Abstract
Background
Spindle cell hemangioma previously known as spindle cell hemangioendothelioma is a benign vascular tumour with rare presentation in head and neck. Presentation in lip is even rarer with three cases reported previously.
Method
This report describes a case of spindle cell hemangioma presented as an asymptomatic growth on lower lip of a 32-year-old male. Clinicopathological characterization of this case along with previously reported 15 cases of spindle cell hemangioma of head and neck were conducted.
Result
Microscopic evaluation shows a well-circumscribed vascular neoplasm of spindled and epithelioid endothelial cells. Large ectatic thin-walled vascular spaces were seen engorged with RBCs. The neoplasm was CD31 positive. Slight predilection for female gender and young age were observed. Minimal possibility of recurrence was also observed.
Conclusion
Spindle cell hemangioma needs to be considered in the differential diagnosis of vascular tumours of head and neck to avoid misdiagnosis of aggressive vascular neoplasms.
Keywords: Spindle cell hemangioma, Hemangioendothelioma, Microscopic evaluation, Vascular tumours
Introduction
Hemangioendothelioma has encompassed multiple vascular proliferations including both benign and malignant neoplasms. With the inception of the terminology ‘hemangioendothelioma’, Mallory in 1908 [1] included all proliferations that arose from endothelial cells. Stout and Kauffman [2] described hemangioendothelioma as a small group of vascular lesions having low metastatic potential and high tendency for local persistence. Weiss and Enzinger [3] proposed that the term hemangioendothelioma should be restricted to those vascular neoplasms showing a borderline biological behaviour, intermediate between benign hemangiomas and highly malignant angiosarcomas.
Spindle cell hemangioendothelioma is one of the several variants of hemangioendothelioma. Weiss and Enzinger [3] described this entity in 1986 as a vascular lesion with intermediate malignancy. However, there exists controversy regarding the hamartomatous, reactive or neoplastic nature of this lesion. Fletcher et al. [4] proposed the reactive nature of the lesion associated with local abnormality of blood flow which may be congenital or acquired. Imayama et al. [5] used ultrasound analysis to suggest the benign nature of the lesion. Perkins and Weiss [6] through a large case series stressed upon the fact that spindle cell hemangioma is a vascular lesion having least metastatic potential but marginal recurrence potential. These evidences have relabelled the lesion as ‘spindle cell hemangioma’ (SCH) which is described as a benign neoplasm or a vascular malformation in which secondary episodes of thrombosis may develop. The usual location of SCH is acral regions. Reports of presentation in the head and neck area are scarce. SCH in lip has been reported in three occasions making this case report as the fourth in English literature. This report, along with presenting the rare case, would also thoroughly review 15 cases of SCH of head and neck regions which are reported till date.
Case Report
A 32-year-old male presented with an asymptomatic firm erythematous and lobulated growth on lower lip (Fig. 1). The patient did not have any other significant systemic diseases. With the clinical differential diagnosis of benign salivary gland lesions and benign mesenchymal tumour, it was excised. Size of the excisional specimen was 2.5 cm × 1.5 cm × 1 cm. Microscopic findings (Fig. 2) of the processed tissue revealed a well-circumscribed lesion. Neoplastic mass showed a lobular growth pattern. There were numerous ectatic thin-walled vessels lined by plump spindle-shaped endothelial cells. Large venous spaces were seen filled with erythrocytes. Clusters of epithelioid cells were also seen. Few atypical endothelial cells and mitotic figures were also observed. Immunohistochemical marker for vascular markers, CD31, was positive in endothelial cells lining the miniature vessels and cavernous spaces as well as spindle cells (Fig. 3). A diagnosis of spindle cell hemangioma was made. No evidence of recurrence was seen after 8 months of follow-up.
Fig. 1.
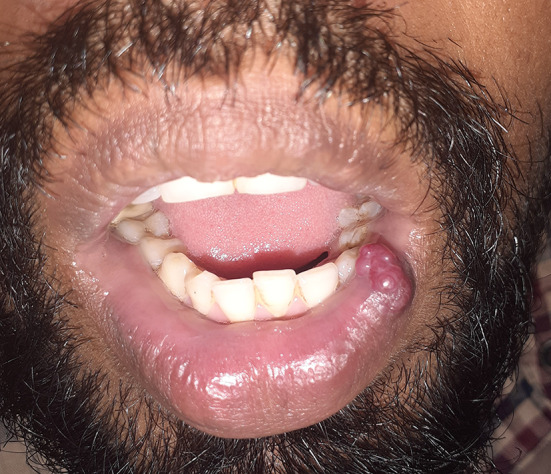
Asymptomatic lobulated growth on lower lip
Fig. 2.
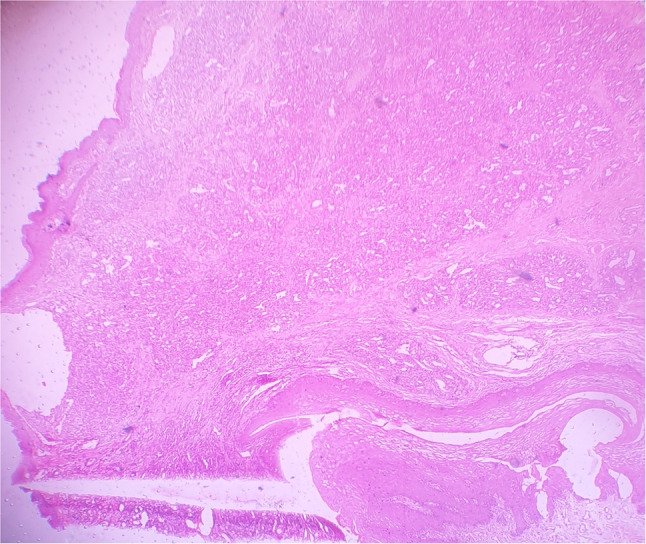
(Haematoxylin and eosin stain, 10X) well-circumscribed lesion showing a lobular growth pattern of numerous ectatic thin-walled vessels lined by plump spindle-shaped endothelial cells and clusters of epithelioid cells
Fig. 3.
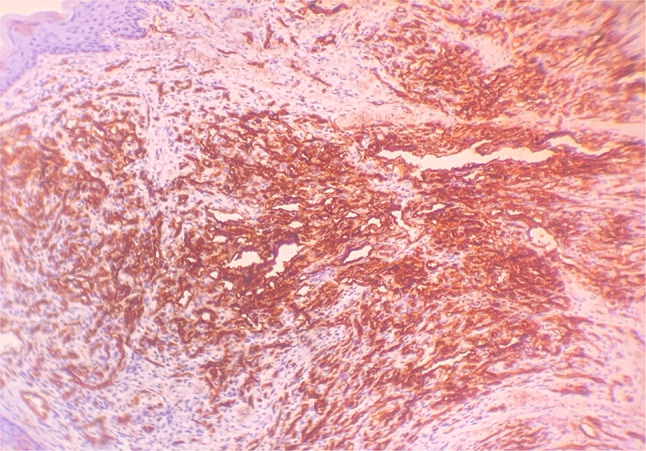
Immunohistochemical marker CD31 was positive in endothelial cells lining the miniature vessels and cavernous spaces as well as spindle cells
Discussion
The rare occurrence of SCH in head and neck region may be attributed to lack of familiarity with the histopathologic features of SCH and overlapping features with hemangiomas. The present case would be the 16th case among all head and neck SCH reported till date in English literature. Among SCH presented on lip, this case would be the fourth one. Considering the paucity of this lesion, we have presented the case in detail as well as critically reviewed the previously presented 15 cases (Table 1).
Table 1.
Cases of SCH head and neck reported till date
| Sl. no | Author/year | Site | Age/gender | Immunohistochemistry | Size of lesion (in cm) | Follow-up |
|---|---|---|---|---|---|---|
| 1. | Scott and Rosei 1988 [7] | Ear | 70/M | NR | NR | NR |
| 2. | Tosios 1995 [8] | Mandibular vestibule | 12/F |
HAM56 and vimentin positive in lining endothelial cells Factor Vlll positive in endothelial cells and round vacuolated cells Vimentin positive in epithelioid cells and majority of spindle cells PCNA positive in less than 3% of the tumour cells |
1 | LF |
| 3. | Baron 2002 [9] | Lateral nasal side wall | 17 months/M | NR | 1 × 1 | 24 FOR |
| 4. | Ide 2004 [10] | Palate | 55/M | CD31 positivity | 1.2 | NR |
| 5. | Lade 2005 [11] | Posterior pharyngeal wall | 25/M | NR | 6 | 48 FOR |
| 6. | Sheehan 2007 | Buccal mucosa | 44/M | CD31 and CD34 positive. | 1.2 | NR |
| 7. | Tosios 2008 [12] | Upper lip | 29/F |
CD34 and factor-VIII positive in endothelial cells and epithelioid cells Smooth-muscle actin positive in endothelial cells and spindle cells Few CD68 positive cells were seen in the solid areas |
1 | 36 FOR |
| 8. | Minagawa 2011 [13] | Temporal muscle | 67/F | NR | 4 | 24 |
| 9. | Cai et al. 2013 [14] | Lower Lip | 34/F | Vimentin and α-SMA positive and CD31, CD34 and D2-40S-100 protein, keratin (AE1/AE3) and CK19 were negative in spindle cell component | 2 | NR |
| 10. | Higashino 2014 [15] | Occipital region | 37/F | NR | 2.5 | NR |
| 11. | Gbolahan 2015 [16] | Orbit | 9/F | CD34 positive | 8 × 6 | 21 FOR |
| 12 | Sapariya 2015 [17] | Thyroid | 30/F |
Thyroid follicles with thyroglobulin positivity Endothelial cells with strong positivity for CD 34 and factor-VIII Spindle cells positive for vimentin and negative for thyroglobulin, CD 34 and factor-VIII |
4.3 × 2.8 | NR |
| 13. | Chhava 2015 [18] | Floor of mouth | 33/M | CD-31, CD-34 positive in spindle cells and endothelial cells | 1 × 1.5 | 6 FOR |
| 14. | French 2016 [19] | Tongue | 52/F | CD31 positive in spindle cells and endothelial cells | 2 | 24 FOR |
| 15. | Murakami 2018 [20] | Upper lip | 41/F | CD34 and SMA positive in most endothelial cells and occasional spindle cells | 3 | 24FOR |
| 16. | Present case 2020 | Lower lip | 32/M | CD 31 positive in endothelial cells and occasional spindle cells. | 2.5 | 8FOR |
NR not reported, FOR Free of recurrence, LF Lost to follow-up
A critical research on pathophysiology of SCH concluded that this may be regarded as a benign vascular neoplasm or malformations similar to angiomatosis in which alterations in blood flow possibly explain the consequential features [5, 21]. Site of abnormal vasculature [4] and injury [5] may predispose initiation of SCH. Areas of diminished blood flow result in vascular collapse with formation of cellular zones, and areas of vascular engorgement with stasis promote thrombosis and organization. Evidencing least metastatic potential, Perkins et al. [6] has suggested that this should be labelled as spindle cell hemangioma for solitary lesions and spindle cell hemangiomatosis for multifocal lesions.
Clinical differential diagnosis in all cases including this was benign salivary gland tumours and other mesenchymal tumours. Vascular lesions were not suspected in any of the cases. The reason may be submucosal location of the lesion [22]. Largest reported size among 15 cases was that of an SCH orbit, 8 cm, and nine out of sixteen cases of SCH occurred in female. However, three cases [12, 14, 23] reported in lip were in female with size ranging from 1 to 3 cm, while this case was in a male with a lesion size of 2.5 cm. Although SCH has a propensity for patients in the second decade or less than that, it may occur at any age [4, 6]. However, out of 15 reported cases eleven patients were in fourth decade and above.
Microscopic evaluation of spindle cell hemangioma describes a well-circumscribed lesion with nodular proliferations of spindled endothelial cells. Thin walled dilated veins supported by endothelial cells are the constant finding. Occasional phlebolith and/or thrombi are also seen which was not observed in our case. In addition to the spindle cells, another group of cells with round to ovoid morphology having vacuolated cytoplasm arranged in solid aggregates are also seen. The histological differential diagnosis centres on several vascular neoplasms. The presence of epithelioid cells raises the possibility of epitheloid hemangioendothelioma which is another vascular neoplasm of intermediate malignancy and a rare occurrence in head and neck [23, 24]. However, lack of infiltrative growth pattern and myxohyaline matrix differentiates spindle cell hemangioma from epithelioid hemangioendothelioma. Kaposi’s sarcoma and cavernous hemangioma are other possible diagnosis for such microscopic findings. Although spindled areas may be mistaken for Kaposi’s sarcoma, lack of features like infiltrative growth, nuclear atypia, higher numbers of mitotic figure and epitheloid endothelial cells excludes Kaposi’s sarcoma. Immunohistochemistry for human herpes virus may help diagnosing certain cases of Kaposi’s sarcoma. However, in the settings of acquired immunodeficiency syndrome and few non-immunocompromised patients, Kaposi’s sarcoma has to be considered as a differential diagnosis. Angiosarcoma can occasionally have spindled morphology. However, lack of infiltrative growth pattern, nuclear atypia and high numbers of mitotic figures again are the exclusion criteria. Lobular capillary hemangioma, a variant of pyogenic granuloma may also need careful exclusion from SCH. Presence of dense inflammatory cells and absence of cavernous spaces may qualify the lesion as pyogenic granuloma [25].
Most cases of cutaneous hemangiomas are sporadic in nature except few which are associated with Mafucci’s syndrome [26], Klippel–Trenaunay syndrome [4], lymphedema [4], early onset of varicose veins and superficial cutaneous lymphatic malformations [4]. Except Cai’s [14] case of SCH on lip, no syndromic association was observed. Recurrence rate is high in cutaneous spindle cell hemangiomas. In fact, Perkins et al. [6] reported 58% recurrence in a series of 78 cases which is attributed to incomplete excision or contiguous spread along or multifocal involvement of a vessel. Reviewing the cases demonstrated no recurrence in eight cases having followed up. Therefore, recurrence rate of head and neck SCH may be far less compared to cutaneous SCH. However, this evidence based upon handful of cases should not defend patients with head and neck SCH from being followed up.
In order to prevent the misinterpretation of a more aggressive vascular tumour, spindle cell hemangioma should be considered as a differential diagnosis among soft tissue neoplasm of head and neck. The clinical and histopathologic features described above may help clinician and pathologist, respectively, to diagnose SCH.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Swagatika Panda, Email: swagatikapanda@soa.ac.in.
Subrat Padhiary, Email: subratkumarpadhiary@soa.ac.in.
Sreepreeti Champatiray, Email: sreepreetichampatiray@soa.ac.in.
Neeta Mohanty, Email: dr.neetamohanty@gmail.com.
Sobhan Mishra, Email: sobhanmishra@soa.ac.in.
References
- 1.Mallory FB. The results of the application of special histological methods to the study of tumors. J Exp Med. 1908;10(5):575–593. doi: 10.1084/jem.10.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kauffman SL, Stout AP. Malignant hemangioendothelioma in infants and children. Cancer. 1961;14:1186–1196. doi: 10.1002/1097-0142(196111/12)14:6<1186::AID-CNCR2820140608>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Weiss S, Enzinger F. Spindle cell hemangioendothelioma. A low-grade angiosarcoma resembling a cavernous hemangioma and Kaposi’s sarcoma. Am J Surg Pathol. 1986;10(8):521–530. doi: 10.1097/00000478-198608000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher CDM, Beham A, Schmid C. Spindle cell haemangioendothelioma: a clinicopathological and immunohistochemical study indicative of a non-neoplastic lesion. Histopathology. 1991;18(4):291–301. doi: 10.1111/j.1365-2559.1991.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 5.Imayama S, Murakamai Y, Hashimoto H, Hori Y. Spindle cell hemangioendothelioma exhibits the ultrastructural features of reactive vascular proliferation rather than of angiosarcoma. Am J Clin Pathol. 1992;97(2):279–287. doi: 10.1093/ajcp/97.2.279. [DOI] [PubMed] [Google Scholar]
- 6.Perkins P, Weiss SW. Spindle cell hemangioendothelioma: an analysis of 78 cases with reassessment of its pathogenesis and biologic behavior. Am J Surg Pathol. 1996;20(10):1196–1204. doi: 10.1097/00000478-199610000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Scott GA, Rosai J. Spindle cell hemangioendothelioma. Report of seven additional cases of a recently described vascular neoplasm. Am J Dermatopathol. 1988;10(4):281–288. doi: 10.1097/00000372-198808000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Tosios K, Koutlas IG, Kapranos N, Papanicolaou SI. Spindle-cell hemangioendothelioma of the oral cavity. A case report. J Oral Pathol Med Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol. 1995;24(8):379–382. doi: 10.1111/j.1600-0714.1995.tb01203.x. [DOI] [PubMed] [Google Scholar]
- 9.Baron JA, Raines J, Bangert J, Hansen RC. Persistent nodule on the nose. Arch Dermatol. 2002;138(2):259–264. doi: 10.1001/archderm.138.2.259-b. [DOI] [PubMed] [Google Scholar]
- 10.Ide F, Obara K, Enatsu K, Mishima K, Saito I. Rare vascular proliferations of the oral mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97(1):75–78. doi: 10.1016/j.tripleo.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Lade H, Gupta N, Singh PP, Dev G. Spindle-cell hemangioendothelioma of the posterior pharyngeal wall. Ear Nose Throat J. 2005;84(6):362–365. doi: 10.1177/014556130508400616. [DOI] [PubMed] [Google Scholar]
- 12.Tosios KI, Gouveris I, Sklavounou A, Koutlas IG. Spindle cell hemangioma (hemangioendothelioma) of the head and neck: case report of an unusual (or underdiagnosed) tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(2):216–221. doi: 10.1016/j.tripleo.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Minagawa T, Yamao T, Shioya R. Spindle cell hemangioendothelioma of the temporal muscle resected with zygomatic osteotomy: a case report of an unusual intramuscular lesion mimicking sarcoma. Case Rep Surg. 2011;2011:481654. doi: 10.1155/2011/481654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai Y, Wang R, Chen X-M, Zhao Y-F, Sun Z-J, Zhao J-H. Maffucci syndrome with the spindle cell hemangiomas in the mucosa of the lower lip: a rare case report and literature review. J Cutan Pathol. 2013;40(7):661–666. doi: 10.1111/cup.12131. [DOI] [PubMed] [Google Scholar]
- 15.Higashino T, Hirai R. A spindle cell hemangioendothelioma on the head resembling an arteriovenous malformation. J Craniofac Surg. 2014;25(4):e312–e313. doi: 10.1097/SCS.0000000000000574. [DOI] [PubMed] [Google Scholar]
- 16.Gbolahan OO, Fasina O, Adisa AO, Fasola OA. Spindle cell hemangioma: unusual presentation of an uncommon tumor. J Oral Maxillofac Pathol JOMFP. 2015;19(3):406. doi: 10.4103/0973-029X.174634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sapariya BJ, Udhreja PR. Spindle cell hemangioma of thyroid. Indian J Cancer. 2015;52(3):349. doi: 10.4103/0019-509X.176716. [DOI] [PubMed] [Google Scholar]
- 18.Chavva S, Priya MH, Garlapati K, Reddy GSP, Gannepalli A. Rare case of spindle cell haemangioma. J Clin Diagn Res JCDR. 2015;9(6):ZD19–ZD21. doi: 10.7860/JCDR/2015/11998.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.French KEM, Felstead AM, Haacke N, Theaker J, Brennan PA, Colbert SD. Spindle cell haemangioma of the tongue. J Cutan Pathol. 2016;43(11):1025–1027. doi: 10.1111/cup.12769. [DOI] [PubMed] [Google Scholar]
- 20.Murakami K, Yamamoto K, Sugiura T, Kirita T. Spindle cell hemangioma in the mucosa of the upper lip: a case report and review of the literature. Case Rep Dent. 2018;2018:1370701. doi: 10.1155/2018/1370701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding J, Hashimoto H, Imayama S, Tsuneyoshi M, Enjoji M. Spindle cell haemangioendothelioma: probably a benign vascular lesion not a low-grade angiosarcoma. A clinicopathological, ultrastructural and immunohistochemical study. Virchows Arch A Pathol Anat Histopathol. 1992;420(1):77–85. doi: 10.1007/BF01605988. [DOI] [PubMed] [Google Scholar]
- 22.Mattassi R, Loose DA, Vaghi M, editors. Hemangiomas and vascular malformations: an atlas of diagnosis and treatment. 2. Mailand: Springer; 2015. [Google Scholar]
- 23.Chi AC, Weathers DR, Folpe AL, Dunlap DT, Rasenberger K, Neville BW. Epithelioid hemangioendothelioma of the oral cavity: report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(6):717–724. doi: 10.1016/j.tripleo.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 24.Orsini G, Fioroni M, Rubini C, Piattelli A. Epithelioid hemangioendothelioma of the oral cavity: report of case. J Oral Maxillofac Surg. 2001;59(3):334–337. doi: 10.1053/joms.2001.21007. [DOI] [PubMed] [Google Scholar]
- 25.Kapadia SB, Heffner DK. Pitfalls in the histopathologic diagnosis of pyogenic granuloma. Eur Arch Otorhinolaryngol. 1992;249(4):195–200. doi: 10.1007/BF00178468. [DOI] [PubMed] [Google Scholar]
- 26.Lawson JP, Scott G. Case report 602: spindle cell hemangioendothelioma (SCH) and enchondromatosis (a form of Maffucci syndrome) in a patient with acute myelocytic leukemia (AML) Skeletal Radiol. 1990;19(2):158–162. doi: 10.1007/BF00197630. [DOI] [PubMed] [Google Scholar]


