Abstract
Aims
This study aimed to assess, in patients with cardiogenic shock secondary to unprotected left main coronary artery‐related myocardial infarction (ULMCA‐related AMICS), the incidence and predictors of no recovery of left ventricular function during the admission.
Methods and results
This was an observational study conducted at two tertiary care centres (2012–20). The main outcome measured was death or requirement for heart transplantation (HT) or left ventricular assist devices (LVAD) during the admission. A total of 70 patients were included. Percutaneous coronary intervention (PCI) was successful in 53/70 patients (75.7%). The combined endpoint of death or requirement of HT or LVAD during the admission occurred in 41/70 patients (58.6%). The highest incidence of the primary endpoint was observed among patients with profound shock and occluded left main coronary artery (LMCA) (20/23, 87%, P < 0.001). Although a successful PCI reduced the incidence of the event in the whole cohort (51.9% vs. 82.4% in failed PCI, P = 0.026), this association was not observed among this last group of complex patients (86.7% vs. 87.5% in failed PCI, P = 0.731). The predictive model included left ventricular ejection fraction, baseline ULMCA Thrombolysis In Myocardial Infarction flow, and severity of shock and showed an optimal ability for predicting death or requirements for HT or LVAD during the admission (area under the curve 0.865, P < 0.001).
Conclusions
ULMCA‐related AMICS was associated with a high in‐hospital mortality or need for HT or LVAD. Prognosis was especially poor among patients with profound shock and baseline occluded LMCA, with a low probability of recovery regardless of successful PCI.
Keywords: Acute myocardial infarction, Cardiogenic shock, Left main coronary artery, Percutaneous coronary intervention, Mechanical circulatory support, Heart transplantation
Introduction
Cardiogenic shock (CS) is a state of severe hypoperfusion due to cardiac dysfunction. It represents approximately 5% of admissions to intensive care units and its incidence has increased in recent years. 1 CS due to acute coronary syndromes (ACS) is described in 5–10% of the cases, and despite the widespread use of revascularization and other therapeutic advances, the in‐hospital mortality of CS in this scenario remains remarkably high (40–60% for most case series). 2 , 3 , 4 Revascularization in the acute phase of ACS‐related CS is practically the only therapeutic measure that has shown a prognostic impact. 2 , 5 , 6 , 7 , 8 For this reason, immediate coronary angiography with the intention of revascularization is strongly recommended in this setting. 9
Culprit ACS lesions located in the left main coronary artery (LMCA) are associated with a faster presentation of CS, more severe systemic organ failure, and slower restoration of the organ function even in cases with successful revascularization (SR). 10 In this sense, information about the clinical course and the probability of recovery and weaning from short‐term mechanical circulatory support (MCS) in patients with LMCA‐related acute myocardial infarction complicated with cardiogenic shock (AMICS) is scarce. 11 , 12 , 13 , 14 , 15 , 16 Recent data describe a high mortality (up to 75%) in those patients. 17 Cardiac arrest, initial Thrombolysis In Myocardial Infarction (TIMI) flow grade, and unsuccessful revascularization have been suggested as predictors of mortality in this setting. 17
Patients with AMICS treated with MCS are usually associated with a significant delay in the decision‐making process as candidates for heart transplantation (HT), because in most cases, short‐term MCS is used as a bridge to recovery after percutaneous coronary intervention (PCI) in this context. 9 In critical patients with LMCA‐related AMICS, this delay can lead to a higher incidence of MCS‐related complications such as bleeding, infections, vascular events, and death.
The aims of this study were to assess the rate of death or requirements for HT and long‐term left ventricular assist devices (LVAD) at hospital discharge in a series of consecutive patients with unprotected left main coronary artery (ULMCA)‐related AMICS undergoing emergent angiography and to investigate the main predictors of no recovery in this complex clinical setting.
Methods
Study design and population
This was an observational, retrospective registry conducted at two tertiary care referral centres, including consecutive patients admitted for LMCA‐related AMICS between February 2012 and December 2020. Inclusion criteria were consecutive patients aged 18 years or older with ACS due to culprit coronary lesion in ULMCA who presented CS before or during coronary angiography. Patients with CS undergoing PCI in another centre that were referred to one of the study centres for continuation of CS management were also included. In order to better assess the impact of PCI on left ventricular recovery, patients with severe brain injury dying from neurological causes (n = 5) were excluded from the present study. The rest of patients with cardiac arrest but without death from brain injury were included in the analysis. No other exclusion criteria were applied. A total of 70 patients were finally included (Figure 1 ).
Figure 1.
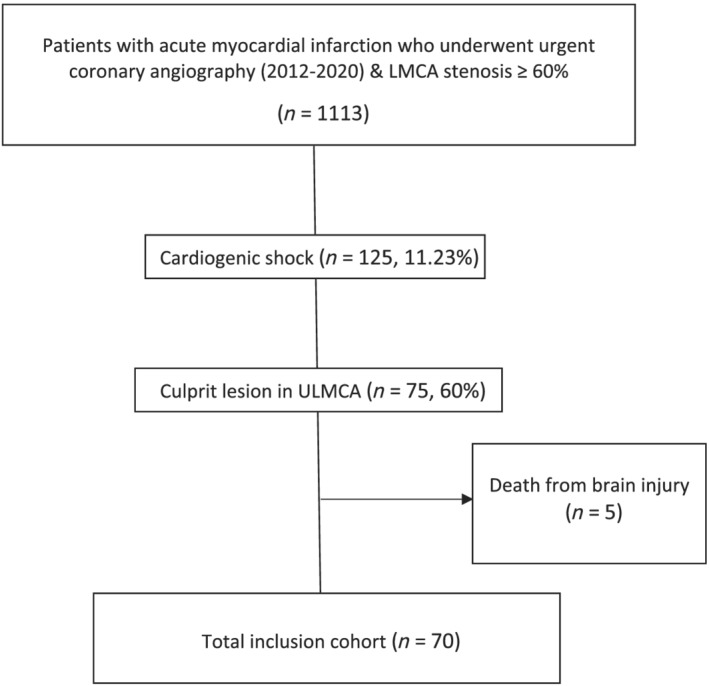
Study flowchart. LMCA, left main coronary artery; ULMCA, unprotected left main coronary artery.
Data collection and definitions
Based on prospective clinical registries of both participating institutions, including clinical condition at hospital admission, all patients with CS were screened for the present study. Baseline demographic, clinical, blood tests, echocardiographic, and angiographic data, as well as variables related to clinical management and in‐hospital complications, were registered for all selected patients.
ACS was defined as the presence of (a) chest pain with persistent elevation of more than 1 mm of the ST segment in two or more contiguous leads on the electrocardiogram or (b) chest pain and one of the following: (1) elevation of markers of myocardial damage (high‐sensitivity troponin) above the corresponding threshold of the laboratory or (2) electrocardiogram suggestive of myocardial ischaemia at the discretion of the treating cardiologist. CS was defined as the presence of (1) systolic blood pressure < 90 mmHg (in the absence of hypovolaemia and after proper fluid resuscitation) for 30 min or need for vasoactive drugs to maintain systolic blood pressure > 90 mmHg and (2) signs of hypoperfusion (altered mental status or confusion, peripheral coldness, oliguria < 0.5 mL/kg/h during the previous 6 h, and blood lactate > 2 mmol/L). The severity of CS was defined by the Society for Cardiovascular Angiography and Interventions (SCAI) classification considering SCAI‐C and D as non‐profound shock and SCAI‐E as profound shock. 18 The definition of culprit lesion in LMCA was at the discretion of the interventional cardiologist responsible for the procedure, based on angiographic criteria such as the presence of thrombus, ulceration, degree of stenosis, distal flow, and anatomical characteristics of the rest of the coronary tree. LMCA was defined as unprotected (ULMCA) when there was no coronary artery bypass grafting (CABG) on left anterior descending artery (LAD) and circumflex artery (Cx). SR was defined as residual stenosis of <30% and final TIMI 3 flow. The remaining definitions are available in the Supporting Information, Appendix S1 .
The decision regarding PCI in non‐culprit lesions was also at the discretion of the interventional cardiologist responsible for the procedure, according to lesion characteristics. The indication of MCS devices (either before or during PCI) was at the discretion of the treating heart team, according to severity of CS, coronary anatomy, and suitability of SR. Intra‐aortic balloon pump (IABP) was used in cases with haemodynamic unstability, especially when multivessel disease was present and complex PCI was anticipated. IABP was positioned sheated per protocol. Impella 2.5® or Impella CP® was used in cases with ongoing CS with high‐risk lesions and complex PCI. Venoarterial extracorporeal membrane oxygenation (VA‐ECMO) was preferably used in cases with profound CS with multiorganic failure criteria. A distal perfusion cannula was used per protocol in cases undergoing femoral VA‐ECMO. Levitronix Centrimag® and Impella 5® were indicated after recovery of multiorganic failure, as a bridge to HT, LVAD, or delayed recovery in cases with initial short‐term MCS weaning failure.
Clinical outcomes
The primary endpoint was the composite of death, HT, or LVAD at hospital discharge. Cause of death adjudication was based on the clinical judgement of the physician at the time of death. Cardiac death was classified when recurrent myocardial infarction, heart failure, or sudden death was registered as main cause of death.
Statistical analysis
The normality of the variables was evaluated using the Shapiro–Wilk test. Continuous variables were expressed, according to their distribution, as mean ± standard deviation (SD) or as median and interquartile range (IQR). The categorical variables were reported as n and percentages. Descriptive statistics were used to present the demographic, clinical, and analytical characteristics of the sample.
The analysis of predictors of ventricular recovery was performed by a logistic regression method, considering the survival free from HT or LVAD as dependent variable and the variables significantly associated (P < 0.2) with the main outcome in the univariate analysis as independent variables. The final model was built by a backward stepwise logistic regression model. The predictive ability of this final model was assessed by the receiver operating characteristic (ROC) curves and the corresponding area under the curve (AUC). SPSS Statistics, Version 21 (SPSS Inc., Chicago, IL, USA) and Stata v14.1 (StataCorp, College Station, TX, USA) were used for the analyses.
Ethics
This observational study was performed in accordance with ethical principles consistent with the Declaration of Helsinki. Management of patients was performed according to current recommendations. Confidential information of the patients was protected according to national normative. The protocol was revised and approved by the reference Clinical Research Ethics Committee (IRB00005523).
Results
Characteristics of patients are shown in Table 1 . The prevalence of comorbidities such as diabetes mellitus, prior acute myocardial infarction (AMI), prior heart failure, chronic kidney disease, or anaemia was significant, with a mean Charlson comorbidity index (CCI) value of 4.53. Likewise, most patients showed a high‐risk profile at admission, with a significant proportion of profound shock (30/70, 42.9%) and cardiac arrest (27/70, 38.6%). High requirements of vasoactive drugs and invasive mechanical ventilation were also described (Table 2 ).
Table 1.
Baseline characteristics
| n = 70 | |
|---|---|
| Age (years), mean (SD) | 66.0 (13.45) |
| Male sex, n (%) | 49 (70.00) |
| Hypertension, n (%) | 36 (50.70) |
| Diabetes mellitus, n (%) | 25 (35.71) |
| Dyslipidaemia, n (%) | 43 (61.43) |
| Ever smokers, n (%) | 49 (70.00) |
| Previous AMI, n (%) | 14 (20.00) |
| Previous PCI, n (%) | 11 (15.71) |
| Previous CABG, n (%) | 0 (0.00) |
| History of AF/atrial flutter, n (%) | 5 (7.14) |
| Previous CHF, n (%) | 14 (20.00) |
| Previous ACVE, n (%) | 8 (11.43) |
| Peripheral vascular disease, n (%) | 6 (8.57) |
| COPD, n (%) | 12 (17.14) |
| OSAHS, n (%) | 6 (8.57) |
| CKD, n (%) | 10 (14.29) |
| Active malignancy, n (%) | 4 (5.71) |
| Anaemia at admission, n (%) | 15 (21.43) |
| Previous clinically significant bleeding, n (%) | 3 (4.29) |
| CCI (points), mean (SD) | 4.53 (2.89) |
Abbreviations: ACVE, acute cerebrovascular event; AF, atrial fibrillation; AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; CCI, Charlson comorbidity index; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; OSAHS, obstructive sleep apnoea/hypopnea syndrome; PCI, percutaneous coronary intervention; SD, standard deviation.
Table 2.
Characteristics of shock
| Severity of cardiogenic shock | |
| SCAI score during PCI, n i/N (%) | |
| C | 22/70 (31.43) |
| D | 18/70 (25.71) |
| E | 30/70 (42.86) |
| Drugs administered during admission, n i/N (%) | |
| Dobutamine | 65/70 (92.86) |
| Norepinephrine | 60/70 (85.71) |
| Epinephrine | 9/70 (12.86) |
| Levosimendan | 5/70 (7.25) |
| Laboratory testing at admission | |
| pH, median (IQR) | 7.26 (0.18) |
| Lactate (mmol/L), median (IQR) | 4.63 (5.11) |
| Haemoglobin (g/dL), mean (SD) | 13.58 (2.33) |
| WBC (×109/L), mean (SD) | 15.51 (6.27) |
| Platelets (×109/L), median (IQR) | 221 (86) |
| Creatinine (μmol/L), median (IQR) | 103 (43) |
| ALT (U/L), median (IQR) | 78 (140.6) |
| INR, median (IQR) | 1.15 (0.26) |
| LVEF at admission (%), mean (SD) | 24.49 (9.97) |
| CPR prior or during PCI | |
| CPR, n i/N (%) | 27/70 (38.57) |
| Total time (min), median (IQR) | 21 (42) |
| Advanced CPR (min), median (IQR) | 17.5 (42) |
| Timing of revascularization | |
| Time from OS to PCI (h), median (IQR) | 3.43 (5.52) |
| Time from OS to FMC (h), median (IQR) | 2.75 (5.37) |
| Time from FMC to PCI (h), median (IQR) | 0.59 (0.65) |
| Respiratory support before PCI | |
| Non‐invasive MV, n i/N (%) | 11/70 (15.71) |
| Invasive MV, n i/N (%) | 45/70 (64.29) |
Abbreviations: ALT, alanine aminotransferase; CPR, cardiopulmonary resuscitation; FMC, first medical contact; INR, international normalized ratio; IQR, interquartile range; LVEF, left ventricular ejection fraction; MV, mechanical ventilation; OS, onset of symptoms; PCI, percutaneous coronary intervention; SD, standard deviation; WBC, white blood cells.
Angiographic data and invasive management
All patients underwent emergent angiography, in most cases (38/70, 54.3%) by femoral approach (Supporting Information, Table S1 ). Most patients (37/70, 52.9%) had TIMI 0 flow in LMCA before PCI, and 47/70 patients (67.1%) had multivessel disease. PCI in LMCA was successful in 53/70 patients (75.7%). PCI in non‐culprit lesions was performed in 19/70 patients (27.1%), in most cases during the first emergent angiography (15/19) and by staged procedures among the rest of cases (4/19). Most patients (57/70, 81.4%) required the use of short‐term MCS devices, being IABP the most commonly used (Table 3 ).
Table 3.
Indication of mechanical circulatory support devices
| Short‐term mechanical circulatory devices | |
| IABP, n i/N (%) | 48/70 (68.57) |
| IABP before PCI, n i/N (%) | 39/48 (81.25) |
| Impella CP/2.5, n i/N (%) | 15/70 (21.43) |
| Impella CP/2.5 before PCI, n i/N (%) | 7/15 (46.67) |
| Impella CP/2.5—removal criteria: complete weaning, n i/N (%) | 6/15 (40.00) |
| Impella CP/2.5—duration of support (days), mean (SD) | 4.4 (3.36) |
| VA‐ECMO, n i/N (%) | 19/70 (27.14) |
| VA‐ECMO before PCI, n i/N (%) | 3/19 (15.79) |
| VA‐ECMO—removal criteria: complete weaning, n i/N (%) | 4/19 (21.05) |
| VA‐ECMO—duration of support (days), median (IQR) | 9 (3) |
| VA‐ECMO—pulmonary congestion during support, n i/N (%) | 10/19 (52.63) |
| IABP for LV unloading in VA‐ECMO patients, n i/N (%) | 3/10 (30.00) |
| Impella CP/2.5 for LV unloading in VA‐ECMO patients, n i/N (%) | 2/10 (20.00) |
| Intermediate and long‐term mechanical ventricular devices | |
| Levitronix Centrimag, n i/N (%) | 8/70 (11.43) |
| Levitronix Centrimag—duration of support (days), mean (SD) | 8 (9.12) |
| Impella 5.0, n i/N (%) | 3/70 (4.29) |
| Impella 5.0—duration of support (days), mean (SD) | 12.67 (7.23) |
| LVAD a , n i/N (%) | 0 (0.00) |
Abbreviations: IABP, intra‐aortic balloon pump; IQR, interquartile range; LVAD, left ventricular assist devices; PCI, percutaneous coronary intervention; SD, standard deviation; VA‐ECMO, venoarterial extracorporeal membrane oxygenation.
Considering HeartMate II, HeartMate3, Heartware, and BerlinHeart Excor.
In‐hospital clinical outcomes
A significant incidence of in‐hospital complications was observed (Table 4 ). In‐hospital bleeding events occurred in 28/70 patients (40%). Most bleeding episodes were non‐device related (23/28, 82.1%), being the most common locations gastrointestinal and respiratory bleeding [6/23 (26.1%) and 6/23 cases (26.1%), respectively].
Table 4.
In‐hospital clinical outcomes
| Infection, n i/N (%) | 27/70 (38.57) |
| RRT, n i/N (%) | 14/70 (20.00) |
| Bleeding, n i/N (%) | 28/70 (40.00) |
| Limb ischaemia, n i/N (%) | 10/70 (14.29) |
| Arterial embolism, n i/N (%) | 7/70 (10.00) |
| Ischaemic ACVE, n i/N (%) | 5/70 (7.14) |
| Duration of IMV a (days), median (IQR) | 1 (8) |
| Duration of IMV b (days), median (IQR) | 5 (15) |
| Prone positioning, n i/N (%) | 3/70 (4.29) |
| Tracheostomy, n i/N (%) | 7/70 (10.00) |
| LMCA stent thrombosis, n i/N (%) | 2/70 (2.86) |
| Repeated PCI on LMCA, n i/N (%) | 1/70 (1.43) |
| PCI on non‐culprit lesion, n i/N (%) | 19/70 (27.14) |
| Death, n i/N (%) | 38/70 (54.29) |
| HT, n i/N (%) | 4/70 (5.71) |
| LVAD implantation, n i/N (%) | 0 (0.00) |
| Combined (death, HT, LVAD), n i/N (%) | 41/70 (58.57) |
| Impella 5.0 implantation, n i/N (%) | 3/70 (4.29) |
| Centrimag implantation, n i/N (%) | 8/70 (11.43) |
| CPC score 1, n i/N (%) c | 20/32 (62.5) |
Abbreviations: ACVE, acute cerebrovascular event; CPC, Cerebral Performance Category; HT, heart transplantation; IMV, invasive mechanical ventilation; IQR, interquartile range; LMCA, left main coronary artery; LVAD, left ventricular assist devices; PCI, percutaneous coronary intervention; RRT, renal replacement therapy.
The entire cohort (n = 71) is considered for calculation.
Only IMV recipients (n = 49) are considered for calculation.
The percentage is calculated considering the number of patients who presented cardiopulmonary resuscitation (CPR) before or during PCI and who did not die from postanoxic encephalopathy during admission.
A total of 27/70 patients (38.6%) presented infectious complications, mostly respiratory infections (12/27, 44.4%) and catheter‐related bacteraemia (6/27, 22.2%). A total of 10/70 patients (14.3%) had limb ischaemia. Overall, a total of 4/70 patients (5.7%) underwent HT during index hospitalization and none received LVAD. The incidence of in‐hospital mortality was 38/70 (54.3%). The combined endpoint of death, HT, or LVAD during the admission occurred in 41/70 patients (58.6%).
Clinical characteristics according to the occurrence of the main endpoint
Patients presenting with any of the components of the primary endpoint during hospitalization were significantly younger and had lower proportion of comorbidities such as diabetes mellitus and dyslipidaemia, with a lower CCI value (Supporting Information, Table S3 ) than patients without the primary endpoint. In contrast, those patients had higher severity of shock during PCI (SCAI‐E class 61% vs. 17%, P < 0.001), higher creatinine, lactate, and transaminases values, lower left ventricular ejection fraction (LVEF) (21% vs. 30%, P < 0.001), and a higher proportion of complete occlusion (TIMI 0 flow) in LMCA before PCI (73% vs. 24%, P < 0.001). Besides, a successful PCI was less commonly achieved among patients presenting with the primary endpoint as compared with recovered patients (65.9% vs. 89.7%, P = 0.026). The use of mechanical support was not significantly associated with the occurrence of the primary endpoint [36/31 (63.2%) in patients with MCS and 21/29 (36.2%) in patients without MCS, P = 0.172].
Patients with cardiac arrest
Among patients with cardiac arrest, ventricular fibrillation was the presenting rhythm in most patients (18/27, 66.6%), followed by pulseless electrical activity. Patients with cardiac arrest were significantly younger and had a lower proportion of hypertension and dyslipidaemia and a lower burden of overall comorbidity. In contrast, they had a much higher proportion of severe shock and a trend towards higher proportion of occluded ULMCA at baseline. These patients had a higher incidence of the primary endpoint [20/27 (74.1%) vs. 21/43 (48.8%), P = 0.028; Supporting Information, Table S4 ].
Predictors of the primary endpoint
The components of the multivariate prediction model of the primary endpoint were LVEF, occluded ULMCA before PCI, and profound shock (SCAI E class) at admission. This predictive model showed an optimal ability for predicting the main outcome (AUC 0.865, 95% CI 0.776–0.953, P < 0.001; Figure 2 ).
Figure 2.
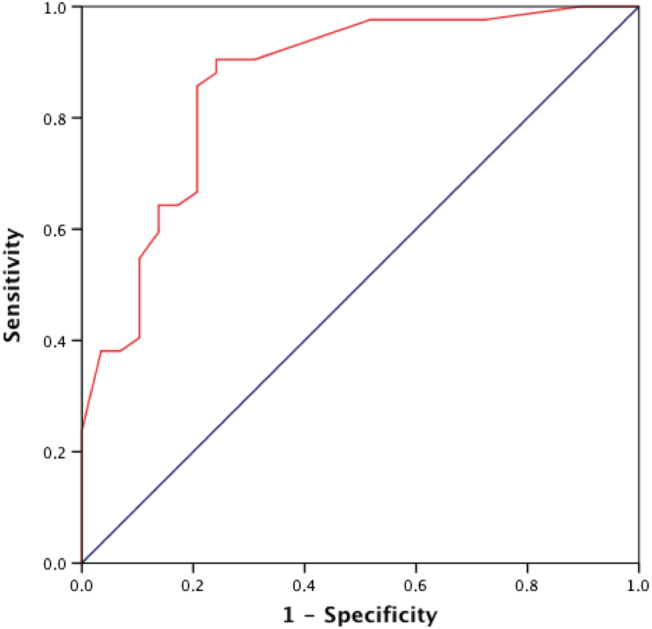
Receiver operating characteristic (ROC) curve for the prediction of mortality or requirement of heart transplantation (HT) or left ventricular assist devices (LVAD) during the admission.
Clinical profile and outcomes according to baseline left main coronary artery Thrombolysis In Myocardial Infarction flow and shock severity
Complete occlusion of the ULMCA (37/70, 52.86%) was observed in younger patients (mean age 63 vs. 69 years, P = 0.049) with a lower burden of comorbidities (mean CCI 3.76 vs. 5.39, P = 0.017). LVEF was statistically significantly lower (mean 21% vs. 29%, P < 0.001) and reperfusion time was significantly shorter (P = 0.027) in those patients. However, this group of patients had a significantly higher proportion of severe shock (62% vs. 21%, P = 0.001).
Likewise, patients with profound shock (30/70, 42.86%) tended to be younger (mean age 62.5 vs. 68.6 years, P = 0.059) and had a lower burden of comorbidities (median CCI 3 vs. 5.5 points, P = 0.002). Lactate levels were significantly higher (P < 0.001) and LVEF was significantly poorer (P = 0.035) in this group.
The occurrence of the primary endpoint progressively increased according to the severity of shock and the presence of an occluded LMCA before PCI (Figure 3 ). In this sense, patients with non‐profound shock (SCAI class C–D) and patent LMCA (initial TIMI flow 1–3) had the lowest incidence of the primary endpoint (23.08%). Both the group of non‐profound shock with occluded LMCA and the group of profound shock with patent LMCA had a higher incidence of the primary endpoint (10/14, 71.43% and 5/7, 71.43%, respectively). However, the highest incidence was observed among patients combining profound shock and occluded LMCA (20/23, 86.96%, P < 0.001). A successful PCI did not significantly reduce the incidence of the event among this last group of complex patients (86.7% in successful PCI vs. 87.5% in failed PCI, P = 0.731).
Figure 3.
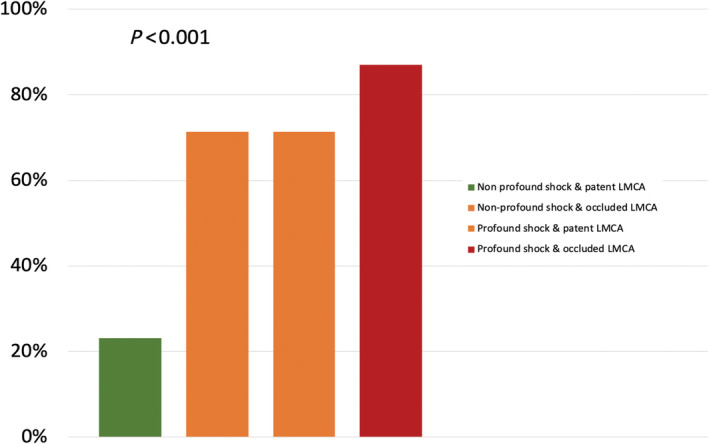
Incidence of mortality or requirement of heart transplantation (HT) or left ventricular assist devices (LVAD) during the admission according to severity of shock and baseline ULMCA Thrombolysis In Myocardial Infarction (TIMI) flow. LMCA, left main coronary artery; ULMCA, unprotected left main coronary artery.
Discussion
The main findings from this study are as follows: (a) Patients with ULMCA‐related AMICS had a high‐risk profile at admission and poor outcomes, with a high mortality and a low probability of survival free from HT or LVAD; (b) the predictors of no recovery were a poorer left ventricular function, profound shock, and baseline occluded ULMCA; and (c) the prognosis was specially poor in patients with occluded ULMCA and profound shock, with a low probability of recovery (<15%) even in cases of successful PCI.
AMICS is an entity associated with high mortality. In the last 20 years, most of the cohorts report a 40–60% in‐hospital or 30 day mortality. 19 This occurs despite the widespread use of emergent coronary revascularization in the context of AMICS. The SHOCK trial showed a trend towards improvement in 30 day mortality and a significant reduction in 6 month mortality among patients with AMICS undergoing urgent revascularization. 20 Concurrent studies demonstrated an improvement in 30 day mortality in AMICS patients admitted to centres with the possibility of performing urgent coronary angiography and coronary angioplasty. 21 Currently, clinical practice guidelines strongly recommend urgent coronary angiography with the intention of revascularization based on this evidence. 22 , 23
However, information on the prognosis according to the culprit coronary artery in patients with AMICS is scarce. In a substudy of the CULPRIT‐SHOCK trial, a worse prognosis was observed among patients with culprit critical lesions in LMCA, LMCA‐equivalent, proximal LAD, or single patent vessel (30 day mortality 55.8% vs. 39.5%, P < 0.001). This finding was observed both in cases in whom PCI was performed only in culprit lesion and in cases with immediate multivessel PCI. 24 Likewise, a higher 30 day mortality has been described in AMICS patients undergoing urgent revascularization with an LMCA as the culprit lesion compared with culprit lesions in other vessels (66% vs. 43–48%). 10 All these data support the idea of a worse prognosis of patients with LMCA‐related AMICS. In addition, different factors of poor prognosis such as initial LMCA TIMI flow or prior cardiac arrest have been proposed in these patients. 25 , 26
Data from our series also showed a very high mortality in this complex setting, with a high incidence of in‐hospital complications. Our data are in line with previous series including high‐risk patients with CS, and this high rate of complications in CS makes especially important the need for centralizing the care of these patients at high‐volume hospitals with full availability of resources for treating CS and its complications. 27
Interestingly, prognosis was especially poor among patients with profound shock and baseline LMCA complete occlusion. These patients had a very low probability of recovery (<15%) despite successful PCI. In this study, we obtained a simple predictive model with three variables showing an optimal ability for predicting in‐hospital mortality or need for HT or LVAD. It is important to note that these factors (LVEF, baseline LMCA TIMI flow, and severity of shock) are easily identifiable upon admission and allow a quick and easy way to characterize the expected probability of recovery in these patients.
Patients with cardiac arrest deserve special comment, because this group has usually a different prognosis, management, and specific causes of death. 28 Interestingly, patients with cardiac arrest from our study were significantly younger and had a lower burden of comorbidities. This fact is probably related to the high probability of death before hospital admission in older sicker patients with cardiac arrest in this complex clinical setting. In addition, patents with cardiac arrest had a higher incidence of death or requirement for HT/LVAD during the admission. However, cardiac arrest was not significantly associated with the primary endpoint in the multivariate analysis, probably due to the strong association between cardiac arrest and other potent predictors of poorer outcomes, especially profound shock at admission.
As stated above, emergent angiography and PCI are strongly recommended in this setting. Despite PCI was performed in a very complex and challenging setting, a successful PCI was achieved in most patients from our series (75.7%), a proportion similar to those observed in studies including ULMCA‐related AMICS cases. 17 Our study showed a significant association between a successful PCI and a lower in‐hospital mortality or need for HT or LVAD in the whole cohort. However, in patients with profound shock and baseline occluded LMCA, a successful PCI did not significantly reduce the incidence of the primary endpoint. In our opinion, this might be one of the main messages from this study. However, these findings should be carefully interpreted. Short‐term MCS devices are commonly needed among critical patients with profound shock to maintain haemodynamics while waiting for recovery or deciding the final strategy. As observed in our series, the incidence of complications is high in this setting, and they are closely related to the severity of shock and the duration of support. To our judgement, emergent angiography and PCI should be highly encouraged also in these patients, but studies about HT or LVAD candidacy should be promptly started to avoid unnecessary delays in patients who finally do not recover. This would be especially important in those patients under MCS in whom the risk of serious complications is greater. Thus, while waiting for future confirmatory and larger studies on the subject, in patients with baseline complete occlusion of the ULMCA and profound shock, the ‘bridge‐to‐transplantation or long‐term LVAD implantation’ strategy might be strongly adopted along with the ‘bridge‐to‐recovery’ strategy.
A total of 38 patients from this study died, and 4 underwent HT. No patient underwent LVAD implantation during the admission. In this sense, it is important to note that this study reflects local clinical practice during a long period of time. During the last years, the indication of LVAD has been very selective in most countries because of lack of expertise and economic issues. The increasing number of potential candidates and the improvement in outcomes of these patients have led to a higher number of indications. ULMCA‐related AMICS is one of most complex CS scenarios, requiring an early stabilization and a thorough assessment for LVAD candidacy. This is probably the main reason for the lack of LVAD indication in our series, which probably may change during the upcoming years.
A significant limitation from this study is that we only assessed ULMCA‐related AMICS who survived until hospital admission. This is a clinically relevant question, because older patients with a higher burden of comorbidities might have a much higher probability of death before arriving at hospital, especially in cases with cardiac arrest. The paradoxical shorter time from symptom onset to PCI in patients with poorer outcomes and the younger age and lower burden of comorbidities among patients with cardiac arrest from this series might support this idea. However, despite this potential selection bias, our findings are clinically relevant, because we assessed the profile of ULMCA‐related AMICS patients usually managed at intensive cardiac care units. As other limitations, this was an observational and retrospective investigation, so it is not possible to rule out other selection biases and unmeasured confounding factors such as emergent or staged PCI of non‐culprit lesions. 29 While most patients underwent MCS, the proportion of use of advanced MCS (VA‐ECMO or Impella® devices) was low, especially before PCI. Taking into account the low number of patients undergoing advanced MCS and the different strategies used, assessing the impact of MCS on outcomes was not feasible. In addition, despite a significant number of events, sample size was relatively small. Finally, this study reflects the picture of LMCA‐related AMICS patients from two tertiary care referral centres. Therefore, this registry should be considered a hypothesis‐generating study, and these findings should be validated in larger series with different clinical profile and management.
Despite these limitations, the results of the present study add novel and interesting data about risk stratification and management of patients with LMCA‐related AMICS, especially related to the low probability of recovery among high‐risk patients (baseline occluded LMCA and profound shock at admission) despite successful PCI. Improving management and outcomes of these critically ill patients may lead to important clinical, economic, and social consequences.
Conclusions
ULMCA‐related AMICS was associated with a high in‐hospital mortality and need of HT or LVAD. LVEF, baseline TIMI flow, and severity of shock at admission were independently associated with a worse prognosis. Prognosis was especially poor among patients with profound shock at admission and baseline occluded LMCA, with a low probability of recovery (<15%), regardless of successful PCI in ULMCA.
Conflict of interest
None declared.
Supporting information
Table S1. Angiographic data and invasive management.
Table S2. Antithrombotic treatment.
Table S3. Comparison of pre‐PCI characteristics between event and non‐event groups.
Table S4. Baseline clinical characteristics, management and outcomes according to the presence of cardiac arrest.
Appendix S1. Definitions of variables.
Acknowledgements
We thank CERCA Programme / Generalitat de Catalunya for institutional support.
Galván‐Román, F. , Fernández‐Herrero, I. , Ariza‐Solé, A. , Sánchez‐Salado, J. C. , Puerto, E. , Lorente, V. , Gómez‐Lara, J. , Martín‐Asenjo, R. , Gómez‐Hospital, J. A. , and Comín‐Colet, J. (2023) Prognosis of cardiogenic shock secondary to culprit left main coronary artery lesion‐related myocardial infarction. ESC Heart Failure, 10: 111–120. 10.1002/ehf2.14128.
Contributor Information
Albert Ariza‐Solé, Email: aariza@bellvitgehospital.cat.
Josep Comín‐Colet, Email: jcomin@bellvitgehospital.cat.
References
- 1. Puymirat E, Fagon JY, Aegerter P, Diehl JL, Monnier A, Hauw‐Berlemont C, Boissier F, Chatellier G, Guidet B, Danchin N, Aissaoui N, Collège des Utilisateurs de Bases de données en Réanimation (CUB‐Réa Group [Intensive Care Database User Group]) . Cardiogenic shock in intensive care units: evolution of prevalence, patient profile, management and outcomes, 1997–2012. Eur J Heart Fail. 2017; 19: 192–200. [DOI] [PubMed] [Google Scholar]
- 2. Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM, Picard MH, Menegus MA, Boland J, Dzavik V, Thompson CR, Wong SC, Steingart R, Forman R, Aylward PE, Godfrey E, Desvigne‐Nickens P, LeJemtel TH. Early revascularization in acute myocardial infarction complicated by cardiogenic shock (SHOCK trial). N Engl J Med. 1999; 341: 625–634. [DOI] [PubMed] [Google Scholar]
- 3. Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Böhm M, Ebelt H, Schneider S, Schuler G, Werdan K. IABP‐SHOCK II Trial Investigators. Intraaortic balloon support for myocardial infarction with cardiogenic shock (IABP‐SHOCK II trial). N Engl J Med. 2012; 367: 1287–1296. [DOI] [PubMed] [Google Scholar]
- 4. Harjola VP, Lassus J, Sionis A, Køber L, Tarvasmäki T, Spinar J, Parissis J, Banaszewski M, Silva‐Cardoso J, Carubelli V, di Somma S, Tolppanen H, Zeymer U, Thiele H, Nieminen MS, Mebazaa A, CardShock Study Investigators , GREAT network . Clinical picture and risk prediction of short‐term mortality in cardiogenic shock. Eur J Heart Fail. 2015; 17: 501–509. [DOI] [PubMed] [Google Scholar]
- 5. Lee L, Erbel R, Brown TM, Laufer N, Meyer J, O'Neill WW. Multicenter registry of angioplasty therapy of cardiogenic shock: initial and long‐term survival. J Am Coll Cardiol. 1991; 17: 599–603. [DOI] [PubMed] [Google Scholar]
- 6. Hochman JS, Sleeper LA, Godfrey E, McKinlay SM, Sanborn T, Col J, LeJemtel T. Should we emergently revascularize occluded coronaries for cardiogenic shock: an international randomized trial of emergency PTCA/CABG‐trial design. The SHOCK Trial Study Group. Am Heart J. 1999; 137: 313–321. [DOI] [PubMed] [Google Scholar]
- 7. Goldberg RJ, Spencer FA, Gore JM, Lessard D, Yarzebski J. Thirty‐year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population‐based perspective. Circulation. 2009; 119: 1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bangalore S, Gupta N, Guo Y, Lala A, Balsam L, Roswell RO, Reyentovich A, Hochman JS. Outcomes with invasive vs conservative management of cardiogenic shock complicating acute myocardial infarction. Am J Med. 2015; 128: 601–608. [DOI] [PubMed] [Google Scholar]
- 9. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group , de Boer RA, Christian Schulze P, Abdelhamid M, Aboyans V, Adamopoulos S, Anker SD, Arbelo E, Asteggiano R, Bauersachs J, Bayes‐Genis A, Borger MA, Budts W, Cikes M, Damman K, Delgado V, Dendale P, Dilaveris P, Drexel H, Ezekowitz J, Falk V, Fauchier L, Filippatos G, Fraser A, Frey N, Gale CP, Gustafsson F, Harris J, Iung B, Janssens S, Jessup M, Konradi A, Kotecha D, Lambrinou E, Lancellotti P, Landmesser U, Leclercq C, Lewis BS, Leyva F, Linhart A, Løchen ML, Lund LH, Mancini D, Masip J, Milicic D, Mueller C, Nef H, Nielsen JC, Neubeck L, Noutsias M, Petersen SE, Sonia Petronio A, Ponikowski P, Prescott E, Rakisheva A, Richter DJ, Schlyakhto E, Seferovic P, Senni M, Sitges M, Sousa‐Uva M, Tocchetti CG, Touyz RM, Tschoepe C, Waltenberger J, Adamo M, Baumbach A, Böhm M, Burri H, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gardner RS, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Piepoli MF, Price S, Rosano GMC, Ruschitzka F, Skibelund AK. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 10. Josiassen J, Helgestad OK, Møller JE, Holmvang L, Jensen LO, Udesen NL, Ravn HB, Hassager C. Prognostic importance of culprit lesion location in cardiogenic shock due to myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2020; 18: 2048872620911848. [DOI] [PubMed] [Google Scholar]
- 11. Higami H, Toyofuku M, Morimoto T, Ohya M, Fuku Y, Yamaji K, Muranishi H, Yamaji Y, Nishida K, Furukawa D, Tada T, Ko E, Ando K, Sakamoto H, Tamura T, Kawai K, Kadota K, Kimura T, AOI‐LMCA Stenting Registry Investigators . Acute coronary syndrome with unprotected left main coronary artery culprit—an observation from the AOI‐LMCA registry. Circ J. 2018; 83: 198–208. [DOI] [PubMed] [Google Scholar]
- 12. Meraj PM, Doshi R, Schreiber T, Maini B, O'Neill WW. Impella 2.5 initiated prior to unprotected left main PCI in acute myocardial infarction complicated by cardiogenic shock improves early survival. J Interv Cardiol. 2017; 30: 256–263. [DOI] [PubMed] [Google Scholar]
- 13. Almudarra SS, Gale CP, Baxter PD, Fleming SJ, Brogan RA, Ludman PF, de Belder MA, Curzen NP, National Institute for Cardiovascular Outcomes Research (NICOR) . Comparative outcomes after unprotected left main stem percutaneous coronary intervention: a national linked cohort study of 5,065 acute and elective cases from the BCIS registry (British Cardiovascular Intervention Society). JACC Cardiovasc Interv. 2014; 7: 717–730. [DOI] [PubMed] [Google Scholar]
- 14. Garcia‐Alvarez A, Arzamendi D, Loma‐Osorio P, Kiamco R, Masotti M, Sionis A, Betriu A, Brugada J, Bosch X. Early risk stratification of patients with cardiogenic shock complicating acute myocardial infarction who undergo percutaneous coronary intervention. Am J Cardiol. 2009; 103: 1073–1077. [DOI] [PubMed] [Google Scholar]
- 15. Hussain F, Nguyen T, Elmayergi N, Ducas J, Minhas K, Vo M, Kass M, Ravandi A, Parmar G, Jassal DS, Tam JW, Freed D, Menkis AH, Philipp RK. The acutely occluded left main coronary artery culprit in cardiogenic shock and initial percutaneous coronary intervention: a substudy of the Manitoba “no option” left main PCI registry. Can J Physiol Pharmacol. 2012; 90: 1325–1331. [DOI] [PubMed] [Google Scholar]
- 16. Barone‐Rochette G, Vanzetto G, Fluttaz A, Marlière S, Bouvaist H, Durand M, Chavanon O, Blin D, Machecourt J. Cardiogenic shock due to unprotected left main coronary artery thrombosis in the era of mechanical circulatory support. Int J Cardiol. 2011; 148: 394–396. [DOI] [PubMed] [Google Scholar]
- 17. Galván‐Román F, Puerto E, Martín‐Asenjo R, Ariza‐Solé A. Cardiogenic shock due to left main related myocardial infarction: is revascularization enough? J Geriatr Cardiol. 2022; 19: 152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, Hollenberg SM, Kapur NK, O'Neill W, Ornato JP, Stelling K, Thiele H, van Diepen S, Naidu SS. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019; 94: 29–37. [DOI] [PubMed] [Google Scholar]
- 19. De Luca L, Savonitto S. Composite trends of cardiogenic shock complicating acute myocardial infarction. Eur J Heart Fail. 2020; 22: 673–675. [DOI] [PubMed] [Google Scholar]
- 20. Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM, Picard MH, Menegus MA, Boland J, Dzavik V, Thompson CR, Wong SC, Steingart R, Forman R, Aylward PE, Godfrey E, Desvigne‐Nickens P, LeJemtel TH. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999; 341: 625–634. [DOI] [PubMed] [Google Scholar]
- 21. Barbash IM, Behar S, Battler A, Hasdai D, Boyko V, Gottlieb S, Leor J. Management and outcome of cardiogenic shock complicating acute myocardial infarction in hospitals with and without on‐site catheterisation facilities. Heart. 2001; 86: 145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neumann FJ, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferović PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO, ESC Scientific Document Group , Wijns W, Glineur D, Aboyans V, Achenbach S, Agewall S, Andreotti F, Barbato E, Baumbach A, Brophy J, Bueno H, Calvert PA, Capodanno D, Davierwala PM, Delgado V, Dudek D, Freemantle N, Funck‐Brentano C, Gaemperli O, Gielen S, Gilard M, Gorenek B, Haasenritter J, Haude M, Ibanez B, Iung B, Jeppsson A, Katritsis D, Knuuti J, Kolh P, Leite‐Moreira A, Lund LH, Maisano F, Mehilli J, Metzler B, Montalescot G, Pagano D, Petronio AS, Piepoli MF, Popescu BA, Sádaba R, Shlyakhto E, Silber S, Simpson IA, Sparv D, Tavilla G, Thiele H, Tousek P, van Belle E, Vranckx P, Witkowski A, Zamorano JL, Roffi M, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet JP, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh TA, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Sousa‐Uva M, Simpson IA, Zamorano JL, Pagano D, Freemantle N, Sousa‐Uva M, Chettibi M, Sisakian H, Metzler B, İbrahimov F, Stelmashok VI, Postadzhiyan A, Skoric B, Eftychiou C, Kala P, Terkelsen CJ, Magdy A, Eha J, Niemelä M, Kedev S, Motreff P, Aladashvili A, Mehilli J, Kanakakis IG, Becker D, Gudnason T, Peace A, Romeo F, Bajraktari G, Kerimkulova A, Rudzītis A, Ghazzal Z, Kibarskis A, Pereira B, Xuereb RG, Hofma SH, Steigen TK, Witkowski A, de Oliveira EI, Mot S, Duplyakov D, Zavatta M, Beleslin B, Kovar F, Bunc M, Ojeda S, Witt N, Jeger R, Addad F, Akdemir R, Parkhomenko A, Henderson R. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019; 40: 87–165. [DOI] [PubMed] [Google Scholar]
- 23. Writing Committee Members , Lawton JS, Tamis‐Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio J, Don CW, Fremes SE, Gaudino MF, Goldberger ZD, Grant MC, Jaswal JB, Kurlansky PA, Mehran R, Metkus TS Jr, Nnacheta LC, Rao SV, Sellke FW, Sharma G, Yong CM, Zwischenberger BA. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022; 79: 197–215. [DOI] [PubMed] [Google Scholar]
- 24. Hauguel‐Moreau M, Barthélémy O, Farhan S, Huber K, Rouanet S, Zeitouni M, Guedeney P, Hage G, Vicaut E, Zeymer U, Desch S, Thiele H, Montalescot G. Culprit lesion location and outcomes in patients with multivessel disease and infarct‐related cardiogenic shock: a core laboratory analysis of the CULPRIT‐SHOCK trial. EuroIntervention. 2021; 17: e418–e424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Homorodean C, Iancu AC, Leucuţa D, Bãlãnescu Ş, Dregoesc IM, Spînu M, Ober M, Tãtaru D, Olinic M, Bindea D, Olinic D. New predictors of early and late outcomes after primary percutaneous coronary intervention in patients with ST‐segment elevation myocardial infarction and unprotected left main coronary artery culprit lesion. J Interv Cardiol. 2019; 2019: 8238972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Édes IF, Ruzsa Z, Lux Á, Gellér L, Molnár L, Nowotta F, Kerülő MC, Becker D, Merkely B. Acute, total occlusion of the left main stem: coronary intervention options, outcomes, and recommendations. Postepy Kardiol Interwencyjnej. 2018; 14: 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shaefi S, O'Gara B, Kociol RD, Joynt K, Mueller A, Nizamuddin J, Mahmood E, Talmor D, Shahul S. Effect of cardiogenic shock hospital volume on mortality in patients with cardiogenic shock. J Am Heart Assoc. 2015; 4: e001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Witten L, Gardner R, Holmberg MJ, Wiberg S, Moskowitz A, Mehta S, Grossestreuer AV, Yankama T, Donnino MW, Berg KM. Reasons for death in patients successfully resuscitated from out‐of‐hospital and in‐hospital cardiac arrest. Resuscitation. 2019; 136: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pedrazzini GB, Radovanovic D, Vassalli G, Sürder D, Moccetti T, Eberli F, Urban P, Windecker S, Rickli H, Erne P, AMIS Plus Investigators . Primary percutaneous coronary intervention for unprotected left main disease in patients with acute ST‐segment elevation myocardial infarction the AMIS (Acute Myocardial Infarction in Switzerland) plus registry experience. JACC Cardiovasc Interv. 2011; 4: 627–633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Angiographic data and invasive management.
Table S2. Antithrombotic treatment.
Table S3. Comparison of pre‐PCI characteristics between event and non‐event groups.
Table S4. Baseline clinical characteristics, management and outcomes according to the presence of cardiac arrest.
Appendix S1. Definitions of variables.


