Abstract
In recent years, non-pharmacology treatments and their effectiveness have gained popularity due to their beneficial properties in the prevention of cardiovascular diseases. Phenolic compounds intake provides a natural means of improving in vivo antioxidant status. Thus, the purpose of this review is to discuss the potential benefits of hydroxytyrosol (HT), a phenolic compound with powerful antioxidant and anti-inflammatory properties, in preventing and reducing cardiovascular risk factors, concretely atherosclerosis. Closer inspection of the studies showed a significant improvement of lipid profile, antioxidant capacity and inflammatory state. A note of caution is due in vitro studies because the lack of validated approaches difficult the goodness of fit with the in vivo and clinical research. However, animal and clinical studies were very encouraging, determining HT supplementation useful on inflammation, oxidative stress, endothelial function and cardiovascular diseases in general.
Keywords: Hydroxytyrosol, Inflammation, Atherosclerosis, Lipid profile
Abbreviations
- CVDs
Cardiovascular diseases
- EVOO
Extra-virgin olive oil
- HT
Hydroxytyrosol
- LDL
Low density lipoproteins
- MPA
Phorbol myristate acetate
- NO
Nitric oxide.
- ROO
Refined olive oil
- ROS
Oxygen-reactive species
1. Introduction
Non-communicable diseases are a continuing concern due to their prevalence. Traditionally, cardiovascular diseases (CVDs) were the main cause of death worldwide by 17.7 million of deaths in 2015 [1]. Concretely, atherosclerosis is an important component in the CVDs and plays a key role in coronary artery pathologies, peripheral artery and cerebrovascular diseases [2]. The most important clinical manifestations of this chronic arterial disease include: ischemic heart disease (IHD), ischemic stroke (IS) and peripheral arterial disease (PAD) [3]. The initial inflammatory cascade of this alteration activates the intracellular signalling pathway of the nuclear factor (NF)-κB which finally regulates inflammatory cytokines (interleukins IL-1, IL-6, IL-8 and TNF-α) and chemokines (monocyte chemotactic protein [MCP-1]) release [2,4], adhesion molecules (platelet [P]-selectin and endothelium [E]-selectin, intercellular adhesion molecule-1 [ICAM-1] and vascular adhesion molecule-1 [VCAM-1]), cyclooxygenase (COX)-2 enzyme and the mitogen-activated protein kinases (MAPK) [4,5].
Extensive research has shown that CVDs are related to several risks factors such as: unhealthy diet, physical inactivity, smoke and alcohol. Data from several studies suggest that combating these inappropriate habits reduces the risk of developing cardiovascular events [1]. Currently, recent developments in the field of CVDs have led to a renewed interest by the prevention with non-pharmacological treatments. Thus, nutritional factors to be influencing the CV system have been explored in several studies to improve CVDs and reduce pharmacological consumption of these patients.
Moreover, there are several important areas where a decrease in medication consumption has been proposed to avoid adverse effects, poor response and emerging resistance among patients. This indicates a need to encourage novel non-pharmacological treatments as a part of a healthy lifestyle. In addition, the response of food industry was strongly engaged by developing functional foods, specifically phenolic compounds that could have an impact over the CV system. These compounds have been attracting a lot of interest because of their potential anti-inflammatory effects, antioxidant activity, effects on signalling cascades and transcription network, immune cells adhesion or the improvement of endothelial dysfunction [6].
In this line, hydroxytyrosol (HT), a phenolic extra-virgin olive oil (EVOO) compound, has been recognised to display different anti-inflammatory, anti-proliferative and anti-microbial [7]. Data from a variety of studies suggest that it targets different cancer types such as breast, prostate, colon or thyroid as well [8]. In addition, recent work has established a neuro-protective role in Parkinson's disease and a critical role in the prevention of atherosclerosis and diabetes [8]. Up to now, far too little attention has been paid to the mechanistic pathways where HT could be involved. As an example, HT could have a role in atherosclerosis’ homeostasis. The initial inflammatory cascade of this disease activates the intracellular signalling pathway of the nuclear factor (NF)-kB which finally regulates inflammatory cytokines (interleukins IL-1, IL-6, IL-8 and TNF-α) and chemokines (monocyte chemotactic protein [MCP-1]) production [2,4], adhesion molecules (platelet [P]-selectin and endothelium [E]-selectin, intercellular adhesion molecule-1 [ICAM-1] and vascular adhesion molecule-1 [VCAM-1]), cyclooxygenase (COX)-2 enzyme and the mitogen-activated protein kinases (MAPK) [4,5]. Indeed, HT induced decrease NF-κB activation and, therefore, would downregulate mRNA levels of cytokines, chemokines, adhesion molecules as well as intracellular signalling pathways [9].
Taking the potential benefits of HT in preventing and reducing the CV risk factors into account, insights into the effects of HT on several processes contributing to CVDs such as inflammation, endothelial dysfunction, immune cells adhesion, among others are here depicted. This review has been divided into three groups characterised by the type of study: in vitro, animal studies and clinical trials. The overall structure provides a clearer understanding of the effects of HT in CVD at various layers of complexity. Understanding the link between HT and the CV system will offer an important issue for future work mainly by deciphering the gaps in knowledge and directions for future research.
2. Materials and methods
2.1. Search strategy
This review was exploratory and interpretative in nature. We designed a comprehensive search strategy from December 2021 to November 2022 that was conducted using electronic databases such as PubMed, SCOPUS and Web of Science regarding HT and CVDs. The search strategy included articles published in the last ten-years to synthesize the most up-to-date evidence relating HT and CVDs. Moreover, there was a restriction of language (English and Spanish). Search strategy was developed by a combination of Boolean operators “OR” and “AND”. The following keywords were used: a first quest of “Hydroxytyrosol” AND “cardiovascular diseases”, followed by a second search of “Hydroxytyrosol” AND “cardiovascular risk”, a third search of “Hydroxytyrosol” AND “atherosclerosis” and a final pursuit of “Hydroxytyrosol” and “heart disease risk factor”.
3. Hydroxytyrosol at the heart of olive oil
As the International Union of Pure and Applied Chemistry (IUPAC) system contemplates, the compound commonly known as HT is also called 3,4-dihydroxyphenylethanol (DOPET), 3,4-dihydroxyphenolethanol (3,4-DHPEA) o 4-(2-Hydroxyethyl)-1,2-benzenediol [10]. The chemical HT form is C8H10O3 and it mainly takes part in EVOO composition acting as a phenol component [10,11]. HT is obtained from olives treatment (by oleuropein hydrolysis) with the purpose of obtaining olive oil, finally producing three sort of phenol-enriched layers (olive mill wastewater, pomace and olive oil) that contain this product. Moreover, fewer levels of HT appear in other substances such as olive trees leaves and wines (red and white) [10].
The pharmacokinetic HT process is initiated by its absorption (depends on age, hormonal status, and gender) [11]. This occurs principally at the small bowel and the colon at percentages which are around 70–100% of absorption. Secondly, it reaches its highest plasma concentration among 8–13 min after oral administration, disappearing from plasma only after 1 h in humans [10,11]. Then, HT suffers a rapid first step metabolism by enterocytes and the liver. In terms of safety profile, no untoward effects have been shown even at very high doses [12]. In fact, microbiota has shown to play an important role by absorbing this compound and its metabolites [10,11]. Finally, 6 h after its ingestion, HT and its metabolites are mainly excreted by the kidneys [10,11].
Different benefits of HT have been decribed in vitro, pre- and clinical human studies. Antimicrobial studies have showed the importance of HT against Escherichia coli, Candida albicans and Clostridium perfringens, among others [8,10]. Increasing attention has been arising towards its antioxidant properties which might have neuroprotective (improving dissociated brain cells’ resistance to oxidative stress), skin (decreasing oxidative stress produced by ultraviolet radiation) and chemo-preventive (blocking cyclin-dependent kinases and messengers involved in cell proliferation) effects [8,10]. A substantial body of clinical and experimental evidence indicates that HT could be useful in several diseases, such as nosocomial diseases, metabolic syndrome, respiratory pathologies, Parkinson and Huntington diseases and multiple types of cancer [8,10].
4. Hydroxytyrosol in vitro studies
Whether it has not been formally demonstrated, current observations support that the key for preventing the atherosclerotic process rests on avoiding some modifiable pro-inflammatory and dyslipidaemia causes (Fig. 1). Human cell culture evidence has suggested the role of HT in the inflammatory process (Table 1). Scoditti et al. evaluated the anti-inflammatory potential of HT in human monocytes by measuring its effects on MMP-9 expression and its relationship with the COX-2/PGE2 pathway [9]. The activation of these cells blended with HT was produced by phorbol myristate acetate (MPA), known as an exogenous protein kinase C (PKC) activator. This activation favoured the expression of the inflammatory mediators MMP-9 and COX-2. At this point, it was found that HT inhibited PMA-induced MMP-9 protein release in a concentration-dependent manner (p < 0.05). Interestingly a reduction of the PMA-induced prostaglandin E2 (PGE2) production (concretely COX-2 reduction) was also found (p < 0.05). Due to the relation of NF-κB with COX-2 and MMP-9 expression, it was confirmed that HT treatment could modify this transcriptional factor (p < 0.05). In addition, HT regulates MCP-1, ICAM-1, IL-β and TNF-α through NF-κB dependent pathway to relieve inflammation (p < 0.05) [9]. Thus, HT showed an important role in the reduction of the main inflammatory effectors.
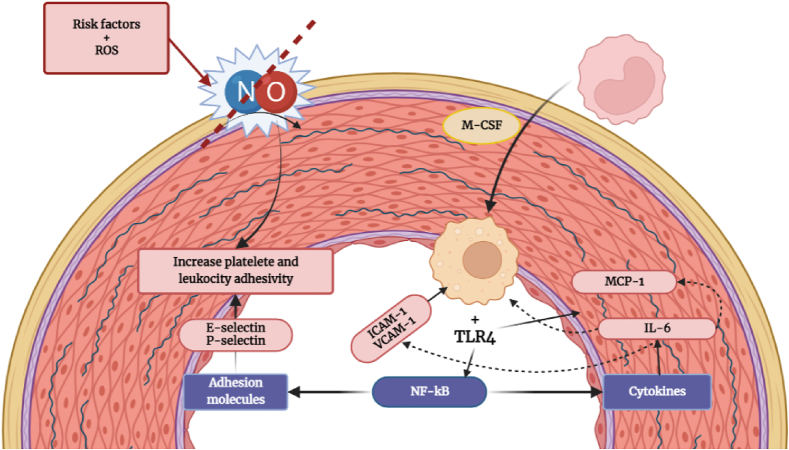
Fig. 1.
The decrease of nitric oxide (NO) synthesis increases leukocytes and platelets adhesivity. Macrophages uptake oxidized LDL (oxLDL), finally triggering inflammation. OxLDL binds to TLR4 which promote the release of interleukine (IL-6) and monocyte chemotactic protein (MCP-1) among others. As a cycle MCP-1 let monocytes to migrate and infiltrate into subendothelial space differentiating into macrophages by the macrophage colony-stimulating factor (M-CSF). TLR activate the necrosis factor kappa B (NF-kB) pathway leading to udregulation of adhesion molecules (Selectin P and E: adhesion of leukocytes; adhesion molecule-1 [ICAM-1] and vascular adhesion molecule-1 [VCAM-1]: macrophage proliferation) and cytokines (IL-6: express VCAM-1 and ICAM-1, inducting MCP-1 and up taking low-density lipoproteins [LDL]).
Table 1.
In vitro studies that investigated the effects of HT at molecular levels.
| Study (year) | Sample | Treatment Groups | Methodology | Outcomes |
|---|---|---|---|---|
| [13] | Caco-2 cells (A) HAEC (B) |
A: HT 10, 15, 20, 25, 50 and 100 μmol/L B: 1, 2, 5 and 10 μM |
A: 21-day cultured cells with 24 h of HT incubation at different concentrations. B: HT or its metabolites and TNF- α or TNF-α alone among 24 h. |
HT reduced E-selectin, P-selectin, VCAM-1 and ICAM-1 at all doses compared with TNF-α alone. |
| [14] | HUVEC | HT and its metabolites 100 μM | HT or its metabolites exposure for 16 h. Then, cells were treated with TNF-α for 24 h. |
HT and sulfate metabolites suppressed the intracellular production of ROS. HT and all the metabolites can downregulated ICAM-1, VCAM-1 and E-selectin genes. |
| [15] | HUVEC HMEC-1 |
HT 0 μmol/L HT 1 μmol/L HT 10 μmol/L HT 30 μmol/L |
Increasing concentrations for 1 h. PMA stimulation. |
HT reduced the inflammatory markers: TNF-α, IL-1β, VCAM-1, ICAM-1. Lipid peroxide production was reduced too. SOD activity was rescued by HT treatment and ATP activity was increased. |
| [16] | HUVEC Raw264.7 cells |
HT 0–80 μM | HT were exposure alone at different concentrations and with acrolein. Cells were exposure to acrolein alone too. |
Suppression of phosphor–NF–kB, IL-1β, TNF- α and IL-6 while the expression of ABCA1 increased. |
| [17] | SGBS adypocytes | HT 1–10 μmol/L | Cells were exposure to HT 1 h before TNF-α stimulation. | Just 1 μmol/L of HT prevents the upregultated mRNA levels of MCP-1, CXCL-10, IL-1β, VEGF, COX-2 and MMP-2. 10 μmol/L prevented IL-6, ICAM-1 and MMP-9 mRNA upregulation too. |
| [19] | Caco-2 cells | HT 1 μM Tyr 1 μM Tyr Sulf 1 μM HT Sulf 1 μM HT Glu 1 μM Tyr Glu 1 μM |
LPS 1 μg/ml treatment + 72 h of incubation. Phenolic compounds’ pretreatment prior to LPS co-exposure for 48 h. |
Phenolic compounds’ pretreatment limits NO release and inhibited iNOS expression. |
| [21] | HUVEC | HT 0–160 μM | Increasing concentrations of HT for 24 and 48 h. | At low HT concentrations, wound healing and cell migration were improved. Tube formation was promoted too. |
| [22] | HUVEC and HREC | 10 ng/ml IL-1β 10 μM HT-30s + IL-1β |
Cells were treated with IL-1β w/o HT-30s every 24 h for 7 days. | HT treatment upregulated CD31 and FGFR1 gene expression while it downregulates α-SMA, Vimentin and TNF-β expression. It prevents SMAD 2/3 translocation too. |
| [18] | HUVEC | HT 10 μmol/L | Cells were exposure to HT 1 h before IL-1β stimulation among 3 h. | HT dowregulated VCAM-1, IL-1A and IL-1B target genes. |
| [23] | HAEC | 1–10 μM of HT-3G, HT-3S TYR-G, TYR-S and MeOH 100 μM of Apocynin and L-NNA |
Increasing concentrations of the compounds + 24 h incubation. Exposed to apocynin, L-NAA, the tested compounds or MeOH for 24 h. |
Phenolic compounds’ treatment decreased superoxide levels while it highly stimulate Akt1 activation. |
Abbreviations: ATP: Adenosine triphosphate; CD31: Cluster of differentiation 31-protein; FGFR1: Fibroblast growth factor receptor-1; Glu: Glucuronide; HAEC: Human Aortic Endothelial Cells; HMEC-1: Human Microvascular Endothelial Cell line; HREC: Human Retinal Endothelial Cells; HT: Hydroxytyrosol; HT-3Os: Hydroxytyrosol-3O sulfate; HUVEC: Human Umbilical Vein Endothelial Cells; ICAM-1: Intercellular adhesion molecule-1; IL-1β: Interleukin-1 β; iNOS: Inducible nitric oxide synthase; L-NNA: L-Nω-nitro-arginine; MeOH: Methanol; PMA: Phorbol myristate acetate; ROS: Reactive Oxygen Species; NO: Nitric oxide; SGBS: Simpson-Golabi-Behmel Syndrome; α-SMA: Smooth muscle actin; SOD: Superoxide dismutase; Sulf: Sulfate; TNF-α: Tumor Necrosis Factor- α; Tyr: Tyrosol; VCAM-1: Vascular cell adhesion molecule-1; VEGF: Vascular endothelial growth factor.
Later, Catalán et al. aimed to elucidate the protective role of HT metabolites against endothelial dysfunction [13]. The human colon cancer cell line, Caco-2, was used to developed HT metabolites while the human aortic endothelial cell line was treated with HT and metabolites and TNF-α. On average, HT was showed to have beneficial properties by reducing E-selectin, P-selectin, VCAM-1 and ICAM-1 (p < 0.05 for all) [13]. The most striking result to emerge from this study is that HT metabolites also display a protective and quantifiable role. Another study evaluated the antioxidant and anti-inflammatory properties of HT and its metabolites over TNF-α stimulated endothelial cells [14]. When human endothelial cells were treated with HT and sulfate metabolites, significant suppression of the production of ROS (p < 0.05) and downregulation of ICAM-1, VCAM-1 and E-selectin genes were detected [14]. With successive stimulation of endothelial cells with PMA, a protective role of HT was found due to a significant reduction in TNF-α, IL-1β, VCAM-1 and ICAM-1 levels (p < 0.05). Moreover, superoxide dismutase (SOD) activity was also measured in order to elucidate a possible improvement from HT into the oxidative status. In fact, HT pre-treatment rescued SOD activity and reduced lipid peroxide production (p < 0.05). Importantly, there was a significant positive effect on the mitochondria due to an increase of the mitochondrial ATP synthase activity when compared with control conditions (p < 0.05) [15]. These results suggest that HT impacts on inflammatory markers but at the mitochondrial level as well (Fig. 2).
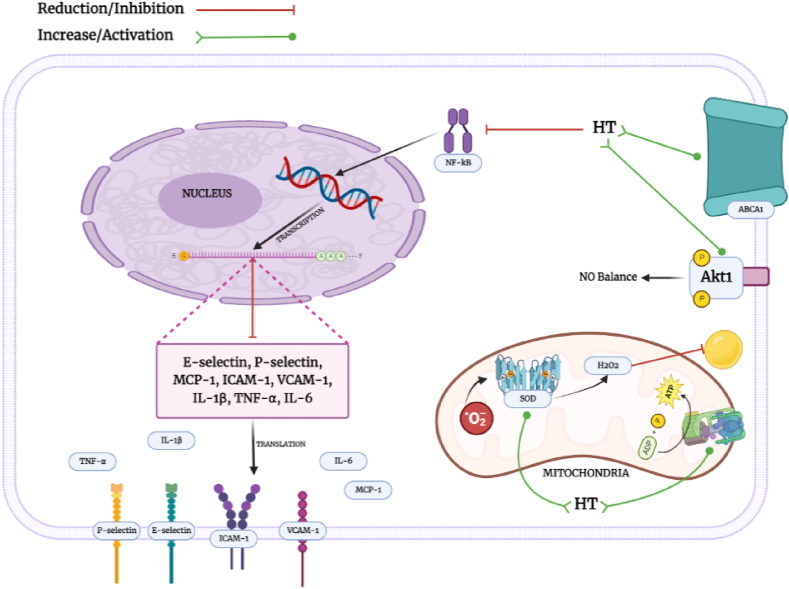
Fig. 2.
Biological effects of HT on inflammation, oxidative stress and cholesterol pathways. (1) Hydroxytyrosol reduces NF-kB activation causing a depletion at mRNA levels of pro-inflammatory genes such as MCP-1, ICAM-1, VCAM-1, IL-1β, IL-6, TNF-α, E-selectin and P-selectin. (2) In addition, HT pre-treatment rescues SOD activity and reduces lipid peroxide production. Importantly, a positive effect on the mitochondria has been manifested by an increase of the mitochondrial ATP synthase activity. (3) Moreover, the expression of ABCA1, a transporter enrolled in cholesterol homeostasis (HDL-chol synthesis), is increased after HT treatment. (4) Finally, Akt1 activation by HT improved the NO levels in endothelial cells.
Furthermore, Wu et al. also attempted to investigate the role of HT in the pro-atherogenic process [16]. Human cells treated with acrolein and HT showed a significant suppression of inflammation markers such as phospho–NF–κB, IL-1β, TNF-α and IL-6 whist the expression of ABCA1 was increased after HT treatment [16]. Thus, strong evidence of the beneficial effects of HT seems to be related to gene expression changes [17]. Indeed, human Simpson-Golabi-Behmel Syndrome (SGBS) adipocytes were treated with HT and TNF-α for transcriptomic analysis. There was a significant difference in HT treatment, where mRNA levels of MCP-1, CXCL-10, IL-1β, vascular endothelial growth factor (VEGF), COX-2 and MMP-2 were found lower (p < 0.05) [17]. Likewise, genomic studies have been also performed after HT treatment [18], endothelial adhesion molecules (VCAM-1) and cytokines (IL-1A and IL-1B) target genes were downregulated after HT treatment in this recent research [18]. Taken together, the results suggest that HT affect gene expression rather than inhibition of receptors.
Elegant experiments using Caco-2 cells treated by a set of phenolic compounds (HT; tyrosol (Tyr); Tyr sulfate (Sulf); HT Sulf; HT-3-glucuronide (Glu); Tyr Glu) and LPS to trigger an inflammatory status demonstrated a significant reduction of NO release after HT in its basic and Sulf form [19]. Concretely, these compounds were able to inhibit the inducible NOS (iNOS) expression, which is also NF-κB-dependent [20]. Thus, it is plausible that HT may act as a potential regulator of NO release which could herald an important avenue of investigation.
Pioneering studies have documented a prominent role of HT in angiogenesis and repair. Although the knowledge of triggering factors is limited, HT has been suggested potential therapeutic compound in the prevention of tissue death [21]. Indeed, HT treatment improved wound healing (p < 0.05) and cell migration (p < 0.01) of endothelial cells [21]. In addition, HT promoted endothelial tube formation, performing an important function in the angiogenesis process. Thus, HT induces a phenotypic switch to a proangiogenic endothelium and this therapeutic alternative should be further investigated.
It has been controversial whether HT by itself or distinct metabolites contribute to the atherosclerotic process. In fact, increasing attention has been arising towards the effectiveness of the distinct metabolites of HT. In this line, Terzuoli et al. investigated the effects of the major plasma metabolite HT-3O sulfate (HT-3Os) in human endothelial vein and retinal cells [22]. It was remarkable that HT-3Os exerted a protective effect by increasing CD31 (p < 0.01) and FGFR1 (p < 0.001) genes (endothelial markers); whereas a depletion of α-SMA (p < 0.001) and Vimentin (p < 0.05) was also found. Interestingly, these last two molecules are mesenchymal markers involved in inflammatory stages. Moreover, the inflammatory status caused by IL-1β was restored by the application of HT-3Os due to the prevention of the nuclear translocation of SMAD 2/3 (p < 0.05) which finally could not trigger the TGF-β activation (p < 0.001). This is a direct demonstration of the importance of focusing on studies with other HT metabolites.
It has also shown that compounds such as Tyr, Tyr Sulf, HT-3 Glu (p < 0.001), HT-3 Sulf, Tyr Glu (p < 0.01), HT and homovanillyl alcohol (HVA; p < 0.05) showed a good efficiency by increasing NO levels [23]. In addition, the exposure of endothelial cells to these compounds reduced superoxide levels with a key role of Tyr (which transforms into HT) (p < 0.001). One of the most significant findings to emerge from this study is that Akt1 activation, widely known as a potential player in the maintenance of NO levels in the endothelial cell, was determined after phenolic compounds’ treatment displaying greater results with Tyr (p < 0.01), HT-3 Sulf (p < 0.05) and HT-3 Glu (p < 0.05) [23]. Thus, the relevance of HT precursors (Tyr) is clearly supported by the current findings.
5. Hydroxytyrosol in animal studies
The in vitro research serves as a base for further in vivo studies. The protective effects of HT, its metabolites and their mechanisms of action already discussed in vitro studies were confirmed in several animal studies (Table 2). In fact, Tabernero et al. focused on clinical markers of the CV status in rats fed with hydroxytyrosol acetate (HT-AC) diet compared to a cholesterol rich diet group [24]. Body and tissue weights, lipid profile, redox status, and biochemical, hormonal, and inflammatory biomarkers were evaluated. Plasma levels of total cholesterol, LDL cholesterol, glucose, insulin and leptin, as well as malondialdehyde in serum increased in cholesterol rich diet group when compared with (HT-AC) diet (p < 0.05, for all of them), thus confirming the metabolic effects of HT, even improved by hydrophobic derivatives, particularly HT-AC.
Table 2.
In vivo studies that investigated the effects of HT in animal models.
| STUDY (year) | Sample (size) | Treatment Group | Methodology | Outcomes |
|---|---|---|---|---|
| [24] | Male Wistar Rats (n = 40) | Control group Cholesterol group HT group HT-Ac group HT-Et group |
During 8 weeks: supplemented diet (2% cholesterol and 0.4% cholic acid) and 0.04% HT, HT-AC and HT-Et if corresponding | LDL-col levels were reduced in Chol group. Serum antioxidant activity were improved in HT and HT-Ac group in relation with Chol group. |
| [27] | Female Wistar Rats (n = 12) | Control group HT group SEC group |
During 21 days: fed with 5 mg/kg SEC or HT. Animals were weighted every 2 days to adjustment. |
HT treatment could play a role in the modulation of several proteins closely related with CVDs. |
| [28] | Wistar Rats (n = 70) | Non-Diabetic rats Diabetic rats Diabetic + 0.5 mg/kg/day HT rats Diabetic + 1 mg/kg/day HT rats Diabetic + 2.5 mg/kg/day HT rats Diabetic + 5 mg/kg/day HT rats Diabetic + 10 mg/kg/day HT rats |
HT was given once per day for 7 days before diabetes’ induction. Then HT was given daily during 2 months. |
HT treatment reduced platelet activity, thromboxane B2 and ox-LDL levels. In addition, the treatment reduced MPOx, VCAM-1 and IL-6. |
| [29] | ApoE−/− mice (n = 20) | Control group SEC treatment group |
Control: standard diet. SEC: standard diet 10 mg/kg SEC extract. |
SEC treatment decreased the stain of E-selectin, MCP-1, ICAM-1, VCAM-1 and F4/80. |
| [30] | BALB/c mice (n = 40) | Control group 1 50 mg/ml LPS group, group 2 40 mg/kg HT group, group 3 80 mg/kg HT group, group 4 80 mg/kg HT at 5 times group, group 5 |
For 3 days: w/o polyphenols Group 5 received HT 2 administrations 8 and 24 h post fasting 1 h after: LPS except group 1 |
At all doses tested of HT, COX-2 gene expression was reduced. Plasma antioxidant power doubled basal levels after HT treatment. |
| [25] | Sirtendo−/− mice and Sirt6flex/flex mice (WT mice) | Control group P-407 group P-407 + HT group p-407 + HT-AC WT group WT + p-407 group WT + p-407 + HT-AC group Sirtendo−/− + p-407 + HT-AC |
During 4 weeks: different concentrations of HT, HT-AC and peritoneal injection with p-407 | HT treatment decreased TNF-α and 1L-1β levels. IL-6 and Ccl2 mRNA levels decreased too. |
| [31] | ApoE−/− mice with the C57BL/6 genetic background (n = 22) | Control group HT group |
Daily HT: HT group Daily saline gavage: Control group |
HT treatment decreased plaque and atherosclerotic lesions while it improves lipid profile. HT treatment upregulated ABCA1 and SR-BI expression while downregulates IL-2 and CRP expression. |
| [26] | ApoE−/− mice in the C57/BL6 background (n = 40) | ND ND + HT-AC HFD HFD + HT-AC |
For 12 weeks: HFD consumed western diet and/or HT-AC | HT treatment decreased plaque formation and suppressed the expression of GSDMD. HT suppressed TNF-α and IL-1β concentrations. HT treatment decreased HDAC11 and HDAC11 mRNA expression. |
| [34] | Wistar rats (n = 60) | Non-Diabetic rats Diabetic rats Diabetic + 5 mg/kg/day HT rats Diabetic + 0.5 mg/kg/day DHPG rats Diabetic + 0.5 mg/kg/day DHPG + HT rats Diabetic + 1 mg/kg/day DHPG + HT rats |
Every treatment were administrated in the drinking water once a day during 7 days before diabetes’ induction. Then, every treatment were administrated daily during 2 months. |
HT increased HDL-chol levels while reduced platelet aggregation, thromboxane B2, MPOx and VCAM-1 levels. |
| [35] | Swiss mice (n = 35) | Control group HT group LPS group 20 mg HT + LPS group 40 mg HT + LPS group |
For 10 days every group received their treatment | HT treatment reduced CRP, MCP-1, MPO, IL-1β, IL-6, TNF-α and NF-kB |
Abbreviations: ABCA1: ATP-binding cassette transporter 1; ApoE−/− mice: Apolipoprotein E knockout mice; Ccl2: Chemokine ligand 2; Con: Control; COX: cyclooxygenase; CRP: C-reactive protein; CVDs: Cardiovascular diesases; DHPG: 3,4-dyhydroxyphenylglycol; HDAC11: Histone deacetylase 11; HFD: High fat diet; HT-AC: Hydroxytyrosol acetate; HT-Et: Hydroxytyrosol ether; ICAM-1: Intercellular adhesion molecule-1; IL: Interleukin; Isop: Isoproterenol; LPS: Lipopolysaccharide; MCP-1: Monocyte chemoattractant protein-1; ND: Normal diet; NF-kB: Nuclear Factor kappa-light-chain-enhancer of activated B cells; OLE: Olive leaf extract; SD: Sprague-Dawley; SEC: Secoiridoids; Sirtendo−/− mice: Endothelium specific Sirt6 knockout; SR-BI: Scavenger receptor class B type 1; TNF-α: Tumor Necrosis Factor- α; VCAM-1: vascular cell adhesion molecule-1; WT: Wild-type.
The impact of HT-AC was examined against the inflammatory status in hypercholesteraemic mice as well [25]. The treatment with HT and HT-AC reduced both, mRNA and protein levels of TNFα, IL-6, Ccl2 and IL-1β (p < 0.05). Interestingly, as observed in rats, HT-AC was found to be better absorbed than HT itself. Similar results were found when ApoE−/− mice fed with high-fat-diet (HFD) had significantly decreased plaque formation after HT-AC treatment (p < 0.05) [26]. Moreover, the inflammatory status improved after this treatment by the reduction of Gasdermin D (GSDMD) (p < 0.01), a protein associated with chemokines production. Furthermore, a pro-inflammation molecule called Histone Deacetylase 11 (HDAC11) (p < 0.01) and its mRNA expression (p < 0.05) decreased by the supplementation of HT-AC [26].
Another study showed the protective effect of phenolic compounds in CVDs after 5 mg/kg of HT supplementation in rats. The clinical relevance of HT is clearly supported by the proteomic data from aorta and heart tissues. Aorta showed an upregulated protein after both treatments (HT and secoiridoids [SEC]), Hexokinase-2 (Hk2), an enzyme involved in cell viability of cardiomyocytes and survival of ventricular myocytes [27]. On the other hand, the most important downregulated proteins were: Kng1, associated with thrombus formation; Gap junction alpha-1 protein (Gja1), implied in endothelial cells proliferation, cardiogenesis and vasoconstriction of blood vessels and Ras-related C3 botulinum toxin substrate 1 (Rac1), involved in endothelial cells migration and heart rate, among others. Returning to one of the hypotheses posed at the beginning of this review, it is now possible to state that HT strongly interfere with reparative, migration and angiogenic potential. Accordingly, we found very suitable the use of proteomic approaches to measure a potential effect of HT. However, no preclinical studies related to other -omics data (metabolomics, lipidomics) were found in the literature.
An in vivo study with diabetic rats (DR) found a dose-dependent reduction of platelet activity, thromboxane B2 concentration and plasma ox-LDL concentration after HT treatment (p < 0.05) [28]. In addition, a significant reduction of MPOx, VCAM-1 and IL-6 was evaluated after HT administration in comparison to untreated rats (p < 0.05) [28]. Consequently, one of the more significant findings to emerge from this study is that HT treatment in hyperglycaemic conditions significantly improved the CV parameters.
The following data were drawn from a study with two different apolipoprotein E knockout mice: control group and SEC group, being the last one supplemented with a mixture of HT and its derivatives [29]. According to the immunohistochemical analysis, the atherosclerosis stages were attenuated in the SEC group by a lesser expression of E-selectin, MCP-1, ICAM-1, VCAM-1 and F4/80 when compared with the control group (p < 0.05). These results in mice fed with a specific HT treatment confirmed the beneficial effects of these compounds in atherosclerosis. A therapeutical implication of these data is the possibility of HT supplementation in early stages of atherosclerosis.
Besides, Fuccelli et al. increased substantially HT supplementation up to 80 mg/kg in mice [30] leading to a 50% decrease in TNF-α levels. Interestingly, elevated doses of HT increased plasma antioxidant activity as well (p < 0.01) [30]. The results of this research support once more the idea that HT supplementation, even at high doses, could exert an antioxidant and antiinflammation effect. Nonetheless, there is a lack of compound doses studies in order to stablish a standard efficient dose in vitro and animals’ studies.
Regarding the HT dosages, the atherosclerotic protective effects of HT were also inferred from an ApoE−/− mice model supplemented with western diet +10 mg/kg/day HT [31]. In this study, lipid profile improved, including serum triglycerides (TG) (p < 0.01), total cholesterol (TC) (p < 0.01), low-density lipoprotein (LDL) (p < 0.01) and high-density lipoprotein (HDL) levels (p < 0.05). Overall, these results indicate a positive association between HT and a higher expression of ABCA1 (associated with cholesterol homeostasis) and SR-BI (an HDL receptor) (Fig. 2). Moreover, the expression of inflammatory cytokines such as IL-2, IL-6 and CRP were also decreased (p < 0.01) [31]. Taken together, these results suggest that there is an association between HT, lipid profile and the inflammatory status. A recent study with induced colitis-mice showed that HT intervention transformed the gut microbiota, leading to a lower abundance of inflammation-related microbes [32]. If the debate is to be moved forward, a better understanding of host-microbiota interactions involving HT supplementation needs to be further developed, since microbiome has also been linked to the pathogenesis of metabolic conditions like diabetes and obesity. In this line, Liu et al. found that HT can improve obesity and insulin resistance in obese mice by altering the composition of the intestinal microbiota and improving integrity of the intestinal wall [33].
Nonetheless, it is also relevant to compared HT effects to other polyphenolic compounds. In this regard, Wistar rats were treated with HT another olive oil polyphenol (DHPG) [34]. The data indicated that HT increased HDL-cholesterol levels (p < 0.05) while reduced platelet aggregation, thromboxane B2, MPOx and VCAM-1 levels (p < 0.05 for all) [34]. Similarly, it was also shown that Swiss mice treated with LPS to stimulate polynuclear cells and proinflammation markers were counteracted by high doses of HT (p < 0.05) [35]. The most obvious finding to emerge from this studies is that all the inflammatory cascade is being impaired by HT administration.
6. The effect of hydroxytyrosol in human studies
Several human studies and clinical trials have been developed showing that HT could be a compound with interest in the prevention or treatment of CV diseases (Table 3). In initial studies comparing the protective effects of HT in humans, dose was 5 mg/day and patients displayed low-moderate CV risk [36]. The nutraceutical supplementation induced significant reductions on LDL-col, Apo B (p < 0.001) and plasma CRP levels (p = 0.021) compared with the placebo group. Since nutraceutical combination contained a mixture of phytosterols, monacolin K from red yeast rice (RYR) and vitamin E, such research was not specifically designed to evaluate the specific effects related to HT.
Table 3.
Clinical trials that investigated the effects of HT in humans.
| Study (year) | Sample (size) | Treatment Group (size) | Methodology | Outcomes |
|---|---|---|---|---|
| [36] | Eligible human patients (n = 40) | Placebo group Aquilea cholesterol group |
2 weeks of a healthy diet. Both placebo and nutraceutical were taken daily before the main meal during 90 days. |
Nutraceutical treatment reduced LDL col, ApoB and CRP levels |
| [37] | Eligible human patients (n = 84) | Placebo Oral supplementation group |
2 treatment sequences including oral supplementation or placebo for 8 weeks, followed by a 4-week wash-out interval and then a crossover. | After oral supplementation, SBP and DBP were reduced while FMD increased. ox-LDL levels were reduced too. |
| [7] | Eligible human patients (n = 32) | Water (control) WW WW + Tyr |
3 intervention periods during 4 weeks preceded by 3 wash-out periods. | After WW + Tyr period increase HDL and AT III while it Hcy decreases. |
| [38] | Eligible human patients (n = 20) | Placebo group Olp group |
Participants in both groups consume 4 tablets/day (30 mg/day HT). A wash-out period of 2 months and then a crossover. |
HT treatment improved TC and LDL cholesterol levels. |
| [39] | Eligible human patients (n = 30) | Control group Extract group |
For 8 weeks 1 group consumed placebo and the other an extract from OO and AS | EG decreased oxLDL levels and it improved oxLDL/LDL-col ratio. |
| [40] | Eligible human patients (n = 84) | SAx supplement Placebo |
3 capsules/day of the assigned product during 20 weeks separated into 8 weeks of treatment (supplement or placebo) by a washout period of 4 weeks. | Supplementation decreased LDL and TG levels while it increases HDL levels. |
Abbreviations: ApoB: Human apolipoprotein B; AS: Almond skin; AT III: antithrombin III; CRP: C-reactive protein; DBP: Diastolic blood pressure; EG: Extract group; FMD: Flow mediated dilatation; Hcy: Homocysteine; HDL: High density lipoproteins; LDL: Low density lipoproteins; Olp: Olive pâté; SBP: Systolic blood pressure; TC: Total cholesterol; TG: triglycerides; Tyr: Tyrosol; OO: Olive oil; oxLDL: oxidized LDL; WW: White wine.
In another randomized, controlled, crossover clinical trial, healthy middle-aged subjects were supplemented with 9.9 mg of HT, 195 mg of Punicalagin and 995.1 mg of maltodextrin [37] (ClinicalTrials.gov, NCT02042742). The bioactive compound mixture displayed better results over the systolic and diastolic blood pressure (SBP and DBP, respectively), flow mediated dilatation (FMD) and circulating oxLDL levels than placebo treatment (p < 0.001, for all) [37]. Similar to the study of Domenech et al. this study did not confirm the direct implication of HT, however, it did partially substantiate its novel effect over the vascular tone, which has not been extensively investigated.
A randomized, crossover, controlled study made several noteworthy contributions as well since it incorporates the conversion of HT from Tyr [7] (ClinicalTrials.gov, NCT02783989), similar to the experimental study of Serreli et al. [19]. The present study provided additional evidence with respect to the increment of Tyr consumption, since it increased, in a dose-dependent manner, the presence of HT and Tyr in urine. Regarding the clinical biomarkers, HDL-col increased after the intervention phase (p = 0.025) [7]. Antithrombin III equally increased in the presence of Tyr (p = 0.005), denoting an improvement in blood coagulation. On the other hand, homocysteine levels decreased (p = 0.028) [7]. Taken together, these findings suggest a role for Tyr in promoting HT release and improving inflammatory, coagulation and vasodilation biomarkers.
Looking for clinical studies with higher HT doses, a placebo-controlled, double-blind crossover trial using an olive pâté (OlP) with 30 mg/day of HT was found. Lipid profile was improved by total (−5.4%) and LDL cholesterol (−11.7%) reduction, together with a decreased in urea levels (−11%) and an increase in calcium levels (+4.3%) (p < 0.05, for all of them) [38]. Thus, the present study confirms previous findings and contributes additional evidence suggesting the high tolerance of this compound. In addition, a recent clinical study with 7.5 mg of HT, 210 mg of almond skin (ASPs) and 33 mg maltodextrins per day (2 capsules/day) vs 800 mg of maltodextrins found a reduction in oxLDL and oxLDL/LDL ratio in the interventional group compared to control conditions (p < 0.001) after 8 weeks of treatment [39] (CrinicalTrials.gov, NCT04029727). Thus, the present study provides additional evidence with respect to the relation between HT, oxLDL levels and the inflammatory status in humans.
Finally, a second study conducted by Quirós et al. aimed to demonstrate whether HT in combination with Punicalagin (PC) improved dyslipidemia. The interventional product contained 3.3 mg of HT, 65 mg of PC and 331.7 mg of maltodextrin. Upon the intervention period, lipid profile improved notoriously by the decrease of LDL (p < 0.004), increase of HDL (p < 0.033) and decrease of TG (p < 0.017) [40] (ClinicalTrials.gov, NCT02042742). Thus, the evidences presented in these clinical trials suggest that HT has a great pharmacological potential.
7. Conclusions and future perspectives
This review assists in our understanding in the effects of HT, from the mechanisms of action in atherosclerosis to the CV risk prevention. In particular, the high range-doses of HT in vitro and in vivo data confirmed an improvement on the lipid profile, plaque formation and inflammatory status after the treatment. However, there is still a lack of human studies to establish the appropriate doses of HT in humans to play a protective role against CVDs. This review has thrown up many questions in need of further investigation such as the involvement of HT in the microbiome and the quantifiable effects in lipidomics and metabolomics. Thus, further -omics studies in human patients are necessary to assess all the profits that HT treatment could exert.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research was funded by the Fundación Séneca de la Región de Murcia (20646/JLI/18, Jóvenes Líderes en Investigación).
Data availability statement
Data included in article/supp. Material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Dr. Orenes-Piñero is supported by a postdoctoral contract from the Instituto Murciano de Investigaciones Biosanitarias Pascual Parrilla (IMIB-Pascual Parrilla, Murcia, Spain). CNN belongs to the “Programa de Doctorado en Ciencias de la Salud” from the Catholic University of Murcia (UCAM) and holds a predoctoral grant from UCAM.
References
- 1.World Health Organization (WHO) https://www.who.int/es/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds
- 2.Wolf D., Ley K. Immunity and inflammation in atherosclerosis. Circ. Res. 2019;124:315–327. doi: 10.1161/CIRCRESAHA.118.313591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herrington W., Lacey B., Sherliker P., Armitage J., Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ. Res. 2016;118:535–546. doi: 10.1161/CIRCRESAHA.115.307611. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y., Xian X., Wang Z., Bi Y., Chen Q., Han X., et al. Research progress on the relationship between atherosclerosis and inflammation. Biomolecules. 2018;8:80. doi: 10.3390/BIOM8030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gimbrone M.A., Jr., García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azambuja Lopes de Souza P., Marcadenti A., Portal V.L. Effects of olive oil phenolic compounds on inflammation in the prevention and treatment of coronary artery disease. Nutrients. 2017;9:1087. doi: 10.3390/NU9101087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boronat A., Mateus J., Soldevila-Domenech N., Guerra M., Rodríguez-Morató J., Varon C., et al. Cardiovascular benefits of tyrosol and its endogenous conversion into hydroxytyrosol in humans. A randomized, controlled trial. Free Radic. Biol. Med. 2019;143:471–481. doi: 10.1016/j.freeradbiomed.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 8.Bertelli M., Kiani A.K., Paolacci S., Manara E., Kurti D., Dhuli K., et al. Hydroxytyrosol: a natural compound with promising pharmacological activities. J. Biotechnol. 2020;309:29–33. doi: 10.1016/j.jbiotec.2019.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Scoditti E., Nestola A., Massaro M., Calabriso N., Storelli C., de Caterina, et al. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCα and PKCβ1 inhibition. Atherosclerosis. 2014;232:17–24. doi: 10.1016/J.ATHEROSCLEROSIS.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Robles-Almazan M., Pulido-Moran M., Moreno-Fernandez J., Ramirez-Tortosa C., Rodriguez-Garcia C., Quiles J.L., et al. Hydroxytyrosol: bioavailability, toxicity, and clinical applications. Food Res. Int. 2018;105:654–667. doi: 10.1016/j.foodres.2017.11.053. [DOI] [PubMed] [Google Scholar]
- 11.D'Angelo C., Franceschelli S., Quiles J.L., Speranza L. Wide biological role of hydroxytyrosol: possible therapeutic and preventive properties in cardiovascular diseases. Cells. 2020;9:1932. doi: 10.3390/CELLS9091932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soni M.G., Burdock G.A., Christian M.S., Bitler C.M., Crea R. Safety assessment of aqueous olive pulp extract as an antioxidant or antimicrobial agent in foods. Food Chem. Toxicol. 2006;44:903–915. doi: 10.1016/j.fct.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Catalán Ú., López de las Hazas M.C., Rubió L., Fernández-Castillejo S., Pedret A., de la Torre R., et al. Protective effect of hydroxytyrosol and its predominant plasmatic human metabolites against endothelial dysfunction in human aortic endothelial cells. Mol. Nutr. Food Res. 2015;59:2523–2536. doi: 10.1002/mnfr.201500361. [DOI] [PubMed] [Google Scholar]
- 14.Lopez S., Montserrat-de la Paz S., Lucas R., Bermudez B., Abia R., Morales J.C., et al. Effect of metabolites of hydroxytyrosol on protection against oxidative stress and inflammation in human endothelial cells. J. Funct.Foods. 2017;29:238–247. doi: 10.1016/j.jff.2016.12.033. [DOI] [Google Scholar]
- 15.Calabriso N., Gnoni A., Stanca E., Cavallo A., Damiano F., Siculella L., et al. Hydroxytyrosol ameliorates endothelial function under inflammatory conditions by preventing mitochondrial dysfunction. Oxid Med Cell Longev. 2018:1–14. doi: 10.1155/2018/9086947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu X., Li C., Mariyam Z., Jiang P., Zhou M., Zeb F., et al. Acrolein‐induced atherogenesis by stimulation of hepatic flavin containing monooxygenase 3 and a protection from hydroxytyrosol. J. Cell. Physiol. 2019;234:475–485. doi: 10.1002/jcp.26600. [DOI] [PubMed] [Google Scholar]
- 17.Scoditti E., Carpi S., Massaro M., Pellegrino M., Polini B., Carluccio M.A., et al. Hydroxytyrosol modulates adipocyte gene and miRNA expression under inflammatory condition. Nutrients. 2019;11:2493. doi: 10.3390/nu11102493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carluccio M.A., Martinelli R., Massaro M., Calabriso N., Scoditti E., Maffia M., et al. Nutrigenomic effect of hydroxytyrosol in vascular endothelial cells: a transcriptomic profile analysis. Nutrients. 2021;13:3990. doi: 10.3390/nu13113990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serreli G., Melis M.P., Corona G., Deiana M. Modulation of LPS-induced nitric oxide production in intestinal cells by hydroxytyrosol and tyrosol metabolites: insight into the mechanism of action. Food Chem. Toxicol. 2019;125:520–527. doi: 10.1016/j.fct.2019.01.039. [DOI] [PubMed] [Google Scholar]
- 20.Katsuyama K., Shichiri M., Marumo F., Hirata Y. NO inhibits cytokine-induced iNOS expression and NF-kappaB activation by interfering with phosphorylation and degradation of IkappaB-alpha. Arterioscler. Thromb. Vasc. Biol. 1998;18:1796–1802. doi: 10.1161/01.ATV.18.11.1796. [DOI] [PubMed] [Google Scholar]
- 21.Abate M., Pisanti S., Caputo M., Citro M., Vecchione C., Martinelli R. 3-Hydroxytyrosol promotes angiogenesis in vitro by stimulating endothelial cell migration. Int. J. Mol. Sci. 2020;21:3657. doi: 10.3390/ijms21103657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terzuoli E., Nannelli G., Giachetti A., Morbidelli L., Ziche M., Donnini S. Targeting endothelial-to-mesenchymal transition: the protective role of hydroxytyrosol sulfate metabolite. Eur. J. Nutr. 2020;59:517–527. doi: 10.1007/s00394-019-01920-x. [DOI] [PubMed] [Google Scholar]
- 23.Serreli G., Le Sayec M., Diotallevi C., Teissier A., Deiana M., Corona G. Conjugated metabolites of hydroxytyrosol and tyrosol contribute to the maintenance of nitric oxide balance in human aortic endothelial cells at physiologically relevant concentrations. Molecules. 2021;26:7480. doi: 10.3390/molecules26247480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabernero M., Sarriá B., Largo C., Martínez-López S., Madrona A., Espartero J.L., et al. Comparative evaluation of the metabolic effects of hydroxytyrosol and its lipophilic derivatives (hydroxytyrosyl acetate and ethyl hydroxytyrosyl ether) in hypercholesterolemic rats. Food Funct. 2014;5:1556–1563. doi: 10.1039/C3FO60677E. [DOI] [PubMed] [Google Scholar]
- 25.Yao F., Yang G., Xian Y., Wang G., Zheng Z., Jin Z., et al. The protective effect of hydroxytyrosol acetate against inflammation of vascular endothelial cells partly through the SIRT6-mediated PKM2 signaling pathway. Food Funct. 2019;10:5789–5803. doi: 10.1039/C9FO00586B. [DOI] [PubMed] [Google Scholar]
- 26.Yao F., Jin Z., Lv X., Zheng Z., Gao H., Deng Y., et al. Hydroxytyrosol acetate inhibits vascular endothelial cell pyroptosis via the HDAC11 signaling pathway in atherosclerosis. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.656272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catalán Ú., Rubió L., López de Las Hazas M.C., Herrero P., Nadal P., Canela N., et al. Hydroxytyrosol and its complex forms (secoiridoids) modulate aorta and heart proteome in healthy rats: potential cardio-protective effects. Mol. Nutr. Food Res. 2016;60:2114–2129. doi: 10.1002/mnfr.201600052. [DOI] [PubMed] [Google Scholar]
- 28.López-Villodres J.A., Abdel-Karim M., De La Cruz J.P., Rodríguez-Pérez M.D., Reyes J.J., Guzmán-Moscoso R., et al. Effects of hydroxytyrosol on cardiovascular biomarkers in experimental diabetes mellitus. J. Nutr. Biochem. 2016;37:94–100. doi: 10.1016/j.jnutbio.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Catalán Ú., López de las Hazas M.C., Piñol C., Rubió L., Motilva M.J., Fernandez-Castillejo S., et al. Hydroxytyrosol and its main plasma circulating metabolites attenuate the initial steps of atherosclerosis through inhibition of the MAPK pathway. J. Funct.Foods. 2018;40:280–291. doi: 10.1016/j.jff.2017.11.007. [DOI] [Google Scholar]
- 30.Fuccelli R., Fabiani R., Rosignoli P. Hydroxytyrosol exerts anti-inflammatory and anti-oxidant activities in a mouse model of systemic inflammation. Molecules. 2018;23:3212. doi: 10.3390/MOLECULES23123212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X., Qin Y., Wan X., Liu H., Iv C., Ruan W., et al. Hydroxytyrosol plays antiatherosclerotic effects through regulating lipid metabolism via inhibiting the p38 signal pathway. Biomed Res Int. 2020:1–12. doi: 10.1155/2020/5036572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q., Wang C., Abdullah Tian W., Qiu Z., Song M., et al. Hydroxytyrosol alleviates dextran sulfate sodium-induced colitis by modulating inflammatory responses, intestinal barrier, and microbiome. J. Agric. Food Chem. 2022;70:2241–2252. doi: 10.1021/acs.jafc.1c07568. [DOI] [PubMed] [Google Scholar]
- 33.Liu Z., Wang N., Ma Y., Wen D. Hydroxytyrosol improves obesity and insulin resistance by modulating gut microbiota in high-fat diet-induced obese mice. Front. Microbiol. 2019;10:390. doi: 10.3389/fmicb.2019.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De La Cruz Cortés J.P., Vallejo-Carmona L., Arrebola M.M., Martín-Aurioles E., Rodriguez-Pérez M.D., Ortega-Hombrados L., et al. Synergistic effect of 3′,4′-dihidroxifenilglicol and hydroxytyrosol on oxidative and nitrosative stress and some cardiovascular biomarkers in an experimental model of type 1 diabetes mellitus. Antioxidants. 2021;10:1983. doi: 10.3390/antiox10121983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alblihed M.A. Hydroxytyrosol ameliorates oxidative challenge and inflammatory response associated with lipopolysaccharide-mediated sepsis in mice. Hum. Exp. Toxicol. 2021;40:342–354. doi: 10.1177/0960327120949618. [DOI] [PubMed] [Google Scholar]
- 36.Domenech M., Casas R., Ruiz-León A.M., Sobrino J., Ros E., Estruch R. Effects of a novel nutraceutical combination (aquilea Colesterol®) on the lipid profile and inflammatory biomarkers: a randomized control trial. Nutrients. 2019;11:949. doi: 10.3390/NU11050949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quirós-Fernández R., López-Plaza B., Bermejo L., Palma-Milla S., Gómez-Candela C. Supplementation with hydroxytyrosol and Punicalagin improves early atherosclerosis markers involved in the asymptomatic phase of atherosclerosis in the adult population: a randomized, placebo-controlled, crossover trial. Nutrients. 2019;11:640. doi: 10.3390/nu11030640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dinu M., Pagliai G., Scavone F., Bellumori M., Cecchi L., Nediani C., et al. Effects of an olive by-product called pâté on cardiovascular risk factors. J. Am. Coll. Nutr. 2021;40:617–623. doi: 10.1080/07315724.2020.1813060. [DOI] [PubMed] [Google Scholar]
- 39.Fonollá J., Maldonado-Lobón J.A., Luque R., Rodríguez C., Bañuelos Ó., López-Larramendi J.L., et al. Effects of a combination of extracts from olive fruit and almonds skin on oxidative and inflammation markers in hypercholesterolemic subjects: a randomized controlled trial. J. Med. Food. 2021;24:479–486. doi: 10.1089/jmf.2020.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quirós-Fernández R., López-Plaza B., Bermejo L.M., Palma-Milla S., Zangara A., Candela C.G. Oral supplement containing hydroxytyrosol and Punicalagin improves dyslipidemia in an adult population without Co-adjuvant treatment: a randomized, double-blind, controlled and crossover trial. Nutrients. 2022;14:1879. doi: 10.3390/nu14091879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. Material/referenced in article.