Abstract
Despite a significant reduction in the burden of malaria in children under five years-old, the efficient implementation of seasonal malaria chemoprevention (SMC) at large scale remains a major concern in areas with long malaria transmission. Low coverage rate in the unattainable areas during the rainy season, a shift in the risk of malaria to older children and the rebound in malaria incidence after stopping drug administration are mainly reported in these areas. These gaps represent a major challenge in the efficient implementation of SMC measures. An open randomized study was conducted to assess the effect of a fifth additional round to current regime of SMC in older children living in Dangassa, a rural malaria endemic area. Poisson regression Model was used to estimate the reduction in malaria incidence in the intervention group compared to the control group including age groups (5–9 and 10–14 years) and the use of long-lasting insecticidal nets (LLINs; Yes or No) with a threshold at 5%. Overall, a downward trend in participation rate was observed from August (94.3%) to November (87.2%). In November (round 4), the risk of malaria incidence was similar in both groups (IRR = 0.66, 95%CI [0.35–1.22]). In December (round 5), a decrease of 51% in malaria incidence was observed in intervention group compared to control group adjusted for age groups and the use of LLINs (IRR = 0.49, 95%CI [0.26–0.94]), of which 17% of reduction is attributable to the 5th round in the intervention group. An additional fifth round of SMC resulted in a significant reduction of malaria incidence in the intervention group. The number of SMC rounds could be adapted to the local condition of malaria transmission.
Keywords: Malaria incidence, SMC, Fifth round, Older children, Mali
1. Introduction
In Mali, the National Malaria Control Program (NMCP) has adopted seasonal malaria chemoprevention (SMC) to prevent malaria illness in children under five years (MS/PNLP, 2013). SMC consists of complete treatment course of Sulfadoxine-Pyrimethamine (SP) plus Amodiaquine (AQ) at a monthly interval during the high transmission period of malaria (OMS, 2012). Previous reports in Mali indicate that SMC implementation was effective in areas where malaria transmission does not exceed 4 months (Diawara et al., 2017; Konate et al., 2020), Senegal (Cisse et al., 2016), Burkina Faso (Druetz et al., 2018) and Ghana (Tagbor et al., 2016). Based on the results of different studies, SMC will be able to significantly enhance the impact of other ongoing interventions in Sub-Saharan African countries.
Efficient implementation of SMC at large scale and long term remains a major concern, particularly in long transmission areas. Usually, SMC was delivered over four monthly rounds from July to October, coinciding with the rainy season. However, malaria transmission season is longer and starts early as June and ends later in December in Dangassa area, increasing malaria incidence during non-SMC implementation periods. Also several factors including, low coverage rate, low compliance to the three-day SP/AQ uptake (Coldiron et al., 2017), a shift in the risk of malaria to older children (Okiro et al., 2009) and the rebound in malaria incidence after stopping drug administration (Greenwood et al., 1995) were major challenges reported in areas with long and intense malaria transmission. These challenges represent major obstacles in the efforts of malaria control and therefore additional measures are required to strengthen SMC implementation in these areas.
The feasibility of an additional round to ordinary SMC delivery was assessed in endemic areas of Sub-Saharan African countries and should be contextualized to adapt its implementation to the local setting of malaria transmission (Malaria Consortium Nigeria: Seasonal Malaria Chemoprevention Programme Start-Up Guide, 2015; Traore et al., 2022). By adding an additional round early at the beginning of rainy season in children under five years of age showed a good adherence with a high coverage rate in Burkina (Traore et al., 2022). In addition, studies conducted in areas with long-transmission reported a decrease in the prevalence of malaria infection and anemia in older children (Konaté et al., 2021; Thera et al., 2018). First pilot non-randomized study conducted in Dangassa in 2019 in collaboration with the National Malaria Control Program (MNCP) showed a reduction in the prevalence of parasitemia in children 5 to 14 years of age (Konaté et al., 2021). The current study assesses the effect of an additional fifth round as well as the extension of SMC to children aged 5–14 years in Dangassa. These results will allow the improvement and efficient implementation of SMC according to the epidemiological setting in Mali.
2. Methods
2.1. Site and population
The study was conducted in Dangassa located 82 Km Southwest of Bamako, in the Region of Koulikoro, Mali. Dangassa is one of West Africa's International Center for Excellence in Malaria Research (WA-ICEMR) study sites since 2010 as previously described by Konate et al. (Konate et al., 2020). Malaria transmission in Dangassa is seasonal, most of the cases occur between July and December (Konate et al., 2020).
From 2010 to 2017, an open dynamic cohort of 1400 participants aged 6 months to 85 years were followed to assess the impact of current control interventions on epidemiological trends of malaria in Mali. In 2018, the follow-up of a second cohort of 1400 participants started (Shaffer et al., 2018). The estimated population in 2020 was 7488 inhabitants (ICEMR census data, April 2020). All children aged 5 to 14 years old living in the village of Dangassa were enrolled in this pilot study in 2020. The ICEMR program has collaborated with the NMCP for SMC routine implementation in Dangassa since 2015.
2.2. Study design
An open randomized study was carried out to estimate the effect of an additional fifth round to the current SMC regimen (four rounds) in children 5–14 years. The study participants were selected from the database of population census.
2.3. Sample size determination and participant recruitment
Based on malaria incidence among children in rural areas (32%) in Mali (Touré et al., 2016), a type I error threshold of 5%, a statistical power 80%, a reduction of 50% in malaria incidence in the intervention group, and a non-response rate of 10%, the minimum sample size was estimated at 161 participants for each study group. Parents of selected children were invited to come to the community health center for enrollment. Inclusion criteria included, being a resident of Dangassa during the 2020 transmission season, no known allergy to SP and AQ, not severely ill, not pregnant and consenting to participate in the study.
2.4. SMC drug administration
Per the NMCP recommendations, SMC is usually given in 4 monthly rounds from July to October. For this pilot study, it was extended to November. During each of the five rounds, the 1st dose of SP/AQ was given under the direct observation of community health workers while the 2nd and 3rd doses were given by the parents/guardians at home. The tablets of AQ (153 mg) per day for three days and SP (500/25 mg) the first day were given to all children in intervention groups.
2.5. Laboratory methods
All children were finger-pricked to collect blood to determine parasitemia and hemoglobin level. Thick smears were prepared from the blood, stained in 10% Giemsa for 15 min, then washed in distilled water and dried at room temperature. They were then sent to the Immunogenetics and Parasitology laboratory at ICER-Mali for examination under 100× magnification using immersion oil. The parasite density (trophozoite per μl of blood sample) was calculated by using this formula (Bejon et al., 2006):
Quality control of 10% of the read slides was done by another reader to validate the microscopy results. HemoCue 301 analyzers (HemoCue AB, Ängelholm, Sweden) were used to measure hemoglobin concentration by putting 10 μl of the collected capillary blood inside a microcuvette containing appropriate reagents and placed in the cuvette holder of HemoCue. The result was directly displayed on the device in g/dl (Adam et al., 2012; Cohen and Seidl-Friedman, 1988).
Malaria infection was defined as parasitemia confirmed by microscopy. Anemia was defined as hemoglobin (Hb) level <11 g/dl. Fever was defined as body temperature over 37.5 °C (Konaté et al., 2021), and clinical malaria as any symptomatic participant with a positive rapid diagnostic test confirmed by microscopy.
2.6. Data collection and statistical analysis
The Research Electronic Data Capture (REDCap) system was used for data management. Data were extracted in Excel format and then exported into STATA statistical package (version 14.2, College Station, Texas) for coding and analysis.
Descriptive analysis was performed to determine the participation rate in the two groups. Bivariate analysis was used to compare the prevalence of malaria infection, anemia, gametocyte carriage, and fever between the two groups. t-test was used to compare the mean hemoglobin level and the parasite density between the two groups, and Poisson regression model to estimate the reduction rate in malaria incidence in the intervention group compared to the control group with a threshold at 5%. This model included age groups (5–9,10–14 years), use of LLINs (Yes or No) and study groups (SMC group control group).
2.7. Effectiveness of the fifth additional round
The effect of the 5th SMC round was assessed at the end of the study by comparing the incidence of malaria between the two groups to the fourth round and fifth round. Any significant reduction in malaria incidence in the intervention group was considered as effects related to the intervention in this group. In Senegal, Ndiaye et al. reported in 2019 a reduction of 8.5 in malaria incidence between round 4 and round 5 (Ndiaye et al., 2019). Based on this result, any difference >10 in malaria incidence between the fourth and the fifth rounds in the intervention group was considered attributable to the effect of the 5th round of SMC in our study.
2.8. Ethics considerations
This pilot study is part of ICEMR research program whose protocol was approved by the Ethical Committee of the Faculty of Medicine, Pharmacy and Odontostomatology (FMPOS) of the University of Sciences, Techniques and Technologies of Bamako (USTTB; No. 2019/04/FMPOS). Community authorization and written informed consent from all study participants were obtained prior to enrollment. All malaria cases diagnosed during this study were treated at the local community health center according to the NMCP guidelines. This study was registered on ClinicalTrials.gov (No. NCT04149106).
2.9. Study procedures
The study protocol was explained to the health authorities as well as the village leaders before the beginning of the study. Study investigators were also trained in good clinical practices. Random allocation of participants to the 2 groups was done by the data management team before enrollment.
The 1st round of SMC was conducted in July 2020 (baseline). During this round, a unique identification number was assigned to all participant enrolled to facilitate their identification during the study. Then, every month, a round was done till the 5th round of SMC in November 2020. During each round, body temperature was recorded as well as venous blood drawn for determining parasitemia and hemoglobin prior to any drug administration. Then, community health workers administered the 1st day treatment to participants in the intervention group. Just after the 1st treatment, treatment for the 2nd and 3rd day of SMC was given to parents/tutors to be administered at home. Rapid diagnostic test (RDT) was performed for any participant with at least one malaria symptom. If the RDT was positive, the participant was treated by artemisinin combination therapy (ACT) as recommended by the NMCP and did not receive SP/AQ drug during this current round. Parents/tutors were encouraged to come with participants to the health center if any adverse event occurred. One month after the 5th round of SMC (December), a survey was done to collect data on temperature, parasitemia and hemoglobin level and comparisons were made to that of the 4th round of SMC. Next, blood smear slides were performed and sent to the Immunogenetics and Parasitology laboratory of ICER-Mali after each round for reading. Quality control of 10% of the slides read was done by another reader for validation.
3. Results
The baseline characteristics were similar between the intervention and control groups. LLINs usage rate the night before the survey (82.6% vs 77.3%), prevalence of malaria infection (13.4% vs 12.3%), anemia (26.2% vs 23.3%), gametocyte carriage (1.2% vs 1.2%) and malaria incidence (1.2% vs 0.6%) were similar between intervention and control groups (Table 1). Overall, a decreasing trend in the participation rate was observed in both intervention and control groups from August (94.3%) to November (90.4%). However, it was similar in both intervention and control groups in August (92.4% vs 96.3%; p = 0.12), September (90.7% vs 95.7%; p = 0.07), October (87.8% vs 93.9%; p = 0.24), and November (89.5% vs 91.4%; p = 0.11; Table 2).
Table 1.
Baseline characteristics of study participants in the two groups.
| Baseline characteristics | Control (N = 163) n (%) |
SMC (N = 172) n (%) |
p |
|---|---|---|---|
| Age groups | |||
| 5–9 years | 91 (51.1) | 87(48.9) | 0.336 |
| 10–14 years | 72 (45.9) | 85(54.1) | |
| ITN use | 126 (77.3) | 142(82.6) | 0.229 |
| Fever | 0 (0.0) | 0(0) | – |
| Malaria infection | 20 (12.3) | 23(13.4) | 0.763 |
| Anemia | 38 (23.3) | 45(26.2) | 0.546 |
| Gametocyte carriage | 2 (1.2) | 2(1.2) | 0.957 |
| RDT+ | 1 (0.6) | 2(1.2) | 0.333 |
Table 2.
Participation rates during 2020 SMC campaign in Dangassa.
| Groups | July | August n(%) |
September n(%) |
October n(%) |
November n(%) |
|---|---|---|---|---|---|
| SMC (N = 172) | 172 | 159(92.4) | 156(90.7) | 151(87.8) | 154(89.5) |
| Control (N = 163) | 163 | 157(96.3) | 156(95.7) | 153(93.9) | 149(91.4) |
| Total | 335 | 316(94.3) | 312(93.1) | 304(90.7) | 303(90.4) |
In November (one month after round 4), the prevalence of anemia was significantly lower in the intervention group than the control for children of ages 5–9 years (25.9% vs 49.3%; p = 0.002) as well for those of ages 10–14 years (9.7% vs 25%; p = 0.018). However, the prevalence of malaria infection, fever, and gametocyte carriage were similar between intervention and control groups in both, 5–9 and 10–14 years olds (all p-values ≥0.05; Table 3). In December, one month after 5th round (additional to routine SMC rounds), the prevalence of malaria infection was significantly lower in the intervention group compared to controls in children aged 5–9 years (8% vs 29.6%; p = 0.001) and 10–14 years (15.7% vs 39.7%; p = 0.002; Table 3).
Table 3.
Prevalence of malaria infection, anemia, and fever after round 4 and round 5 of SMC in Dangassa.
| Outcome | ||||||
|---|---|---|---|---|---|---|
| 5–9 years |
10–14 years |
|||||
| Control n(%) |
SMC n(%) |
p | Control n(%) |
SMC n(%) |
p | |
| Round 4 | ||||||
| Malaria infection | 17(20.5) | 8(10.7) | 0.091 | 14(22.6) | 12(17.9) | 0.509 |
| Anemia | 43(49.3) | 21(25.9) | 0.002 | 16(25.0) | 7(9.7) | 0.018 |
| Gametocyte carriage | 2(2.4) | 2(2.7) | 0.918 | 3(4.8) | 2(3.0) | 0.586 |
| Fever | 7(8.1) | 10(12.4) | 0.356 | 2(3.1) | 5(6.9) | 0.314 |
| Round 5 | ||||||
| Malaria infection | 24(29.6) | 6(8.0) | 0.001 | 23(39.7) | 11(15.7) | 0.002 |
| Anemia | 31(38.3) | 21(28.0) | 0.174 | 18(31.0) | 18(25.7) | 0.505 |
| Gametocyte carriage | 2(2.5) | 3(4.0) | 0.588 | 2(3.5) | 1(1.4) | 0.452 |
| Fever | 4(4.7) | 4(5.1) | 0.915 | 7(10.9) | 5(6.7) | 0.372 |
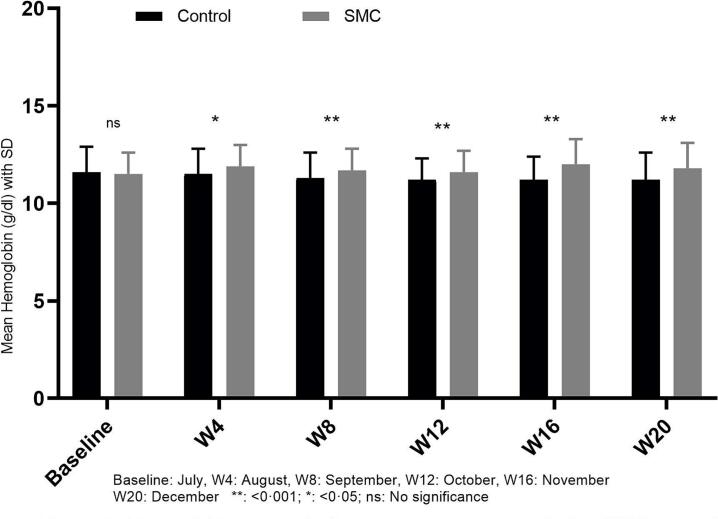
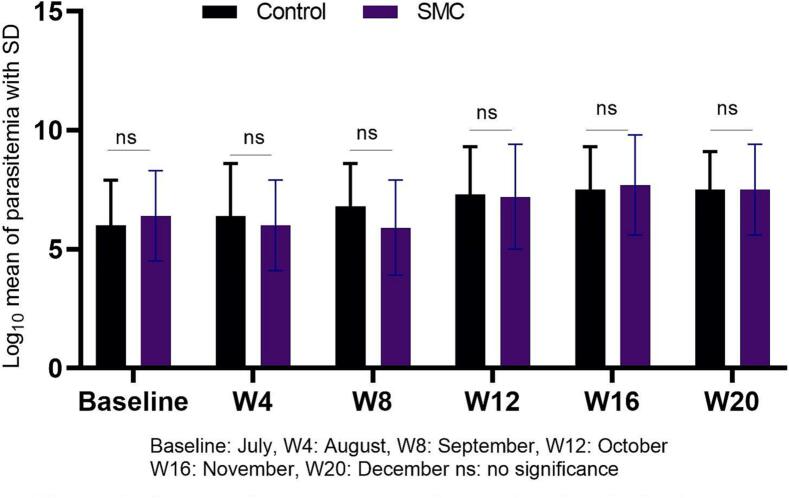
The mean of hemoglobin level was significantly higher in the intervention group compared to control group in August (11.9 ± 1.1 vs 11.5 ± 1.3; p = 0.005), September (11.7 ± 1.1 vs 11.3 ± 1.3; p = 0.006), October (11.6 ± 1.1 vs 11.2 ± 1.1; p = 0.0045), November (12 ± 1.3 vs 11.2 ± 1.2; p = 0.001) and December (11.8 ± 1.3 vs 11.2 ± 1.4; p = 0.002; Fig. 1). No significant difference was found in the mean of parasitemia between intervention and control groups (Fig. 2). In November, the risk of malaria was similar in both groups (IRR = 0.66, 95%CI [0.35–1.22]). In December, after adjusting for age groups and use of ITNs, a reduction of 51% in malaria incidence was observed in intervention (IRR = 0.49, 95%CI [0.26–0.94]) of which 17% attributed to the 5th round (Table 4).
Fig. 1.
Hemoglobin means in the comparison groups during SMC campaign in Dangassa.
Fig. 2.
Geometric mean asexual parasite density in the comparison groups during SMC campaign in Dangassa.
Table 4.
Estimating incidence rate ratios using Poisson regression models by comparison groups.
| Independent variables | 4th round |
5th round |
||||
|---|---|---|---|---|---|---|
| IRR | 95% CI | p | IRR | 95% CI | p | |
| Age groups | 0.84 | [0.451.56] | 0.586 | 0.81 | [0.43–1.51] | 0.502 |
| Use of INT | 0.88 | [0.12–6.67] | 0.903 | 0.71 | [0.36–1.41] | 0.334 |
| SMC group | 0.66 | [0.35–1.22] | 0.186 | 0.49 | [0.26–0.94] | 0.032 |
4. Discussion
This pilot study is part of a collaboration between ICEMR program and the Mali's NMCP to assess the feasibility and effect of a fifth additional round of SMC in older children. Since 2015, ICEMR program started implementing SMC in children under five in Dangassa with a significant reduction in malaria incidence (Konate et al., 2020). The rationale of this pilot study is based on age shift in the risk of malaria incidence to older children and the rebound in malaria incidence after the 4th round of SMC in areas with long malaria transmission (Coulibaly et al., 2021). Indeed, Mali has different eco-climatic zones with different length of malaria transmission. The NMCP in collaboration with ICEMR program and its other partners decided to investigate other approaches for efficient implementation of SMC according to local conditions of malaria transmission. In 2019, a pilot study was conducted in Dangassa to assess the feasibility of SMC by including children 5–14 years into routine SMC (Konaté et al., 2021). Based on the limitations of this study (non-randomized trial and design not considering clinical malaria), a randomized trial was conducted in 2020 transmission season to assess the effect of a 5th round of SMC on malaria incidence in 5–14 years old.
A total of 335 children aged 5–14 years were included and randomly assigned to the intervention (n = 172) and control groups (n = 163). At enrollment in July, baseline characteristics were similar in both comparison groups. High participation rates were observed during 2020 SMC campaign with the highest in August (94.3%). Despite a downward trend, the participation rate was similar in the 2 groups. Those lost during the follow up had multiple reasons. However, the major ones were helping parents for fieldwork and animals breeding because SMC campaigns coincide to rainy season. Also, as the SMC rounds are going on, some parents decline to participate due to adverse events occurrence (Diawara et al., 2017; Konate et al., 2020; Loua and Milligan, 2019).
One month after the ordinary 4th round of SMC, no significant difference was observed between the 2 groups regarding the prevalence of malaria infection, fever, and gametocyte carriage. However, prevalence of anemia was significantly lower in the intervention in comparison to the control group (p = 0.001). One month after a 5th additional round, the prevalence of malaria infection was significantly lower in the intervention compared to control group in both 5–9 years (p = 0.001) and 10–14 years olds (p = 0.002), however, the prevalence of anemia, fever and gametocyte carriage were comparable. Our studies corroborate previous studies that investigated the effectiveness of SMC (Dicko et al., 2011; Druetz et al., 2018; Konaté et al., 2021). Monthly administration of antimalarial drugs to cover the high transmission period seeks to maintain an optimal plasmatic concentration of the drugs, which contribute to significantly reduce parasite carriage.
In endemic areas, anemia represents the clinical phenotype of malaria (White, 2018). The reduction in parasitemia could also lead to a reduction in the prevalence of anemia as previously reported (Ambe et al., 2020; Druetz et al., 2018). In this study, we did not observe a significant reduction in the prevalence of anemia after the 5th round. This was in contrast to the 4th round where the prevalence of anemia was lower in the intervention group. Given that anemia in multifactorial in Africa, especially in children, other factors such as the the presence of other parasitic diseases such as intestinal parasitosis (Njunda et al., 2015), iron deficiency (Nacher et al., 2001) and/ antimalarial treatment of uncomplicated malaria (Sowunmi et al., 2017) could account for the observed anemia in children.
A significant reduction in the mean hemoglobin level was observed in the control as compared to the intervention group to both the 4th round (12 ± 1.3 vs. 11 ± 1.2; p = 0.001) and 5th round (11.8 ± 1.3 vs. 11.2 ± 1.4; p = 0.002). Beneficial effects of SMC on hemoglobin concentration improvement has previously been reported in children under five years in Nigeria (Ambe et al., 2020), and in Senegal in children up to 10 years (Ndiaye et al., 2019). Despite the reduction in the prevalence and incidence of malaria during the 5th round in the intervention group compared to control group, there was no significant difference in the mean of parasitemia between the 2 groups as previously reported in Burkina-Faso (Yaméogo et al., 2021). This study did not find any significant difference in malaria incidence between the 2 comparison groups (IRR = 0.66; 95% CI [0.35–1.22]) after the ordinary 4th round. However, a significant reduction of 51% was observed in the intervention group to 5th round of SMC (IRR = 0.49; 95% CI [0.26–0.94]), with 17% attributable to this additional round. The reduction in malaria incidence was already reported in several studies in sub-Saharan Africa (Baba Hamade Kivumbi Marasciulo Maxwell Moroso Roca et al., 2020; Konate et al., 2020) and this may be due to the cumulative effect of drugs that significantly reduces the parasitemia and prevents the onset of malaria disease as expected. Overall, the implementation of SMC during the high transmission period resulted in a significant reduction in the prevalence and incidence of malaria. In this study, we did not find a significant reduction in malaria incidence during the 4th ordinary round, likely because the four rounds did not fully cover the transmission season in Dangassa. Administration of SP/AQ to children to cover the period of high transmission season led to a significant reduction in malaria incidence. These results demonstrate the need to adapt the SMC implementation to local conditions of malaria transmission to maximize its effects on malaria reduction and fortify the impact of the current control interventions.
4.1. Challenges of an additional SMC
Despite the community acceptance and effectiveness of an additional round of SMC in malaria incidence among the target population, there are challenges for its effective implementation at large scale. An additional 5th round to the ordinary SMC could result in additional costs in terms of number or workload of community health workers, and other costs related for its addition. Beyond that, at the community level, the availability of older children during the SMC campaign which coincides with the fieldwork, approaches of SMC drug delivery, and possible changes in the beginning or the duration of rainy season remains an additional challenge that must be addressed for the efficient implementation of this 5th round and SMC implementation overall (Pitt et al., 2017; Traore et al., 2022). Although there is no clear evidence of increases in the frequency of antimalarial drug resistance or modification of the genetic diversity of Plasmodium parasite population during SMC in children (Cairns et al., 2021; Plowe, 2022), an additional round of SMC requires the monitoring of P. falciparum resistance to antimalarial drug used in chemoprophylaxis.
4.2. Limitations of the study
The limitations noted during this pilot study were the unavailability of information on the cost and other expenses related to this additional round of SMC, P. falciparum resistance to SP/AQ and changes in P. falciparum population. Further studies are needed to assess cost-effectiveness, monitor P. falciparum resistance to antimalarial drugs used during SMC campaign, and investigate alternative approaches to this additional round according to the timing of the rainy season (5th round at the beginning or end of the rainy season).
5. Conclusion
Results of this pilot study has shown that no significant difference in the malaria infection and incidence between the two groups at the 4th ordinary round, while the 5th additional round resulted in a significant reduction in malaria incidence and prevalence. The number of the round during SMC campaign should be adapted to the local conditions of malaria transmission including school-age children.
Funding
This study was supported by the National Institutes of Health Cooperative Agreements U19AI089696 and U19AI129387 for the West African Center of Excellence for Malaria Research.
Availability of data and materials
All data requests should be addressed to the data management team through the corresponding author.
Authors' contributions
Conception and design: SD, NS, SID, DK.
Acquisition, analysis, and interpretation of data: DK, SID, BK, AC, AT, SK, AD, FK, LD, IS, AD.
Drafting the article: DK, SID,
Revising article: DK, SID, NS, MAG, BK, SB, AC, AT, SK, AD, FK, LD, MT, VS, JM, CJW, JS, ST, SASD, KT, ID, IS, GWA.
Final approval: SD, MD.
Declaration of Competing Interest
We declare any competing interests here. All authors read and approved the version submitted.
Acknowledgements
Acknowledgements to community of Dangassa, ICEMR program all study team, Mali's National Malaria Control Program, Jules Mihigo and Celia Jane Woodfill from the President's Malaria Initiative (PMI). Dr. Konaté received support from the Fogarty International Center of the National Institutes of Health of the United States under Grant D43TW008652. We thank H3Abionet (U41HG006941) for its support.
References
- Adam I., Ahmed S., Mahmoud M.H., Yassin M.I. Comparison of HemoCue® hemoglobin-meter and automated hematology analyzer in measurement of hemoglobin levels in pregnant women at Khartoum hospital, Sudan. Diagn. Pathol. 2012;7(1):30. doi: 10.1186/1746-1596-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambe J.P., Balogun S.T., Waziri M.B., Nglass I.N., Saddiq A. Impacts of seasonal malaria chemoprevention on malaria burden among under five-year-old children in Borno State, Nigeria. Trop. Med. 2020:9. doi: 10.1155/2020/9372457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejon P., Andrews L., Hunt-Cooke A., Sanderson F., Gilbert S.C., Hill A.V. Thick blood film examination for plasmodium falciparum malaria has reduced sensitivity and underestimates parasite density. Malar. J. 2006;5:104. doi: 10.1186/1475-2875-5-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns M., Ceesay S.J., Sagara I., Zongo I., Kessely H., Gamougam K., et al. Effectiveness of seasonal malaria chemoprevention (SMC) treatments when SMC is implemented at scale: case–control studies in 5 countries. PLoS Med. 2021;18(9) doi: 10.1371/journal.pmed.1003727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisse B., Ba E.H., Sokhna C., JL N.D., Gomis J.F., Dial Y., et al. Effectiveness of seasonal malaria chemoprevention in children under ten years of age in Senegal: a stepped-wedge cluster-randomised trial. PLoS Med. 2016;13(11) doi: 10.1371/journal.pmed.1002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A.R., Seidl-Friedman J. HemoCue system for hemoglobin measurement. Evaluation in anemic and nonanemic children. Am. J. Clin. Pathol. 1988;90(3):302–305. doi: 10.1093/ajcp/90.3.302. [DOI] [PubMed] [Google Scholar]
- Coldiron M.E., Von Seidlein L., Grais R.F. Seasonal malaria chemoprevention: successes and missed opportunities. Malar. J. 2017;16(1):481. doi: 10.1186/s12936-017-2132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulibaly D., Guindo B., Niangaly A., Maiga F., Konate S., Kodio A., et al. A decline and age shift in malaria incidence in rural Mali following implementation of seasonal malaria chemoprevention and indoor residual spraying. Am. J. Trop. Med. Hyg. 2021;104(4):1342–1347. doi: 10.4269/ajtmh.20-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diawara F., Steinhardt L.C., Mahamar A., Traore T., Kone D.T., Diawara H., et al. Measuring the impact of seasonal malaria chemoprevention as part of routine malaria control in Kita, Mali. Malar. J. 2017;16(1):325. doi: 10.1186/s12936-017-1974-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicko A., Diallo A.I., Tembine I., Dicko Y., Dara N., Sidibe Y., et al. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Mali: a randomised, double-blind, placebo-controlled trial. PLoS Med. 2011;8(2) doi: 10.1371/journal.pmed.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druetz T., Corneau-Tremblay N., Millogo T., Kouanda S., Ly A., Bicaba A., et al. Impact evaluation of seasonal malaria chemoprevention under routine program implementation: a quasi-experimental study in Burkina Faso. Am. J. Trop. Med. Hyg. 2018;98(2):524–533. doi: 10.4269/ajtmh.17-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood B.M., David P.H., Otoo-Forbes L.N., Allen S.J., Alonso P.L., Armstrong Schellenberg J.R., et al. Mortality and morbidity from malaria after stopping malaria chemoprophylaxis. Trans. R. Soc. Trop. Med. Hyg. 1995;89(6):629–633. doi: 10.1016/0035-9203(95)90419-0. [DOI] [PubMed] [Google Scholar]
- Konate D., Diawara S.I., Toure M., Diakite S.A.S., Guindo A., Traore K., et al. Effect of routine seasonal malaria chemoprevention on malaria trends in children under 5 years in Dangassa, Mali. Malar. J. 2020;19(1):137. doi: 10.1186/s12936-020-03202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konaté D., Diawara S.I., Keita B., Sogoba N., Fayiçal M., Guindo A., et al. Effectiveness and community acceptance of extending seasonal malaria chemoprevention to children 5 to 14 years of age in Dangassa, Mali. Am. J. Trop. Med. Hyg. 2021 doi: 10.4269/ajtmh.21-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loua K., Milligan P. 2019. Seasonal Malaria Chemoprevention Coverage Survey in Guinea; p. 2018. [Google Scholar]
- Malaria Consortium Nigeria: Seasonal Malaria Chemoprevention Programme Start-Up Guide. 2015. [Google Scholar]
- MS/PNLP Plan stratégique de lutte contre le paludisme 2013–2017. 2013. https://www.severemalaria.org/sites/mmv-smo/files/content/attachments/2017-07-25/Mali%20malaria%20PStrag%202013-17PNLP_0.pdf from.
- Nacher M., Singhasivanon P., Gay F., Phumratanaprapin W., Silachamroon U., Looareesuwan S. Association of helminth infection with decreased reticulocyte counts and hemoglobin concentration in Thai falciparum malaria. Am. J. Trop. Med. Hyg. 2001;65(4):335–337. doi: 10.4269/ajtmh.2001.65.335. [DOI] [PubMed] [Google Scholar]
- Ndiaye J.L.A., Ndiaye Y., Ba M.S., Faye B., Ndiaye M., Seck A., et al. Seasonal malaria chemoprevention combined with community case management of malaria in children under 10 years of age, over 5 months, in south-east Senegal: A cluster-randomised trial. 2019;16(3) doi: 10.1371/journal.pmed.1002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njunda A.L., Fon S.G., Assob J.C.N., Nsagha D.S., Kwenti T.D.B., Kwenti T.E. Coinfection with malaria and intestinal parasites, and its association with anaemia in children in Cameroon. Infect. Dis. Poverty. 2015;4(1):43. doi: 10.1186/s40249-015-0078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okiro E.A., Al-Taiar A., Reyburn H., Idro R., Berkley J.A., Snow R.W. Age patterns of severe paediatric malaria and their relationship to plasmodium falciparum transmission intensity. Malar. J. 2009;8:4. doi: 10.1186/1475-2875-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMS . Organisation mondiale de la Santé; 2012. Recommandation de politique générale de l’OMS : chimioprévention du paludisme saisonnier pour lutter contre le paludisme à plasmodium falciparum en zone de forte transmission saisonnière dans la sous-région du Sahel en Afrique.https://apps.who.int/iris/handle/10665/337982 from. (consulté le 04/08/2021) [Google Scholar]
- Pitt C., Ndiaye M., Conteh L., Sy O., Hadj Ba E., Cissé B., et al. Large-scale delivery of seasonal malaria chemoprevention to children under 10 in Senegal: an economic analysis. Health Policy Plan. 2017;32(9):1256–1266. doi: 10.1093/heapol/czx084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowe C.V. Malaria chemoprevention and drug resistance: a review of the literature and policy implications. Malar. J. 2022;21(1):104. doi: 10.1186/s12936-022-04115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca Baba Hamade Kivumbi Marasciulo Maxwell Moroso, Adam E.P.H.M.K.D.A., Baba E., Hamade P., Kivumbi H., Marasciulo M., et al. Effectiveness of seasonal malaria chemoprevention at scale in west and Central Africa: an observational study. Lancet (London, England) 2020;396:1829–1840. doi: 10.1016/S0140-6736(20)32227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer J.G., Doumbia S.O., Ndiaye D., Diarra A., Gomis J.F., Nwakanma D., et al. Development of a data collection and management system in West Africa: challenges and sustainability. Infect. Dis. Poverty. 2018;7(1):125. doi: 10.1186/s40249-018-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowunmi A., Akano K., Ntadom G., Ayede A., Oguche S., Agomo C., et al. Anaemia following artemisinin-based combination treatments of uncomplicated plasmodium falciparum malaria in children: temporal patterns of haematocrit and the use of uncomplicated Hyperparasitaemia as a model for evaluating late-appearing anaemia. Chemotherapy. 2017;62(4):231–238. doi: 10.1159/000449366. [DOI] [PubMed] [Google Scholar]
- Tagbor H., Antwi G.D., Acheampong P.R., Bart Plange C., Chandramohan D., Cairns M. Seasonal malaria chemoprevention in an area of extended seasonal transmission in Ashanti, Ghana: an individually randomised clinical trial. Tropical Med. Int. Health. 2016;21(2):224–235. doi: 10.1111/tmi.12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thera M.A., Kone A.K., Tangara B., Diarra E., Niare S., Dembele A., et al. School-aged children based seasonal malaria chemoprevention using artesunate-amodiaquine in Mali. Parasite Epidemiol. Control. 2018;3(2):96–105. doi: 10.1016/j.parepi.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touré M., Sanogo D., Dembele S., Diawara S.I., Oppfeldt K., Schiøler K.L., et al. Seasonality and shift in age-specific malaria prevalence and incidence in Binko and Carrière villages close to the lake in Selingué, Mali. Malar. J. 2016;15(1):219. doi: 10.1186/s12936-016-1251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traore A., Donovan L., Sawadogo B., Ward C., Smith H., Rassi C., et al. Extending seasonal malaria chemoprevention to five cycles: a pilot study of feasibility and acceptability in Mangodara district, Burkina Faso. BMC Public Health. 2022;22 doi: 10.1186/s12889-022-12741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N.J. Anaemia and malaria. Malar. J. 2018;17(1):371. doi: 10.1186/s12936-018-2509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaméogo K.B., Yerbanga R.S., Ouattara S.B., Yao F.A., Lefèvre T., Zongo I., et al. Effect of seasonal malaria chemoprevention plus azithromycin on plasmodium falciparum transmission: gametocyte infectivity and mosquito fitness. Malar. J. 2021;20(1) doi: 10.1186/s12936-021-03855-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data requests should be addressed to the data management team through the corresponding author.