fornieles J. Relationship between cognition and age at onset of first-episode psychosis: comparative study between adolescents, young adults, and adults. Eur Child Adolesc Psychiatry. 2021 doi: 10.1007/s00787-021-01901-8r. [DOI] [PubMed] [Google Scholar]
- Fagerlund B, Pantelis C, Jepsen JRM, Raghava JM, Rostrup E, Thomas MB, Glenthøj BY. Differential effects of age at illness onset on verbal memory functions in antipsychotic-naïve schizophrenia patients aged 12–43 years. Psychol Med. 2021;51(9):1570–1580. doi: 10.1017/s0033291720000409. [DOI] [PubMed] [Google Scholar]
- Fett AJ, Velthorst E, Reichenberg A, Ruggero CJ, Callahan JL, Fochtmann LJ, Kotov R. Long-term changes in cognitive functioning in individuals with psychotic disorders: findings from the suffolk county mental health project. JAMA Psychiat. 2019;77(4):387–396. doi: 10.1001/jamapsychiatry.2019.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett A-KJ, Reichenberg A, Velthorst E. Lifespan evolution of neurocognitive impairment in schizophrenia - a narrative review. Schizophr Res Cogn. 2022 doi: 10.1016/j.scog.2022.100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Czajkowski N, Rund BR, Torgalsboen AK. The relationship between level of cognitive impairments and functional outcome trajectories in first-episode schizophrenia. Schizophr Res. 2017;190:144–149. doi: 10.1016/j.schres.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Vyas NS, Testa R, Wood SJ, Pantelis C. Age of onset of schizophrenia: perspectives from structural neuroimaging studies. Schizophr Bull. 2011;37(3):504–513. doi: 10.1093/schbul/sbr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Harris JG, Nuechterlein KH. The matrics consensus cognitive battery: what we know 6 years later. Am J Psychiatry. 2014;171(11):1151–1154. doi: 10.1176/appi.ajp.2014.14070936. [DOI] [PubMed] [Google Scholar]
- Grover S, Sahoo S, Nehra R. A comparative study of childhood/adolescent and adult onset schizophrenia: does the neurocognitive and psychosocial outcome differ? Asian J Psychiatry. 2019;43:160–169. doi: 10.1016/j.ajp.2019.05.031. [DOI] [PubMed] [Google Scholar]
- Holmén A, Juuhl-Langseth M, Thormodsen R, Ueland T, Agartz I, Sundet K, Melle I. Executive function in early- and adult onset schizophrenia. Schizophr Res. 2012;142(1–3):177–182. doi: 10.1016/j.schres.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Howard R, Rabins PV, Seeman MV, Jeste DV. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. The international late-onset schizophrenia group. Am J Psychiatry. 2000;157(2):172–178. doi: 10.1176/appi.ajp.157.2.172. [DOI] [PubMed] [Google Scholar]
- Immonen J, Jääskeläinen E, Korpela H, Miettunen J. Age at onset and the outcomes of schizophrenia: a systematic review and meta-analysis. Early Interv Psychiatry. 2017;11(6):453–460. doi: 10.1111/eip.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, Marder SR. The MATRICS consensus cognitive battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165(2):214–220. doi: 10.1176/appi.ajp.2007.07010043. [DOI] [PubMed] [Google Scholar]
- Kharawala S, Hastedt C, Podhorna J, Shukla H, Kappelhoff B, Harvey PD. The relationship between cognition and functioning in schizophrenia: a semi-systematic review. Schizophr Res Cogn. 2022;27:100217. doi: 10.1016/j.scog.2021.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Reichenberg A, Keefe RS, Fisher HL, Harrington H, Moffitt TE. Neuropsychological decline in schizophrenia from the premorbid to the postonset period: evidence from a population-representative longitudinal study. Am J Psychiatry. 2014;171(1):91–101. doi: 10.1176/appi.ajp.2013.12111438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Marder SR. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Ventura J, Subotnik KL, Bartzokis G. The early longitudinal course of cognitive deficits in schizophrenia. J Clin Psychiatry. 2014;75(Suppl 2):25–29. doi: 10.4088/JCP.13065.su1.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. Br J Psychiatry. 2009;195(4):286–293. doi: 10.1192/bjp.bp.108.060723. [DOI] [PubMed] [Google Scholar]
- Sánchez-Torres AM, Moreno-Izco L, Lorente-Omeñaca R, Cabrera B, Lobo A, González-Pinto AM, Cuesta MJ. Individual trajectories of cognitive performance in first episode psychosis: a 2-year follow-up study. Eur Arch Psychiatry Clin Neurosci. 2018;268(7):699–711. doi: 10.1007/s00406-017-0857-z. [DOI] [PubMed] [Google Scholar]
- Santesteban-Echarri O, Paino M, Rice S, González-Blanch C, McGorry P, Gleeson J, Alvarez-Jimenez M. Predictors of functional recovery in first-episode psychosis: a systematic review and meta-analysis of longitudinal studies. Clin Psychol Rev. 2017;58:59–75. doi: 10.1016/j.cpr.2017.09.007. [DOI] [PubMed] [Google Scholar]
- Sharma M, Acharya UR. Automated detection of schizophrenia using optimal wavelet-based l 1 norm features extracted from single-channel eeg. Cogn Neurodyn. 2021;15(4):661–674. doi: 10.1007/s11571-020-09655-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solmi M, Radua J, Olivola M, Croce E, Soardo L, Salazar de Pablo G, Fusar-Poli P. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry. 2021 doi: 10.1038/s41380-021-01161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen YN, Wong SMY, Hui CLM, Chan SKW, Lee EHM, Chang WC, Chen EYH. Late-onset psychosis and very-late-onset-schizophrenia-like-psychosis: an updated systematic review. Int Rev Psychiatry. 2019;31(5–6):523–542. doi: 10.1080/09540261.2019.1670624. [DOI] [PubMed] [Google Scholar]
- Tuulio-Henriksson A, Partonen T, Suvisaari J, Haukka J, Lönnqvist J. Age at onset and cognitive functioning in schizophrenia. Br J Psychiatry. 2004;185:215–219. doi: 10.1192/bjp.185.3.215. [DOI] [PubMed] [Google Scholar]
- Vahia IV, Palmer BW, Depp C, Fellows I, Golshan S, Kraemer HC, Jeste DV. Is late-onset schizophrenia a subtype of schizophrenia? Acta Psychiatr Scand. 2010;122(5):414–426. doi: 10.1111/j.1600-0447.2010.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werf M, Köhler S, Verkaaik M, Verhey F, van Os J. Cognitive functioning and age at onset in non-affective psychotic disorder. Acta Psychiatr Scand. 2012;126(4):274–281. doi: 10.1111/j.1600-0447.2012.01873.x. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhang Y. Schizophrenia in mid-adulthood after prenatal exposure to the Chinese famine of 1959–1961. Schizophr Res. 2017;184:21–25. doi: 10.1016/j.schres.2016.11.030. [DOI] [PubMed] [Google Scholar]
- White T, Ho BC, Ward J, O'Leary D, Andreasen NC. Neuropsychological performance in first-episode adolescents with schizophrenia: a comparison with first-episode adults and adolescent control subjects. Biol Psychiatry. 2006;60(5):463–471. doi: 10.1016/j.biopsych.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Woolston AL, Hsiao PC, Kuo PH, Wang SH, Lien YJ, Liu CM, Chen WJ. Genetic loci associated with an earlier age at onset in multiplex schizophrenia. Sci Rep. 2017;7(1):6486. doi: 10.1038/s41598-017-06795-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Fan H, Cui J, Yang G, Song H, Tan S, Zou Y. Yi xing he yang xing zheng zhuang liang biao de xin 5 yin zi mu xing. Chin Ment Health J. 2015;29(04):264–266. [Google Scholar]
- Yu Q, McCall DM, Homayouni R, Tang L, Chen Z, Schoff D, Ofen N. Age-associated increase in mnemonic strategy use is linked to prefrontal cortex development. Neuroimage. 2018;181:162–169. doi: 10.1016/j.neuroimage.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Pantelis C, Cropley V, Fornito A, Cocchi L, McAdams H, Gogtay N. Delayed development of brain connectivity in adolescents with schizophrenia and their unaffected siblings. JAMA Psychiat. 2015;72(9):900–908. doi: 10.1001/jamapsychiatry.2015.0226. [DOI] [PubMed] [Google Scholar]
- Zanelli J, Mollon J, Sandin S, Morgan C, Dazzan P, Pilecka I, Reichenberg A. Cognitive change in schizophrenia and other psychoses in the decade following the first episode. Am J Psychiatry. 2019;176(10):811–819. doi: 10.1176/appi.ajp.2019.18091088. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wang Y, Hu Y, Zhu Y, Zhang T, Wang J, Li C. Meta-analysis of cognitive function in Chinese first-episode schizophrenia: MATRICS Consensus Cognitive Battery (MCCB) profile of impairment. Gen Psychiatry. 2019;32(3):e100043. doi: 10.1136/gpsych-2018-100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Cui J, Wang J, Chen N, Tan S, Zhang D, Xu Z. Jing shen fen lie zheng ren zhi gong neng cheng tao ce yan zhong wen ban lin chuang xin du ji xiao du de yan jiu[clinical reliability and validity of the Chinese version of measurement and treatment research to improve cognition in schizophrenia consensus cognitive battery] Chin J Psychiatry. 2009;42(1):29–33. doi: 10.3760/cma.j.issn.1006-7884.2009.01.009. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact YL Tan for reasonable request.
Not applicable.


