Abstract
Myeloproliferative neoplasms (MPNs) with concurrent BCR‐ABL1 fusion gene and CALR mutation are especially rare. We report a patient with coexisting BCR‐ABL1 fusion gene, CALR, and TET2 mutations who was treated with the combination of the second‐generation TKI nilotinib and JAK1/JAK2 inhibitor ruxolitinib.
Keywords: BCR‐ABL1 fusion gene, CALR mutation, myeloproliferative neoplasms, nilotinib, ruxolitinib
1. INTRODUCTION
Myeloproliferative neoplasms (MPNs) are divided into two categories: chronic myeloid leukemia (CML) characterized by the presence of the BCR‐ABL1 fusion gene and BCR‐ABL1‐negative MPNs. Somatic mutations in Janus kinase 2 (JAK2)‐V617F, exon 10 of thrombopoietin receptor/myeloproliferative leukemia (MPL) or exon 9 of calreticulin (CALR), are found in >90% of patients with classical BCR‐ABL1‐negative MPNs. 1 BCR‐ABL1 fusion gene and JAK2/MPL/CALR mutations are usually considered to be mutually exclusive. 2 However, several recent case reports have described patients with coexisting BCR‐ABL1 fusion gene and JAK2/MPL/CALR mutations. 3 , 4 , 5 Among these isolated cases, patients with concurrent BCR‐ABL1 fusion gene and CALR mutation are especially rare. The clonal relationship between two disorders and other genetic events that might contribute to the cooccurrence of more than one type of MPN in a patient are uncertain. Moreover, the safety and efficacy of the combination of tyrosine kinase inhibitor (TKI) and JAK1/JAK2 inhibitor for these patients are still unclear. Here, we report a patient with coexisting BCR‐ABL1 fusion gene, CALR and ten‐eleven translocation oncogene family member 2 (TET2) mutations who was treated with the combination of the second‐generation TKI nilotinib and JAK1/JAK2 inhibitor ruxolitinib.
2. CASE REPORT
In December 2014, a 42‐year‐old woman presented to our hospital with massive splenomegaly (9 cm below the costal margin). The initial complete blood count analysis showed a white blood cell count of 165 × 109/L, a hemoglobin (Hb) level of 68 g/L, and a platelet count of 156 × 109/L. Bone marrow aspiration showed marked granulocytic proliferation with an increased myeloid: erythroid ratio. Cytogenetic analysis revealed t(9;22)(q34;q11) in 10 out of 10 metaphase cells, and fluorescence in situ hybridization (FISH) for BCR‐ABL1 was also positive (93%). Reverse transcriptase polymerase chain reaction (RT‐PCR) confirmed a BCR‐ABL1 fusion gene, and the BCR‐ABL1 transcript level, which was shown as the ratio of BCR‐ABL1 to ABL1 on an international scale (BCR‐ABL1 IS), was 17.035% by quantitative RT‐PCR (qRT‐PCR). Thus, the patient was diagnosed with chronic‐phase CML and was initially treated with hydroxyurea, which caused a rapid reduction in the leucocyte count to a normal level. A week later, hydroxyurea was substituted with nilotinib at a dosage of 600 mg/day. Although splenomegaly and anemia persisted (6 cm below the costal margin and Hb: 87 g/L after 1 year of nilotinib treatment), the patient achieved a complete cytogenetic response (CCyR) after 3 months of nilotinib treatment and achieved a major molecular response 12 months later (Figure 1A–D).
FIGURE 1.
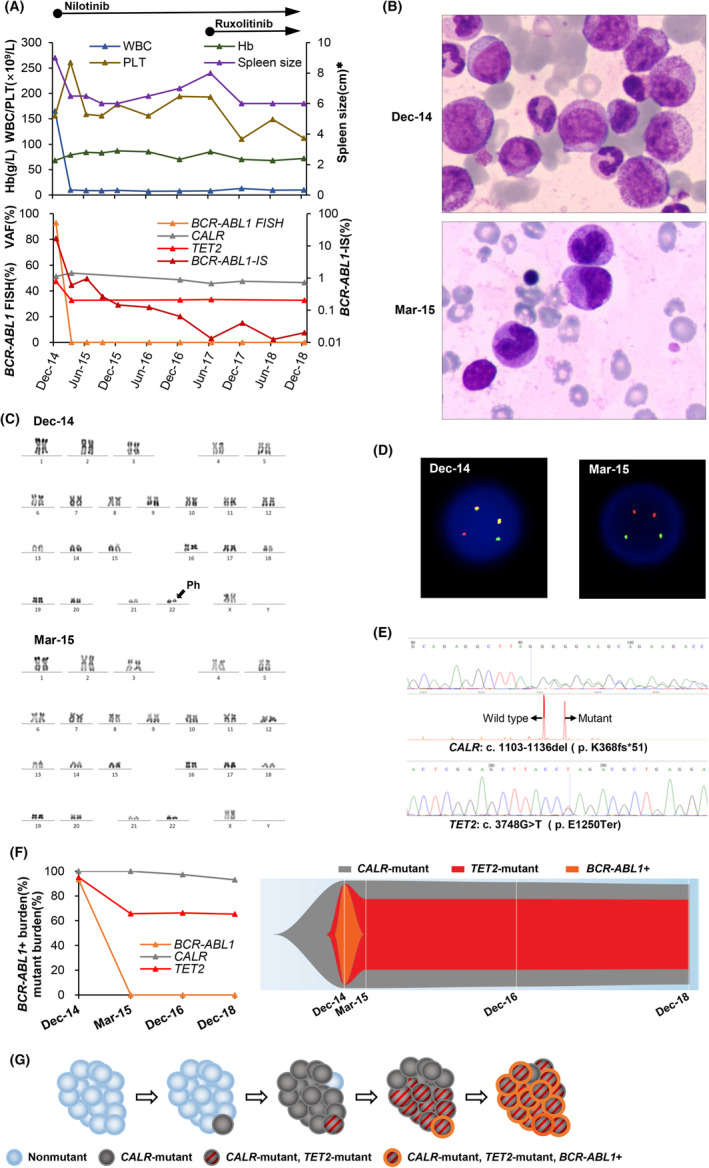
(A) Spleen size, hematological, cytogenetic, and molecular data acquired during the follow‐up of this patient with myeloproliferative neoplasm with coexisting BCR‐ABL1 fusion gene, CALR, and TET2 mutations. BCR‐ABL1 transcript levels are shown as the ratio of BCR‐ABL1 to ABL1, expressed as a percentage on the international scale (BCR‐ABL1‐IS). The CALR and TET2 mutations were detected by next‐generation sequencing and confirmed by Sanger sequencing. CALR mutation monitoring was performed using polymerase chain reaction followed by capillary electrophoresis. The variant allele frequency (VAF) of the CALR mutation was calculated by dividing the mutant peak area by the sum of the mutant and wild‐type peak areas. TET2 mutation monitoring was performed using next‐generation sequencing. (B) Bone marrow aspirate smears of this patient at the first diagnosis of CML and 3 months after treatment. The bone marrow aspirate smear at the first diagnosis of CML showed expansion of the neutrophil lineage and increased basophiles. (C) Chromosome karyotypes of this patient at the first diagnosis of CML and 3 months after treatment. At the first diagnosis of CML, the patient had the characteristic t(9;22)(q34;q11) reciprocal translocation which resulted in Ph chromosome, while the cytogenetic analysis revealed the normal karyotype after 3 months of treatment. (D) Fluorescence in situ hybridization (FISH) with dual‐color and dual‐fusion translocation probes for ABL1 (red) and BCR (green) of this patient at the first diagnosis of CML and 3 months after treatment. BCR‐ABL1‐positive cells at the first diagnosis of CML showed two fusion (red/green, yellow), one red and one green signals (2F1R1G), while the normal cells showed two red and two green signals (2R2G) after 3 months of treatment. (E) Sanger sequencing of CALR confirmed a frameshift mutation (c.1103_1136del, p.K368fs*51). Fragment length analysis by capillary electrophoresis showed concurrent amplification of the wild‐type allele and a mutant allele. Sanger sequencing of TET2 confirmed a nonsense mutation (c.3748G > T, p.E1259Ter). (F) Clonal evolution from the first diagnosis of CML to the last follow‐up. Mutant burdens were estimated according to the percentage of BCR‐ABL1‐positive cells by FISH and the VAF of CALR/TET2 mutations. Fish plot 15 showed that the CALR‐mutant, TET2‐mutant, and BCR‐ABL1‐positive subclone disappeared immediately after nilotinib treatment, while the CALR‐mutant, TET2‐mutant, BCR‐ABL1‐negative subclone and the initial dominant CALR‐mutant, TET2‐wild, BCR‐ABL1‐negative clone persisted even after treatment with a combination of nilotinib and ruxolitinib. (G) Schematic diagram of speculative clonal architecture and the order of alteration acquisition in this MPN patient. WBC, white blood cell; Hb, hemoglobin; PLT, platelet; *, below the costal margin. December 14, first diagnosis of CML; March 15, 3 months of nilotinib treatment; December 16, diagnosis of primary myelofibrosis; December 18, 18 months of combination therapy with nilotinib and ruxolitinib
In December 2016, the patient arrived at our hospital with appetite loss, fatigue, and increased splenomegaly (7 cm below the costal margin). Cytogenetic analysis demonstrated a CCyR, while BCR‐ABL IS was 0.06% by qRT‐PCR. The bone marrow biopsy showed granulocytic and erythrocytic hyperplasia accompanied by reticulin fibrosis grade 3. Molecular studies detected a 34‐bp deletion in CALR exon 9 (c.1103‐1136del, K368fs*51; Figure 1E), which was also observed in a previous study, 1 with a variant allele frequency (VAF) of 51.3% in the absence of JAK2 V617F and MPL mutations. Retrospective analysis of historical samples showed that the CALR mutation had been present since the initial diagnosis of CML with a stable allele burden regardless of the decrease in BCR‐ABL1 IS (Figure 1A). To investigate other genetic mutations that might contribute to this rare event of the concurrent BCR‐ABL1 fusion gene and CALR mutation, we also retrospectively studied the mutational status of 51 genes (Table S1) that are usually mutated in hematologic malignancies by next‐generation sequencing. A mutation in TET2 (c. 3748G > T, p. E1250Ter; Figure 1E) was detected in all historical samples analyzed, and the VAFs were slightly lower than that of the CALR mutation (Figure 1A). Ruxolitinib was administered at a dosage of 10 mg twice daily with the continuation of nilotinib, when the patient's constitutional symptoms worsened with progressive splenomegaly (8 cm below the costal margin) in June 2017. After 6 months of combination therapy, which the patient tolerated well, there was significant improvement in the constitutional symptoms and reduction in splenomegaly (6 cm below the costal margin). At the last follow‐up at our hospital in December 2018, the patient continued the combination treatment with nilotinib and ruxolitinib and maintained the original doses. Her constitutional symptoms had disappeared, while the spleen size remained 6 cm below the costal margin. Within 18 months of combination therapy, the VAF of CALR mutation fluctuated from 45.7% to 46.5%, and BCR‐ABL1 IS decreased to 0.01% (Figure 1A). Since 2019, this patient returned to the local hospital for treatment. As the telephone follow‐up in October 2020, she continued the combination treatment, splenomegaly and anemia still existed, BCR‐ABL1 IS was lower than 0.01%, and CALR mutation and TET2 mutation were not analyzed.
3. RESULTS AND DISCUSSION
To the best of our knowledge, there have been 21 patients with coexisting BCR‐ABL1 fusion gene and the CALR mutation reported in the literature, including our patient (Table 1). Only two of these patients had both the BCR‐ABL1 fusion gene and CALR mutation identified simultaneously at initial diagnosis (Table 1). Five of the seven patients with the initial diagnosis of BCR‐ABL1‐negative MPN had no detectable BCR‐ABL1 fusion gene and/or Ph + chromosome at initial diagnosis and then acquired this molecular alteration later with a median time to the second diagnosis of 48 months (range, 30–336 months); the other two patients had no available data (Table 1). Otherwise, 9/12 patients with the initial diagnosis of BCR‐ABL1‐positive CML had not only the BCR‐ABL1 fusion gene but also the CALR mutation by retrospective analysis of initial samples at the first diagnosis, and three patients did not undergo retrospective analysis of the CALR mutation (Table 1). These isolated cases suggested that the CALR mutation usually occurred earlier than the BCR‐ABL1 fusion gene. As observed in other case reports, 2 , 6 , 7 in our patient, the mutant CALR retained a high VAF of 45.7%–53.8%, while the BCR‐ABL1 IS decreased to 0.01% after nilotinib treatment. Therefore, we speculate that the CALR‐mutant, BCR‐ABL1‐positive subclone sensitive to nilotinib, arose from the initial dominant CALR‐mutant, BCR‐ABL1‐negative clone, which was resistant to nilotinib (Figure 1F,G). Additionally, we also detected the TET2 mutation in our patient, which was found in 10%–20% of all MPN subtype. 8 At the initial diagnosis, the VAF of TET2 mutation was 47.5%; together with the VAF of the CALR mutation (51.3%) and the BCR‐ABL1‐positive cells determined by FISH (93%), this suggests that the CALR mutation, TET2 mutation, and BCR‐ABL1 fusion gene coexisted in nearly all cells at this time. As the BCR‐ABL1 IS decreased to 0.597% after 3 months of nilotinib treatment, the VAF of the TET2 mutation also decreased to 32.8% and was maintained at a similar level, which was lower than that of the CALR mutation (Figure 1A). This result suggested that the BCR‐ABL1‐positive subclone arose from the CALR‐mutant, TET2‐mutant clone, and the TET2 mutation might have occurred after CALR mutation (Figure 1F,G). A previous study, 9 which found that patients in whom the JAK2 mutation was acquired first presented at a younger age than patients in whom the TET2 mutation was acquired first (60.71 years vs. 71.17 years), also supported our hypothesis, as our patient was only 42 years old at initial diagnosis. The clinical and morphologic appearance of our case at the initial presentation was more suggestive of a diagnosis of CML, despite the coexistence of BCR‐ABL1 and CALR mutation. With the decrease in BCR‐ABL1 IS after nilotinib therapy, the patient showed constitutional symptoms, increased splenomegaly, and myelofibrosis, which suggested the presence of a CALR mutation and supported the diagnosis of primary myelofibrosis (MF). This finding suggested that the clinical and morphologic features of patients with coexisting BCR‐ABL1 and CALR mutation might be dominated by BCR‐ABL1, which was also observed in previous reports. 6 , 7 Given the overlapping clinical and morphologic features of MPN, it is important to test all of the mutations in JAK2/MPL/CALR and the BCR‐ABL1 status in patients suspected of having MPNs.
TABLE 1.
Characteristics of reported patients of cooccurrence of BCR‐ABL1 fusion gene and CALR mutation
| Pt. | Age at 1st Dx, y | Sex | 1st Dx | 2nd Dx | Interval between Dx, m | Phase of CML at Dx | Driver mutations at 1st Dx | Driver mutations at 2nd Dx | Type of CALR mutation | Type of BCR‐ABL1 transcript | TKIs | RUX | Other therapy | Best response to TKIs | Status | OS from 1st Dx, m | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CALR | BCR‐ABL1 | CALR | BCR‐ABL1 | REF | |||||||||||||||
| 1 | 78 | F | CML + MPN, NOS | — | concurrent | BP | pos | pos | — | — | 52‐bp deletion (L367fs) | e1a2 | ND | ND | ND | ND | ND | ND | 16 |
| 2 | 65 | M | CML + MF | — | concurrent | CP | pos | pos | — | — | 52‐bp deletion (L367fs) | e13a2 | Dasat | N | N | CMR | alive | 27+ | 4 |
| 3 | 26 | M | ET | CML | 43 | CP | pos | neg | pos | pos | 5‐bp insertion (K385fs) | e14a2 | Nilot | N | IFN | CHR | alive | 48+ | 6 |
| 4 | 70 | M | Post‐ET MF | CML | 48 | ND | ND | ND | pos | pos | E364fs | ND | IM/Dasat | Y | Anagr | CHR | alive | 60+ | 3 |
| 5 | 63 | F | PMF | CML | 30 | CP | ND | neg | pos | pos | ND | e14a2 | IM | Y |
Hu/ allo‐SCT |
ND | alive | 60+ | 4 |
| 6 | 55 | F | MPN, NOS | CML | 336 | CP | ND | neg | pos | pos | 52‐bp deletion (L367fs) | ND | IM/Nilot | N | Hu | CHR | alive | 420+ | 17 |
| 7 | 46 | F | ET | CML | 216 | CP | ND | neg | pos | pos | 5‐bp insertion (K385fs) | e13a2/e14a2 | IM | Y | Hu/Anagr | MMR | alive | 240+ | 5 |
| 8 | 65 | F | ET | CML | 48 | CP | ND | neg | pos | pos | 52‐bp deletion (L367fs) | e13a2/e14a2 | Dasat | N | Aspirin | ND | alive | 68+ | 18 |
| 9 | 46 | M | ET | CML | 132 | CP | ND | ND | pos | pos | c.1182_1215del (L368fs) | e13a2/e14a2 | IM | N | IFN | MMR | alive | 156+ | 19 |
| 10 | 73 | F | CML | MPN, NOS | 90 | CP | pos | pos | pos | pos | 52‐bp deletion (L367fs) | ND | IM/Dasat | N | Peg‐IFN/Hu/EPO | MMR | alive | 91+ | 2 |
| 11 | 67 | M | CML | PMF | 7 | CP | pos | pos | pos | pos | 52‐bp deletion (L367fs) | ND | Dasat | N | N | CCyR | alive | 10+ | 7 |
| 12 | 50 | F | CML | PMF | 48 | CP | pos | pos | pos | MR4.0 | 52‐bp deletion (L367fs) | e14a2 | IM | N | N | MR4.5 | alive | 60+ | 20 |
| 13 | 54 | M | CML | MF | 24 | ND | ND | pos | pos | MMR | ND | e13a2/e14a2 | Dasat | N | N | MMR | alive | 48+ | 3 |
| 14 | 61 | F | CML | ET | 165 | CP | pos | pos | pos | MR4.5 | 52‐bp deletion (L367fs) | ND | IM | N | Hu/Peg‐IFN/CML VAX | MR4.5 | alive | 181+ | 21 |
| 15 | 67 | F | CML | ET | 3 | CP | pos | pos | pos | pos | 52‐bp deletion (L367fs) | ND | IM | N | N | ND | alive | 21+ | 22 |
| 16 | 55 | F | CML | MPN, NOS | 120 | AP | ND | pos | pos | MMR | c.1095_1140del (L367fs) | ND | IM/Nilot/Dasat | N | Hu/IFN+Ara‐C | MMR | alive | 180+ | 23 |
| 17 | 54 | M | CML | ET | 120 | CP | pos | pos | pos | pos | ND | ND | IM | N | Hu | CMR | alive | 120+ | 24 |
| 18 | 73 | F | CML | ET | 3 | CP | pos | pos | pos | pos | 52‐bp deletion (L367fs) | e13a2/e14a2 | IM | N | Hu/Anagr/Peg‐IFN | MR4.0 | alive | 26+ | 25 |
| 19 | 80 | M | CML | MPN, NOS | 3 | CP | ND | pos | pos | pos | 52‐bp deletion (L367fs) | ND | IM/Bosut | N | Hu | MMR | alive | ND | 26 |
| 20 | 33 | F | CML | ET | 51 | CP | Pos | Pos | Pos | MR4.0 | c.1099_1136delinsAGGT | ND | IM/Nilot | N | Hu | MR4.0 | alive | 51+ | 27 |
| 21 | 42 | F | CML | PMF | 24 | CP | pos | pos | pos | MMR | 34‐bp deletion (K368fs) | e13a2/e14a2 | Nilot | Y | Hu | MR4.0 | alive | 70+ | Our Pt. |
Abbreviations: allo‐SCT, allogeneic stem cell transplantation; Anagr, anagrelide; AP, accelerated phase; Ara‐C, cytosine arabinoside; Bosut, bosutinib; BP, blast phase; CCyR, complete cytogenetic response; CHR, complete hematologic response; CML VAX, a peptide vaccination protocol; CML, chronic myeloid leukemia; CMR, complete molecular response; CP, chronic phase; Dasat, dasatinib; Dx, diagnosis; EPO, erythropoietin; ET, essential thrombocythemia; F, female; Hu, hydroxyurea; IFN, interferon; IM, imatinib; M, male; m, months; MF, myelofibrosis; MMR, major molecular response; MPN, NOS, Myeloproliferative neoplasm, not otherwise specified; MR, molecular response; N, no; ND, no data; neg, negative; Nilot, nilotinib; OS, over survival; Peg‐IFN, peginterferon; PMF, primary myelofibrosis; pos, positive; Pt, patient; REF, reference; RUX, ruxolitinib; TKIs, tyrosine kinase inhibitors; y, years; Y, yes; —, not applicable.
As observed in CML patients 10 and patients with coexistent BCR‐ABL1 and JAK2 mutation, 11 combination treatment with TKI and ruxolitinib in our patient was safe and effective, which was also confirmed by three previous patients with coexistent BCR‐ABL1 and CALR mutation, one treated with imatinib/dasatinib and ruxolitinib 3 and the others with imatinib and ruxolitinib 4 , 5 Moreover, recent studies found that the combination of ruxolitinib and nilotinib had a synergistic effect against both CML stem cells 12 and MF cells, 13 so our combination of nilotinib and ruxolitinib may have been better for this patient. However, the CALR mutation allele burden was not decreased and the splenomegaly persisted after 18 months of ruxolitinib therapy. Other choices should be considered for this patient. Interferon‐α was reported to induce molecular responses in CALR‐mutated essential thrombocythemia, but not for the patients with additional nondriver mutations (including TET2 mutation). 14 Allogeneic stem cell transplant, if eligible for this procedure, may be a superior option. Summarizing such rare cases had important clinical significance. Firstly, with the continuous development of sequencing technology, more and more patients were reported, but its pathogenesis and clonal evolution were still inconclusive, which were under further research. Secondly, as for treatment, in addition to the traditional treatment, TKIs combined with ruxolitinib had considerable efficacy and safety, which provided more possibilities for the treatment of such patients. However, due to the scarcity of cases, the optimal treatment modality for patients with coexisting BCR‐ABL1 and JAK2/MPL/CALR mutations still remains uncertain, and a multicenter study with a large patient population may help address this unknown issue.
AUTHOR CONTRIBUTIONS
Li Huo: Data curation; formal analysis; visualization; writing – original draft. Jundan Xie: Formal analysis; methodology; visualization. Qian Wang: Formal analysis; visualization; writing – review and editing. Hongjie Shen: Methodology. Zixuan Ding: Methodology. Lijun Wen: Methodology. Zhao Zeng: Methodology. Yi Xu: Methodology. Changgeng Ruan: Supervision; writing – review and editing. Suning Chen: Conceptualization; data curation; funding acquisition; supervision; writing – review and editing. Mengxing Xue: Conceptualization; data curation; formal analysis; funding acquisition; visualization; writing – original draft.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICAL APPROVAL
This study was approved by the Ethics committee of the First Affiliated Hospital of Soochow University.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Supporting information
Table S1
ACKNOWLEDGMENTS
This study was supported by grants from the National Key R&D Program of China (2019YFA0111000), the National Natural Science Foundation of China (81900130, 81970136, 81970142, 82000132, 8217011130, and 8210010924), the Natural Science Foundation of the Jiangsu Higher Education Institution of China (18KJA320005), the Natural Science Foundation of Jiangsu Province (BK20190180), priority academic program development of Jiangsu Higher Education Institution, the Innovation Capability Development Project of Jiangsu Province (BM215004), the Translational Research Grant of NCRCH (2020WSB03, 2020WSB11, and 2020WSB13), and the Open Project of Jiangsu Biobank of Clinical Resources (SBK202003001, and SBK202003003).
Huo L, Xie J, Wang Q, et al. Insights from a rare myeloproliferative neoplasm with coexisting BCR‐ABL1 fusion gene, CALR, and TET2 mutations treated with nilotinib and ruxolitinib. Clin Case Rep. 2023;11:e06801. doi: 10.1002/ccr3.6801
Li Huo, Jundan Xie, Qian Wang contributed equally.
Contributor Information
Suning Chen, Email: chensuning@suda.edu.cn.
Mengxing Xue, Email: xuemengxing@suda.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379‐2390. [DOI] [PubMed] [Google Scholar]
- 2. Cabagnols X, Cayuela JM, Vainchenker W. A CALR mutation preceding BCR‐ABL1 in an atypical myeloproliferative neoplasm. N Engl J Med. 2015;372(7):688‐690. [DOI] [PubMed] [Google Scholar]
- 3. Kandarpa M, Wu Y, Robinson D, Burke PW, Chinnaiyan AM, Talpaz M. Clinical characteristics and whole exome/transcriptome sequencing of coexisting chronic myeloid leukemia and myelofibrosis. Am J Hematol. 2017;92(6):555‐561. [DOI] [PubMed] [Google Scholar]
- 4. Boddu P, Chihara D, Masarova L, Pemmaraju N, Patel KP, Verstovsek S. The co‐occurrence of driver mutations in chronic myeloproliferative neoplasms. Ann Hematol. 2018;97(11):2071‐2080. [DOI] [PubMed] [Google Scholar]
- 5. De Roeck L, Michaux L, Debackere K, Lierman E, Vandenberghe P, Devos T. Coexisting driver mutations in MPN: clinical and molecular characteristics of a series of 11 patients. Hematology. 2018;23(10):785‐792. [DOI] [PubMed] [Google Scholar]
- 6. Bonzheim I, Mankel B, Klapthor P, et al. CALR‐mutated essential thrombocythemia evolving to chronic myeloid leukemia with coexistent CALR mutation and BCR‐ABL translocation. Blood. 2015;125(14):2309‐2311. [DOI] [PubMed] [Google Scholar]
- 7. Loghavi S, Pemmaraju N, Kanagal‐Shamanna R, et al. Insights from response to tyrosine kinase inhibitor therapy in a rare myeloproliferative neoplasm with CALR mutation and BCR‐ABL1. Blood. 2015;125(21):3360‐3363. [DOI] [PubMed] [Google Scholar]
- 8. Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017;129(6):667‐679. [DOI] [PubMed] [Google Scholar]
- 9. Ortmann CA, Kent DG, Nangalia J, et al. Effect of mutation order on myeloproliferative neoplasms. N Engl J Med. 2015;372(7):601‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sweet K, Hazlehurst L, Sahakian E, et al. A phase I clinical trial of ruxolitinib in combination with nilotinib in chronic myeloid leukemia patients with molecular evidence of disease. Leuk Res. 2018;74:89‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iurlo A, Gianelli U, Rapezzi D, et al. Imatinib and ruxolitinib association: first experience in two patients. Haematologica. 2014;99(6):e76‐e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gallipoli P, Cook A, Rhodes S, et al. JAK2/STAT5 inhibition by nilotinib with ruxolitinib contributes to the elimination of CML CD34+ cells in vitro and in vivo. Blood. 2014;124(9):1492‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cortés AA, Diaz RA, Hernández‐Campo P, et al. Ruxolitinib in combination with prednisone and nilotinib exhibit synergistic effects in human cells lines and primary cells from myeloproliferative neoplasms. Haematologica. 2019;104(5):937‐946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verger E, Cassinat B, Chauveau A, et al. Clinical and molecular response to interferon‐α therapy in essential thrombocythemia patients with CALR mutations. Blood. 2015;126(24):2585‐2591. [DOI] [PubMed] [Google Scholar]
- 15. Miller CA, McMichael J, Dang HX, et al. Visualizing tumor evolution with the fishplot package for R. BMC Genomics. 2016;17(1):880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seghatoleslami M, Ketabchi N, Ordo A, Asl JM, Golchin N, Saki N. Coexistence of p190 BCR/ABL transcript and CALR 52‐bp deletion in chronic myeloid leukemia blast crisis: a case report. Mediterr J Hematol Infect Dis. 2016;8(1):e2016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klairmont MM, Cheng J, Schwartzberg L, Ho HH, Gradowski JF. Chronic myeloid leukemia, BCR‐ABL1‐positive with CALR and MPL mutations. Int J Lab Hematol. 2018;40(3):e41‐e42. [DOI] [PubMed] [Google Scholar]
- 18. Xia D, Hsi ED, Dal Cin P, Hasserjian RP. Composite chronic myeloid leukemia and essential thrombocythemia with BCR‐ABL1 fusion and CALR mutation. Am J Hematol. 2019;94(4):504‐505. [DOI] [PubMed] [Google Scholar]
- 19. Liu C, Hu R, Du Z, Abecasis M, Wang C. Atypical myeloproliferative neoplasm with concurrent BCR‐ABL1 fusion and CALR mutation: a case report and literature review. Medicine (Baltimore). 2020;99(5):e18811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diamond JMS, de Almeida AM, Belo HJLMR, Cabeçadas JMVS, de Sousa Ferreira Abecasis MM. CALR‐mutated primary myelofibrosis evolving to chronic myeloid leukemia with both CALR mutation and BCR‐ABL1 fusion gene. Ann Hematol. 2016;95(12):2101‐2104. [DOI] [PubMed] [Google Scholar]
- 21. Dogliotti I, Fava C, Serra A, et al. CALR‐positive myeloproliferative disorder in a patient with Ph‐positive chronic myeloid leukemia in durable treatment‐free remission: a case report. Stem Cell Investig. 2017;4:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gilles SR, Baughn LB, Schomaker ML, Courville EL, Nelson AC, Sachs Z. Buccal epithelial cells display somatic, bone marrow–derived CALR mutation. Blood Adv. 2017;1(25):2302‐2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lewandowski K, Gniot M, Wojtaszewska M, et al. Coexistence of JAK2 or CALR mutation is a rare but clinically important event in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Int J Lab Hematol. 2018;40(3):366‐371. [DOI] [PubMed] [Google Scholar]
- 24. da Costa VEF, de Oliveira RD, Traina F, Chahud F, Palma LC, de Figueiredo‐Pontes LL. Co‐occurrence of BCR–ABL1‐positive chronic myeloid leukaemia and CALR‐mutated essential thrombocythaemia. Br J Haematol. 2020;188(3):e21‐e23. [DOI] [PubMed] [Google Scholar]
- 25. Balducci E, Sanekli S, Hugues P, et al. Co‐occurrence of BCR‐ABL1 rearrangement and CALR mutation in a single leukemic stem cell: evidence that BCR‐ABL1 oncogenic addiction prevails over CALR signaling. Leuk Lymphoma. 2020;61(1):209‐212. [DOI] [PubMed] [Google Scholar]
- 26. Guidotti F, Gardellini A, Feltri M, et al. Concurrent chronic myeloid leukemia and CALR‐mutated chronic myeloproliferative neoplasm. Blood Cells Mol Dis. 2019;81:102395. [DOI] [PubMed] [Google Scholar]
- 27. Yoon SY, Jeong SY, Kim C, et al. Philadelphia+ chronic myeloid leukemia with CALR mutation: a case report and literature review. Cancer Res Treat. 2020;52(3):987‐991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


