Abstract
Application of polyester‐degrading microorganisms or enzymes should be considered as an eco‐friendly alternative to chemical recycling due to the huge plastic waste disposal nowadays. However, current impranil DLN‐based screening of polyester‐degrading microorganisms is time‐consuming, labour‐intensive and unable to distinguish polyesterases from other protease‐ or amidase‐like enzymes. Herein, we present an approach that combined a novel synthetic fluorescent polyurethane analogue probe (FPAP), along with the droplet‐based microfluidics to screen polyurethane‐degrading microorganisms through fluorescence‐activated droplet sorting (FADS) pipeline. The fluorescent probe FPAP exhibited a fluorescence enhancement effect once hydrolysed by polyesterases, along with a strong specificity in discriminating polyesterases from other non‐active enzymes. Application of FPAP in a microfluidic droplet system demonstrated that this probe exhibited high sensitivity and efficiency in selecting positive droplets containing leaf‐branch compost cutinase (LCC) enzymes. This novel fluorogenic probe, FPAP, combined with the droplet microfluidic system has the potential to be used in the exploitation of novel PUR‐biocatalysts for biotechnological and environmental applications.
A complete FADS pipeline using FPAP as the fluorogenic probe for screening polyester‐degrading microorganisms was developed.
INTRODUCTION
Polyester‐based plastics are considered a solution to the current plastic waste problem as well as leading polymers in terms of biodegradability and sustainability (Satti & Shah, 2020). Microorganisms and their enzymes involved in the process have emerged as important biocatalysts for polyester biodegradation or recycling via breaking ester bonds (Singh et al., 2021). However, up to now, only a few polyester‐depolymerizing enzymes with polyethylene glycol terephthalate (PET)‐degrading ability was reported, like PETase from Ideonella sakaiensis, and LCC cutinase (Cui et al., 2021; Tournier et al., 2020; Yoshida et al., 2016). Despite these, polyester‐depolymerizing enzymes, especially polyurethane (PUR)‐degrading enzymes, have been rarely reported (Liu et al., 2021). It can be expected that much more microbial polyester‐depolymerizing enzymes have not yet been discovered based on the great abundance of microorganisms in nature.
Currently, most of the screening methods for polyester‐degrading microorganisms are based on the transparent zones formed on impranil DLN‐based agar plates (Bollinger et al., 2020; Molitor et al., 2020). Impranil DLN is an anionic aliphatic polyester‐PUR, which appears as a white, milky suspension containing 40% of polymer particles (Howard et al., 2001). Bacterial colonies which have PUR‐degrading ability show clear zones on the impranil DLN containing agar plate (Russell et al., 2011). This screening method is limited to a maximum sample size of ~104 isolates and requires several days to complete, which is time‐consuming and labour‐intensive. Besides, the method may also yield false positive hits, due to those clones having protease‐ or amidase‐like enzymes also degrade impranil DLN to form clear halos (Magnin et al., 2020). Thus, construction of high‐throughput screening strategies with high efficiency targeting polyester‐degrading microorganisms is currently urgent.
Recently, the development of fluorescence‐activated droplet sorting (FADS) using microfluidic technologies has quickly emerged as a powerful tool for screening with ultra‐high throughput (Baret et al., 2009; Perez‐Rodriguez et al., 2022; Tu et al., 2021). The droplets‐based sorting enables cell proliferation inside droplets, achieving a considerable cell density to express desired enzymes, whereas the fluorescence‐activated cell sorting (FACS) technique is not suitable to do this. FADS technology has been successfully used for the directed evolution of enzymes (Qiao et al., 2018), single‐cell analysis (Vallejo et al., 2019) and screening for improved microbial production of extracellular compounds (Oberpaul et al., 2022; Xu et al., 2022). For most of the FADS screening, it is essential to convert the desired phenotype into a detectable signal. To address this problem, several strategies were created by the use of fluorescent dyes, through improved growth identified via green fluorescent protein (GFP) or red fluorescent protein (RFP) tagging of microbes, or with the use of chemical or enzyme‐linked reactions (Bowman & Alper, 2020; Chung et al., 2017). However, microfluidic‐based droplet sorting of polyester‐degrading microorganisms has been rarely explored due to the lack of highly efficient and specific fluorescent probes. A recent study by Qiao et al. applied a fluorescein dibenzoate (FDBz) for screening of PET‐degrading microorganisms by FADS (Qiao et al., 2022), this probe exhibited a fluorescence enhancement once the ester bond was hydrolysed by such as PETases and PET‐degrading microbes, allowing a significant difference in fluorescence for sorting (Qiao et al., 2022).
In this work, based on the molecular structure of PUR plastic monomer, we designed a novel fluorogenic probe and its related droplet‐based FADS pipeline for the high‐throughput polyester‐degrading microorganisms screening. This probe exhibited a fluorescence enhancement effect once degraded by the microorganisms and/or their enzymes, along with high specificity for targeting polyester hydrolases, enabling an easy identification of positive clones in droplets containing polyester‐degrading microorganisms from background environmental colonies. This novel PUR‐structure‐based fluorogenic probe not only facilitates a more specific, reliable and rapid screening of PUR‐degrading microbes but also gives strong inspiration for the application of fluorescent probes in other plastic‐degrading microorganisms screening.
RESULTS AND DISCUSSION
Design and synthesis of the fluorescent PUR analogue probe, FPAP
To develop a rapid, sensitive and specific fluorogenic assay for droplet‐based single‐cell sorting of PUR‐degrading microorganisms, we selected FPAP, a f luorescent p olyurethane a nalogue p robe, as the candidate fluorogenic probe and evaluated its specificity and sensitivity for fluorescence detection in picolitre droplets. A key difference between FPAP and the reported PET probe, such as FDBz, was that PFAP was synthesized based on the PUR monomer structure, by tagging the fluorogenic molecule to both sides of the PUR monomer (Figure 1). More importantly, the probe would show a strong increased fluorescent signal when degraded by polyesterase to release fluorescein, which is the fundamental basis for applying this probe in microfluidic droplet sorting. The FPAP itself emits a weak fluorescence, due to the low solubility of the probe, thus generating an aggregation‐caused quenching (ACQ) effect (Kisiel et al., 2020). ACQ is a known phenomenon responsible for low emission of dyes when in the form of solid‐state (e.g. in nanostructures, nano‐precipitates), although in solution (in appropriate solvent) these are characterized by bright emission (Kisiel et al., 2020). Based on the principle of ACQ, when PFAP is degraded by polyesterases, the two fluorescein molecules are released from FPAP, resulting in a significant enhancement of fluorescent intensity. Consistent with this, a high concentration of FPAP decreased the overall fluorescence signal output, and the optimal concentration of FPAP was 50 μM, with Ex = 440 nm and Em = 537 nm (Figure 2). We further tested the toxin effect of FPAP, and the results show that FPAP does not inhibit the growth of common model microorganisms, including Escherichia coli, Pseudomonas aeruginosa and Saccharomyces cerevisiae (Figure 3), suggesting a low biotoxicity and good biocompatibility of FPAP. Overall, FPAP was synthesized based on the structure of PUR plastic monomer, along with a fluorescence enhancement effect during screening, which will greatly facilitate the discovery of new polyester‐degrading microorganisms and/or enzymes.
FIGURE 1.
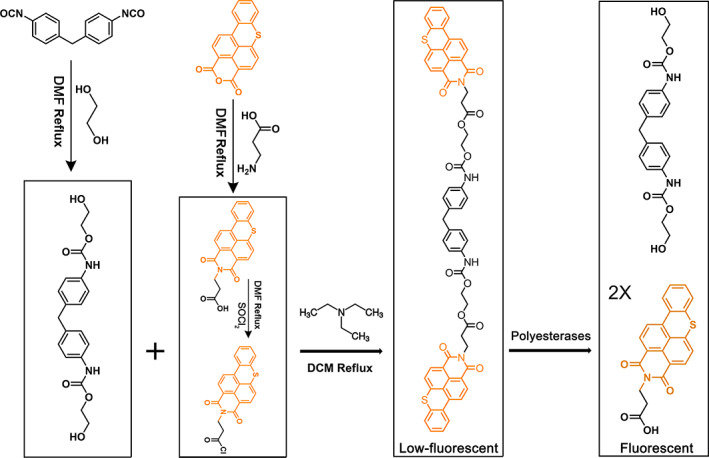
Synthesis route of the fluorescent polyurethane analogue probe, PFAP. PUR analogue monomer was first synthesized with 4,4′‐methylene diphenyl diisocyanate (4,4′‐MDI) and 1,4‐butanediol (1,4‐BDO); then, the fluorogenic substrate was reacted with 3‐aminopropionic acid and further tagged into PUR monomer. The synthetic probe was confirmed by 1H NMR spectroscopy. PFAP was characterized by low‐fluorescence when the structure is unbroken, this is due to the low solubility of the probe generating an aggregation‐caused quenching effect. Once PFAP is degraded by polyesterases, the two molecules fluorescent were released from the FPAP; thus, the fluorescence intensity was enhanced.
FIGURE 2.
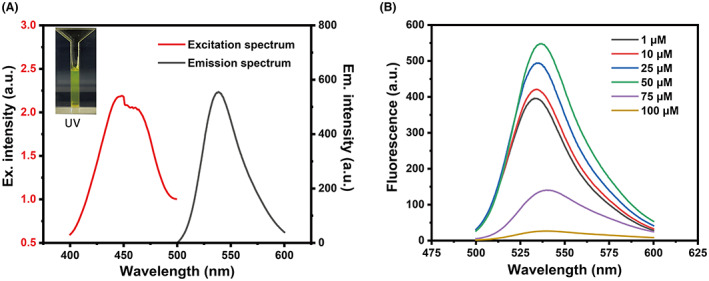
Fluorescent properties of the probe. (A) The maximum absorption peak appeared at 440 nm and the maximum wavelength of fluorescence emission was at 537 nm. (B) Optimized concentration of PFAP used for screening. PFAP exhibited an ACQ effect, a high concentration of the probe leads to aggregation while a low concentration of PFAP results in low fluorescence intensity, thus an optimized concentration for the probe used in this study is 50 μM.
FIGURE 3.
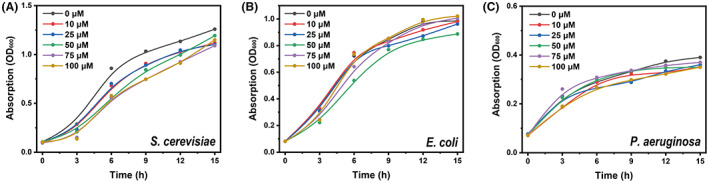
Toxicity analysis of the fluorescent probe during microbial growth. (A) Growth curves of S. cerevisiae (A), E. coli (B) and P. aeruginosa (C) with 0, 10, 25, 50, 75, 100 μM fluorescent probes show no significant inhibition of the growth.
FPAP exhibits a high‐level specificity in targeting polyesterases
One disadvantage of the conventional DLN‐based agar plate screening was the yield of false positive clones, which express protease‐ or amidase‐like enzymes rather than esterases, and these two enzymes are also able to hydrolyse DLN to form halos (Biffinger et al., 2015). Thus, a key aspect of whether PFAP could be used in FADS was the specificity in targeting polyesterases. To test this, we used lipase (Cat: L3126; Sigma) and protease (Cat: P5147; Sigma) to see whether these two enzymes were able to transform FPAP, at the same time, the LCC enzyme was used as a positive control, which has been reported to have the biodegradability of PUR in addition to PET. LCC could effectively break the ester bond and release the fluorescent molecules from the FPAP probe, resulting in a significant enhancement in the fluorescence signal (Figure 4A). As two references, the exogenous addition of lipase and protease did not alter the chemical structure of the probe, leading to overlapped fluorescence curves compared with FPAP as the sole substrate (Figure 4B). Overall, these data suggested that the synthetic fluorogenic probe exhibited a high level of specificity in discriminating polyesterases from other non‐active enzymes.
FIGURE 4.
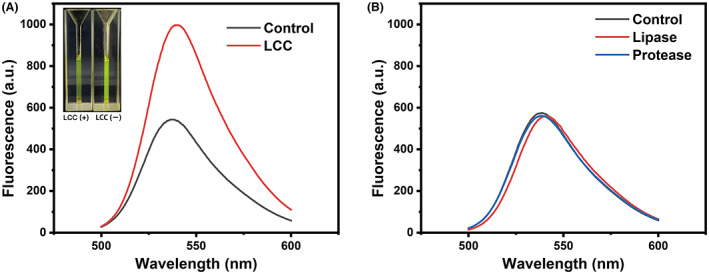
Specificity of PFAP in discriminating polyesterases. (A) Variation of the fluorescence intensity of the FPAP when depolymerize by LCC and PBS control. The Insert panel was the fluorescent images treated by UV irradiation. (B) Common commercial lipases and proteases do not affect the structure of PFAP.
It is worth noting that whether the FPAP probe is specifically targeting the PUR‐ polyesterases is currently unknown. This is caused by the fact that most of the reported plastic‐degrading enzymes were PET‐polyesterases, such as LCC, PETase, TfCut2, etc., polyesterases with exclusively PUR depolymerization ability have never been reported so far. Thus, we are not able to compare the activity between PET‐ and PUR‐polyesterases by using the FPAP substrate. Given that the probe was synthesized based on the structure of PUR monomer, we posit that FPAP would give a high possibility in selecting PUR‐degrading enzymes.
Application of FPAP in microfluidic droplet system through a FADS pipeline
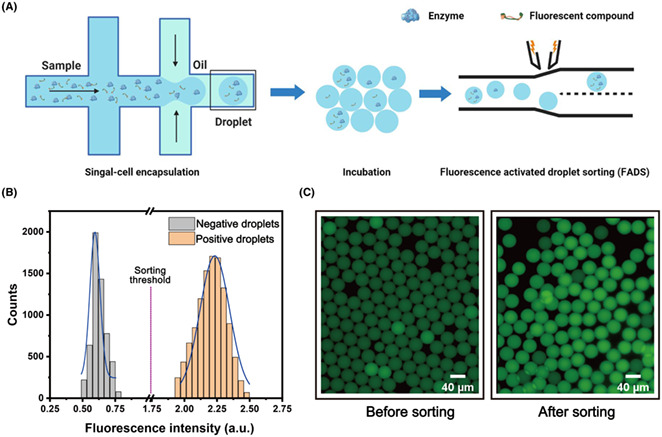
We then applied the fluorescent probe FPAP in a droplet‐based system by a FADS pipeline. We first encapsulated FPAP in droplets with the LCC enzymes to make positive droplets, droplets contain only FPAP as negative droplets. As the size of the droplets is consistent, every single microdroplet can be considered an individual microreactor and the catalytic abilities of the LCC enzyme can be quantitatively evaluated within droplets (Figure 5A). As a proof of concept, we prepared a mixed population droplet, containing 50% of the positive droplets along with 50% of negative droplets. After 6 h of incubation, those droplets were re‐injected into a microfluidic sorting device for analysis and sorting. When each microdroplet passed through the device, the fluorescence was detected by a photomultiplier tube (PMT), followed by the recording in a data acquisition card. As shown in Figure 5B, two distinct sub‐population with different fluorescent signals were found in 50 seconds recording (Figure 5B), suggesting a highly sensitive of our pipeline in selecting positive droplets containing LCC enzymes.
FIGURE 5.
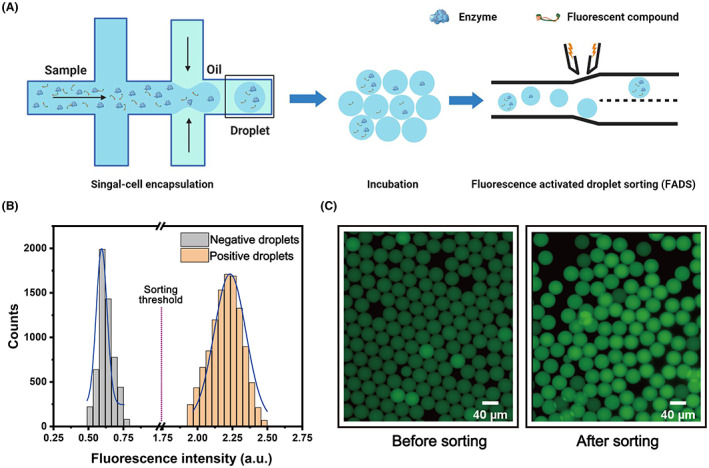
Application of FPAP in the microfluidic droplet system. (A) Schematic representation of enzyme encapsulation, probe decomposition by polyesterases during culturing, and the fluorescence activated droplet sorting system to select droplets with enhanced fluorescence. When the droplet passes the laser excitation point, the detection object in the droplet is excited to produce fluorescence. The signal generator receives the electrical signal and transmits the signal to the high‐voltage amplifier to amplify and generate a sorting pulse to generate the dielectrophoretic force required to deflect the target droplet into the collection channel. (B) The fluorescence intensity distribution of different droplets with or without LCC enzyme. (C) Images of the droplets containing LCC enzyme before and after sorting, showing a high efficiency of this probe in discriminating LCC polyesterase.
Next, we prepared 5% droplets containing the positive droplets to check the screening efficiency of our pipeline. We set a threshold at 1.75 a.u., which is the medium fluorescence intensities of positive and negative droplets. As a result, the droplets with the higher fluorescent signal will be dragged into a collection channel. Upon sorting, the microdroplets with higher fluorescence accounted for 95% of the total sorted droplets, indicating a high efficiency of this probe in discriminating LCC polyesterase (Figure 5C). In conclusion, these results indicate that the designed fluorescent probe, FPAP, combined with the droplet microfluidic system has the potential to be used in the screening of polyester‐degrading microorganisms or their corresponding enzymes.
CONCLUSIONS
In summary, we developed a complete FADS pipeline using FPAP as the fluorogenic probe for screening polyester‐degrading microorganisms to advance the discovery of PUR‐degrading enzymes for biodegradation and sustainable recycling of PUR. One of the highlights of the study was the fluorogenic polyurethane analogue probe, FPAP, an analogue of PUR plastic monomer containing the ester bond, which is the target site for various polyesterases. We believe this probe will greatly contribute to the exploitation of novel PUR‐biocatalysts.
FUNDING INFORMATION
The authors are supported by the National Key R & D Program of China (2021YFC2103600), the National Natural Science Foundation of China (31961133017, 21978129), the Natural Science Foundation of Jiangsu Province of China for Excellent Young Scholars (BK20211591), and the Jiangsu Association for Science and Technology Young Scientific and Technological Talents Support Project (TJ‐2021‐092). These grants are part of the ‘MIXed plastics biodegradation and UPcycling using microbial communities’ MIX‐UP research project, which is a joint NSFC and EU H2020 collaboration. In Europe, MIX‐UP has received funding from the European Union‘s Horizon 2020 research and innovation programme under grant agreement No 870294.
CONFLICT OF INTEREST
There are no conflicts to declare.
ACKNOWLEDGEMENTS
The authors are supported by the National Key R & D Program of China (2021YFC2103600), the National Natural Science Foundation of China (31961133017, 21978129), the Natural Science Foundation of Jiangsu Province of China for Excellent Young Scholars (BK20211591) and the Jiangsu Association for Science and Technology Young Scientific and Technological Talents Support Project (TJ‐2021‐092). These grants are part of the ‘MIXed plastics biodegradation and UPcycling using microbial communities’ MIX‐UP research project, which is a joint NSFC and EU H2020 collaboration. In Europe, MIX‐UP has received funding from the European Union‘s Horizon 2020 research and innovation programme under grant agreement No 870294.
Xu, A. , Liu, J. , Cao, S. , Xu, B. , Guo, C. & Yu, Z. et al. (2023) Application of a novel fluorogenic polyurethane analogue probe in polyester‐degrading microorganisms screening by microfluidic droplet. Microbial Biotechnology, 16, 474–480. Available from: 10.1111/1751-7915.14121
Anming Xu and Jiawei Liu contributed equally to this work.
Contributor Information
Jie Zhou, Email: jayzhou@njtech.edu.cn.
Weiliang Dong, Email: dwl@njtech.edu.cn.
REFERENCES
- Baret, J.C. , Miller, O.J. , Taly, V. , Ryckelynck, M. , El‐Harrak, A. , Frenz, L. et al. (2009) Fluorescence‐activated droplet sorting (FADS): efficient microfluidic cell sorting based on enzymatic activity. Lab on a Chip, 9, 1850–1858. [DOI] [PubMed] [Google Scholar]
- Biffinger, J.C. , Barlow, D.E. , Cockrell, A.L. , Cusick, K.D. , Hervey, W.J. , Fitzgerald, L.A. et al. (2015) The applicability of Impranil (R) DLN for gauging the biodegradation of polyurethanes. Polymer Degradation and Stability, 120, 178–185. [Google Scholar]
- Bollinger, A. , Thies, S. , Knieps‐Grunhagen, E. , Gertzen, C. , Kobus, S. , Hoppner, A. et al. (2020) A novel polyester hydrolase from the marine bacterium Pseudomonas aestusnigri ‐ structural and functional insights. Frontiers in Microbiology, 11, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, E.K. & Alper, H.S. (2020) Microdroplet‐assisted screening of biomolecule production for metabolic engineering applications. Trends in Biotechnology, 38, 701–714. [DOI] [PubMed] [Google Scholar]
- Chung, M.T. , Nunez, D. , Cai, D.W. & Kurabayashi, K. (2017) Deterministic droplet‐based co‐encapsulation and pairing of microparticles via active sorting and downstream merging. Lab on a Chip, 17, 3664–3671. [DOI] [PubMed] [Google Scholar]
- Cui, Y.L. , Chen, Y.C. , Liu, X.Y. , Dong, S.J. , Tian, Y.E. , Qiao, Y.X. et al. (2021) Computational redesign of a PETase for plastic biodegradation under ambient condition by the GRAPE strategy. ACS Catalysis, 11, 1340–1350. [Google Scholar]
- Howard, G.T. , Vicknair, J. & Mackie, R.I. (2001) Sensitive plate assay for screening and detection of bacterial polyurethanase activity. Letters in Applied Microbiology, 32, 211–214. [DOI] [PubMed] [Google Scholar]
- Kisiel, A. , Baniak, B. , Maksymiuk, K. & Michalska, A. (2020) Ion‐selective reversing aggregation‐caused quenching‐ maximizing optodes signal stability. Talanta, 220, 121358. [DOI] [PubMed] [Google Scholar]
- Liu, J.W. , He, J. , Xue, R. , Xu, B. , Qian, X.J. , Xin, F.X. et al. (2021) Biodegradation and up‐cycling of polyurethanes: progress, challenges, and prospects. Biotechnology Advances, 48, 107730. [DOI] [PubMed] [Google Scholar]
- Magnin, A. , Pollet, E. , Phalip, V. & Averous, L. (2020) Evaluation of biological degradation of polyurethanes. Biotechnology Advances, 39, 107457. [DOI] [PubMed] [Google Scholar]
- Molitor, R. , Bollinger, A. , Kubicki, S. , Loeschcke, A. , Jaeger, K.E. & Thies, S. (2020) Agar plate‐based screening methods for the identification of polyester hydrolysis by pseudomonas species. Microbial Biotechnology, 13, 274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberpaul, M. , Brinkmann, S. , Marner, M. , Mihajlovic, S. , Leis, B. , Patras, M.A. et al. (2022) Combination of high‐throughput microfluidics and FACS technologies to leverage the numbers game in natural product discovery. Microbial Biotechnology, 15, 415–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Rodriguez, S. , Garcia‐Aznar, J.M. & Gonzalo‐Asensio, J. (2022) Microfluidic devices for studying bacterial taxis, drug testing and biofilm formation. Microbial Biotechnology, 15, 395–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao, Y.X. , Zha, X.Y. , Zhu, J. , Tu, R. , Dong, L.B. , Wang, L. et al. (2018) Fluorescence‐activated droplet sorting of lipolytic microorganisms using a compact optical system. Lab on a Chip, 18, 190–196. [DOI] [PubMed] [Google Scholar]
- Qiao, Y.X. , Hu, R. , Chen, D.W. , Wang, L. , Wang, Z.Y. , Yu, H.Y. et al. (2022) Fluorescence‐activated droplet sorting of PET degrading microorganisms. Journal of Hazardous Materials, 424, 127417. [DOI] [PubMed] [Google Scholar]
- Russell, J.R. , Huang, J. , Anand, P. , Kucera, K. , Sandoval, A.G. , Dantzler, K.W. et al. (2011) Biodegradation of polyester polyurethane by endophytic fungi. Applied and Environmental Microbiology, 77, 6076–6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satti, S.M. & Shah, A.A. (2020) Polyester‐based biodegradable plastics: an approach towards sustainable development. Letters in Applied Microbiology, 70, 413–430. [DOI] [PubMed] [Google Scholar]
- Singh, A. , Rorrer, N.A. , Nicholson, S.R. , Erickson, E. , DesVeaux, J.S. , Avelino, A.F.T. et al. (2021) Techno‐economic, life‐cycle, and socioeconomic impact analysis of enzymatic recycling of poly(ethylene terephthalate). Joule, 5, 2479–2503. [Google Scholar]
- Tournier, V. , Topham, C.M. , Gilles, A. , David, B. , Folgoas, C. , Moya‐Leclair, E. et al. (2020) An engineered PET depolymerase to break down and recycle plastic bottles. Nature, 580, 216. [DOI] [PubMed] [Google Scholar]
- Tu, R. , Zhang, Y. , Hua, E.B. , Bai, L.K. , Huang, H.M. , Yun, K.Y. et al. (2021) Droplet‐based microfluidic platform for high‐throughput screening of Streptomyces . Communications Biology, 4, 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo, D. , Nikoomanzar, A. , Paegel, B.M. & Chaput, J.C. (2019) Fluorescence‐activated droplet sorting for single‐cell directed evolution. ACS Synthetic Biology, 8, 1430–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, A. , Zhang, X. , Cao, S. , Zhou, X. , Yu, Z. , Qian, X. et al. (2022) Transcription‐associated fluorescence‐activated droplet sorting for Di‐rhamnolipid hyperproducers. ACS Synthetic Biology, 11, 1992–2000. [DOI] [PubMed] [Google Scholar]
- Yoshida, S. , Hiraga, K. , Takehana, T. , Taniguchi, I. , Yamaji, H. , Maeda, Y. et al. (2016) A bacterium that degrades and assimilates poly(ethylene terephthalate). Science, 351, 1196–1199. [DOI] [PubMed] [Google Scholar]


