Abstract
Recently, we reported that Campylobacter jejuni, an important gastrointestinal pathogen, has the genetic determinants to produce a capsular polysaccharide (Karlyshev et al., Mol. Microbiol. 35:529–541, 2000). Despite these data, the presence of a capsule in these bacteria has remained controversial. In this study we stain C. jejuni cells with the cationic dye Alcian blue and demonstrate for the first time by electron microscopy that C. jejuni cells produce a polysaccharide capsule that is retained in the coccoid form but is absent in a kpsM mutant.
Campylobacter jejuni is currently a major cause of bacterial gastrointestinal disease in humans in many countries (13). Despite deciphering the genomic sequence of one isolate, NCTC 11168 (12), many questions relating to the biochemistry, physiology, and pathogenicity of the microorganism remain unanswered (20). Capsules frequently play key roles in the survival of bacteria and in immune evasion of several bacterial pathogens (16). Recently, it has been suggested that the high-molecular-weight fractions of C. jejuni lipopolysaccharide may be capsular polysaccharide (CPS) (3). Using the NCTC 11168 genome sequence data, we reported the genetic and biochemical characterization of these molecules and found that they are related to group II and III CPS found in other pathogenic bacteria (8). Despite detection of CPS in C. jejuni, to date there have been no reports on the direct visualization of capsules in this species. In this study, using electron microscopy (EM), we demonstrate for the first time that this important pathogen does produce a polysaccharide capsule surrounding the surface of the cell. Capsule detection was facilitated by our recent discovery that CPS of C. jejuni has a strong affinity to Alcian blue dye (9).
Bacterial polysaccharide capsules are usually heavily hydrated polymers loosely attached to the bacterial cell surface (16). Because of their fragile nature, they may be lost during sample preparation for EM. A number of different approaches to stabilize capsules for EM visualization have been used, including treatment with antibodies, different dyes, and chemical cross-linking (2). Alcian blue has been used for staining capsules in several bacterial species, including Klebsiella pneumoniae (2), Neisseria gonorrhoeae (6, 14), Bordetella bronchiseptica (10), Pasteurella multocida (5), and Haemophilus pleuropneumoniae (7). Both whole bacteria (10) and thin sections (2, 5, 6, 7, 14) were used successfully. To avoid disturbing fragile capsular material, bacteria are often fixed and stained directly on agar plates, followed by thin-section analyses (7, 14). Ruthenium red dye has also been used for staining bacterial CPS, for example, in Vibrio parahaemolyticus (4, 5). Alcian blue may stabilize the capsule, since treatment of cells only during the initial fixation step but not at subsequent stages facilitated capsule detection (10). Although Alcian blue is known to have a high affinity for acidic polysaccharides (1), the mechanism by which the dye binds is unclear.
Bacterial strains G1 (isolate from a patient with Guillain-Barré syndrome), X (gastrointestinal isolate) (8), and NCTC 11168 were grown at 37°C on agar plates (Columbia agar; Oxoid, Basingstoke, United Kingdom) containing growth and selective supplements (Oxoid) in a microaerobic atmosphere for 2 days. Agar pieces containing bacteria were cut, fixed, and treated with Alcian blue (17) or ruthenium red (11).
Fixation and processing steps were performed within a fume cupboard at room temperature in vials on a rotary mixer. Agar pieces with bacteria were put into fixative containing 1% Alcian blue 8GX (or 0.1% ruthenium red) and 3% glutaraldehyde prepared in 0.075 M sodium cacodylate buffer (pH 7.4) and incubated for 2.5 h. The samples were then washed with four changes of 0.2 M sucrose in 0.075 M sodium cacodylate buffer for 1.5 h and left for a further 1.5 h in 1% osmium tetroxide. The samples were incubated in buffered 0.2 M sucrose solution overnight in a refrigerator at 4°C, washed in ultrapure MilliQ water, and dehydrated through a graded series of 30 to 100% ethanol for 10 min per step, transferred sequentially into 3:1, 1:1, and 1:3 ethanol-TAAB resin (TAAB Laboratories, Reading, United Kingdom) mixtures for 1 h per step, and finally into 100% TAAB resin overnight. The samples were incubated in fresh 100% TAAB resin for 3 h, embedded in silicone flat molds, and placed into a 60°C oven to polymerize for 2 days. The blocks were removed from the molds and trimmed and 100-nm ultrathin sections were cut using a glass knife and a Leica Ultracut R ultramicrotome, (Leica Microsystems, Milton Keynes, England). The sections were floated onto MilliQ water and then placed onto copper grids coated with a thin Pioloform support film. The grid-mounted sections were then stained with 2% alcoholic uranyl acetate for 10 min in the dark, thoroughly washed in MilliQ water, then stained for a further 10 min on a drop of Reynolds' lead citrate (15) in a CO2-free atmosphere, washed in MilliQ water, and allowed to air dry before examination and photography on a Jeol 1200EX transmission electron microscope at 80 kV (Jeol Ltd, Welwyn Garden City, England). Micrographs were taken on Agfa Scienta EM film, and the resulting images were printed on Agfa multicontrast resin-coated paper using an Agfa Rapidoprint processor and Agfa chemistry.
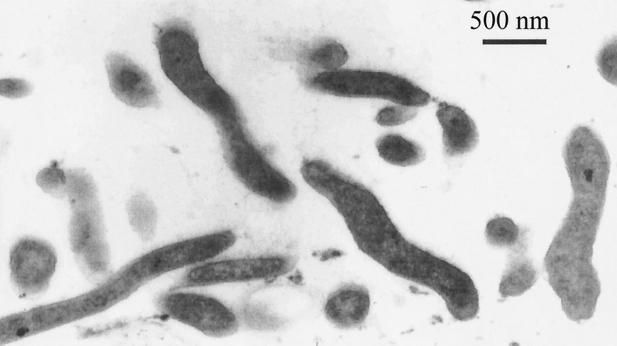
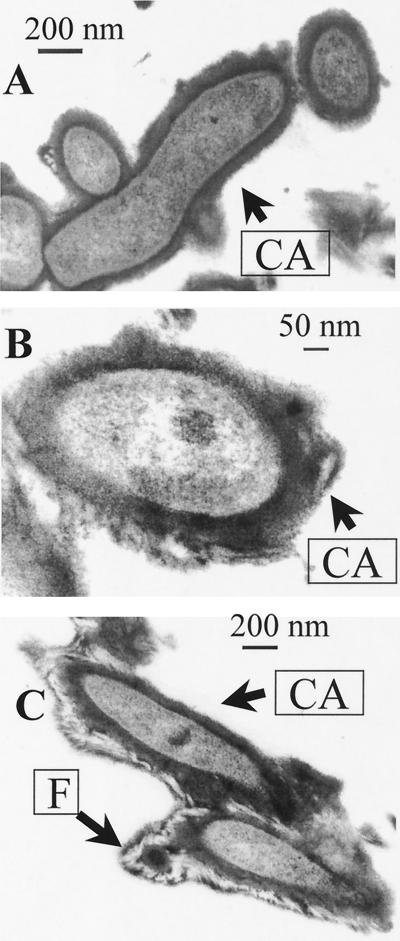
Ruthenium red dye was unable to stain C. jejuni cells (Fig. 1). By contrast, clearly defined capsules were detected after cells on agar blocks were fixed in the presence of Alcian blue (Fig. 2A and 2B). The capsule was presented as a thick amorphous structure covering bacterial cells. The thickness of the capsule usually varied between 70 and 100 nm, whereas the diameter of cross-cut cells was in the range of 200 to 250 nm. Some capsules were extended by a distinct massive fibrous layer (F) containing a characteristic bright internal area (Fig. 2C). As F structures have been reported during Alcian blue EM visualization of capsules in other microorganisms (2, 5), we investigated their nature. Surprisingly, such F structures could also be observed after treatment of blank agar blocks with Alcian blue. We suggest that F structures visible in EM are aggregates of Alcian blue dye. Such aggregates, which can often be observed on EM photographs between the cells, stick to encapsulated cells (Fig. 2C) and can easily be differentiated from amorphous capsule layer with irregular structure (Fig. 2A and B).
FIG. 1.
Cells of strain G1 treated with ruthenium red dye.
FIG. 2.
EM at different magnifications (A and B) of cells of strain G1 treated with Alcian blue. Capsulated cells with fibrilla-like structures are also shown (C). CA, capsule; F, fibrillae.
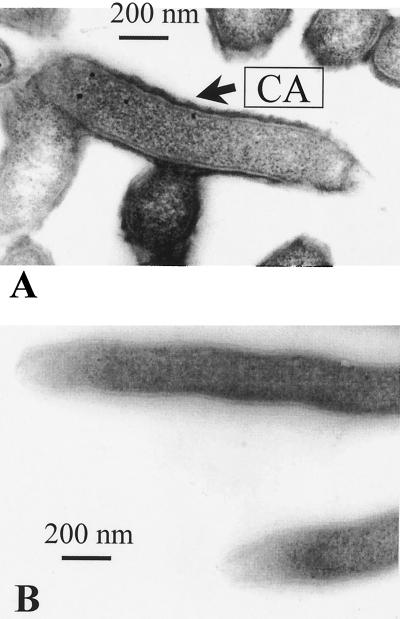
To investigate whether the capsule in the G1 strain consists of CPS, Alcian blue staining and EM were performed on defined insertional G1 mutants. One of the mutations, pldA, does not affect CPS production (9), and this mutant was used as a positive control. By contrast, the kpsM mutation was found to abolish production of CPS molecules (8). A capsule was seen in the pldA mutant, but it was somewhat thinner than the capsule observed in the wild-type strain (Fig. 3A). The reason for this difference remains unclear, although one cannot exclude that capsule formation can be modulated by the outer membrane phospholipase product of the pldA gene. As expected, no capsule was detected in the kpsM mutant by Alcian blue staining (Fig. 3B), confirming that the structure detectable in wild-type G1 cells is CPS.
FIG. 3.
Alcian blue-treated cells of pldA (A) and kpsM (B) mutants of strain G1.
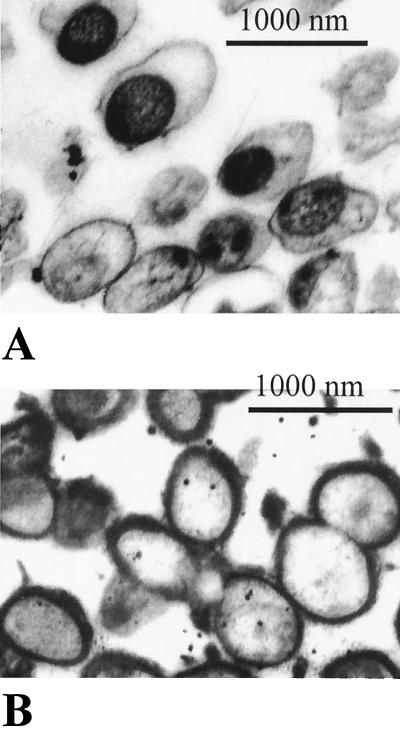
The results presented in Fig. 1 to 3 were from a 2-day-old culture. It is known that C. jejuni cells are prone to undergo transformation from a spiral to a coccoid morphology after prolonged incubation on a growth medium (19). We investigated whether this transformation has any effect on capsule staining. Four-day agar cultures of strain G1 were analyzed in EM after staining with either ruthenium red or Alcian blue dye. Most cells after 4 days of growth had become oval, with an average size of about 900 nm, i.e., about three times the cross-section of the spiral forms (Fig. 4A and B). As with 2-day-old cultures, no capsule could be detected with ruthenium red (Fig. 4A). A dark internal area presumably consisting of a nucleoid could be observed in the older culture. Interestingly, in contrast to 2-day-old cultures, the space between the nucleoid and cell wall is nonstainable. This probably indicates dramatic reduction in biochemical (transcriptional) activity in the coccoid cells. Similar observations were noticed when the same culture was treated with Alcian blue. A difference in this case, however, was the presence of a capsule (Fig. 4B).
FIG. 4.
Cells of 4-day agar culture of strain G1 after treatment with ruthenium red (A) and Alcian blue (B).
As in strain G1, a capsule was detected in wild-type strain X but not in the X kpsM::kan mutant (data not shown). The use of kpsM mutants in both cases was essential in eliminating the possibility of false-positive results due to a potential of Alcian blue to form aggregates and interact with other molecules on the cell surface (14).
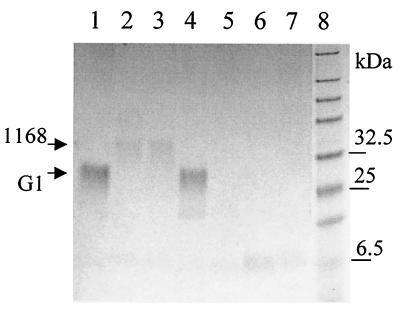
We also attempted to detect a capsule in the sequenced strain NCTC 11168 (12). A gene cluster related to CPS production is present in this strain (8). However, attempts to detect capsule in this strain have not been successful despite the observation that CPS of this strain is stainable with Alcian blue (9). One possibility is that the CPS of NCTC 11168 has lower affinity for Alcian blue and staining conditions on a gel (acid pH) are different from those used for staining-fixation for EM. This correlated with the lower intensity of CPS in the NCTC 11168 strain compared to that of strain G1 (Fig. 5). It is possible that despite CPS production in NCTC 11168, the mechanism of capsule formation in this strain is impaired or specific environmental signals are required for capsule formation. Reversible loss of capsule has been reported in some bacteria (4). As the kpsM gene is present in all nine C. jejuni strains tested (data not shown) and this gene is involved in both CPS biosynthesis and capsule formation in strain G1, we suggest that the majority of C. jejuni strains make or have the potential to make a polysaccharide capsule.
FIG. 5.
Alcian blue staining of heat extracts of different C. jejuni strains and their mutants after electrophoresis in an acrylamide gel as described (9). Lanes: 1, G1; 2, NCTC 11168; 3, 11168H (hypermotile clonal isolate of NCTC 11168); 4, G1 pldA::kan; 5, G1 kpsM::kan; 6, X kpsM::kan; 7, NCTC 11168 kpsM::kan; 8, prestained protein markers (New England BioLabs). The arrows indicate locations of CPSs of G1 and NCTC 11168 strains.
Through a combination of Alcian blue staining and EM, we have shown for the first time visualization of a C. jejuni capsule. This finding has confirmed the prediction from sequence analysis that C. jejuni cells may produce CPS and paves the way for the full genetic, biochemical, and structural analysis of this subcellular organelle. We have also demonstrated that despite apparent significant structural and biochemical changes, the coccoid form of C. jejuni retains the capsule. By similarity to the polysaccharide capsules of other microorganisms, C. jejuni capsules may be involved in enhancing survival of the microorganism in the environment by affording resistance to desiccation and increased adherence to various surfaces via biofilm formation, as well as in colonization and resistance to nonspecific and specific host immunity (reviewed in reference 16).
C. jejuni remains a major conundrum in microbiology. One paradox is how a microorganism that is difficult to culture and that has reduced viability when cultivated in vitro is able to survive in the environment to be the most frequently isolated bacterial food-borne pathogen (13, 18). The polysaccharide capsule described in this study might be an important new factor essential for the survival and pathogenicity of C. jejuni. Its discovery should facilitate future investigations on the survival, transmission, and pathogenesis of this problematic food-borne pathogen and may assist in the development of improved intervention strategies to reduce the burden of C. jejuni infection.
Acknowledgments
This work was supported by the BBSRC and the Wellcome Trust, United Kingdom.
REFERENCES
- 1.Bonnell B S, Smith S G, Hedrick J L. Dichromatic staining of electrophoretically separated extracellular matrix macromolecules. Anal Biochem. 1999;271:91–93. doi: 10.1006/abio.1999.4121. [DOI] [PubMed] [Google Scholar]
- 2.Cassone A, Garaci E. The capsular network of Klebsiella pneumoniae. Can J Microbiol. 1977;23:684–689. doi: 10.1139/m77-102. [DOI] [PubMed] [Google Scholar]
- 3.Chart H, Frost J A, Oza A, Thwaites R, Gillanders S, Rowe B. Heat-stable serotyping antigens expressed by strains of Campylobacter jejuni are probably capsular and not long-chain lipopolysaccharide. J Appl Bacteriol. 1996;81:635–640. doi: 10.1111/j.1365-2672.1996.tb03558.x. [DOI] [PubMed] [Google Scholar]
- 4.Enos-Berlage J L, McCarter L L. Relation of capsular polysaccharide production and colonial cell organization to colony morphology in Vibrio parahaemolyticus. J Bacteriol. 2000;182:5513–5520. doi: 10.1128/jb.182.19.5513-5520.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunther H, Rosner H, Godat M, Erler W. Electron microscopic visualization of the capsule of Pasteurella multocida. Acta Histochem Suppl. 1986;33:293–296. [PubMed] [Google Scholar]
- 6.Hendley J O, Powell K R, Salomonsky N L, Rodewald R R. Electron microscopy of the gonococcal capsule. J Infect Dis. 1981;143:796–802. doi: 10.1093/infdis/143.6.796. [DOI] [PubMed] [Google Scholar]
- 7.Jensen A E, Bertram T A. Morphological and biochemical comparison of virulent and avirulent isolates of Haemophilus pleuropneumoniae serotype 5. Infect Immun. 1986;51:419–424. doi: 10.1128/iai.51.2.419-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlyshev A V, Linton D, Gregson N A, Lastovica A J, Wren B W. Genetic and biochemical evidence of a Campylobacter jejuni capsular polysaccharide that accounts for Penner serotype specificity. Mol Microbiol. 2000;35:529–541. doi: 10.1046/j.1365-2958.2000.01717.x. [DOI] [PubMed] [Google Scholar]
- 9.Karlyshev A V, Wren B W. Detection and initial characterization of novel capsular polysaccharide among diverse Campylobacter jejuni strains using Alcian blue dye. J Clin Microbiol. 2001;39:279–284. doi: 10.1128/JCM.39.1.279-284.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kludas U, Jr, Rudolph W. A new method for the demonstration of the Bordetella bronchiseptica capsule in electron microscopy. Z Allg Mikrobiol. 1984;24:85–92. doi: 10.1002/jobm.3630240206. [DOI] [PubMed] [Google Scholar]
- 11.Luft J H. Ruthenium and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971;171:347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- 12.Parkhill J, Wren B W, Mungall K, Ketley J M, Churcher C, Basham D, Chillingworth T, Davies R M, Feltwell T, Holroyd S, Jagels K, Karlyshev A V, Moule S, Pallen M J, Penn C W, Quail M A, Rajandream M A, Rutherford K M, van Vliet A H, Whitehead S, Barrell B G. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 13.Rautelin H, Hanninen M L. Campylobacters: the most common bacterial enteropathogens in the Nordic countries. Ann Med. 2000;32:440–445. doi: 10.3109/07853890009002018. [DOI] [PubMed] [Google Scholar]
- 14.Reimann K, Heise H, Blom J. Attempts to demonstrate a polysaccharide capsule in Neisseria gonorrhoeae. APMIS. 1988;96:735–740. doi: 10.1111/j.1699-0463.1988.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 15.Reinolds E S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts I S. The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu Rev Microbiol. 1996;50:285–315. doi: 10.1146/annurev.micro.50.1.285. [DOI] [PubMed] [Google Scholar]
- 17.Shea S M. Lanthanum staining of the surface coat of cells. Its enhancement by the use of fixatives containing Alcian blue or cetylpyridinium chloride. J Cell Biol. 1971;51:611–620. doi: 10.1083/jcb.51.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solomon E B, Hoover D G. Campylobacter jejuni: a bacterial paradox. J Food Safety. 1999;19:121–136. [Google Scholar]
- 19.Thomas C, Hill D J, Mabey M. Morphological changes of synchronized Campylobacter jejuni populations during growth in single phase liquid culture. Lett Appl Microbiol. 1999;28:194–198. doi: 10.1046/j.1365-2672.1999.00504.x. [DOI] [PubMed] [Google Scholar]
- 20.Wassenaar T M, Blaser M J. Pathophysiology of Campylobacter jejuni infections of humans. Microbes Infect. 1999;1:1023–1033. doi: 10.1016/s1286-4579(99)80520-6. [DOI] [PubMed] [Google Scholar]