Abstract
Curcumae Rhizoma is the dry rhizome coming from Curcuma longa L. which grow widely in tropical south and southwest Asia. It has been used to treat conditions such as dermatoses, infections, stress, and depression. Moreover, in China, Curcumae Rhizoma and its active constituents have been made into different pharmaceutical preparations. Growing evidence suggests that these preparations can exert antioxidant, anti-inflammatory, and anti-cancer effects, which may play crucial roles in the treatment of various diseases, including cancer, infectious-, autoimmune-, neurological-, and cardiovascular diseases, as well as diabetes. The anti-infective effect of Curcumae Rhizoma has become a popular field of research around the world, including for the treatment of COVID-19, influenza virus, hepatitis B virus, human immunodeficiency virus, and human papilloma virus, among others. In this paper, the basic characteristics of Curcumae Rhizoma and its active constituents are briefly introduced, and we also give an overview on their applications and mechanisms in infectious diseases.
Keywords: curcumin, zedoary turmeric oil, infectious diseases, antibacterial effect, antiviral effect, COVID-19, Curcumae Rhizoma
1 Introduction
According to Chinese Pharmacopoeia, Curcuma longa L. has different species: Curcuma phaeocaulis Valeton, Curcuma kwangsiensis S. G. Lee, and C. F. Liang, Curcuma wenyujin Y. H. Chen and C. Ling. Curcumae Rhizoma is the dry rhizome coming from the three above. Curcumae Rhizoma commonly known as “zedoary” or “turmeric” in English and “ezhu” or “yujin” in Chinese, they are similar in source but different in medicinal parts. It is extensively cultivated in tropical and subtropical regions and used in food as a spice or ingredient in South-East Asia (Serpa Guerra et al., 2020). It was first mentioned in the theory of Medicinal Properties in China 1,400 years ago (Guo et al., 2022). Modern pharmacological research has shown that Curcumae Rhizoma has diverse pharmacological effects, including, but not limited to, antineoplastic, antibacterial, antiviral, antihyperlipidemic, anti-thrombus, and anti-liver fibrosis (Aggarwal and Harikumar, 2009; Akaberi et al., 2021).
Curcumae Rhizoma has been formulated into different traditional Chinese medicine decoctions, such as Sanleng Ezhu Decoction (Tang et al., 2021). Further, it has also been made into related pharmaceutical preparations, such as zedoary turmeric oil (ZTO) injection, antiviral oral liquid, and ZTO vaginal suppository, among others. However, because of its poor water solubility and upper gastrointestinal irritation, novel preparations are in constant development. Examples include, solid lipid nanoparticles and nanostructured lipid carriers, sustained-release microspheres, β-cyclodextrin, and inclusion complexes (Zhao et al., 2010; Li et al., 2017).
Currently, these preparations are widely and safely used in diseases caused by infection with microorganisms (Soleimani et al., 2018). Indeed, due to synergistic multi-component and multi-target effects, the pharmacological effects of Curcumae Rhizoma cannot be fully explained. Therefore, to further explore the role of Curcumae Rhizoma and its possible clinical applications, we searched relevant articles to systematically summarize the biological functions of these compounds and elucidate its mechanisms of action against microbial infectious diseases. The objective of this paper is to provide a reference for scientists to carry out further research on Curcumae Rhizoma.
2 Active constituents
Curcumae Rhizoma generally contain many components: including curcuminoid and ZTO, among others (e.g., sugars, sterols, fatty acids, alkaloids, resins, lignans, peptides, trace elements, and flavonoids) (Yuan et al., 2018). The main active components of Curcumae Rhizoma are ZTO and the non-volatile curcuminoids (Table 1).
TABLE 1.
Main active constituents of Curcumae Rhizoma.
| Component | Main chemical compound | Character | Composition |
|---|---|---|---|
| Zedoary turmeric oil | Sesquiterpenoids, monoterpenoids, diterpenoids | Volatile oils | Curcumol, germacrone, curdione, etc. |
| Curcuminoids a | Polyphenolic derivatives (diphenylheptane hydrocarbons, amyl hydrocarbons) | Yellow-orange powder | Curcumin (77%), demethoxycurcumin (17%) and bisdemethoxycurcumin (6%) |
Curcuminoids include cyclocurcumin, but it has poor biological activity.
2.1 Zedoary turmeric oil
ZTO is the volatile oil extracted from Curcumae Rhizoma which is light yellow oil with special aroma (Jankasem et al., 2013; Yang et al., 2022). It was included in the Chinese Pharmacopoeia in 1977. Currently, the commonly extraction methods are steam distillation, Soxhlet extraction, microwave-assisted extraction, supercritical CO2 extraction and ultrasound-assisted extraction (Cai et al., 2022).
ZTO is one of the main chemical constituents in Curcumae Rhizoma and belongs to the terpenoid family, which include sesquiterpenoids, monoterpenoids, and diterpenoids, among which sesquiterpenoids are the most abundant (Li et al., 2022). Dried Curcumae Rhizoma typically contains 1.5%–5% volatile oils. Generally, the ZTO of Curcumae Rhizoma species possesses a wide variety of pharmacological properties, especially antimicrobial and antiviral properties (Dosoky and Setzer, 2018).
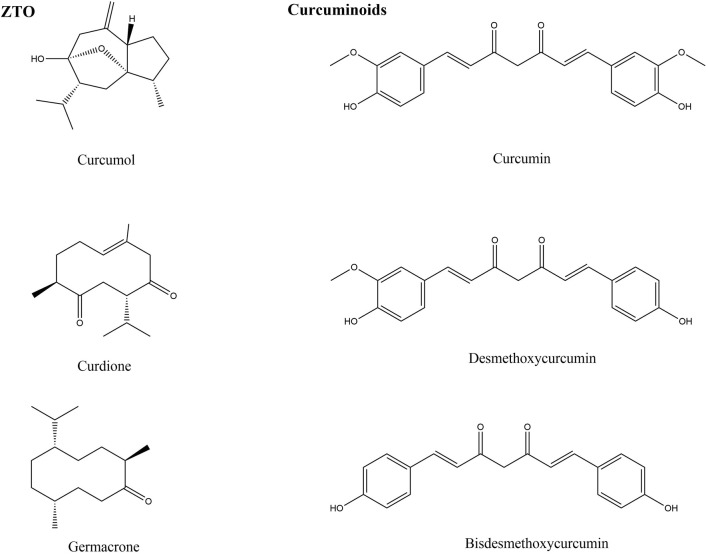
Hundreds of constituents have been identified from ZTO, the main compounds with antimicrobial activity are curcumol, curdione, and germacrone. The chemical structures of these compounds are shown in Figure 1. These constituents have different curative effects and mechanisms for treating infectious diseases. Modern pharmacological studies have found that curcumol, curdione and germacrone suppress viral replication. Particularly, germacrone showed increased in vitro and in vivo antiviral therapeutic potential because it increased expression of antiviral proteins and it significantly reduced intracellular viral load (Li et al., 2020).
FIGURE 1.
The chemical structures of active components in ZTO and curcuminoids.
2.2 Curcuminoids
Curcuminoids are non-toxic polyphenolic derivatives (yellow-orange powder), which mainly refer to diphenylheptane hydrocarbons, but also include some amyl hydrocarbons. There are 20 types of curcumin compounds found in Curcumae Rhizoma. The main active components of curcuminoids are curcumin (77%), demethoxycurcumin (DMC, 17%), and bisdemethoxycurcumin (BDMC, 6%) (Aggarwal and Sung, 2009). The chemical structures of these curcuminoids are shown in Figure 1.
Importantly, curcumin is a lipophilic polyphenol substance and the most extensively studied component. DMC and BDMC are natural analogues of curcumin with similar biological activity to curcumin. One study found that curcumin inhibits NF-κB much more effectively than others (curcumin > DMC > BDMC), this result may be related to the important role of the methoxy group on the phenyl ring of curcumin (Sandur et al., 2007). Curcumin has been determined to be safe and tolerable by the United States Food and Drug Administration, even at a dose of 12 g per day (Devassy et al., 2015; Hewlings and Kalman, 2017).
3 Pharmacokinetics
Despite a long history of clinical application, Curcumae Rhizoma and its active constituents have faced many barriers due to low bioavailability after administration. Low water solubility, hydrophobicity, and poor stability lead to the poor absorption, limited tissue distribution, and extensive metabolism. Therefore, researchers are constantly looking for ways to increase the bioavailability of these constituents. Currently, the medicinal products on the market are limited to ZTO injection, freeze-dried powder, and vaginal suppositories. Therefore, more efforts are required to develop novel ZTO drug delivery systems to enhance their overall quality. To address the issue of poor water solubility and low bioavailability of ZTO and its main components in vivo, several studies have been conducted on nanoparticle delivery systems, which has resulted in remarkable data in the last decade (Chen et al., 2011). Liposomes (Chen et al., 2021), solid lipid nanoparticles (Yang et al., 2020), and microspheres (Song and Sun, 2016) are examples of delivery systems which have been studied. In addition, one study showed that preparation of nanocapsules of curdione using the melting method increases curdione solubility. Further, in vitro release experiments showed that curdione-nanocapsules have a sustained release effect (Wu et al., 2011). A pharmacokinetic study on ZTO and a ZTO-b-cyclodextrin inclusion complex in pigs indicated that Cmax, Tmax, AUC, and MRT were significantly different in the ZTO-b-cyclodextrin group compared to the ZTO group. Moreover, the relative bioavailability of ZTO-b-cyclodextrin was significantly higher compared to the ZTO group (Yong-Xue et al., 2012). An in vitro study of the absorption mechanism of ZTO and some monomers (i.e., curcumol, germacrone, curdione) showed that curdione had a high apparent permeability coefficient, which was greater than curcumol and germacrone (Leng et al., 2007). The structure of curcudione can be modified by micro-biological transformation technology to improve its water solubility and pharmacological activity. Thus, Ma et al., 2007 used platycodonum platyflorum suspension cells to convert curcudione and isolated 7 gemmarane sesquiterpene alcohol compounds.
Over the past 3 decades, many studies have concentrated on the absorption, metabolism, and tissue distribution of curcumin, all of which reached the conclusion that curcuminoids are poorly absorbed and metabolized quickly in vivo. Phase I clinical trials demonstrated poor bioavailability of curcumin even at high doses (Anand et al., 2007). The key factor affecting the effectiveness of oral doses was absorption degree and absorption speed in the digestive tract. When taken orally, most of the curcumin was passed out of the body in the stool, and only a small amount was absorbed in the intestine and rapidly metabolized in the plasma and liver. One study investigated the absorption behavior of curcumin in mice by gavage and intraperitoneal (i.p.) injection (Schiborr et al., 2010), the results showed that the curcumin concentration in the plasma, liver, and brain of mice in the gavage group (50 mg kg−1) were all below the detection limit, while the curcumin content in the brain of the injection group (100 mg kg−1) was 4–5 μg g−1. Compared to oral administration, i.p. injection can significantly increase plasma curcumin concentration (Pan and Wang, 2012). This suggests that the pharmacokinetic parameters may be affected by different routes of administration. Co-administrating with other drugs could also increase the bioavailability of curcumin. In one study, volunteers took 2 g curcumin on fasting for 1 h, and the plasma level of curcumin was less than 10 ng mL−1. However, curcumin bioavailability increased by 2,000% after co-administrating with 20 mg piperine (Amit and Ben-Neriah, 2003).
Due to its extremely low water solubility, to improve the bioavailability, prolong bioactivity, and pharmacokinetic properties in vivo and in vitro, many researchers have studied the effect of different forms of curcumin, including, but not limited to, solid dispersions, liposomes, cyclodextrin inclusion complexes, microemulsions, micelles, nanoparticles, alcohol bodies, microspheres, microcapsules, and precursor drugs (Liu et al., 2016; Ma et al., 2019; Li et al., 2021). In one study using a curcumin nanoparticle suspension, the AUC0-∞ of the preparation was 5.85 times higher than that of free drug and the bioavailability was significantly increased. This suggests that the bioavailability of curcumin is significantly increased by improving its water solubility (Ipar et al., 2019). Chen Jing found that DMC and BDMC, the main components of curcuminoids, had improved bioavailability compared to curcuminoids. Further, compared to DMC, the bioavailability of the DMC-nanoemulsion was significantly improved (Chen et al., 2016). Although significant progress has been achieved over the past few decades, there are still various drawbacks in improving bioavailability. Moving forward, further studies are required to investigate additional strategies that could be applied to enhance the bioavailability of Curcumae Rhizoma and its active constituents.
4 Pharmacological actions of Curcumae Rhizoma
4.1 Antibacterial effect
Both Curcumae Rhizoma and its active components have strong antibacterial activities against a variety of bacteria through different signaling pathways and targets. A graphical presentation of the antibacterial effect of Curcumae Rhizoma and its active constituents is provided in Figure 2.
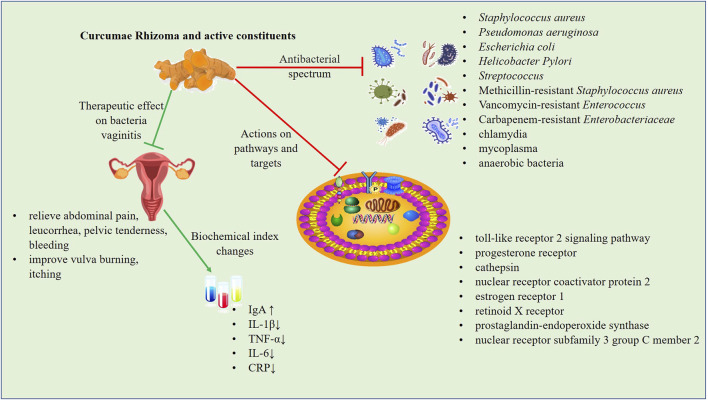
FIGURE 2.
The antibacterial mechanism of Curcumae Rhizoma and its active constituents. Both Curcumae Rhizoma and its active constituents have strong antibacterial activities against a variety of bacteria through different signaling pathways and targets. The ZTO vaginal suppository has definite clinical efficacy in the treatment of bacterial vaginitis, which can significantly decrease inflammatory factors (TNF-α, IL-6, and CRP), and ameliorate clinical symptoms.
4.1.1 Curcumae Rhizoma has good antibacterial activity
Curcumae Rhizoma may be an alternative antimicrobial agent to fight fatal bacterial infections (Sindhu et al., 2011). Moreover, dried Curcumae Rhizoma powder has been used in traditional Chinese medicine to treat infections. Curcumin, a bioactive compound derived from Curcumae Rhizoma, is known for its antibacterial properties. Curcumin possesses strong antimicrobial activity against many Gram-positive and Gram-negative bacteria (Moghadamtousi et al., 2014; Teow et al., 2016; Zheng et al., 2020).
A recent study showed that curcumin had antimicrobial activity against strains of Staphylococcus aureus and Pseudomonas aeruginosa, and improved the effect of ciprofloxacin (Kamurai et al., 2020). Huang reported that curcumin presented good activity against Escherichia coli and S. aureus with MIC values of 44.4 mg L−1 (Huang et al., 2015). In an in vivo study of pylori-infected C57BL/6 mice, curcumin showed significant therapeutic potential and significantly eradicated Helicobacter Pylori (HP) and ameliorated gastric damage caused by HP. In addition, curcumin also played a significant role against 65 clinical HP strains in the absence of an antimicrobial, with the minimum MIC values ranging from 5 to 50 μg/mL (De et al., 2009). Interestingly, Curcumae Rhizoma mouthwash can be successfully used as an adjunct to mechanical plaque management measures to prevent plaque and gingivitis, and it significantly reduces total microbial load (Waghmare et al., 2011).
The resistance of pathogenic bacteria to various antibiotics has led to the need for new and effective strategies to tackle antimicrobial resistance (El-Kattan et al., 2022). In clinical settings, common multidrug-resistant bacteria include Methicillin-resistant S. aureus (MRSA), Vancomycin-resistant Enterococcus (VRE), ultra-broad spectrum β-lactamase (ESBLs), and Carbapenem-resistant Enterobacteriaceae, among others, which pose a significant threat to patients (Wang Z. et al., 2022). An in vitro study testing the antibacterial and synergistic effects of curcumin against MRSA strains showed that curcumin had strong antibacterial activity and synergistic effects either alone or combination with certain antibiotics, namely oxacillin, ampicillin, ciprofloxacin, and norfloxacin) (Mun et al., 2013). Additionally, another study demonstrated the synergistic effect of curcumin with antibiotics (e.g., imipenem and colistin) on Carbapenem-resistant Enterobacteriaceae in vitro and activity against Galleria mellonella larvae in vivo (Ozturk, 2022). Those studies reveal the potential role of curcumin on multi-drug resistance and the possibility to drastically reduce the use of existing antibiotics.
4.1.2 ZTO vaginal suppository is effective at treating bacteria vaginitis
The ZTO vaginal suppository contains ZTO and borneol. The preparation can promote vaginal epithelial cell proliferation by inhibiting the proliferation of hemolytic Streptococcus and S. aureus in the vagina and can also enhance the self-cleaning function of the vagina, improve vaginal resistance to infection, and vaginal microcirculation (Peng, 2019). As a byproduct of Curcumae Rhizoma, ZTO is effective against S. aureus, Streptococcus, E. coli, P. aeruginosa, chlamydia, mycoplasma, and anaerobes (Negi et al., 1999; Peng, 2019; Zeng, 2019).
In the treatment of bacterial vaginitis, Wang (2022) found that ZTO vaginal suppository combined with azithromycin (1 g/day) had a better effect than oral azithromycin dispersive tablets alone. This combination treatment ameliorated pH, bacterial community concentration, bacterial community diversity, increased the level of IgA, and decreased interleukin (IL)-1β expression. Another study on the treatment of bacterial vaginitis combined with pelvic inflammation by ZTO vaginal suppository showed that the relief time of lower abdominal pain, abnormal leucorrhea, pelvic tenderness, and vaginal bleeding were shortened. Further, TNF-α, IL-6, and CRP were decreased, the Pittsburgh Sleep index (PSQI) score was lower than pre-treatment, and the Short Form Health Life Scale (SF-36) score was higher than before treatment (Lan and Yang, 2022). Therefore, the ZTO vaginal suppository has definite clinical efficacy in the treatment of bacterial vaginitis with pelvic inflammatory disease, which can effectively decrease inflammatory factors, shorten the time of symptom improvement, and improve the quality of life of affected individuals. As women age ovarian function declines, leading to a reduction in the secretion of female hormones, resulting in vaginal wall atrophy and damage to the resistance to vaginal function. This may lead to a situation whereby infection with pathogenic bacteria, including E. coli and Staphylococcus, happens more readily. Co-administration of a ZTO vaginal suppository with estrogen ointment has been shown to have a synergistic effect and also improved vulva burning, itching, and other symptoms (Zhang et al., 2017). Mixed infectious vaginitis refers to two or more types of mixed infection, partly for chlamydia, mycoplasma, anaerobic bacteria, and aerobic bacteria mixed infection (Zeng, 2019). In addition, studies have shown that the clinical efficacy of lactobacillus vagina capsule combined with roxithromycin and ZTO vaginal suppository was relatively accurate, and the recurrence rate was effectively reduced in patients with mixed infectious vaginitis.
In mechanistic studies, ZTO was found to attenuate or prevent the strong inflammatory reaction of the vaginal mucosa by downregulating the toll-like receptor 2 (TLR-2) signaling pathway, leading to a reduction in inflammation and improved function of the vaginal mucosa (Li et al., 2021). Moreover, the potential regulatory network of Curcumae Rhizoma against vaginitis was preliminarily revealed through network pharmacology. Curcumae Rhizoma has been shown to directly act on progesterone receptor (PGR), cathepsin (CTSD), nuclear receptor coactivator protein 2 (NCOA2), estrogen receptor (ESR1), retinoid X receptor (RXRA), and other protein targets, and achieve its therapeutic purpose by regulating estrogen and thyroid hormone signaling pathways (Huang et al., 2019). In another network pharmacological study, it was revealed that the key targets of Curcumae Rhizoma include PGR and ESR1, as well as prostaglandin-endoperoxide synthase 1 (PTGS1), prostaglandin-endoperoxide synthase 2 (PTGS2), and nuclear receptor subfamily three group C member 2 (NR3C2) (Zhen et al., 2020). This suggests that the pharmacological anti-bacterial vaginitis effects of Curcumae Rhizoma may be achieved through multi-component, multi-target, and multi-pathway mechanisms.
4.2 Antiviral effect
Curcumae Rhizoma and its active components have remarkable antiviral properties, and the associated mechanism(s) of action are complex and have been investigated in depth. We collected data from different studies, including those conducted in vitro, in vivo, in clinical trials, as well as network pharmacology studies. These studies all had a focus on the activity, performance, and mechanism of Curcumae Rhizoma and its active components in viruses (herpesvirus, hepatitis B virus, human immunodeficiency virus, and human papilloma virus) and virosis (COVID-19 and respiratory infection diseases). The specific mechanisms of action are shown in Table 2.
TABLE 2.
Antiviral activities of Curcumae Rhizoma and its active constituents.
| Virus | Antiviral substances | Description of antiviral activity type | References |
|---|---|---|---|
| COVID-19 | Turmeric | Improve the severity of clinical symptoms (e.g., cough, anosmia and ageusia) | Chabot and Huntwork, (2021) |
| COVID-19 | ZTO injection | Promote absorption of lung lesions, reduce lung injury, and reduce the fatality rate in severe patients | Jiang et al. (2020) |
| COVID-19 | Curcumae Rhizoma | The mechanism of action of Curcumae Rhizoma against COVID-19 may be related to inhibition of inflammatory molecules | Qin et al. (2020) |
| COVID-19 | Curcuminoids | Curcuminoids is an excellent candidate for COVID-19 prevention and treatment | Fu et al. (2022) |
| COVID-19 | Curcumin | Curcumin blocks SARS-CoV-2 entry into cells as well as viral replication; both bromelacin and curcumin have anti-inflammatory properties including inhibition of transcription factors, leading to subsequent downregulation od pro-inflammatory mediators | Kritis et al. (2020) |
| COVID-19 | Curcumin | Curcumin exhibits good binding affinity at the active site of the SARS-CoV-2 RdRP complex | Singh et al. (2021) |
| COVID-19 | Turmeric | Turmeric has a potential to inhibit the SARS-CoV-2 vital proteins | Emirik, (2022) |
| COVID-19 | ZTO | ZTO shows strong dose-dependent inhibitory effects on SARS-CoV-2 virus | Zhou et al. (2022) |
| COVID-19 | Curcumin | Curcumin inhibits trans-activation of various AIDS-related kinases, including tyrosine kinase, protein kinase 1, mitogen-activated protein kinase, protein kinase C, and cyclin kinase | Prasad and Tyagi, (2015) |
| COVID-19 (pulmonary fibrosis) | Curcumin and Curcumenol | Effectively regulate the expression of TGF-β1, α -smooth muscle actin (α-SMA) and collagen-III; reduces extracellular matrix and collagen fiber deposition, and relieves bleomycin-induced pulmonary fibrosis in mice; promoting the expression of autophagy related proteins LC3B-II and Beclin1 and inhibiting the PI3K/Akt/mTOR signaling pathway may | Hu et al. (2020) |
| COVID-19 (pulmonary fibrosis) | Astragalus-Turmeric Mixture | Astragalus-Turmeric Mixture can significantly inhibit bleomycin-induced pulmonary fibrosis in rats, and inhibition of TGF-β1 mRNA expression is one of the possible mechanisms | Sun et al. (2007) |
| COVID-19 | Curcumin | Regulate the intracellular signal transduction pathways involved in inflammation, including IBB, NF-kBERK1,2, AP-1, TGF-β, TXNIP, STAT3, PPARγ, JAK2-STAT3, NLRP3, p38MAPK, Nrf2, Notch-1, AMPK, TLR-4 and MyD-88 | Saeedi-Boroujeni et al. (2021) |
| Influenza virus | ZTO | The effects of ZTO against influenza virus have been confirmed | Xia et al. (2004) |
| Influenza virus | ZTO | ZTO has a direct inactivation effect to influenza virus A1 and influenza virus A3, which can restore the patient’s body temperature to normal and relieve symptoms such as cough and sore throat | Lin, (2011) |
| Influenza virus (H1N1) | ZTO | ZTO inhibits the replication of H1N1 | Li et al. (2020) |
| Influenza virus (H3N2) | ZTO | ZTO inhibits the replication of H3N2 | Liao et al. (2013) |
| Influenza virus (H5N1) | ZTO | ZTO inhibits the replication of H5N1 | Cai et al., 2020 |
| Huang et al. (2009) | |||
| Influenza virus | ZTO combined with oseltamivir | Has significantly effect in treatment of children with viral pneumonia, significantly decreased the serum levels of IL-8, C-reactive protein, creatine kinase, and cardiac troponin | Wang et al. (2019b) |
| Influenza virus (H5N1) | The active components of ZTO (curcumol and hypericin) | Inactivate H5N1 virus on MDCK cells, block virus infection, inhibit virus adsorption, and treat virus invaded cells | Huang et al. (2009) |
| Influenza virus | Curcumin | Curcumin inhibits influenza viruses by disrupting the integrity of the viral envelope and liposome membranes | Chen et al. (2013) |
| Influenza virus (H1N1, H5N1) | ZTO | Curcumol, curdione, and germacrone, which are the three main active ingredients from ZTO, inhibit the replication of H1N1 effectively, among which germacrone has the best effect | Li et al. (2020) |
| Influenza virus (H1N1, H3N2, influenza A and influenza B viruses) | The active components of ZTO (germacrone) | germacrone inhibited the attachment/entry step and early stage of replication cycle of H1N1, H3N2, influenza A and influenza B viruses by inhibiting the expression of viral proteins and RNA synthesis of progeny viruses on MDCK and A549 cells | Liao et al. (2013) |
| Influenza virus (H1N1 and H6N1) | Curcumin | Directly affects replication of H1N1 and H6N1 subtypes of influenza virus by inhibiting hemagglutinin | Chen et al. (2013) |
| Influenza virus | Curcumin | Curcumin may interfere with influenza virus entry by interacting with hemagglutination protein in the receptor-binding region of the virus | Ou et al. (2013) |
| Influenza virus | Anti-influenza active ingredients of Curcumae Rhizoma | May be achieved by regulating phosphatidylinositol-3-hydroxykinase (PI3K) protein kinase B (Akt) and tyrosine kinase (Jak)-transcription factor (STAT) signaling pathway. The effective components of Curcuma longa L have binding and inhibitory effects on influenza virus protein targets and inflammatory related targets, which indicate the pattern of multi-targeting anti-influenza action | Ti et al. (2021) |
| RSV | Antiviral oral liquid | Has a remarkable effect on respiratory diseases caused by RSV infection | Liu, (2018) |
| RSV | ZTO | The pulmonary index value also decreased correspondingly, presenting a significant dose correlation | Xia et al. (2004) |
| RSV | Uniform and stable silver nanoparticles (AgNPs) with antiviral properties by using curcumin | Had an efficient inhibitory effect on respiratory syncytial virus infection and had no toxicity to host cells; AgNPs can directly inactivate RSV and prevent RSV from infecting host cells | Yang et al. (2016) |
| Adenovirus | Curcumin | Reduced adenovirus replication, gene expression, and virus production at curcumin concentrations that had little effect on cell viability | Jennings and Parks, (2020) |
| HSV | Curcumin | Curcumin affects vp16-mediated recruitment of RNA polymerase II to the IE gene promoter through a mechanism that depends on the activity of histone acetyltransferases of p300/CBP, leading to suppression of gene expression and blocking viral infection | Kutluay et al. (2008) |
| HSV-2 | Curcumin | Curcumin effectively inhibited HSV-2 replication | Bourne et al. (1999) |
| HSV-2 | Curcumin | Inhibiting HSV-2 replication is related to the transcription factor NF-κB | Flores et al. (2016) |
| HSV-2 | Curcumin nanoparticles | Inhibit HSV-2 infection and replication | Vitali et al. (2020) |
| HBV | Aqueous extract of Curcumae Rhizoma | Increased the level of p53 protein in vitro, which inhibits the HBV enhancer and X promoter that mediate HBV replication | Kim et al. (2009) |
| HBV | Curcumae Rhizoma | Reduce the expression of HBx in the liver in vivo, increase the expression of p21, cyclin D1 and the level of p-p53; effect on the early and late stages of liver disease, and prevent and delay the occurrence of liver cancer | Kim et al. (2011) |
| HBV | Curcumin | Downregulating the acetylation of CCcdNA-bound histones, thereby inhibiting HBV gene replication | Wei et al. (2017) |
| HBV | Curcumin exerts | Targeting multiple cellular and molecular pathways such as Wnt/β-catenin, Ap1, STAT3, MAPK and NF-κB signaling pathways | Hesari et al. (2018) |
| HBV | KCT-01 (a new botanical drug formula containing Curcumae Rhizoma) | Inhibit HBV replication and IL-6 in vitro and in vivo models without showing toxicity, indicating that KCT-01 potential as an antiviral drug alone or in combination with entecavir | Kim et al. (2018) |
| HIV | Curcumin and curcumin A | Curcumin and curcumin A exhibit similar inhibitory effects on of HIV-1 infection in cultured lymphoblastoid T cells; curcumin A inhibited HIV-1 reverse transcription at low concentrations; curcumin A induced HO-1 expression and reduced the progression of the HIV-1 infection cycle | Kumari et al. (2015) |
| HIV/AIDS | Oral curcumin | Oral curcumin supplementation positively regulates energy metabolism in HIV/AIDS patients receiving antiretroviral therapy | da Silva et al. (2019) |
| HIV | Curcumin | No antiviral effect in clinical trial, but patients prefer to take curcumin to endure mild gastrointestinal pain and feel better | Pawar et al. (2021) |
| HIV | Curcumin | Inhibition of LTR activity to block HIV-1 replication and inhibits HIV-1 LTR-directed gene expression | Nabel et al. (1988) |
| Li et al. (1993) | |||
| HIV | Curcumin | Inhibit trans-activator of transcription protein transactivation of the HIV1-LTR genome, inflammatory molecules (interleukins, TNF-α, NF-κB, COX-2) and HIV associated various kinases including tyrosine kinase, PAK1, MAPK, and PKC. | Prasad and Tyagi, (2015) |
| HIV | Turmeric | Inhibit nucleocapsid proteins which play a key role in controlling HIV viral replication | Wang et al. (2021) |
| HPV | ZTO vaginal suppository | The total effective rate and the recovery rate were improved significantly | Wang et al. (2022b) |
| Hu et al. (2021) | |||
| HPV | ZTO vaginal suppository | Significantly decreased HPV-DNA viral load, down-regulated IL-6, TNF-α, hs-CRP, and increased CD3+, CD4+, CD8+ and CD4+/CD8+ water level | Hu et al. (2021) |
| HPV | ZTO vaginal suppository | Execute its anti-HPV activity primarily by decreasing the expression of HPV16E6E7 in cervical cancer cell line and inhibit the growth of cervical epithelial cell | Zhang et al. (2007) |
| HPV | ZTO vaginal suppository | Regulation of immunity and the reduction of inflammatory response | Yin et al. (2021) |
| HPV | Curcumin | Selectively downregulate HPV18 transcription and AP-1 binding activity in HeLa cells; the complete down-regulation of AP-1 binding activity and reversal of c-fos/FRA1 transcription to normal state was observed in tumorigenic HeLa cells mediated | Prusty and Das, (2005) |
| HPV | Curcumin | Selectively inhibit the expression of viral oncogenes E6 and E7; downregulated activation of NF-kB induced by TNF-a; blocks the phosphorylation and degradation of IkBa; down-regulate the expression of NFkB-regulated COX-2 gene, and selectively down-regulate AP-1 binding | Divya and Pillai, (2006) |
4.2.1 Respiratory infection
4.2.1.1 Influenza virus
Influenza virus is one of the most commonly detected viruses around the world. Human influenza viruses are classified into types A, B, and C, with influenza A virus causing the most damage. Influenza A viruses are further divided into subtypes based on different proteins expressed on the surface of the viruses, including H1N1, H3N2, H5N1, and H7N9 (Zhou et al., 2015).
In China, many Chinese patent medicines, which claim to treat influenza viruses, contain Curcumae Rhizoma. Further, oral solutions extracted from Curcumae Rhizoma have been shown to resist influenza A virus and parainfluenza II virus (Mao et al., 2018). In vivo and in vitro studies have confirmed the effect of Curcumae Rhizoma oil against influenza virus (Xia et al., 2004). Clinical studies have also shown that ZTO has a direct inactivation effect on influenza virus A1 and influenza virus A3, which restores the patient’s body temperature to normal and relieves symptoms such as cough and sore throat (Zhang et al., 2007; Lin, 2011). In some reports, ZTO has been shown to inhibit replication of H1N1 (Li et al., 2020), H3N2 (Liao et al., 2013), and H5N1 (Huang et al., 2009; Cai et al., 2020). Further, ZTO has also been shown to have an effect in children with viral pneumonia by significantly decreasing serum levels of IL-8, CRP, creatine kinase, and cardiac troponin (Wang L. et al., 2019).
In vitro studies have demonstrated that curcumin extract directly kills H1N1 and H3N2 subtypes in Madin-Darby canine kidney (MDCK) cells (Lai et al., 2020; Dound and Sehgal 2021). In mice with influenza virus model, curcumin (25–100 mg/kg) has been shown to improve the degree of lung lesions, reduce the lung index, significantly prolong the average survival days of infected mice, and decrease mortality (Xu et al., 2009). In addition, the active component of ZTO was found to block viral infection, inhibit viral adsorption, and prevent viral invasion into cells, thus inactivating the H5N1 virus in MDCK cells. (Huang et al., 2009). Furthermore, Chen showed that curcumin inhibits influenza viruses by disrupting the integrity of the viral envelope and liposome membranes (Chen et al., 2013). Furthermore, Li and others confirmed that curcumol, curdione, and germacrone, which are the three main active ingredients from ZTO, can effectively inhibit H1N1 replication, among which germacrone has the best effect (Li et al., 2020). Liao et al. (2013) reported that germacrone inhibits the attachment/entry step at the early stage of the replication cycle of H1N1, H3N2, influenza A, and influenza B viruses by inhibiting the expression of viral proteins and RNA synthesis of progeny viruses in MDCK and A549 cells (Liao et al., 2013). In addition, there is growing evidence to suggest that curcumin directly affects the replication of influenza viruses by inhibiting hemagglutinin, or by interfering with the entry of influenza virus by interacting with hemagglutinin in the receptor binding region of the virus (Chen et al., 2013; Ou et al., 2013).
Based on systems pharmacology studies, Ti et al. (2021) discovered that the anti-influenza activities of Curcumae Rhizoma may be achieved by regulating phosphatidylinositol-3-hydroxykinase (PI3K) protein kinase B (Akt) and tyrosine kinase (Jak)-transcription factor (STAT) signaling pathways. The effective components of Curcumae Rhizoma have a binding and inhibitory effect on influenza virus proteins and inflammatory related targets, which indicate the pattern of multi-targeting anti-influenza actions (Ti et al., 2021).
4.2.1.2 Respiratory syncytial virus (RSV) and adenovirus
Respiratory syncytial virus (RSV) is a common pathogen that causes viral pneumonia in children. Antiviral oral liquid, which is composed of Curcumae Rhizoma, anemone, Rehmannia glutinosa, and patchouli, combined with chemical drugs, have a prominent effect on respiratory diseases driven by RSV infection (Liu, 2018). In mice experiments have demonstrated that ZTO injection has an inhibitory effect on RSV. Moreover, an increase in the dose (20, 40, 80 mg/kg) leads to an enhanced therapeutic effect as well as a reduction in the pulmonary index value, presenting a significant dose correlation (Xia et al., 2004). Some researchers have prepared uniform and stable silver nanoparticles (AgNPs) with antiviral properties using curcumin, which has an efficient inhibitory effect on RSV infection and had no toxicity to host cells. Mechanistic studies showed that AgNPs could directly inactivate RSV and prevent viral infection of host cells, which make this a potentially promising novel highly effective RSV fungicide (Yang et al., 2016).
Adenoviruses are double-stranded DNA non-enveloped viruses, which can infect the respiratory tract, intestines, eyes, and liver. Adenoviruses can also cause respiratory infection in children and with high mortality. A study focusing on the effect of curcumin on adenovirus replication demonstrated that treatment of cultured cells with curcumin reduced adenovirus replication, gene expression, and virus production, while having little effect on cell viability. Thus, curcumin represents a promising compound for further investigation as a potential treatment for adenovirus infections (Jennings and Parks, 2020).
4.2.1.3 COVID-19
Since COVID-19 emerged as a global public health crisis, many agents have been studied as potential treatments for the disease. As a novel coronavirus, SARS-CoV-2 showed a high degree of sequence similarity with SARS (Wang et al., 2020; Emirik, 2022). During the SARS pandemic, Curcumae Rhizoma combined with Western medicine was used in patients with the asthmatic stage of the disease and showed good therapeutic effect. An in vitro study showed that ZTO had a strong dose-dependent inhibitory effects on the SARS-CoV-2 virus (Zhou et al., 2022). In patients with COVID-19, Curcumae Rhizoma and its active constituents (ZTO and curcumin) significantly improved the severity of clinical symptoms, such as cough, anosmia, and ageusia (Chabot and Huntwork, 2021) to promote absorption of lung lesions and reduce lung injury leading to a significant reduction in mortality in severe patients (Jiang et al., 2020).
Qin et al. (2020) hypothesized that the mechanism of action of Curcumae Rhizoma against COVID-19 may be related to inhibition of inflammatory molecules. Multiple lines of evidence suggest that curcumin, which is isolated from Curcumae Rhizoma is an excellent candidate for COVID-19 prevention and treatment (Fu et al., 2022). One study highlighted the potential therapeutic role of the combination of bromelacin and curcumin in preventing COVID-19. This study showed that curcumin blocks SARS-CoV-2 entry into cells as well as viral replication, inhibits transcription factors, and downregulates pro-inflammatory mediators (Kritis et al., 2020). RNA dependent RNA polymerase (RdRP) is a defined molecular target for inhibition of SARS-CoV-2. Interestingly, Prasad and Tyagi found that curcumin inhibits trans-activation of various Acquired Immune Deficiency Syndrome (AIDS)-related kinases, including tyrosine kinase, protein kinase 1, mitogen-activated protein kinase, protein kinase C, and cyclin kinase (Prasad and Tyagi, 2015). This explains why anti-AIDS drugs, such as lopinavir and ritonavir, were found to be effective against COVID-19 during the early stages of the pandemic (Saeedi-Boroujeni et al., 2021).
The mechanism of COVID-19 treatment may also be related to the anti-pulmonary fibrosis effect. Curcumae Rhizoma may play a prominent role in improving cough, fever, lung rales, and the degree of lung function injury by anti-pulmonary fibrosis (Qiao et al., 2020). In vivo studies with bleomycin-induced pulmonary fibrosis mice, curcumin was shown to be highly effective at regulating the expression of TGF-β1, α-smooth muscle actin (α-SMA), and collagen-III, as well as in the reduction of extracellular matrix and collagen fiber deposition (Sun et al., 2007; Hu et al., 2020). In vitro studies also showed that curcumin promotes the expression of autophagy related proteins LC3B-II and Beclin1, and inhibits the phosphatidylinositol-3-kinase/Akt/mammalian target of the rapamycin (PI3K/Akt/mTOR) signaling pathway, which is one of the most important signal transduction mechanisms in alleviating pulmonary fibrosis (Di Tu et al., 2020; Yu et al., 2020).
As a key player in signal transduction pathways within inflammatory cells, curcumin regulates multiple targets involved in intracellular signal transduction pathways of inflammation, signal transducers and activators including PI3K, ERK1, AP-1, TGF-β, TXNIP, STAT3, PPARγ, JAK2-STAT3, p38MAPK, and AMPK, as well as myeloid differentiation primary response gene 88 (MyD-88). Curcumin also downregulates the expression of inflammatory enzymes such as COX2 and inducible nitric oxide synthase (iNOS), as well as CRP and inflammatory factors such as TNF-α, IL-6, and IL-8 (Patel et al., 2020; Saeedi-Boroujeni et al., 2021). Therefore, one hypothesis suggests that Curcumae Rhizoma and its active constituents act on COVID-19 through multi-targets and multi-inflammatory signaling pathways (Figure 3).
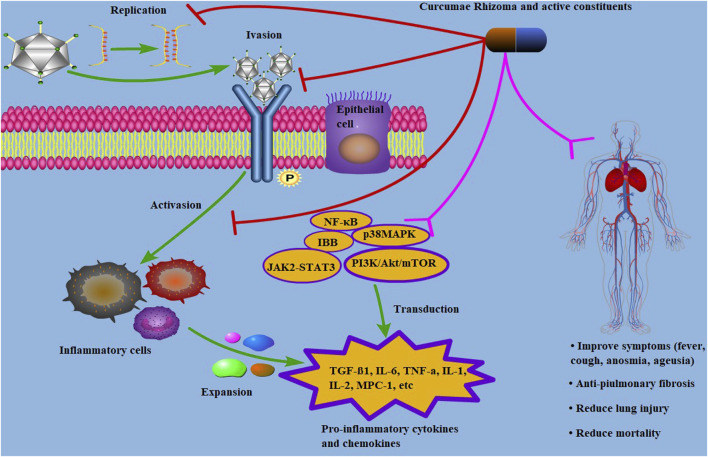
FIGURE 3.
Mechanism of Curcumae Rhizoma and its active constituents against COVID-19. Antiviral mechanism of Curcumae Rhizoma and its active constituents in treating with COVID-19. Curcumae Rhizoma and its active constituents exhibit effects on COVID-19 by “multi-component, multi-target, multi-pathway”. The steps include preventing viral replication and invasion, suppressing the activations of inflammatory cells, and regulating the signaling pathways by acting on the targets, leading to improvement of respiratory symptoms.
4.2.2 Herpesvirus (HSV)
Herpesvirus (HSV) is a large DNA virus that commonly infects humans and animals, which has the capacity to induce latent and lytic infection in the host, leading to serious health complications. There are two types of HSV: HSV-1 and HSV-2. HSV-1 can be widely spread by mouth-to-mouth contact, causing herpes labialis, herpetic whitlow, keratitis, and genital herpes. Genital herpes is a common sexually transmitted disease caused by HSV-2. Considering the antiviral capabilities of curcumin, the main biological functions of which is to defeat viral infections by targeting viral entry and ingredients critical for viral replication and other cellular or molecular processes involved in the viral life/infection cycle.
The mechanism of curcumin attributable to the action of anti-HSV effects has been thoroughly investigated through various in vivo and in vitro experiments (LoTempio et al., 2005; Kutluay et al., 2008). These mechanisms are associated with curcumin’s capacity to block a series of cellular and molecular events which are essential for viral replication, viral gene expression, and pathogenesis. Curcumin affects vp16-mediated recruitment of RNA polymerase II to the IE gene promoter through a mechanism that depends on the activity of histone acetyltransferases of p300/CBP, leading to suppression of gene expression and viral infection (Kutluay et al., 2008). Preclinical studies have shown that curcumin effectively inhibits HSV-2 replication (Bourne et al., 1999). The mechanism of curcumin has been revealed by inhibition of HSV-1 and HSV-2 adsorption in vitro (Flores et al., 2016). Other mechanisms have also confirmed that curcumin inhibits HSV-2 replication is related to the transcription factor, NF-κB (Ferreira et al., 2015). Inhibition of HSV-2 infection and replication by curcumin nanoparticles has been suggested as one of the mechanisms responsible for their anti-inflammatory properties (Vitali et al., 2020). Thus, more studies are required to fully elucidate these mechanisms.
4.2.3 Hepatitis B virus (HBV)
Hepatitis B virus (HBV) affects millions of people worldwide, sometimes dramatically evolving into chronic invasive infections, cirrhosis, and hepatocellular carcinoma. In China, Curcumae Rhizoma has been used as a traditional medicine to treat liver disease caused by HBV infection (Ailioaie and Litscher, 2020).
Kim et al. (2009) found that Curcumae Rhizoma extract increased the level of p53 protein in vitro, which inhibits the HBV enhancer and X promoter that mediate HBV replication. Furthermore, Curcumae Rhizoma was also found to reduce HBV-X protein (HBx) expression in the liver of HBx transgenic mice in vivo, increase the expression of p21, cyclin D1, and the level of p-p53. Taken together, these data imply that Curcumae Rhizoma has an effect on the early and late stages of liver disease, and that it prevents and delays the occurrence of liver cancer. Therefore, Curcumae Rhizoma should be considered as a potential chemo-preventive agent for HBV-related hepatocarcinogenesis (Kim et al., 2011).
Previous studies have shown that curcumin has important pharmacological effects in HBV (Rechtman et al., 2010). Experimental studies highlight the potential of curcumin as an antiviral drug via its downregulation of the acetylation of CCcdNA-bound histones, thereby inhibiting HBV gene replication (Wei et al., 2017). More recent studies suggest that curcumin exerts therapeutic effects on HBV patients by targeting multiple cellular and molecular pathways such as Wnt/β-catenin, Ap1, STAT3, MAPK, and NF-κB signaling pathways (Hesari et al., 2018). Kim and his team revealed that KCT-01 (a new botanical drug formula containing Curcumae Rhizoma) inhibits HBV replication and inflammatory cytokine production (IL-6) in vitro and in vivo with no toxicity, indicating the potential of KCT-01 as an antiviral drug alone or in combination with entecavir (Kim et al., 2018). However, whether curcumin can inhibit HCV replication remains unknown. A recent study found that curcumin reduces HCV gene expression by inhibiting the activation of Akt-sterol regulatory element-binding proteins-1. This suggest that curcumin inhibits HCV replication in vitro and may act as a new anti-HCV agent (Kim et al., 2010).
4.2.4 Human immunodeficiency virus (HIV)
The antiviral effectiveness of curcumin against HIV has been studied extensively. One in vitro study evaluated the biological effectiveness on curcumin activity by comparing it to the synthetic analogue, curcumin A. The aim of this study was to assess HIV-1 activity at specific stages of replication (Kumari et al., 2015). The results revealed that curcumin and curcumin A produce similar inhibitory effects on HIV-1 activity in cultured lymphoblastoid T cells. HIV-1 reverse transcription was restrained by curcumin A at low concentrations, but had no role in long terminal repeats (LTRs), trans-activator of transcription protein, or transcription factor NF-κB. The same experiments were performed in lymphoblastoid cultures and the results showed that curcumin A induced HO-1 expression and slowed the progression of the HIV-1 infection cycle. By analyzing this group of activities, the authors concluded that the maintenance of anti-HIV-1 properties was associated with improved stability of curcumin A compared to curcumin, enabling its selection as an antiretroviral candidate.
In one case study, a 42 years old female infected with HIV and receiving antiretroviral treatment was evaluated for the impact of curcumin supplementation on her energy metabolism (da Silva et al., 2019). Improvements in lipid profile and insulin sensitivity, as well as positive regulation of substrate oxidation at rest, were observed during intervention with curcumin. Therefore, oral curcumin supplements positively regulate energy metabolism in HIV patients with antiretroviral therapy. In a clinical trial involving in 40 patients over 8 weeks who were treated with curcumin as anti-HIV compound, patients claimed that they preferred to take curcumin to endure mild gastrointestinal pain and feel better (Pawar et al., 2021).
Viral LTRs are implicated in the transcription of the HIV-1 provirus. Inhibition of LTR activity may be a possible pathway for antiviral agent candidates to block HIV-1 replication (Nabel et al., 1988). Curcumin is a potent compound that inhibits HIV-1 LTR-directed gene expression without any significant effect on cell viability (Li et al., 1993). Recent studies show that curcumin inhibits trans-activator of transcription protein transactivation of the HIV1-LTR genome, inflammatory molecules (interleukins, TNF-α, NF-κB, COX-2) and various HIV associated kinases including tyrosine kinase, PAK1, MAPK, and PKC (Prasad and Tyagi, 2015). In addition, Curcumae Rhizoma inhibits nucleocapsid proteins which play a key role in controlling HIV viral replication (Wang et al., 2021).
4.2.5 Human papilloma virus (HPV)
Cervical cancer is the third most common female cancers in the world. It is highly connected to infection with the HPV. Among the 200 HPV types, more than 50% and 13% of cervical cancers are associated with HPV 16 and 18, respectively (Lim et al., 2010). In clinical studies, it was found that the recovery rate and total effective rate of ZTO vaginal suppository group in the treatment of HPV infection were clearly higher than the blank control group (Wang Q. et al., 2022). Recently, A meta-analysis indicated that the total effective rate and the recovery rate following treatment with a ZTO vaginal suppository was better than interferon-α2b in cervical HPV infection therapy. This treatment markedly reduced HPV-DNA viral load, downregulated IL-6, TNF-α, hs-CRP, and increased CD3+, CD4+, CD8+, and CD4+/CD8+ expression (Hu et al., 2021). Further, in vitro experimental studies have demonstrated that ZTO vaginal suppositories execute their anti-HPV activity primarily by decreasing HPV16E6E7 expression in cervical cancer cells to inhibit cervical epithelial cell proliferation (Zhang et al., 2007). The mechanism behind this might be linked to the regulation of immunity and the reduction of inflammatory responses (Yin et al., 2021).
Transcription factor AP-1 plays an essential role in the transcriptional regulation of specific types of high-risk human papillomaviruses (HPV16 and HPV18) which are etiologically associated with the development of cervical cancer in women. Prusty and Das (2005) reported that AP-1 had high binding activity, and that most of its members were highly expressed in tumor tissues, but c-fos expression gradually increased while fra1 expression decreased, which was consistent with the progression of cervical lesions. Moreover, in vivo and in vitro studies have demonstrated that curcumin selectively downregulates HPV18 transcription and AP-1 binding activity in HeLa cells.
Infection with high-risk HPV causes cervical cancer, mainly through the action of viral oncoproteins E6 and E7. Importantly, curcumin has been shown to selectively inhibit the expression of these viral oncogenes by RT-PCR and western blotting. Electrophoretic mobility shift assays have shown that curcumin downregulates activation of TNF-α-induced NF-κB, and that curcumin also inhibits the phosphorylation and degradation of IkBa, resulting in ineffective NF-κB activation. In addition, curcumin downregulates the expression of the NF-κB-regulated COX-2 gene, and selectively downregulates AP-1 binding (Divya and Pillai, 2006). Singh and his teammates speculated that the possible molecular targets of curcumin in cervical cancer cells include inhibition of telomerase, inhibition of Ras, ERK pathway finally resulting in inhibition of cyclin D1, c-Myc, Hsp 70, activation of AIF, release of cytochrome c, and triggering of apoptosis via the mitochondrial pathway (Singh and Singh, 2009).
4.3 Fungal
Curcumae Rhizoma has gained increased interest in the field of antifungal research (Romoli et al., 2022; Zahra et al., 2022). Chinese researchers have reported that the volatile oil of Curcumae Rhizoma has a strong antifungal effect with a minimum inhibitory concentration (Wang, 2015). The prepared luliconazole cream, which includes Curcumae Rhizoma oil, is an effective antifungal of infection caused by dermatophytes namely, Candida albicans and Trichophyton spp. This antifungal effect may be credited to Curcumae Rhizoma oil in the cream formulation which enhances drug delivery into the skin and may also have a synergistic effect with luliconazole to treat athlete’s foot and ringworm (Singh et al., 2021).
In vitro experiments have shown that curcumol, which is the main ingredient in the volatile oil of Curcumae Rhizoma, has strong antifungal activity (Yang et al., 2007). In the clinic, ZTO vaginal suppositories are effective in the treatment of vulvovaginal and vaginal candidiasis (VVC) (Cao, 2016) and recurrent vulvovaginal and vaginal candidiasis (RVVC), with a low recurrence rate. After treatment, the symptoms, including pubic pruritus, leucorrhea, and a peculiar smell, were significantly improved (Shang, 2017). This treatment can also be administrated safely in pregnant women with fungal vaginitis (Lin et al., 2021). In vitro studies into the anti-candida activity of ZTO found that the mechanism of action may be through the destruction of fungal cell membrane and mitochondria (Wei and Luo, 2005). Moreover, the mechanism may also be related to repressing functions of ergosterol synthesis, mitochondrial ATPase, malate dehydrogenase, and succinate dehydrogenase activities, as well as downregulation of the relative expression of mycotoxin genes in the biosynthetic pathway. Thus, ZTO is an environmentally friendly antifungal agent with great potential (Hu et al., 2017).
4.4 Other infectious diseases
In addition to the abovementioned effects, Curcumae Rhizoma and its active constituents also have applications in other diseases. Curcumin has a protective effect on myocardial injury induced by Coxsackievirus B group 3 (CVB3) in mice with viral myocarditis, which may improve CVB3 virus-induced serum levels of TNF-α, IL-1β, IL-6, CK-MB, and LDH in mice by inhibiting the protein expression of caspase-3, NF-κB, and iNOS (Lu et al., 2021). Furthermore, a meta-analysis on the efficacy and safety of ZTO injection showed that the ZTO injection group had significantly higher efficiency in the treatment of hand, foot and mouth, herpetic angina and other diseases (Ma et al., 2019). In addition, Wang reported that ZTO had a strong inhibitory effect on rotavirus, it was found that the short-term efficacy of ZTO injection in the treatment of rotavirus enteritis was superior to ribavirin, which inhibits the virus and also activate blood circulation to remove blood stasis, eliminate accumulation and relieve pain, aroma and appetite, thereby improving microcirculation, promoting the regeneration of damaged epithelial cells, and promoting intestinal absorption of water and electrolyte (Wang P. L. et al., 2019).
5 Conclusion
The treatment of infectious diseases, especially those caused by microorganisms, has long been an issue of concern. Long-term use of antimicrobial agents leads to various adverse reactions and drug resistance. Therefore, novel drug candidates for a therapeutic agent are urgently needed. Botanical drugs and their active components have few adverse drug reactions, making them are ideal libraries for antimicrobial drug screening.
Recently, Curcumae Rhizoma has received increased attention due to its multiple therapeutic benefits against various biological targets. The main active components in Curcumae Rhizoma are non-volatile curcuminoids (curcumin) and ZTO, which act to inhibit bacterial, viral, and fungal growth. Based on our summary of the anti-microbial mechanisms of Curcumae Rhizoma, the therapeutic mechanisms of Curcumae Rhizoma may be achieved through inhibition of viral NP protein, regulation of the PI3K/Akt/mTOR signaling pathway, direct inactivation of the virus, inhibition of relevant inflammatory factors, and anti-pulmonary fibrosis. However, the mechanism responsible for microbial infection is very complex and requires further study. Studies have confirmed that a reduction in inflammatory responses suppresses viral replication and infection, inhibits concurrent bacterial infection, improves immunity, and alleviates the degree of lung function injury, with good efficacy and good safety margins, making this an ideal treatment for infected patients. Moreover, this paper highlights in vitro studies of Curcumae Rhizoma to verify whether it has the anti-SARS-COV-2 effect. However, these investigations are still limited and/or have yet to be proven in vivo. If the anti-SARS-COV-2 effect is significant, the above speculation on the mechanism of treating COVID-19 could be further verified.
In conclusion, we hypothesize that Curcumae Rhizoma will attract increased global research interest as a potentially potent drug for the treatment of infectious diseases, as well as for overcoming the associated challenges.
Acknowledgments
The authors sincerely appreciate Mr. Shuo Zhou who provide thought in manuscript design.
Author contributions
Y-QW and TT prepared the manuscript. TT conceived the manuscript and critically reviewed the manuscript. All authors have reviewed and approved the submitting version of manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Aggarwal B. B., Harikumar K. B. (2009). Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 41 (1), 40–59. 10.1016/j.biocel.2008.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal B. B., Sung B. (2009). Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Pharmacol. Sci. 30 (2), 85–94. 10.1016/j.tips.2008.11.002 [DOI] [PubMed] [Google Scholar]
- Ailioaie L. M., Litscher G. (2020). Curcumin and photobiomodulation in chronic viral hepatitis and hepatocellular carcinoma. Int. J. Mol. Sci. 21 (19), 7150. 10.3390/ijms21197150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaberi M., Sahebkar A., Emami S. A. (2021). Turmeric and curcumin: From traditional to modern medicine. Adv. Exp. Med. Biol. 1291, 15–39. 10.1007/978-3-030-56153-6_2 [DOI] [PubMed] [Google Scholar]
- Amit S., Ben-Neriah Y. (2003). NF-kappaB activation in cancer: A challenge for ubiquitination- and proteasome-based therapeutic approach. Semin. Cancer Biol. 13 (1), 15–28. 10.1016/s1044-579x(02)00096-2 [DOI] [PubMed] [Google Scholar]
- Anand P., Kunnumakkara A. B., Newman R. A., Aggarwal B. B. (2007). Bioavailability of curcumin: Problems and promises. Mol. Pharm. 4 (6), 807–818. 10.1021/mp700113r [DOI] [PubMed] [Google Scholar]
- Bourne K. Z., Bourne N., Reising S. F., Stanberry L. R. (1999). Plant products as topical microbicide candidates: Assessment of in Vitro and in vivo activity against herpes simplex virus type 2. Antivir. Res. 42 (3), 219–226. 10.1016/s0166-3542(99)00020-0 [DOI] [PubMed] [Google Scholar]
- Cai J. X., Huang L. Y., Yan Q. W., Qin L. Q., Zhang P., Zhao H. Y. (2022). Optimization of microwave-assisted extraction process and antioxidant activity of Curcuma kwangsiensis oil. China Food Addit. 33 (04), 136–143. 10.19804/j.issn1006-2513.2022.04.018 [DOI] [Google Scholar]
- Cai W., Wen H., Zhou Q., Wu L., Chen Y., Zhou H., et al. (2020). 14-Deoxy-11, 12-didehydroandrographolide inhibits apoptosis in influenza A(H5N1) virus-infected human lung epithelial cells via the caspase-9-dependent intrinsic apoptotic pathway which contributes to its antiviral activity. Antivir. Res. 181, 104885. 10.1016/j.antiviral.2020.104885 [DOI] [PubMed] [Google Scholar]
- Cao Y. J. (2016). Clinical effect of integrated traditional Chinese and western medicine on Candida vaginitis. China's Naturop. 24 (12), 73. 10.19621/j.cnki.11-3555/r.2016.12.060 [DOI] [Google Scholar]
- Chabot A. B., Huntwork M. P. (2021). Turmeric as a possible treatment for COVID-19-induced anosmia and ageusia. Cureus 13 (9), e17829. 10.7759/cureus.17829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Ma Y., Tao Y., Zhao X., Xiong Y., Chen Z., et al. (2021). Formulation and evaluation of a topical liposomal gel containing a combination of zedoary turmeric oil and tretinoin for psoriasis activity. J. Liposome Res. 31 (2), 130–144. 10.1080/08982104.2020.1748646 [DOI] [PubMed] [Google Scholar]
- Chen J., Zhang J. Q., Yang M., Jiang X. H., Hu X. Y. (2016). Comparative study of pharmacokinetics between monomer bisdemethoxycurcumin and curcumin. J. Food Sci. Biotechnol. (08), 890–895. 10.3969/j.issn.1673-1689.2016.08.016 [DOI] [Google Scholar]
- Chen M., Wang S., Tan M., Wang Y. (2011). Applications of nanoparticles in herbal medicine: Zedoary turmeric oil and its active compound β-elemene. Am. J. Chin. Med. 39 (6), 1093–1102. 10.1142/S0192415X11009421 [DOI] [PubMed] [Google Scholar]
- Chen T. Y., Chen D. Y., Wen H. W., Ou J. L., Chiou S. S., Chen J. M., et al. (2013). Inhibition of enveloped viruses infectivity by curcumin. Plos One 8 (5), e62482. 10.1371/journal.pone.0062482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva T., Medeiros R., de Medeiros D. C., Medeiros R., de Medeiros J. A., de Medeiros G., et al. (2019). Impact of curcumin on energy metabolism in HIV infection: A case study. Phytother. Res. 33 (3), 856–858. 10.1002/ptr.6258 [DOI] [PubMed] [Google Scholar]
- De R., Kundu P., Swarnakar S., Ramamurthy T., Chowdhury A., Nair G. B., et al. (2009). Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob. Agents Chemother. 53 (4), 1592–1597. 10.1128/AAC.01242-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devassy J. G., Nwachukwu I. D., Jones P. J. (2015). Curcumin and cancer: Barriers to obtaining a health claim. Nutr. Rev. 73 (3), 155–165. 10.1093/nutrit/nuu064 [DOI] [PubMed] [Google Scholar]
- Di Tu Q., Jin J., Hu X., Ren Y., Zhao L., He Q. (2020). Curcumin improves the renal autophagy in rat experimental membranous nephropathy via regulating the PI3K/AKT/mTOR and Nrf2/HO-1 signaling pathways. Biomed. Res. Int. 2020, 7069052. 10.1155/2020/7069052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divya C. S., Pillai M. R. (2006). Antitumor action of curcumin in human papillomavirus associated cells involves downregulation of viral oncogenes, prevention of NFkB and AP-1 translocation, and modulation of apoptosis. Mol. Carcinog. 45 (5), 320–332. 10.1002/mc.20170 [DOI] [PubMed] [Google Scholar]
- Dosoky N. S., Setzer W. N. (2018). Chemical composition and biological activities of essential oils of Curcuma species. Nutrients 10 (9), 1196. 10.3390/nu10091196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dound Y. A., Sehgal R. (2021). Preclinical efficacy and safety studies of formulation SSV-003, a potent anti-viral herbal formulation. J. Exp. Pharmacol. 13, 913–921. 10.2147/JEP.S310452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kattan N., Emam A. N., Mansour A. S., Ibrahim M. A., Abd El-Razik A. B., Allam K., et al. (2022). Curcumin assisted green synthesis of silver and zinc oxide nanostructures and their antibacterial activity against some clinical pathogenic multi-drug resistant bacteria. RSC Adv. 12 (28), 18022–18038. 10.1039/d2ra00231k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emirik M. (2022). Potential therapeutic effect of turmeric contents against SARS-CoV-2 compared with experimental COVID-19 therapies: In silico study. J. Biomol. Struct. Dyn. 40 (5), 2024–2037. 10.1080/07391102.2020.1835719 [DOI] [PubMed] [Google Scholar]
- Ferreira V. H., Nazli A., Dizzell S. E., Mueller K., Kaushic C. (2015). The anti-inflammatory activity of curcumin protects the genital mucosal epithelial barrier from disruption and blocks replication of HIV-1 and HSV-2. Plos One 10 (4), e0124903. 10.1371/journal.pone.0124903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores D. J., Lee L. H., Adams S. D. (2016). Inhibition of curcumin-treated herpes simplex virus 1 and 2 in vero cells. Adv. Microbiol. 6, 276–287. 10.4236/aim.2016.64027 [DOI] [Google Scholar]
- Fu Y. S., Ho W. Y., Kang N., Tsai M. J., Wu J., Huang L., et al. (2022). Pharmaceutical prospects of curcuminoids for the remedy of COVID-19: Truth or myth. Front. Pharmacol. 13, 863082. 10.3389/fphar.2022.863082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X. Y., Mao M., Zhao J. C. (2022). Textual research on yujin in angong niuhuang pills. Chin. J. Ration. Drug Use 19 (03), 83–88. 10.3969/j.issn.2096-3327.2022.03.014 [DOI] [Google Scholar]
- Hesari A., Ghasemi F., Salarinia R., Biglari H., Tabar Molla Hassan A., Abdoli V., et al. (2018). Effects of curcumin on NF-κB, AP-1, and wnt/β-catenin signaling pathway in hepatitis B virus infection. J. Cell Biochem. 119 (10), 7898–7904. 10.1002/jcb.26829 [DOI] [PubMed] [Google Scholar]
- Hewlings S. J., Kalman D. S. (2017). Curcumin: A review of its effects on human health. Foods 6 (10), 92. 10.3390/foods6100092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G., Zhou X. N., Deng W., Wang X. H., Zhou J. J., Hu K. (2021). Comparison of zedoary turmeric oil vaginal suppository and interferon α2b in the treatment of cervical HPV infection: A meta-analysis. Jiangxi Med. J. 56 (10), 1692–1697. 10.3969/j.issn.1006-2238.2021.10.034 [DOI] [Google Scholar]
- Hu X., Xu Q., Wan H., Hu Y., Xing S., Yang H., et al. (2020). PI3K-Akt-mTOR/PFKFB3 pathway mediated lung fibroblast aerobic glycolysis and collagen synthesis in lipopolysaccharide-induced pulmonary fibrosis. Lab. Invest. 100 (6), 801–811. 10.1038/s41374-020-0404-9 [DOI] [PubMed] [Google Scholar]
- Hu Y., Zhang J., Kong W., Zhao G., Yang M. (2017). Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 220, 1–8. 10.1016/j.foodchem.2016.09.179 [DOI] [PubMed] [Google Scholar]
- Huang H. F., Zhen C. J., Chen G. Y., Yin W. Q., Mo Z. R., Zhang P., et al. (2015). Comparison of chemical composition and antibacterial activity between zedoary turmeric oil and dregs. Chin. J. Exp. Traditional Med. Formulae 21 (20), 67–71. 10.13422/j.cnki.syfjx.2015200067 [DOI] [Google Scholar]
- Huang Y. D., Li Y. M., Xiang Q., Su Z. J., Wang L. C., Li X. K., et al. (2009). Antiviral effect on H5N1 avian influenza virus of compound of Curcuma zedoaria volatile oil and hypericin perforatum extract liquid. J. China Pharm. Univ. 40 (2), 166–172. 10.3321/j.issn:1000-5048.2009.02.015 [DOI] [Google Scholar]
- Huang Z. Q., Liu L., Yang J., Chen J. J., Lin W. J., Wen G. J., et al. (2019). Study on anti-vaginitis mechanism of Curcuma zedoary based on network pharmacology. J. Math. Med. 32 (09), 1265–1268. 10.3969/j.issn.1004-4337.2019.09.001 [DOI] [Google Scholar]
- Ipar V. S., Dsouza A., Devarajan P. V. (2019). Enhancing curcumin oral bioavailability through nanoformulations. Eur. J. Drug Metab. Pharmacokinet. 44 (4), 459–480. 10.1007/s13318-019-00545-z [DOI] [PubMed] [Google Scholar]
- Jankasem M., Wuthi-Udomlert M., Gritsanapan W. (2013). Antidermatophytic properties of Ar-turmerone, turmeric oil, and Curcuma longa preparations. ISRN Dermatol 2013, 250597. 10.1155/2013/250597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings M. R., Parks R. J. (2020). Antiviral effects of curcumin on adenovirus replication. Microorganisms 8 (10), 1524. 10.3390/microorganisms8101524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C. X., Guo Y. Q., Zhang S. J., Cui Y., Li X. X., Ren X. Y., et al. (2020). Feasibility analysis of coronavirus disease 2019 Co-treated by zedoary turmeric oil injection. Chin. Traditional Herb. Drugs 51 (11), 3062–3069. 10.7501/j.issn.0253-2670.2020.11.026 [DOI] [Google Scholar]
- Kamurai B., Mombeshora M., Mukanganyama S. (2020). Repurposing of drugs for antibacterial activities on selected ESKAPE bacteria Staphylococcus aureus and Pseudomonas aeruginosa . Int. J. Microbiol. 2020, 8885338. 10.1155/2020/8885338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Jang E., Kim S. Y., Choi J. Y., Lee N. R., Kim D. S., et al. (2018). Preclinical evaluation of in vitro and in vivo antiviral activities of KCT-01, a new herbal formula against hepatitis B virus. Evid. Based Complement. Altern. Med. 2018, 1073509. 10.1155/2018/1073509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J., Yoo H. S., Kim J. C., Park C. S., Choi M. S., Kim M., et al. (2009). Antiviral effect of Curcuma longa linn extract against hepatitis B virus replication. J. Ethnopharmacol. 124 (2), 189–196. 10.1016/j.jep.2009.04.046 [DOI] [PubMed] [Google Scholar]
- Kim J., Ha H. L., Moon H. B., Lee Y. W., Cho C. K., Yoo H. S., et al. (2011). Chemopreventive effect of Curcuma longa linn on liver pathology in HBx transgenic mice. Integr. Cancer Ther. 10 (2), 168–177. 10.1177/1534735410380613 [DOI] [PubMed] [Google Scholar]
- Kim K., Kim K. H., Kim H. Y., Cho H. K., Sakamoto N., Cheong J. (2010). Curcumin inhibits hepatitis C virus replication via suppressing the akt-SREBP-1 pathway. FEBS Lett. 584 (4), 707–712. 10.1016/j.febslet.2009.12.019 [DOI] [PubMed] [Google Scholar]
- Kritis P., Karampela I., Kokoris S., Dalamaga M. (2020). The combination of bromelain and curcumin as an immune-boosting nutraceutical in the prevention of severe COVID-19. Metabol. Open 8, 100066. 10.1016/j.metop.2020.100066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari N., Kulkarni A. A., Lin X., McLean C., Ammosova T., Ivanov A., et al. (2015). Inhibition of HIV-1 by curcumin A, a novel curcumin analog. Drug Des. 9, 5051–5060. 10.2147/DDDT.S86558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutluay S. B., Doroghazi J., Roemer M. E., Triezenberg S. J. (2008). Curcumin inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology 373 (2), 239–247. 10.1016/j.virol.2007.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y., Yan Y., Liao S., Li Y., Ye Y., Liu N., et al. (2020). 3D-quantitative structure-activity relationship and antiviral effects of curcumin derivatives as potent inhibitors of influenza H1N1 neuraminidase. Arch. Pharm. Res. 43 (5), 489–502. 10.1007/s12272-020-01230-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan M. Y., Yang C. (2022). Clinical efficacy of guizhi fuling capsule combined with zedoary turmeric oil vaginal suppository in the treatment of bacterial vaginitis with pelvic inflammatory disease. Chin. J. Clin. Ration. Drug Use 15 (6), 127–130. 10.15887/j.cnki.13-1389/r.2022.06.040 [DOI] [Google Scholar]
- Leng W., Zhen Y., Li S. P., Yang F. Q., Wang Y. T. (2007). Study on absorption mechanisms of zedoary turmeric oil by caco-2 cell model. Chin. Pharm. J. (16), 1228–1232. 10.3321/j.issn:1001-2494.2007.16.009 [DOI] [Google Scholar]
- Li C. J., Zhang L. J., Dezube B. J., Crumpacker C. S., Pardee A. B. (1993). Three inhibitors of type 1 human immunodeficiency virus long terminal repeat-directed gene expression and virus replication. Proc. Natl. Acad. Sci. U. S. A. 90 (5), 1839–1842. 10.1073/pnas.90.5.1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Y., Wang C. L., Wang Y. X., Qi X. J. (2021). Research advances on pharmacokinetics and anti-breast cancer effect of curcumin. Traditional Chin. Drug Res. Clin. Pharmacol. (05), 744–750. 10.19378/j.issn.1003-9783.2021.05.022 [DOI] [Google Scholar]
- Li L., Xie Q., Bian G., Zhang B., Wang M., Wang Y., et al. (2020). Anti-H1N1 viral activity of three main active ingredients from zedoary oil. Fitoterapia 142, 104489. 10.1016/j.fitote.2020.104489 [DOI] [PubMed] [Google Scholar]
- Li T., Bai H. H., Zong X. N., Zhang Z., Fan L. Y., Liu Z. H. (2021). Experimental study on the repair mechanism of zedoary turmeric oil vaginal suppository on vaginal epithelial cells in atrophic vaginitis. Chin. J. Pract. Gynecol. Obstetrics 37 (05), 595–597. 10.19538/j.fk2021050122 [DOI] [Google Scholar]
- Li Y., Wang H., Wang H., Wu Y., Li Y., Guo F. (2022). Nine new sesquiterpenes from Curcuma wenyujin rhizomes. Fitoterapia 158, 105167. 10.1016/j.fitote.2022.105167 [DOI] [PubMed] [Google Scholar]
- Li Y., Wu J. H., Xie Y. H. (2017). On research progress of zedoary turmeric oil. J. Shaanxi Univ. Chin. Med. 40 (3), 118–121. 10.13424/j.cnki.jsctcm.2017.03.036 [DOI] [Google Scholar]
- Liao Q., Qian Z., Liu R., An L., Chen X. (2013). Germacrone inhibits early stages of influenza virus infection. Antivir. Res. 100 (3), 578–588. 10.1016/j.antiviral.2013.09.021 [DOI] [PubMed] [Google Scholar]
- Lim C. B., Ky N., Ng H. M., Hamza M. S., Zhao Y. (2010). Curcuma wenyujin extract induces apoptosis and inhibits proliferation of human cervical cancer cells in vitro and in vivo . Integr. Cancer Ther. 9 (1), 36–49. 10.1177/1534735409359773 [DOI] [PubMed] [Google Scholar]
- Lin M. H. (2011). Curative effect of zedoary turmeric oil injection on 80 cases of acute upper respiratory tract infection in children. Youjiang Med. J. 39 (2), 189. 10.3969/j.issn.1003-1383.2011.02.033 [DOI] [Google Scholar]
- Lin M., Li F. L., Lin H. Y., Li X. H. (2021). Effect analysis of zedoary turmeric oil vaginal suppository in treating mycotic vaginitis during pregnancy. Contemp. Med. Forum 19 (1), 80–82. 10.3969/j.issn.2095-7629.2021.01.055 [DOI] [Google Scholar]
- Liu W., Zhai Y., Heng X., Che F. Y., Chen W., Sun D., et al. (2016). Oral bioavailability of curcumin: Problems and advancements. J. Drug Target 24 (8), 694–702. 10.3109/1061186X.2016.1157883 [DOI] [PubMed] [Google Scholar]
- Liu X., Wang M., Zhou C., Zhou W., Cheng K., Kang J., et al. (2018). Selective transformation of carbon dioxide into lower olefins with a bifunctional catalyst composed of ZnGa2O4 and SAPO-34. China Contin. Med. Educ. 10 (4), 140–143. 10.1039/c7cc08642c [DOI] [PubMed] [Google Scholar]
- LoTempio M. M., Veena M. S., Steele H. L., Ramamurthy B., Ramalingam T. S., Cohen A. N., et al. (2005). Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin. Cancer Res. 11, 6994–7002. 10.1158/1078-0432.CCR-05-0301 [DOI] [PubMed] [Google Scholar]
- Lu D., Chen Q., Yi H. P. (2021). Regulatory mechanism of curcumin on CVB3-induced viral myocarditis in mice. J. Guangxi Med. Univ. 38 (7), 1363–1368. 10.16190/j.cnki.45-1211/r.2021.07.018 [DOI] [Google Scholar]
- Ma Q. Y., Jing W. Z., Liu Y., Xu Z. Y., Li W., Liu M. (2019). The meta-analysis on efficacy and safety of zedoary turmeric oil injection in the treatment of hand, foot, and mouth disease. Int. J. Virology 26 (1), 54–59. 10.3760/cma.j.issn.1673-4092.2019.01.016 [DOI] [Google Scholar]
- Ma X. C., Zheng J., Wu L. J., Guo D. A. (2007). Structural determination of three new germacrane-type sesquiterpene alcohols from curdione by microbial transformation. Magn. Reson Chem. 45 (1), 90–92. 10.1002/mrc.1922 [DOI] [PubMed] [Google Scholar]
- Ma Z., Wang N., He H., Tang X. (2019). Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. J. Control Release 316, 359–380. 10.1016/j.jconrel.2019.10.053 [DOI] [PubMed] [Google Scholar]
- Mao X., Yao R. M., Gao Y. J., Sun J., Bao L., Qu T. G., et al. (2018). Treatment effects and mechanisms of kangbingdu oral liquid against the infection of influenza A (H1N1). Chin. J. Pharmacovigil. 15 (9), 518–522. 10.3969/j.issn.1672-8629.2018.09.002 [DOI] [Google Scholar]
- Moghadamtousi S. Z., Kadir H. A., Hassandarvish P., Tajik H., Abubakar S., Zandi K. (2014). A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed. Res. Int. 2014, 186864. 10.1155/2014/186864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun S. H., Joung D. K., Kim Y. S., Kang O. H., Kim S. B., Seo Y. S., et al. (2013). Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus . Phytomedicine 20 (8-9), 714–718. 10.1016/j.phymed.2013.02.006 [DOI] [PubMed] [Google Scholar]
- Nabel G. J., Rice S. A., Knipe D. M., Baltimore D. (1988). Alternative mechanisms for activation of human immunodeficiency virus enhancer in T cells. Science 239 (4845), 1299–1302. 10.1126/science.2830675 [DOI] [PubMed] [Google Scholar]
- Negi P. S., Jayaprakasha G. K., Jagan Mohan Rao L., Sakariah K. K. (1999). Antibacterial activity of turmeric oil: A byproduct from curcumin manufacture. J. Agric. Food Chem. 47 (10), 4297–4300. 10.1021/jf990308d [DOI] [PubMed] [Google Scholar]
- Ou J. L., Mizushina Y., Wang S. Y., Chuang D. Y., Nadar M., Hsu W. L. (2013). Structure-activity relationship analysis of curcumin analogues on anti-influenza virus activity. FEBS J. 280 (22), 5829–5840. 10.1111/febs.12503 [DOI] [PubMed] [Google Scholar]
- Ozturk S. (2022). Demonstration of the efficacy of curcumin on carbapenem-resistant Pseudomonas aeruginosa with Galleria mellonella larvae model. Arch. Microbiol. 204 (8), 524. 10.1007/s00203-022-03135-x [DOI] [PubMed] [Google Scholar]
- Pan W. H., Wang X. Z. (2012). Progress in pharmacokinetics of curcumin. China Pharm. 21 (10), 95–96. 10.3969/j.issn.1006-4931.2012.10.060 [DOI] [Google Scholar]
- Patel S. S., Acharya A., Ray R. S., Agrawal R., Raghuwanshi R., Jain P. (2020). Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit. Rev. Food Sci. Nutr. 60 (6), 887–939. 10.1080/10408398.2018.1552244 [DOI] [PubMed] [Google Scholar]
- Pawar K. S., Mastud R. N., Pawar S. K., Pawar S. S., Bhoite R. R., Bhoite R. R., et al. (2021). Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19: A randomized clinical trial. Front. Pharmacol. 12, 669362. 10.3389/fphar.2021.669362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y. Y. (2019). Clinical effect of nifuratel tablet combined with zedoary turmeric oil vaginal suppository and ozone therapy instrument on patients with vaginitis during pregnancy. Med. Equip. 32 (20), 116–117. 10.3969/j.issn.1002-2376.2019.20.078 [DOI] [Google Scholar]
- Prasad S., Tyagi A. K. (2015). Curcumin and its analogues: A potential natural compound against HIV infection and AIDS. Food Funct. 6 (11), 3412–3419. 10.1039/c5fo00485c [DOI] [PubMed] [Google Scholar]
- Prusty B. K., Das B. C. (2005). Constitutive activation of transcription factor AP-1 in cervical cancer and suppression of human papillomavirus (HPV) transcription and AP-1 activity in HeLa cells by curcumin. Int. J. Cancer 113 (6), 951–960. 10.1002/ijc.20668 [DOI] [PubMed] [Google Scholar]
- Qiao B., Wu Y. Y., Li X. Y., Hu Y. N., Jia W. Q., Xing H. J., et al. (2020). Likely mechanisms of yifei sanjie prescription in treating pulmonary fibrosis. J. Yunnan Univ. Traditional Chin. Med. 43 (2), 97–102. 10.19288/j.cnki.issn.1000-2723.2020.02.018 [DOI] [Google Scholar]
- Qin Y. W., Zhao Q., Zhao Y. S., Ren X. Y., Bao K. D., Li X. K., et al. (2020). Exploring mechanism of Curcuma wenyujin against COVID-19. (2020). Chin. Traditional Herb. Drugs 51 (8), 1977–1983. 10.7501/j.issn.0253-2670.2020.08.001 [DOI] [Google Scholar]
- Rechtman M. M., Har-Noy O., Bar-Yishay I., Fishman S., Adamovich Y., Shaul Y., et al. (2010). Curcumin inhibits hepatitis B virus via down-regulation of the metabolic coactivator PGC-1alpha. Febs. Lett. 584 (11), 2485–2490. 10.1016/j.febslet.2010.04.067 [DOI] [PubMed] [Google Scholar]
- Romoli J., Silva M. V., Pante G. C., Hoeltgebaum D., Castro J. C., Oliveira da Rocha G. H., et al. (2022). Anti-mycotoxigenic and antifungal activity of ginger, turmeric, thyme and rosemary essential oils in deoxynivalenol (DON) and zearalenone (ZEA) producing Fusarium graminearum. Food Addit. Contam. Part B Surveill. 39 (2), 362–372. 10.1080/19440049.2021.1996636 [DOI] [PubMed] [Google Scholar]
- Saeedi-Boroujeni A., Mahmoudian-Sani M. R., Bahadoram M., Alghasi A. (2021). COVID-19: A case for inhibiting NLRP3 inflammasome, suppression of inflammation with curcumin? Basic Clin. Pharmacol. Toxicol. 128 (1), 37–45. 10.1111/bcpt.13503 [DOI] [PubMed] [Google Scholar]
- Sandur S. K., Pandey M. K., Sung B., Ahn K. S., Murakami A., Sethi G., et al. (2007). Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 28 (8), 1765–1773. 10.1093/carcin/bgm123 [DOI] [PubMed] [Google Scholar]
- Schiborr C., Eckert G. P., Rimbach G., Frank J. (2010). A validated method for the quantification of curcumin in plasma and brain tissue by fast narrow-bore high-performance liquid chromatography with fluorescence detection. Anal. Bioanal. Chem. 397 (5), 1917–1925. 10.1007/s00216-010-3719-3 [DOI] [PubMed] [Google Scholar]
- Serpa Guerra A. M., Gómez Hoyos C., Velásquez-Cock J. A., Vélez Acosta L., Gañán Rojo P., Velásquez Giraldo A. M., et al. (2020). The nanotech potential of turmeric (Curcuma longa L.) in food technology: A review. Crit. Rev. Food Sci. Nutr. 60 (11), 1842–1854. 10.1080/10408398.2019.1604490 [DOI] [PubMed] [Google Scholar]
- Shang J. L. (2017). Clinical efficacy of amphotericin B vaginal effervescent tablet combined with zedoary turmeric oil vaginal suppository in the treatment of recurrent vulvar and vaginal Candida albicans patients. Anti-infection Pharm. 14 (07), 1411–1412. 10.13493/j.issn.1672-7878.2017.07-051 [DOI] [Google Scholar]
- Sindhu K., Indra R., Rajaram A., Sreeram K. J., Rajaram R. (2011). Investigations on the interaction of gold-curcumin nanoparticles with human peripheral blood lymphocytes. J. Biomed. Nanotechnol. 7 (1), 56. 10.1166/jbn.2011.1199 [DOI] [PubMed] [Google Scholar]
- Singh M., AlkaLee K. E., Kumar P., Kang S. G. (2021). Curcuma turmeric oil enhanced anti-dermatophytic drug activity against Candida albicans and Trichophyton mentagrophytes. Curr. Drug Deliv. 18 (10), 1494–1504. 10.2174/1567201818666210729105220 [DOI] [PubMed] [Google Scholar]
- Singh M., Singh N. (2009). Molecular mechanism of curcumin induced cytotoxicity in human cervical carcinoma cells. Mol. Cell Biochem. 325 (1-2), 107–119. 10.1007/s11010-009-0025-5 [DOI] [PubMed] [Google Scholar]
- Soleimani V., Sahebkar A., Hosseinzadeh H. (2018). Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother. Res. 32 (6), 985–995. 10.1002/ptr.6054 [DOI] [PubMed] [Google Scholar]
- Song T., Sun R. (2016). Pharmacodynamics study of zedoary turmeric oil chitosan microspheres administered via arterial embolization. Artif. Cells Nanomed Biotechnol. 44 (8), 1958–1963. 10.3109/21691401.2015.1115411 [DOI] [PubMed] [Google Scholar]
- Sun Z. T., Lian F., Li X. J., Liu E. S., Feng J. H. (2007). Interventional effect of huangqi ezhu heji on the expression of transforming growth factor-beta 1 and transforming growth factor-beta 1 mRNA in the lung tissues of rats with pulmonary fibrosis. J. Clin. Rehabilitative Tissue Eng. Res. 11 (14), 2645–2656. 10.3321/j.issn.1673-8225.2007.14.019 [DOI] [Google Scholar]
- Tang R. S., Shi Z., Li J. Q., Chen L. (2021). Progress in the study of sparagnii rhizoma and Curcumae rhizoma medicine pair. J. Clin. Med. 18 (05), 226–229. 10.3969/j.issn.1672-6170.2021.05.062 [DOI] [Google Scholar]
- Teow S. Y., Liew K., Ali S. A., Khoo A. S., Peh S. C. (2016). Antibacterial action of curcumin against Staphylococcus aureus: A brief review. J. Trop. Med. 2016, 2853045. 10.1155/2016/2853045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ti H., Mai Z., Wang Z., Zhang W., Xiao M., Yang Z., et al. (2021). Bisabolane-type sesquiterpenoids from Curcuma longa L. Exert anti-influenza and anti-inflammatory activities through NF-κB/MAPK and RIG-1/STAT1/2 signaling pathways. Food Funct. 12 (15), 6697–6711. 10.1039/d1fo01212f [DOI] [PubMed] [Google Scholar]
- Vitali D., Bagri P., Wessels J. M., Arora M., Ganugula R., Parikh A., et al. (2020). Curcumin can decrease tissue inflammation and the severity of HSV-2 infection in the female reproductive mucosa. Int. J. Mol. Sci. 21 (1), 337. 10.3390/ijms21010337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghmare P. F., Chaudhari A. U., Karhadkar V. M., Jamkhande A. S. (2011). Comparative evaluation of turmeric and chlorhexidine gluconate mouthwash in prevention of plaque formation and gingivitis: A clinical and microbiological study. J. Contemp. Dent. Pract. 12 (4), 221–224. 10.5005/jp-journals-10024-1038 [DOI] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. JAMA 323 (11), 1061–1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Tang S. W., Xu H., Ge W. H. (2019a). Efficacy and safety of zedoray turmeric oil injection in the treatment of rotavirus enteritis: A meta-analysis. China Pharm. 28 (7), 65–68. 10.3969/j.issn.1006-4931.2019.07.021 [DOI] [Google Scholar]
- Wang P. L., Zhu M., Zhang W. (2019b). Clinical study of zedoary turmeric oil injection combined with oseltamivir in treatment of children with viral pneumonia. Drugs Clin. 34 (5), 1406–1409. 10.7501/j.issn.1674-5515.2019.05.030 [DOI] [Google Scholar]
- Wang Q., Guo J., Wang Y. N., Zhao L., Chen X. J., Song D. R. (2022b). Clinical observation on zedoary turmeric oil vaginal suppository in the treatment of cervical HPV infection. Guangming J. Chin. Med. 37 (10), 1761–1765. 10.3969/j.issn.1003-8914.2022.10.021 [DOI] [Google Scholar]
- Wang R., Wei Y., Wang M., Yan P., Jiang H., Du Z. (2021). Interaction of natural compounds in licorice and turmeric with HIV-NCp7 zinc finger domain: Potential relevance to the mechanism of antiviral activity. Molecules 26 (12), 3563. 10.3390/molecules26123563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. M. (2022). Effect of zedoary turmeric oil vaginal suppository combined with azithromycin on bacterial vaginitis and its influence on vaginal flora and Immune environment. Chin. Med. J. Metallurgical Industry 39 (2), 248. 10.13586/j.cnki.yjyx1984.2022.02.117 [DOI] [Google Scholar]
- Wang Y. (2015). Effect of compound zedoary turmeric oil suppository in clinical treatment of Candida vaginitis. World Latest Med. Inf. (28), 121. 10.3969/j.issn.1671-3141.2015.28.093 [DOI] [Google Scholar]
- Wang Z., Yin C., Gao Y., Liao Z., Li Y., Wang W., et al. (2022a). Novel functionalized selenium nanowires as antibiotic adjuvants in multiple ways to overcome drug resistance of multidrug-resistant bacteria. Biomater. Adv. 137, 212815. 10.1016/j.bioadv.2022.212815 [DOI] [PubMed] [Google Scholar]
- Wei Y. P., Luo Z. C. (2005). Study on anticandida activity of Curcuma zedoaria volatile oil in vitro . China J. Lepr. Skin Dis. 21 (7), 524–526. 10.3969/j.issn.1009-1157.2005.07.009 [DOI] [Google Scholar]
- Wei Z. Q., Zhang Y. H., Ke C. Z., Chen H. X., Ren P., He Y. L., et al. (2017). Curcumin inhibits hepatitis B virus infection by down-regulating cccDNA-bound histone acetylation. World J. Gastroenterol. 23 (34), 6252–6260. 10.3748/wjg.v23.i34.6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. M., Xu Z., Gu Q. Q., Gao H. C., Huang K. X. (2011). Research progress of curdione. Chin. J. Pharm. Biotechnol. (04), 369–371. 10.19526/j.cnki.1005-8915.2011.04.022 [DOI] [Google Scholar]
- Xia Q., Huang Z. G., Li S. P., Zhang P., Wang J., He L. N. (2004). The eExperiment study of the anti-virus effects of zedoary oil on influenza virus and respiratory syncytial virus. Chin. Pharmacol. Bull. 20 (3), 357–358. 10.3321/j.issn:1001-1978.2004.03.033 [DOI] [Google Scholar]
- Xu P. P., Fu L. C., Zhang F. X., Li J. Q., Zhu Y. T. (2009). The antiviral effect of curcumin on influenza virus. J. Trop. Dis. Parasitol. 7 (3), 127–128. 10.3969/j.issn.1672-2302.2009.03.002 [DOI] [Google Scholar]
- Yang B., Jiang J., Jiang L., Zheng P., Wang F., Zhou Y., et al. (2020). Chitosan mediated solid lipid nanoparticles for enhanced liver delivery of zedoary turmeric oil in vivo . Int. J. Biol. Macromol. 149, 108–115. 10.1016/j.ijbiomac.2020.01.222 [DOI] [PubMed] [Google Scholar]
- Yang R. L., Liu H., Ren H. (2007). Determination of curcumol in zedoary turmeric vaginal suppository by reversed-phase HPLC. J. Nant. Univ. Med. Sci. 27 (3), 187–188. 10.3969/j.issn.1674-7887.2007.03.018 [DOI] [Google Scholar]
- Yang X. X., Li C. M., Huang C. Z. (2016). Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale 8 (5), 3040–3048. 10.1039/c5nr07918g [DOI] [PubMed] [Google Scholar]
- Yang Z., Wang Z., Li J., Long J., Peng C., Yan D. (2022). Network pharmacology-based dissection of the underlying mechanisms of dyspnoea induced by zedoary turmeric oil. Basic Clin. Pharmacol. Toxicol. 130 (5), 606–617. 10.1111/bcpt.13722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin B. Z., Zhou L. L., Wang F., Sun X. L. (2021). Efficacy and mechanism of zedoary turmeric oil vaginal suppository combined with xinfuning for treating women with human papilloma virus infection. Chin. J. Fam. Plan. 29 (9), 1858–1863. 10.3969/j.issn.1004-8189.2021.09.018 [DOI] [Google Scholar]
- Yong-Xue S., Yongjin L., Dongping Z., Gang W., Zhichang L., Haiyan Z. (2012). Pharmacokinetic study of rhizoma Curcumae oil and rhizoma Curcumae oil-β-cyclodextrin inclusion complex in pigs after oral administration. J. Vet. Pharmacol. Ther. 35 (1), 47–51. 10.1111/j.1365-2885.2011.01286.x [DOI] [PubMed] [Google Scholar]
- Yu W., Qin X., Zhang Y., Qiu P., Wang L., Zha W., et al. (2020). Curcumin suppresses doxorubicin-induced cardiomyocyte pyroptosis via a PI3K/Akt/mTOR-dependent manner. Cardiovasc. Diagnosis Ther. 10 (4), 752–769. 10.21037/cdt-19-707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W., Qin Y. W., Lu T. L., Ji D., Jiang C. X. (2018). History evolution and modern research on processing technology of Curcumae radix, Curcumae rhizoma, and wenyujin rhizoma concisum. Chin. Traditional Herb. Drugs 49 (5), 1192–1200. 10.7501/j.issn.0253-2670.2018.05.031 [DOI] [Google Scholar]
- Zahra M., Mostafa T., Yasaman H., Amin D., Mostafa S., Seyed Sina N., et al. (2022). Review on the effects curcumin on tumors of the reproductive system: Curcumin and reproductive tumors. Galen. Med. J. 10, 1–8. 10.31661/gmj.v10i0.2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y. P. (2019). Clinical efficacy evaluation of roxithromycin combined with lactobacillus vaginal capsule and zedoary turmeric oil vaginal suppository in patients with mixed infectious vaginitis. Anti-Infection Pharm. 16 (5), 834–836. 10.13493/j.issn.1672-7878.2019.05-033 [DOI] [Google Scholar]
- Zhang J., Wei H., Zhang H. J. (2017). Clinical research on nifuratel nystatin suppository combined with zedoary turmeric oil vaginal suppository for atrophic vaginitis before colposcopy. Pract. Pharm. Clin. Remedies 20 (6), 676–678. 10.14053/j.cnki.ppcr.201706017 [DOI] [Google Scholar]
- Zhang X. Y., Bian M. L., Fang Q., Chen Q. Y., Chen Z. H., Xu M. (2007). Study on the mechanism of the proliferative inhibition of zedoary turmeric oil vaginal suppository on human papillomavirus in vitro . J. China-Japan Friendsh. Hosp. 21 (4), 216–219+259. 10.3969/j.issn.1001-0025.2007.04.008 [DOI] [Google Scholar]
- Zhao X. L., Yang C. R., Yang K. L., Li K. X., Hu H. Y., Chen D. W. (2010). Preparation and characterization of nanostructured lipid carriers loaded traditional Chinese medicine, zedoary turmeric oil. Drug Dev. Ind. Pharm. 36 (7), 773–780. 10.3109/03639040903485716 [DOI] [PubMed] [Google Scholar]
- Zhen Y., Wang J. H., Liang T. J., Wang J. R., Zhao T. J. (2020). Mechanism of Curcuma based on network pharmacology. Chin. Archives Traditional Chin. Med. 38 (2), 15–18. 10.13193/j.issn.1673-7717.2020.02.005 [DOI] [Google Scholar]
- Zheng D., Huang C., Huang H., Zhao Y., Khan M., Zhao H., et al. (2020). Antibacterial mechanism of curcumin: A review. Chem. Biodivers. 17 (8), e2000171. 10.1002/cbdv.202000171 [DOI] [PubMed] [Google Scholar]
- Zhou Y. Y., Dai Z. J., Zhang S. J., Li Y. C., Dai Y. R., Wang H., et al. (2022). Effect of zedoary turmeric oil injection on novel coronavirus in vivo and in vitro . Acta Univ. Med. Anhui 57 (04), 664–667+672. 10.19405/j.cnki.issn1000-1492.2022.04.030 [DOI] [Google Scholar]
- Zhou Z., Li X., Liu J., Dong L., Chen Q., Liu J., et al. (2015). Honeysuckle-encoded atypical MicroRNA2911 directly targets influenza A viruses. Cell Res. 25 (1), 39–49. 10.1038/cr.2014.130 [DOI] [PMC free article] [PubMed] [Google Scholar]