Graphical abstract
Keywords: Transcatheter edge-to-edge repair, Percutaneous mitral valve repair, Mitral regurgitation, Cleft leaflet, Down syndrome
Highlights
-
•
TEER of mitral leaflet clefts has been reported sparingly.
-
•
Patients with DS may have left AVV disease including clefts.
-
•
TEER may be feasible in high surgical risk patients with AVV cleft.
Introduction
Transcatheter mitral valve edge-to-edge repair (TEER) has an established role in the management of severe mitral regurgitation (MR), with proven benefits to patients with primary MR at high risk for surgery as well as select patients with secondary MR.1 As experience with the use of the MitraClip (Abbott, Abbott Park, IL) for TEER continues to grow, the use of these devices is increasingly expanding to cases with unconventional and challenging anatomy. Cleft mitral leaflets, a typically rare finding, represent a situation featuring complex valve anatomy where the role of TEER has not been clearly established and surgery has been the default consideration for management.2,3 Patients with Down syndrome (DS) have a relatively higher incidence of atrioventricular septal defects (AVSDs) and concomitant cleft mitral/atrioventricular valve (AVV) leaflets potentially requiring multiple surgical repairs in childhood, thus placing them at higher risk of additional complications in adulthood.4, 5, 6, 7 Prior case reports have demonstrated the use of TEER in cases of cleft mitral leaflets, highlighting possible interventional approaches to achieve an effective repair.8, 9, 10, 11, 12, 13 Here we present a case with prior surgical repair of a complete atrioventricular canal (CAVC) defect with left AVV cleft who underwent successful TEER for severe regurgitation.
Case Presentation
A 33-year-old man with a history of DS with surgically repaired CAVC in the first year of life and repeat surgical intervention 11 years later, bicuspid aortic valve, symptomatic sinus bradycardia with permanent pacemaker implantation, nonsustained ventricular tachycardia, supraventricular tachycardia, obstructive sleep apnea, chronic kidney disease, and seizure disorder was evaluated for progressively worsening dyspnea on exertion and fatigue. Subsequent evaluation with transesophageal echocardiogram (TEE) confirmed surgical repair with inlet ventricular septal defect (VSD) patch and a common AVV with the presence of anterior left AVV cleft and severe regurgitation. Given the progressively worsening nature of the patient's symptoms resulting in significant functional decline, the patient was evaluated by cardiac surgery for consideration of repeat surgical intervention. The patient was thought to be prohibitively high risk for repeat surgery in the setting of multiple prior sternotomies and presence of significant medical comorbidities. He was referred to the structural heart team for exploration of percutaneous therapeutic options. The decision was made to proceed with percutaneous valve repair with TEER.
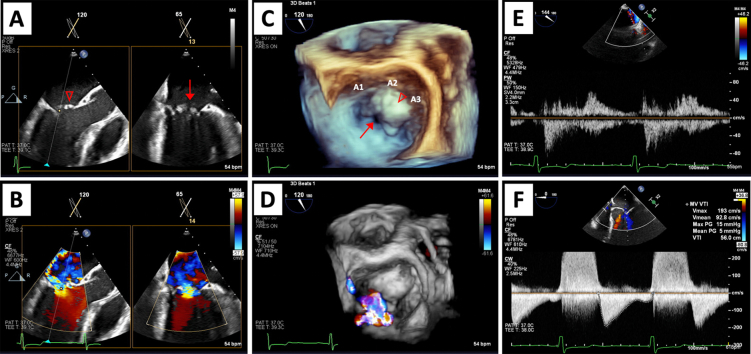
Intraoperative TEE demonstrated normal biventricular size and function, with a left ventricular (LV) end-diastolic internal diameter of 4.6 cm and an estimated LV ejection fraction of 55% to 60%. There was no evidence of flow across the site of inlet VSD repair by color and spectral flow Doppler. An anterior cleft of the left AVV was located at the lateral aspect of the A2 scallop with prolapse of the A2 and A3 scallops. There was severe left AVV regurgitation originating at the site of the anterior cleft with a posteromedially directed jet. The effective regurgitant orifice area was 0.59 cm2, with estimated regurgitant volume of 99 mL and regurgitant fraction of 74% along with systolic flow reversal in the right-sided pulmonary veins consistent with severe regurgitation. The peak and mean transvalvular gradients were 15 mm Hg and 5 mm Hg, respectively (Figure 1, panel A in Videos 1-4). The estimated mitral valve area by two-dimensional (2D) planimetry in the transgastric view was approximately 3.8 cm2, suggesting that the elevation in transmitral gradients was likely due to significant regurgitant flow. Posterior leaflet length was assessed preintervention to guide device selection and measured approximately 9 mm. The anterior leaflet measured 14 mm in length.
Figure 1.
Baseline intraprocedural TEE images. Midesophageal biplane imaging of a long-axis and bicommissural view without and with color Doppler (A, B), three-dimensional surgeon's view without and with color Doppler demonstrating severe regurgitation (C, D), systolic flow reversal in the right upper pulmonary vein (E), and elevated transvalvular inflow gradients (F). Anterior leaflet cleft (↓) and A2/A3 prolapse (∇) are shown in 2D (A) and three-dimensional (C) imaging.
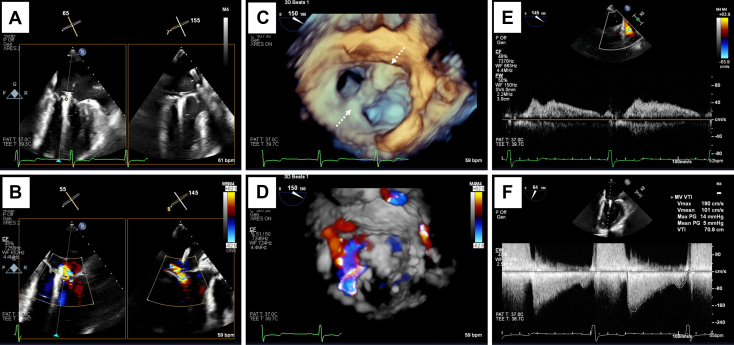
With TEE guidance, transseptal puncture was performed using a radiofrequency wire, and the delivery sheath was advanced into the left atrium. Posterior leaflet length was important in determining appropriate device selection, with 6 mm of leaflet insertion needed for NT/NTW MitraClips, according to the manufacturer's recommendations, and 9 mm of leaflet insertion needed for XT/XTW MitraClips. An NTW MitraClip was selected due to a posterior leaflet length of only 9 mm. The device was subsequently advanced into the left ventricle, and bileaflet capture was confirmed. Initially, the device was positioned at the A2/P2 scallops with orientation of the clip arms at 12 and 6 o'clock, essentially perpendicular to the valve plane. However, significant left AVV regurgitation remained with this approach, with difficulty in capturing the anterior leaflet within the clip arms due to the presence of the anterior cleft near the 12 o'clock position (Video 5). The device was repositioned with slight angulation with clip arms adjusted to 2 and 8 o'clock so that there was capture of the A2 scallop just medial to the cleft and capture of the lateral aspect of the P2 scallop (panel B in Videos 1-4). This approach resulted in significant reduction in left AVV regurgitation with estimated residual mild-moderate regurgitation based on an effective regurgitant orifice area of 0.33 cm2 and regurgitant volume of 44 mL. There was subsequently systolic dominant flow in all 4 pulmonary veins with no evidence of flow reversal. The peak and mean transvalvular gradients remained stable from baseline at 14 and 5 mm Hg, respectively (Figure 2).
Figure 2.
Post-TEER intraprocedural TEE images. Midesophageal biplane imaging of a long-axis and bicommissural view without and with color Doppler demonstrating bileaflet capture with reduction to mild-moderate regurgitation (A, B), three-dimensional surgeon's view demonstrating angulated device positioning (dashed arrows) with tissue bridge and color Doppler (C, D), right upper pulmonary vein subsequently with systolic dominant flow (E), and stable transvalvular inflow gradients following device release (F).
Discussion
Isolated mitral clefts are a rare occurrence, but the presence of left AVV or mitral valve clefts in patients with DS with AVSDs is well described.14 Due to failure of endocardial cushion fusion, left AVV/mitral valve deformities such as a cleft can be present and result in significant valve regurgitation. It is estimated that 40% to 60% of patients with DS will have a major congenital heart defect, representing a notable patient population at risk for AVV issues with complex anatomical considerations.4 Despite early surgical repair of AVSDs in childhood, patients with DS can present later in life with significant left AVV regurgitation. Minimally invasive surgical repair has been described; however, many patients are prohibitive surgical risk due to the presence of significant medical comorbidities and prior extensive cardiac surgical histories.15 Data on percutaneous options for treatment of these valvular conditions are limited and there is a crucial unmet need for these data in the high-risk DS population.
The presence of a cleft leaflet poses unique challenges to deployment of the TEER device, which relies on grasping the leading edges of the leaflets to approximate and improve leaflet coaptation. Various methods have been successfully employed, including an orthogonal 2-clip approach; however, few published reports in patients with DS with a history of AVSD exist.9, 10, 11 In this case, we demonstrate the successful use of a single TEER device to percutaneously repair a left AVV cleft in a patient with DS and prior surgical interventions for CAVC deemed to not be a suitable surgical candidate due to extensive surgical history and comorbidities. The severe left AVV regurgitation resulting in significant symptoms and functional limitation was substantially reduced with TEER with clear evidence of improvement by hemodynamic parameters. Using an approach of angulating the device such that the A2 scallop medial to the cleft was grasped with lateral tension resulted in more successful reduction of regurgitation. This technique allowed a redundant portion of the A2 scallop to essentially “cover” a portion of the cleft with a resultant decrease in the regurgitant orifice area and allowed for the most optimal result with a single clip. The elevated transvalvular gradients ultimately precluded the use of a second clip.
Conclusion
Percutaneous repair in cases of mitral cleft has been described only sparingly in the past. The population of patients with DS with relatively higher incidence of AVSDs and left AVV/mitral disease represents an important population where this may prove to be an important technique. This case demonstrates the feasibility of achieving a successful result with percutaneous repair in left AVV cleft with a single MitraClip approach in this congenital heart disease population.
Ethics Statement
The authors declare that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Consent Statement
The authors declare that since this was a non-interventional, retrospective, observational study utilizing de-identified data, informed consent was not required from the patient under an IRB exemption status.
Funding Statement
The authors declare that this report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure Statement
The authors report no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.case.2022.09.008.
Supplementary Data
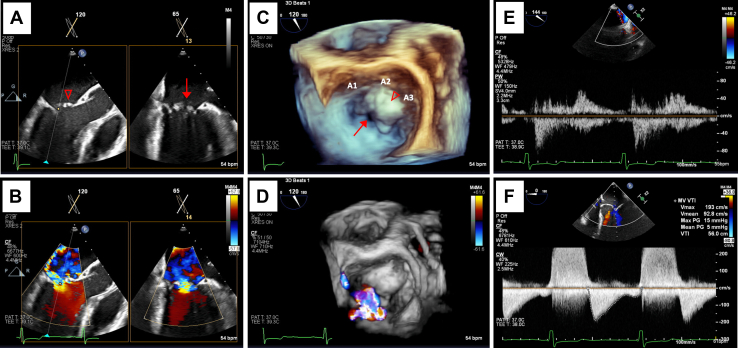
Intraprocedural TEE with 2D views pre- and post-TEER without color Doppler. Midesophageal biplane imaging of a long-axis (120°-155°) and bicommissural view (55°-65°) without color Doppler (A) demonstrating A2/A3 prolapse (∇) and the location of the anterior cleft (↓). Similar views are shown (B) that demonstrate 2D placement of the device at the location of the anterior cleft.
Intraprocedural TEE with two-dimensional views pre- and post-TEER with color Doppler. Midesophageal biplane imaging of a long-axis (120°-155°) and bicommissural view (55°-65°) with color Doppler (A) demonstrating severe left AVV regurgitation originating at the site of the anterior cleft (↓) and A2/A3 prolapse (∇) with a posteromedially directed jet. Similar views are shown (B) that demonstrate 2D placement of the device at the site of regurgitation.
Three-dimensional surgeon's view of the left AVV pre- and post-TEER without color Doppler. Pre-TEER imaging (A) demonstrates anterior leaflet cleft (↓) with A2/A3 prolapse (∇). Post-TEER imaging (B) shows angulated positioning of the device at the 2 and 8 o'clock positions (dashed arrows) with adequate tissue bridge above and below the device.
Three-dimensional surgeon's view of the left AVV pre- and post-TEER with color Doppler. Pre-TEER imaging (A) demonstrates severe left AVV regurgitation originating at the site of the anterior cleft and A2/A3 prolapse with a posteromedially directed jet. Post-TEER imaging (B) shows angulated positioning of the device at the 2 and 8 o'clock positions with significant reduction in the degree of regurgitation.
Three-dimensional surgeon's view of the left AVV with attempted TEER at the 6 and 12 o'clock positions (dashed arrows) without (A) and with color Doppler (B) demonstrating significant residual A2/A3 prolapse medial to the device (∇) and severe left AVV regurgitation.
References
- 1.Otto C.M., Nishimura R.A., Bonow R.O., Carabello B.A., Erwin J.P., 3rd, Gentile F., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 2.Artrip J.H., Rumball E.M., Finucane K. Repair of left atrioventricular valve cleft defects with patch augmentation. Ann Thorac Surg. 2012;93:2081–2083. doi: 10.1016/j.athoracsur.2011.12.074. [DOI] [PubMed] [Google Scholar]
- 3.Van Praagh S., Porras D., Oppido G., Geva T., Van Praagh R. Cleft mitral valve without ostium primum defect: anatomic data and surgical considerations based on 41 cases. Ann Thorac Surg. 2003;75:1752–1762. doi: 10.1016/s0003-4975(03)00167-x. [DOI] [PubMed] [Google Scholar]
- 4.Fudge J.C., Li S., Jaggers J., O’Brien S.M., Peterson E.D., Jacobs J.P., et al. Congenital heart surgery outcomes in Down syndrome: analysis of a national clinical database. Pediatrics. 2010;126:315–322. doi: 10.1542/peds.2009-3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calabrò R., Limongelli G. Complete atrioventricular canal. Orphanet J Rare Dis. 2006;1:8. doi: 10.1186/1750-1172-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodge-Khatami A., Herger S., Rousson V., Comber M., Knirsch W., Bauersfeld U., et al. Outcomes and reoperations after total correction of complete atrio-ventricular septal defect. Eur J Cardio-Thorac Surg. 2008;34:745–750. doi: 10.1016/j.ejcts.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 7.Azzab S., Samy A., Singab H., Zeinah M., Musollari G., Axiaq A., et al. The effect of surgical technique, age, and trisomy 21 on early outcome of surgical management of complete atrioventricular canal defect. Cardiol Young. 2022;32:869–873. doi: 10.1017/S1047951121003139. [DOI] [PubMed] [Google Scholar]
- 8.Cheng R., Kar S., Siegel R.J., Nakamura M. Cleft mitral leaflets and severe mitral regurgitation: testing the limits of percutaneous mitral valve repair. Catheter Cardiovasc Interv. 2019;93:1161–1164. doi: 10.1002/ccd.27980. [DOI] [PubMed] [Google Scholar]
- 9.Russo M.J., Garg A., Okoh A., Chaudhary A., Hakeem A., Lee L.Y., et al. MitraClip implantation in a patient with post-surgical repair of primum atrial septal defect and residual mitral cleft. JACC Case Rep. 2020;2:2027–2029. doi: 10.1016/j.jaccas.2020.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hakeem A., Chen C., Russo M. Transcatheter mitral valve repair for severe mitral regurgitation in Down’s syndrome. J Invasive Cardiol. 2021;33:E1008. doi: 10.25270/jic/21.00229. [DOI] [PubMed] [Google Scholar]
- 11.Willemsen H.M., van den Heuvel A., Schurer R., van Melle J., Natour E. Mitral cleft repair by mitraclipping. Eur Heart J. 2014;35:1021. doi: 10.1093/eurheartj/eht475. [DOI] [PubMed] [Google Scholar]
- 12.Melillo E., Ancona F., Buzzatti N., Denti P., Agricola E. A challenging mitral valve anatomy for percutaneous repair with MitraClip: cleft posterior leaflet. Eur Heart J Cardiovasc Imaging. 2019;20:1433–1434. doi: 10.1093/ehjci/jez175. [DOI] [PubMed] [Google Scholar]
- 13.Öztürk C., Schueler R., Werner N., Nickenig G., Hammerstingl C. MitraClip procedure for the treatment of a pseudo-cleft in the posterior mitral leaflet. Eur Heart J Cardiovasc Imaging. 2015;16:112. doi: 10.1093/ehjci/jeu154. [DOI] [PubMed] [Google Scholar]
- 14.Craig B. Atrioventricular septal defect: from fetus to adult. Heart. 2006;92:1879–1885. doi: 10.1136/hrt.2006.093344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsangaris A., Pasala T.K.R., Jelnin V., Anderson M., Ruiz C.E. A case of left sided valve defects in a patient with repaired partial atrioventricular canal. Int J Cardiovasc Imaging. 2019;35:1037–1038. doi: 10.1007/s10554-019-01594-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intraprocedural TEE with 2D views pre- and post-TEER without color Doppler. Midesophageal biplane imaging of a long-axis (120°-155°) and bicommissural view (55°-65°) without color Doppler (A) demonstrating A2/A3 prolapse (∇) and the location of the anterior cleft (↓). Similar views are shown (B) that demonstrate 2D placement of the device at the location of the anterior cleft.
Intraprocedural TEE with two-dimensional views pre- and post-TEER with color Doppler. Midesophageal biplane imaging of a long-axis (120°-155°) and bicommissural view (55°-65°) with color Doppler (A) demonstrating severe left AVV regurgitation originating at the site of the anterior cleft (↓) and A2/A3 prolapse (∇) with a posteromedially directed jet. Similar views are shown (B) that demonstrate 2D placement of the device at the site of regurgitation.
Three-dimensional surgeon's view of the left AVV pre- and post-TEER without color Doppler. Pre-TEER imaging (A) demonstrates anterior leaflet cleft (↓) with A2/A3 prolapse (∇). Post-TEER imaging (B) shows angulated positioning of the device at the 2 and 8 o'clock positions (dashed arrows) with adequate tissue bridge above and below the device.
Three-dimensional surgeon's view of the left AVV pre- and post-TEER with color Doppler. Pre-TEER imaging (A) demonstrates severe left AVV regurgitation originating at the site of the anterior cleft and A2/A3 prolapse with a posteromedially directed jet. Post-TEER imaging (B) shows angulated positioning of the device at the 2 and 8 o'clock positions with significant reduction in the degree of regurgitation.
Three-dimensional surgeon's view of the left AVV with attempted TEER at the 6 and 12 o'clock positions (dashed arrows) without (A) and with color Doppler (B) demonstrating significant residual A2/A3 prolapse medial to the device (∇) and severe left AVV regurgitation.