Abstract
Nowadays the importance of vitamins is clear for everyone. However, many patients are suffering from insufficient intake of vitamins. Incomplete intake of different vitamins from food sources due to their destruction during food processing or decrease in their bioavailability when mixing with other food materials, are factors resulting in vitamin deficiency in the body. Therefore, various lipid based nanocarriers such as nanoliposomes were developed to increase the bioavailability of bioactive compounds. Since the function of nanoliposomes containing vitamins on the body has a direct relationship with the quality of produced nanoliposomes, this review study was planned to investigate the several aspects of liposomal characteristics such as size, polydispersity index, zeta potential, and encapsulation efficiency on the quality of synthesized vitamin-loaded nanoliposomes.
Keywords: Encapsulation, Vitamins, Nanoliposomes, Nanoliposome preparation, Nutraceuticals
Introduction
Vitamins are essential micronutrients considered as a class of organic compounds which are not synthesized in the human body (except vitamin D and B2), and are usually deprived of providing energy, however, they are extremely required for proper performance of body.1 There are thirteen vitamins which are categorized based on their solubility. Lipophilic vitamins are composed of A, E, K, and D vitamins along with carotenoids showing the functional traits of vitamin A, and hydrophilic vitamins are comprised of vitamin C and the group of B vitamins, including thiamin (B1), riboflavin (B2), nicotinic acid (B3), pyridoxine (B6), pantothenic acid (B5), folate, and cyanocobalamin (B12).2
The importance of vitamins has been discussed in a separate section in this paper. In addition to the function of vitamins in the body, these micronutrients are able to prevent many diseases. The most important example that can be mentioned is COVID-19. Although, there are not adequate data regarding the function of vitamins against COVID-19, a certain number of recent studies have reported that some vitamins, particularly vitamin D may have an important role in improving the immune system of the body to act against the coronavirus.3-6
In spite of many advantages of vitamins, they have poor bio-accessibility and bioavailability, and they are highly vulnerable to degradation. Different parameters during the process and storage may cause degradation of vitamins such as oxygen, temperature, light, water activity, moisture content, pH, metal trace elements especially iron and copper, and degradative enzymes (lipase, proteases, and nucleases).7 Encapsulation techniques can surround vitamins and protect them against sever conditions such as exposure to oxygen, heat, pro-oxidants, or UV light, throughout the storage and can also enhance their functional properties like solubility, stability and controlled release. Vitamin D and K have lipophilic nature with a week solubility in the aqueous media. To improve their stability, solubility, and targeting feature, some modifications are needed to be made.8 Thus, a wide range of delivery systems have been developed.
Nanoliposome is considered as one of the lipid-based carriers, which is used for delivery of different bioactive compounds.9 Due to the presence of hydrophilic and hydrophobic components in the structure of nanoliposomes, they are considered as suitable encapsulants for loading and delivering different types of vitamins to target cells.10 Although, there are some studies related to the delivery of vitamins using lipid carriers such as the work of Hsu et al,7 the main purpose of this study is more detailed and comprehensive investigation of recent literature pertaining to the application and efficacy of nanoliposomes to encapsulate vitamins, especially in different foods and environmental conditions, is conducted.
An overview of vitamins
Vitamins are considered as one of the essential organic and micronutrient compounds with bioactive characteristics which are necessary for preserving the normal performance of human body through adjusting enzymatic and chemical reactions and physiological effects on various biological responses, including host immunity.11,12 Moreover, they play primary roles in different basic metabolic pathways, supporting the fundamental function of human cells. In particular, their participation in DNA synthesis, neuronal functions, energy-yielding metabolism, and oxygen transport makes them essential for muscular and brain functions. All of these factors can directly or indirectly affect the psychological and cognitive processes, including physical and mental fatigue.1 They can be extracted from food and supplements, or in some cases, synthesized by our body or gut microbiome.13
Vitamins are classified into fat-soluble vitamins (vitamins A, D, E, and K) and water-soluble vitamins (vitamins B and C). Fat-soluble vitamins usually bind to cellular nuclear receptors and have an effect on the expression of particular genes.14 In addition, adequate amount of bile flow and micelle formation enhance the absorption of fat-soluble vitamins. In recent decades, insufficiencies of these vitamins have been the ground for expanded danger of tumor, type II diabetes mellitus and other various invulnerable framework issues.15 Water-soluble vitamins are mostly considered as cofactors for enzymes, influencing the enzymatic activity which can also improve the energy metabolism. Water-soluble vitamins are not stored in the body and any excess amount of them will be excreted in the urine.16 However, fat-soluble vitamins, can be stored in greasy tissues.17 Moreover, there are several techniques, such as chromatography and capillary electrophoresis to isolate and purify vitamins.15 Biological functions of vitamins are included in Table 1.
Table 1. Biological functions of vitamins .
| Vitamin | Source | Recommended dietary allowance (per day) | Some of functions in the body | Reference |
| A | Retinoic acid: Animal tissues Carotenoids: Production by plants, fungi, algae, and bacteria |
600-800 (μg retinol activity equivalents) |
Anti‐aging, Skin therapy, cytokine modulation, antioxidant, melonocyte function modulation, sunscreen effect, role in vision, bone growth, reproduction, and function of the immune system |
7,18,19 |
| D | D2: Plant products such as mushrooms D3: liver, fatty fish, fish oils, egg yolks and human skin by exposure to UV radiation |
14000 IU | Skeletal functions including, calcium absorption and bone health, inhibition of diabetes, cancer, and cardiovascular diseases, prevention of postmenopausal osteoporosis, therapy of inflammatory, cell proliferation modulation |
20-22 |
| K | K1 (phylloquinone): Green, leafy vegetables like spinach, collards, and broccoli, and soybean oil and canola oil. K2 (menaquinones): Animal/bacteria-derived products |
90-120 mg | Activation of particular proteins in atherosclerosis prevention and bone metabolism, procoagulant | 23 |
| E | In leafy vegetables and fortified cereals | 15 mg | Anti-inflammatory activity, Treatment of cancers and cardiovascular disorders, antioxidant, supporting eyes and neurological function, inhibition of platelet coagulation |
24,25 |
| B12 | Meat and fish, dairy products, cheese, egg white, and boiled haddock | 2.4 μg | Vital co-enzyme for growth of cells, DNA synthesis, nerve cell maintenance | 7,13,26-28 |
| C | Fruits and vegetables, such as papaya, mango, kiwi, spinach, tomato. lettuce, strawberry, peppers potatoes, broccoli, lemons, cabbage, peas, pears, brussels cauliflower, sprouts, meat organs (heart liver, and kidney), seafood, chicken, and pork | 500 mg | Anti-inflammatory and depigmenting effects | 29,30 |
Characterization and role of vitamins in the body
Vitamin B
The B family vitamins include B1 (thiamine), B2 (riboflavin), B3 (niacin), B4 (Choline), B5 (pantothenic acid), B6 (pyridoxine), B8 (biotin), B9 (folate), and B12 (cobalamin).31 Mammals cannot synthesize B vitamins on their own; thus, they need to take them up in sufficient amounts from dietary or microbial sources, such as the intestinal microbiota. Although the majority of them are produced by plants, yeasts, and bacteria, they can be found in animal‐derived foods such as eggs, meat, and dairy, through plant consumption by mammals.11 Some main sources of B vitamins are broccoli, bananas, potatoes, dates, spinach, asparagus, nuts, figs, and dairy products.15 Plants are not able to produce vitamin B12; however, the bacteria located in the ruminants foregut or humans colon can produce this vitamin.32 According to the WHO reports, vitamin B12 deficiency will probably be the most prevalent malnutrition problem in the future.33
The B vitamins aim in digestion, boosting immune system and metabolism, and repairing cells.15 Vitamins B have an important role as cofactor of enzymes in all tissues, through numerous biochemical pathways. They can enhance the function of the nervous and immune systems,34 regulate metabolisms, and improve the cell division and growth. Most of these B vitamins are essential bioactive compounds which are dependent on the diet supply, excluding niacin that can likewise be produced from tryptophan. Vitamin B deficiencies can be frequently seen in elderly, children, pregnant women, vegetarians, and patients with gastrointestinal disorders.35 A higher risk of mood and behavioral disorders, increased serum homocysteine levels, and heart diseases were recently connected to vitamin B deficiencies.36 B vitamins, specifically B9, B12, and B6 are involved in removing homocysteine from the body, pertaining to the dementia via direct vascular or neurotoxic mechanisms.37 For proper neuronal performances, having an adequate quantity of folic acid, vitamin B12, and vitamin B6 is essential. These vitamins possess critical roles in donation of methyl group for synthesizing lipids, proteins, nucleic acids, hormones, and neurotransmitters. However, their deficiencies have been reported to be related to an increased risk of dementia, neurodevelopmental, and psychiatric diseases. Moreover, improper absorption, function, and metabolism of such vitamins are attributed to gene polymorphisms related to the increased occurrence of cognitive disorders.38
Vitamin C
Vitamin C, also known as ascorbate, is an essential vitamin broadly distributed in many tissues. This nutrient is plentiful in fruits, vegetables, and animal livers. Vitamin C is comprised of two molecular forms: the oxidized form (dehydroascorbic acid) and the reduced form (ascorbic acid). This vitamin is principal for the physiological performance of the nervous system and antioxidative functions of body through reducing lipid peroxidation, scavenging free radicals, and inhibiting oxidative stress. Furthermore, it participates in several non-oxidative stress processes, such as production of cholesterol, collagen, carnitine, amino acids, catecholamines, and some hormones.12 Vitamin C is necessary for the function of two dioxygenase enzymes responsible for the biosynthesis of carnitine, an important cofactor that transports long-chain fatty acids into the mitochondria. Therefore, it has an effect on generating energy via beta-oxidation.39,40 Vitamin C is involved in biochemical reactions catalyzed by monooxygenases, dioxygenases and mixed function oxygenases. A lack of vitamin C hampers the activity of a range of enzymes and may lead to scurvy in humans.41
Vitamin D
Vitamin D is classified in to two main groups: ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3), which are different in terms of physicochemical traits, molecular structures, and biological effects.42 Vitamin D3 can be mainly found in animal foods. Vitamin D2 is mostly found in some wild mushrooms, in which it is converted from a provitamin called ergosterol. Plants utilized as food may have ergosterol, but it is not transformed to vitamin D2 in nature.43 Vitamin D has significant impact on brain function and development, mood regulation, dopamine ontogeny, axonal connectivity, neuronal differentiation, immunological modulation, and transcriptional control of a huge number of genes. Vitamin D has been proved to protect neurons from inflammation damage via clearance of gathered amyloid β.37 Its deficiency has been attributed to the pathogenesis of some psychiatric disorders, such as depression and autism spectrum disorder.44
Vitamin A
Vitamin A is a collection of organic compounds, including retinoic acid, retinol, retinal, and provitamin A called carotenoids.13 Vitamin A is a constituent of the pigment rhodopsin situated in the retina, contributing in visual processes and prevention of blindness. It is also involved in the function of gut microbiota, plasma retinol, CD38 (cluster of differentiation 38), and RORA (Retinoic acid receptor-related orphan receptor alpha4) mRNA.1 Insufficient levels of vitamin A can cause decreased CARS (Childhood Autism Rating Scale) score, increased level of serum 5-hydroxytryptamine (5-HT), and reduced development of the central nervous system.45 Vitamin A is necessary to maintain epithelial integrity and cellular differentiation, production of red blood cell, as well as increase in body resistance against infections. Severe deficiencies of vitamin A lead to vision issues (xerophthalmia). It has been reported that even moderate deficiency of vitamin A may damage vaccine elicited immunity for some types of vaccines.13
Vitamin E
Vitamin E (tocopherols and tocotrienols) exists in all membranes of cells and plasma lipoproteins, particularly in human red blood cells. Vitamin E can protect DNA, fatty acids, and low-density lipoproteins from oxidation. It, also, has a role in biosynthesis of hemoglobin, stabilization of the membranes structure, and modulation of immune responses.41 Fine sources of vitamin E are vegetable oils, oil seeds, nuts, cheese, egg yolk, margarine, soya beans, oatmeal, wheat germ, avocados, and green leafy vegetables, etc. Deficiency of vitamin E is rare in humans, though it can be seen in premature infants as well as in people with chronic fat malabsorption, mild anemia, and ataxia.24
Vitamin K
Vitamin K exists in two natural forms. Vitamin K1 (phylloquinone) is ample in leafy green vegetables, such as lettuce, cabbage, and spinach.46 The other natural form, vitamin K2 (menaquinone) is predominantly from microbial origin.47 Vitamin K2 is mostly present in fermented foodstuff such as natto and cheese; however, gut microbiota (Escherichia coli, Mycobacterium phlei, and Bacillus subtilis) is able to produce vitamin K2, as well.48 There is also a synthetic form of vitamin K, which is known as vitamin K3 (Menadione).49
Vitamin K has an important promoting role in controlling the bone formation and blood clotting. Vitamin K deficiency may cause hemorrhagic diseases in babies, in addition to muscle hematomas, postoperative bleeding, and intracranial hemorrhages in grown person.41 Some food sources containing vitamin K are liver, meat, egg yolk, whole grain, brussels sprouts, vegetables, parsley, celery, iceberg lettuce, cabbage, peas, asparagus, broccoli, cucumbers, and soya bean.15
Nanoencapsulation
Generally, encapsulation is defined as a process to incorporate bioactive compounds into another compound named cover material, shielding them from environmental and gastrointestinal conditions.50 The coated substances (active agents) are also called as fill, core, or internal phase, whereas the coating substances (carrier agents) are known as shell, membrane, wall material, matrix, capsule, or external phase.51
Encapsulation method is widely used to improve the shelf life and bioavailability of bioactive compounds. Encapsulation of food ingredients within nano-capsules, can elevate the stability and bioavailability of bioactive compounds, thus improving food products quality.52 Several food-grade materials such as polysaccharides, lipids, and proteins are used to encapsulate bioactive compounds. However, carbohydrates and proteins are not appropriate for industrial aims because of the utilization of complex chemical materials or heat processing that cannot be completely controlled. On the other hand, lipid-based nanocarriers have advantages such as more loading efficiency, biocompatibility, targeted effect, low toxicity, modified release, and ease of constant production.53 In the following sections, a summary of different lipid-based nanocarrier properties is given.
Lipid-based nanocarriers for vitamin delivery
Lipid-based nanocarriers bear excellent functionality in film formation, emulsification, and encapsulation of a variety of substances. These systems are generally categorized into two groups. First group is liquid lipid nanoparticles including nanoemulsions, colloidosomes, nanoliposomes, and multiple nanoemulsions. The second group is solid lipid-based nanocarriers including solid lipid nanoparticles (SLNs), and nanostructured lipid carriers (NLCs).54 Some important numbers of such delivery systems are briefly discussed in the following sections.
Nanoemulsion
Nanoemulsions are defined as liquid dispersions with droplet sizes of 50 to 500 nm.55 This kind of nanocarriers are formulated by water, oil, and surfactants/biopolymers in several types of single water-in-oil (W/O) or oil-in-water (O/W) nanoemulsions, double water-in-oil-in-water (W/O/W) or oil-in-water-in-oil (O/W/O) nanoemulsions, Pickering nanoemulsions formed by biopolymer nanoparticles, and structural nanoemulsions covered by one or two layers of biopolymer materials.54 A detailed information pertained to different nanoemulsion structures can be found in different studies.56-59 Oil-in-water nanoemulsions are particularly appropriate for nanoencapsulation and carrying lipophilic vitamins due to their reliable physicochemical stability along with acceptable oral bioavailability.60 For example, vitamin D3 was encapsulated in O/W nanoemulsions. In this work both in vitro and in vivo studies demonstrated that the nanoemulsion-based delivery system improves the bioavailability of vitamin D3 absorption.61 The capability of W/O/W emulsions to provide an effective delivery system for vitamin B12 into skim milk was evaluated and 88.85% encapsulation efficiency was obtained by this W/O/W emulsion.26 Nanoemulsions are promising carriers owing to their easy preparation, rapid release traits, and high stability. They can be fabricated to meet the particular requirements needed for certain bioactive compounds.62
Nanoemulsions are appropriate for encapsulating, shielding, and carrying both hydrophilic and lipophilic bioactive compounds. Bioavailability and bioaccessibility of bioactive components encapsulated with nanoemulsions are affected by several parameters, such as the type and the physical state of lipid, the size of nanoemulsions, and the nature of surfactants.63 Normally, nanoemulsions are created from GRAS (generally recognized as safe) compounds. To increase the stability and decrease the toxicity, surfactants or co-surfactants such as peptides, proteins, polysaccharides, phospholipids, or nonionic surfactants (Tween and Span) are being used in the structure of nanoemulsions.62,64
Solid lipid nanoparticles
SLNs are often mentioned in texts as the first lipid-based nanocarrier group which were designed at early 1990s as a replacement to emulsions, liposomes, and polymeric nanoparticles.65 SLNs are a particular kind of nanoemulsions (with diameter range of 50 to 1000 nm), which are fabricated by substituting the oil phase in an O/W nanoemulsion with a solid lipid or a mixture of solid lipids (such as paraffin, waxes, and triacylglycerol). Contrary to nanoemulsions, lipid droplets in SLN systems have high crystallinity, increasing the stability of encapsulated bioactive and prolonging the release process due to much lower diffusion rate.18,52,66
It has been demonstrated that, poly (vinyl alcohol) films containing SLNs with entrapped α-tocopherol, had a higher control on the release of α-tocopherol compared to the neat films, confirming the more controlled release of bioactive components in this system and the possibility of its usage in active packages for foodstuff conservation.67
Owing to the solid based of SLNs, a sustained release of bioactive compounds can be provided. Nevertheless, the most important issue related to SLNs is their low bioactive compound loading capacity as well as the possibility of expulsion throughout the storage.68 For example in a study by Couto et al,69 only 12% encapsulation efficiency was observed for vitamin B2 loaded within SLNs. However, reaching to a higher loading efficiency have also been reported. In this regard, vitamin A-loaded SLNs were successfully prepared using the hot homogenization method with the help of cetyl alcohol and Gelucire 44/14® as carrier materials, which showed more than 90% entrapment efficiency.70
Nanostructured lipid carriers
To overcome some of the problems related to SLNs such as low encapsulation efficiency, NLCs were suggested as the second generation of SLNs.71 Nanostructured lipid carriers are spherical shape particles with mean diameters of 50 nm to 500 nm, constituted of a blend of liquid and solid lipids, dispersed within the aqueous media and stabilized using an external layer of surfactants. The NLCs containing vitamin E have been prepared by medium-chain triglycerides, avocado oil or coconut oil as liquid lipids, stearic acid or beeswax as solid lipids, and some nonionic surfactants.72,73 The incorporation of oil within a solid matrix results in the creation of amorphous nanostructures with numerous imperfections inside its matrix, granting NLCs to have a higher bioactive capacity and a lower degree of expulsion through the storage, compared to SLNs.74 High encapsulation efficiency related to vitamin E (EE:86.6%)75 and Vitamin D3 (EE:90.4%)76 has been observed in literatures. Important attributes of NLCs such as size, particle distribution, and stability, depend on the components of NLC and the type of the process that is being used to synthesize the particles.72
Lipid–polymer hybrid nanoparticle
Lipomers, lipid– polymer hybrid nanoparticles, have been developed as promising nanocarriers and have gained considerable interest owing to the corresponding beneficial properties of both polymeric nanoparticles and phospholipid shell. This core-shell kind of nanocarriers, in which a lipid monolayer or a liposomal bilayer envelops the polymeric core, has great structural stability provided by the polymer core rigidity, sustained-release ability, biocompatibility, and surface functionality.77 However, due to the complexity of their structure, designing of lipomers needs more works and accuracy. Thus, lipomers have not been developed in the industrial scale. Contrary to other lipid-based nanocarriers, lipomers offer some exclusive features such as the variety in structural components, controlled bioactive release, higher encapsulation, improved stability profile, enhanced cellular uptake.78 Unlike SLNs and NLCs which are mostly utilized to encapsulate hydrophobic bioactive compounds, Lipid–polymer hybrid nanoparticles are capable of loading several hydrophilic and hydrophobic bioactive compounds due to the coexistence of polymer and lipid providing different material properties, such as hydrophobicity and water-solubility.79 In a research study, a protein-lipid composite lipomers with three layers, including barley protein layer, phospholipid layer, and α-tocopherol layer and an inner aqueous partition was fabricated and successfully entrapped vitamin B12 with encapsulation efficiency of 69% and prolonged release behavior in a simulated gastrointestinal medium.80 Application of different lipid-based nanocarriers for encapsulation of vitamins is summarized in Table 2.
Table 2. Application of different lipid-based nanocarriers for encapsulation of vitamins .
| Nanocarrier | Synthesizing method | Formulation ingredients | Vitamin | Encapsulation efficiency (%) | Reference |
| Nano-emulsions | Phase-inversion based hot water dilution |
Monegyl caprylic-/capric triglyceride, polyethylene glycol hydroxyl stearate, water |
D3 | Up to 90 | 81 |
| Sonication | Pea protein isolate, canola oil, ethanol, water | D3 | 93 | 21 | |
| Solid lipid nanoparticles (SLNs) | High-speed homogenization/solvent diffusion | Phosphatidylcholine, stearic acid, ethanol, water | E | 90 | 82 |
| microemulsion method using stirring | Glyceryl monostearate, tween 80, butanol, water | E | 84 | 83 | |
| Nanostructured lipid carriers (NLCs) | Melt-emulsification | Stearic and oleic acids, glyceryl monostearate, beeswax, chitosan, tween 80, water | A | 98 | 84 |
| Homogenization and sonication | Phosphatidylcholine, ascorbic acid, glycerol monostearate, Phosphatidyl serine sodium, chloroform-toluene | D3 | 63 | 22 | |
| Lipid-polymer hybrid nanoparticles (LPHNs) | Solvent evaporation emulsification | Poly(lactic-co-glycolic acid), ethyl acetate, poly(vinyl alcohol), cholesterol, phosphatidylcholine, chloroform |
D | > 60 | 85 |
Nanoliposomes
Characteristics of nanoliposomes
Nanoliposome as a vesicular lipid bilayer nanocarrier, is a developing structure capable of encapsulating the biologically active ingredients, ensuring their safe delivery. In general, there are similar chemical, physical and thermodynamic properties between liposomes and nanoliposomes. However, smaller particle size, which means larger surface-to-volume ratio is the advantage of nanoliposomes over liposomes. This feature provides other benefits such as improved bioavailability, increased solubility, exact targeting, and better controlled release of the encapsulated material.86 A clinical comparison study on non-liposomal and liposomal vitamin C, demonstrated that the liposomal encapsulated vitamin C has uniform particle size, and well-organized morphological pattern, providing highly efficient encapsulation resulting in an improved bioavailability.87 Nanoliposomes are artificial vesicles of spherical shape with small size that are made of natural non-toxic phospholipids and cholesterol. In these systems, water-soluble drugs are encapsulated in the aqueous core which consists of hydrophilic parts of the phospholipids, and the insoluble agents are entrapped in the hydrophobic domain which contains lipid part of the phospholipids bilayer.88,89
Nanoliposomes have different structural sizes and shapes based on environmental circumstances, their constituents, and their production techniques.90 Liposomes can be categorized into five types based on their diameter: (1) multilamellar vesicles - 0.5–5 nm with 5 to 20 lipid bilayers, (2) small unilamellar one lipid bilayer vesicles - 20–200 nm, (3) large unilamellar vesicles with one lipid bilayer - 200 nm, (4) giant unilamellar vesicles with one lipid bilayer - 1 nm, and (5) multi vesicular vesicles with multi lipid bilayer - 1 nm.91
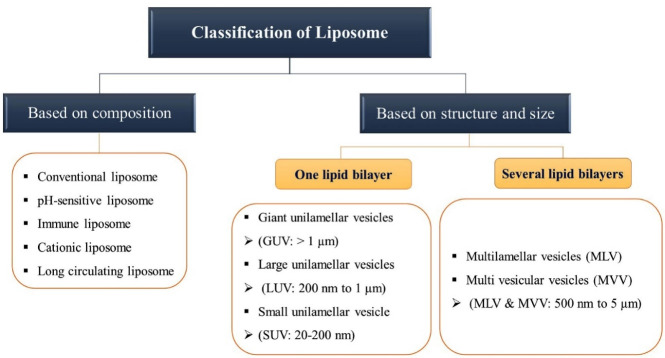
Nanoliposomes with a unilamellar state show a balloon-like structure with a simple monolayer, whereas liposomes that are multilamellar have onion like structures consisting of several single-layer cases. Unilamellar liposomes can be categorized as small unilamellar vesicles or large unilamellar vesicles, with diameters below 100 nm and over 100 nm, respectively. Several smaller vesicles are entrapped in a bigger one in multivesicular vesicles.92 Another classification of nanoliposomes is: (1) pH-sensitive liposomes: External changes in pH may destroy the lipid composition of liposomes, (2) conventional liposomes: the lipid layer of liposomes is composed of positively and negatively charged phospholipids and cholesterol, which are attached to the aqueous core, (3) immunoliposomes: with antibody molecules on the surface of liposomes, (4) cationic liposomes: with one positive charged lipid or phospholipid in the structure of liposomes, which can interact with nucleic acids and compounds with negative charge, through a simple mixing process, (5) long circulating liposomes: synthetic polymers, glycoproteins, oligosaccharides, and polysaccharides can make a hydrophilic layer on the surface of liposome, which result in the prolonged circulation of the liposomal components in drug delivery systems.93 A chart of main types of liposomes and their characteristics is given as Figure 1.
Figure 1.
A chart of main types of liposomes and their characteristics.
Characterization of phospholipids used in the structure of nanoliposomes
In pharmaceutical and food industries, these systems are formed from surfactants such as phospholipids with relative ratios of hydrophilic-lipophilic balance (HLB) and ideal curvatures about zero. Phospholipids are derived from natural sources such as milk, soy, and egg, so it is safe to use them in food-grade products. In recent years, liposomes were generally produced by phospholipids from eggs and soy94; however, milk phospholipids have a higher quantity of glycolipids and sphingolipids in comparison to other phospholipids. Moreover, they have low membrane permeability, thick walls, higher heat transfer, and good stability while storing at 4–35°C temperatures, compared to phospholipids prepared from soybean.95 Commercial lecithin is comprised of a blend of several phospholipids and other components such as triglycerides, free fatty acids, and sterols.92,96 Phosphatidyl inositol and phosphatidyl ethanolamine can be considered as examples of the most significant phospholipids forming lecithin. Natural lecithins are usually used beside other surfactants in nanoliposome systems due to their HLB of 8, which makes them unsuitable to be used alone for this purpose. Lecithin as zwitterionic surfactant, can have negative, neutral, or positive charges depending on its pH value and electrolyte content. However, these compounds are not stable against oxidation which is probably due to their large amounts of unsaturated fatty acids.97,98 Marine phospholipids are other kind of phospholipids which are being used in the structure of nanoliposomes. These phospholipids which contain eicosapentaenoic acid or docosahexaenoic acid, have better resistance to oxidation, high bioavailability, and high nutraceutical properties.99,100
Characterization of hydrophobic and hydrophilic parts of nanoliposomes
Nanoliposomes are able to increase the solubility, bioavailability, and controlled release of the encapsulated material to a larger extent. Hydrophobic bioactive compounds are entrapped in the lipid bilayer of these systems, during their formation process. The compounds inside them can be released when they diffuse through the membranes, or when their membrane is disrupted due to the alterations in temperature or pH, and etc.101 These systems can entrap different molecules such as amphiphilics, hydrophilics, and lipophilics which are entrapped into the lipid/water bilayer, into the interior, and within the hydrophobic bilayer, respectively. These heterogenous particles are obtained by placing phospholipids (such as lecithin) in the water or organic solutions and applying enough energy which results in the formation of unilamellar (one bilayer) or multilamellar (series of bilayers) structures.
While phospholipids can spontaneously turn into unilamellars by applying them in the aqueous mediums, their structure do not show desirable properties and good stability. Suitable production processes should be applied for production of liposomes to obtain appropriate properties such as smaller particle size, high loading capacity, and a proper encapsulation entrapment.102-104 Several factors such as temperature, ionic strength, and pH, determine the physicochemical properties of nanoliposomes. Lipid and phospholipid vesicles display low permeability to the entrapped or encapsulated material. Nevertheless, at increased temperatures, a phase transition occurs in them which can have impact on their permeability characteristics. Phospholipids of nanoliposomes have a substantial thermal characteristic in which phase transition (Tc) occurs at temperatures lower than their final melting point (Tm). At Tc, known as gel to liquid crystalline transition temperature, much of the ordered packing arrangement of phospholipid bilayers are being lost, whereas there is an upsurge in its fluidity.105 By placing the amphiphilic molecules such as phospholipids in an aqueous phase, they can form aggregated complexes to protect their hydrophobic parts from water molecules; however, they keep their contact with the aqueous phase via the hydrophilic head groups. By presence of enough level of energy, the aggregated phospholipids can organize themselves in the form of closed bilayer vesicles such as liposomes or nanoliposomes. In this way, liposomes can entrap hydrophilic, lipophilic molecules, or lipid soluble compounds such as nutrients, drugs, and certain vitamins.106,107 Moreover, there can be other compounds in the structure of nanoliposomes, such as sterols. Sterol incorporation into bilayers of nanoliposome can cause major changes in the properties of these carriers. Cholesterol is the most commonly used sterol in the production of the lipid vesicles. However, it forms bilayer structures by itself. High concentrations of this compound can be incorporated into phospholipid membranes, to modulate the fluidity of the lipid bilayer which results in an increase in the stability of vesicles, and reduced permeability of the lipid membrane to solutes.108
Other sterols have been scarcely used in the structure of nanoliposomes containing vitamins; however, in a study by Amiri et al10 the effect of cholesterol and phytosterol (Campesterol) powder was investigated to synthesize a new formulation of nanoliposome for entrapment of vitamin C. The findings showed that the highest stability of vitamin C in a period of 20 days was obtained in phospholipids to campesterol ratio of 75:25. A positive impact of cholesterol substitution with campesterol on control release, encapsulation efficiency, and stability of vitamin C in nanoliposomes was reported. Regardless of current studies, further researches are needed to be done in this field.
Methods of nanoliposome preparation
Several factors such as concentration of the encapsulated material, nature of the medium, physicochemical characteristics of the ingredients, polydispersity, size, and shelf life of the liposome are significant factors to be considered for preparation of liposome or nanoliposome. Preparation method of these systems can vary depending on their lipid composition.
The preparation methods of liposomes and nanoliposomes usually include the utilization of toxic or non-food grade solvents such as ether solution, ethanol, hexane, chloroform, methanol and detergents, such as alkyl glycoside, cholate, alkyl benzene sulfonates substances and triton X-100 for the solubilization of hydrophobic and/or hydrophilic ingredients.109,110 However, there are several techniques to reduce or completely eliminate the use of organic solvents for the formation of liposomes, requiring skilled technical knowledge and high investment for being industrialized such as heating metho.110
Several techniques such as emulsion method, freeze drying double emulsion method, membrane contactor technology, supercritical fluid technology, solvent-nonsolvent method, high-pressure homogenization, dual asymmetric centrifugation, cross-flow filtration with detergent depletion method, thin film hydration, reverse-phase evaporation, solvent/surfactant displacement, heating method, microfluidization, sonication, and extrusion method, are being used for liposome and nanoliposome production.
In spite of several techniques being used for production of liposomes and nanoliposomes, due to the complexity of most of these methods, their application in the industrial scale is difficult and very challenging. For instance, thin film hydration method followed by sonication was used for the production nanoliposomes to encapsulate different kinds of compounds such as vitamins, previously.111 In spite of the production of nano-size liposomes with this method, it is impossible to scale up the process. Some other modifications are needed to overcome the scale up problems of these methods. For instance, to scale up the sonication assisted homogenization process, different parameters should be considered. The cycle of sonication time should be kept constant and a 6 mm tip and 100% amplitude is needed to be used. The power which is delivered to the sample as a result of the number of sonication cycles is also an important parameter, because the sound wave should be amplified on the whole batch volume at the same dimensional properties.112 Some of the most important methods of nanoliposome production are summarized below.
Emulsion and freeze-drying double emulsion method
In the emulsification method, appropriate surfactants are being used to produce oil-in-water and water-in-oil emulsions. This method is a traditional method; however, freeze-drying double emulsion method is a novel trend, in which cryoprotectants are added to the liposome formulation and W/O/W emulsion will be formed followed by a sterilization step.
Membrane contactor technology
In this method, organic phase is placed in the pressurized vessel. A pump directs the aqueous phase to a module and the nitrogen gas is used to push the oil phase into the system. The aqueous phase is subsequently pumped through the membrane contactor module, and liposomes will be formed spontaneously at the time that the lipid and aqueous phases meet. Liposomes prepared using this technology are homogeneous with small particle size and high encapsulation efficiency for lipophilic compounds. Moreover, this method is simple to scale up.113,114
Supercritical fluid technology
In supercritical anti-solvent method, lipids are readily dissolved in supercritical carbon dioxide and then are precipitated, so that ultrafine particles will be formed. In the supercritical reverse phase evaporation method, which is another supercritical fluid technology, aqueous phase and solid lipid materials are put into a sealed viewing cell and the pressure and temperature are adjusted and then the CO2 gas is introduced. CO2 is then removed, and liposomes are formed. These methods of liposome preparation do not contain any organic solvents showing their higher advantage compared to conventional methods.115,116
Solvent-nonsolvent method
In the conventional solvent-nonsolvent method, particles are produced due to the lack of solvent. In this method, firstly solution of the material that is going to be encapsulated is prepared and then the solvent is removed through diafiltration or an evaporation procedure after dispersion in its nonsolvent, and liposomes will be produced due to the lack of solvent.117
Homogenization
In homogenization method of liposome production, the suspension of drug or any compound to be encapsulated, suddenly passes through a homogenization gap causing high streaming velocity, and in this way liposomes are being produced.118 The main drawback of this method is its extremely high operating pressure.119
Dual asymmetric centrifugation
This method of liposome production includes an additional sample rotation around its own vertical axis which pushes sample toward the center of the centrifuge, while in the conventional centrifugation sample is constantly being pushed outwards. Therefore, two overlaying movements of the materials occur in the centrifugation vial, which provides shear forces and an efficient homogenization for even a concentrated and viscous blend of lipids.120 This method is easy to operate and no organic solvent is being used for production of liposomes with small particle size.114,120
Cross-flow filtration detergent depletion method
One of the production methods of liposomes is based on detergent addition to solubilize lipids which is being removed through next steps. In this technique membrane can still contain a huge amount of detergent which is difficult to remove. Therefore, a technique, which can solve this problem and reduce the preparation time and heterogeneous liposome lamellarity is preferred. Combination of cross-flow technique and conventional detergent depletion method is an economical technique which is used to overcome the problems related to the detergent removal.121 This method is consisted of a filtration device, a pump, a starting reservoir, tubing, an integrated rotary slide valve and a manometer for monitoring the pressure of retentate.114
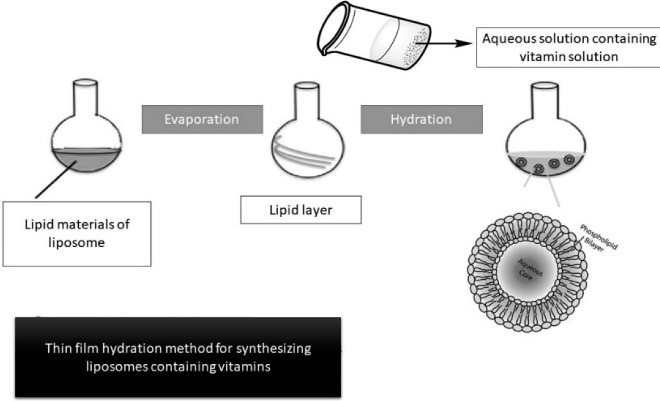
Thin film hydration method
Thin-film hydration method is one of the simplest methods to form liposomes which is followed by extrusion. In this method by removing the organic solvent in a round-bottom flask a thin lipid film is being made. At first step, heterogenous liposomes are made, then homogeneous small liposomes are obtained after extrusion through polycarbonate membranes.122
Schematic thin film hydration method to produce nanoliposomes is illustrated as Figure 2.
Figure 2.
A schematic of thin film hydration method to produce nanoliposomes.
Reverse-phase evaporation method
In reverse-phase evaporation method, several pure or mixed phospholipids can be used. The solvent is removed by rotary evaporator. At next step by purging nitrogen into system, lipid is dissolved in the organic phase again and the reverse phase vesicles will be made in this step. After this step, the aqueous phase is added which contains materials that are going to be encapsulated, followed by sonication and evaporation of solvent until a gel is formed.123
Solvent/surfactant displacement method
In solvent/surfactant displacement method, firstly phospholipids are dissolved in an amphiphilic organic solvent, then the mixture is inserted into the aqueous solution (containing surfactant), and the nanoliposomes are created. Sonication and homogenization are also used to decrease the liposomes formed by different methods.124
Heating method
In this method, high pressures or toxic solvents are not used. All of the compounds including lipids are added to a heating flask and are heated at high temperatures for about 30 min to let lipids are dissolved. Depending on the liposomal compounds, nanovesicles are formed.105
Microfluidization
Microfluidization is a common type of homogenization method which is widely used in food industry. This technique generates high pressures, directing the flow stream to the impingement area through microchannels. This technique provides acceptable costs for large scale productions and does not include toxic solvents, which favors food regulatory requirements. However, there are three drawbacks for it such as contamination, material loss, and hard scale-up.125,126
Sonication method
Sonication is a simple way for production of nanoliposomes from liposomes. In this method, hydrated vesicles are treated by a titanium-tipped probe sonicator for a few minutes with determined seconds of on- and off- intervals in a temperature-controlled environment. At this stage, nanoliposomes in the form of small unilamellar vesicles are formed.105
Extrusion method
In the extrusion method of liposome or nanoliposome preparation, micrometric liposomes are modified to large unilamellar vesicles or nanoliposomes which depends on the pore-size of filters.127 Vesicles are extruded by passing through polycarbonate filters with defined pore sizes for several times. At the end, a homogenous sample (nanoliposome) is produced.105
Advantages and disadvantages of nanoliposomes
Generally, liposomes and nanoliposomes are systems contributing to the recent food trend. These systems can improve the efficiency of food additives and reduce the amounts of required bioactive compounds. They favor the green chemistry, and they can increase the use of natural compounds instead of synthetic constituents.
Comparing the lipid based nanocarriers with other encapsulation materials such as carriers based on polymeric compounds, shows that lipid based nanocarriers have distinctive advantages, such as their ability to provide a protective cover for biological compounds which protects them from degradation, their capability of entrapping the compounds with broad range of solubility, and their high potential for industrial production due to the natural food components being used in their formulations, as well as their minimum production costs. In addition, they have good biocompatibility and biodegradability.128 According to USFDA (United States Food and Drug Administration) guidance, liposomes are different than drug-lipid complexes, emulsions, and microemulsions. Thus, developing a liposomal product needs suitable certification for its chemistry, production, and constituents, beside bioavailability in human pharmacokinetics and labeling (FDA 2018). Nanoliposomes have several advantageous and useful features in the formulation, application and delivery; however, they have limited drawbacks such as poor encapsulation efficiency, lack of enough parameters for continuous industrial production and stability, excessive cost of food components, and the use of high force pressure, homogenization, and sonication.129
In contrary to micron-sized particles, nanoliposomes have remarkable properties such as being metastable and dilatable with water, without any changes in their size distribution. Moreover, nanoliposomes can encapsulate and liberate two materials with different solubilities such as vitamin C and vitamin E, simultaneously. Nanoliposomes are able to include and deliver both vitamin C and vitamin E to an oxidation site, resulting in a synergistic effect. Lipid based vesicles incorporating two bioactive agents are called bifunctional vesicles.130-132 In other study, Chaves et al133 showed that it is feasible to produce “co-encapsulated liposome” vesicle containing two curcumin and vitamin D3, which is stable over the storage time of more than 40 days.
One of the most useful characteristics of liposomes and nanoliposomes is their targetability. Directing bioactive molecules to a specific part in which they can apply their optimum efficacy, is very important. An appropriate and directed release enhances the efficacy of bioactive material, certifies optimal dosage, expands the range of their application, and thus increases the cost-effectiveness of the product.102,134 Moreover, nanoliposomes provide a delivery option for multiple drug molecules at the same time.135,136
Structural appearance and stability of the nanoliposomes are highly associated with the technology adopted for their synthesis. Several problems related to physicochemical properties and stability of conventional nanoliposomes have been reported. Studies showed that liposomes might not be able to provide good physical and chemical stability resulting in low encapsulation efficiency, lipid oxidation, aggregation of vesicles or fast release of bioactive compounds.137,138 Moreover, rapid clearance of most of the liposomes from circulation by the reticuloendothelial system, and fast leakage of water-soluble drugs under improper storage conditions, are some other problems related to these formulations.139,140
Thus, modification of nanoliposomes using methods such as addition of charged substances on their surface, coating their surfaces with polymers, or using some drying techniques after their formation, have emerged as efficient approaches to boost their physicochemical properties and stability.141-143 In addition to the formation of biopolymer-associated liposomes, another promising method to improve the bioactivity, stability, and bioavailability of liposomes is to accomplish additional processes, referred to as “post-processing techniques”, to the aqueous liposome. Some common methods of post-processing include spray drying, freeze drying, and spray freeze drying.144
Important properties of vitamin-loaded nanoliposomes
Particle size, PDI and zeta potential of nanoliposomes
Generally, the average particle size, and polydispersity index (PDI) are parameters that are of high importance in liposomes and nanoliposomes. PDI, shows the quality of the formulated system with regard to the size distribution. The suitable application of nanocarriers including nanoliposomes for a specific route of drug administration is highly dependent on their average particle size, PDI and size stability. Size variations of nano sized systems are of high importance in the formulation of nanocarriers and to achieve ideal results, constant and narrow size distribution should be considered. It should be considered that for nanocarriers, size stability is more important, in comparison to micro size systems, and this is due to their larger specific surface area.145 In a study on the liposomal formulation of glucosamine and vitamin D, it was shown that the encapsulated liposomes have average particle size of 840 nm with great stability.146
PDI is known as a parameter indicating uniformity of nanocarriers. When this parameter is more than 1, it means that different sizes of particles exist per volume unite in the solution. Particle aggregation increases the PDI, so factors causing aggregation can affect PDI. Studies on lipid-based nanoparticles have shown that several factors such as surfactant type and its concentration, instrumental conditions, and lipids used in their formulations can have impact on PDI and particle size.147
A study on encapsulation of vitamin E in nanoliposome revealed that the formulation of liposomes by hydrophobic stabilizers such as gamma oryzanol and lauric acid leads to an increase in the particle size. Though, hydrophilic stabilizer such as PEG 400 did not have significant effect on mean size. In addition, it was shown that by using hydrophobic stabilizers, particle size distribution (PDI) decreases in colloidal system which indicates their stabilizing effect during storage time while PEG had only a slight effect on PDI.148
Results of a study showed that particle sizes of vitamin C-folic acid liposomes and chitosan coated vitamin C- folic acid liposomes are 138.58 nm and 249.13 nm, respectively. Also the reported PDIs were 0.18 and 0.31, respectively, indicating the similarity in the size of nanoliposomes.149 El Adawy et al150 could produce nanoliposomes encapsulating ascorbic acid with particle size of 421.6 nm and PDI of 0.539, showing narrow and homogenous particle size distributions.
Zeta potential is known as the electrokinetic potential between particles which are dispersed in a solution. This parameter shows the surface electrical charges of particles as well as their stability. Zeta potential and the size distribution are both measured by dynamic light scattering (DLS) technique. A large positive or negative zeta potential of particles present in the suspension results in less tendency of them to aggregate, so they will have higher stability.151 To broaden the zeta potential of nanoliposomes, providing stronger repulsion, some researchers use charged substances such as biopolymers as an extra layer covering the surface of nanoliposomes. The results of a study on chitosan-coated nanoliposomes of vitamin D3 showed that coating the nanoliposomes with 0.01% (w/v) chitosan can improve favorable properties of nanoliposomes. The PDI of this covered nanoliposome was close to the monodisperse distribution. Moreover, the increase in size and zeta potential verified the interaction of chitosan and liposome, showing a successful coverage.152 The increase in zeta potential is due to the more adsorbed cationic polymers on the surface of nanoliposomes. Since chitosan possess a high positive charge, the adsorption of chitosan seems to increase the density of positive charge, resulting in a more positive zeta potential.
Encapsulation efficiency
Encapsulation of vitamins into nanoliposomes provides many advantages, which has been highlighted in this review. One of the most significant benefits of encapsulation of vitamins within liposomes is improving their bioavailability in the human body. The encapsulation efficiency (EE) can be considered as the most important factor to determine the capability of nanoliposomes to encapsulate bioactive compounds. Encapsulation efficiency can also be stated as trapping efficiency, incorporation efficiency, or encapsulation percentage.153 The EE depends on the composition of nanoliposomes and the characteristics and concentration of the encapsulated bioactive compound.154 Dalmoro,Bochicchio 152 reported that the EE of D3 and K2-loaded in cholesterol and phosphatidylcholine nanoliposomes was 88.4% and 94.7%, respectively. The chitosan coverage for each nanoliposomal formulation of both vitamins, led to up to 98% increase in the entrapment efficiency. Lee, Park155 have reported that multilamellar vesicle nanoliposomes of retinol, composed of L-α-phosphatidylcholine (PC) and 10% sterol (w/w), could increase the EE of retinol up to 99%.
Since higher EE is correlated with longer shelf-life and better stability of the nanocarrier, it is always favorable to elevate the EE by selecting the best possible mixture of core-wall ratio, processing steps such as drying methods, homogenization process, and other processing variables.156 The EE of an entrapped bioactive in a nanoliposome depends on its partition coefficient and polarity. If loaded ingredients are hydrophobic in nature, they reside in the hydrocarbon chain of nanoliposome. However, if loaded bioactive compounds are polar, they are likely to be positioned in the aqueous core or next to the water–lipid interface, adjacent to the nanoliposome polar head groups.157 Overall, the EE of lipophilic materials is usually higher than hydrophilic ones in nanoliposomes, since they can be tightly positioned in the membrane.158 For instance, in a recent study, two vitamins were simultaneously co-encapsulated within synthesized nanoliposomes: vitamin C as a hydrophilic and vitamin E as a lipophilic bioactive. Vitamin E, and vitamin C displayed an average EE of 95.1% and 77.8 %, respectively. Thus, vitamin E demonstrated the highest EE, which can be attributed to its high lipid affinity.159 A summarized application of nanoliposome for encapsulation of vitamins is given in Table 3.
Table 3. Application of nanoliposome for encapsulation of vitamins .
| Formulation ingredients | Fabrication method | Coating compounds | Encapsulated vitamin | Optimum specifications | Nano-encapsulation efficiency (%) | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Emulsifier | Sterol | Organic solvent | Size (nm) | PDI | Zeta potential (mv) | |||||
| MFGM phospholipid concentrate, SFPC |
Cholesterol | SC-CO2 | Vent-RESS | - | C/E | MFGM-based: 533 SFPC-based: 761 |
- | MFGM-based: −57 SFPC-based: −37 |
MFGM-based: C: 65 E: 77 SFPC-based: C: 72 E: 88 |
160 |
| Phosphatidylcholine | Cholesterol | SC-CO2 | Vent-RESS | - | C/E | 580 | - | −40 | C: 60 E: 88 |
161 |
| Phosphatidylcholine | Cholesterol | SC-CO2 | Vent-RESS | C/E | 2130 | - | −30 | C: 78 E: 95 |
159 | |
| Soybean L-α- phosphatidylcholine | Cholesterol | Ethanol | Novel simil-microfluidic | - | D3/K2/E | D3: 87 K2: 145 E: 118 |
D3: 0.40 K2: 0.31 E: 0.38 |
D3: −38 K2: −36 E: −27 |
D3: 88 K2: 95 E: 93 |
162 |
| Soybean phosphatidylcholine, rapeseed lecithin | - | - | - | - | C | 180 | - | - | - | 163 |
| Phosphatidylcholine | - | Ethanol | Thin-film evaporation | - | C | Below 100 | - | −40 | 66 | 164 |
| Egg yolk phosphatidylcholine | Cholesterol | Dichloromethane: Ethanol | Ethanol injection | - | C | 229 | 0.32 | −26 | 71 | 165 |
| Marine phospholipid | - | Ethanol | Thin-film evaporation | - | C | 237 | 0.20 | −39 | 52 | 128 |
| Phosphatidylcholine | - | - | Thermal method with stirring, homogenization, and sonication | Milk protein concentrate, modified starch, gum Arabic, and maltodextrin | D | 120 | - | - | 74 | 166 |
| Egg yolk Phosphatidylcholine | - | - | Thin-film dispersion | Maltodextrin, gum Arabic, modified starch, and whey protein | D3 | 140 | - | - | - | 167 |
| Phosphatidylcholine | Cholesterol | Ethanol | Thin-film evaporation | β-Lac | A | - | - | - | With β-Lac: 56 Without β-Lac: 51 |
168 |
| Sesame phospholipids | - | Chloroform | Thin layer formation, ultra-sonication and hydration in phosphate buffer solution |
- | C | - | - | - | 80 | 169 |
| Soybean L-α-Phosphatidylcholine | Cholesterol | Ethanol | Microfluidic method | CS | D3/K2 | D3: 87 K2:145 |
D3: 0.24 K2: 0.28 |
D3: –38 K2: –36 |
D3: 88 K2: 95 |
152 |
| Soybean phosphatidylcholine | Cholesterol | Ethanol, Tween 80 | Ethanol injection | - | C/FA |
C-Lip: 145 FA-Lip: 109 |
C-Lip: 0.17 FA-Lip: 0.15 |
C-Lip: 25 FA-Lip: −28 |
C-Lip = 32 FA-Lip = 49 |
149 |
| Soybean phosphatidylcholine | Cholesterol | Ethanol, Tween 80 | Ethanol injection | CS | C/FA |
CFA-Lip: 139 CS-CFA-Lip: 249 |
CFA-Lip: 0.18 CS-CFA-Lip: 0.31 |
CFA-Lip: 34 CS-CFA-Lip: +25 |
CFA-Lip:
C = 35 FA = 64 CS-CFA-Lip: C = 81 FA = 88 |
170 |
| Phospholipid | - | Ethanol | Microfluidic method | Xanthan and guar gums | D3 | 1131 | 0.20 | −49 | 91 | 171 |
| Hydrated Phosphatidylcholine | - | - | Thermal method with sonication treatment | Gamma oryzanol, lauric acid, polyethylene glycol 400 | E |
With vitamin E:
111 to 138 Without vitamin E: 122 to 153 |
0.19 to 0.38 | −37 to −78 | >75 | 148 |
| DPPC | - | Chloroform | Thin film hydration | - | Ascorbic Acid | 422 | 0.54 | - | 23 | 150 |
| Soybean phosphatidylcholine | Cholesterol | Absolute ether | Reversed-phase evaporation | - | Thiamine (TH)/ Niacin (NA) |
NA-Lip: 136 TH-Lip: 126 |
NA-Lip: 0.28 TH-Lip: 0.22 |
- |
NA-Lip: 30 TH-Lip: 30 |
172 |
| Soybean L-α- phosphatidylcholine | Cholesterol | Chloroform | Thin film hydration | Sorbitol | C | 3050 | - | - | 52 | 173 |
| Soybean phosphatidylcholine | Cholesterol | Anhydrous ethanol and chloroform | EI, FH, MFH, and RP | - | Pyridoxine hydrochloride (B6) | 154 with RP method | 0.18 with RP method | - | 44 with MFH method | 174 |
| Soy phosphatidylcholine | Cholesterol | Chloroform | Thin-film hydration | - | E | 181 | - | −22 | - | 175 |
PDI, Poly dispersity index; MFGM, Milk fat globule membrane; SFPC, Sunflower phosphatidylcholine; SC-CO2, Supercritical carbon dioxide; β-Lac, β-lactoglobulin; FA, Folic acid; DPPC, 1,2 Dipalmitoyl-sn-glycero-3-phosphatidylcholine; Vent-RESS, Venturi-based rapid expansion of supercritical solutions; EI, ethanol injection; FH, film hydration; MFH, Modified film hydration; RP, Reverse-phase evaporation; CS, Chitosan; PDI, poly dispersity index; Lip, liposome.
Biocompatibility
Biocompatibility shows the ability of nanoliposomes to apply their proposed function without having any negative effect on the targeted tissues. There are several studies mentioning the biocompatibility and biodegradability of nanoliposomes loaded with bioactive compounds. Liposomes are comprised of natural lipids which are biodegradable, biocompatible, and less immunogenic. Preliminary skin toxicity study of oleic acid liposomes showed that no epidermal cell apoptosis occurs in the skin that is treated with these liposomes, indicating good biocompatibility of them with mouse skin.176 In a study by Al-Ogaidi, chitosan and alginate which are biocompatible compounds were used to manufacture biodegradable and biocompatible nanoliposomes of vitamin C.140
There are several compounds which can be used in the formulation of nanoliposomes to provide biocompatible liposomes. For instance, chitosan has been used in combination with sodium tripolyphosphate to provide core-shell of nanoliposomes for vitamin E encapsulation.177,178 Chitosan provides an outer hydrophilic barrier by formation of a coating layer onto membrane, which can prevent the interaction between liposomes, increase their drug delivery efficiency, improve their structural properties, and biocompatibility.179
In other study, L-α-phosphatidylcholine which packages with 1α,25(OH)2D3, and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino-(polyethylene glycol)2000], were used to produce non-toxic and biocompatible vitamin D nanoliposomes.180
Moreover, in spite of biocompatibility properties of liposomes, these formulations could decrease the toxicity of antimicrobial agent which are potentially toxic.176
Controlled release
The nanoencapsulation systems compared to the direct application of bioactive compounds provide better functional properties such as controlled-release, and higher bioavailability due to their high surface area, in comparison to large particles.181,182 Encapsulation provides a surrounding for bioactive compounds or drugs, which protects them against environmental stresses and controls their release over time.183,184 Several studies have shown the controlled release of different bioactive compounds using nanoliposomes.185,186 Hydrophilic and hydrophobic compounds can simultaneously and efficiently be trapped in the phospholipids bilayer membrane structure of nanoliposome through various physical and chemical interactios.187 Therefore, nanoliposome is able to extend the residence time of compounds or drugs in the ambient, causing the controlled in vivo release of these compounds, which enables the activity of compounds for a longer time.188
The control release of loaded compounds in nanoliposomes can be improved by doing some modifications on the surface of conventional nanoliposomes.189 Researchers have reported that the surface decoration of nanoliposomes of neohesperidin by chitosan and pectin loaded, improve the controlled release of this compound.190 Chitosan nanoparticles were shown to improve the controlled release vitamin C in several studies.191,192 In a study by Liu et al,193 the release behavior of vitamin C was observed for the chitosan and alginate coated nanoliposomes. It was indicated that addition of these polymers onto the surface of anionic nanoliposomes improves the control release of vitamin C during 90 days of storage at 4°C, by a steric barrier on the surface.
Bioavailability of nanoliposomes containing vitamins
Bioavailability is a key parameter of pharmacokinetic, which states the proportion of a bioactive compound or a drug, administered through any non-vascular route which reaches to the systemic circulation.194 A number of studies have indicated the increased bioavailability of vitamins by loading them in nanoliposomes. A review of recent literature shows a growing trend to increase the bioavailability of vitamins by loading them in nanoliposomes. Łukawski et al195 conducted a study with the aim of comparing the profiles of serum concentration of vitamin C in 20 healthy volunteers, after the oral administration either as an aqueous solution or as a liposomal suspension. Their results showed that in the nanoliposomal vitamin C treatment group, Cmax of vitamin gets to higher values compared to the vitamin C solution treatment group (303 µµ compared to 180 µµ). Moreover, for nanoliposomal formulation, the maximum vitamin C blood concentration delay time (Tmax) is longer by about 1 h in comparison to the solution (Tmax=180 min vs. 96 min). The incremented half-life (t1/2>6 h vs. t1/2=4 h) and increased AUC (81 570 µµ* min vs. 45 330 µµ* min) shows that loading vitamin C in nanoliposomes improve the bioavailability of this vitamin. The same result was reported by Davis et al196 who compared the bioavailability of vitamin C in free and liposomal forms.
Moreover, in another study conducted on the bioavailability of encapsulated and free vitamin C, Gopi and Balakrishnan197 indicated that nanoliposomal vitamin C is 1.77 times more bioavailable compared to the free vitamin C. The nanoliposomal vitamin C showed higher values of Cmax (5.23 vs 2.17 mg/dL), AUC0-t (55.86 vs 31.53 mg.h/dL), and AUC0-∞ (78.90 vs 57.12 mg.h/dL), compared to the aqueous solution of vitamin C.
Conclusion
In this review, the application of nanoliposomes to encapsulate vitamins was investigated. Regarding to vitamin encapsulation, nanoliposomes are known as the most used lipid based nanocarriers. One of the most principal reasons for the high use of nanoliposomes to deliver vitamins is related to their ability to encapsulate both hydrophilic and hydrophobic vitamins as well as the ease of their production in industrial scale. However, the quality of produced nanoliposomes is very important. The fabrication of a high quality nanoliposome containing vitamins mainly depends on the production method, utilized materials, and characteristics of liposomes such as particle size, PDI, zeta potential, controlled release, and encapsulation efficiency. Lack of attention to these parameters, while producing nanoliposomes, can easily lead to the fabrication of systems with several drawbacks such as fast release, deposition, low stability, and imperfect protection of vitamins. Recent trends demonstrate that researchers are interested to synthesize nanoliposomes with the highest encapsulation efficiency of vitamins, thus many of the recently published studies have reported reaching to the higher than 70% encapsulation efficiency for different vitamins.
In addition to inherent properties of nanoliposomes, the bioavailability of loaded vitamins into nanoliposomes is another significant factor. Increased bioavailability can be considered as the final goal for the application of nanoliposomes to cover different vitamins. As mentioned in this review, there are clear recent evidences showing that nanoliposomes are able to improve the bioavailability of vitamin C. Moreover, based on our knowledge, some variables still need more investigations. For instance, the replacement of cholesterol with other sterols and their effects on different factors of vitamin-loaded nanoliposomes require more studies. Moreover, interaction of vitamins with other compounds that are incorporated into nanoliposomes, and their effects on several parameters of nanoliposomes such as stability, zeta potential, and encapsulation efficiency should be investigated.
Acknowledgments
This study is related to the project NO. 1399/62182 From Student Research Committee, Shahid Beheshti University of Medical Sciences,Tehran,Iran. We also appreciate the “Student Research Committee” and “Research & Technology Chancellor” in Shahid Beheshti University of Medical Sciences for their financial support of this study.
Author Contributions
Conceptualization: MasoudAman Mohammadi, Parastou Farshi.
Data curation: Parastou Farshi.
Formal Analysis: MasoudAman Mohammadi.
Funding acquisition:No one.
Investigation: Azam Ahmadi, Parisa Ahmadi.
Methodology: MasoudAman Mohammadi, Parastou Farshi.
Project administration: Marjan Ghorbano, Seyede Marzieh Hosseni.
Resources: Masoud Aman Mohammadi.
Software: Masoud Aman Mohammadi.
Supervision: Marjan Ghorbani.
Validation: Mohammad Yousefi.
Visualization: Mohammad Yousefi.
Writing – original draft: Masoud Aman Mohammadi, Parastou Farshi, Azam Ahmadi, Parisa Ahmadi.
Writing – review & editing:Marjan Ghorbani, Seyede Marzieh Hosseini.
Ethical Issues
Not applicable.
Conflict of Interest
The authors declare that they have no competing interests.
References
- 1.Tardy AL, Pouteau E, Marquez D, Yilmaz C, Scholey A. Vitamins and minerals for energy, fatigue and cognition: a narrative review of the biochemical and clinical evidence. Nutrients. 2020;12(1):228. doi: 10.3390/nu12010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gupta M, Aggarwal R, Raina N, Khan A. Vitamin-loaded nanocarriers as nutraceuticals in healthcare applications. In: Rahman M, Beg S, Kumar V, Ahmad FJ, eds. Nanomedicine for Bioactives. Singapore: Springer; 2020. p. 451-70. 10.1007/978-981-15-1664-1_18. [DOI]
- 3.Dehghani-Samani A, Kamali M, Hoseinzadeh-Chahkandak F. The role of vitamins on the prevention and/or treatment of COVID-19 infection; a systematic review. Mod Care J. 2020;17(3):e104740. doi: 10.5812/modernc.104740. [DOI] [Google Scholar]
- 4.Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCartney DM, Byrne DG. Optimisation of vitamin D status for enhanced immuno-protection against COVID-19. Ir Med J. 2020;113(4):58. [PubMed] [Google Scholar]
- 6.Ali N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J Infect Public Health. 2020;13(10):1373–80. doi: 10.1016/j.jiph.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu CY, Wang PW, Alalaiwe A, Lin ZC, Fang JY. Use of lipid nanocarriers to improve oral delivery of vitamins. Nutrients. 2019;11(1):68. doi: 10.3390/nu11010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glowka E, Stasiak J, Lulek J. Drug delivery systems for vitamin D supplementation and therapy. Pharmaceutics. 2019;11(7):347. doi: 10.3390/pharmaceutics11070347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ajeeshkumar KK, Aneesh PA, Raju N, Suseela M, Ravishankar CN, Benjakul S. Advancements in liposome technology: preparation techniques and applications in food, functional foods, and bioactive delivery: a review. Compr Rev Food Sci Food Saf. 2021;20(2):1280–306. doi: 10.1111/1541-4337.12725. [DOI] [PubMed] [Google Scholar]
- 10.Amiri S, Rezazadeh-Bari M, Alizadeh-Khaledabad M, Amiri S. New formulation of vitamin C encapsulation by nanoliposomes: production and evaluation of particle size, stability and control release. Food Sci Biotechnol. 2019;28(2):423–32. doi: 10.1007/s10068-018-0493-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshii K, Hosomi K, Sawane K, Kunisawa J. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front Nutr. 2019;6:48. doi: 10.3389/fnut.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao X, Zhang M, Li C, Jiang X, Su Y, Zhang Y. Benefits of vitamins in the treatment of Parkinson’s disease. Oxid Med Cell Longev. 2019;2019:9426867. doi: 10.1155/2019/9426867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutierrez Gossweiler A, Martinez-Mier EA. Vitamins and oral health. Monogr Oral Sci. 2020;28:59–67. doi: 10.1159/000455372. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez-Hernández D, Anderson GH, Poon AN, Pannia E, Cho CE, Huot PSP, et al. Maternal fat-soluble vitamins, brain development, and regulation of feeding behavior: an overview of research. Nutr Res. 2016;36(10):1045–54. doi: 10.1016/j.nutres.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 15. Kumar PS, Joshiba GJ. Separation and purification of vitamins: vitamins B1, B2, B6, C and K1. In: Inamuddin, ed. Applications of Ion Exchange Materials in Biomedical Industries. Cham: Springer; 2019. p. 177-87. 10.1007/978-3-030-06082-4_9. [DOI]
- 16. Chawla J, Kvarnberg D. Hydrosoluble vitamins. In: Biller J, Ferro JM, eds. Handbook of Clinical Neurology. Elsevier; 2014. p. 891-914. 10.1016/b978-0-7020-4087-0.00059-0. [DOI] [PubMed]
- 17. Cole LA, Kramer PR. Human Physiology, Biochemistry and Basic Medicine. Academic Press; 2015.
- 18.AlZahabi S, Sakr OS, Ramadan AA. Nanostructured lipid carriers incorporating prickly pear seed oil for the encapsulation of vitamin A. J Cosmet Dermatol. 2019;18(6):1875–84. doi: 10.1111/jocd.12891. [DOI] [PubMed] [Google Scholar]
- 19.He W, Guo X, Feng M, Mao N. In vitro and in vivo studies on ocular vitamin A palmitate cationic liposomal in situ gels. Int J Pharm. 2013;458(2):305–14. doi: 10.1016/j.ijpharm.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 20.Moeller H, Martin D, Schrader K, Hoffmann W, Lorenzen PC. Spray-or freeze-drying of casein micelles loaded with vitamin D2: studies on storage stability and in vitro digestibility. LWT. 2018;97:87–93. doi: 10.1016/j.lwt.2018.04.003. [DOI] [Google Scholar]
- 21.Tan Y, Liu J, Zhou H, Muriel Mundo J, McClements DJ. Impact of an indigestible oil phase (mineral oil) on the bioaccessibility of vitamin D3 encapsulated in whey protein-stabilized nanoemulsions. Food Res Int. 2019;120:264–74. doi: 10.1016/j.foodres.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 22.Zai K, Hirota M, Yamada T, Ishihara N, Mori T, Kishimura A, et al. Therapeutic effect of vitamin D3-containing nanostructured lipid carriers on inflammatory bowel disease. J Control Release. 2018;286:94–102. doi: 10.1016/j.jconrel.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Shearer MJ, Okano T. Key pathways and regulators of vitamin K function and intermediary metabolism. Annu Rev Nutr. 2018;38:127–51. doi: 10.1146/annurev-nutr-082117-051741. [DOI] [PubMed] [Google Scholar]
- 24.Colombo ML. An update on vitamin E, tocopherol and tocotrienol-perspectives. Molecules. 2010;15(4):2103–13. doi: 10.3390/molecules15042103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med. 2014;72:76–90. doi: 10.1016/j.freeradbiomed.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaghian N, Goli M. Optimization of the production conditions of primary (W1/O) and double (W1/O/W2) nano-emulsions containing vitamin B12 in skim milk using ultrasound wave by response surface methodology. J Food Meas Charact. 2020;14(6):3216–26. doi: 10.1007/s11694-020-00567-1. [DOI] [Google Scholar]
- 27.Cavalcoli F, Zilli A, Conte D, Massironi S. Micronutrient deficiencies in patients with chronic atrophic autoimmune gastritis: a review. World J Gastroenterol. 2017;23(4):563–72. doi: 10.3748/wjg.v23.i4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li R, Yu K, Wang Q, Wang L, Mao J, Qian J. Pellagra secondary to medication and alcoholism: a case report and review of the literature. Nutr Clin Pract. 2016;31(6):785–9. doi: 10.1177/0884533616660991. [DOI] [PubMed] [Google Scholar]
- 29.Maione-Silva L, de Castro EG, Nascimento TL, Cintra ER, Moreira LC, Cintra BAS, et al. Ascorbic acid encapsulated into negatively charged liposomes exhibits increased skin permeation, retention and enhances collagen synthesis by fibroblasts. Sci Rep. 2019;9(1):522. doi: 10.1038/s41598-018-36682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prantl L, Eigenberger A, Gehmert S, Haerteis S, Aung T, Rachel R, et al. Enhanced resorption of liposomal packed vitamin C monitored by ultrasound. J Clin Med. 2020;9(6):1616. doi: 10.3390/jcm9061616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calderón-Ospina CA, Nava-Mesa MO. B Vitamins in the nervous system: current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS NeurosciTher. 2020;26(1):5–13. doi: 10.1111/cns.13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennedy DO. B vitamins and the brain: mechanisms, dose and efficacy--a review. Nutrients. 2016;8(2):68. doi: 10.3390/nu8020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen LH, Miller JW, de Groot L, Rosenberg IH, Smith AD, Refsum H, et al. Biomarkers of nutrition for development (BOND): vitamin B-12 review. J Nutr. 2018;148(Suppl 4):1995S–2027S. doi: 10.1093/jn/nxy201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosomi K, Kunisawa J. The specific roles of vitamins in the regulation of immunosurveillance and maintenance of immunologic homeostasis in the gut. Immune Netw. 2017;17(1):13–9. doi: 10.4110/in.2017.17.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mikkelsen K, Stojanovska L, Tangalakis K, Bosevski M, Apostolopoulos V. Cognitive decline: a vitamin B perspective. Maturitas. 2016;93:108–13. doi: 10.1016/j.maturitas.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Fluegge K. Propionic acid metabolism, ASD, and vitamin B12: is there a role for environmental nitrous oxide? Int J Dev Neurosci. 2017;57:21–3. doi: 10.1016/j.ijdevneu.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Suh SW, Kim HS, Han JH, Bae JB, Oh DJ, Han JW, et al. Efficacy of vitamins on cognitive function of non-demented people: a systematic review and meta-analysis. Nutrients. 2020;12(4):1168. doi: 10.3390/nu12041168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maillet D, Rajah MN. Age-related differences in brain activity in the subsequent memory paradigm: a meta-analysis. NeurosciBiobehav Rev. 2014;45:246–57. doi: 10.1016/j.neubiorev.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 39. Erdman JW Jr, Macdonald IA, Zeisel SH. Present Knowledge in Nutrition. John Wiley & Sons; 2012.
- 40.Jang S, Han JW, Shin J, Kim TH, Kwak KP, Kim K, et al. Normal-but-low serum folate levels and the risks for cognitive impairment. Psychiatry Investig. 2019;16(7):532–8. doi: 10.30773/pi.2019.05.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shashirekha MN, Mallikarjuna SE, Rajarathnam S. Status of bioactive compounds in foods, with focus on fruits and vegetables. Crit Rev Food Sci Nutr. 2015;55(10):1324–39. doi: 10.1080/10408398.2012.692736. [DOI] [PubMed] [Google Scholar]
- 42.Borel P, Caillaud D, Cano NJ. Vitamin D bioavailability: state of the art. Crit Rev Food Sci Nutr. 2015;55(9):1193–205. doi: 10.1080/10408398.2012.688897. [DOI] [PubMed] [Google Scholar]
- 43.Lamberg-Allardt C. Vitamin D in foods and as supplements. Prog Biophys Mol Biol. 2006;92(1):33–8. doi: 10.1016/j.pbiomolbio.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 44.Naas H, de Oliveira AA, Karpova T, Nunes KP. Toll-like receptor 4 (TLR4) as a possible pathological mechanism in hyperglycemia-associated testicular dysfunction. Med Hypotheses. 2019;127:116–9. doi: 10.1016/j.mehy.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 45.Guo M, Zhu J, Yang T, Lai X, Liu X, Liu J, et al. Vitamin A improves the symptoms of autism spectrum disorders and decreases 5-hydroxytryptamine (5-HT): a pilot study. Brain Res Bull. 2018;137:35–40. doi: 10.1016/j.brainresbull.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 46. Booth SL. Vitamin K: food composition and dietary intakes. Food Nutr Res 2012;56. 10.3402/fnr.v56i0.5505. [DOI] [PMC free article] [PubMed]
- 47. Walther B, Chollet M. Menaquinones, bacteria, and foods: vitamin K2 in the diet. In: Gordeladze JO, ed. Vitamin K2-Vital for Health and Wellbeing. IntechOpen; 2017. p. 63-82. 10.5772/63712. [DOI]
- 48.Shahzad S, Ashraf MA, Sajid M, Shahzad A, Rafique A, Mahmood MS. Evaluation of synergistic antimicrobial effect of vitamins (A, B1, B2, B6, B12, C, D, E and K) with antibiotics against resistant bacterial strains. J Glob Antimicrob Resist. 2018;13:231–6. doi: 10.1016/j.jgar.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Tintino SR, Souza VCA, Silva J, Oliveira-Tintino CDM, Pereira PS, Leal-Balbino TC, et al. Effect of vitamin K3 inhibiting the function of NorA efflux pump and its gene expression on Staphylococcus aureus. Membranes (Basel) 2020;10(6):130. doi: 10.3390/membranes10060130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pimentel-Moral S, Teixeira MC, Fernandes AR, Arráez-Román D, Martínez-Férez A, Segura-Carretero A, et al. Lipid nanocarriers for the loading of polyphenols - a comprehensive review. Adv Colloid Interface Sci. 2018;260:85–94. doi: 10.1016/j.cis.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Devi N, Sarmah M, Khatun B, Maji TK. Encapsulation of active ingredients in polysaccharide-protein complex coacervates. Adv Colloid Interface Sci. 2017;239:136–45. doi: 10.1016/j.cis.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 52.Katouzian I, Faridi Esfanjani A, Jafari SM, Akhavan S. Formulation and application of a new generation of lipid nano-carriers for the food bioactive ingredients. Trends Food Sci Technol. 2017;68:14–25. doi: 10.1016/j.tifs.2017.07.017. [DOI] [Google Scholar]
- 53.Fathi M, Mozafari MR, Mohebbi M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci Technol. 2012;23(1):13–27. doi: 10.1016/j.tifs.2011.08.003. [DOI] [Google Scholar]
- 54. Mohammadi M, Assadpour E, Jafari SM. Encapsulation of food ingredients by nanostructured lipid carriers (NLCs). In: Jafari SM, ed. Lipid-Based Nanostructures for Food Encapsulation Purposes. Academic Press; 2019. p. 217-70. 10.1016/b978-0-12-815673-5.00007-6. [DOI]
- 55.Aboalnaja KO, Yaghmoor S, Kumosani TA, McClements DJ. Utilization of nanoemulsions to enhance bioactivity of pharmaceuticals, supplements, and nutraceuticals: nanoemulsion delivery systems and nanoemulsion excipient systems. Expert Opin Drug Deliv. 2016;13(9):1327–36. doi: 10.1517/17425247.2016.1162154. [DOI] [PubMed] [Google Scholar]
- 56. Shaddel R, Akbari-Alavijeh S, Jafari SM. Encapsulation of food ingredients by Pickering nanoemulsions. In: Jafari SM, ed. Lipid-Based Nanostructures for Food Encapsulation Purposes. Academic Press; 2019. p. 151-76. 10.1016/B978-0-12-815673-5.00005-2. [DOI]
- 57.Salvia-Trujillo L, Soliva-Fortuny R, Rojas-Graü MA, McClements DJ, Martín-Belloso O. Edible nanoemulsions as carriers of active ingredients: a review. Annu Rev Food Sci Technol. 2017;8:439–66. doi: 10.1146/annurev-food-030216-025908. [DOI] [PubMed] [Google Scholar]
- 58.Naseema A, Kovooru L, Behera AK, Kumar KPP, Srivastava P. A critical review of synthesis procedures, applications and future potential of nanoemulsions. Adv Colloid Interface Sci. 2021;287:102318. doi: 10.1016/j.cis.2020.102318. [DOI] [PubMed] [Google Scholar]
- 59. Nejatian M, Saberian H, Jafari SM. Encapsulation of food ingredients by double nanoemulsions. In: Jafari SM, ed. Lipid-Based Nanostructures for Food Encapsulation Purposes. Academic Press; 2019. p. 89-128. 10.1016/b978-0-12-815673-5.00003-9. [DOI]
- 60.Guttoff M, Saberi AH, McClements DJ. Formation of vitamin D nanoemulsion-based delivery systems by spontaneous emulsification: factors affecting particle size and stability. Food Chem. 2015;171:117–22. doi: 10.1016/j.foodchem.2014.08.087. [DOI] [PubMed] [Google Scholar]
- 61.Kadappan AS, Guo C, Gumus CE, Bessey A, Wood RJ, McClements DJ, et al. The efficacy of nanoemulsion-based delivery to improve vitamin D absorption: comparison of in vitro and in vivo studies. Mol Nutr Food Res. 2018;62(4):1700836. doi: 10.1002/mnfr.201700836. [DOI] [PubMed] [Google Scholar]
- 62.Lohith Kumar DH, Sarkar P. Encapsulation of bioactive compounds using nanoemulsions. Environ Chem Lett. 2018;16(1):59–70. doi: 10.1007/s10311-017-0663-x. [DOI] [Google Scholar]
- 63.Rao J, Decker EA, Xiao H, McClements DJ. Nutraceutical nanoemulsions: influence of carrier oil composition (digestible versus indigestible oil) on β-carotene bioavailability. J Sci Food Agric. 2013;93(13):3175–83. doi: 10.1002/jsfa.6215. [DOI] [PubMed] [Google Scholar]
- 64.Wibowo D, Zhao CX, Middelberg AP. Emulsion-templated silica nanocapsules formed using bio-inspired silicification. Chem Commun (Camb) 2014;50(77):11325–8. doi: 10.1039/c4cc04904g. [DOI] [PubMed] [Google Scholar]
- 65.Boskabadi M, Saeedi M, Akbari J, Morteza-Semnani K, Hashemi SMH, Babaei A. Topical gel of vitamin A solid lipid nanoparticles: a hopeful promise as a dermal delivery system. Adv Pharm Bull. 2021;11(4):663–74. doi: 10.34172/apb.2021.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Katouzian I, Jafari SM. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci Technol. 2016;53:34–48. doi: 10.1016/j.tifs.2016.05.002. [DOI] [Google Scholar]
- 67.de Carvalho SM, Noronha CM, da Rosa CG, Sganzerla WG, Bellettini IC, Nunes MR, et al. PVA antioxidant nanocomposite films functionalized with alpha-tocopherol loaded solid lipid nanoparticles. Colloids Surf A PhysicochemEng Asp. 2019;581:123793. doi: 10.1016/j.colsurfa.2019.123793. [DOI] [Google Scholar]
- 68.Geszke-Moritz M, Moritz M. Solid lipid nanoparticles as attractive drug vehicles: composition, properties and therapeutic strategies. Mater Sci Eng C Mater Biol Appl. 2016;68:982–94. doi: 10.1016/j.msec.2016.05.119. [DOI] [PubMed] [Google Scholar]
- 69.Couto R, Alvarez V, Temelli F. Encapsulation of vitamin B2 in solid lipid nanoparticles using supercritical CO2. J Supercrit Fluids. 2017;120(Pt 2):432–42. doi: 10.1016/j.supflu.2016.05.036. [DOI] [Google Scholar]
- 70.Argimón M, Romero M, Miranda P, Mombrú ÁW, Miraballes I, Zimet P, et al. Development and characterization of vitamin A-loaded solid lipid nanoparticles for topical application. J Braz Chem Soc. 2017;28(7):1177–84. doi: 10.21577/0103-5053.20160276. [DOI] [Google Scholar]
- 71.Aditya NP, Macedo AS, Doktorovova S, Souto EB, Kim S, Chang PS, et al. Development and evaluation of lipid nanocarriers for quercetin delivery: a comparative study of solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and lipid nanoemulsions (LNE) LWT. 2014;59(1):115–21. doi: 10.1016/j.lwt.2014.04.058. [DOI] [Google Scholar]
- 72.de Souza IDL, Saez V, de Campos VEB, Nascimento MR, Mansur CRE. Multiple response optimization of beeswax-based nanostructured lipid carriers for the controlled release of vitamin E. J NanosciNanotechnol. 2020;20(1):31–41. doi: 10.1166/jnn.2020.16875. [DOI] [PubMed] [Google Scholar]
- 73.de Souza IDL, Saez V, de Campos VEB, Mansur CRE. Size and vitamin E release of nanostructured lipid carriers with different liquid lipids, surfactants and preparation methods. MacromolSymp. 2019;383(1):1800011. doi: 10.1002/masy.201800011. [DOI] [Google Scholar]
- 74.Pinto F, de Barros DPC, Fonseca LP. Design of multifunctional nanostructured lipid carriers enriched with α-tocopherol using vegetable oils. Ind Crops Prod. 2018;118:149–59. doi: 10.1016/j.indcrop.2018.03.042. [DOI] [Google Scholar]
- 75.Suner S, Yazici O, Rezaie F, Maleki Dizaj E. New synthesized nanostructured lipid carriers (NLCs) for the delivery of vitamin E: production and physicochemical characterization. Sci J Adv Chem Pharm Mater. 2019;2(1):85–8. [Google Scholar]
- 76.Seo TR, Lee I, Chun YG, Park DJ, Lee SH, Kim BK. Improved stability of polyglycerol polyricinoleate-substituted nanostructured lipid carrier cholecalciferol emulsions with different carrier oils. J Food Sci. 2019;84(4):782–91. doi: 10.1111/1750-3841.14423. [DOI] [PubMed] [Google Scholar]
- 77.Zhang M, He J, Zhang W, Liu J. Fabrication of TPGS-stabilized liposome-PLGA hybrid nanoparticle via a new modified nanoprecipitation approach: in vitro and in vivo evaluation. Pharm Res. 2018;35(11):199. doi: 10.1007/s11095-018-2485-3. [DOI] [PubMed] [Google Scholar]
- 78.Bose RJC, Ravikumar R, Karuppagounder V, Bennet D, Rangasamy S, Thandavarayan RA. Lipid-polymer hybrid nanoparticle-mediated therapeutics delivery: advances and challenges. Drug Discov Today. 2017;22(8):1258–65. doi: 10.1016/j.drudis.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 79.Wu XY. Strategies for optimizing polymer-lipid hybrid nanoparticle-mediated drug delivery. Expert Opin Drug Deliv. 2016;13(5):609–12. doi: 10.1517/17425247.2016.1165662. [DOI] [PubMed] [Google Scholar]
- 80.Liu G, Huang W, Babii O, Gong X, Tian Z, Yang J, et al. Novel protein-lipid composite nanoparticles with an inner aqueous compartment as delivery systems of hydrophilic nutraceutical compounds. Nanoscale. 2018;10(22):10629–40. doi: 10.1039/c8nr01009a. [DOI] [PubMed] [Google Scholar]
- 81.Maurya VK, Aggarwal M. A phase inversion based nanoemulsion fabrication process to encapsulate vitamin D3 for food applications. J Steroid Biochem Mol Biol. 2019;190:88–98. doi: 10.1016/j.jsbmb.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 82.Nasiri F, Faghfouri L, Hamidi M. Preparation, optimization, and in-vitro characterization of α-tocopherol-loaded solid lipid nanoparticles (SLNs) Drug Dev Ind Pharm. 2020;46(1):159–71. doi: 10.1080/03639045.2019.1711388. [DOI] [PubMed] [Google Scholar]
- 83.Gupta S, Wairkar S, Bhatt LK. Isotretinoin and α-tocopherol acetate-loaded solid lipid nanoparticle topical gel for the treatment of acne. J Microencapsul. 2020;37(8):557–65. doi: 10.1080/02652048.2020.1823499. [DOI] [PubMed] [Google Scholar]
- 84.Rabelo RS, Oliveira IF, da Silva VM, Prata AS, Hubinger MD. Chitosan coated nanostructured lipid carriers (NLCs) for loading vitamin D: a physical stability study. Int J Biol Macromol. 2018;119:902–12. doi: 10.1016/j.ijbiomac.2018.07.174. [DOI] [PubMed] [Google Scholar]
- 85.Scopel R, Falcão MA, Cappellari AR, Morrone FB, Guterres SS, Cassel E, et al. Lipid-polymer hybrid nanoparticles as a targeted drug delivery system for melanoma treatment. Int J Polym Mater PolymBiomater. 2022;71(2):127–38. doi: 10.1080/00914037.2020.1809406. [DOI] [Google Scholar]
- 86.Sun Y, Chi J, Ye X, Wang S, Liang J, Yue P, et al. Nanoliposomes as delivery system for anthocyanins: physicochemical characterization, cellular uptake, and antioxidant properties. LWT. 2021;139:110554. doi: 10.1016/j.lwt.2020.110554. [DOI] [Google Scholar]
- 87.Yin X, Chen K, Cheng H, et al. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants (Basel) 2022;11(1):153. doi: 10.3390/antiox11010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Deb PK, Al-Attraqchi O, Chandrasekaran B, Paradkar A, Tekade RK. Protein/peptide drug delivery systems: practical considerations in pharmaceutical product development. In: Tekade RK, ed. Basic Fundamentals of Drug Delivery. Academic Press; 2019. p. 651-84. 10.1016/b978-0-12-817909-3.00016-9. [DOI]
- 89. van Rooijen N. Liposomes. In: Delves PJ, ed. Encyclopedia of immunology. 2nd ed. Oxford: Elsevier; 1998. p. 1588-92.
- 90.Sarabandi K, Sadeghi Mahoonak A, Hamishehkar H, Ghorbani M, Jafari SM. Protection of casein hydrolysates within nanoliposomes: antioxidant and stability characterization. J Food Eng. 2019;251:19–28. doi: 10.1016/j.jfoodeng.2019.02.004. [DOI] [Google Scholar]
- 91.Lasic DD. Novel applications of liposomes. Trends Biotechnol. 1998;16(7):307–21. doi: 10.1016/s0167-7799(98)01220-7. [DOI] [PubMed] [Google Scholar]
- 92.Taylor TM, Davidson PM, Bruce BD, Weiss J. Liposomal nanocapsules in food science and agriculture. Crit Rev Food Sci Nutr. 2005;45(7-8):587–605. doi: 10.1080/10408390591001135. [DOI] [PubMed] [Google Scholar]
- 93.Sharma A, Sharma US. Liposomes in drug delivery: progress and limitations. Int J Pharm. 1997;154(2):123–40. doi: 10.1016/s0378-5173(97)00135-x. [DOI] [Google Scholar]
- 94.Thompson AK, Hindmarsh JP, Haisman D, Rades T, Singh H. Comparison of the structure and properties of liposomes prepared from milk fat globule membrane and soy phospholipids. J Agric Food Chem. 2006;54(10):3704–11. doi: 10.1021/jf052859b. [DOI] [PubMed] [Google Scholar]
- 95.Farhang B, Kakuda Y, Corredig M. Encapsulation of ascorbic acid in liposomes prepared with milk fat globule membrane-derived phospholipids. Dairy Sci Technol. 2012;92(4):353–66. doi: 10.1007/s13594-012-0072-7. [DOI] [Google Scholar]
- 96.Mozafari MR, Johnson C, Hatziantoniou S, Demetzos C. Nanoliposomes and their applications in food nanotechnology. J Liposome Res. 2008;18(4):309–27. doi: 10.1080/08982100802465941. [DOI] [PubMed] [Google Scholar]
- 97.Ghorbanzade T, Jafari SM, Akhavan S, Hadavi R. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chem. 2017;216:146–52. doi: 10.1016/j.foodchem.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 98.Tavakoli H, Hosseini O, Jafari SM, Katouzian I. Evaluation of physicochemical and antioxidant properties of yogurt enriched by olive leaf phenolics within nanoliposomes. J Agric Food Chem. 2018;66(35):9231–40. doi: 10.1021/acs.jafc.8b02759. [DOI] [PubMed] [Google Scholar]
- 99.Cansell M, Moussaoui N, Petit AP, Denizot A, Combe N. Feeding rats with liposomes or fish oil differently affects their lipid metabolism. Eur J Lipid Sci Technol. 2006;108(6):459–67. doi: 10.1002/ejlt.200500337. [DOI] [Google Scholar]
- 100.Takahashi K, Inoue Y. Marine by-product phospholipids as booster of medicinal compounds. Adv Food Nutr Res. 2012;65:31–46. doi: 10.1016/b978-0-12-416003-3.00003-2. [DOI] [PubMed] [Google Scholar]
- 101.Thompson AK, Couchoud A, Singh H. Comparison of hydrophobic and hydrophilic encapsulation using liposomes prepared from milk fat globule-derived phospholipids and soya phospholipids. Dairy Sci Technol. 2009;89(1):99–113. doi: 10.1051/dst/2008036. [DOI] [Google Scholar]
- 102.Maherani B, Arab-Tehrany E, Mozafari MR, Gaiani C, Linder M. Liposomes: a review of manufacturing techniques and targeting strategies. CurrNanosci. 2011;7(3):436–52. doi: 10.2174/157341311795542453. [DOI] [Google Scholar]
- 103. McClements DJ. Nanoparticle-and Microparticle-Based Delivery Systems: Encapsulation, Protection and Release of Active Compounds. CRC Press; 2014.
- 104. Rafiee Z, Jafari SM. Application of lipid nanocarriers for the food industry. In: Mérillon J, Ramawat KG, eds. Bioactive Molecules in Food. Cham: Springer; 2019. p. 623-65. 10.1007/978-3-319-78030-6_93. [DOI]
- 105. Mozafari MR. Nanoliposomes: preparation and analysis. In: Weissig V, ed. Liposomes. Totowa, NJ: Humana Press; 2010. p. 29-50. 10.1007/978-1-60327-360-2_2. [DOI] [PubMed]
- 106.Bochicchio S, Barba AA, Grassi G, Lamberti G. Vitamin delivery: carriers based on nanoliposomes produced via ultrasonic irradiation. LWT. 2016;69:9–16. doi: 10.1016/j.lwt.2016.01.025. [DOI] [Google Scholar]
- 107.Barba AA, Bochicchio S, Lamberti G, Dalmoro A. Ultrasonic energy in liposome production: process modelling and size calculation. Soft Matter. 2014;10(15):2574–81. doi: 10.1039/c3sm52879k. [DOI] [PubMed] [Google Scholar]
- 108. Reineccius GA. Liposomes for controlled release in the food industry. In: Encapsulation and Controlled Release of Food Ingredients. American Chemical Society; 1995. p. 113-31. 10.1021/bk-1995-0590.ch011. [DOI]
- 109. Lakkis JM. Encapsulation and Controlled Release Technologies in Food Systems. Wiley Online Library; 2007.
- 110.Jahadi M, Khosravi-Darani K, Ehsani MR, Mozafari MR, Saboury AA, Pourhosseini PS. The encapsulation of flavourzyme in nanoliposome by heating method. J Food Sci Technol. 2015;52(4):2063–72. doi: 10.1007/s13197-013-1243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Panigrahi SS, Syed I, Sivabalan S, Sarkar P. Nanoencapsulation strategies for lipid-soluble vitamins. Chemical Papers. 2019;73(1):1–6. doi: 10.1007/s11696-018-0559-7. [DOI] [Google Scholar]
- 112.Bochicchio S, Dalmoro A, Lamberti G, Barba AA. Advances in nanoliposomes production for ferrous sulfate delivery. Pharmaceutics. 2020;12(5):445. doi: 10.3390/pharmaceutics12050445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Laouini A, Jaafar-Maalej C, Sfar S, Charcosset C, Fessi H. Liposome preparation using a hollow fiber membrane contactor--application to spironolactone encapsulation. Int J Pharm. 2011;415(1-2):53–61. doi: 10.1016/j.ijpharm.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 114.Huang Z, Li X, Zhang T, Song Y, She Z, Li J, et al. Progress involving new techniques for liposome preparation. Asian J Pharm Sci. 2014;9(4):176–82. doi: 10.1016/j.ajps.2014.06.001. [DOI] [Google Scholar]
- 115.Lesoin L, Boutin O, Crampon C, Badens E. CO2/water/surfactant ternary systems and liposome formation using supercritical CO2: a review. Colloids Surf APhysicochemEng Asp. 2011;377(1-3):1–14. doi: 10.1016/j.colsurfa.2011.01.027. [DOI] [Google Scholar]
- 116.Lesoin L, Crampon C, Boutin O, Badens E. Preparation of liposomes using the supercritical anti-solvent (SAS) process and comparison with a conventional method. J Supercrit Fluids. 2011;57(2):162–74. doi: 10.1016/j.supflu.2011.01.006. [DOI] [Google Scholar]
- 117.Aguilar-Pérez KM, Avilés-Castrillo JI, Medina DI, Parra-Saldivar R, Iqbal HMN. Insight Into Nanoliposomes as Smart Nanocarriers for Greening the Twenty-First Century Biomedical Settings. Front Bioeng Biotechnol. 2020 Dec 15;8:579536. doi: 10.3389/fbioe.2020.579536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Perrie Y, Kastner E, Khadke S, Roces CB, Stone P. Manufacturing methods for liposome adjuvants. In: Fox CB, ed. Vaccine Adjuvants. New York, NY: Springer; 2017. p. 127-44. 10.1007/978-1-4939-6445-1_9. [DOI] [PubMed]
- 119.Patil YP, Jadhav S. Novel methods for liposome preparation. Chem Phys Lipids. 2014;177:8–18. doi: 10.1016/j.chemphyslip.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 120.Massing U, Cicko S, Ziroli V. Dual asymmetric centrifugation (DAC)--a new technique for liposome preparation. J Control Release. 2008;125(1):16–24. doi: 10.1016/j.jconrel.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 121.Schubert R, Beyer K, Wolburg H, Schmidt KH. Structural changes in membranes of large unilamellar vesicles after binding of sodium cholate. Biochemistry. 1986;25(18):5263–9. doi: 10.1021/bi00366a042. [DOI] [PubMed] [Google Scholar]
- 122. Zhang H. Thin-film hydration followed by extrusion method for liposome preparation. In: D’Souza GG, ed. Liposomes. New York, NY: Springer; 2017. p. 17-22. 10.1007/978-1-4939-6591-5_2. [DOI] [PubMed]
- 123.Treat J, Huang C, Damjanov N, Walker S, Drobins P, Gokhale P, et al. Phase I trial in advanced malignancies with liposome encapsulated paclitaxel (LEP) Lung Cancer. 2000;29(1 Suppl 1):64. doi: 10.1016/s0169-5002(00)80206-8. [DOI] [Google Scholar]
- 124. Sarabandi K, Rafiee Z, Khodaei D, Jafari SM. Encapsulation of food ingredients by nanoliposomes. In: Jafari SM, ed. Lipid-Based Nanostructures for Food Encapsulation Purposes. Academic Press; 2019. p. 347-404. 10.1016/b978-0-12-815673-5.00009-x. [DOI]
- 125.Anwekar H, Patel S, Singhai AK. Liposome-as drug carriers. Int J Pharm Life Sci. 2011;2(7):945–51. [Google Scholar]
- 126.Kataria S, Sandhu P, Bilandi A, Akanksha M, Kapoor B. Stealth liposomes: a review. Int J Res Ayurveda Pharm. 2011;2(5):1534–8. [Google Scholar]
- 127.Berger N, Sachse A, Bender J, Schubert R, Brandl M. Filter extrusion of liposomes using different devices: comparison of liposome size, encapsulation efficiency, and process characteristics. Int J Pharm. 2001;223(1-2):55–68. doi: 10.1016/s0378-5173(01)00721-9. [DOI] [PubMed] [Google Scholar]
- 128.Hassane Hamadou A, Huang WC, Xue C, Mao X. Formulation of vitamin C encapsulation in marine phospholipids nanoliposomes: characterization and stability evaluation during long term storage. LWT. 2020;127:109439. doi: 10.1016/j.lwt.2020.109439. [DOI] [Google Scholar]
- 129.Khorasani S, Danaei M, Mozafari MR. Nanoliposome technology for the food and nutraceutical industries. Trends Food Sci Technol. 2018;79:106–15. doi: 10.1016/j.tifs.2018.07.009. [DOI] [Google Scholar]
- 130.Agrawal M, Ajazuddin Ajazuddin, Tripathi DK, Saraf S, Saraf S, Antimisiaris SG, et al. Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer’s disease. J Control Release. 2017;260:61–77. doi: 10.1016/j.jconrel.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 131. Grumezescu A, Oprea AE. Nanotechnology Applications in Food: Flavor, Stability, Nutrition and Safety. Academic Press; 2017.
- 132.Xu H, Zhang W, Li Y, Ye FF, Yin PP, Yu X, et al. The bifunctional liposomes constructed by poly(2-ethyl-oxazoline)-cholesteryl methyl carbonate: an effectual approach to enhance liposomal circulation time, pH-sensitivity and endosomal escape. Pharm Res. 2014;31(11):3038–50. doi: 10.1007/s11095-014-1397-0. [DOI] [PubMed] [Google Scholar]
- 133.Chaves MA, Oseliero Filho PL, Jange CG, Sinigaglia-Coimbra R, Oliveira CLP, Pinho SC. Structural characterization of multilamellar liposomes coencapsulating curcumin and vitamin D3. Colloids Surf A PhysicochemEng Asp. 2018;549:112–21. doi: 10.1016/j.colsurfa.2018.04.018. [DOI] [Google Scholar]
- 134.Mozafari MR. Liposomes: an overview of manufacturing techniques. Cell Mol Biol Lett. 2005;10(4):711–9. [PubMed] [Google Scholar]
- 135.Aveling E, Zhou J, Lim YF, Mozafari MR. Targeting lipidic nanocarriers: current strategies and problems. Pharmakeftiki. 2006;19(4):101–9. [Google Scholar]
- 136.Stephenson SM, Low PS, Lee RJ. Folate receptor-mediated targeting of liposomal drugs to cancer cells. Methods Enzymol. 2004;387:33–50. doi: 10.1016/s0076-6879(04)87003-4. [DOI] [PubMed] [Google Scholar]
- 137.Gibbs BF, Kermasha S, Alli I, Mulligan CN. Encapsulation in the food industry: a review. Int J Food Sci Nutr. 1999;50(3):213–24. doi: 10.1080/096374899101256. [DOI] [PubMed] [Google Scholar]
- 138.Tan C, Xue J, Lou X, Abbas S, Guan Y, Feng B, et al. Liposomes as delivery systems for carotenoids: comparative studies of loading ability, storage stability and in vitro release. Food Funct. 2014;5(6):1232–40. doi: 10.1039/c3fo60498e. [DOI] [PubMed] [Google Scholar]
- 139.Gregoriadis G. Overview of liposomes. Journal of antimicrobial chemotherapy. 1991 Jan 1;28(suppl_B):39–48. doi: 10.1093/jac/28.suppl_b.39. [DOI] [PubMed] [Google Scholar]
- 140.Al-Ogaidi I. Al-Ogaidi IEvaluation of the Antioxidant and Anticancer Effects of Biodegradable/Biocompatible Chitosan–Alginate Nanoparticles Loaded withIntJPharmResAllied Sci. 2018 Jan. 1;7(3):189–97. [Google Scholar]
- 141.Hsieh YF, Chen TL, Wang YT, Chang JH, Chang HM. Properties of liposomes prepared with various lipids. J Food Sci. 2002;67(8):2808–13. doi: 10.1111/j.1365-2621.2002.tb08820.x. [DOI] [Google Scholar]
- 142.Park SN, Jo NR, Jeon SH. Chitosan-coated liposomes for enhanced skin permeation of resveratrol. J Ind Eng Chem. 2014;20(4):1481–5. doi: 10.1016/j.jiec.2013.07.035. [DOI] [Google Scholar]
- 143.Takeuchi H, Yamamoto H, Toyoda T, Toyobuku H, Hino T, Kawashima Y. Physical stability of size controlled small unilameller liposomes coated with a modified polyvinyl alcohol. Int J Pharm. 1998;164(1-2):103–11. doi: 10.1016/s0378-5173(97)00404-3. [DOI] [Google Scholar]
- 144.Yu JY, Chuesiang P, Shin GH, Park HJ. Post-processing techniques for the improvement of liposome stability. Pharmaceutics. 2021;13(7):1023. doi: 10.3390/pharmaceutics13071023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):57. doi: 10.3390/pharmaceutics10020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Madrigal Redondo G, Vargas Zúñiga R, Chavarría Rojas M, Sibaja Rodríguez S, Chaves Noguera S. Physicochemical characterization of a liposomal formulation based on glucosamine and vitamin D, commercialized as a nutritional supplement. J Pharm Res Int. 2018;22(2):1–6. doi: 10.9734/jpri/2018/39746. [DOI] [Google Scholar]
- 147.Baviskar A, Hiremath S, Devanna N, Akul M. Influence of various process variables and formulation excipients on the engineering of sertaconazole solid lipid nanoparticles. IOSR J Pharm. 2016;6(7):51–63. doi: 10.9790/3013-06715163. [DOI] [Google Scholar]
- 148.Amiri S, Ghanbarzadeh B, Hamishehkar H, Hosein M, Babazadeh A, Adun P. Vitamin E loaded nanoliposomes: effects of gammaoryzanol, polyethylene glycol and lauric acid on physicochemical properties. Colloid Interface Sci Commun. 2018;26:1–6. doi: 10.1016/j.colcom.2018.07.003. [DOI] [Google Scholar]
- 149.Jiao Z, Wang X, Yin Y, Xia J. Preparation and evaluation of vitamin C and folic acid-coloaded antioxidant liposomes. Part Sci Technol. 2019;37(4):453–9. doi: 10.1080/02726351.2017.1391907. [DOI] [Google Scholar]
- 150.El Adawy B, Salama A, Gaber MH, El Saeid A. The role of liposome-encapsulated ascorbic acid on hemoglobin damage by gamma irraidation. Egypt J Biomed EngBiophys. 2018;19(1):13–24. doi: 10.21608/ejbbe.2018.3301.1017. [DOI] [Google Scholar]
- 151. Paolino D, Sinha P, Fresta M, Ferrari M. Drug delivery systems. In: Webster JG, ed. Encyclopedia of Medical Devices and Instrumentation. Wiley; 2006. 10.1002/0471732877.emd274. [DOI]
- 152.Dalmoro A, Bochicchio S, Lamberti G, Bertoncin P, Janssens B, Barba AA. Micronutrients encapsulation in enhanced nanoliposomal carriers by a novel preparative technology. RSC Adv. 2019;9(34):19800–12. doi: 10.1039/c9ra03022k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kanásová M, Nesměrák K. Systematic review of liposomes’ characterization methods. Monatsh Chem. 2017;148(9):1581–93. doi: 10.1007/s00706-017-1994-9. [DOI] [Google Scholar]
- 154.Park SJ, Garcia CV, Shin GH, Kim JT. Development of nanostructured lipid carriers for the encapsulation and controlled release of vitamin D3. Food Chem. 2017;225:213–9. doi: 10.1016/j.foodchem.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 155.Lee DU, Park HW, Lee SC. Comparing the stability of retinol in liposomes with cholesterol, β-sitosterol, and stigmasterol. Food Sci Biotechnol. 2021;30(3):389–94. doi: 10.1007/s10068-020-00871-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Timilsena YP, Haque MA, Adhikari B. Encapsulation in the food industry: a brief historical overview to recent developments. Food Nutr Sci. 2020;11(6):481–508. doi: 10.4236/fns.2020.116035. [DOI] [Google Scholar]
- 157.Mohan A, Narayanan S, Sethuraman S, Krishnan UM. Novel resveratrol and 5-fluorouracil coencapsulated in PEGylated nanoliposomes improve chemotherapeutic efficacy of combination against head and neck squamous cell carcinoma. Biomed Res Int. 2014;2014:424239. doi: 10.1155/2014/424239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Has C, Sunthar P. A comprehensive review on recent preparation techniques of liposomes. J Liposome Res. 2020;30(4):336–65. doi: 10.1080/08982104.2019.1668010. [DOI] [PubMed] [Google Scholar]
- 159.Jash A, Hatami T, Rizvi SSH. Phosphatidylcholine solubility in supercritical carbon dioxide: experimental data, thermodynamic modeling, and application in bioactive-encapsulated liposome synthesis. J Supercrit Fluids. 2020;158:104720. doi: 10.1016/j.supflu.2019.104720. [DOI] [Google Scholar]
- 160.Jash A, Ubeyitogullari A, Rizvi SSH. Synthesis of multivitamin-loaded heat stable liposomes from milk fat globule membrane phospholipids by using a supercritical-CO2 based system. Green Chem. 2020;22(16):5345–56. doi: 10.1039/d0gc01674h. [DOI] [Google Scholar]
- 161.Sharifi F, Jash A, Abbaspourrad A, Rizvi SSH. Generation of ironized and multivitamin-loaded liposomes using venturi-based rapid expansion of a supercritical solution (Vent-RESS) Green Chem. 2020;22(5):1618–29. doi: 10.1039/c9gc04018h. [DOI] [Google Scholar]
- 162.Bochicchio S, Dalmoro A, De Simone V, Bertoncin P, Lamberti G, Barba AA. Simil-microfluidic nanotechnology in manufacturing of liposomes as hydrophobic antioxidants skin release systems. Cosmetics. 2020;7(2):22. doi: 10.3390/cosmetics7020022. [DOI] [Google Scholar]
- 163. Davis JL, Paris HL, Beals JW, et al. Liposomal-encapsulated Ascorbic Acid: Influence on Vitamin C Bioavailability and Capacity to Protect Against Ischemia-Reperfusion Injury. Nutr Metab Insights 2016;9:25-30. Published 2016 Jun 20. 10.4137/NMI.S39764. [DOI] [PMC free article] [PubMed]
- 164. Maghsoudnia N, Edalat M, Hajimolaali M, Iranpour S, Khoshkhat P, Inanloo P, Baradaran Eftekhari R, Antimisiaris SG, Abedin Dorkoosh F. Liposomal Supplements. In Liposomes for Functional Foods and Nutraceuticals, Apple Academic Press 2022: 235-278.. 10.1201/9781003277361-8. [DOI]
- 165.Liu X, Wang P, Zou YX, Luo ZG, Tamer TM. Co-encapsulation of vitamin C and β-carotene in liposomes: storage stability, antioxidant activity, and in vitro gastrointestinal digestion. Food Res Int. 2020;136:109587. doi: 10.1016/j.foodres.2020.109587. [DOI] [PubMed] [Google Scholar]
- 166.Jafari SM, Vakili S, Dehnad D. Production of a functional yogurt powder fortified with nanoliposomal vitamin D through spray drying. Food Bioproc Tech. 2019;12(7):1220–31. doi: 10.1007/s11947-019-02289-9. [DOI] [Google Scholar]
- 167.Jafari SM, Masoudi S, Bahrami A. A Taguchi approach production of spray-dried whey powder enriched with nanoencapsulated vitamin D3. Dry Technol. 2019;37(16):2059–71. doi: 10.1080/07373937.2018.1552598. [DOI] [Google Scholar]
- 168.Rovoli M, Pappas I, Lalas S, Gortzi O, Kontopidis G. In vitro and in vivo assessment of vitamin A encapsulation in a liposome-protein delivery system. J Liposome Res. 2019;29(2):142–52. doi: 10.1080/08982104.2018.1502314. [DOI] [PubMed] [Google Scholar]
- 169.Hudiyanti D, Hamidi NI, Anugrah DS, Salimah SN, Siahaan P. Encapsulation of vitamin C in sesame liposomes: computational and experimental studies. Open Chem. 2019;17(1):537–43. doi: 10.1515/chem-2019-0061. [DOI] [Google Scholar]
- 170.Jiao Z, Wang X, Yin Y, Xia J, Mei Y. Preparation and evaluation of a chitosan-coated antioxidant liposome containing vitamin C and folic acid. J Microencapsul. 2018;35(3):272–80. doi: 10.1080/02652048.2018.1467509. [DOI] [PubMed] [Google Scholar]
- 171.Chaves MA, Oseliero Filho PL, Jange CG, Sinigaglia-Coimbra R, Oliveira CLP, Pinho SC. Structural characterization of multilamellar liposomes coencapsulating curcumin and vitamin D3. Colloids Surf A PhysicochemEng Asp. 2018;549:112–21. doi: 10.1016/j.colsurfa.2018.04.018. [DOI] [Google Scholar]
- 172.He H, Lu Y, Qi J, Zhao W, Dong X, Wu W. Biomimetic thiamine- and niacin-decorated liposomes for enhanced oral delivery of insulin. Acta Pharm Sin B. 2018;8(1):97–105. doi: 10.1016/j.apsb.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Parhizkar E, Rashedinia M, Karimi M, Alipour S. Design and development of vitamin C-encapsulated proliposome with improved in-vitro and ex-vivo antioxidant efficacy. J Microencapsul. 2018;35(3):301–11. doi: 10.1080/02652048.2018.1477845. [DOI] [PubMed] [Google Scholar]
- 174.Abd-El-Azim H, Ramadan A, Nafee N, Khalafallah N. Entrapment efficiency of pyridoxine hydrochloride in unilamellar liposomes: experimental versus model-generated data. J Liposome Res. 2018;28(2):112–6. doi: 10.1080/08982104.2016.1275679. [DOI] [PubMed] [Google Scholar]
- 175.Vicario-de-la-Torre M, Caballo-González M, Vico E, Morales-Fernández L, Arriola-Villalobos P, De Las Heras B, et al. Novel nano-liposome formulation for dry eyes with components similar to the preocular tear film. Polymers (Basel) 2018;10(4):425. doi: 10.3390/polym10040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sadrieh N, Davis CD, Snyderwine EG. N-acetyltransferase expression and metabolic activation of the food-derived heterocyclic amines in the human mammary gland. Cancer Res. 1996;56(12):2683–7. [PubMed] [Google Scholar]
- 177.Gan Q, Wang T, Cochrane C, McCarron P. Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surf B Biointerfaces. 2005;44(2-3):65–73. doi: 10.1016/j.colsurfb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 178.Tsai ML, Chen RH, Bai SW, Chen WY. The storage stability of chitosan/tripolyphosphate nanoparticles in a phosphate buffer. Carbohydr Polym. 2011;84(2):756–61. doi: 10.1016/j.carbpol.2010.04.040. [DOI] [Google Scholar]
- 179.Takeuchi H, Matsui Y, Sugihara H, Yamamoto H, Kawashima Y. Effectiveness of submicron-sized, chitosan-coated liposomes in oral administration of peptide drugs. Int J Pharm. 2005;303(1-2):160–70. doi: 10.1016/j.ijpharm.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 180.Lee YC, Huang CT, Cheng FY, Hung SY, Lin TM, Tsai YC, et al. The clinical implication of vitamin D nanomedicine for peritoneal dialysis-related peritoneal damage. Int J Nanomedicine. 2019;14:9665–75. doi: 10.2147/ijn.s215717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Assadpour E, Jafari SM. Nanoencapsulation: techniques and developments for food applications. In: López Rubio A, Fabra Rovira MJ, Martínez Sanz M, Gómez-Mascaraque LG, eds. Nanomaterials for Food Applications. Elsevier; 2019. p. 35-61. 10.1016/b978-0-12-814130-4.00003-8. [DOI]
- 182.Jafari SM, McClements DJ. Nanotechnology approaches for increasing nutrient bioavailability. Adv Food Nutr Res. 2017;81:1–30. doi: 10.1016/bs.afnr.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 183.Faridi Esfanjani A, Jafari SM. Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids Surf B Biointerfaces. 2016;146:532–43. doi: 10.1016/j.colsurfb.2016.06.053. [DOI] [PubMed] [Google Scholar]
- 184.Assadpour E, Mahdi Jafari S. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit Rev Food Sci Nutr. 2019;59(19):3129–51. doi: 10.1080/10408398.2018.1484687. [DOI] [PubMed] [Google Scholar]
- 185.Bahrami A, Delshadi R, Assadpour E, Jafari SM, Williams L. Antimicrobial-loaded nanocarriers for food packaging applications. Adv Colloid Interface Sci. 2020;278:102140. doi: 10.1016/j.cis.2020.102140. [DOI] [PubMed] [Google Scholar]
- 186.Pabast M, Shariatifar N, Beikzadeh S, Jahed G. Effects of chitosan coatings incorporating with free or nano-encapsulated Satureja plant essential oil on quality characteristics of lamb meat. Food Control. 2018;91:185–92. doi: 10.1016/j.foodcont.2018.03.047. [DOI] [Google Scholar]
- 187.Ma QH, Kuang YZ, Hao XZ, Gu N. Preparation and characterization of tea polyphenols and vitamin E loaded nanoscale complex liposome. J NanosciNanotechnol. 2009;9(2):1379–83. doi: 10.1166/jnn.2009.c161. [DOI] [PubMed] [Google Scholar]
- 188.Lee JS, Chung D, Lee HG. Preparation and characterization of calcium pectinate gel beads entrapping catechin-loaded liposomes. Int J Biol Macromol. 2008;42(2):178–84. doi: 10.1016/j.ijbiomac.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 189.Aguilar-Pérez KM, Avilés-Castrillo JI, Medina DI, Parra-Saldivar R, Iqbal HMN. Insight into nanoliposomes as smart nanocarriers for greening the twenty-first century biomedical settings. Front BioengBiotechnol. 2020;8:579536. doi: 10.3389/fbioe.2020.579536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Karim N, Shishir MRI, Chen W. Surface decoration of neohesperidin-loaded nanoliposome using chitosan and pectin for improving stability and controlled release. Int J Biol Macromol. 2020;164:2903–14. doi: 10.1016/j.ijbiomac.2020.08.174. [DOI] [PubMed] [Google Scholar]
- 191.Cho Y, Kim JT, Park HJ. Size-controlled self-aggregated N-acyl chitosan nanoparticles as a vitamin C carrier. CarbohydrPolym. 2012;88(3):1087–92. doi: 10.1016/j.carbpol.2012.01.074. [DOI] [Google Scholar]
- 192.Aresta A, Calvano CD, Trapani A, Cellamare S, Zambonin CG, De Giglio E. Development and analytical characterization of vitamin(s)-loaded chitosan nanoparticles for potential food packaging applications. J Nanopart Res. 2013;15(4):1592. doi: 10.1007/s11051-013-1592-7. [DOI] [Google Scholar]
- 193.Liu W, Tian M, Kong Y, Lu J, Li N, Han J. Multilayered vitamin C nanoliposomes by self-assembly of alginate and chitosan: long-term stability and feasibility application in mandarin juice. LWT. 2017;75:608–15. doi: 10.1016/j.lwt.2016.10.010. [DOI] [Google Scholar]
- 194.Toutain PL, Bousquet-Mélou A. Bioavailability and its assessment. J Vet PharmacolTher. 2004;27(6):455–66. doi: 10.1111/j.1365-2885.2004.00604.x. [DOI] [PubMed] [Google Scholar]
- 195.Łukawski M, Dałek P, Borowik T, Foryś A, Langner M, Witkiewicz W, et al. New oral liposomal vitamin C formulation: properties and bioavailability. J Liposome Res. 2020;30(3):227–34. doi: 10.1080/08982104.2019.1630642. [DOI] [PubMed] [Google Scholar]
- 196.Davis JL, Paris HL, Beals JW, Binns SE, Giordano GR, Scalzo RL, et al. Liposomal-encapsulated ascorbic acid: influence on vitamin C bioavailability and capacity to protect against ischemia-reperfusion injury. NutrMetab Insights. 2016;9:25–30. doi: 10.4137/nmi.s39764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gopi S, Balakrishnan P. Evaluation and clinical comparison studies on liposomal and non-liposomal ascorbic acid (vitamin C) and their enhanced bioavailability. J Liposome Res. 2021;31(4):356–64. doi: 10.1080/08982104.2020.1820521. [DOI] [PubMed] [Google Scholar]