Abstract
Recent evidence suggests that the human genitourinary microbiome plays a significant role in mediating the development and progression of urological tumors, including bladder cancer (BC). Clinicians widely recognize the role of Bacille Calmette Guérin (BCG), an attenuated Mycobacterium tuberculosis vaccine, in the management of intermediate- and high-risk NMIBC. However, compared to the large body of evidence on the gut microbiota and gastrointestinal tumors, limited information is available about the interaction between BC and the genitourinary microbiome. This is an expanding field that merits further investigation. Urologists will need to consider the potential impact of the microbiome in BC diagnosis, prevention of recurrence and progression, and treatment prospects in the future. This review highlights the approaches adopted for microbiome research and the findings and inadequacies of current research on BC.
Keywords: Bladder cancer, Microbiota, Recurrence, Prognosis, Therapeutics
1. Introduction
In the past decades, the urine of healthy individuals was considered to be sterile, based on findings from traditional bacterial culture experiments. However, traditional microbiological methods cannot be used to isolate or characterize all urinary bacterial species owing to the limitations of the culture medium. In the past decade, this view has been challenged by the development of next-generation sequencing technologies. Researchers believe that urine has a unique microbiota, even that of healthy individuals [1]. The microbiota of healthy individuals and patients with urological diseases have significant differences. Alterations in the urinary microbiota have been identified in association with many urological diseases, such as interstitial cystitis, urinary incontinence, and neurogenic bladder dysfunction [[2], [3], [4], [5]].
Bladder cancer (BC) is one of the most common tumors of the urological tract, and in Europe and the US, the incidence of BC is second only to that of prostate cancer [6]. BC can be categorized as non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC) based on the different histological manifestations and biological characteristics. Approximately 70% of BC are NMIBC, whereas 30% are MIBC with potential for invasion and metastasis [7]. Most patients with NMIBC undergo transurethral resection of the bladder (TURBT), but the recurrence rates are as high as 40%–80%. Twenty-five percent of NMIBC cases progress to MIBC or even distant tumor metastases. MIBC has few early symptoms, progresses rapidly, and has a poor prognosis [8].
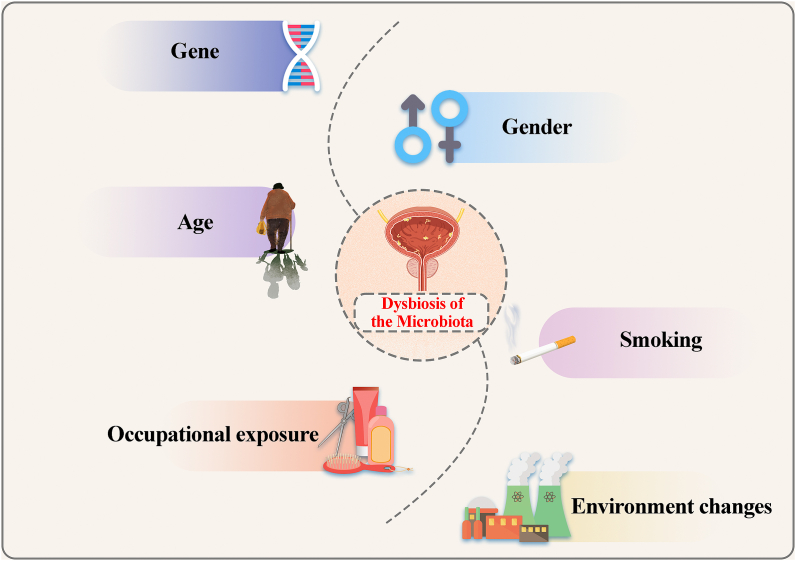
Although BC is a common urinary tract tumor in clinical practice, there is a lack of sufficient insights into the occurrence, diagnosis, and treatment of BC. In addition to genetic factors, environmental factors play a key role in the development of BC, such as smoking and occupational exposure [[9], [10], [11]]. Interestingly, these factors can also affect changes in the microbiota (Fig. 1). Increasing evidence indicates that the microbiota is strongly associated with human cancers, such as schistosomes and bladder squamous cell carcinoma [12]. BučevićPopović et al. showed that 20% of malignant tumor tissues were infiltrated with microbes. This indicated the possibility of bacterial infiltration in BC tissues [13]. Bacteria, fungi, or viruses in the genitourinary tract may be a causative factor or cofactor in the progression of urological tumors. The microbiota may also play an important role in BC treatment. For example, the Bacille Calmette Guérin (BCG) vaccine is widely used to prevent the recurrence of high-risk NMIBC via the induction and enhancement of immune responses [14].
Fig. 1.
Alterations in the urinary tract microbiota are associated with multiple factors. Alterations in the urinary tract microbiota, including the bladder, are closely related to factors such as genetic mutations, gender, age, smoking, occupational exposure, and environmental changes.
Therefore, the potential role of microbiota is only being elucidated in the tumorigenesis, progression, immunomodulation, and drug efficacy of BC. Here, we aim to summarize findings on the role of the microbiota in BC in the last decade (Table 1).
Table 1.
Microbiome studies in BC.
| Authors | Years | Patient (Bca vs Control) | Methods | Gender | Samples | NMIBC vs MIBC | Microbes showing an increase in abundance | Microbes showing a decrease in their abundance |
|---|---|---|---|---|---|---|---|---|
| Xu et al. [15] | 2014 | 8 vs 6 | 16S | Not mentioned | Urine | Not mentioned | Streptoccocus, Pseudomonas, Anaerococcus | / |
| Wu et al. [16] | 2018 | 31 vs 18 | 16S | 49 male | Urine | 26 vs 5 | Acinetobacter, Anaerococcus, Sphingobacterium, Atopostipes, and Geobacillus | Serratia, Proteus, Roseomonas and Ruminiclostridium-6 and Eubacterium-x |
| Bučević Popović et al. [13] | 2018 | 12 vs 11 | 16S | 33 male | Urine | 12 vs 0 | Fusobacterium nucleatum, Actinobaculum, Facklamia, Campylobacter, Subdoligranulum, and Ruminococcaceae | Veillonella, Streptococcus, and Corynebacterium |
| Bi et al. [17] | 2019 | 29 vs 16 | 16S | 35 vs 20 | Urine | 20 vs 9 | Actinomyces | Streptococcus, Bifidobacterium, Lactobacillus, Veillonella |
| Liu et al. [18] | 2019 | 22 vs 12 | 16S | 22 male | Tissue | 5 vs 17 |
Cupriavidus spp., Acinetobacter, Anoxybacillus, Escherichia-Shigella, Geobacillus, Pelomonas, Ralstonia, and Sphingomonas |
Lactobacillus, Prevotella, and Ruminococcus |
| Mai et al. [19] | 2019 | 24 Bca | 16S | 18 vs 6 | Urine | Not mentioned |
Enterobacteriaceae, Streptococcus, Lactobacillus, Ureaplasma, Corynebacterium, Stenotrophomonas, Enterococcus, Staphylococcus |
/ |
| Moynihan et al. [20] | 2019 | 8 vs 33 | 16S | 41 male | Urine | Not mentioned | No significant differences (24 non-smokers and 17 smokers) | |
| Chipollini et al. [21] | 2020 | 38 vs 10 | 16S | 42 vs 6 | Urine | 22 vs 16 | Bacteroides and Faecalibacterium | Lachnoclostridium, Burkholderiaceae |
| He et al. [22] | 2020 | 26 vs 16 | 16S | 18 vs 8 | Feces | 16 vs 10 | / | Clostridium XI and Prevotella |
| Hourigan et al. [23] | 2020 | 22 Bca | 16S | 14 vs 8 | Urine | 22 vs 0 | (Male)Tepidimonas | (Male) Prevotella, Veillonella |
| Mansour et al. [24] | 2020 | 10 Bca | 16S | 5 vs 5 | Urine and Tissue | 6 vs 4 | Tissue: Akkermansia, Bacteroides, Clostridium sensu stricto, Enterobacter and Klebsiella |
/ |
| Pederzoli et al. [25] | 2020 | 49 vs 59 | 16S | 70 vs 38 | Urine and Tissue | Not mentioned | Tissue: Burkholderia; Urine (Male): Acidobacteria-6, Opitutaceae; Urine (Female): Klebsiella |
Urine (Male): Tissierellaceae, Alphaproteobacteria, Rhizobiales, Sphingomonadales, Pasteurellales, Streptococcaceae, Corynebacteriaceae, Patulibaceteraceae; Urine (Female): Betaproteobacteria, Burkholderiales, Pseudomonadales, Comamonadaceae, Moraxellaceae, Coriobacteriaceae, Coriobacteriia. |
| Zeng et al. [26] | 2020 | 62 vs 19 | 16S | Not mentioned | Urine | 51 vs 11 | Micrococcus and Brachybacterium in recurrence group | / |
| Ma et al. [27] | 2021 | 15 vs 11 | 16S | Not mentioned | Urine | 7 vs 8 | Smoker group: bacteroidaceae, erysipelotrichales and lachnospiraceae | / |
| Hussein et al. [28] | 2021 | 43 vs 10 | 16S | 36 vs 7 | Urine | 29 vs 14 | MIBC: Haemophilus and Veillonela NMIBC: Cupriavidus, Serratia, Brochothrix, Negativicoccus, Escherichia-Shigella, and Pseudomonas |
/ |
| Qiu et al. [29] | 2022 | 40 Bca | 16S and IHC | 40 male | Urine | 40 vs 0 | Recurrence group: Pseudomonas, Staphylococcus, Corynebacterium, and Acinetobacter genera. |
/ |
2. Urinary microbiota and its characterization
The human body contains trillions of microbes, which include bacteria, archaea, fungi, and viruses that constitute the human microbiome [30]. These microbes host the human body and form different micro-ecologies in different parts of the body, including the gastrointestinal tract, skin, oral cavity, and genitourinary tract. The host and its microbiota collectively form a superorganism. The dysbiosis or disruption of the human microbiome has several consequences for human health and can lead to disease [31]. Changes in microbial composition may be influenced by genetic and environmental factors, including diet, geographic location, metabolites, and carcinogens, among others [32]. The microbiota can induce tumorigenesis/or promote tumor development through various mechanisms: (i) direct DNA-damaging effects of bacterial toxins, (ii) microbial metabolites that may act as potential carcinogens, (iii) inflammation-cancer transformation owing to bacteria-induced inflammation or invasive biofilm formation, and (iv) microbiome-mediated driving of the cellular microenvironment [33,34].
The urinary microbiome is defined as the genes and genomes of the microbiota as well as the metabolites of the microbiota and host environment. Variations in the urinary microbiome are usually characterized in terms of the α diversity (diversity of populations in a sample) and β diversity (population differences between samples) [35]. The resolution of species composition in microbial communities relies primarily on two high-throughput tools: amplicon sequencing (16S rRNA sequencing and 18S rRNA sequencing, among others) and shotgun metagenomic sequencing. These techniques can be used to identify bacteria that have not been detected previously using traditional culture techniques [36].
Amplicon sequencing is limited by amplification preference, off-target amplification, and low species resolution, and is often unable to detect bacteria, archaea, and fungi simultaneously [37]. Shotgun metagenomic sequencing can be used for macrogenomic analysis, in which all DNA for a sample can be sequenced. Although it addresses the issues of amplicon sequencing, it requires high-quality sample DNA in large quantities, and it is challenging to analyze microscopic, highly degraded, or highly contaminated samples using this method [38]. Sun et al. developed a new technique known as 2bRAD-M, which can effectively overcome the shortcomings of amplicon sequencing and shotgun metagenomic sequencing. However, this technique requires higher volumes and different types of samples to validate its effectiveness [39,40]. In addition, microbiome analyses combined with metabolomics for studying small molecules produced by host and microbes can be used to explore the interaction between cells and microbiota, reshape the cellular metabolic microenvironment, and regulate cell proliferation, differentiation, and programmed death [41]. In BC, the continuous development of emerging technologies can facilitate the characterization of the tumor-related microbiome, thus providing a basis for the identification of specific microorganisms and exploring the etiology and treatment of BC.
3. Urine or tissues
The bladder is the organ that stores urine. Thus, urine may be the most appropriate sample for identifying the BC microbiome. Urine contains a high concentration of salts, which allows the urinary microbiome and nucleic acids to be preserved for a longer period. However, separating the microbial precipitate by centrifugation is more important. Meanwhile, different ways of collecting urine samples, such as transurethral catheterization, suprapubic aspiration, urina sanguinis, or midstream urine collection, may lead to differences in the microbiota [42,43]. Hourigan et al. found no differences between the alpha diversities of urine samples collected from various patients or men and women. Meanwhile, the β diversity of the urinary microbiome varied according to the method of urine collection in males (cystoscopy or midstream urine collection) [23]. Evidence from studies also indicates that midstream urine may be unsuitable for detecting bacteria in bladder biofilms that adhere to the mucosa, which can directly interact with urothelial cells [44,45]. However, because catheterization and cystoscopy are invasive methods, midstream urine samples are still used in most studies on the BC microbiome, which is considered to yield a broader representation of the bladder microbiome.
At present, standardized procedures for urine collection, preservation, and storage for microbiome research have not been established. Researchers studied the effects of different storage conditions (including temperature, storage time and whether preservatives were added) on the microbial diversity of urine. The results showed that lower temperature, shorter storage time and the addition of preservatives were most beneficial to the reproducibility of the urine microbiome [46,47].
However, can urinary microbes accurately characterize the microbiome in BC tissues? Studies on the BC tissue microbiome are insufficient. For the first time, Liu et al. used BC tissues for 16S sequencing. In this study, we confirmed the presence of microbiota in BC tissues [18]. Pederzoli showed that urine collected from patients with BC shares more than 80% of the microbiome with BC tissues. The findings of this study indicated that the urinary microbiome may appropriately reflect the microbiome in the BC environment [25]. Meanwhile, in another study, the abundance of the microbiome in urine and tissues from four patients with BC was analyzed. The results indicated a greater abundance of Akkermansia, Bacteroides, Clostridium sensu stricto, Enterobacter, and Klebsiella in tissue samples than in urine, although the number of samples in this study was small [24].
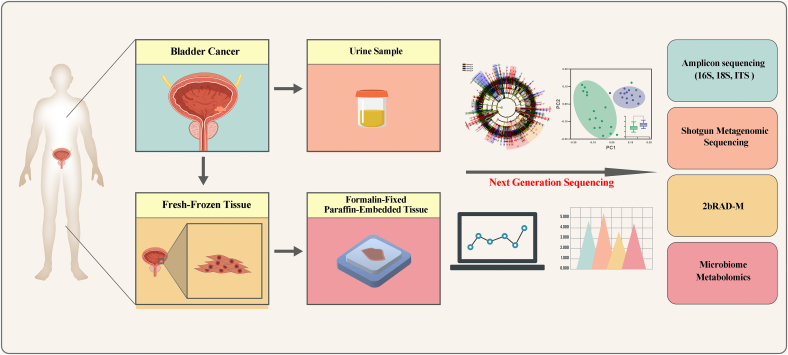
Another issue to be aware of is the difference between fresh-frozen (FF) tissue and formalin-fixed paraffin-embedded (FFPE) tissue. FF tissue is considered the “gold standard” for high-throughput sequencing of the microbiome and is advantageous for preserving DNA, because it can be frozen immediately, causes less fragmentation, and has lower contamination. FFPE tissues can be stored at room temperature for long periods and can be used in retrospective studies. However, the tissues are not sterile during paraffin embedding, and DNA degradation may occur within the paraffin-embedded specimens. Therefore, the use of paraffinized tissues for high-throughput microbiome sequencing requires the elimination of the “noise” from paraffin [48,49]. Studies comparing FF and FFPE tissues have been performed in other cancer types [50]. However, the quality of FFPE and FF tissues of BC in the analysis of the difference in microbiota has not been investigated. Thus, this topic warrants investigation. Meanwhile, FFPE tissues can also be analyzed using new technologies, such as 2bRAD-M and RNA-ISH, to assess the abundance of the microbiome in BC tissues (Fig. 2).
Fig. 2.
Bladder cancer-associated microbiota in different sample types. Urine, fresh frozen tissue, and formalin-fixed paraffin-embedded tissue from patients with BC can be used for microbiome analysis. Meanwhile, with the development of next-generation sequencing (NGS) technology, various methods for microbiome analysis are available: amplicon sequencing (16S, 18S), shotgun metagenomic sequencing, 2bRAD-M, and microbiome metabolomics.
4. Microbiota and bladder tumorigenesis
The association among bladder schistosomiasis infection, inflammation, and BC is well confirmed. Schistosomes were shown to be associated with a very high incidence of BC in Egypt [51]. Schistosome eggs can colonize the bladder wall and stimulate a host immune response, causing granulomatous inflammation. Meanwhile, adult schistosomes can parasitize the bladder venous plexus and induce severe metaplasia [52,53]. A recent study has also shown that the urinary microbiota affects schistosome-induced BC. Adebayo et al. performed 16S microbiome sequencing using 70 urine samples from schistosome-infected patients in southwestern Nigeria. Patients with BC caused by schistosomiasis, patients with only schistosomiasis infection, and healthy individuals could be distinguished based on the presence of different bacteria, such as Fusobacterium, Sphingobacterium, and Enterococcus [54].
Meanwhile, bacteria can lead to inflammation, which can increase the risk of tumorigenesis. Inflammation triggers the generation of intracellular reactive oxygen species (ROS), which cause DNA breaks, inhibit DNA damage repair, suppress the expression of related RNA and proteins, and promote microenvironmental angiogenesis [55,56]. Bacteria also disrupt the extracellular matrix, modulate cell surface receptors, affect cytokine secretion, and weaken the immune response of the body through its protease activity [57]. Cystitis was shown to be positively associated with the risk of BC, and a history of three or more occurrences of urinary tract infections (UTIs) was considered a risk factor for BC [58]. In another study, the risk of squamous BC was specifically associated with UTIs and was strongly dose-dependent with UTI-specific antibiotics [59]. Xu et al. found that the ratio of Pseudomonas to Streptococcus is closely related to the occurrence of BC [15]. Streptococcus are highly invasive and can produce various enzymes and exotoxins, which promote inflammation [60].
Bacterial aggregates, also known as biofilms, cause chronic inflammation in the genitourinary system. The interaction of biofilms with epithelial cells was shown to be associated with a higher risk of bladder tumorigenesis [61,62]. Biofilms often adhere to the apical epithelial cells of the bladder (also known as umbrella cells), which have a protective layer of sulfated polysaccharide aminoglycans in their physiological state. The disruption of the protective layer may lead to pathological changes in the bladder and causes chronic inflammation in the genitourinary system. The interaction of biofilms with epithelial cells has been shown to promote bladder tumorigenesis [63]. Nadler et al. investigated the presence of biofilms in FFPE tissues from ten patients with BC by FISH. In two samples, a dense biofilm was observed near the apical surface of the urothelium, and one sample was confirmed to contain spherical bacteria. Interestingly, both patients had negative preoperative urine cultures [44].
Findings from epidemiological surveys confirmed that the occurrence of BC is closely related to the patients' age and gender [64]. There are significant differences in urinary microbiota between different age groups. Jonquetella, Parvimonas, Proteniphilum, and Saccharofermentans were primarily detected in the elderly population aged more than 70 years. Concurrently, men have a much higher risk of cancer than women, with a ratio of 3:1 (men:women), but women with BC tend to have a more aggressive form of the disease. These biological characteristics of BC may be attributed to differences in the male and female urinary tract microbiota [65]. Lewis et al. showed that Actinobacteria, including Mycobacterium and Bacteroidetes, were detected in the female urinary tract. BCG is also prepared from a species of Mycobacterium. Therefore, Actinobacteria present in the female urinary microbiota may play a role in preventing BC [66]. Pederzoli et al. described differences in the urinary microbiome of female and male patients with BC. In males, the abundance of Opitutales and the subordinate family Opitutaceae increased significantly. Klebsiella species among Enterobacteriaceae were more abundant in females. Klebsiella produces toxins (e.g., colibactin) that directly contribute to bladder tumorigenesis [25].
4.1. Microbiota and the diagnosis of BC
Cystoscopic biopsy with pathological diagnosis remains the gold standard for BC diagnosis, but the method is invasive [67]. DNA methylation in specific genes extracted from urine samples is also evaluated in clinical applications, but the validity of the results is not satisfactory [68,69]. With the development of the BC microbiome, differential microbiome analysis of the urine microbiome, a painless diagnostic method, has garnered the attention of researchers.
At present, most studies on the urine or tissue microbiota of patients with BC are retrospective in nature, with a small sample size and conducted at a single institution. Wu et al. analyzed the microbiome in the midstream urine of 31 male patients with BC and 18 healthy controls. The results showed that the microbiota abundance increased significantly in patients with BC. The researchers found that patients with BC had higher abundances of Acinetobacter, Anaerococcus and Sphingobacterium, but lower abundances of Serratia, Proteus, and Roseomonas. The abundances of microbiota were greater in the urine of patients with a higher risk of clinical progression and recurrence [16]. Bi et al. showed that the dysbiosis of urinary microbiota may play an important role in BC development. The researchers found that the abundance of Streptococcus, Bifidobacterium, Lactobacillus, Veillonella, and Actinobacteria differed significantly between patients with tumors and healthy controls. Additionally, the abundance of Actinomycetes was higher in patients with BC. These results suggest that the higher abundance of Actinomycetes may serve as a diagnostic marker for BC [17]. Hussein et al. compared the differences in the NMIBC and MIBC microbiota. The researchers observed a significant increase in the abundance of Haemophilus and Veillonella in the urine of patients with MIBC. Meanwhile, Cupriavidus was significantly more abundant in the urine of patients with NMIBC [28]. BučevićPopović et al. analyzed the midstream urine of 12 patients with BC and 11 age-matched healthy controls. They found Fusobacterium nucleatum in 26% of BC samples [13]. These results suggest that a higher microbial load may be a potential risk factor for BC recurrence or progression.
Meanwhile, Chipollini et al. arrived at a contradictory result. They performed 16S sequencing on samples from 38 patients with BC and ten healthy controls. The microbial diversity in the urine of patients with BC was significantly lower, and Bacteroides and Faecalibacterium were significantly enriched [21]. The results of BC tissue sequencing by Liu et al. [18] also suggested that BC tissues have a low species richness and diversity. Therefore, based on the varying results reported in different studies, we should conduct prospective studies on different ethnic groups and larger samples.
Smoking is a clear inducer of BC occurrence and development [70,71]. We conducted a retrospective study of 15 patients with BC and 11 healthy controls. The results showed a significant difference in the abundance of microbiota between smokers and non-smokers (P < 0.001). The α-diversity in smokers was significantly higher among patients with BC, and the abundance of bacteroidaceae, erysipelotrichales and lachnospiraceae was considerably higher in the urine samples of smokers than in those of non-smokers [27]. However, Moynihan et al. performed a 16S RNA analysis of 43 patients with hematuria in the US, including eight patients diagnosed with BC. The results indicated no significant difference in microbial diversities between patients with BC and other hematuria patients. In a subgroup analysis of eight patients with BC, no significant differences were observed in the urinary microbial diversity among patients, regardless of the smoking status [20]. Based on these findings, we are conducting more in-depth research on the correlation between smoking, the bladder microbiome, and BC.
The frequent recurrence of non-muscle-invasive BC and the easy progression of muscle-invasive BC are important biological characteristics of BC [72]. This is an interesting topic that warrants further investigation: Are there specific microbiotas that influence BC recurrence and progression? Mai et al. investigated the common core microbiota in the urine of 24 patients with BC in combination with data from published research datasets. Compared to those of healthy people, the urine samples of patients with BC had significantly higher abundances of some common core microbes, such as Acinetobacter. Acinetobacter can promote tumor metastasis by participating in biofilm formation and epithelial cell adhesion and invasion and promoting phospholipid degradation in the mucosal barrier [19]. Zeng et al. studied 62 patients with BC and conducted 16S rRNA analysis to detect changes in the patient’s microbiome. Species diversity in the microbiome was significantly higher in patients with BC recurrence after 1-year follow-up, and the abundances of Micrococcus and Brevibacterium were higher in patients with recurrence [26]. Likewise, an interesting follow-up study was conducted by Herr et al. They observed 387 cases of low-grade NMIBC and assessed the association of asymptomatic bacteriuria (ABU) with tumor recurrence. Seventy-five percent of patients with ABU survived tumor-free at 3 years, compared with only 40% of uninfected patients. This suggests that prolonged asymptomatic bacteriuria may activate the immune system [73]. Another study on Escherichia coli showed that it can promote the progression of BC T24 cells through epithelial-mesenchymal transformation and metabolic reprogramming [74]. As our understanding of the molecular subtypes of BC continues to expand, we anticipate a certain correlation between microbiome composition or diversity and BC molecular subtypes; this theory requires further research.
4.2. Microbiota and the treatment of BC
BC is second only to lung cancer with respect to the tumor mutational burden (TMB) [75]. Increasing evidence suggests that the microbiota and its bioactive metabolites exhibit immunomodulatory functions and may be involved in regulating the immune microenvironment of solid tumors, including BC [76]. The immune system rarely triggers early carcinogenesis but may promote tumor progression through the immune surveillance of tumor interstitial feedback loops, inflammation, or dysfunction [77]. Chen et al. investigated the relationship between urinary microbiota and PD-L1 expression in 28 patients with NMIBC. Urinary microbiota was shown to exhibit greater species abundance in nine PD-L1-positive patients. The abundance of microbial species gradually increased with the number of PD-L1-positive cells. The expanded microbiota included Leptotrichia, Roseomonas, and Propionibacterium. Concurrently, the researchers also observed a decline in the abundance of Prevotella and Massilia. This result showed a significant correlation between microbiota enrichment and PD-L1 expression in NMIBC [78]. Qiu et al. observed whether the urinary microbiota influences FoxP3+ regulatory T cell infiltration and Ki-67 expression and assessed the impact on clinical recurrence and prognosis in 40 male patients with NMIBC. The results showed that Pseudomonas, Staphylococcus, Corynebacterium, and Acinetobacter were significantly enriched in patients with tumor recurrence. Patients with lower urinary microbial diversity had longer recurrence-free survival. FoxP3+ T cell infiltration and Ki-67 expression were significantly higher in patients with tumor recurrence. However, this study revealed that microbial diversity was not associated with the infiltration of FoxP3+ T cells, and the authors proposed that this finding could be related to the small cohort size and the recruitment of only patients with NMIBC [29]. However, this result cannot negate the role of the microbiota in immune regulation in BC. Pederzoli et al. studied the effect of antibiotics on the outcome of immunotherapy in 149 patients with MIBC, the results showed that concurrent use of antibiotics with immunotherapy drugs was associated with lower complete response rates and relapse-free survival. These results also indirectly support the role of microbiota in the immunotherapy of BC [79]. Thus, prospective studies with more samples should be conducted.
For nearly 50 years, the intravesical instillation of BCG (attenuated M. tuberculosis vaccine) has been the mainstay for preventing recurrence in high-risk NMIBC [80]. However, the mechanism by which it prevents tumor recurrence is unclear, and almost 50% of patients show failure in BCG therapy and may even show progression [81]. Recent studies have shown that the potential mechanism of action of BCG in BC treatment may involve BCG promoting the expansion of BCG-specific T cells in peri-vesical lymph nodes and recruiting them to the tumor microenvironment. Meanwhile, BCG can promote the proliferation, activation, and differentiation of CD4+ T cells (but not CD8+ T cells) [82,83]. Chen et al. proposed that BCG needs to interact with the bladder wall to show effectiveness in patients. α5β1, fibronectin, and integrin play key roles in this interaction. α5β1 integrin induces tumor cell cycle arrest. BCG binds to fibronectin through specific binding proteins, thereby inducing NK cells to kill tumors [84,85]. Hussein et al. also assessed differences in the urinary microbiota of patients with BC who received BCG treatment. The results showed that Serratia, Brochothrix, Negativicoccus, Escherichia-Shigella, and Pseudomonas were significantly more abundant in patients with NMIBC responsive to BCG [28].
An important factor affecting the therapeutic effect of BCG is that other microbes potentially colonizing the bladder may interact with the urinary urothelium, thereby affecting the binding of BCG to tumor cells. McMillan et al. observed the enrichment of Lactobacillus iners in urinary microbiota. This bacteria binds to fibronectin with higher affinity than other lactobacilli from vaginal samples or other probiotic strains [86]. Another study showed that commensals and probiotics in humans could inhibit the NF-κB pathway and suppress the ability to release cytokines such as IL-6 to reduce mucosal inflammation. However, this interaction may reduce the efficacy of BCG therapy [87]. It is generally believed that commensal microbiota can protect the host from pathogens, but it may be a “double-edged sword” in case of BCG regulating the immune microenvironment in patients with BC. In 2022, Bieri et al. initiated a prospective study to assess differences in the urinary microbiota of patients (BCG responders vs. non-responders) using 16S rRNA sequencing; the findings of this prospective trial are currently pending [88]. Therefore, in future clinical trials, we should assess the pre-treatment, on-treatment, and post-treatment microbiome.
Given the efficiency of BCG treatment, researchers from the “pre-microbiome” era have attempted to use probiotics as an alternative to BCG treatment, such as intestinal Lactobacillus casei Shirota strain, to reduce BC recurrence [89,90]. However, further clinical research was hindered by confounding factors such as high discontinuation rates and ineffective study design. Another probiotic-based study showed that supplementation with Butyricoccus pullicaecorum, a probiotic that produces butyrate, increased the anti-inflammatory potential of cells. Butyrate mediates antitumor effects on bladder uroepithelial cells via two short-chain fatty acid G protein-coupled receptors, GPR43 and GPR109B. However, the limitation of this study was that the findings were only validated in BC cell lines and mouse models [91]. Meanwhile, He et al. also observed changes in butyrate levels while evaluating the effect of the gut microbiota in BC. These findings indicated gut dysbiosis, reduction of butyrate concentrations, and impairment of the structural integrity of the gut in patients with BC [22,92]. The findings from these studies suggest a potential role of butyrate in the treatment of BC. With the continuous development of microbiome sequencing technologies and advances in basic research, clinical and basic research on probiotic microorganisms such as Lactobacillus should be emphasized.
In recent years, researchers have explored new ways to use microbes to treat BC. Researchers have used bacterial tumor tropism and gene editing techniques to achieve the programmed delivery of recombinant bacteria encoding cytotoxic molecules to tumor-homing bacteria to facilitate their accumulation in tumors [34,93]. Fragelli et al. used recombinant Salmonella to treat BC in mice. The researchers inserted IL-2 or/and TRAIL genes into the genome of attenuated Salmonella strain SL3261 and examined the effect of this Salmonella strain on BC in mice. The recombinant SL3261 strain caused cell membrane damage, increased NO production, and promoted apoptosis in mouse BC M49 cells [94]. Butler et al. also showed that uropathogenic E. coli degraded c-MYC protein and attenuated MYC expression in tumor cells. The investigators identified and vernalized the bacterial protease Lon, a c-MYC-specific protease, in bacterial culture supernatants. They further demonstrated the therapeutic efficacy of recombinant Lon (rLon) protease in a mouse BC model. Lon protease was found to slow tumor progression and improve the survival of patients with BC. New techniques for bacterial recombination or the identification of bacteria-derived proteins offer promising approaches for treating BC [95].
5. Perspectives on the BC microbiome
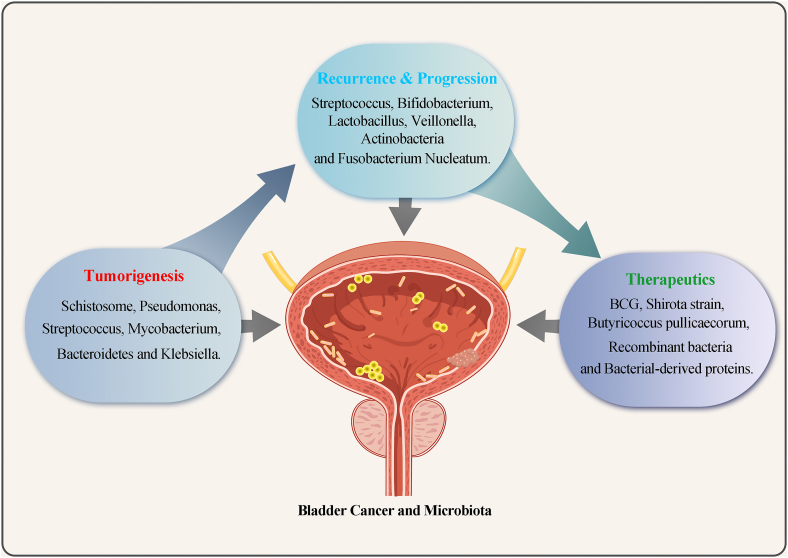
As a “forgotten organ,” the potential role of the microbiota in the development and treatment of BC has only been focused on in the last 10 years (Fig. 3). Improvements and innovations in methods such as NGS, 2bRAD-M, and metabolomics serve as powerful tools for research on the bladder microbiome. The composition and function of the microbiota during tumorigenesis and development, as well as the underlying mechanisms of action, are gradually being elucidated. In the future, with a better understanding of the BC tumor microbiome, we can use the urine microbiome to non-invasively and accurately diagnose tumors and detect recurrence as well as to use and modify the microbiome to treat BC.
Fig. 3.
The bladder microbiota is strongly associated with different statuses of bladder cancer. The increased abundance of microbes such as Schistosoma, Pseudomonas, Streptococcus, Mycobacterium, Bacteroidetes, and Klebsiella was associated with the development of BC during tumorigenesis. Microbes such as Streptococcus, Bifidobacterium, Lactobacillus, Veillonella, Actinobacteria, and Fusobacterium nucleatum may promote the recurrence and progression of BC. BCG, Shirota strain, Butyricoccus pullicaecorum, and others can be beneficial for the treatment of BC, e.g., by preventing recurrence. Recombinant bacteria and bacteria-derived proteins are emerging novel microbiome-based treatment agents for BC.
However, it is important to note that microbiota alterations in tumors may be obscure owing to tumor heterogeneity or the complexity of the tumor microenvironment. Concurrently, we should further investigate the effects of smoking, environmental changes, and antibiotic use on the human microbiome and develop methods to manipulate the urinary microbiome for cancer treatment and improve patient outcomes.
Authors' contributions
ZWT, YFH wrote the manuscript, MSY and WRL Collected Studies. CHT, RYF and ZJF contributed to Figures, WPF and LSH design the table, LW, YY and YXD contributed to ideas of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported in part by a grant from the National Natural Science Foundation of China (#81472389); National Natural Science Foundation of China, Youth Project (#81602469; #81802554, #82101838); Shanghai Pujiang Talent Program (#20PJ1412400) ; General Project of Shanghai Natural Science Foundation of China (#20ZR1443000) and Experimental Animal Fund of Shanghai Science and Technology Commission (#22140903800).
Data availability statement
No data was used for the research described in the article.
Declaration of interest’s statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank OE Biotech Co., Ltd for excellent technical support. We thank Bullet Edits Limited for the linguistic editing of the manuscript.
Contributor Information
Wei Li, Email: weili06@tongji.edu.cn.
Junfeng Zhang, Email: zhangjunfeng@alumni.tongji.edu.cn.
Xudong Yao, Email: yxd@tongji.edu.cn.
Abbreviation
- BC
bladder cancer
- NGS
next generation sequencing
- NMIBC
non-muscle invasive bladder cancer
- MIBC
muscle invasive bladder cancer
- BCG
Bacille Calmette Guérin
- FF
Fresh-Frozen
- FFPE
Formalin-Fixed Paraffin-Embedded
- ABU
asymptomatic bacteriuria
- ROS
reactive oxygen species
- UTIs
urinary tract infections
References
- 1.Hilt E.E., McKinley K., Pearce M.M., Rosenfeld A.B., Zilliox M.J., Mueller E.R., Brubaker L., Gai X., Wolfe A.J., Schreckenberger P.C. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J. Clin. Microbiol. 2014;52:871–876. doi: 10.1128/jcm.02876-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhide A., Tailor V., Khullar V. Interstitial cystitis/bladder pain syndrome and recurrent urinary tract infection and the potential role of the urinary microbiome. Post Reprod. Health. 2020;26:87–90. doi: 10.1177/2053369120936426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Govender Y., Gabriel I., Minassian V., Fichorova R. The current evidence on the association between the urinary microbiome and urinary incontinence in women. Front. Cell. Infect. Microbiol. 2019;9:133. doi: 10.3389/fcimb.2019.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knippel R.J., Drewes J.L., Sears C.L. The cancer microbiome: recent highlights and knowledge gaps. Cancer Discov. 2021;11:2378–2395. doi: 10.1158/2159-8290.Cd-21-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lane G., Gracely A., Bassis C., Greiman S.E., Romo P.B., Clemens J.Q., Gupta P., O’Dell D., Stoffel J.T., Cameron A.P. Distinguishing features of the urinary bacterial microbiome in patients with neurogenic lower urinary tract dysfunction. J. Urol. 2022;207:627–634. doi: 10.1097/ju.0000000000002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 7.Grayson M. Bladder cancer. Nature. 2017;551:S33. doi: 10.1038/551S33a. [DOI] [PubMed] [Google Scholar]
- 8.Antoni S., Ferlay J., Soerjomataram I., Znaor A., Jemal A., Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur. Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Bassett J.C., Gore J.L., Chi A.C., Kwan L., McCarthy W., Chamie K., Saigal C.S. Impact of a bladder cancer diagnosis on smoking behavior. J. Clin. Oncol. 2012;30:1871–1878. doi: 10.1200/jco.2011.36.6518. [DOI] [PubMed] [Google Scholar]
- 10.Hadkhale K., Martinsen J.I., Weiderpass E., Kjaerheim K., Sparen P., Tryggvadottir L., Lynge E., Pukkala E. Occupational exposure to solvents and bladder cancer: a population-based case control study in Nordic countries. Int. J. Cancer. 2017;140:1736–1746. doi: 10.1002/ijc.30593. [DOI] [PubMed] [Google Scholar]
- 11.Lipunova N., Wesselius A., Cheng K.K., van Schooten F.J., Bryan R.T., Cazier J.B., Zeegers M.P. Gene-environment interaction with smoking for increased non-muscle-invasive bladder cancer tumor size. Transl. Androl. Urol. 2020;9:1329–1337. doi: 10.21037/tau-19-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sholl J., Sepich-Poore G.D., Knight R., Pradeu T. Redrawing therapeutic boundaries: microbiota and cancer. Trends Cancer. 2022;8:87–97. doi: 10.1016/j.trecan.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bučević Popović V., Šitum M., Chow C.T., Chan L.S., Roje B., Terzić J. The urinary microbiome associated with bladder cancer. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-29054-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babjuk M., Burger M., Capoun O., Cohen D., Compérat E.M., Dominguez Escrig J.L., Gontero P., Liedberg F., Masson-Lecomte A., Mostafid A.H., et al. European association of urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ) Eur. Urol. 2022;81:75–94. doi: 10.1016/j.eururo.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Xu W., Yang L., Lee P., Huang W.C., Nossa C., Ma Y., Deng F.M., Zhou M., Melamed J., Pei Z. Mini-review: perspective of the microbiome in the pathogenesis of urothelial carcinoma. Am. J. Clin. Exp. Urol. 2014;2:57–61. [PMC free article] [PubMed] [Google Scholar]
- 16.Wu P., Zhang G., Zhao J., Chen J., Chen Y., Huang W., Zhong J., Zeng J. Profiling the urinary microbiota in male patients with bladder cancer in China. Front. Cell. Infect. Microbiol. 2018;8:167. doi: 10.3389/fcimb.2018.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bi H., Tian Y., Song C., Li J., Liu T., Chen Z., Chen C., Huang Y., Zhang Y. Urinary microbiota - a potential biomarker and therapeutic target for bladder cancer. J. Med. Microbiol. 2019;68:1471–1478. doi: 10.1099/jmm.0.001058. [DOI] [PubMed] [Google Scholar]
- 18.Liu F., Liu A., Lu X., Zhang Z., Xue Y., Xu J., Zeng S., Xiong Q., Tan H., He X., et al. Dysbiosis signatures of the microbial profile in tissue from bladder cancer. Cancer Med. 2019;8:6904–6914. doi: 10.1002/cam4.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mai G., Chen L., Li R., Liu Q., Zhang H., Ma Y. Common core bacterial biomarkers of bladder cancer based on multiple datasets. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/4824909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moynihan M., Sullivan T., Provenzano K., Rieger-Christ K. Urinary microbiome evaluation in patients presenting with hematuria with a focus on exposure to tobacco smoke. Res. Rep. Urol. 2019;11:359–367. doi: 10.2147/rru.S233386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chipollini J., Wright J.R., Nwanosike H., Kepler C.Y., Batai K., Lee B.R., Spiess P.E., Stewart D.B., Lamendella R. Characterization of urinary microbiome in patients with bladder cancer: results from a single-institution, feasibility study. Urol. Oncol. 2020;38:615–621. doi: 10.1016/j.urolonc.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 22.He C., Li B., Huang L., Teng C., Bao Y., Ren M., Shan Y. Gut microbial composition changes in bladder cancer patients: a case-control study in Harbin, China. Asia. Pac. J. Clin. Nutr. 2020;29:395–403. doi: 10.6133/apjcn.202007_29(2).0022. [DOI] [PubMed] [Google Scholar]
- 23.Hourigan S.K., Zhu W., W S.W.W., Clemency N.C., Provenzano M., Vilboux T., Niederhuber J.E., Deeken J., Chung S., McDaniel-Wiley K., Trump D. Studying the urine microbiome in superficial bladder cancer: samples obtained by midstream voiding versus cystoscopy. BMC Urol. 2020;20:5. doi: 10.1186/s12894-020-0576-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansour B., Monyók Á., Makra N., Gajdács M., Vadnay I., Ligeti B., Juhász J., Szabó D., Ostorházi E. Bladder cancer-related microbiota: examining differences in urine and tissue samples. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-67443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pederzoli F., Ferrarese R., Amato V., Locatelli I., Alchera E., Lucianò R., Nebuloni M., Briganti A., Gallina A., Colombo R., et al. Sex-specific alterations in the urinary and tissue microbiome in therapy-naïve urothelial bladder cancer patients. Eur. Urol. Oncol. 2020;3:784–788. doi: 10.1016/j.euo.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Zeng J., Zhang G., Chen C., Li K., Wen Y., Zhao J., Wu P. Alterations in urobiome in patients with bladder cancer and implications for clinical outcome: a single-institution study. Front. Cell. Infect. Microbiol. 2020;10 doi: 10.3389/fcimb.2020.555508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma W., Zhang W., Shen L., Liu J., Yang F., Maskey N., Wang H., Zhang J., Yan Y., Yao X. Can smoking cause differences in urine microbiome in male patients with bladder cancer? A retrospective study. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.677605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hussein A.A., Elsayed A.S., Durrani M., Jing Z., Iqbal U., Gomez E.C., Singh P.K., Liu S., Smith G., Tang L., Guru K.A. Investigating the association between the urinary microbiome and bladder cancer: an exploratory study. Urol. Oncol. 2021;39:370.e379. doi: 10.1016/j.urolonc.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Qiu Y., Gao Y., Chen C., Xie M., Huang P., Sun Q., Zhou Z., Li B., Zhao J., Wu P. Deciphering the influence of urinary microbiota on FoxP3+ regulatory T cell infiltration and prognosis in Chinese patients with non-muscle-invasive bladder cancer. Hum. Cell. 2022;35:511–521. doi: 10.1007/s13577-021-00659-0. [DOI] [PubMed] [Google Scholar]
- 30.Locey K.J., Lennon J.T. Scaling laws predict global microbial diversity. Proc. Natl. Acad. Sci. U. S. A. 2016;113:5970–5975. doi: 10.1073/pnas.1521291113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nejman D., Livyatan I., Fuks G., Gavert N., Zwang Y., Geller L.T., Rotter-Maskowitz A., Weiser R., Mallel G., Gigi E., et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368:973–980. doi: 10.1126/science.aay9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majnik A.V., Lane R.H. The relationship between early-life environment, the epigenome and the microbiota. Epigenomics. 2015;7:1173–1184. doi: 10.2217/epi.15.74. [DOI] [PubMed] [Google Scholar]
- 33.Poore G.D., Kopylova E., Zhu Q., Carpenter C., Fraraccio S., Wandro S., Kosciolek T., Janssen S., Metcalf J., Song S.J., et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature. 2020;579:567–574. doi: 10.1038/s41586-020-2095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sepich-Poore G.D., Zitvogel L., Straussman R., Hasty J., Wargo J.A., Knight R. The microbiome and human cancer. Science. 2021;371 doi: 10.1126/science.abc4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wensel C.R., Pluznick J.L., Salzberg S.L., Sears C.L. Next-generation sequencing: insights to advance clinical investigations of the microbiome. J. Clin. Invest. 2022;132 doi: 10.1172/jci154944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bharti R., Grimm D.G. Current challenges and best-practice protocols for microbiome analysis. Briefings Bioinf. 2021;22:178–193. doi: 10.1093/bib/bbz155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y., Qian P.Y. Conservative fragments in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA amplicons in metagenomic studies. PLoS One. 2009;4 doi: 10.1371/journal.pone.0007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jovel J., Patterson J., Wang W., Hotte N., O’Keefe S., Mitchel T., Perry T., Kao D., Mason A.L., Madsen K.L., Wong G.K. Characterization of the gut microbiome using 16S or shotgun metagenomics. Front. Microbiol. 2016;7:459. doi: 10.3389/fmicb.2016.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lam T., Chew D., Zhao H., Zhu P., Zhang L., Dai Y., Liu J., Xu J. Species-resolved metagenomics of kindergarten microbiomes reveal microbial admixture within sites and potential microbial hazards. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.871017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Z., Huang S., Zhu P., Tzehau L., Zhao H., Lv J., Zhang R., Zhou L., Niu Q., Wang X., et al. Species-resolved sequencing of low-biomass or degraded microbiomes using 2bRAD-M. Genome Biol. 2022;23:36. doi: 10.1186/s13059-021-02576-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nallanchakravarthula S., Amruta N., Ramamurthy C. Cancer microbiome; opportunities and challenges. Endocr., Metab. Immune Disord.: Drug Targets. 2021;21:215–229. doi: 10.2174/1871530320999200818134942. [DOI] [PubMed] [Google Scholar]
- 42.Bao Y., Al K.F., Chanyi R.M., Whiteside S., Dewar M., Razvi H., Reid G., Burton J.P. Questions and challenges associated with studying the microbiome of the urinary tract. Ann. Transl. Med. 2017;5:33. doi: 10.21037/atm.2016.12.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oresta B., Braga D., Lazzeri M., Frego N., Saita A., Faccani C., Fasulo V., Colombo P., Guazzoni G., Hurle R., Rescigno M. The microbiome of catheter collected urine in males with bladder cancer according to disease stage. J. Urol. 2021;205:86–93. doi: 10.1097/ju.0000000000001336. [DOI] [PubMed] [Google Scholar]
- 44.Nadler N., Kvich L., Bjarnsholt T., Jensen J.B., Gögenur I., Azawi N. The discovery of bacterial biofilm in patients with muscle invasive bladder cancer. Apmis. 2021;129:265–270. doi: 10.1111/apm.13097. [DOI] [PubMed] [Google Scholar]
- 45.Ozer M.S., Yildiz H.A., Incir C., Deger M.D., Bozkurt O., Ergor G., Tuncok Y., Esen N., Esen A.A. Urinary microbiota; Which non-ınvasive urine collection method should we use? Int. J. Clin. Pract. 2021;75 doi: 10.1111/ijcp.14193. [DOI] [PubMed] [Google Scholar]
- 46.Bundgaard-Nielsen C., Ammitzbøll N., Isse Y.A., Muqtar A., Jensen A.M., Leutscher P.D.C., Arenholt L.T.S., Hagstrøm S., Sørensen S. Voided urinary microbiota is stable over time but impacted by post void storage. Front. Cell. Infect. Microbiol. 2020;10:435. doi: 10.3389/fcimb.2020.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung C.E., Chopyk J., Shin J.H., Lukacz E.S., Brubaker L., Schwanemann L.K., Knight R., Wolfe A.J., Pride D.T. Benchmarking urine storage and collection conditions for evaluating the female urinary microbiome. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-49823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cruz-Flores R., López-Carvallo J.A., Cáceres-Martínez J., Dhar A.K. Microbiome analysis from formalin-fixed paraffin-embedded tissues: current challenges and future perspectives. J. Microbiol. Methods. 2022;196 doi: 10.1016/j.mimet.2022.106476. [DOI] [PubMed] [Google Scholar]
- 49.Flores Bueso Y., Walker S.P., Tangney M. Characterization of FFPE-induced bacterial DNA damage and development of a repair method. Biol. Methods Protoc. 2020;5 doi: 10.1093/biomethods/bpaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borgognone A., Serna G., Noguera-Julian M., Alonso L., Parera M., Català-Moll F., Sanchez L., Fasani R., Paredes R., Nuciforo P. Performance of 16S metagenomic profiling in formalin-fixed paraffin-embedded versus fresh-frozen colorectal cancer tissues. Cancers(Basel) 2021;13 doi: 10.3390/cancers13215421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mostafa M.H., Sheweita S.A., O’Connor P.J. Relationship between schistosomiasis and bladder cancer. Clin. Microbiol. Rev. 1999;12:97–111. doi: 10.1128/cmr.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Honeycutt J., Hammam O., Fu C.L., Hsieh M.H. Controversies and challenges in research on urogenital schistosomiasis-associated bladder cancer. Trends Parasitol. 2014;30:324–332. doi: 10.1016/j.pt.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mantica G., Terrone C., Der Merwe A.V. Bladder cancer and associated risk factors: the african panorama. Eur. Urol. 2021;79:568–570. doi: 10.1016/j.eururo.2020.11.041. [DOI] [PubMed] [Google Scholar]
- 54.Adebayo A.S., Suryavanshi M.V., Bhute S., Agunloye A.M., Isokpehi R.D., Anumudu C.I., Shouche Y.S. The microbiome in urogenital schistosomiasis and induced bladder pathologies. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elinav E., Nowarski R., Thaiss C.A., Hu B., Jin C., Flavell R.A. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer. 2013;13:759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 56.Michaud D.S. Chronic inflammation and bladder cancer. Urol. Oncol. 2007;25:260–268. doi: 10.1016/j.urolonc.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Alfano M., Canducci F., Nebuloni M., Clementi M., Montorsi F., Salonia A. The interplay of extracellular matrix and microbiome in urothelial bladder cancer. Nat. Rev. Urol. 2016;13:77–90. doi: 10.1038/nrurol.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vermeulen S.H., Hanum N., Grotenhuis A.J., Castaño-Vinyals G., van der Heijden A.G., Aben K.K., Mysorekar I.U., Kiemeney L.A. Recurrent urinary tract infection and risk of bladder cancer in the Nijmegen bladder cancer study. Br. J. Cancer. 2015;112:594–600. doi: 10.1038/bjc.2014.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pottegård A., Kristensen K.B., Friis S., Hallas J., Jensen J.B., Nørgaard M. Urinary tract infections and risk of squamous cell carcinoma bladder cancer: a Danish nationwide case-control study. Int. J. Cancer. 2020;146:1930–1936. doi: 10.1002/ijc.32842. [DOI] [PubMed] [Google Scholar]
- 60.Sumitomo T., Nakata M., Higashino M., Terao Y., Kawabata S. Group A streptococcal cysteine protease cleaves epithelial junctions and contributes to bacterial translocation. J. Biol. Chem. 2013;288:13317–13324. doi: 10.1074/jbc.M113.459875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson G.G., Palermo J.J., Schilling J.D., Roth R., Heuser J., Hultgren S.J. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 62.Shoemaker R., Kim J. Urobiome: an outlook on the metagenome of urological diseases. Investig. Clin. Urol. 2021;62:611–622. doi: 10.4111/icu.20210312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wyndaele J.J.J., Riedl C., Taneja R., Lovász S., Ueda T., Cervigni M. GAG replenishment therapy for bladder pain syndrome/interstitial cystitis. Neurourol. Urodyn. 2019;38:535–544. doi: 10.1002/nau.23900. [DOI] [PubMed] [Google Scholar]
- 64.Bilski K., Dobruch J., Kozikowski M., Skrzypczyk M.A., Oszczudłowski M., Ostrowski J. Urobiome in gender-related diversities of bladder cancer. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21124488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pearce M.M., Zilliox M.J., Rosenfeld A.B., Thomas-White K.J., Richter H.E., Nager C.W., Visco A.G., Nygaard I.E., Barber M.D., Schaffer J., et al. The female urinary microbiome in urgency urinary incontinence. Am. J. Obstet. Gynecol. 2015;213 doi: 10.1016/j.ajog.2015.07.009. 347.e341-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lewis D.A., Brown R., Williams J., White P., Jacobson S.K., Marchesi J.R., Drake M.J. The human urinary microbiome; bacterial DNA in voided urine of asymptomatic adults. Front. Cell. Infect. Microbiol. 2013;3:41. doi: 10.3389/fcimb.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tran L., Xiao J.F., Agarwal N., Duex J.E., Theodorescu D. Advances in bladder cancer biology and therapy. Nat. Rev. Cancer. 2021;21:104–121. doi: 10.1038/s41568-020-00313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chai C.A., Yeoh W.S., Rajandram R., Aung K.P., Ong T.A., Kuppusamy S., Nazran A., Kumaran K., Razack A.H.A., Teoh J.Y. Comparing CxBladder to urine cytology as adjunct to cystoscopy in surveillance of non-muscle invasive bladder cancer-A pilot study. Front Surg. 2021;8 doi: 10.3389/fsurg.2021.659292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gontero P., Montanari E., Roupret M., Longo F., Stockley J., Kennedy A., Rodriguez O., McCracken S.R.C., Dudderidge T., Sieverink C., et al. Comparison of the performances of the ADXBLADDER test and urinary cytology in the follow-up of non-muscle-invasive bladder cancer: a blinded prospective multicentric study. BJU Int. 2021;127:198–204. doi: 10.1111/bju.15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freedman N.D., Silverman D.T., Hollenbeck A.R., Schatzkin A., Abnet C.C. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306:737–745. doi: 10.1001/jama.2011.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teoh J.Y., Huang J., Ko W.Y., Lok V., Choi P., Ng C.F., Sengupta S., Mostafid H., Kamat A.M., Black P.C., et al. Global trends of bladder cancer incidence and mortality, and their associations with tobacco use and gross domestic product per capita. Eur. Urol. 2020;78:893–906. doi: 10.1016/j.eururo.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 72.Chamie K., Litwin M.S., Bassett J.C., Daskivich T.J., Lai J., Hanley J.M., Konety B.R., Saigal C.S. Recurrence of high-risk bladder cancer: a population-based analysis. Cancer. 2013;119:3219–3227. doi: 10.1002/cncr.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herr H., Donat M. Reduced recurrence of low-grade papillary bladder tumors associated with asymptomatic bacteriuria. Urology. 2019;124:179–182. doi: 10.1016/j.urology.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 74.Abd-El-Raouf R., Ouf S.A., Gabr M.M., Zakaria M.M., El-Yasergy K.F., Ali-El-Dein B. Escherichia coli foster bladder cancer cell line progression via epithelial mesenchymal transition, stemness and metabolic reprogramming. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-74390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McGrail D.J., Pilié P.G., Rashid N.U., Voorwerk L., Slagter M., Kok M., Jonasch E., Khasraw M., Heimberger A.B., Lim B., et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 2021;32:661–672. doi: 10.1016/j.annonc.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bersanelli M., Santoni M., Ticinesi A., Buti S. The urinary microbiome and anticancer immunotherapy: the potentially hidden role of unculturable microbes. Targeted Oncol. 2019;14:247–252. doi: 10.1007/s11523-019-00643-7. [DOI] [PubMed] [Google Scholar]
- 77.Huang X., Pan T., Yan L., Jin T., Zhang R., Chen B., Feng J., Duan T., Xiang Y., Zhang M., et al. The inflammatory microenvironment and the urinary microbiome in the initiation and progression of bladder cancer. Genes Dis. 2021;8:781–797. doi: 10.1016/j.gendis.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen C., Huang Z., Huang P., Li K., Zeng J., Wen Y., Li B., Zhao J., Wu P. Urogenital microbiota:potentially important determinant of PD-L1 expression in male patients with non-muscle invasive bladder cancer. BMC Microbiol. 2022;22:7. doi: 10.1186/s12866-021-02407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pederzoli F., Bandini M., Raggi D., Marandino L., Basile G., Alfano M., Colombo R., Salonia A., Briganti A., Gallina A., et al. Is there a detrimental effect of antibiotic therapy in patients with muscle-invasive bladder cancer treated with neoadjuvant pembrolizumab? Eur. Urol. 2021;80:319–322. doi: 10.1016/j.eururo.2021.05.018. [DOI] [PubMed] [Google Scholar]
- 80.Redelman-Sidi G., Glickman M.S., Bochner B.H. The mechanism of action of BCG therapy for bladder cancer-a current perspective. Nat. Rev. Urol. 2014;11:153–162. doi: 10.1038/nrurol.2014.15. [DOI] [PubMed] [Google Scholar]
- 81.Li R., Lerner S.P., Kamat A.M. The who, what, when, where, and why of Bacillus calmette-guérin-unresponsive bladder cancer. Eur. Urol. 2021;79:437–439. doi: 10.1016/j.eururo.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 82.Biot C., Rentsch C.A., Gsponer J.R., Birkhäuser F.D., Jusforgues-Saklani H., Lemaître F., Auriau C., Bachmann A., Bousso P., Demangel C., et al. Preexisting BCG-specific T cells improve intravesical immunotherapy for bladder cancer. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3003586. [DOI] [PubMed] [Google Scholar]
- 83.Li R., Gilbert S.M., Kamat A.M. Unraveling the mechanism of the antitumor activity of Bacillus calmette-guérin. Eur. Urol. 2021;80:1–3. doi: 10.1016/j.eururo.2020.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen F., Zhang G., Cao Y., Wakim B., See W.A. A synthetic polyvalent ligand for α5β1 integrin activates components of the urothelial carcinoma cell response to bacillus Calmette-Guérin. J. Urol. 2013;189:1104–1109. doi: 10.1016/j.juro.2012.08.218. [DOI] [PubMed] [Google Scholar]
- 85.Whiteside S.A., Razvi H., Dave S., Reid G., Burton J.P. The microbiome of the urinary tract-a role beyond infection. Nat. Rev. Urol. 2015;12:81–90. doi: 10.1038/nrurol.2014.361. [DOI] [PubMed] [Google Scholar]
- 86.McMillan A., Macklaim J.M., Burton J.P., Reid G. Adhesion of Lactobacillus iners AB-1 to human fibronectin: a key mediator for persistence in the vagina? Reprod. Sci. 2013;20:791–796. doi: 10.1177/1933719112466306. [DOI] [PubMed] [Google Scholar]
- 87.Cosseau C., Devine D.A., Dullaghan E., Gardy J.L., Chikatamarla A., Gellatly S., Yu L.L., Pistolic J., Falsafi R., Tagg J., Hancock R.E. The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect. Immun. 2008;76:4163–4175. doi: 10.1128/iai.00188-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bieri U., Scharl M., Sigg S., Szczerba B.M., Morsy Y., Rüschoff J.H., Schraml P.H., Krauthammer M., Hefermehl L.J., Eberli D., Poyet C. Prospective observational study of the role of the microbiome in BCG responsiveness prediction (SILENT-EMPIRE): a study protocol. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2022-061421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Naito S., Koga H., Yamaguchi A., Fujimoto N., Hasui Y., Kuramoto H., Iguchi A., Kinukawa N. Prevention of recurrence with epirubicin and lactobacillus casei after transurethral resection of bladder cancer. J. Urol. 2008;179:485–490. doi: 10.1016/j.juro.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 90.Seow S.W., Rahmat J.N., Mohamed A.A., Mahendran R., Lee Y.K., Bay B.H. Lactobacillus species is more cytotoxic to human bladder cancer cells than Mycobacterium Bovis (bacillus Calmette-Guerin) J. Urol. 2002;168:2236–2239. doi: 10.1097/01.ju.0000034353.97729.69. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y.C., Ku W.C., Liu C.Y., Cheng Y.C., Chien C.C., Chang K.W., Huang C.J. Supplementation of probiotic butyricicoccus pullicaecorum mediates anticancer effect on bladder urothelial cells by regulating butyrate-responsive molecular signatures. Diagnostics. 2021;11 doi: 10.3390/diagnostics11122270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mirzaei R., Afaghi A., Babakhani S., Sohrabi M.R., Hosseini-Fard S.R., Babolhavaeji K., Khani Ali Akbari S., Yousefimashouf R., Karampoor S. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed. Pharmacother. 2021;139 doi: 10.1016/j.biopha.2021.111619. [DOI] [PubMed] [Google Scholar]
- 93.Zheng J.H., Nguyen V.H., Jiang S.N., Park S.H., Tan W., Hong S.H., Shin M.G., Chung I.J., Hong Y., Bom H.S., et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aak9537. [DOI] [PubMed] [Google Scholar]
- 94.de Lima Fragelli B.D., Camillo L., de Almeida Rodolpho J.M., de Godoy K.F., de Castro C.A., Brassolatti P., da Silva A.J., Borra R.C., de Freitas Anibal F. Antitumor effect of IL-2 and TRAIL proteins expressed by recombinant Salmonella in murine bladder cancer cells. Cell. Physiol. Biochem. 2021;55:460–476. doi: 10.33594/000000398. [DOI] [PubMed] [Google Scholar]
- 95.Butler D.S.C., Cafaro C., Putze J., Wan M.L.Y., Tran T.H., Ambite I., Ahmadi S., Kjellström S., Welinder C., Chao S.M., et al. A bacterial protease depletes c-MYC and increases survival in mouse models of bladder and colon cancer. Nat. Biotechnol. 2021;39:754–764. doi: 10.1038/s41587-020-00805-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.