Abstract
Aims
Peripartum cardiomyopathy (PPCM) are more vulnerable to intracardiac thrombus than other types of cardiomyopathies, although explicit anticoagulant strategy is not sure. Too aggressive anticoagulation therapy can lead to severe bleeding events. Hence, we want to construct a risk stratification model for intracardiac thrombus in PPCM patients.
Methods and results
A total of 159 suspected PPCM cases were initially screened, whereas 123 confirmed cases were enrolled in the final analysis. The study population was randomly assigned as derivation group (N = 83) and validation group (N = 40). The derivation cohort was utilized to develop the model, and the validation cohort was used to internal validate the discriminatory ability of the model. Formation of intracardiac thrombus was detected in 22 patients. After adjusted by multivariable logistic regression analysis, left ventricle ejection fraction (LVEF, OR 0.772, 95% CI 0.665–0.897, P = 0.001), haemoglobin levels (OR 1.050, 95% CI 1.003–1.099, P = 0.038), and thrombocyte counts (OR 1.018, 95% CI 1.006–1.029, P = 0.003) were identified as risk factors independently associated with intracardiac thrombus and were finally included in the tentative risk stratification model with a C‐indexes of 0.916 (95% CI: 0.850–0.982, P < 0.001). A score of ≤7 was regarded as low risk, 8–10 defined intermediate risk, and ≥11 defined high risk in our model. Internal validation showed good discriminatory ability of the model with a C‐indexes of 0.790 (95% CI: 0.644–0.936, P = 0.017).
Conclusions
In our retrospective study, impaired LVEF, elevated haemoglobin levels, and high thrombocyte counts were regarded as independent risk factors for intracardiac thrombus in PPCM. A risk stratification model derived from these risk factors, which was economic and easily applicable in clinical practice, could rapidly and accurately identify PPCM patients with higher‐risk of intracardiac thrombus.
Keywords: Peripartum cardiomyopathy, Intracardiac thrombus, Risk stratification, Anticoagulant therapy, Heart failure
Introduction
Peripartum cardiomyopathy (PPCM) is a specific and rare form of cardiomyopathy occurring towards the end of pregnancy or in the months following delivery, abortion, or miscarriage, where no other aetiology of heart failure (HF) is identified. 1 The clinical manifestation of PPCM is characterized by impaired cardiac systolic function, reduced left ventricle ejection fraction (LVEF), and dilated left ventricle diameter. The epidemiology of PPCM varies between different regions and countries, and the incidence of PPCM is relatively low in China, at one in about 346–912 deliveries. 2 , 3
Compared with other types of cardiomyopathies or HF induced by other aetiologies, higher incidence of intracardiac thrombus, peripheral arterial and venous embolism has been observed in PPCM. 4 According to data from the world registry study on PPCM, the rate of thromboembolism events (TEEs) in PPCM is 6.8%, whereas the estimated incidence is only 2.7% patient‐year in overall congestive HF population. 5 , 6 As for TEEs, intracardiac thrombus is the most important and distinctive one for PPCM, which can lead to severe cardiovascular and cerebrovascular events. During peripartum period, the coagulation system tends to be hyper‐coagulated for the increase of various clotting factors, decrease of quantity of anticoagulants, and hypofunction of fibrinolytic system. 7 Besides, reduced LVEF, cardiac dilation, and endothelial dysfunction predispose PPCM to prothrombotic states, which makes PPCM patients more vulnerable to TEEs, especially intracardiac thrombus.
However, anticoagulate drug administration must be initiated after deliberation of potential benefits weighed against by haemorrhagic risk. Despites the recommendation proposed by the European Society of Cardiology (ESC) study group on PPCM that the prophylactic dose of oral anticoagulation drugs or low molecule weight heparin should be administrated in PPCM patients with reduced LVEF, aggressive initiation of anticoagulant therapy will escalate haemorrhage risk in individuals without TEEs. However, the firm indications of anticoagulant therapy for PPCM patients are intracardiac thrombus detected by echocardiograph as well as complication of atrial fibrillation. 8 Thus, identification of risk factors for intracardiac thrombus is relatively important and practical to discriminate individuals with higher risk of intracardiac thrombus. The objective of our study is to construct a tentative simple integer risk score to discriminate PPCM patients who are vulnerable to intracardiac mural thrombus.
Methods
Study population
The study was approved by the Ethics Committee of Qilu Hospital of Shandong University (2020SDUCRCA009), and participants' clinical data were retrospectively reviewed without written informed consent.
Inclusion criteria included (i) primary diagnosed PPCM with clinical manifestation of heart failure with reduced ejection fraction (HFrEF) occurring in the last month of pregnancy or within 5 months after delivery, including orthopnoea, peripheral oedema, and paroxysmal nocturnal dyspnoea; (ii) left ventricle dysfunction and reduced LVEF (less than 45%) validated by transthoracic echocardiography; and (iii) retrievable necessary clinical data, including echocardiogram and laboratory tests. The major exclusion criteria included (i) other previously identifiable aetiologies of HF, such as coronary heart disease, other types of cardiomyopathies, and congenital or organic valvular heart disease; (ii) patients younger than 18 years; and (iii) malignant diseases such as neoplasm.
Consecutive patients (n = 159) suspectedly diagnosed as PPCM at Qilu Hospital from January 2010 to December 2021 were screened in our retrospective and observational case–control study. Thirteen patients with an LVEF ranged from 45 to 50%, 9 patients with HF induced by other aetiologies, 11 patients missing necessary clinical data, and 3 patients with an age less than 18 years old were excluded. Finally, 123 eligible patients were enrolled in our analysis. Among the participants, intracardiac mural thrombus was detected in 22 PPCM patients (Figure 1 ).
Figure 1.
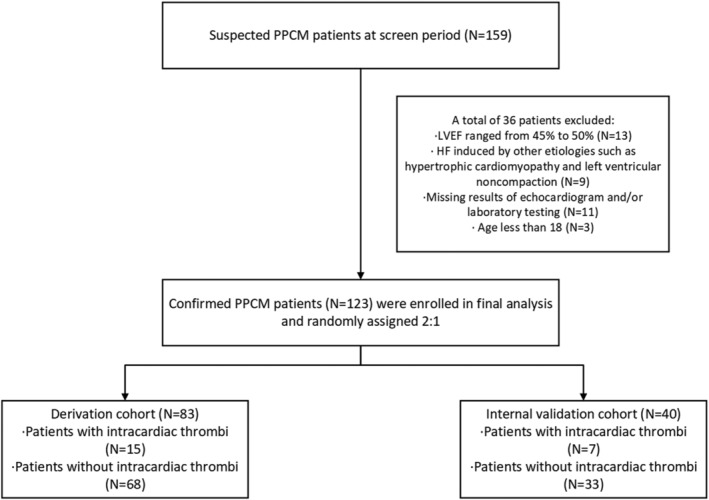
Study flow chart and cohort development. HF, heart failure; LVEF, left ventricular ejection fraction; PPCM, peripartum cardiomyopathy.
Data collection
The data of the patients enrolled in the clinical trial, including (i) obstetric information, including maternal age at presentation, parity, multifetal pregnancies, pregnancy complication (such as gestational hypertension and diabetes), and percentage of postpartum presentation; (ii) echocardiography parameters, including LVEF, left ventricular end‐diastolic dimension (LVEDD), right ventricular end‐diastolic dimension (RVEDD), left atrial diameter, and interventricular septum thickness; (iii) routine laboratory testing, including complete blood count, potassium, alanine aminotransferase, aspartate aminotransferase, creatinine, and N‐terminal B‐type natriuretic peptide (NT‐proBNP); (iv) physical examination information, including blood pressure and heart rate measured at the first day after admission and New York Heart Association (NYHA) functional class, were collected.
Echocardiography examination
Transthoracic echocardiography was performed on patients at their admission. Two‐dimensional and targeted M‐mode echocardiography with Doppler colour flow mapping were performed by Philips EPIQ7C system (Philips Ultrasound, Bothell, WA, USA). Routine parameters, including LVEF, LVEDD, and left atrial diameter, were measured by echocardiograph according to the American Society of Echocardiography and the European Association of Cardiovascular Imaging guidelines. LVEF is calculated via biplane modified Simpson's method. Intracardiac thrombus was confirmed by echocardiography. The presence of an echo‐dense mass protruding into the atrial and/or ventricular chamber with margins distinct from the atrial and/or ventricular wall, endocardium, and papillary muscles was identified as intracardiac thrombus (Figure 2 ). To avoid as far as possible the artefacts or potential false positive findings, multiple views, including apical four‐chamber view and left parasternal long‐axis view, were explored. Each echocardiographic image was read and checked by at least two experienced echocardiographic cardiologists for internal controls.
Figure 2.
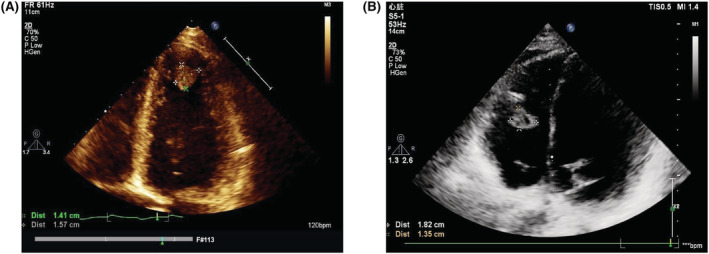
Transthoracic echocardiogram images of left ventricle (A) and right ventricle (B) intracardiac mural thrombus.
Blood tests
The blood samples were all taken at the second day of admission. Laboratory examinations, such as complete blood count, potassium, alanine aminotransferase, and NT‐proBNP, were conducted in the clinical chemistry laboratories of Qilu Hospital.
Statistical methods
The data were analysed via IBM SPSS Statistics version 25, 2017 (IBM, Armonk, New York), R (version 4.1.0) software, and GraphPad Prism version 8, 2018 (GraphPad Software, Inc.). Continuous data are expressed as mean ± standard deviation (SD) or median and interquartile range (IQR) and categorical data as frequencies (%). Chi‐square test or Fisher's exact probability tests were conducted to investigate the presence of differences between categorical variables, whereas Kruskal–Wallis test or Mann–Whitney U test was performed for continuous variables as appropriate.
To explore the consistency of the tentative risk stratification score system, two‐thirds of the whole study population (n = 83), which included 15 patients with intracardiac thrombi, was randomly allocated as a derivation cohort using a random number generator, whereas the remaining 1/3 (n = 40) was utilized as internal validation cohort. For the derivation group, univariable logistic regression analysis was conducted to select potential candidate risk factors. Those with statistical significance (P < 0.05) or potential statistical significance with a P value at 0.05 would be further enrolled in the multivariable logistic regression to construct the risk stratification model. Stepwise forward multiple‐regression analysis was performed to evaluate the odds ratio (OR) and 95% CIs of intracardiac thrombus for all potential predictors separately and together, adjusting for confounding factors. Thus, candidate factors with most risk discriminated value for intracardiac thrombus, which were selected and identified by multivariable logistic regression analysis, would be enrolled for the model development.
The risk stratification model was developed according to the beta coefficients and standard errors in the multivariable logistic regression. Receiver operating characteristic (ROC) was performed to assess the C‐statistic, which reflects the model discrimination ability. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were also calculated to assess the diagnostic value of this model. Hosmer–Lemeshow goodness‐of‐fit test statistic was conducted to assess model calibration. Decision curve analysis (DCA) was also performed to assess the net clinical benefits of our risk stratification model. The discriminatory ability of the model was tested in the internal validation group. All tests were two tailed and a P value < 0.05 was considered significant.
Results
Population characteristics
The clinical and laboratory characteristics of the whole cohort summarized in Table 1 . The median age at diagnosis was 30 years old. The patients presented with a median heart rate of 110 bpm (IQR 87–113) and a median systolic and diastolic blood pressure of 125 mmHg (IQR 110–143) and 82 mmHg (IQR 70–96). The majority (86.2%) of patients had a NYHA functional class III or IV. For echocardiography parameters, the median LVEF was 32% (IQR 22–40), and the median LVEDD was 57 mm (IQR 53–62). For laboratory examination results, the median haemoglobin was 115 g/L (IQR 102–128), and the median thrombocyte count was 257 × 103 μL (IQR 186–308). Besides, the median plasm NT‐proBNP concentration at diagnosis was 2702 pg/mL (IQR 965–7372). All patients were sinus rhythm and did not have atrial fibrillation. For HF therapy, angiotensin‐converting enzyme (ACE) inhibitor, angiotensin receptor blocker (ARB) or angiotensin receptor/neprilysin inhibitor (ARNI) (78.9%), beta‐blocker (83.7%), and mineralocorticoid receptor antagonist (MRA) (69.9%) were prescribed. No patient received bromocriptine therapy.
Table 1.
Clinical characteristics of PPCM patients with or without intracardiac mural thrombus
| Variables | Total (n = 123) | Intracardiac thrombus group (n = 22) | Non‐intracardiac thrombus group (n = 101) | P value |
|---|---|---|---|---|
| Maternal age at diagnosis, years | 30 (25–34) | 28 (24–34) | 30 (26–34) | 0.263 |
| Postpartum presentation, n (%) | 38 (30.9) | 12 (54.5) | 26 (25.7) | 0.008 |
| Primiparity, n (%) | 52 (42.3) | 11 (50.0) | 41 (40.6) | 0.418 |
| Multifoetal pregnancies, n (%) | 17 (13.8) | 1 (4.5) | 16 (15.8) | 0.294 |
| Gestational hypertension, n (%) | 44 (35.8) | 4 (18.2) | 40 (39.6) | 0.057 |
| Systolic blood pressure, mmHg | 125 (110–143) | 108 (98–125) | 129 (114–146) | <0.001 |
| Diastolic blood pressure, mmHg | 82 (70–96) | 72 (60–84) | 85 (73–99) | 0.003 |
| Heart rate, bpm | 110 (87–113) | 100 (79–126) | 100 (89–110) | 0.992 |
| NYHA function class, n (%) | 0.246 | |||
| II | 17 (13.8) | 1 (4.5) | 16 (15.8) | |
| III | 52 (42.3) | 9 (40.9) | 43 (42.6) | |
| IV | 54 (43.9) | 12 (54.5) | 42 (41.6) | |
| Echocardiogram parameters | ||||
| LVEF | 32 (22–40) | 22 (16–27) | 35 (25–40) | <0.001 |
| LVEDD | 57 (53–62) | 58 (53–68) | 57 (53–62) | 0.191 |
| LAD | 40 (37–43) | 41 (38–46) | 40 (37–43) | 0.231 |
| RVEDD | 23 (21–26) | 26 (23–30) | 22 (20–25) | <0.001 |
| IVS | 9 (8–10) | 9 (7–9) | 9 (8–10) | 0.030 |
| Laboratory results | ||||
| White cell count, ×103 μL | 825 (592–1018) | 870 (618–1211) | 813 (577–990) | 0.417 |
| Haemoglobin, g/L | 115 (102–128) | 121 (111–139) | 113 (100–127) | 0.049 |
| Platelet count, ×103 μL | 257 (186–308) | 278 (229–398) | 252 (177–306) | 0.055 |
| Alanine aminotransferase, mmol/L | 17 (10–33) | 19 (14–34) | 17 (10–32) | 0.210 |
| Total bilirubin, mmol/L | 9.3 (6.0–16.0) | 15.0 (11.0–26.4) | 8.3 (5.5–13.2) | <0.001 |
| Albumin, g/L | 33.7 (28.5–38.9) | 32.8 (30.2–39.7) | 34.0 (28.4–38.9) | 0.882 |
| eGFR, mL/min/1.73 m2 | 111.8 (96.1–121.6) | 112.0 (98.4–123.4) | 111.6 (94.1–121.6) | 0.965 |
| Potassium, mmol/L | 4.06 (3.80–4.48) | 4.10 (3.74–4.40) | 4.06 (3.82–4.48) | 0.682 |
| NT‐proBNP, pg/mL | 2702 (965–7372) | 7273 (2596–10 137) | 2298 (661–5022) | <0.001 |
| Medication, n (%) | ||||
| RAASi/ARNI | 97 (78.9) | 17 (77.3) | 80 (79.2) | 0.840 |
| β‐Blockers | 103 (83.7) | 17 (77.3) | 86 (85.1) | 0.556 |
| MRA | 86 (69.9) | 17 (77.3) | 69 (68.3) | 0.566 |
ANRI, angiotensin receptor/neprilysin inhibitor; eGFR, estimated glomerular filtration rate; IVS, interventricular septum; LAD, left atrium diameter; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; RAASi, renin–angiotensin–aldosterone inhibitors; RVEDD, right ventricular end‐diastolic dimension.
P value indicated the difference between patients with or without intracardiac thrombus.
Association of clinical characteristics and intracardiac thrombus in PPCM
During their admission, intracardiac thrombus was observed in 22 patients. Among the PPCM patients with intracardiac thrombus, left ventricular (LV) thrombus was confirmed in 19 patients, right ventricular thrombus was detected in one patient, and biventricular thrombi was identified in two patients. The anticoagulation therapy was immediately initiated once intracardiac thrombi was confirmed. The anticoagulation strategies varied from individuals. Warfarin was prescribed in ten patients. Low molecular weight heparins were administered in seven patients. Rivaroxaban was given in four patients, whereas one patient received isolated anti‐platelet therapy without any anticoagulant administration for unknown reason. No severe bleeding event was observed during their hospitalization, whereas cerebral infarction was detected in one patient with LV mural thrombus, and pulmonary embolism was observed in one patient with biventricular thrombi.
Compared with individuals without intracardiac thrombus, those with intracardiac thrombus tended to be delay‐onset and postpartum presentation accounted for half (54.5% vs 25.7%, P = 0.008). Those with intracardiac thrombus tended to have lower systolic blood pressure (median = 108 mmHg, IQR 98–125 mmHg vs. median = 129 mmHg, IQR 114–146 mmHg, P < 0.001) and diastolic blood pressure (median = 72 mmHg, IQR 60–84 mmHg vs. median = 85 mmHg, IQR 73–99 mmHg, P = 0.003). Overall, patients with intracardiac thrombus tended to have poor systolic function. Compared with patients without intracardiac thrombus, lower LVEF (median = 22%, IQR 16–27% vs. median = 35%, IQR 25–40%, P < 0.001) and larger RVEDD (median = 26 mm, IQR 23–30 mm vs. median = 22 mm, IQR 20‐25 mm, P < 0.001) were also detected in patients with intracardiac thrombus, whereas the LVEDD (median = 58 mm, IQR 53‐68 mm vs. median = 57 mm, IQR 53‐62 mm, P = 0.191) was not statistically significant between two groups. Furthermore, intracardiac thrombus group had higher plasm NT‐proBNP concentrations (median = 7273 pg/mL, IQR 2956–10 137 pg/mL vs median = 2298 pg/mL, IQR 661–5022 pg/mL, P < 0.001) than non‐intracardiac thrombus group. For PPCM patients, those judged to have intracardiac thrombus had a significantly higher haemoglobin (median = 121 g/L, IQR 111–139 g/L vs. median = 113 g/L, IQR 100–127 g/L, P = 0.049) and total bilirubin (median = 15.0 mmol/L, IQR 11.0–26.4 mmol/L vs. median = 8.3 mmol/L, IQR 5.5–13.2 mmol/L, P < 0.001) vs. those without.
Risk factors of intracardiac thrombus for PPCM patients
Table 2 showed the homogeneity between the derivation cohort and internal validation cohort. No statistically significant difference was observed between two cohorts. The development of risk stratification model was based on the derivation cohort (N = 83). When univariate logistic regression analysis was conducted, as shown in Table 3 , lower systolic (OR 0.944, 95% CI 0.911–0.978, P = 0.001) and diastolic blood pressure (OR 0.948, 95% CI 0.911–0.986, P = 0.008), lower LVEF (OR 0.848, 95% CI 0.778–0.926, P < 0.001), larger RVEDD (OR 1.187 95% CI 1.030–1.368, P = 0.018), and higher thrombocyte count (OR 1.010, 95% CI 1.003–1.016, P = 0.004) were significantly associated with the formation of intracardiac thrombus. Besides, high haemoglobin levels (OR 1.031, 95% CI 0.999–1.063, P = 0.058) and history of gestational hypertension (OR 0.220, 95% CI 0.046–1.051, P = 0.058) were also candidate risk factors for intracardiac thrombus in PPCM. For that the variance inflation factors were less than 0.2 for all candidate risk factors, there was no multicollinearity examined by multiple linear regression analysis was conducted to examine and eliminate.
Table 2.
Clinical characteristics and laboratory results between the derivation and validation cohorts
| Variables | Derivation group (n = 83) | Validation group (n = 40) | P value |
|---|---|---|---|
| Maternal age at diagnosis, years | 30 (25–34) | 29 (25–33) | 0.269 |
| Postpartum presentation, n (%) | 25 (30.1) | 13 (32.5) | 0.789 |
| Primiparity, n (%) | 32 (37.3) | 21 (52.5) | 0.143 |
| Multifoetal pregnancies, n (%) | 10 (12.0) | 7 (17.5) | 0.412 |
| Gestational hypertension, n (%) | 30 (36.1) | 14 (35.0) | 0.901 |
| Systolic blood pressure, mmHg | 127 (114–155) | 117 (100–136) | 0.089 |
| Diastolic blood pressure, mmHg | 85 (72–96) | 80 (64–96) | 0.231 |
| Heart rate, bpm | 100 (87–110) | 104 (85–115) | 0.880 |
| NYHA function class, n (%) | 0.521 | ||
| II | 11 (13.3) | 6 (15.0) | |
| III | 38 (45.8) | 14 (35.0) | |
| IV | 34 (41.0) | 20 (50.0) | |
| Echocardiogram parameters | |||
| LVEF | 33 (24–40) | 29 (18–40) | 0.192 |
| LVEDD | 57 (53–62) | 59 (51–63) | 0.570 |
| LAD | 40 (37–43) | 41 (37–43) | 0.970 |
| RVEDD | 22 (21–25) | 24 (21–28) | 0.082 |
| IVS | 9 (8–10) | 9 (8–10) | 0.056 |
| Laboratory results | |||
| White cell count, ×103 μL | 825 (606–994) | 803 (545–1021) | 0.808 |
| Haemoglobin, g/L | 117 (100–130) | 114 (107–124) | 0.840 |
| Platelet count, ×103 μL | 251 (178–307) | 268 (222–319) | 0.208 |
| Alanine aminotransferase, mmol/L | 17 (10–33) | 19 (10–34) | 0.300 |
| Total bilirubin, mmol/L | 9.4 (6.0–16.2) | 9.1 (5.8–14.9) | 0.852 |
| Albumin, g/L | 34.0 (28.4–38.8) | 33.0 (29.2–39.1) | 0.880 |
| eGFR, mL/min/1.73 m2 | 109.3 (97.6–131.2) | 113.7 (95.3–129.0) | 0.873 |
| Potassium, mmol/L | 4.09 (3.79–4.49) | 4.06 (3.90–4.38) | 0.966 |
| NT‐proBNP, pg/mL | 2698 (943–7174) | 2736 (1210–8812) | 0.574 |
eGFR, estimated glomerular filtration rate; IVS, interventricular septum; LAD, left atrium diameter; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; RVEDD, right ventricular end‐diastolic dimension.
Table 3.
Univariable and multivariable analysis of risk factors for intracardiac thrombus in the derivation cohort (N = 83)
| Characteristics | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| β‐Coefficient | OR (95% CI) | P value | β‐Coefficient | OR (95% CI) | P value | |
| Systolic blood pressure | −0.058 | 0.944 (0.911–0.978) | 0.001 | |||
| Diastolic blood pressure | −0.053 | 0.948 (0.911–0.986) | 0.008 | |||
| Gestational hypertension | −1.515 | 0.220 (0.046–1.051) | 0.058 | |||
| LVEF | −0.164 | 0.848 (0.778–0.926) | <0.001 | −0.259 | 0.772 (0.665–0.897) | 0.001 |
| RVEDD | 0.171 | 1.187 (1.030–1.368) | 0.018 | |||
| Haemoglobin | 0.030 | 1.031 (0.999–1.063) | 0.058 | 0.048 | 1.050 (1.003–1.099) | 0.038 |
| Platelet count | 0.010 | 1.010 (1.003–1.016) | 0.004 | 0.017 | 1.018 (1.006–1.029) | 0.003 |
LVEF, left ventricular ejection fraction; RVEDD, right ventricular end‐diastolic dimension.
Finally, the history of gestational hypertension, LVEF, RVEDD, haemoglobin, and thrombocyte count were adopted in the multivariable logistic regression analysis for further filtration. After adjusted by multivariable logistic regression analysis using stepwise regression, LVEF (OR 0.772, 95% CI 0.665–0.897, P = 0.001), haemoglobin levels (OR 1.050, 95% CI 1.003–1.099, P = 0.038), and thrombocyte counts (OR 1.018, 95% CI 1.006–1.029, P = 0.003) were statistically significant and included in the final risk stratification model (Figure 3 ).
Figure 3.
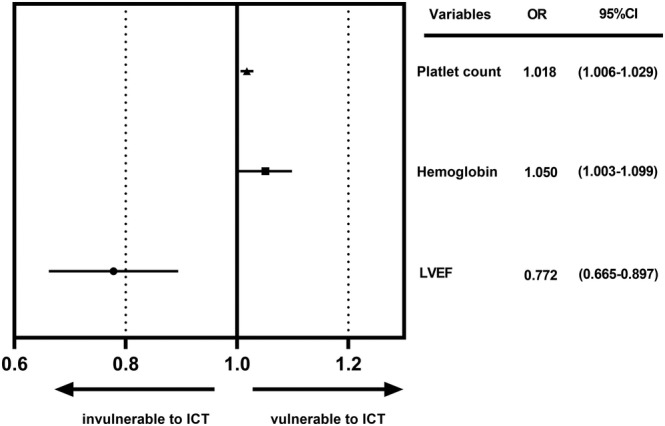
Risk factors enrolled in the risk stratification model after adjusted by multivariable logistic regression analysis. ICT, intracardiac thrombus; LVEF, left ventricular ejection fraction; OR, odd ratios.
Development and internal validation of the risk stratification model
The risk stratification model was developed according to the beta coefficients in the multivariable logistic regression analysis (Table 4 ). The possible total points ranged from 0 to 20, and patients with higher scores tended to have intracardiac thrombus. The ROC curve was conducted to assess the discrimination ability of the tentative risk stratification model. The C‐indexes, which is the area under the ROC, was 0.916 (95% CI: 0.850–0.982, P < 0.001), which indicated an excellent calibration performance (Figure 4 A ). The Hosmer–Lemeshow goodness‐of‐fit test statistic was 2.717 (P = 0.951), which demonstrated a good fitness of the model.
Table 4.
Intracardiac thrombus risk stratification model development and corresponding risk scores
| Variables | Scores | Variables | Scores |
|---|---|---|---|
| LVEF, % | 110–129 | 2 | |
| 36–44 | 0 | 130–149 | 3 |
| 28–35 | 2 | >150 | 4 |
| 20–27 | 4 | Platelet count, ×103 μL | |
| 12–19 | 6 | <200 | 0 |
| <12 | 8 | 200–320 | 2 |
| Haemoglobin, g/L | 320–440 | 4 | |
| <90 | 0 | 440–660 | 6 |
| 90–109 | 1 | >660 | 8 |
LVEF, left ventricular ejection fraction.
Figure 4.
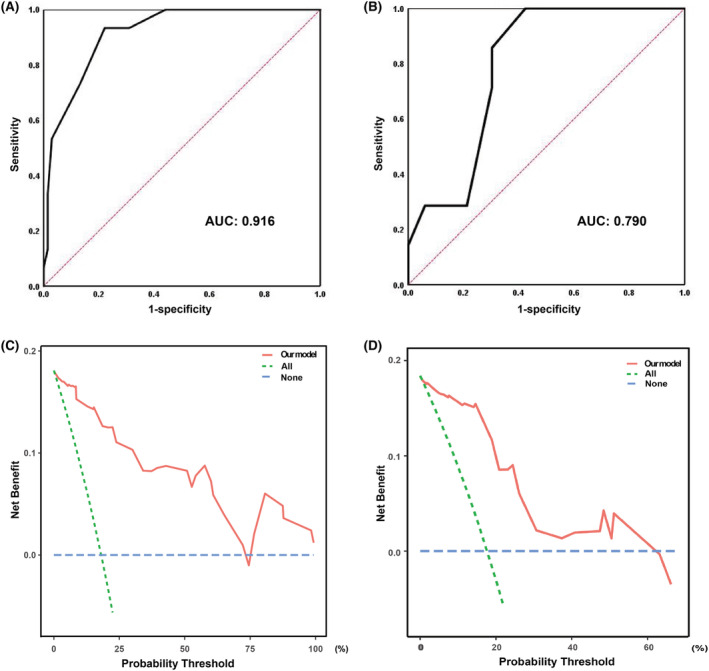
ROC curve and DCA curve in the derivation cohort and validation cohort. (A) and (C) for derivation group, (B) and (D) for validation group. AUC, area under curve; DCA, decision curve analysis; ROC, receiver operating characteristic.
A score of 8 was the optimal cut‐off score (sensitivity = 93.3%, specificity = 77.9%, PPV = 48.3%, NPV = 98.1%, accuracy = 80.7%) for the risk stratification. Based on the risk stratification model, the risk classification of intracardiac thrombus was illustrated in Table 5 . A score of ≤7 was regarded as low risk, 8–10 defined intermediate risk, and ≥11 defined high risk. The incidence of intracardiac thrombus in different risk categories was similar in the internal validation group (Table 5 ). This model showed a good discriminatory ability with a C‐indexes of 0.790 (95% CI: 0.644–0.936, P = 0.017; Figure 4 B ). For the potential for clinical decision making of our risk stratification model, significant clinical net benefits could be achieved in both derivation and validation group as shown by DCA curves (Figure 4 C,D ).
Table 5.
The risk classification for intracardiac thrombus based on the risk stratification model according to the proportion of patients with intracardiac thrombus in each risk group
| Group | Low risk | Intermediate risk | High risk |
|---|---|---|---|
| Cumulative risk scores | <8 | 8–10 | >10 |
| Derivation group, n (%) | 1 (1.9) | 9 (39.1) | 5 (83.3) |
| Validation group, n (%) | 2 (8.0) | 3 (30.0) | 2 (40.0) |
Discussion
The highlight of our study was that we tentatively develop a risk stratification model to assess the cumulative risk of intracardiac thrombus in PPCM patients. To our knowledge, this is the first retrospective observational study concerning intracardiac thrombus in PPCM patients, and no other study has focused on the risk assessment and stratification for intracardiac thrombus formation in PPCM before our research.
Intracardiac thrombus in PPCM
PPCM is a specific cardiomyopathy with low morbidity, approximately one in 1000 deliveries worldwide. PPCM can increase the pregnancy‐related mortality. 9 The maternal mortality of PPCM, which extremely depends on local perinatal healthcare levels and economic conditions, correlates with the prevalence and varies in different countries. The highest mortality was found in Nigeria, at 814 in per 100 000 live births. The lowest mortality of four in per 100 000 deliveries was found in Sweden. 2 Intracardiac thrombus were more prevalent in PPCM patients than any other types of cardiomyopathies, which had been validated by numerous multicentral studies. On the one hand, peripartum period is inclined to a state of hypercoagulability. To maintain the normal function of utero‐placental unit and to minimize the bleeding risk and maternal blood loss during delivery, numerous coagulation factors (including factors VIII, X, and XII) and fibrinogen increase during pregnancy and puerperium. Furthermore, the quantity of natural anticoagulants (including tissue pathway factor inhibitor, protein C, and protein S), thrombocyte count, and fibrinolysis decrease for the parturient. 7 , 10 Thus, the risk of thromboembolism surges during peripartum period. On the other hand, there is a predisposition to high TEEs risks for patients with HFrEF. Cardiac systolic dysfunction, cardiac chambers dilatation, disturbance of intracardiac haemodynamics, decreased myocardial compliance, and increased blood viscosity facilitate the clotting propensity in HFrEF patients. Besides, chronic oxidative stress, endothelial injury, relative hypoxia states, and proinflammatory changes also contribute to the pathophysiological change. 11 Multifactorial pathophysiology changes promote the formation of intracardiac thrombus in PPCM patients.
Despites that the explicit incidence of intracardiac thrombi has not been unequivocally demonstrated, various clinical trials have validated that PPCM patients were more vulnerable to intracardiac thrombus. In a PPCM registry study in Nigeria (PEACE Registry), cardiac mural thrombus was detected in 26 (6.4%) patients. 12 In a nationwide population‐based study in the USA, which recruited a total of 34 219 patients, the incidence of thromboembolism, the most common serious complication, was 6.6%. 13 In some sporadic small‐scale clinical studies, intracardiac thrombi were more prevalent. For example, in a German PPCM cohort study, thrombotic events were detected in nine (13.6%) patients. 14 In a forward‐looking study enrolled 33 PPCM patients in Senegal, the incidence of intracardiac thrombi could go as high as 30%. 3 Besides, scattered case about intracardiac thrombus in PPCM have been reported. Whereas for common HF patients, based on the database of Veterans Affairs Vasodilator‐Heart Failure Trials (V‐HeFT I and II), the average rate of all thromboembolic events was only 2.7% patient‐year. 6 In our retrospective study, the incidence of intracardiac thrombi was 22.4%, which was some higher than previous studies, which may be due to the limited population in our study. Overall, intracardiac thrombus in PPCM is a noticeable problem.
Risk factors for intracardiac thrombus in PPCM
In our study, LVEF, hemoglobin, and thrombocyte count were finally included in the tentative risk stratification model after adjusted by multivariable logistic regression analysis. The evidence that severe cardiac systolic dysfunction is strong predictor for intracardiac thrombus has been validated by various studies. Reduced LVEF strongly correlates to TEEs in HF patients, which may attribute to the relatively low‐flow states induced by impaired cardiac output. The Studies of Left Ventricular Dysfunction (SOLVD) illustrated that decreased LVEF was associated with higher vascular TEEs risk in women (relative risk per 10% decrease in ejection fraction 1.53, 95% CI 1.06–2.20, P = 0.02). In the retrospective case–control study conducted by Sharma et al., patients with LV thrombus had lower LVEF (17.5 ± 5.5 vs. 20.0 ± 6.9, P = 0.08) compared with those without. Besides, the proportion of patients with a LVEF ≤20% was higher in patients with LV thrombus than those without (57.1% vs. 36.2%, P = 0.04). 15 In addition, Al Rawahi et al. had observed that HF is predictor for left atrial appendage thrombus (OR, 2.2; 95% CI, 1.0–4.7). 16
Decreased LVEF is the direct manifestation of impaired cardiac systolic function, whereas reduced systolic blood pressure is the indirect form. In our study, in the univariable analysis, both systolic blood pressure and diastolic blood pressure correlated to intracardiac thrombus. Patients with lower systolic blood pressure tend to have impaired myocardial contractility and lower cardiac output, which aggravate the blood stasis in cardiac chamber and chaos of intracardiac haemodynamics. Thus, intracardiac thrombus generated more easily in individuals with lower blood pressure. However, considering that the value of blood pressure was single measurement, neither the average of three measurements in a row nor the continuous monitoring data during hospitalization could be obtained, which may cause inevitable bias and made it hard to manipulate the model in the clinical practice. Under this circumstance, blood pressure should not be considered as a good independent parameter for our risk model. Thus, blood pressure was not enrolled in our multivariable logistic regression analysis despite its statistical significance in univariable logistic analysis. Besides, blood pressure could be influenced by numerous factors including medication and transient hypovolemia, which can lead to misinterpretation of our results. Considers these factors, we did not adopt blood pressure as an independent parameter to construct our final model. The correlation of blood pressure and intracardiac thrombus should be explored in well‐designed prospective studies.
Platelet abnormalities are also commonplace in HF and have been elaborated in previous literature. For HF patients, Platelet is over‐activated via various substances such as p‐selectin, beta‐thromboglobulin, and platelet/endothelial cell adhesion molecule‐1. 17 Furthermore, platelet is itself an important member in the physiopathologic mechanism of coagulation. Elevated thrombocyte counts escalated both blood viscosity and platelet–vessel wall adhesion; thus, higher risk of intracardiac thrombus will be observed in patients with high platelet count.
Although few study pay attention on the relationship of haemoglobin concentration and intracardiac thrombus in HF patients, researches in other fields have demonstrated the strong correlation between elevated haemoglobin and increased risk of arterial vascular events. A large‐scale clinic study in Sweden and Denmark which recruited 1 538 019 blood donors has illustrated that the risk of myocardial infarction and ischaemic stroke surges as haemoglobin concentrations elevated (HR = 3.52, 95% CI 2.85–4.36 for myocardial infarction, HR = 2.36, 95% CI 1.63–3.43 for ischaemic stroke). 18 The explicit mechanism behind the haemoglobin‐associated thrombotic risks is still ambiguous. Blood low‐flow states, increased blood viscosity, and increased platelet–vessel wall adhesion may partly elucidate the aetiologies.
Bromocriptine, a potent efficient drug for PPCM, may aggravate abnormality of coagulation during peripartum period. In vitro and vivo experiments, hyperprolactinaemia may induce hypercoagulability by enhanced ADP‐stimulated platelet activation and increased platelet aggregation. Elevated plasm fibrinogen and antithrombin III concentrations may also exacerbate the coagulation disorder induced by hyperprolactinaemia. 19 , 20 However, the influence of bromocriptine on TEEs is still controversial. Mon et al. found the neutral impact of dopaminergic treatment on TEEs, whereas several case reports revealed the potential correlation between bromocriptine therapy and atrial/venous thromboses. 21 , 22 , 23 The underlying mechanism is still unclear. However, significant variances of bromocriptine administration exist worldwide. The highest prescription rate of 87.5% was found in Europe, whereas in North America, Africa, and the Middle East, the prescription rates of bromocriptine have been as low as 0–20%. 24 Different to medical centres in Europe, bromocriptine has not been widely prescribed for PPCM patients in China for lack of large‐scale clinical trials, which explained no use of bromocriptine in our study. Considering that the prescription rate of bromocriptine varies greatly between different countries and bromocriptine is widely prescribed in European countries based on the positive results of several clinical trials and ESC recommendation, for PPCM populations receiving bromocriptine treatment, the risk factors for intracardiac thrombus may should be adjusted, and further evaluation is indispensable. 24 For the limited sample size in our retrospective study, lack of population on bromocriptine treatment was an inescapable limitation. For further studies involved bromocriptine administration, essential comparation should be conducted, and the relationship between bromocriptine and intracardiac thrombus in PPCM should be explored.
Risk stratification model and its pros and cons
As shown in the results of our study, patients with intracardiac thrombus tended to have worse cardiac systolic function, which reflected by lower LVEF and more dilatated cardiac chambers. Thus, poor prognosis may be observed in individuals with intracardiac thrombi. Besides, the presence of cardiac mural thrombus increases the risk of systemic thromboembolism. Effective and accurate anticoagulate therapy are relatively significant for PPCM patients. The definite indication of anticoagulate therapy is still unclear. According to the position statement from the Heart Failure Association of the ESC on PPCM, prophylactic dose of low molecular weight heparin or oral anticoagulation is recommended for PPCM patients with reduced LVEF. But therapeutic anticoagulation is firmly endorsed in those with intracardiac thrombus validated by imaging or evidence of systemic embolism, as well as in patients with paroxysmal or persistent atrial fibrillation. 8 However, overly aggressive anticoagulant therapy may increase bleeding risk, which may result in catastrophic adverse events. Anticoagulate drug potential benefits must be initiated after thorough consideration of haemorrhagic risk and potential benefit.
To our knowledge of current literature, no risk stratification model for intracardiac thrombus has been established to guide anticoagulate therapy before our study. Our work innovatively constructed a tentative risk evaluating model, which enrolled several risk predicting factors, to assess the cumulative risk for intracardiac thrombi in PPCM patients. Through our study, we provided additional value to timely identify high‐risk patients who may suffer intracardiac thrombus. Individuals who have an accumulative score ≥8, especially for those with scores ≥11, are more susceptible to intracardiac thrombus. For the cons of our model, the enrolled population in our study had numerous limitations for our study was just a small sample retrospective study. As described hereinbefore, compared with other researches, no patient in our cohort received bromocriptine therapy. Besides, for culture differences, no patient in our cohort had smoking, alcohol abuse, and drug abuse history, which may cause bias in result interpretations. To sum up, our stratification model was not fully suitable to influence and guide clinical practice for that our study was just a retrospective study in specific population with limited clinical characteristics. For the pros, the discrimination ability of our model is superb, and the clinical parameters enrolled in our model were easily accessed by clinicians. Furthermore, our method was economic, simple, and practicable, which was the highlighted advantage in our study.
Study limitations
First, our study was just a single‐centre retrospective study, and the sample size of study population was limited. The conclusion of our study was drawn based on the patients with limited clinical characteristics, and the criteria of ≥10 events per variable did not be met when multivariable logistic regression was conducted. Second, neither LV opacification techniques with contrast agents, cardiac magnetic resonance imaging, nor cardiac computed tomography was applied in the diagnosis of intracardiac mural thrombus. False positive findings and diagnosis bias were inevitable in our retrospective study. Third, although laboratory parameters indicative of increased coagulation is important to assess the formation of intracardiac thrombus, we did not include these biomarkers, such as fibrinogen and D‐dimer, into our risk stratification model for that coagulation status is different for antepartum and postpartum patients to some extent. Besides, some obstetricians empirically administrated anticoagulation agents to pregnant women before PPCM was confirmed, even in those without intracardiac thrombus or embolic events, which would influence the accuracy of factors indicating coagulation. Fourth, for no access of databases of pervious international studies, internal validation rather than external validation was used in our study. Confounders factors and bias are inevitable on this occasion. Overall, this is just an exploratory study to construct a tentative risk stratification model for intracardiac thrombus in PPCM patients. We will conduct large‐scale multicentre study to externally validate the efficiency of the risk stratification model.
Conclusions
In our retrospective study, impaired LVEF, elevated haemoglobin levels and high thrombocyte counts were regarded as independent risk factors for intracardiac thrombus in PPCM. A risk stratification model integrates these readily available clinical variables into a neat and simple‐to‐use scale to estimate the risk of intracardiac thrombus for PPCM patients. This tentative risk stratification model can stratify the risk of intracardiac thrombus into low‐risk, intermediate‐risk, and high‐risk categories, which will help clinicians identify PPCM patients with high risk of intracardiac thrombus more easily.
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding
This study was supported by the National Natural Science Foundation of China (81873516, 81873522, and 81900444), the National Key Research and Development Program of China (2017YFC1308303 and 2021YFF0501400), and the Shandong Provincial Natural Science Foundation of China (ZR2019PH030). Besides, this study was funded by Clinical Research Center of Shandong University (2020SDUCRCA009).
Acknowledgements
The authors thank Qiang Liu and Yunfei Guo for their technical assistance in R software.
Fu, K. , Zhang, H. , Chen, N. , Hu, Y. , Xiao, J. , Zhang, X. , Lin, Z. , Lu, H. , and Ji, X. (2023) Risk factors for intracardiac thrombus in peripartum cardiomyopathy: a retrospective study in China. ESC Heart Failure, 10: 148–158. 10.1002/ehf2.14158.
Contributor Information
Huixia Lu, Email: luhuixia@sdu.edu.cn.
Xiaoping Ji, Email: jixiaoping@sdu.edu.cn.
References
- 1. Davis MB, Arany Z, McNamara DM, Goland S, Elkayam U. Peripartum cardiomyopathy: JACC state‐of‐the‐art review. J Am Coll Cardiol 2020; 75: 207–221. [DOI] [PubMed] [Google Scholar]
- 2. Isogai T, Kamiya CA. Worldwide incidence of peripartum cardiomyopathy and overall maternal mortality. Int Heart J 2019; 60: 503–511. [DOI] [PubMed] [Google Scholar]
- 3. Ricke‐Hoch M, Pfeffer TJ, Hilfiker‐Kleiner D. Peripartum cardiomyopathy: Basic mechanisms and hope for new therapies. Cardiovasc Res 2020; 116: 520–531. [DOI] [PubMed] [Google Scholar]
- 4. Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation 2016; 133: 1397–1409. [DOI] [PubMed] [Google Scholar]
- 5. Sliwa K, Mebazaa A, Hilfiker‐Kleiner D, Petrie MC, Maggioni AP, Laroche C, Regitz‐Zagrosek V, Schaufelberger M, Tavazzi L, van der Meer P, Roos‐Hesselink JW, Seferovic P, van Spandonck‐Zwarts K, Mbakwem A, Böhm M, Mouquet F, Pieske B, Hall R, Ponikowski P, Bauersachs J. Clinical characteristics of patients from the worldwide registry on peripartum cardiomyopathy (PPCM): EURObservational research programme in conjunction with the Heart Failure Association of the European Society of Cardiology Study Group on PPCM. Eur J Heart Fail 2017; 19: 1131–1141. [DOI] [PubMed] [Google Scholar]
- 6. Dunkman WB, Johnson GR, Carson PE, Bhat G, Farrell L, Cohn JN. Incidence of thromboembolic events in congestive heart failure. The V‐HeFT VA cooperative studies group. Circulation 1993; 87: VI94–VI101. [PubMed] [Google Scholar]
- 7. Thornton P, Douglas J. Coagulation in pregnancy. Best Pract Res Clin Obstet Gynaecol 2010; 24: 339–352. [DOI] [PubMed] [Google Scholar]
- 8. Bauersachs J, König T, van der Meer P, Petrie MC, Hilfiker‐Kleiner D, Mbakwem A, Hamdan R, Jackson AM, Forsyth P, Boer RA, Mueller C, Lyon AR, Lund LH, Piepoli MF, Heymans S, Chioncel O, Anker SD, Ponikowski P, Seferovic PM, Johnson MR, Mebazaa A, Sliwa K. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail 2019; 21: 827–843. [DOI] [PubMed] [Google Scholar]
- 9. Cunningham FG, Byrne JJ, Nelson DB. Peripartum cardiomyopathy. Obstet Gynecol 2019; 133: 167–179. [DOI] [PubMed] [Google Scholar]
- 10. Hellgren M. Hemostasis during normal pregnancy and puerperium. Semin Thromb Hemost 2003; 29: 125–130. [DOI] [PubMed] [Google Scholar]
- 11. Dotsenko O, Kakkar VV. Antithrombotic therapy in patients with chronic heart failure: Rationale, clinical evidence and practical implications. J Thromb Haemost 2007; 5: 224–231. [DOI] [PubMed] [Google Scholar]
- 12. Karaye KM, Ishaq NA, Sa'idu H, Balarabe SA, Talle MA, Isa MS, Adamu UG, Umar H, Okolie HI, Shehu MN, Mohammed IY, Sanni B, Ogah OS, Oboirien I, Umuerri EM, Mankwe AC, Shidali VY, Njoku P, Dodiyi‐Manuel S, Shogade TT, Olunuga T, Ojji D, Josephs V, Mbakwem AC, Tukur J, Isezuo SA, PEACE Registry Investigators . Incidence, clinical characteristics, and risk factors of peripartum cardiomyopathy in Nigeria: Results from the PEACE registry. ESC Heart Fail 2020; 7: 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kolte D, Khera S, Aronow WS, Palaniswamy C, Mujib M, Ahn C, Jain D, Gass A, Ahmed A, Panza JA, Fonarow GC. Temporal trends in incidence and outcomes of peripartum cardiomyopathy in the United States: A nationwide population‐based study. J Am Heart Assoc 2014; 3: e001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moulig V, Pfeffer TJ, Ricke‐Hoch M, Schlothauer S, Koenig T, Schwab J, Berliner D, Pfister R, Michels G, Haghikia A, Falk CS, Duncker D, Veltmann C, Hilfiker‐Kleiner D, Bauersachs J. Long‐term follow‐up in peripartum cardiomyopathy patients with contemporary treatment: Low mortality, high cardiac recovery, but significant cardiovascular co‐morbidities. Eur J Heart Fail 2019; 21: 1534–1542. [DOI] [PubMed] [Google Scholar]
- 15. Sharma ND, McCullough PA, Philbin EF, Weaver WD. Left ventricular thrombus and subsequent thromboembolism in patients with severe systolic dysfunction. Chest 2000; 117: 314–320. [DOI] [PubMed] [Google Scholar]
- 16. Al Rawahi M, Samuel M, Galatas C, Joza J, Lima PY, Barbosa R, Thanassoulis G, Bernier ML, Huynh T, Essebag V. Incidence and predictors of intracardiac thrombus on pre‐electrophysiological procedure transesophageal echocardiography. CJC Open 2019; 1: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim JH, Shah P, Tantry US, Gurbel PA. Coagulation abnormalities in heart failure: Pathophysiology and therapeutic implications. Curr Heart Fail Rep 2016; 13: 319–328. [DOI] [PubMed] [Google Scholar]
- 18. Hultcrantz M, Modlitba A, Vasan SK, Sjölander A, Rostgaard K, Landgren O, Hjalgrim H, Ullum H, Erikstrup C, Kristinsson SY, Edgren G. Hemoglobin concentration and risk of arterial and venous thrombosis in 1.5 million Swedish and Danish blood donors. Thromb Res 2020; 186: 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Erem C, Kocak M, Nuhoglu I, Yılmaz M, Ucuncu O. Blood coagulation, fibrinolysis and lipid profile in patients with prolactinoma. Clin Endocrinol (Oxf) 2010; 73: 502–507. [DOI] [PubMed] [Google Scholar]
- 20. Wallaschofski H, Donné M, Eigenthaler M, Hentschel B, Faber R, Stepan H, Koksch M, Lohmann T. PRL as a novel potent cofactor for platelet aggregation. J Clin Endocrinol Metab 2001; 86: 5912–5919. [DOI] [PubMed] [Google Scholar]
- 21. Mon SY, Alkabbani A, Hamrahian A, Thorton JN, Kennedy L, Weil R, Olansky L, Doshi K, Makin V, Hatipoglu B. Risk of thromboembolic events in patients with prolactinomas compared with patients with nonfunctional pituitary adenomas. Pituitary 2013; 16: 523–527. [DOI] [PubMed] [Google Scholar]
- 22. Maurel C, Abhay K, Schaeffer A, Lange F, Castot A, Melon E. Acute thrombotic accident in the postpartum period in a patient receiving bromocriptine. Crit Care Med 1990; 18: 1180–1181. [DOI] [PubMed] [Google Scholar]
- 23. Dargaud Y, Pariset C, Pinede L, Rugeri L, Mohammedi I, Trzeciak C, Negrier C, Ninet J. Multiple arterial thromboses in a patient with primary antiphospholipid syndrome receiving a bromocriptine therapy. Lupus 2004; 13: 957–960. [DOI] [PubMed] [Google Scholar]
- 24. Hoevelmann J, Engel ME, Muller E, Hohlfeld A, Böhm M, Sliwa K, Viljoen C. A global perspective on the management and outcomes of peripartum cardiomyopathy: A systematic review and meta‐analysis. Eur J Heart Fail 2022. [DOI] [PubMed] [Google Scholar]


