Abstract
Mineral bone disorder (MBD) is a frequent consequence of chronic kidney disease, more so in patients with kidney failure treated by kidney replacement therapy. Despite the wide availability of interventions to control serum phosphate and parathyroid hormone levels, unmet gaps remain on optimal targets and best practices, leading to international practice pattern variations over time. In this Special Report, we describe international trends from the Dialysis Outcomes and Practice Patterns Study (DOPPS) for MBD biomarkers and treatments from 2002-2021, including data from a group of 7 European countries (Belgium, France, Germany, Italy, Spain, Sweden, United Kingdom), Japan, and the United States. From 2002-2012, mean phosphate levels declined in Japan (5.6 to 5.2 mg/dL), Europe (5.5 to 4.9 mg/dL), and the United States (5.7 to 5.0 mg/dL). Since then, levels rose in the United States (to mean 5.6 mg/dL, 2021), were stable in Japan (5.3 mg/dL), and declined in Europe (4.8 mg/dL). In 2021, 52% (United States), 27% (Europe), and 39% (Japan) had phosphate >5.5 mg/dL. In the United States, overall phosphate binder use was stable (80%-84% over 2015-2021), and parathyroid hormone levels rose only modestly. Although these results potentially stem from pervasive knowledge gaps in clinical practice, the noteworthy steady increase in serum phosphate in the United States over the past decades may be consequential to patient outcomes, an uncertainty that hopefully will soon be addressed by ongoing clinical trials. The DOPPS will continue to monitor international trends as new interventions and strategies ensue for MBD management in chronic kidney disease.
Biomarkers of mineral bone disorder (MBD) in chronic kidney disease (CKD) are associated with worse clinical1 and patient-reported outcomes2 among in-center hemodialysis (ICHD) patients. Practice patterns for MBD management in ICHD vary globally, and current evidence for pharmacological or non-pharmacological interventions in MBD is limited. The description of international trends in biomarkers of MBD, such as PTH and serum phosphate, is key to the understanding of distinct practice patterns and country policies that can impact ICHD patient outcomes, as well as to the recognition of challenges in the real-world implementation of medical interventions. We report international trends from The Dialysis Outcomes and Practice Patterns Study (DOPPS) for MBD biomarkers and treatments, focusing on serum phosphate, from 2002-2021, including data from a group of 7 European countries (Belgium, France, Germany, Italy, Spain, Sweden, United Kingdom), Japan, and the US, highlighting the rise in average serum phosphate level in the US over the last decade.
The DOPPS and The US DOPPS Practice Monitor (DPM)
The Dialysis Outcomes and Practice Patterns Study (DOPPS) is an international prospective study of in-center hemodialysis (HD) patients, practices, and outcomes.1 Participating dialysis sites and patients are enrolled in DOPPS based on stratified random selection from comprehensive lists of each country’s in-center HD facilities. Since 1996, the DOPPS has been carried out via 7 study phases, typically with 300-500 facilities per phase, with detailed data reported thus far from >150,000 in-center HD patients, and in recent years from >20 countries. In the United States, DOPPS data have been applied to create the DOPPS Practice Monitor-HD (DPM-HD) (https://www.dopps.org/DPM-HD/), which has provided publicly available, contemporary data and trends regarding common in-center HD practices and biochemical markers since 2010. The DPM-HD has been shown to provide nationally representative statistics regarding numerous US in-center HD practice measures.3
Serum Phosphate and Parathyroid Hormone (PTH): International and US Trends
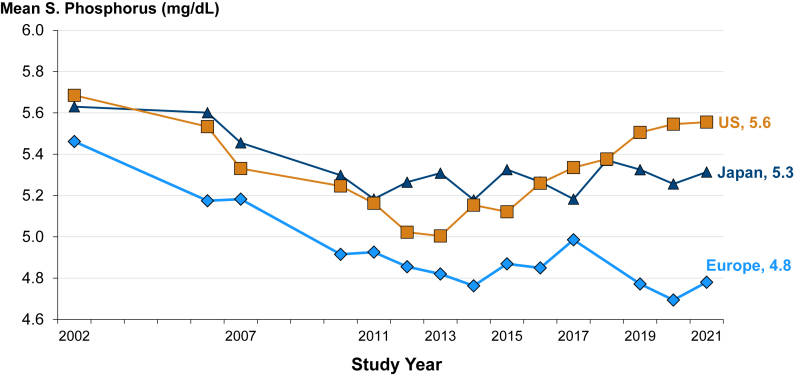
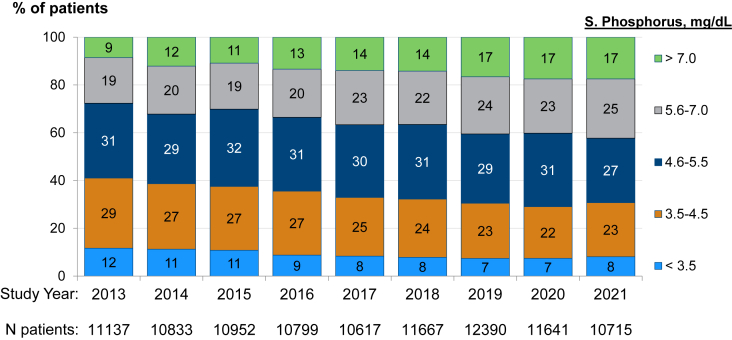
Trends in serum phosphate levels by region among patients receiving in-center HD are shown in Fig 1. In 2002, mean phosphate levels were 5.5 (Europe), 5.6 (Japan), and 5.7 (United States) mg/dL. Mean levels declined notably from 2002-2012, by 0.6 (Europe), 0.4 (Japan), and 0.7 (United States) mg/dL. Over the decade since (through 2021), mean phosphate rose steadily in the United States (to 5.6 mg/dL in 2021), was stable in Japan (5.3), and stable or lower in Europe (4.8 mg/dL). In the United States, the proportion of in-center HD patients with phosphate >5.5 mg/dL increased from 28% in 2013 to 42% by 2021 (Fig 2), whereas in 2021, only 18% in Europe and 28% in Japan had serum phosphate > 5.5 mg/dL. In the United States, mean phosphate levels were comparable between Black and non-Black patients.
Figure 1.
International serum phosphate trends among in-center hemodialysis patients, from 2002-2021.
[DOPPS phases 2-7 included facilities from the US, Japan, and 7 European countries (Belgium, France, Germany, Italy, Spain, Sweden, United Kingdom). The period covers 123,997 patient-months from the United States, 23,789 from Japan, and 34,208 from Europe.]
Figure 2.
Proportion of United States in-center hemodialysis patients with distinct serum phosphate categorical levels, by year (2013-2021).
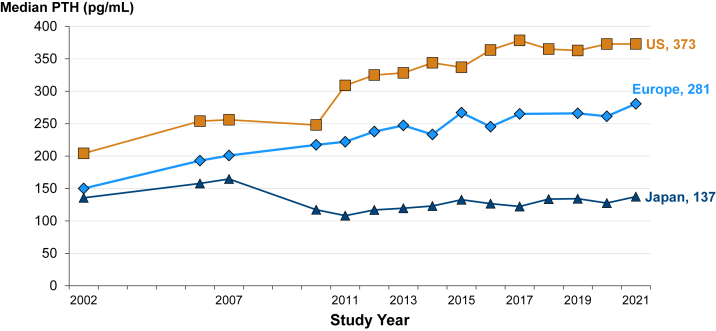
Figure 3 summarizes trends in median PTH by region. Over the past 2 decades, median PTH levels have been consistently higher in the United States than in Europe and Japan. In the United States, median PTH nearly doubled, from 200 pg/mL in 2002 to 373 pg/mL in 2021, whereas rising in Europe from 150 to 278 pg/mL. In Japan, median PTH has been stable and consistently much lower than in the United States and Europe, at 100-150 pg/mL. In the United States, median PTH levels were consistently higher in Black versus non-Black in-center HD patients during this period. Of note is an increase in this disparity over time, with Black US in-center HD patients consequently more likely to exceed the Kidney Disease: Improving Global Outcomes (KDIGO) recommended PTH level targets.
Figure 3.
International parathyroid hormone (PTH) trends among in-center hemodialysis patients, from 2002-2021.
[DOPPS phases 2-7 included facilities from the United States, Japan, and 7 European countries (Belgium, France, Germany, Italy, Spain, Sweden, United Kingdom). The period covers 121,898 patient-months from the United States, 18,845 from Japan, and 18,845 from Europe.]
Medication Trends in the United States
No substantial changes in phosphate binder types have been observed that readily explain the increase in serum phosphate in the United States over recent years. Approximately 80% of in-center HD patients in the United States have been prescribed phosphate binders each year since 2015. Sevelamer and calcium-based phosphate binders have been the most often prescribed phosphate binder types, with a small absolute increase in the latter’s uptake since 2017. In 2021, as single agents, sevelamer and calcium-based phosphate binders accounted for 23% and 44% of all phosphate binder prescriptions, respectively, while sevelamer in combination with calcium-based phosphate binders comprised 11% of all phosphate binder prescriptions. The use of any iron-containing phosphate binder rose steadily from 2% in 2015 to 14% in 2021.
The use of vitamin D analogs (intravenous or oral) has remained stable in the United States since 2015 but with oral vitamin D analogs increasing from 10% in 2015 to 35% in 2021 of all patients. Calcimimetic use has remained generally unchanged over the past decade, at approximately 27% in 2010 and 2021 (https://www.dopps.org/DPM-HD/).
Hyperphosphatemia and Mortality Risk in Dialysis
In chronic kidney disease (CKD), phosphate accumulation ensues as the glomerular filtration rate declines, resulting in a wide range of direct and indirect deleterious effects. Clinically, hyperphosphatemia in CKD is often accompanied by abnormalities in PTH and calcium, which are the 2 other most commonly followed laboratory measures of mineral bone disorder (MBD) in CKD. In vitro and in vivo models have demonstrated that higher phosphate levels lead to endothelial cell dysfunction, accelerated atherosclerosis, and vascular calcification, suggesting a direct role of phosphate in the vascular pathogenesis in CKD patients with MBD.4 Numerous cohort studies among in-center HD patients, adjusted for multiple potential confounders, have reported elevated risk of all-cause mortality in patients with serum phosphorus levels around 5.5 mg/dL or higher.5, 6, 7, 8, 9, 10, 11 This elevated, non-monotonic risk appears to be largely due to higher cardiovascular-related mortality.11 Based on biologic plausibility and corroborating observational data, despite the lack of high-quality randomized controlled trial (RCT) evidence,12 the current KDIGO CKD-MBD guidelines, published in 2017 and consistent with the 2009 recommendations,13 emphasize that phosphate-lowering treatment should be based on serial phosphate measurements and targeted toward the normal range, notably with specific targets not mentioned. For adults, the normal serum phosphate range is approximately 2.8-4.5 mg/dL, with variations between laboratories.
Phosphate Control in HD: Where From Here?
Despite strong biological plausibility and observational evidence documenting the potential harms that stem from high phosphate levels, well-designed RCTs have yet to establish optimal serum phosphate targets in the in-center HD population. Challenges to achieve the recommended ranges further complicate adequate phosphate control. Phosphate binders pose a high pill burden on in-center HD patients, who are frequently prescribed 10 or more pharmacological agents to treat their complex medical conditions. Lack of adherence to phosphate binder use and high pill burden are associated with lower phosphate control,14 and as such are important barriers to the real-world implementation of pharmacological therapy.
RCT data on non-pharmacological interventions show that dietary phosphate restriction is feasible and modestly reduces phosphate levels in in-center HD patients15 and that lower phosphate intake can be achieved without nutritional compromise. However, adherence to a low-phosphate diet is characteristically suboptimal among in-center HD patients.16 Finally, phosphate removal can be optimized by increases in the dialysis dose, and evidence suggests high-volume hemodiafiltration (HDF) results in greater phosphate removal compared to high-flux HD.17,18 The use of HDF varies considerably across regions worldwide, with HDF use having increased greatly in western Europe and Japan over the last several years as seen in the DOPPS.19
A decline in serum phosphate levels from 2002-2013 has been completely reversed in the United States since then, while Europe and Japan maintained lower serum phosphate levels over time. Despite requiring additional investigation, it can be speculated that serum phosphate can be efficiently controlled on an in-center HD population level, and practice patterns in terms of serum phosphate management differ between the United States and Europe and Japan.
It is not apparent thus far what practice changes caused the increase in serum phosphate, and to some extent PTH, in the United States during the past years. Some non-mutually exclusive possibilities are: (1) whether the uncertainties ensuing from the lack of RCT data evaluating patient-relevant outcomes have contributed to differences in the international perspectives regarding the value of stricter versus more lenient phosphate control; (2) counseling in the United States toward a less stringent dietary phosphorus control (perhaps based on the perception that this can improve nutrition or quality of life); (3) lower effective daily dosing of phosphate binders in the United States (percent phosphate binder use has not changed over the years); (4) increasing the phosphate target limits or reducing the emphasis on achieving a lower phosphate target level; (5) a decline in patient adherence in taking phosphate binders or reducing phosphate intake in the United States compared to other regions; (6) distinct practice patterns for vitamin D analog prescriptions resulting in higher intestinal phosphate absorption,20 despite the flat overall use of such drugs in the United States since 2015; or (7) a potential misinterpretation of the 2009 KDIGO Guidelines leading clinicians to not begin treatment for hyperparathyroidism until PTH levels were above 9 times the upper limit of normal (˜600 pg/mL), which, particularly in the United States, may have prompted the prescription of higher doses of vitamin D analogs, thus increasing phosphate levels over time. In Europe, where PTH levels are lower than in the United States, similar interpretations of the KDIGO Guidelines may have led to higher PTH levels over time.21 More use of calcimimetics or fewer prescriptions (or lower doses) of vitamin D analogs in Europe compared to the United States may explain the differences in phosphate trends across these regions. Finally, our authors also speculate that the large uptake of HDF in Europe and Japan over the past decade may have aided the ability of European and Japanese centers to achieve and maintain a lower serum phosphate level.19 However, this would not explain the large US increase in serum phosphate, where use of HDF has been negligible.
On the horizon, 2 major pragmatic RCTs, the Hi-Lo22 and “Pragmatic randomised trial of high or standard phosphate targets in end-stage kidney disease” (PHOSPHATE)23 trials, are designed to evaluate phosphate targets’ effects on clinical outcomes in dialysis patients, with projected completion in 2025 (Table 1). Both trials compare generally permissive serum phosphate targets (6.5-7.0 mg/dL in the Hi-Lo and 6.2-7.7 mg/dL in the PHOSPHATE) to either a target approximating KDIGO guidance (near the normal phosphate range, PHOSPHATE) or serum phosphate <5.5 mg/dL (Hi-Lo). These RCTs are powered to detect differences in patient-relevant clinical outcomes, from cardiovascular events to mortality and hospitalizations. As secondary or exploratory endpoints, these studies will evaluate patient-reported outcomes (PHOSPHATE) and nutritional surrogates (Hi-Lo). Because of their pragmatic design, the type of phosphate binder will be at the physician’s discretion, increasing practice pattern variation in the study and the external validity thereof.
Table 1.
Study Characteristics of the Hi-Lo and PHOSPHATE Clinical Trials
| Hi-Lo Trial | PHOSPHATE Trial | |
|---|---|---|
| Participating countries | United States | Australia, Canada, New Zealand, United Kingdom |
| Population | Adult in-center HD patients | Adult in-center HD or PD patients receiving at least one PB agent |
| High phosphate target | 6.5-7.0 mg/dL | 6.2-7.7 mg/dL |
| Low-phosphate target | < 5.5 mg/dL | ≤ 4.6 mg/dL |
| Primary outcome | Hierarchical composite of mortality and all-cause hospitalization | Major adverse cardiovascular eventsa |
| Secondary outcomes | Individual components of the primary outcome; inpatient hospital days; serum albumin and protein catabolic rates | Individual components of the primary outcome; all-cause mortality; quality of life (EQ5D-5L) |
| Sample size | 4,400 | 3,600 |
| No. of study sites | 120-150 | - |
| Design features | Open-label, cluster-level randomization | Open-label, individual-level randomization |
| Start of enrollment | March 13, 2020 | December 10, 2019 |
| Projected end of study | April 30, 2025 | December 31, 2025 |
| Primary sponsor | National Institutes of Health | The University of Queensland |
Abbreviations: HD, hemodialysis; PB, phosphate binder; PD, peritoneal dialysis.
Composite of cardiovascular death, non-fatal myocardial infarction or coronary revascularization, stroke or peripheral arterial events.
Lately, data have emerged from RCTs assessing the use of novel phosphate-lowering agents in CKD. Tenapanor safely reduces serum phosphate levels in kidney failure patients.24 Tenapanor blocks absorption of phosphate, rather than binds phosphate, in the intestine and can substantially decrease pill burden compared to most phosphate binders; the Food and Drug Administration will discuss tenapanor’s approval for the dialysis population soon.
Within several years, the landscape regarding approaches to optimal phosphate management may be clearer, as data from large clinical trials and the availability of novel pharmacological interventions may clarify remaining uncertainties that have exacerbated wide practice variation across the globe. Proximally, addressing unmet evidence gaps would be expected to reduce variation in phosphate levels and management across regions. As a bellwether for the community, the DOPPS and DOPPS Practice Monitor will continue to monitor these and other changes. From the patients’ perspective, we can hope that better evidence will lead to gains in quality of life, including reduced pill burden and more palatable nutritional guidance, and plausibly help to extend survival and limit medical complications.
Article Information
Authors’ Full Names and Academic Degrees
Murilo Guedes, MD, Brian Bieber, MS, Indranil Dasgupta, MD, Almudena Vega, MD, Kosaku Nitta, MD, Steven Brunelli, MD, John Hartman, MD, Jochen G. Raimann, MD, PhD, Bruce M. Robinson, MD, and Ronald L. Pisoni, PhD
Support
Global support for the ongoing DOPPS Programs is provided without restriction on publications by a variety of funders. For details see https://www.dopps.org/AboutUs/Support.aspx.
Financial Disclosure
Dr Guedes has consultancy fees from AstraZeneca and a postdoctoral scholarship from Pontificia Universidade Católica do Paraná. Dr Dasgupta has received payment or reimbursement of travel/accommodation expenses for expert testimony or lectures (including service on speakers bureaus) from GSK, AstraZeneca, Vifor, and Sanofi. Dr Vega has received payment or reimbursement of travel/accommodation expenses for expert testimony or lectures (including service on speakers bureaus) from Baxter, Viphor, and Braun. Dr Nitta has no conflicts of interest to declare. Dr Brunelli is an employee of DaVita, Inc; his spouse is employed by AstraZeneca. Dr Hartman is on the board and owns equity in Visonex. Dr Raimann is an employee of the Renal Research Institute, a wholly owned subsidiary of Fresenius Medical Care. Mr Bieber, Dr Pisoni, and Dr Robinson are employees of Arbor Research Collaborative for Health, which administers the DOPPS Program. Dr Robinson has received consultancy fees or travel reimbursement since 2019 from AstraZeneca, GlaxoSmithKline, Kyowa Kirin Co, and Monogram Health, all paid directly to his institution of employment.
Peer Review
Received August 2, 2022. Evaluated by 1 external peer reviewer, with direct editorial input from the Editor-in-Chief. Accepted in revised form October 9, 2022.
Footnotes
Complete author and article information provided before references.
References
- 1.Pisoni R.L., Gillespie B.W., Dickinson D.M., Chen K., Kutner M.H., Wolfe R.A. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis. 2004;44(5 suppl 2):7–15. doi: 10.1053/j.ajkd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka M., Yamazaki S., Hayashino Y., et al. Hypercalcaemia is associated with poor mental health in haemodialysis patients: results from Japan DOPPS. Nephrol Dial Transplant. 2007;22(6):1658–1664. doi: 10.1093/ndt/gfm008. [DOI] [PubMed] [Google Scholar]
- 3.Robinson B.M., Bieber B., Pisoni R.L., Port F.K. Dialysis Outcomes and Practice Patterns Study (DOPPS): its strengths, limitations, and role in informing practices and policies. Clin J Am Soc Nephrol. 2012;7(11):1897–1905. doi: 10.2215/CJN.04940512. [DOI] [PubMed] [Google Scholar]
- 4.Gross P., Six I., Kamel S., Massy Z.A. Vascular toxicity of phosphate in chronic kidney disease: beyond vascular calcification. Circ J. 2014;78(10):2339–2346. doi: 10.1253/circj.cj-14-0735. [DOI] [PubMed] [Google Scholar]
- 5.Tentori F., Blayney M.J., Albert J.M., et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52(3):519–530. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Block G.A., Klassen P.S., Lazarus J.M., Ofsthun N., Lowrie E.G., Chertow G.M. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 7.Kalantar-Zadeh K., Kuwae N., Regidor D.L., et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70(4):771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 8.Young E.W., Albert J.M., Satayathum S., et al. Predictors and consequences of altered mineral metabolism: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2005;67(3):1179–1187. doi: 10.1111/j.1523-1755.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- 9.Kimata N., Albert J.M., Akiba T., et al. Association of mineral metabolism factors with all-cause and cardiovascular mortality in hemodialysis patients: the Japan dialysis outcomes and practice patterns study. Hemodial Int. 2007;11(3):340–348. doi: 10.1111/j.1542-4758.2007.00190.x. [DOI] [PubMed] [Google Scholar]
- 10.Block G.A., Hulbert-Shearon T.E., Levin N.W., Port F.K. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31(4):607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 11.Lopes M.B., Karaboyas A., Bieber B., et al. Impact of longer term phosphorus control on cardiovascular mortality in hemodialysis patients using an area under the curve approach: results from the DOPPS. Nephrol Dial Transplant. 2020;35(10):1794–1801. doi: 10.1093/ndt/gfaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int Suppl (2011) 2017;7(1):1–59. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2009;113(113):S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 14.Fissell R.B., Karaboyas A., Bieber B.A., et al. Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: findings from the DOPPS. Hemodial Int. 2016;20(1):38–49. doi: 10.1111/hdi.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan C., Sayre S.S., Leon J.B., et al. Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: a randomized controlled trial. JAMA. 2009;301(6):629–635. doi: 10.1001/jama.2009.96. [DOI] [PubMed] [Google Scholar]
- 16.Zeller K., Whittaker E., Sullivan L., Raskin P., Jacobson H.R. Effect of restricting dietary protein on the progression of renal failure in patients with insulin-dependent diabetes mellitus. N Engl J Med. 1991;324(2):78–84. doi: 10.1056/NEJM199101103240202. [DOI] [PubMed] [Google Scholar]
- 17.Oates T., Pinney J.H., Davenport A. Haemodiafiltration versus high-flux haemodialysis: effects on phosphate control and erythropoietin response. Am J Nephrol. 2011;33(1):70–75. doi: 10.1159/000322834. [DOI] [PubMed] [Google Scholar]
- 18.Pecoits-Filho R., Larkin J., Poli-de-Figueiredo C.E., et al. Effect of hemodiafiltration on measured physical activity: primary results of the HDFIT randomized controlled trial. Nephrol Dial Transplant. 2021;36(6):1057–1070. doi: 10.1093/ndt/gfaa173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Locatelli F., Karaboyas A., Pisoni R.L., et al. Mortality risk in patients on hemodiafiltration versus hemodialysis: a 'real-world' comparison from the DOPPS. Nephrol Dial Transplant. 2018;33(4):683–689. doi: 10.1093/ndt/gfx277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukumoto S. Phosphate metabolism and vitamin D. BoneKEy Rep. 2014;3:497. doi: 10.1038/bonekey.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldsmith D.J., Covic A., Fouque D., et al. Endorsement of the Kidney Disease Improving Global Outcomes (KDIGO) chronic kidney disease-mineral and bone disorder (CKD-MBD) guidelines: a European Renal Best Practice (ERBP) commentary statement. Nephrol Dial Transplant. 2010;25(12):3823–3831. doi: 10.1093/ndt/gfq513. [DOI] [PubMed] [Google Scholar]
- 22.Edmonston D.L., Isakova T., Dember L.M., et al. Design and rationale of HiLo: a pragmatic, randomized trial of phosphate management for patients receiving maintenance hemodialysis. Am J Kidney Dis. 2021;77(6):920–930.e1. doi: 10.1053/j.ajkd.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pragmatic Randomised Trial of High Or Standard PHosphAte Targets in End-stage Kidney Disease (PHOSPHATE) (PHOSPHATE) ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03573089 identifier: NCT03573089.
- 24.Block G.A., Rosenbaum D.P., Yan A., Chertow G.M. Efficacy and safety of tenapanor in patients with hyperphosphatemia receiving maintenance hemodialysis: a randomized phase 3 trial. J Am Soc Nephrol. 2019;30(4):641–652. doi: 10.1681/ASN.2018080832. [DOI] [PMC free article] [PubMed] [Google Scholar]