Abstract
One of the central signaling pathways with a regulatory effect on cell proliferation and survival is Akt/mTOR. In many human cancer types, for instance, lung cancer, the overexpression of Akt/mTOR has been reported. For this reason, either targeting cancer cells by synthetic or natural products affecting the Akt/mTOR pathway down-regulation is a useful strategy in cancer therapy. Direct inhibition of the signaling pathway or modulation of each related molecule could have significant feedback on the growth and proliferation of cancer cells. A variety of secondary metabolites has been identified to directly inhibit the AKT/mTOR signaling, which is important in the field of drug discovery. Naturally occurring nitrogenous and phenolic compounds can emerge as two pivotal classes of natural products possessing anticancer abilities. Herein, we have summarized the alkaloids and flavonoids for lung cancer treatment together with all the possible mechanisms of action relying on the Akt/mTOR pathway down-regulation. This review suggested that in search of new drugs, phytochemicals could be considered as promising scaffolds to be developed into efficient drugs for the treatment of cancer. In this review, the terms "Akt/mTOR", "Alkaloid", "flavonoid", and "lung cancer" were searched without any limitation in search criteria in Scopus, PubMed, Web of Science, and Google scholar engines.
Keywords: Alkaloids, Cancer treatment, Drug discovery, Flavonoids, Lung cancer, PI3K/Akt/mTOR
Introduction
Lung cancer is now the most commonly identified solid tumor and the most common cause of cancer death worldwide. It is estimated that there are 1.59 million deaths from lung cancer every year.1 Despite the use of various strategies for treating lung cancer, these strategies are not satisfactory and the recovery of these patients is not as expected.2,3 There are two main forms of lung cancer: small-cell lung cancer (SCLC; about 15% of all lung cancer) and non-small-cell lung cancer (NSCLC; about 85% of all lung cancer). Despite developments in standard treatment and early detection, NSCLC has a weak prognosis and is mostly diagnosed at an advanced stage. This kind of lung cancer includes three major histologic subtypes: large-cell, adenocarcinoma, and squamous cell carcinoma.4,5 Protein kinase B (PKB), or Akt, is a serine/threonine protein kinase. Akt molecule as an essential effector has a critical role in specific cellular processes such as metabolism, proliferation, regulating cell growth, and survival.6 Activated Akt agitates various processes including translation of proteins and their modification via activating its downstream proteins.7 Reducing cell cycle inhibitors by activated Akt increases cell processes and cell cycle activity. This can reduce apoptosis by inactivating cell death protease and suppressing pro-apoptotic proteins.8,9 Activated Akt is one of the important kinases that contribute to various cancers as well as lung cancer. Lung cancer treatment through various strategies and compounds has been investigated in different studies. Plants have provided phytochemicals or secondary metabolites that are increasingly used against different cancers. Among the secondary compounds, flavonoids and alkaloids are of interest in terms of therapeutic activities. Flavonoids are polyphenolic compounds found in plant-based food and many medicinal plants. They are divided into several subclasses including anthocyanins, flavonols, flavan-3-ols, flavones, flavanones, proanthocyanidins, and isoflavones.10 Flavonoids indicated potent anti-cancer activity against various cancer models in vitro and in vivo, mediated through regulating key signaling pathways involved in the migration and invasion of cancer cells and the metastatic process.11 They also play a key role in preventing lung cancer. Two clinical trials were undertaken on the patients with lung cancer who received flavonoids epigallocatechin gallate and polyphenon E and, treatment with these compounds resulted in decreased radiation therapy oncology group (RTOG) scores and pain scores compared to the baseline.12 Alkaloids represent a diverse chemical group with at least one basic nitrogen atom in their structures. They are specifically distributed in higher plants. These alkaloids are used for drug discovery and most of them show anti-proliferative and anticancer effects on various cancers, both in vivo and in vitro.13,14 Alkaloids may promote cytostatics in drug-resistant cancer cells, induce cell cycle arrest, and be involved in inhibiting tumor metastasis and proliferation.15 Some of them, such as camptothecin and vinblastine, have been used as chemotherapeutic agents. Moreover, the National Cancer Institute of Canada JBR.10 trial showed that a Vinca alkaloid, vinorelbine plus cisplatin, improved overall survival for stage IB–II NSCLC. Cisplatin is a chemotherapeutic drug commonly used for the first-line treatment of cancers including lung, bladder, ovarian, testicular, etc.16 One of the most attributed mechanisms to alkaloids and flavonoids is their ability in the down-regulation of the Akt/mTOR signaling pathway in cancer cells. Although there are review articles aimed at underacting the role of this pathway in cancer, there has been no comprehensive review on the secondary metabolites that affect this target in a specific type of cancer up to now. In this review, therefore, we pointed out the inhibitors of the Akt/mTOR signaling pathway (by focusing on alkaloids and flavonoids) in different lung cancer cell lines in detail. Moreover, the mentioned bioactive compounds were structurally classified here, which that can give insight to drug discovery researchers to find out chemical scaffolds with the Akt/mTOR inhibitory property.
Akt/mTOR signaling pathway and its role in lung cancer cells
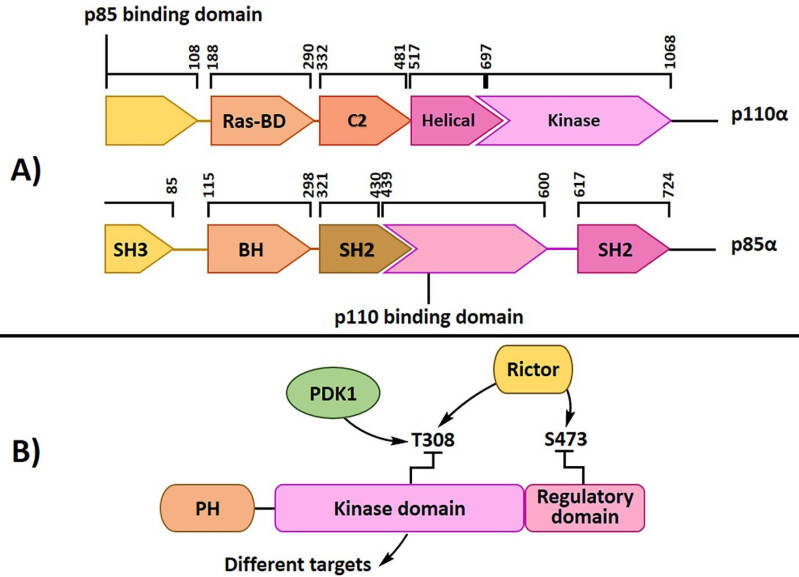
Phosphatidylinositol 3-kinase (PI3-K) is a heterodimer enzyme with p110 catalytic and p85 regulatory subunits (Figure 1A). There are two types of this enzyme (IA and IB), which are different in their subunits. Receptor tyrosine kinase activates class I PI3K with the association of its through one or two SH2 domains in the adaptor unit binding to phosphotyrosine consensus motifs. PI3-K catalyzes the phosphorylation of lipid substrate phosphatidylinositols, such as PI (4,5), P2, and PI (4) P, and the conversion into PI-3,4,5-P3 (PI3P), which activates downstream signaling pathways by the phosphorylation of various kinases such as PDK and Akt.17 PKB, or Akt, is a serine/threonine-protein kinase that regulates cell survival, proliferation, growth, apoptosis, and glycogen metabolism.18,19 Akt has a short carboxyl-terminal regulatory domain (RT), a crucial catalytic domain, and an amino-terminal Pleckstrin-homology (PH) domain (Figure 1B).2,20 Activated PI3K recruits Akt through direct interaction with its PH domain; then, another PH domain-containing 3-phosphoinositide-dependent protein kinase (PDK), serine/threonine kinase, phosphorylates Akt on serine 473 (S473) and threonine 308 (T308) and causes AKT activation.21,22 Afterwards, Akt can phosphorylate a variety of downstream protein substrates, including Bcl-2-associated death promoter (BAD), glycogen synthase kinase-3β (GSK-3β), forkhead in rhabdomyosarcoma, and mouse double minute 2 homolog (Mdm2).23 Activated Akt stimulates protein translation and cell cycle processing; it also reduces apoptosis by suppressing pro-apoptotic proteins.8 According to various studies, Akt is one of the most hyperactive kinases in different human cancers such as lung cancer. Akt phosphorylation was detected in human bronchial epithelial cells before malignancy24; activated Akt was also discovered in pre-neoplastic bronchial lesions and an increase in the incidence and progression of lung cancer.25 Phosphorylation and hyperactivation of Akt were detected in 30-75% of NSCLCs.26 Furthermore, phospho-Akt (p-Akt) was shown in 70% of SCLCs patients by immune-histochemical analysis,27 which proved the role of Akt hyperactivation in lung cancer progression. Various hereditary and environmental factors are involved in developing lung cancer. Different studies have shown that Akt has an essential role in lung cancer cells. Malanga et al reported that Akt1 hyperactivity due to E17K point mutation might cause significant progression in these cancers.28 Smoking is the leading cause of lung cancer and 85-90% of patients use tobacco.29 Tobacco carcinogen prompts PI3K-dependent activation of Akt in the lung epithelial cells.30 Moreover, tobacco constituents can activate the PI3K/Akt pathway by activating various upstream signals of PI3K, containing Ras, growth factor tyrosine kinase receptor, and phosphatase tensin homolog deleted on chromosome ten (PTEN). Epidermal growth factor receptor (EGFR) is a main upstream signal of PI3K. Overexpression of EGFR has a close interaction with tobacco use and was detected in bronchial epithelial cells of smokers.31 Over-expression of EGFR is also specified in 40-80% of patients with NSCLCs. Some studies have shown that some mutations in EGFR can lead to its overexpression32 such as point mutation in exon 18, insertion in exon 20, deletion in exon 19, and point mutation in exon 21,33 among which the last two are very important. According to various studies, there is an important linkage between PI3K/Akt and Ras signaling.34 K-ras mutation is responsible for 25% of smoking-associated human lung adenocarcinomas and boosts motility and invasiveness of lung adenocarcinoma cells through Akt activation.35 PTEN is a lipid phosphatase that can negatively regulate the PI3K/Akt pathway via PIP3 dephosphorylation at the plasma membrane.36 When this phosphatase is mutated, it cannot convert PI3P into PIP and the PI3K/Akt signaling pathway will be hyperactive. PTEN mutations are involved in almost 70% of NSCLCs through inactivating mutations37 and cause constitutively activated the PI3K/Akt signaling pathway. Overexpression of various microRNAs, such as miR-21, miR-221, and miR-222,38,39 as well as methylation of PTEN promoter and homogenous deletion of PTEN gene, are the most significant mechanisms for reducing PTEN activity in lung cancer.40 Therefore, different pathways correlate with the hyperactivation of Akt signaling and the growth and progression of lung cancer cells. Regulating various cellular processes plays an important role in lung tumorigenesis by Akt hyperactivation. One of the most essential downstream proteins associated with Akt is the mammalian target of rapamycin (mTOR). The interaction of Akt and mTOR stimulates the growth and survival of cancer cells.41 Activation of the Akt/mTOR signaling pathway induces tumorigenesis via regulating cell growth and progression, protein synthesis, and metabolism. Hyperactivation of Akt/mTOR was shown in 74% of NSCLCs.41 mTOR is composed of two separate complexes, mTOR complex 1 and 2 (mTORC1 and mTORC2).42 Akt activation by various factors is due to the inhibition of tuberous sclerosis complex (TSC 1/2) and activation of mTORC1. By the inactivation of eIF4E binding protein and activation of p70 ribosomal protein S6 kinase (S6K1), mTORC1 can then increase ribosome biogenesis and various protein synthesis that are essential for proliferation, cell cycle growth, angiogenesis, and survival pathways.42 Besides, mTORC2 phosphorylated protein kinase B (Akt) at serine-473 can contribute to cell growth and progression.43 One of the utmost mechanisms for cell cycle progression is the increase in cell cycle promoter cyclin D1 and the inactivation of cell cycle inhibitors, p27 and p21. Cyclin D1 is involved in the transition of G1 to S in the cell cycle. Overexpression of cyclin D1 has been shown in some studies and it has a close correlation with NSCLCs tumorigenesis from the early stage, hence, it can be a molecular biomarker in cancer.44 Activation of the Akt signaling pathway has been shown to increase cell survival through inhibiting some pro-apoptotic proteins, such as BAD, and increasing various anti-apoptotic factors such as survival.45 On the other hand, Akt can inhibit apoptosis by influencing FOXO protein. Activated FOXO proteins are transferred to the cytoplasm from the nucleus and separate from their pro-apoptotic gene targets.46 Activated Akt can also induce FOXO protein degradation through its phosphorylation.47 Based on previous studies, neo-angiogenesis in lung cancer has a close correlation with activated Akt. Hypoxia is an important factor for solid tumors enhancing the hypoxia-inducible factor-1 (HIF-1).48-50 HIF-1 can increase vascular endothelial growth factor (VEGF) expression that is an essential component of endothelial vascular permeability and cell proliferation.51 One of the leading causes of cancer mortality is metastasis that occurs at different rates in various cancers.52 Numerous factors are involved in the development and metastasis. Matrix metalloproteinases (MMPs) show a specific function in cancer metastasis.53 Several types of MMPs, such as MMP-2, MMP-7, and MMP-9, increase in patients with lung cancer.54,55 In some studies, it is observed that Akt signaling activation has the main link to MMPs expression and its regulation.55 Accordingly, the Akt/mTOR pathway targeting lung cancer cells can have useful effects on growth control and proliferation. Herbal compounds with the least side effects and maximum impression on cancer cell progression have been observed by various studies. This review summarizes two main groups of natural compounds, covering diverse structural alkaloids and flavonoids with anticancer properties and their possible mechanisms are reviewed relying on the down-regulation of the Akt/mTOR pathway in lung cancer cells.
Figure 1.
PI3K subunits and domains (A) and Akt domains (B)
Alkaloids and flavonoids as biologically effective metabolites in lung cancer treatment
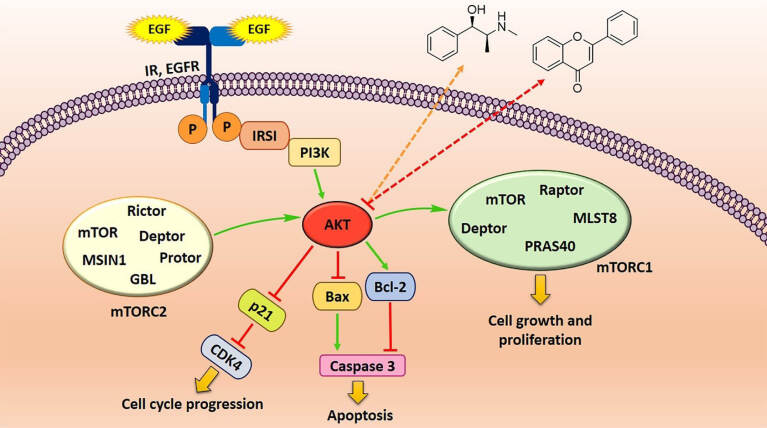
Natural products have a long history in cancer therapy. Several clinically applied phytochemicals or their semisynthetic derivatives include vincristine, vinblastine, etoposide, teniposide, and taxol.56 Flavonoid compounds belong to the polyphenol class of natural products consisting of two aromatic rings (A & B) and an oxygenated heterocyclic ring (C). Depending on the structure of the C ring, they are classified as flavonols, flavones, catechin/condensed tannins, antocyanidine, and isoflavones.57 Flavonoids have been investigated to be potential compounds with a broad spectrum of pharmacological effects including their capacities as anticancer agents. Ligand-receptor interaction and antioxidants activities are considered as the main properties of flavonoids as beneficial agents in health.58 Cancer chemoprevention of flavonoids in many types of cancer, such as lung cancer, arises from their various features including anti-oxidant, anti-proliferation, anti-angiogenesis, anti-metastasis, and immunoregulatory activities.59 Alkaloids are a different group of natural compounds with a cyclic structure containing at least one nitrogen atom. They are distributed in many of the plants belonging to Ranunculaceae, Leguminosae, Loganiaceae, Menispermaceae, and Papaveraceae families.13 A wide variety of biological activities, including anti-malarial, anti-asthmatic, anti-cancer, vasodilatory, anti-arrhythmic, analgesic, anti-bacterial, and anti-hyperglycemic properties, have been reported for alkaloids. Many of alkaloids have been tested for their anticancer activity and some have even been approved by FDA for cancer therapy. Several mechanisms, including DNA cleavage mediated by the inhibition of topoisomerase I & II, induction of mitochondrial permeabilization, mitotic arrest, and inhibition of enzymes, are involved in cell signaling.60 One of the important mechanisms for the function of these compounds in cancer cells is the down-regulation of the PI3K/AKT/mTOR signaling pathway (Figure 2). Therefore, examining their molecular aspects could open a new perspective on the use of these compounds. In the following, we introduce various kinds of natural subclasses belonging to the alkaloid and flavonoid backbones.
Figure 2.
Down-regulation of Akt/mTOR signaling pathway in lung cancer cells by alkaloids and flavonoids
Alkaloids regulate the PI3K/Akt/mTOR pathway in lung cancer
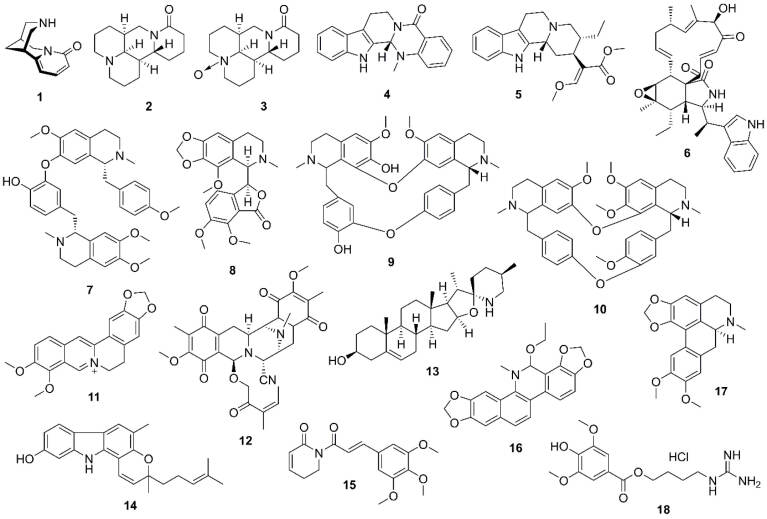
To have a comprehensive literature review, the anti-cancer natural alkaloids targeting the PI3K/Akt/mTOR signaling pathway in lung cancer are categorized according to their chemical classes (Figure 3) while the mechanisms of actions are also summarized for each class of alkaloids where available (Table S1; Supplementary file 1).
Figure 3.
Chemical structures of natural alkaloids (1-18)
Quinolizidine alkaloids
The roots of Papilionaceae and Caesalpinioideaefamiliesare used in traditional Chinese medicine, which contains a quinolizidine alkaloid, cytisine (1) (Figure 3). To study the anti-tumor effects of compound 1 on lung cancer, Xu et al found that 1 caused cell cycle arrest and apoptosis in A549 cells mediated through ROS generation via MAPK/STAT/NF-кB and Akt signaling pathways, respectively.61 Matrine (2) (Figure 3) isolated from the Chinese medicinal plant, Sophora flavescens Aiton (Fabaceae), is responsible for the induction of apoptosis in 95D and A549 cells via Akt inactivation. The total and phosphorylated Akt expression levels were down-regulated by treatment with 2 in a dose-dependent manner. Compound 2 also promoted apoptosis via reducing the expression of c-IAP1 and c-IAP2, the anti-apoptotic proteins.62 Another potential compound found in Sophora species was oxymatrine (3) (Figure 3), which exhibited G0/G1 cell cycle arrest in HCC827 cells by inhibiting EGFR as a tumorigenesis agent in lung cancer cells. Interestingly, Akt inhibition partially required oxymatrine-mediated apoptosis.63
Indole alkaloids
The sonic hedgehog/GLI family zinc finger 1 (SHH/GLI1) signaling pathway is an oncogenesis pathway activated in lung cancer. Evodiamine (4) (Figure 3), an indole alkaloid obtained from Evodia rutaecarpa (Juss.) Benth. (Rutaceae),not only inhibited A549 cell proliferation by decreasing the SHH/GLI1 signaling pathway but also blocked the inhibition of the Akt/NF-кB signaling pathway.64,65 Hirsutine (5) (Figure 3), extracted from Uncaria rhyncophylla (Miq.) Jacks (Rubiaceae), is believed to show anticancer activity in lung cancer cell lines via the mitochondrial apoptosis process. Furthermore, compound 5 interrupted the ROCK1/PTEN/P13K/Akt signaling pathway and consequently led to GSK-3β-mediated mitochondria apoptosis when examined in A549 cells and A549 xenograft mouse model.66 Chaetoglobosin K (6) (Figure 3) is a cytochalasin-derived alkaloid isolated from Diplodia macrospora Earle. It has been reported that 6 inhibits Akt and JNK pathways in H1299 and H2009 cells. Compound 6 also reduces the phosphorylation of Akt (ser473 active site) together with JNK (thr183/tyr185 active site) without any alteration in total Akt kinase and total JNK levels.67
Isoquinoline alkaloids
Neferine (7) (Figure 3) is a major bisbenzylisoquinoline alkaloid of Nelumbo nucifera Gaertn. (also known as Lotus; Nelumbonaceae). Compound 7 induced autophagy in human lung adenocarcinoma cells (A549) through the following two pathways, P13K/Akt/mTOR and ROS hypergeneration. It also caused the acidic vesicular organelle formation and conversion of autophagosome markers, LC3B-І and LC3B-II, which are involved in the canonical form of autophagy. Neferine-induced non-canonical autophagy was conducted through the down-regulation of P13K, Akt, and mTOR, in addition to inhibition of P13K/Akt/mTOR pathway.68 However, the expression of P13K/Akt/mTOR genes was down-regulated by 7 treatment in vivo diethylnitrosamine-induced lung carcinogenesis.69 Moreover, 7 could increase cisplatin-induced autophagy in A549. Significant pieces of evidence were observed when a combination of neferine and cisplatin down-regulated the expression of protein and mRNA of the P13K/Akt/mTOR pathway. These effects not only significantly reduced the phosphorylation of amino acids, Ser473, and Thr308 in Akt protein but also decreased the expression of p85 subunit of P13K protein and mTOR levels.70 Liu et al showed that co-treatment with neferine and ethoxysanguinarine, enhanced respectively cisplatin-induced autophagy and apoptosis in lung cancer cells, associated with down-regulation of PI3K/AKT/mTOR pathway.71 It has been shown that the synergistic effect of cisplatin and noscapine (8) (Figure 3), an opium alkaloid from Papaver somniferumL., the Papaveraceae family, resulted in decreased the Akt expression level, survived proteins, and increased the expression of proapoptotic proteins in H460 cells.72 Mutations of proto-oncogene KRAS are found in 20-30% of patients with NSCLC that can activate downstream pathways, most significantly the RAS-P13K-Akt and RAS-RAF-ERK. Krukovine (9) (Figure 3), which is a bisbenzylisoquinoline alkaloid of Abuta grandifolia (C.Mart.) Sandwith (Menispermaceae),15 effectively targeted the downstream pathways in A549 and H460 cell lines with KRAS mutations. Compound 9 down-regulated RAF-ERK and inhibited the Akt phosphorylation pathway resulting in inducing the G1 apoptosis and arrest in KRAS-mutated lung cancer cells.73 Tetrandrine (10) (Figure 3), a bis-benzylisoquinoline component yielded from Stephania tetrandra S. Moore (Menispermaceae), induced apoptosis and inhibited proliferation in A549 cells by the down-regulation of ERK and Akt phosphorylation.74 Another useful isoquinoline alkaloid of Berberis sp. (Berberidaceae) namely berberine (11) (Figure 3), is used in various biological activities, especially in the field of cancer therapy. It has been studied that compound 11 inhibits the growth of A549 and H1299 cells through several different mechanisms including AP-2/hTERT, Raf/MEK/ERK, NF-кB/COX-2, P13K/Akt, HIF-1α/VEGF, and cytochrome c/caspase.75 Anoikis is known as a process, during which apoptotic cells are detached from the extracellular matrix and neighboring cells. Anoikis-resistant agents are found to develop in metastatic cancer cells, which primarily occur through survival and apoptotic mechanisms including ERK and Akt. Reniermycin M (12) (Figure 3), a bistetrahydroisoquinolinequinone alkaloid isolated from a marine blue sponge, Xestospongia sp., was sensitive to Anoikis-resistant H460 lung cancer cells through suppressing p-ERK, Akt, and total Akt and decreased levels of anti-apoptotic proteins including MCL1 and BCL2.76
Steroidal alkaloids
Overexpression of oncogenic microRNA-21 in lung cancer cells resulted in the invasion of cancerous cells by targeting reversion-inducing cysteine-rich protein with kazal motifs (RECK). Solasodine (13) (Figure 3) is a terpenic aglycone of solanine alkaloid found in eggplant, which inhibited lung cancer cell invasion. Compound 13 potentially down-regulated miR-21 expression and elevated RECK in A549 through the suppression of the P13K/Akt pathway.77
Carbazole alkaloids
Mahanine (14) (Figure 3), a carbazole alkaloid of Murraya koenigii (L.) Sprengel (Rutaceae), defected the gene expression of rapamycin component of m-TORC2 (rictor) in A549 and H1299 cells, and the consequent inhibition of rictor expression caused the reduction of p-Akt and p-mTOR levels.78
Miscellaneous alkaloids
Wang et al studied the effects of Piperlongumine (15) (Figure 3), found in long pepper, against A549 and docetaxel-resistant A549 (A549/DTX) cells. Compound 15 not only induced apoptosis in both cell lines through modulating the P13K/Akt/mTOR79 but also exhibited an anti-proliferative activity in A549 cells by multiple processes, such as decreased expression of CDK6, CDK4, and cyclin D1, declined generation of ROS, inhibition of Akt phosphorylation, and NF-кB inactivation.80 Several kinds of cancers, including NSCLC, are associated with the overexpression of the cancerous inhibitor of protein phosphatase 2A (CIP2A) oncoprotein. CIP2A regulates c-Myc stability through inactivating protein phosphatase 2A (PPA2) and causes pAkt activity. Ethoxysanguinarine (16) (Figure 3) is a benzophenanthridine alkaloid found in Papaveraceae plants such as Macleaya cordata (Willd) R. Br. A study on the effects of 16 towards H1975 and A549 cells showed a down-regulation of CIP2A and CIP2A downstream molecules, pAkt and c-Myc, and activated PP2A. In addition, combining 16 with cisplatin increased apoptosis in lung cancer cell lines.71 Tumor necrosis factor α (TNF-α) promotes the survival and metastasis progressions in lung cancer; therefore, identifying the natural compounds with the ability to suppress TNF-α-induced survival signaling is viable in cancer therapy. Dicentrine (17) (Figure 3), an aporphin alkaloid in various medicinal plants such as Lindera megaphyllaHemsl., significantly enhanced TNF-α-mediated apoptosis in A549 cells through the cleavage of the caspase family and PARP and reduced the expression of anti-apoptotic proteins including cIAP2, c-FLIP, and Bcl-xl. It could also decrease the expression enhancement of TNF-α-induced metastasis-associated proteins and inhibited TNF-α-induced AP-1 and NF-кB activation. In addition, compound 17 blocked the phosphorylation of TNF-α-activated ERK1/2 and Akt signaling pathways.81 Leonurine hydrochloride (18) (Figure 3), the major alkaloid of Leonurus japonicus Houtt. (Lamiaceae), induced apoptosis in H292 cells by the mitochondrial-dependent pathway associated with ROS production and loss of MMP. In addition, it reduced the phosphorylated Akt level.79
Flavonoids regulate the PI3K/Akt/mTOR signaling in lung cancer
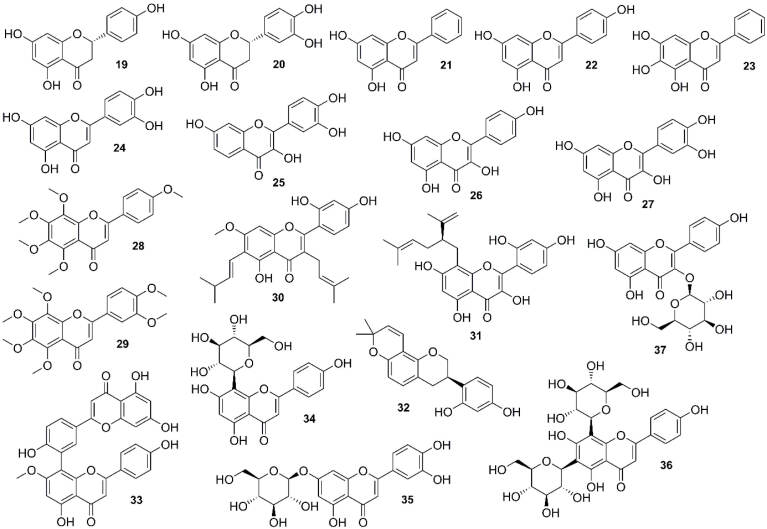
To have a comprehensive literature review, the anti-cancer natural flavonoids targeting the PI3K/Akt/mTOR signaling pathway in lung cancer are categorized according to their chemical classes (Figure 4) while the mechanisms of actions are also summarized for each class of flavonoids where available (Table S2; Supplementary file 1).
Figure 4.
Chemical structures of natural flavonoids (19-37)
Flavanones
An experimental study on a Citrus flavonoid, naringenin (19) (Figure 4), revealed an anti-migration activity in A549 cells, which was associated with reducing MMP-9 and MMP-2 activities and inhibition of Akt activity in a dose-dependent manner.82,83 Eriodictyol (a bitter-masking flavanone; 20) is a common flavonoid of the plant kingdom. Zhang et al showed that compound 20 (Figure 4) constructed A549 cell death by inducing the G2/M cell cycle arrest, mitochondrial apoptosis, and the mTOR/PI3K/Akt pathway suppression.84
Flavones
Chrysin (21) (Figure 4), a widely distributed flavone in medicinal plants, promoted A549 cells’ apoptosis and growth inhibition of Akt/mTOR contributed by AMPK activation in A549 cells.85 Apigenin (22) (Figure 4), another common flavone found in vegetables and fruits, has been considered as a potential agent with anti-invasion, anti-migration, and anti-proliferation activities in A549 cells. It was indicated that apigenin inhibited phosphorylation and activation of Akt as well as the gene expression of Akt downstream including MMP-9, GSK-3β, and HEF1.86 In addition, combined apigenin and TRAIL up-regulated death receptors 4 and 5 levels in a p53-dependent manner resulted in apoptosis in NSCLC cells. This combined product could also suppress ERK activation and translocation of NF-кB and PI3K/Akt pathways.87 Compound 22 could suppress the invasion and migration of lung cancer cells with different EGFRs via inhibiting Snail/Slug-mediated EMT attributed to its capacity in Akt inactivation.88 Inhibition of epithelial-mesenchymal transition (EMT) and induction of apoptosis via PI3K/Akt/NF-кB involved two mechanisms induced by baicalein (23) (Figure 4), a bioactive flavone of Scutellariaspecies (Lamiaceae), to overcome the resistance of lung adenocarcinoma cells to cisplatin.89 EMT is a key process in promoting cancer progression, metastasis, and invasion, promoted through TGF-β, a multifunctional cytokine overexpressed in many types of cancers. One study showed that luteolin (24) (Figure 4), a common dietary flavone, acted as anti-adhesive molecules, and E-cadherin attenuated the activation of the PI3K–Akt–IκBa–NF-κB–Snail pathway.90
Flavonols
Fisetin (25) (Figure 4) is a dietary flavonol, which has shown a selective inhibition of mTOR signaling and PI3K/Akt in NSCLC cells without side effects on normal human bronchial epithelial cells.91 Likewise, effects of kaempferol (26) (Figure 4) were evaluated on A549 cells by Han et al. The authors claimed that compound 26 inhibited proliferation and enhanced autophagy and apoptosis through the PTEN/PI3K/Akt pathway; however, another study reported that the inactivation of MEK/ERK was necessary for kaempferol-induced apoptosis in lung cancer cells.92,93 Another abundant bioflavonoid, quercetin (27) (Figure 4), was claimed to be an anti-metastatic agent on NSCLC cells through Snail-dependent Akt activity up-regulated by maspin and Snail-independent ADAM9 pathways.94 Inhibition of Akt was also involved in sensitizing TRAIL-induced cytotoxicity in NSCLC by 27.95 A study carried out by Nguyen et al revealed quercetin-induced apoptosis in A549 cells by inactivating MEK/ERK and Akt-1 and influencing the expression of the Bcl-2 family of proteins.96 In addition, quercetin-metabolite-enriched plasma obtained from Mongolian gerbils fed with quercetin for 24 hours (100 mg/kg body/wt/week) was shown to reduce the growth of A549 cells and increase PARP-γ expression, which was associated with reduced phosphorylation of Akt.97
Polymethoxy flavones
Polymethoxylated flavone of Citrus fruits peel, tangeretin (28) (Figure 4), suppressed COX-2 expression induced by IL-1β in A549 cells. This effect might be mediated through inhibiting the phosphorylation of JNK, p38 MAPK, and AKT as well as blocking NF-кB translocation.98 Another Citrus polymethoxy flavone, nobiletin (29) (Figure 4), revealed an ability to improve Adriamycin (ADR) resistance of A549/ADR cells. It might also increase ADR accumulation by inhibiting the MRP1 expression via down-regulating the expression of β-catenin, GSK-3β, Akt, and MYCN.83
Isoprenylated flavonoids
Artocarpin (30) (Figure 4), a major flavonoid of Artocarpus species (Moraceae), has been observed to induce apoptosis through Nox2/p47phox activation and enhance ROS generation, which caused PI3K/Akts473/p53-independent activation of the NF-кB/c-Myc/Noxa pathway in H1299 and A549 cells.99 One isolated flavonoid of Sophora flavescens Aiton (Fabaceae), kushenol z (31) (Figure 4), demonstrated an anti-proliferative effect by inhibiting the cAMP-PDE pathway and, subsequently, increasing PKA activity and led to the mTOR pathway inhibition in A549 cells. Additionally, down-regulation of Akt was involved in the inhibition of cell proliferation by 31.100
Prenylated isoflavanes
Glabridin (32) (Figure 4), the flavonoid compound of licorice (Glycyrrhiza glabra L.; Fabaceae), has been reported to have anti-metastasis, anti-migration, and anti-invasion effects on A549 cells, and cooperation of FAK/Src with Akt was considered to have a key role in glabridin-mediated cell migration.101
Biflavones
Wang et al evaluated the anti-metastasis and anti-invasion activity of sotetsuflavone (33) (Figure 4), an isolated flavonoid of Cycas revoluta Thunb. (Cycadaceae), in A549 cells, which occurred by reversing EMT and inhibiting angiogenesis. Compound 33 suppressed TNF-α/NF-кB and PI3K/Akt pathways involved in the down-regulation of HIF-1α with a key role in the anti-transfer activity of 33.102
Flavone and flavonol glycosides
In an experimental study, Liu et alshowed that vitexin (34) (Figure 4), a flavonoid of Crataegus pinnatifida Bunge (Rosaceae), induced apoptosis in A549 cells, partly through PI3K/Akt/mTOR signaling.103 Luteoloside (Figure 4) (also known as cynaroside; 35), found in Gentiana macrophylla Pall. (Gentianaceae), induced autophagy in A549 cells by inhibiting p-p70S6K, p-mTOR, and p-Akt, which were correlated with ROS generation.104 A flavonoid glycoside extracted from Dendrobium officinale Kimura et Migo (Orchidaceae), vicenin II (Figure 4) (vitexin 8-C-glycosyl flavone; 36), inhibited metastasis by suppressing EMT activated by TGF-β1 as the promotion of EMT in A549 cells. Deactivation of PI3K/Akt/mTOR and TGF-β/SMAD pathways was involved in this anti-metastatic activity.105,106
One of the mechanisms involved in the induced apoptosis of lung cancer cells by astragaline (37) (Figure 4), a flavonoid of Rosa agrestis Savi (Rosaceae), is the inhibition of PI3K and Akt phosphorylation, which could inhibit IкBα degradation, p56 translocation, and Bcl-xl and Bcl-2 expression.107
Feature prospective and conclusions
The Akt/mTOR signaling pathway plays an essential role in cell survival, growth, and proliferation. During cancer cell development and tumorigenesis, the up-regulation of Akt/mTOR signaling occurs by either carcinogens or several genetic mutations of upstream regulators. In addition, this pathway could be considered as the main core of all signaling pathways in cancer cells. Associated with various aspects of cancer cells, the Akt/mTOR pathway also includes the malignant progression and radio/chemotherapy resistance in patients with lung cancer. Therefore, targeting the pivotal pathway is one of the most efficient strategies for cancer therapy that can guide researchers to select the potential natural metabolites for further investigations. Numerous clinically approved inhibitors of the Akt/mTOR signaling components are capable of regulating this pathway; however, they are connected with unwanted side effects. Down-regulation of the Akt/mTOR pathway in the early stages of tumor growth could lead to tumor growth inhibition. Targeting each of the upstream proteins of Akt can inhibit or reduce the activity of the Akt/mTOR pathway. Herbal medicines along with their preparations are attractive and increasingly popular in various health care systems. Natural metabolites are the most potential agents because of their eminent efficacy and safety. To the best of our knowledge, we have summarized some classes of natural products, such as alkaloids and flavonoids, possessing anticancer properties through the various mechanisms, especially targeting Akt/mTOR signaling. The most significant flavonoids discussed in the present study showed an Akt regulation in various kinds of lung cancer cells. In addition to the effective activity of aglycone, the activity of flavonoids might be related to their structural features bearing ortho hydroxy groups in the B ring along with meta hydroxy groups in the A ring as well as the carbonyl group in ring C (C-4).108 On the other hand, the backbone of quinolizidine, indole, and isoquinoline alkaloids could be selected as promising compounds for further studies. Taken together, naturally occurring metabolites are now used for developing alternative therapeutic strategies as they increase the action of traditional treatment methods through their multi-targeting capacity. Some studies revealed that the secondary metabolites showed stronger effects against cancer cells in the presence of cisplatin. Hence, a smart combination approach can target the PI3K/AKT/mTOR pathway in lung cancer cells. However, there are significant challenges associated with bioactive the PI3-kinase/Akt/mTOR inhibitors, which have limited their uses in cancer chemoprevention development. For instance, problems associated with flavonoids included their poor extraction yield, complicated purification methods, and pharmacokinetic/pharmacodynamic properties such as bioavailability, drug–drug interactions, and metabolic instability, which can be improved by effective drug delivery systems.109 In the case of alkaloids, inappropriate water solubility and low bioavailability are major challenges limiting their uses in oral administration. Nevertheless, water solubility and efficacy can increase by using semisynthetic and biochemical transformation approaches.13
Overall, the PI3K/Akt/mTOR regulators are of more interest to researchers to unravel the real power of the Akt/mTOR targeting strategy in lung cancer therapy. In the next steps, clinical trials using natural compounds targeting Akt/mTOR in combination with standard treatments are vigorously suggested for future studies.
Acknowledgments
The authors with to thank University of Tehran for logistical supports.
Author Contributions
Conceptualization: Sommayeh Ghareghomi, Shahin Ahmadian.
Investigation: Sommayeh Ghareghomi, Vahideh Atabaki, Naseh Abdollahzadeh.
Project administration: Shahin Ahmadian, Salar Hafez Ghoran.
Resources: Sommayeh Ghareghomi, Vahideh Atabaki, Salar Hafez Ghoran.
Software: Salar Hafez Ghoran.
Supervision: Shahin Ahmadian, Salar Hafez Ghoran
Validation: Shahin Ahmadian, Salar Hafez Ghoran
Visualization: Sommayeh Ghareghomi, Vahideh Atabaki, Salar Hafez Ghoran.
Writing – original draft: Sommayeh Ghareghomi, Vahideh Atabaki, Naseh Abdollahzadeh.
Writing – review & editing: Shahin Ahmadian, Salar Hafez Ghoran.
Ethical Issues
Not applicable.
Conflict of Interests
The authors declare no potential competing interest.
Supplementary Files
Supplementary file 1 contains Tables S1 and S2.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Thomas A, Liu SV, Subramaniam DS, Giaccone G. Refining the treatment of NSCLC according to histological and molecular subtypes. Nat Rev Clin Oncol. 2015;12(9):511–26. doi: 10.1038/nrclinonc.2015.90. [DOI] [PubMed] [Google Scholar]
- 3.Iacono D, Chiari R, Metro G, Bennati C, Bellezza G, Cenci M, et al. Future options for ALK-positive non-small cell lung cancer. Lung Cancer. 2015;87(3):211–9. doi: 10.1016/j.lungcan.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Rahmati-Yamchi M, Ghareghomi S, Haddadchi G, Milani M, Aghazadeh M, Daroushnejad H. Fenugreek extract diosgenin and pure diosgenin inhibit the hTERT gene expression in A549 lung cancer cell line. Mol Biol Rep. 2014;41(9):6247–52. doi: 10.1007/s11033-014-3505-y. [DOI] [PubMed] [Google Scholar]
- 5.Rahmati-Yamchi M, Ghareghomi S, Haddadchi G, Mobasseri M, Rasmi Y. Diosgenin inhibits hTERT gene expression in the A549 lung cancer cell line. Asian Pac J Cancer Prev. 2013;14(11):6945–8. doi: 10.7314/apjcp.2013.14.11.6945. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez E, McGraw TE. The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle. 2009;8(16):2502–8. doi: 10.4161/cc.8.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruggero D, Sonenberg N. The Akt of translational control. Oncogene. 2005;24(50):7426–34. doi: 10.1038/sj.onc.1209098. [DOI] [PubMed] [Google Scholar]
- 8.Kizilboga T, Baskale EA, Yildiz J, Akcay IM, Zemheri E, Can ND, et al. Bag-1 stimulates Bad phosphorylation through activation of Akt and Raf kinases to mediate cell survival in breast cancer. BMC Cancer. 2019;19(1):1254. doi: 10.1186/s12885-019-6477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serrano BP, Hardy JA. Phosphorylation by protein kinase A disassembles the caspase-9 core. Cell Death Differ. 2018;25(6):1025–39. doi: 10.1038/s41418-017-0052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George VC, Dellaire G, Rupasinghe HPV. Plant flavonoids in cancer chemoprevention: role in genome stability. J NutrBiochem. 2017;45:1–14. doi: 10.1016/j.jnutbio.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Liskova A, Koklesova L, Samec M, Smejkal K, Samuel SM, Varghese E, et al. Flavonoids in Cancer Metastasis. Cancers (Basel) 2020;12(6):1498. doi: 10.3390/cancers12061498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zanoaga O, Braicu C, Jurj A, Rusu A, Buiga R, Berindan-Neagoe I. Progress in research on the role of flavonoids in lung cancer. Int J Mol Sci. 2019;20(17):4291. doi: 10.3390/ijms20174291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mondal A, Gandhi A, Fimognari C, Atanasov AG, Bishayee A. Alkaloids for cancer prevention and therapy: current progress and future perspectives. Eur J Pharmacol. 2019;858:172472. doi: 10.1016/j.ejphar.2019.172472. [DOI] [PubMed] [Google Scholar]
- 14.Khattak S, Khan H. Anti-cancer potential of phyto-alkaloids: a prospective review. Curr Cancer Ther Rev. 2016;12(1):66–75. doi: 10.2174/1573394712666160617081638. [DOI] [Google Scholar]
- 15.Hałas-Wiśniewska M, Zielińska W, Izdebska M, Grzanka A. The synergistic effect of piperlongumine and sanguinarine on the non-small lung cancer. Molecules. 2020;25(13):3045. doi: 10.3390/molecules25133045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzáles-Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7(9):719–27. doi: 10.1016/s1470-2045(06)70804-x. [DOI] [PubMed] [Google Scholar]
- 17.Paraskevopoulou MD, Tsichlis PN. A perspective on AKT 25-plus years after its discovery. Sci Signal. 2017;10(486):eaan8791. doi: 10.1126/scisignal.aan8791. [DOI] [PubMed] [Google Scholar]
- 18.Zhang D, Wang A, Feng J, Zhang Q, Liu L, Ren H. Ginsenoside Rg5 induces apoptosis in human esophageal cancer cells through the phosphoinositide-3 kinase/protein kinase B signaling pathway. Mol Med Rep. 2019;19(5):4019–26. doi: 10.3892/mmr.2019.10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le HT, Lee HJ, Cho J, Min HY, Lee JS, Lee SJ, et al. Insulin-like growth factor binding protein-3 exerts its anti-metastatic effect in aerodigestive tract cancers by disrupting the protein stability of vimentin. Cancers (Basel) 2021;13(5):1041. doi: 10.3390/cancers13051041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: at the bench and bedside. Semin Cancer Biol. 2019;59:125–32. doi: 10.1016/j.semcancer.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Xu CX, Jin H, Shin JY, Kim JE, Cho MH. Roles of protein kinase B/Akt in lung cancer. Front Biosci (Elite Ed) 2010;2(4):1472–84. doi: 10.2741/e206. [DOI] [PubMed] [Google Scholar]
- 22.Ghareghomi S, Ahmadian S, Zarghami N, Kahroba H. Fundamental insights into the interaction between telomerase/TERT and intracellular signaling pathways. Biochimie. 2021;181:12–24. doi: 10.1016/j.biochi.2020.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Song M, Bode AM, Dong Z, Lee MH. AKT as a therapeutic target for cancer. Cancer Res. 2019;79(6):1019–31. doi: 10.1158/0008-5472.can-18-2738. [DOI] [PubMed] [Google Scholar]
- 24.Hsiao YT, Fan MJ, Huang AC, Lien JC, Lin JJ, Chen JC, et al. Deguelin impairs cell adhesion, migration and invasion of human lung cancer cells through the NF-κ B signaling pathways. Am J Chin Med. 2018;46(1):209–29. doi: 10.1142/s0192415x1850012x. [DOI] [PubMed] [Google Scholar]
- 25.Zheng X, Lu G, Yao Y, Gu W. An autocrine IL-6/IGF-1R loop mediates EMT and promotes tumor growth in non-small cell lung cancer. Int J Biol Sci. 2019;15(9):1882–91. doi: 10.7150/ijbs.31999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Padala RR, Karnawat R, Viswanathan SB, Thakkar AV, Das AB. Cancerous perturbations within the ERK, PI3K/Akt, and Wnt/β-catenin signaling network constitutively activate inter-pathway positive feedback loops. Mol Biosyst. 2017;13(5):830–40. doi: 10.1039/c6mb00786d. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Xu C, Xu B, Li L, Li W, Wang W, et al. Xiaoai Jiedu Recipe inhibits proliferation and metastasis of non-small cell lung cancer cells by blocking the P38 mitogen-activated protein kinase (MAPK) pathway. Med Sci Monit. 2019;25:7538–46. doi: 10.12659/msm.917115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malanga D, Scrima M, De Marco C, Fabiani F, De Rosa N, De Gisi S, et al. Activating E17K mutation in the gene encoding the protein kinase AKT1 in a subset of squamous cell carcinoma of the lung. Cell Cycle. 2008;7(5):665–9. doi: 10.4161/cc.7.5.5485. [DOI] [PubMed] [Google Scholar]
- 29.Gridelli C, Maione P, Rossi A. The potential role of mTOR inhibitors in non-small cell lung cancer. Oncologist. 2008;13(2):139–47. doi: 10.1634/theoncologist.2007-0171. [DOI] [PubMed] [Google Scholar]
- 30.Du X, Qi F, Lu S, Li Y, Han W. Nicotine upregulates FGFR3 and RB1 expression and promotes non-small cell lung cancer cell proliferation and epithelial-to-mesenchymal transition via downregulation of miR-99b and miR-192. Biomed Pharmacother. 2018;101:656–62. doi: 10.1016/j.biopha.2018.02.113. [DOI] [PubMed] [Google Scholar]
- 31.Merikallio H, Kaarteenaho R, Lindén S, Padra M, Karimi R, Li CX, et al. Smoking-associated increase in mucins 1 and 4 in human airways. Respir Res. 2020;21(1):239. doi: 10.1186/s12931-020-01498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.An Z, Aksoy O, Zheng T, Fan QW, Weiss WA. Epidermal growth factor receptor and EGFRvIII in glioblastoma: signaling pathways and targeted therapies. Oncogene. 2018;37(12):1561–75. doi: 10.1038/s41388-017-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inamura K, Ninomiya H, Ishikawa Y, Matsubara O. Is the epidermal growth factor receptor status in lung cancers reflected in clinicopathologic features? Arch Pathol Lab Med. 2010;134(1):66–72. doi: 10.5858/2008-0586-rar1.1. [DOI] [PubMed] [Google Scholar]
- 34.Memmott RM, Dennis PA. The role of the Akt/mTOR pathway in tobacco carcinogen-induced lung tumorigenesis. Clin Cancer Res. 2010;16(1):4–10. doi: 10.1158/1078-0432.ccr-09-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Román M, Baraibar I, López I, Nadal E, Rolfo C, Vicent S, et al. KRAS oncogene in non-small cell lung cancer: clinical perspectives on the treatment of an old target. Mol Cancer. 2018;17(1):33. doi: 10.1186/s12943-018-0789-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuurbiers OC, Kaanders JH, van der Heijden HF, Dekhuijzen RP, Oyen WJ, Bussink J. The PI3-K/AKT-pathway and radiation resistance mechanisms in non-small cell lung cancer. J Thorac Oncol. 2009;4(6):761–7. doi: 10.1097/JTO.0b013e3181a1084f. [DOI] [PubMed] [Google Scholar]
- 37.Perumal E, So Youn K, Sun S, Seung-Hyun J, Suji M, Jieying L, et al. PTEN inactivation induces epithelial-mesenchymal transition and metastasis by intranuclear translocation of β-catenin and snail/slug in non-small cell lung carcinoma cells. Lung Cancer. 2019;130:25–34. doi: 10.1016/j.lungcan.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 38.Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K, Yang GH. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC) Clin Chim Acta. 2010;411(11-12):846–52. doi: 10.1016/j.cca.2010.02.074. [DOI] [PubMed] [Google Scholar]
- 39.Mayoral RJ, Pipkin ME, Pachkov M, van Nimwegen E, Rao A, Monticelli S. MicroRNA-221-222 regulate the cell cycle in mast cells. J Immunol. 2009;182(1):433–45. doi: 10.4049/jimmunol.182.1.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lapthanasupkul P, Klongnoi B, Mutirangura A, Kitkumthorn N. Investigation of PTEN promoter methylation in ameloblastoma. Med Oral Patol Oral Cir Bucal. 2020;25(4):e481–e7. doi: 10.4317/medoral.23498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montor WR, Salas A, Melo FHM. Receptor tyrosine kinases and downstream pathways as druggable targets for cancer treatment: the current arsenal of inhibitors. Mol Cancer. 2018;17(1):55. doi: 10.1186/s12943-018-0792-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibbons JJ, Abraham RT, Yu K. Mammalian target of rapamycin: discovery of rapamycin reveals a signaling pathway important for normal and cancer cell growth. Semin Oncol. 2009;36 Suppl 3:S3–S17. doi: 10.1053/j.seminoncol.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 43.Li X, Wu C, Chen N, Gu H, Yen A, Cao L, et al. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget. 2016;7(22):33440–50. doi: 10.18632/oncotarget.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, Yang MQ, Lei L, Fei LR, Zheng YW, Huang WJ, et al. Overexpression of KRT17 promotes proliferation and invasion of non-small cell lung cancer and indicates poor prognosis. Cancer Manag Res. 2019;11:7485–97. doi: 10.2147/cmar.s218926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martínez-García D, Manero-Rupérez N, Quesada R, Korrodi-Gregório L, Soto-Cerrato V. Therapeutic strategies involving survivin inhibition in cancer. Med Res Rev. 2019;39(3):887–909. doi: 10.1002/med.21547. [DOI] [PubMed] [Google Scholar]
- 46.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24(50):7410–25. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Ao X, Ding W, Ponnusamy M, Wu W, Hao X, et al. Critical role of FOXO3a in carcinogenesis. Mol Cancer. 2018;17(1):104. doi: 10.1186/s12943-018-0856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol (1985) 2000;88(4):1474–80. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 49.Huang J, Gao L, Li B, Liu C, Hong S, Min J, et al. Knockdown of hypoxia-inducible factor 1α (HIF-1α) promotes autophagy and inhibits phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway in ovarian cancer cells. Med Sci Monit. 2019;25:4250–63. doi: 10.12659/msm.915730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soni S, Padwad YS. HIF-1 in cancer therapy: two decade long story of a transcription factor. Acta Oncol. 2017;56(4):503–15. doi: 10.1080/0284186x.2017.1301680. [DOI] [PubMed] [Google Scholar]
- 51.Jiang BH, Liu LZ. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res. 2009;102:19–65. doi: 10.1016/s0065-230x(09)02002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Wang L, Cao L, Zhang Q, Song Q, Meng Z, et al. Inhibition of autophagy potentiates the anti-metastasis effect of phenethyl isothiocyanate through JAK2/STAT3 pathway in lung cancer cells. Mol Carcinog. 2018;57(4):522–35. doi: 10.1002/mc.22777. [DOI] [PubMed] [Google Scholar]
- 53.Li X, Wu JF. Recent developments in patent anti-cancer agents targeting the matrix metalloproteinases (MMPs) Recent Pat Anticancer Drug Discov. 2010;5(2):109–41. doi: 10.2174/157489210790936234. [DOI] [PubMed] [Google Scholar]
- 54.Rath B, Klameth L, Plangger A, Hochmair M, Ulsperger E, Huk I, et al. Expression of proteolytic enzymes by small cell lung cancer circulating tumor cell lines. Cancers (Basel) 2019;11(1):114. doi: 10.3390/cancers11010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang H. Matrix metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: recent advances. Sensors (Basel) 2018;18(10):3249. doi: 10.3390/s18103249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. BiochimBiophys Acta. 2013;1830(6):3670–95. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amararathna M, Johnston MR, Rupasinghe HP. Plant polyphenols as chemopreventive agents for lung cancer. Int J Mol Sci. 2016;17(8):1352. doi: 10.3390/ijms17081352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Batra P, Sharma AK. Anti-cancer potential of flavonoids: recent trends and future perspectives. 3 Biotech. 2013;3(6):439–59. doi: 10.1007/s13205-013-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Costea T, Vlad OC, Miclea LC, Ganea C, Szöllősi J, Mocanu MM. Alleviation of multidrug resistance by flavonoid and non-flavonoid compounds in breast, lung, colorectal and prostate cancer. Int J Mol Sci. 2020;21(2):401. doi: 10.3390/ijms21020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ballout F, Habli Z, Monzer A, Rahal ON, Fatfat M, Gali-Muhtasib H. Anticancer alkaloids: molecular mechanisms and clinical manifestations. In: Sharma AK, ed. Bioactive Natural Products for the Management of Cancer: From Bench to Bedside. Singapore: Springer; 2019. p. 1-35. 10.1007/978-981-13-7607-8_1. [DOI]
- 61.Xu WT, Li TZ, Li SM, Wang C, Wang H, Luo YH, et al. Cytisine exerts anti-tumour effects on lung cancer cells by modulating reactive oxygen species-mediated signalling pathways. Artif Cells NanomedBiotechnol. 2020;48(1):84–95. doi: 10.1080/21691401.2019.1699813. [DOI] [PubMed] [Google Scholar]
- 62.Niu H, Zhang Y, Wu B, Zhang Y, Jiang H, He P. Matrine induces the apoptosis of lung cancer cells through downregulation of inhibitor of apoptosis proteins and the Akt signaling pathway. Oncol Rep. 2014;32(3):1087–93. doi: 10.3892/or.2014.3273. [DOI] [PubMed] [Google Scholar]
- 63.Li W, Yu X, Tan S, Liu W, Zhou L, Liu H. Oxymatrine inhibits non-small cell lung cancer via suppression of EGFR signaling pathway. Cancer Med. 2018;7(1):208–18. doi: 10.1002/cam4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin L, Ren L, Wen L, Wang Y, Qi J. Effect of evodiamine on the proliferation and apoptosis of A549 human lung cancer cells. Mol Med Rep. 2016;14(3):2832–8. doi: 10.3892/mmr.2016.5575. [DOI] [PubMed] [Google Scholar]
- 65.Mohan V, Agarwal R, Singh RP. A novel alkaloid, evodiamine causes nuclear localization of cytochrome-c and induces apoptosis independent of p53 in human lung cancer cells. BiochemBiophys Res Commun. 2016;477(4):1065–71. doi: 10.1016/j.bbrc.2016.07.037. [DOI] [PubMed] [Google Scholar]
- 66.Zhang R, Li G, Zhang Q, Tang Q, Huang J, Hu C, et al. Hirsutine induces mPTP-dependent apoptosis through ROCK1/PTEN/PI3K/GSK3β pathway in human lung cancer cells. Cell Death Dis. 2018;9(6):598. doi: 10.1038/s41419-018-0641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ali A, Sidorova TS, Matesic DF. Dual modulation of JNK and Akt signaling pathways by chaetoglobosin K in human lung carcinoma and ras-transformed epithelial cells. Invest New Drugs. 2013;31(3):525–34. doi: 10.1007/s10637-012-9883-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poornima P, Weng CF, Padma VV. Neferine from Nelumbo nucifera induces autophagy through the inhibition of PI3K/Akt/mTOR pathway and ROS hyper generation in A549 cells. Food Chem. 2013;141(4):3598–605. doi: 10.1016/j.foodchem.2013.05.138. [DOI] [PubMed] [Google Scholar]
- 69.Sivalingam K, Amirthalingam V, Ganasan K, Huang CY, Viswanadha VP. Neferine suppresses diethylnitrosamine-induced lung carcinogenesis in Wistar rats. Food Chem Toxicol. 2019;123:385–98. doi: 10.1016/j.fct.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 70.Kalai Selvi S, Vinoth A, Varadharajan T, Weng CF, Vijaya Padma V. Neferine augments therapeutic efficacy of cisplatin through ROS- mediated non-canonical autophagy in human lung adenocarcinoma (A549 cells) Food Chem Toxicol. 2017;103:28–40. doi: 10.1016/j.fct.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 71.Liu Z, Ma L, Wen ZS, Cheng YX, Zhou GB. Ethoxysanguinarine induces inhibitory effects and downregulates CIP2A in lung cancer cells. ACS Med Chem Lett. 2014;5(2):113–8. doi: 10.1021/ml400341k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chougule M, Patel AR, Sachdeva P, Jackson T, Singh M. Anticancer activity of noscapine, an opioid alkaloid in combination with cisplatin in human non-small cell lung cancer. Lung Cancer. 2011;71(3):271–82. doi: 10.1016/j.lungcan.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lai H, Wang Y, Duan F, Li Y, Jiang Z, Luo L, et al. Krukovine suppresses KRAS-mutated lung cancer cell growth and proliferation by inhibiting the RAF-ERK pathway and inactivating AKT pathway. Front Pharmacol. 2018;9:958. doi: 10.3389/fphar.2018.00958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cho HS, Chang SH, Chung YS, Shin JY, Park SJ, Lee ES, et al. Synergistic effect of ERK inhibition on tetrandrine-induced apoptosis in A549 human lung carcinoma cells. J Vet Sci. 2009;10(1):23–8. doi: 10.4142/jvs.2009.10.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fu L, Chen W, Guo W, Wang J, Tian Y, Shi D, et al. Berberine targets AP-2/hTERT, NF-κB/COX-2, HIF-1α/VEGF and cytochrome-c/caspase signaling to suppress human cancer cell growth. PLoS One. 2013;8(7):e69240. doi: 10.1371/journal.pone.0069240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sirimangkalakitti N, Chamni S, Suwanborirux K, Chanvorachote P. Renieramycin M sensitizes anoikis-resistant H460 lung cancer cells to anoikis. Anticancer Res. 2016;36(4):1665–71. [PubMed] [Google Scholar]
- 77.Shen KH, Hung JH, Chang CW, Weng YT, Wu MJ, Chen PS. Solasodine inhibits invasion of human lung cancer cell through downregulation of miR-21 and MMPs expression. Chem Biol Interact. 2017;268:129–35. doi: 10.1016/j.cbi.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 78.Chatterjee P, Seal S, Mukherjee S, Kundu R, Bhuyan M, Barua NC, et al. A carbazole alkaloid deactivates mTOR through the suppression of rictor and that induces apoptosis in lung cancer cells. Mol Cell Biochem. 2015;405(1-2):149–58. doi: 10.1007/s11010-015-2406-2. [DOI] [PubMed] [Google Scholar]
- 79.Wang F, Mao Y, You Q, Hua D, Cai D. Piperlongumine induces apoptosis and autophagy in human lung cancer cells through inhibition of PI3K/Akt/mTOR pathway. Int J ImmunopatholPharmacol. 2015;28(3):362–73. doi: 10.1177/0394632015598849. [DOI] [PubMed] [Google Scholar]
- 80.Seok JS, Jeong CH, Petriello MC, Seo HG, Yoo H, Hong K, et al. Piperlongumine decreases cell proliferation and the expression of cell cycle-associated proteins by inhibiting Akt pathway in human lung cancer cells. Food Chem Toxicol. 2018;111:9–18. doi: 10.1016/j.fct.2017.10.058. [DOI] [PubMed] [Google Scholar]
- 81.Ooppachai C, Limtrakul Dejkriengkraikul P, Yodkeeree S. Dicentrine potentiates TNF-α-induced apoptosis and suppresses invasion of A549 lung adenocarcinoma cells via modulation of NF-κB and AP-1 activation. Molecules. 2019;24(22):3100. doi: 10.3390/molecules24224100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang HL, Chang YM, Lai SC, Chen KM, Wang KC, Chiu TT, et al. Naringenin inhibits migration of lung cancer cells via the inhibition of matrix metalloproteinases-2 and -9. Exp Ther Med. 2017;13(2):739–44. doi: 10.3892/etm.2016.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moon JY, Manh Hung LV, Unno T, Cho SK. Nobiletin enhances chemosensitivity to adriamycin through modulation of the Akt/GSK3β/β-catenin /MYCN/MRP1 signaling pathway in A549 human non-small-cell lung cancer cells. Nutrients. 2018;10(12):1829. doi: 10.3390/nu10121829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Y, Zhang R, Ni H. Eriodictyol exerts potent anticancer activity against A549 human lung cancer cell line by inducing mitochondrial-mediated apoptosis, G2/M cell cycle arrest and inhibition of m-TOR/PI3K/Akt signalling pathway. Arch Med Sci. 2020;16(2):446–52. doi: 10.5114/aoms.2019.85152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shao JJ, Zhang AP, Qin W, Zheng L, Zhu YF, Chen X. AMP-activated protein kinase (AMPK) activation is involved in chrysin-induced growth inhibition and apoptosis in cultured A549 lung cancer cells. BiochemBiophys Res Commun. 2012;423(3):448–53. doi: 10.1016/j.bbrc.2012.05.123. [DOI] [PubMed] [Google Scholar]
- 86.Zhou Z, Tang M, Liu Y, Zhang Z, Lu R, Lu J. Apigenin inhibits cell proliferation, migration, and invasion by targeting Akt in the A549 human lung cancer cell line. Anticancer Drugs. 2017;28(4):446–56. doi: 10.1097/cad.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 87.Chen M, Wang X, Zha D, Cai F, Zhang W, He Y, et al. Apigenin potentiates TRAIL therapy of non-small cell lung cancer via upregulating DR4/DR5 expression in a p53-dependent manner. Sci Rep. 2016;6:35468. doi: 10.1038/srep35468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang JH, Cheng CW, Yang YC, Chen WS, Hung WY, Chow JM, et al. Downregulating CD26/DPPIV by apigenin modulates the interplay between Akt and Snail/Slug signaling to restrain metastasis of lung cancer with multiple EGFR statuses. J Exp Clin Cancer Res. 2018;37(1):199. doi: 10.1186/s13046-018-0869-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu M, Qi B, Xiaoxiang W, Xu J, Liu X. Baicalein increases cisplatin sensitivity of A549 lung adenocarcinoma cells via PI3K/Akt/NF-κB pathway. Biomed Pharmacother. 2017;90:677–85. doi: 10.1016/j.biopha.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 90.Chen KC, Chen CY, Lin CR, Yang TY, Chen TH, Wu LC, et al. Luteolin attenuates TGF-β1-induced epithelial-mesenchymal transition of lung cancer cells by interfering in the PI3K/Akt-NF-κB-Snail pathway. Life Sci. 2013;93(24):924–33. doi: 10.1016/j.lfs.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 91.Khan N, Afaq F, Khusro FH, Mustafa Adhami V, Suh Y, Mukhtar H. Dual inhibition of phosphatidylinositol 3-kinase/Akt and mammalian target of rapamycin signaling in human nonsmall cell lung cancer cells by a dietary flavonoid fisetin. Int J Cancer. 2012;130(7):1695–705. doi: 10.1002/ijc.26178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Han X, Liu CF, Gao N, Zhao J, Xu J. Kaempferol suppresses proliferation but increases apoptosis and autophagy by up-regulating microRNA-340 in human lung cancer cells. Biomed Pharmacother. 2018;108:809–16. doi: 10.1016/j.biopha.2018.09.087. [DOI] [PubMed] [Google Scholar]
- 93.Nguyen TT, Tran E, Ong CK, Lee SK, Do PT, Huynh TT, et al. Kaempferol-induced growth inhibition and apoptosis in A549 lung cancer cells is mediated by activation of MEK-MAPK. J Cell Physiol. 2003;197(1):110–21. doi: 10.1002/jcp.10340. [DOI] [PubMed] [Google Scholar]
- 94.Chang JH, Lai SL, Chen WS, Hung WY, Chow JM, Hsiao M, et al. Quercetin suppresses the metastatic ability of lung cancer through inhibiting Snail-dependent Akt activation and Snail-independent ADAM9 expression pathways. BiochimBiophys Acta Mol Cell Res. 2017;1864(10):1746–58. doi: 10.1016/j.bbamcr.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 95.Chen W, Wang X, Zhuang J, Zhang L, Lin Y. Induction of death receptor 5 and suppression of survivin contribute to sensitization of TRAIL-induced cytotoxicity by quercetin in non-small cell lung cancer cells. Carcinogenesis. 2007;28(10):2114–21. doi: 10.1093/carcin/bgm133. [DOI] [PubMed] [Google Scholar]
- 96.Nguyen TT, Tran E, Nguyen TH, Do PT, Huynh TH, Huynh H. The role of activated MEK-ERK pathway in quercetin-induced growth inhibition and apoptosis in A549 lung cancer cells. Carcinogenesis. 2004;25(5):647–59. doi: 10.1093/carcin/bgh052. [DOI] [PubMed] [Google Scholar]
- 97.Yeh SL, Yeh CL, Chan ST, Chuang CH. Plasma rich in quercetin metabolites induces G2/M arrest by upregulating PPAR-γ expression in human A549 lung cancer cells. Planta Med. 2011;77(10):992–8. doi: 10.1055/s-0030-1250735. [DOI] [PubMed] [Google Scholar]
- 98.Chen KH, Weng MS, Lin JK. Tangeretin suppresses IL-1beta-induced cyclooxygenase (COX)-2 expression through inhibition of p38 MAPK, JNK, and AKT activation in human lung carcinoma cells. BiochemPharmacol. 2007;73(2):215–27. doi: 10.1016/j.bcp.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 99.Tsai MH, Liu JF, Chiang YC, Hu SC, Hsu LF, Lin YC, et al. Artocarpin, an isoprenyl flavonoid, induces p53-dependent or independent apoptosis via ROS-mediated MAPKs and Akt activation in non-small cell lung cancer cells. Oncotarget. 2017;8(17):28342–58. doi: 10.18632/oncotarget.16058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen H, Yang J, Hao J, Lv Y, Chen L, Lin Q, et al. A novel flavonoid kushenol Z from Sophora flavescens mediates mTOR pathway by inhibiting phosphodiesterase and Akt activity to induce apoptosis in non-small-cell lung cancer cells. Molecules. 2019;24(24):4425. doi: 10.3390/molecules24244425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tsai YM, Yang CJ, Hsu YL, Wu LY, Tsai YC, Hung JY, et al. Glabridin inhibits migration, invasion, and angiogenesis of human non-small cell lung cancer A549 cells by inhibiting the FAK/rho signaling pathway. Integr Cancer Ther. 2011;10(4):341–9. doi: 10.1177/1534735410384860. [DOI] [PubMed] [Google Scholar]
- 102.Wang S, Yan Y, Cheng Z, Hu Y, Liu T. Sotetsuflavone suppresses invasion and metastasis in non-small-cell lung cancer A549 cells by reversing EMT via the TNF-α/NF-κB and PI3K/AKT signaling pathway. Cell Death Discov. 2018;4:26. doi: 10.1038/s41420-018-0026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu X, Jiang Q, Liu H, Luo S. Vitexin induces apoptosis through mitochondrial pathway and PI3K/Akt/mTOR signaling in human non-small cell lung cancer A549 cells. Biol Res. 2019;52(1):7. doi: 10.1186/s40659-019-0214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou M, Shen S, Zhao X, Gong X. Luteoloside induces G0/G1 arrest and pro-death autophagy through the ROS-mediated AKT/mTOR/p70S6K signalling pathway in human non-small cell lung cancer cell lines. BiochemBiophys Res Commun. 2017;494(1-2):263–9. doi: 10.1016/j.bbrc.2017.10.042. [DOI] [PubMed] [Google Scholar]
- 105.Luo Y, Ren Z, Du B, Xing S, Huang S, Li Y, et al. Structure identification of viceninii extracted from Dendrobium officinale and the reversal of TGF-β1-induced epithelial⁻mesenchymal transition in lung adenocarcinoma cells through TGF-β/Smad and PI3K/Akt/mTOR signaling pathways. Molecules. 2019;24(1):144. doi: 10.3390/molecules24010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baruah TJ, Kma L. Vicenin-2 acts as a radiosensitizer of the non-small cell lung cancer by lowering Akt expression. Biofactors. 2019;45(2):200–10. doi: 10.1002/biof.1472. [DOI] [PubMed] [Google Scholar]
- 107.Chen M, Cai F, Zha D, Wang X, Zhang W, He Y, et al. Astragalin-induced cell death is caspase-dependent and enhances the susceptibility of lung cancer cells to tumor necrosis factor by inhibiting the NF-кB pathway. Oncotarget. 2017;8(16):26941–58. doi: 10.18632/oncotarget.15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lago JH, Toledo-Arruda AC, Mernak M, Barrosa KH, Martins MA, Tibério IF, et al. Structure-activity association of flavonoids in lung diseases. Molecules. 2014;19(3):3570–95. doi: 10.3390/molecules19033570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Amawi H, Ashby CR Jr, Tiwari AK. Cancer chemoprevention through dietary flavonoids: what’s limiting? Chin J Cancer. 2017;36(1):50. doi: 10.1186/s40880-017-0217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 1 contains Tables S1 and S2.