Abstract
Aims
We aim to investigate the correlation between high levels of the systemic immune‐inflammation index (SII) and long‐term mortality and major cardiovascular adverse events in advanced chronic heart failure patients with renal dysfunction.
Methods and results
Seven hundred seventeen advanced chronic heart failure patients with renal dysfunction, who visited the First affiliated hospital of Zhengzhou University from September 2019 to December 2020, were included. All‐cause mortalities (ACM) were selected as primary endpoints and major cardiovascular adverse events (MACEs) as the secondary endpoints. Based on the receiver operating characteristic (ROC) curve and the Youden index, the optimal cut‐off values of SII for ACM and MACEs were 1228 and 1406. In the group where ACM were the primary endpoint, patients were categorized into the low‐SII group (n = 479) and the high‐SII group (n = 238). Patients in the group using MACEs as the secondary endpoint were also categorized into the low‐SII groups (n = 514) and the high‐SII groups (n = 203). Univariate and multivariate COX regression were used to screen the independent predictors for ACM and MACEs, revealing the relationship between SII levels and endpoints. According to the univariate COX analysis, SII was the risk factor (hazard ratio [HR] = 2.144, 95% confidence interval [CI]: 1.565–2.938, P < 0.001) for the ACM subgroup. It was also the risk factor (HR = 1.625, CI: 1.261–2.905, P < 0.001) for the MACEs subgroup. Multivariate COX regression analysis indicated that the occurrence of ACM and MACEs in high‐level SII and low‐level SII patients had statistical differences. The incidence of ACM increased by 70.3% (HR = 1.703; 95% CI: 1.200–2.337; P = 0.002) in patients of the high SII level group, the incidence of MACEs increased by 58.3% (HR = 1.583, 95% CI: 1.213–2.065, P = 0.001). Kaplan–Meier (K‐M) survival analysis further suggested that patients with a high SII level had an increased risk of having ACM (log‐rank P < 0.001) and MACEs (log‐rank P < 0.001) within 30 months. SII could be considered as a novel predictor of the occurrence of ACM and MACEs for patients with advanced chronic heart failure and renal dysfunction.
Conclusions
This study suggested that SII is a novel independent predictor of mortality in advanced chronic heart failure patients with renal dysfunction, and it should be considered in current clinical management.
Keywords: advanced chronic heart failure, prognosis, MACEs, mortality, renal dysfunction, systemic immune‐inflammation index
1. Introduction
Chronic heart failure (CHF) is the end‐stage manifestation of many cardiovascular diseases, leading to premature death. Recent studies indicate that renal function and cardiac function interact with each other. More importantly, the renal function in patients with chronic heart failure is usually associated with increased readmission rate and mortality, resulting in a poor prognosis. 1 , 2 , 3 The progression of heart failure often accelerates the decline of renal function, which in turn affects cardiac function, forming a vicious cycle. Therefore, renal dysfunction is recognized as a higher risk factor for CHF patients. 4 , 5
In CHF, the activation of the immune cells and responses results in the secretion of proinflammatory cytokines, activation of the complement system, the production of autoantibodies, and the upregulated expression of adhesion molecules, further enhancing the inflammatory state. 6 In fact, the inflammatory response is now considered as one of the main contributors to the development of chronic heart failure in addition to the original hypothesis that insufficient circulation volume and the insufficient organ perfusion caused by the reduced cardiac output are the main causes of CHF. Furthermore, because tubular epithelial cells are sensitive to reactive oxygen species, inflammatory responses and immune‐induced oxidative damage may contribute to renal dysfunction, which has been observed in tubular cell injury. 7 Taken together, immune responses may play an important role in the poor prognosis of CHF patients with renal dysfunction. Hence, biomarkers that reflect the immune response would benefit clinical diagnostic purposes. Given that the systemic immune‐inflammation index (SII) is a comprehensive inflammatory index that reflects three kinds of important immune cells, including neutrophils, lymphocytes, and platelets, indicating the local immune response and systemic inflammation. Therefore, SII would be considered as a biomarker. 8 , 9 However, limited study has explored the correlation between SII and the prognosis and mortality of CHF patients with renal dysfunction.
Herein, in this study, we investigated the correlation between SII and the prognosis and occurrence of mortality in CHF patients with renal dysfunction, shining a light on a biomarker that can potentially contribute to the current clinical management.
2. Method
2.1. Data source
Patients with chronic heart failure who were admitted to the First Affiliated Hospital of Zhengzhou University from September 2019 to December 2020 were selected for this study, and the inclusion and exclusion criteria were as follows:
Inclusion criteria were as follows: (1) have an advanced heart failure by diagnosis, and the diagnostic criteria refer to the 2021 ESC advanced heart failure diagnostic criteria. 10 To be more specific about the CHF definition, at least two of the following four criteria must be met despite the treatment: (a) Severe and persistent heart failure symptoms (grade NYHA III or IV). (b) Severe cardiac insufficiency was defined by at least one of the following: (i) LEVF ejection fraction of 30%; (ii) isolated right heart insufficiency; (iii) inoperable severe valvular anomalies, inoperable severe congenital anomalies. (iv) persistent high BNP or NT‐proBNP values; (v) severe LV diastolic dysfunction or structural abnormalities (according to the HFpEF definition). (c) Pulmonary or systemic congestion episodes requiring high intravenous dose diuretics or low output paroxysmal HF requiring positive inotropes or vasoactive drugs or malignant arrhythmia resulting in >1 unscheduled visit or hospitalization in the past 12 months. (d) Impaired exercise capacity for cardiac causes, inability to exercise or a 6‐min walking test with low distance (<300 m) or peak oxygen consumption <12 mL/kg/min or <50% predictive value; (2) was diagnosed with renal insufficiency, and with eGFR < 90 mL/(min/1.73 m2). (3) Adult patients were over 18 years old; (4) Patients were hospitalized for heart failure twice.
Exclusion criteria were as follows: (1) first onset of acute heart failure; (2) with primary renal disease; (3) with infectious disease or malignant tumour; (4) glomerular filtration rate <15 mL/(min/1.73 m2) or receiving renal dialysis treatment; (5) hospitalization time <2 days; (6) incomplete clinical case data.
According to the above criteria, a total of 815 patients were selected, 98 patients were lost contact during the follow‐up, and 717 patients were included. The flow chart demonstrated the general information and laboratory data of these patients were collected. The general information included age, sex, hypertension history, diabetes history. Meanwhile, the laboratory data included blood routine, blood and biochemical indicators, and markers of myocardial injury, such as white blood cell count (WBC), lymphocyte count (LY), neutrophil count (NEUT), serum creatinine (Scr), blood urea nitrogen (BUN), serum albumin (ALB), fasting blood glucose (FPG), amino‐terminal brain sodium peptide precursor (N terminal pro B type natriuretic peptide, NT‐proBNP), troponin and so on.
2.2. Diagnostic criteria and definitions
According to European Society of Cardiology (ESC) recommendations, the criteria for the diagnosis of hypertension 11 are a clear history of hypertension and active antihypertensive medication, or a systolic blood pressure ≥140 mmHg and/or a diastolic blood pressure ≥90 mmHg at least three remeasurements during at least two separate occasions. The diagnostic criteria for diabetes 12 were the presence of typical diabetic symptoms, arbitrary blood glucose ≥11.1 mmol/L, or FPG ≥7.0 mmol/L, or being treated with hypoglycaemic drugs. Smoking status was one of the following conditions: current smokers or previous smokers. Drinking status was defined as any consumption of alcohol in the previous 6 months.
2.3. Endpoints
The primary endpoint event was all‐cause mortality (ACM), which was further categorized as cardiac death and non‐cardiac death, where cardiac death was caused by heart failure, myocardial infarction or malignant arrhythmia. Secondary endpoints were major adverse cardiovascular events (MACEs), defined as the combination of cardiac death, non‐cardiac death, readmitted for heart failure, the use of mechanical circulatory support and implementation of heart transplantation.
2.4. Follow‐up
The follow‐up methods mainly include extracting case system records, outpatient records, and telephone interviews. The final follow‐up time of this study is in March 2022. Among the 815 patients who met the inclusion criteria, 717 patients were contacted, and 98 were lost contact during the follow‐up period, with a loss rate of 12.0%. The median follow‐up time was 21.58 (17.98–25.39) months. Overall, a total of 156 patients died and 244 patients had MACEs events. To specific, 133 patients had cardiac deaths, and 111 had recurrent heart failure admissions (Figure 1).
Figure 1.

Flow chart of patient inclusion.
2.5. Statistical analysis
SPSS version 26.0 (IBM Corp., Armonk, NY, USA) was used to analyse data. The ROC curves were drawn with the two endpoint events as state variables (Figures 2 and 3), and the corresponding Youden index was obtained, thus finding the cut‐off value of each endpoint event to divide the patients into low SII and high SII groups. The best cut‐off value with all‐cause death as the status variable was 1228 (AUC = 0.740, P < 0.001), and the best cut‐off value with secondary key MACEs events as the status variable was 1406 (AUC = 0.728, P < 0.001). Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range). A t‐test was used if the continuous variables met both a normal distribution and homogeneity of variance, and a Mann–Whitney U‐test was applied if a normal distribution or homogeneity of variance was not satisfied. Categorical variables were presented as the number of cases (percentage) and analysed using the χ 2 test (or Fisher's exact method). Furthermore, Kaplan–Meier analysis was employed to calculate the cumulative incidence of long‐term outcomes and used the log‐rank test, which visually demonstrated the correlation between SII and patient survival and MACEs event incidence on Kaplan–Meier (K‐M) curves. Two multivariate COX regression models were constructed with two different endpoint events: first using univariate COX regression to screen for covariates, including statistically significant variables with univariate COX regression into multivariate COX regression equations, and using backward stepwise regression to screen for independent predictors of HF outcome. P < 0.05 was considered to be statistically significant. GraphPad Prism8 was used to draw the forest map to intuitively demonstrate the relationship between SII and various independent influencing factors and CHF mortality and MACEs in the COX regression model.
Figure 2.
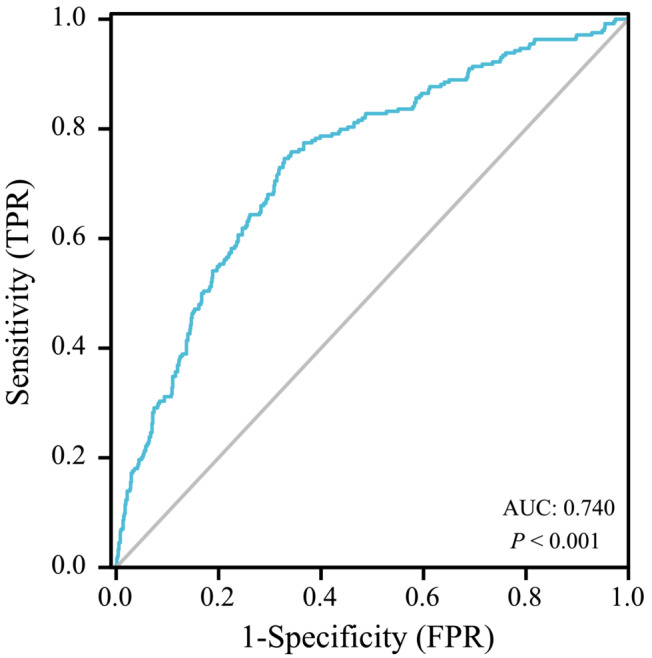
ROC curve of SII predicting long‐term mortality in patients with advanced chronic heart failure with renal insufficiency.
Figure 3.
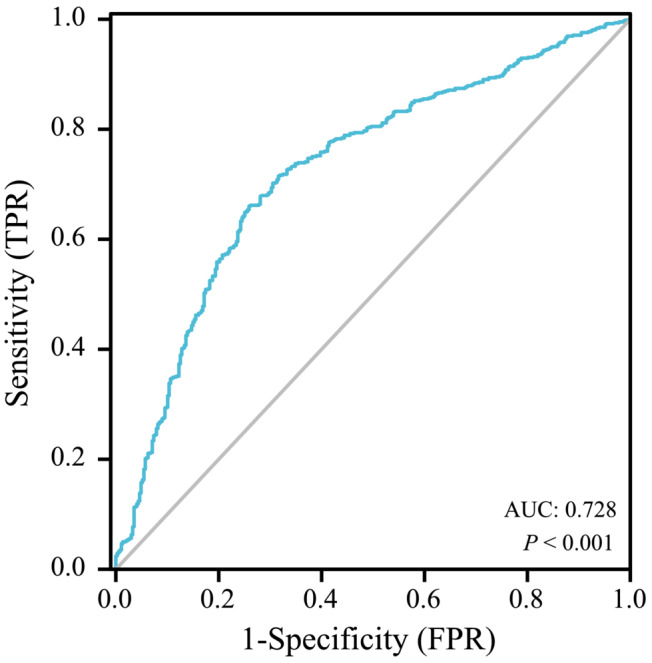
The ROC curve of SII predicting the incidence of long‐term MACEs events in patients with advanced chronic heart failure and renal insufficiency.
3. Results
3.1. Baseline characteristics
Based on the inclusion criteria, exclusion criteria and follow‐up data, 717 patients were included. In the subgroups with ACM as the primary endpoint, sensitivity and specificity were 78.2% and 60.5%, respectively, and Youden's index was 0.177. The area under the curve (AUC) was 0.740, and the cut‐off value was 1228 (Figure 2). Participants were categorized into the low‐SII group (SII < 1228, n = 479) and the high‐SII group (SII ≥ 1228, n = 238). All of the baseline data are shown in Table 1. Statistical differences in WBC, RBC, platelets, neutrophils, lymphocytes, BNP and CRP (P<0.05) were observed the between two groups. Interestingly, the insignificant differences in age and sex were identified (P ≥ 0.05).
Table 1.
Clinical and laboratory characteristics according to the SII (mortality)
| Characteristics | SII < 1228 (n = 479) | SII > 1228 (n = 238) | χ 2/t/z | P |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 57.88 ± 16.55 | 60.01 ± 16.63 | −1.744 | 0.082 |
| Male, n (%) | 301 (62.8) | 135 (56.7) | 2.496 | 0.067 |
| Smoke, n (%) | 135 (28.2) | 63 (26.5) | 0.426 | 0.514 |
| Alcohol, n (%) | 83 (17.3) | 44 (18.5) | 0.147 | 0.702 |
| Co‐morbidities, n (%) | ||||
| Hypertension | 335 (69.9) | 161 (67.6) | 7.723 | 0.052 |
| Diabetes mellitus | 160 (33.4) | 88 (37.0) | 2.814 | 0.093 |
| Arrhythmia | 141 (29.4) | 81 (36.5) | 1.527 | 0.210 |
| PVD | 71 (14.8) | 36 (15.1) | 0.993 | 0.334 |
| Thyroid disease | 102 (21.3) | 43 (18.1) | 1.026 | 0.311 |
| CVD | 80 (16.7) | 49 (20.6) | 1.628 | 0.202 |
| Laboratory parameters | ||||
| WBC, 109/L | 6.03 (4.92–7.33) | 9.00 (6.73–12.67) | 12.560 | <0.001 |
| RBC, 109/L | 3.61 ± 0.93 | 3.44 ± 0.91 | 2.200 | 0.028 |
| PLT, 109/L | 177 ± 72 | 232 ± 103 | −7.110 | <0.001 |
| Neut, 109/L | 4.09 (3.25–4.10) | 7.62 (5.45–10.74) | 15.821 | <0.001 |
| Lymph, 109/L | 1.15 (0.85–1.59) | 0.81 (0.57–1.17) | −8.764 | <0.001 |
| BUN, mmol/L | 15.4 (7.6–24.8) | 17.7 (10.1–27.1) | 1.900 | 0.057 |
| Creatinine, μmol/L | 334 (101–734) | 232 (99–657) | −1.439 | 0.150 |
| TC, mmol/L | 5.55 (4.47–6.36) | 5.68 (4.72–6.36) | 1.572 | 0.116 |
| TG, mmol/L | 2.80 (1.92–3.64) | 2.55 (1.84–3.16) | −1.052 | 0.293 |
| HDL‐C, mmol/L | 1.45 (1.17–1.70) | 1.55 (1.30–1.76) | 0.846 | 0.397 |
| LDL‐C, mmol/L | 3.53 (2.72–4.19) | 3.72 (2.95–4.38) | 1.223 | 0.221 |
| NT‐proBNP, ng/ml | 7932 (2018–28753) | 12 297 (3678–33857) | 2.808 | 0.005 |
| eGFR, mL/(min/1.73 m2) | 52.962 (31.183–72.146) | 47.214 (29.225–66.068) | 2.072 | 0.043 |
| CRP, mg/L | 7.10 (2.42–17.89) | 22.08 (8.41–63.34) | 9.428 | <0.001 |
| LVEF, % | 52 (47–55) | 51 (40–54) | 2.023 | 0.039 |
| LVD, mm | 51.17 ± 10.18 | 50.44 ± 10.68 | 0.982 | 0.327 |
| Medication, n (%) | ||||
| Antiplatelet drugs | 193 (40.3) | 42 (17.6) | 0.770 | 0.401 |
| ARNI | 119 (24.8) | 64 (26.9) | 0.351 | 0.554 |
| ACEI | 212 (44.3) | 103 (43.3) | 0.062 | 0.803 |
| Beta‐blocker | 270 (56.4) | 130 (54.6) | 0.196 | 0.658 |
| Statin | 206 (43.0) | 119 (50.0) | 2.175 | 0.140 |
| Calcium channel blocker | 142 (29.6) | 96 (40.3) | 0.022 | 0.936 |
| Digoxin | 31 (13.0) | 51 (10.6) | 0.784 | 0.376 |
| Diuretics | 191 (39.9) | 111 (46.6) | 2.984 | 0.084 |
Abbreviations: PVD, peripheral vascular disease; CVD, cerebro‐vascular disease; WBC, white blood cell; Neut, neutrophils; Lymph, lymphocyte; PLT, platelet; TC, total cholesterol; TG, triglyceride; HDL‐C, high density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; CRP, C‐reactive protein; LVD, left ventricle diameter; ACEI, angiotensin‐converting enzyme inhibitors; BUN, blood urea nitrogen.
Similarly, in the subgroups with MACEs as the secondary endpoint, sensitivity and specificity were 87.7% and 63.9%, respectively, and Youden's index was 0.238. The area under the curve (AUC) was 0.728 (P < 0.001), and the cut‐off value was 1406 (Figure 3). Participants were divided into the low‐SII group (SII < 1406, n = 514) and the high‐SII group (SII ≥ 1406, n = 203). All of the baseline data were included in Table 2. Factors including age, WBC, RBC, platelets, neutrophils, lymphocytes, BUN, BNP, and CRP were statistically significant between the two groups (P < 0.05), but not in other factors (P ≥ 0.05).
Table 2.
Clinical and laboratory characteristics according to the SII (MACEs)
| Characteristics | SII < 1406 (n = 514) | SII > 1406 (n = 203) | χ 2/t/z | P |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 57.84 ± 16.59 | 60.45 ± 16.53 | 0.314 | 0.037 |
| Male, n (%) | 323 (62.8) | 113 (55.7) | 3.144 | 0.076 |
| Smoke, n (%) | 145 (28.2) | 53 (26.1) | 0.322 | 0.571 |
| Alcohol, n (%) | 90 (17.5) | 37 (18.2) | 0.051 | 0.821 |
| Co‐morbidities, n (%) | ||||
| CHD | 233 (45.3) | 107 (52.7) | 3.178 | 0.075 |
| Hypertension | 356 (69.2) | 140 (69.0) | 4.182 | 0.242 |
| Diabetes mellitus | 172 (33.5) | 76 (37.4) | 1.017 | 0.313 |
| Arrhythmia | 154 (30.0) | 68 (33.5) | 0.851 | 0.356 |
| PVD | 76 (14.8) | 31 (15.3) | 0.027 | 0.87 |
| Thyroid disease | 109 (21.2) | 36 (17.7) | 1.088 | 0.297 |
| CVD | 86 (16.7) | 43 (21.2) | 1.954 | 0.162 |
| Laboratory parameters | ||||
| WBC, 109/L | 6.05 (4.99–7.50) | 9.39 (7.03–12.85) | 12.388 | <0.001 |
| RBC, 109/L | 3.61 ± 0.93 | 3.43 ± 0.96 | 0.157 | 0.024 |
| PLT, 109/L | 181 ± 76 | 231 ± 104 | 17.605 | <0.001 |
| Neut, 109/L | 4.15 (3.27–5.29) | 7.81 (5.75–11.00) | 15.528 | <0.001 |
| Lymph, 109/L | 1.15 (0.85–1.57) | 0.77 (0.53–1.07) | −9.021 | <0.001 |
| BUN, mmol/L | 15.3 (8.5–24.7) | 17.7 (10.3–27.2) | 2.387 | 0.017 |
| Creatinine, μmol/L | 331 (101–716) | 257 (98–690) | −1.024 | 0.306 |
| TC, mmol/L | 3.63 (2.99–2.50) | 3.78 (3.07–4.71) | 0.904 | 0.366 |
| TG, mmol/L | 1.34 (0.86–1.92) | 1.20 (0.83–1.84) | −1.490 | 0.136 |
| HDL‐C, mmol/L | 0.97 (0.78–1.18) | 0.99 (0.79–1.31) | 1.286 | 0.199 |
| LDL‐C, mmol/L | 2.12 (1.60–2.79) | 2.19 (1.46–2.92) | 0.369 | 0.712 |
| NT‐proBNP, ng/mL | 7723 (1946–28 218) | 13 487 (4735–35 000) | 2.667 | <0.001 |
| eGFR, mL/(min/1.73 m2) | 53.422 (32.931–71.567) | 44.769 (26.840–65.456) | 1.973 | 0.049 |
| CRP, mg/L | 7.20 (2.44–19.65) | 23.76 (8.70–64.10) | 8.994 | <0.001 |
| LVEF, % | 52 (45–55) | 51 (40–54) | 2.473 | 0.014 |
| LVD, mm | 51.18 ± 10.27 | 50.37 ± 10.78 | 0.103 | 0.348 |
| Medication, n (%) | ||||
| Antiplatelet drugs | 81 (15.8) | 42 (20.7) | 2.490 | 0.115 |
| ARNI | 130 (25.2) | 53 (26.7) | 0.051 | 0.821 |
| ACEI | 230 (44.7) | 85 (41.9) | 0.488 | 0.485 |
| Beta‐blocker | 294 (57.2) | 106 (52.2) | 1.646 | 0.226 |
| Statin | 225 (43.8) | 100 (49.3) | 1.768 | 0.184 |
| Calcium channel blocker | 209 (40.7) | 83 (40.9) | 0.003 | 0.956 |
| Digoxin | 58 (11.3) | 24 (11.8) | 0.042 | 0.838 |
| Diuretics | 207 (40.3) | 95 (46.8) | 2.524 | 0.111 |
Abbreviations: SII, systemic immune‐inflammation index; PVD, peripheral vascular disease; CVD, cerebro‐vascular disease; WBC, white blood cell; Neut, neutrophils; Lymph, lymphocyte; PLT, platelet; TC, total cholesterol; TG, triglyceride; HDL‐C, high density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; CRP, C‐reactive protein; LVD, left ventricle diameter; ACEI, angiotensin‐converting enzyme inhibitors; BUN, blood urea nitrogen.
3.2. The relationship between SII and all‐cause mortality (ACM)
In the subgroups with ACM as the primary endpoint, we created a univariate COX model (Table 3) for each of the predictor variables and entered these variables that were significant (P < 0.05) in the univariate COX models into multivariate COX regression analysis (Figure 4). The univariate COX analysis results showed that age ≥ 60 years, gender, history of coronary artery disease, hypertension and arrhythmia, WBC, neutrophils, total cholesterol, NT‐pro BNP, eGFR, troponin, CRP, SII, use of digoxin and antiplatelet agents were risk factors for ACM in CHF patients with renal dysfunction (P < 0.05), and the haemoglobin, use of Entresto and calcium channel blockers were protective factors (P < 0.05).
Table 3.
Univariate COX regression analysis results for ACM
| Variables | Univariate analysis | ||||
|---|---|---|---|---|---|
| β | SE | Wald χ 2 | Crude HR (95% CI) | Crude P‐value | |
| Age | 1.123 | 0.181 | 38.596 | 3.075 (2.157–4.383) | <0.001 |
| Gender | −0.484 | 0.161 | 9.072 | 0.616 (0.450–0.844) | 0.003 |
| CHD | 0.638 | 0.165 | 15.039 | 1.893 (1.371–2.613) | <0.001 |
| Hypertension | 0.831 | 0.172 | 23.326 | 1.436 (1.311–1.610) | <0.001 |
| Arrhythmia | 0.712 | 0.162 | 19.434 | 2.039 (1.485–2.799) | <0.001 |
| Diabetes mellitus | 0.015 | 0.169 | 0.008 | 0.985 (0.707–1.373) | 0.929 |
| CVD | 0.222 | 0.199 | 1.255 | 1.249 (0.846–1.843) | 0.263 |
| PVD | 0.066 | 0.230 | 0.082 | 1.608 (0.680–1.677) | 0.775 |
| Thyroid disease | 0.447 | 0.230 | 3.779 | 0.639 (0.407–1.004) | 0.052 |
| Smoke | 0.087 | 0.182 | 0.229 | 0.916 (0.641–1.310) | 0.632 |
| Alcohol | 0.119 | 0.218 | 0.296 | 0.888 (0.579–1.362) | 0.586 |
| WBC | 0.618 | 0.161 | 14.801 | 0.539 (0.393–0.738) | <0.001 |
| RBC | −0.236 | 0.172 | 1.887 | 1.266 (0.904–1.773) | 0.169 |
| HB | −0.335 | 0.166 | 4.076 | 1.398 (1.010–1.936) | 0.043 |
| PLT | −0.307 | 0.215 | 2.041 | 0.736 (0.483–1.121) | 0.153 |
| Neut | 0.732 | 0.173 | 17.96 | 2.080 (1.482–2.918) | <0.001 |
| Lymph | −0.296 | 0.507 | 0.341 | 0.744 (0.276–2.008) | 0.559 |
| TC | 0.397 | 0.171 | 5.385 | 0.672 (0.481–0.940) | 0.020 |
| TG | 0.345 | 0.177 | 3.804 | 0.708 (0.501–1.002) | 0.050 |
| NT‐proBNP | 0.819 | 0.192 | 18.149 | 2.268(1.556–3.305) | <0.001 |
| eGFR, mL/(min/1.73 m2) | 0.705 | 0.187 | 14.214 | 2.033 (1.403–2.918) | <0.001 |
| Troponin | 0.864 | 0.162 | 28.433 | 2.372 (1.727–3.258) | <0.001 |
| CRP | 1.038 | 0.167 | 38.452 | 2.822 (1.033–3.918) | <0.001 |
| SII | 0.763 | 0.161 | 22.513 | 2.144 (1.565–2.938) | <0.001 |
| ARNI | −0.393 | 0.206 | 3.632 | 0.675 (0.450–1.011) | 0.07 |
| Beta‐blocker | −0.297 | 0.161 | 3.414 | 0.743 (0.542–1.018) | 0.065 |
| Digoxin | 0.539 | 0.212 | 6.479 | 1.715 (1.132–2.597) | 0.011 |
| Calcium channel blocker | −0.872 | 0.189 | 21.385 | 0.418 (0.289–0.605) | <0.001 |
| Antiplatelet drugs | 0.628 | 0.184 | 11.667 | 1.873 (1.307–2.685) | 0.001 |
Abbreviations: PVD, peripheral vascular disease; CVD, cerebro‐vascular disease; WBC, white blood cell; Neut, neutrophils; Lymph, lymphocyte; PLT, platelet; TC, total cholesterol; TG, triglyceride; SII, systemic immune‐inflammation index; HDL‐C, high density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; CRP, C‐reactive protein; BUN, blood urea nitrogen; ACEI, angiotensin‐converting enzyme inhibitors.
Figure 4.
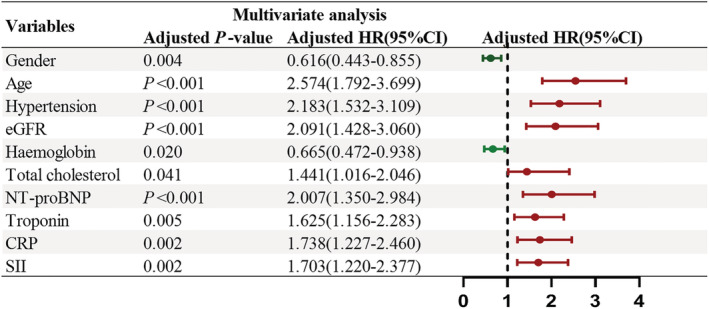
Multivariate Cox regression analysis results for ACM.
In the multivariate COX regression analysis, using the backward stepwise regression method with a justification of traditional clinical prognostic factors including male, age, history of hypertension, total cholesterol, NT‐pro BNP, eGFR, troponin and CRP, high‐level SII indicated a poor clinical outcome (P < 0.05), were the independent risk factors for ACM, and the male, HB were the protect factors. However, after justification in multivariate Cox regression analysis, insignificant differences in the risk factors of history of arrhythmia was observed between the two groups. During long‐term follow‐up, the risk of ACM in high SII group increased (Figure 4) by 70.3% (hazard ratio [HR] = 1.703; 95% confidence interval [CI], 1.200–2.337; P = 0.002).
3.3. The relationship between SII and MACEs events
When MACEs were selected as the secondary endpoint, a univariate COX model (Table 4) for each of the predictor variables were created. and entered The significant variables (P < 0.05) in the univariate Cox models were recorded the multivariate Cox regression analysis (Figure 5). The univariate COX analysis results showed that age ≥ 60 years, male, history of coronary artery disease, hypertension and arrhythmia, WBC, neutrophils, troponin, CRP, SII, use of digoxin and antiplatelet agents were risk factors for MACE in CHF patients with renal dysfunction (P < 0.05), and the use of ARNI and calcium channel blockers were protective factors (P < 0.05).
Table 4.
Univariate Cox regression analysis results for MACEs.
| Variables | Univariate analysis | ||||
|---|---|---|---|---|---|
| β | SE | Wald χ 2 | Crude HR (95% CI) | Crude P‐value | |
| Age | 0.783 | 0.134 | 34.079 | 2.189 (1.683–2.847) | <0.001 |
| Gender | −0.386 | 0.128 | 9.066 | 0.680 (0.529–0.874) | 0.003 |
| CHD | 0.733 | 0.132 | 31.012 | 2.081 (1.608–2.694) | <0.001 |
| Hypertension | 0.546 | 0.137 | 15.912 | 1.579 (1.443–1.758) | <0.001 |
| Arrhythmia | 0.727 | 0.13 | 31.482 | 2.069 (1.605–2.667) | <0.001 |
| Diabetes mellitus | 0.013 | 0.135 | 0.010 | 0.987 (0.757–1.286) | 0.922 |
| CVD | 0.122 | 0.162 | 0.561 | 1.129 (0.821–1.553) | 0.454 |
| PVD | 0.067 | 0.181 | 0.136 | 0.936 (0.657–1.333) | 0.712 |
| Thyroid disease | 0.336 | 0.175 | 3.688 | 0.715 (0.508–1.007) | 0.055 |
| Smoke | 0.040 | 0.142 | 0.080 | 1.041 (0.7888–1.376) | 0.777 |
| Alcohol | 0.001 | 0.17 | 0.000 | 0.999 (0.716–1.393) | 0.995 |
| WBC | 0.469 | 0.129 | 13.265 | 1.699 (1.242–2.508) | <0.001 |
| RBC | 0.37 | 0.14 | 7.000 | 1.448 (1.101–1.904) | 0.008 |
| HB | 0.509 | 0.135 | 14.262 | 1.664 (1.278–2.167) | <0.001 |
| PLT | −0.275 | 0.175 | 2.466 | 1.316 (0.934–1.854) | 0.116 |
| Neut | 0.595 | 0.134 | 19.653 | 1.812 (1.393–2.357) | <0.001 |
| Lymph | −0.296 | 0.507 | 0.341 | 0.744 (0.276–2.008) | 0.559 |
| TC | 0.224 | 0.133 | 2.854 | 0.799 (0.616–1.037) | 0.091 |
| TG | 0.244 | 0.138 | 3.310 | 0.784 (0.598–1.027) | 0.077 |
| NT‐proBNP | 0.281 | 0.136 | 4.274 | 1.325 (1.015–1.730) | 0.039 |
| Troponin | 0.603 | 0.133 | 20.53 | 1.828 (1.408–2.373) | <0.001 |
| CRP | 0.538 | 0.128 | 17.576 | 1.712 (1.332–2.201) | <0.001 |
| SII | 0.486 | 0.130 | 14.041 | 1.625 (1.261–2.905) | <0.001 |
| ARNI | −0.329 | 0.163 | 4.072 | 0.720 (0.523–0.991) | 0.044 |
| Beta‐blocker | −0.039 | 0.129 | 0.090 | 0.962 (0.747–1.239) | 0.764 |
| Digoxin | 0.514 | 0.173 | 8.810 | 1.672 (1.191–2.347) | 0.003 |
| Calcium channel blocker | −0.734 | 0.144 | 26.050 | 0.480 (0.362–0.636) | <0.001 |
| Antiplatelet drugs | 0.734 | 0.147 | 25.054 | 2.084 (1.563–2.777) | <0.001 |
Abbreviations: PVD, peripheral vascular disease; CVD, cerebro‐vascular disease; WBC, white blood cell; Neut, neutrophils; Lymph, lymphocyte; PLT, platelet; TC, total cholesterol; TG, triglyceride; HDL‐C, high density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; CRP, C‐reactive protein; BUN, blood urea nitrogen; SII, systemic immune‐inflammation index; ACEI, angiotensin‐converting enzyme inhibitors.
Figure 5.

Multivariate Cox regression analysis results for MACEs.
In the multivariate Cox regression analysis, using the backward stepwise regression method with the justification of traditional clinical prognostic factors including male, age, history of hypertension and CHD, haemoglobin, NT‐pro BNP, troponin and use of digoxin, ARNI and calcium channel blockers and antiplatelet agents, SII level could predict poor clinical outcomes (P < 0.05), where male, age ≥ 60 years, history of coronary heart disease, haemoglobin, NT‐proBNP, troponin, use of anti‐platelet agents and calcium channel blockers and SII were independent risk factors for ACM. Meanwhile, the risk factors of history of hypertension, RBC and ARNI drugs were insignificant between the two groups. More importantly, the risk of MACEs in high SII group increased (Figure 5) by 58.3% (HR = 1.583, 95% CI: 1.213–2.065, P = 0.001).
3.4. Kaplan–Meier survival analysis
The Kaplan–Meier survival analysis was shown in Figure 6. The cumulative long‐term ACM rate after discharge in the patients of the high SII group was significantly higher than that in the low SII group (log rank P < 0.001). In addition, the incidence of MACEs was also higher in the high SII group.
Figure 6.
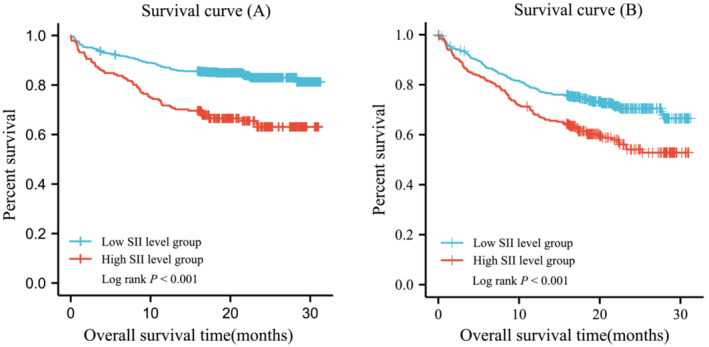
Cumulative Kaplan–Meier estimates of the time to the first adjudicated occurrence of ACM (A) and MACEs (B).
4. Discussion
This study assessed the prognostic value of SII in patients with CHF combined renal dysfunction, concluding the potential of SII as an independent risk factor for ACM and MACEs. ACM increased by 70.3% (HR = 1.703; 95% CI, 1.200–2.337; P = 0.002) in high SII group. The incidence of events in MACEs also increased by 58.3% (HR = 1.583, 95% CI: 1.213–2.065, P = 0.001) in the high SII level group. This study pioneeringly explored the relationship between SII and the prognosis of CHF patients with renal dysfunction.
4.1. Bidirectional pathway between heart and kidney
Previous study 13 has shown that approximately 40%–50% of patients with HF have chronic renal dysfunction. Even a slight reduction in eGFR can significantly affect ACM in patients with HF. Cardiac and renal diseases interact in a complex bidirectional and interdependent manner acutely and chronically, resulting in high mortality. In HF patients (LEVF < 40) with dialysis‐dependent and CKD stage 5, kidney transplantation can lead to a significant increase in mean LVEF and even normalization of LVEF in a considerable number of patients. This improvement in LVEF highlights the key role of renal dysfunction in cardiac remodelling. 14
From the pathophysiology point of view, cardiac and renal diseases share many common pathogeneses. Several non‐haemodynamic pathways can exacerbate cardiac and renal injury in cardiac insufficiency, such as sympathetic nervous system (SNS) activation, persistent renin‐angiotensin‐aldosterone system activation, and inflammation‐induced tissue damage. 15 Meanwhile, in haemodynamics, cardiac output decreases due to ventricular systolic or diastolic dysfunction in CHF patients. A significant reduction in cardiac output not only leads to tubular hypoxia and acute tubular necrosis but also contributes to the reduction in renal perfusion pressure, the increase in the glomerular hydrostatic pressure, elevated intratubular pressure, and diminished net filtration pressure, 16 , 17 resulting in increased circulatory load. The subsequent progression of long‐term renal hypoperfusion triggers baroreceptors, paragerular renin release and rein‐angiotensin‐aldosterone‐system (RAAS) activation, which may lead to renal vasoconstriction impacting on glomerular and tubular reabsorption, and contributing to the aggravation of the renal injury. 18 Among the common HF medications, diuretics are fundamental in heart failure treatment, given they are only category drugs than could control fluid retention diuretics. However, when diuretics are used to correct circulatory volume overload, the renal excretion of sodium is significantly increased, which promotes the secretion of renin, increases the activity of renin in the plasma, and activates the RAAS system, resulting in the reduction of renal function. 19 , 20 Apart from RAAS system activation, heart failure also induces arginine vasopressin (AVP) and other hormones in the body. Elevated level of AVP induces free water reabsorption, a mechanism that may further cause water retention, leading to elevated systemic pressure and aggravating the severity of heart failure. To this end, the progression of heart failure accelerates the decline of renal function, which in turn affects the cardiac function, forming a vicious circle, resulting in catastrophic consequence for the patients.
4.2. Inflammatory response in heart failure combined with renal dysfunction
Systemic chronic low‐grade inflammation is the key trigger for CHF and renal dysfunction, 21 as chronic inflammation significantly increases the general cardiovascular risk and infection rates in patients with CHF and renal dysfunction. In fact, inflammation‐induced cardiovascular disease is considered the leading cause of death. 22 Therefore, the degree of chronic inflammation in chronic kidney diseases (CKD) and CHF could predict MACEs and ACM. 23
There are a variety of mechanisms inducing chronic low‐grade inflammation of HF with renal dysfunction, including sympathetic activation and other hormonal effects induced by stress responses, visceral oedema due to water and sodium retention and vasodilation, chronic activation of the adaptive immune system, activation of endothelial cells and oxidative stress factors, and so on. 24 It has been suggested that the innate and adaptive immune systems play an important role in the bidirectional pathway between HF and chronic renal dysfunction. 25 Another study showed that 26 during the healing phase of myocardial necrosis occurring CHF progression, the initiation of the innate immune system leads to the activation of T lymphocytes and antigen‐presenting cells, together with the mobilization and infiltration of proinflammatory monocytes and macrophages into necrotic myocardial sites, promoting myocardial remodelling. Myocardial remodelling can lead to decreased cardiac output, decreased heart function, and further decreased renal function. Hence, chronic low‐grade inflammation in HF with renal dysfunction can activate a harmful immune response to the body, resulting in cardiac and renal impairment.
In patients with co‐morbidity of HF and renal, angiotensinogen (Ang) II level elevates, which increases the levels of reactive oxygen species by promoting the activity of NADH and NADPH oxidase, resulting in the enhanced oxidative damage response, thereby promoting oxidative damage and endothelial dysfunction. 27 Therefore, chronic inflammation and associated changes in cellular immunity play a key role in driving vascular lesions and tissue remodelling in the heart and kidney. In addition, the inflammatory responses are also the key factors that accelerate the progression of HF and renal dysfunction.
4.3. The manifestation of SII in inflammation in CHF patients with renal dysfunction
SII could reflect the inflammatory responses and partially indicate the overall immune system. Given the close correlation between inflammatory response and heart failure with renal dysfunction, and the SII level indicating an enhanced inflammatory response and weakened immune response, the SII level could be used to predict the prognosis of heart failure with renal dysfunction. SII was calculated based on three peripheral blood cell counts: neutrophil, platelet and lymphocyte count, This ratio was first proposed by Hu et al., 28 who pointed out that SII is a strong predictor of poor prognosis in patients with hepatocellular carcinoma (HCC) after liver resection, it has been widely used in coronary atherosclerosis, and oesophageal cancer, 29 non‐small‐cell lung cancer, 30 osteosarcoma and other malignant tumours. 31 Despite its popularity as a biomarker, the correlation between SII and co‐morbidity of CHF and renal insufficiency remains elusive.
As the SII reflects the neutrophil, platelet and lymphocyte, the elevated SII indicates the increased number of neutrophils and platelets, as well as the reduced lymphocytes. In fact, the infiltration of neutrophils has been observed in the development of cardiac disease evidenced by the Physicians' Health Study. 32 A significant amount of neutrophils was found in myocardial tissue in an animal model of HF. 33 , 34 Numerous studies, such as the Canakinumab Anti‐inflammatory Thrombosis Outcomes Study (CANTOS) 35 trial, have shown that elevated neutrophils are associated with cardiovascular outcomes, leading to myocardial infarction and cardiac death. Moreover, multiple studies have reported that in CHF, 36 myocarditis, 37 and atrial fibrillation, 38 the serum levels of calcium‐binding proteins A8 and A9 (S100A8/S100A9) derived from neutrophil‐secreted are increased, indicating the upregulation of neutrophil. More importantly, it has been found that neutrophils contribute to tissue damage in the heart and kidney, 39 , 40 due to the release of damage‐associated molecular patterns (DAMPs) from necrotic cells and inflammatory cells. Circulating neutrophils are recruited to the site of injury, resulting in the elevation of neutrophil levels and the formation of neutrophil extracellular traps (NETs) 41 and expansion loops. At the same time, the neutrophil can also accelerate the aggregation of platelets and promote thrombosis, causing coronary and renal vasospasm to constrict, affecting myocardial contractility and renal function, leading to HF and renal dysfunction. 42 In addition, the study conducted by Nakazawa et al. 40 demonstrated that NETs induced damage to remote organs such as the heart during renal ischemia/reperfusion injury (IRI). It has also been shown that neutrophils can migrate outside the blood vessels and then return to the bloodstream, 43 , 44 , 45 an unexpected ability known as reverse migration or abluminal‐to‐luminal migration. This migration was first observed by intravital microscopy during IRI, which may also be a possibility for elevated circulating neutrophils following cardiorenal inflammation.
Apart from the upregulated neutrophils, the increased amount of platelet is another contributor to the SII elevation. The activation and adhesion of platelets have been widely reported in CVD patients. For example, Mehta et al. 46 first reported that patients with heart failure had significantly more circulating platelet (PLT) aggregates than the normal population in 1979. Elevated levels of PLT are associated with atherosclerosis, coronary artery disease (CAD), cerebrovascular disease (CD), and systemic inflammatory diseases, 47 , 48 , 49 which play a key role in the pathophysiology of HF. 50 , 51 , 52 , 53 Studies have shown that high activity of PLT can independently predict cardiac death and adverse cardiovascular events in patients with CAD, 50 PDW levels, which reflect PLT activity, 54 , 55 and can predict the occurrence of cardiac death, coronary infarction recurrence, and repeat revascularization. 56 Apart from the CADs, the upregulation of platelet is also identified in the renal dysfunction patients. For instance, Xie et al. 57 found that patients with chronic kidney disease had elevated levels of the platelet activity marker CD40L compared with healthy controls. More studies have shown that the coagulation curve of patients with renal dysfunction shows normal or elevated levels of coagulation factors, 58 suggesting that platelets may be elevated in patients with renal insufficiency.
In addition, severe alterations induced by the HF also contribute to the reduced lymphocytes, resulting in the increased SII level. Firstly, the elevated level of endogenous cortisol hormone caused by CHD‐induced inflammation leads to the development of immune disorders such as lymphopaenia. More importantly, chronic inflammation also aggravates the apoptosis of lymphocytes. 59 Both result in a declined number of lymphocytes. For instance, a 36 month follow‐up study of 549 patients with ST‐elevation myocardial infarction showed patients with reduced lymphocytes within 96 h of admission had an increased risk of recurrence of adverse cardiovascular events. 60
Taken together, SII, as a biomarker, has an enhanced predictive value as compared to a single leukocyte sub‐type indicator, due to its capability to reflect two different and complementary immune pathways in patients with co‐morbidity of CHF and renal insufficiency. It can indicate the balance state of platelets and lymphocytes in the body together with coagulation function and systemic immune response. More importantly, it is relatively consistent despite the alteration in physiological conditions, making it a perfect biomarker. The study successfully proved that SII level was an independent risk factor for mortality and MACEs events in CHF patients with renal insufficiency, which would be beneficial for clinical management. That being said, because this study was a retrospective and single‐centre study, multi‐centre studies are required for further validation.
5. Conclusions
In summary, we demonstrated that SII levels are an independent risk factor for mortality and MACEs events in advanced CHF with renal dysfunction. More important, it is sensitive and reliable with great clinical potential.
Conflict of interest
None declared.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82170281, 82222007 and U2004203), the Henan Thousand Talents Program (No. ZYQR201912131), Excellent Youth Science Foundation of Henan Province (No. 202300410362), New Zealand Health Research Council (Explorer grant 19/779), New Zealand Ministry for Business, Employment and Innovation (MBIE Science Whitinga Fellowship, MWF‐UOO2103), National Heart Foundation of New Zealand (1896 and 1891), The Chinese University of Hong Kong (Shenzhen) startup funding (K10120220254), and Henan Province Medical Science and Technology Key Joint Project (SBGJ202101012).
Acknowledgement
The authors wish to acknowledge the assistance of the First Affiliated Hospital of Zhengzhou University with the data extraction.
Wang, Z. , Qin, Z. , Yuan, R. , Guo, J. , Xu, S. , Lv, Y. , Xu, Y. , Lu, Y. , Gao, J. , Yu, F. , Tang, L. , Zhang, L. , Bai, J. , Cui, X. , Zhang, J. , and Tang, J. (2023) Systemic immune‐inflammation index as a prognostic marker for advanced chronic heart failure with renal dysfunction. ESC Heart Failure, 10: 478–491. 10.1002/ehf2.14217.
Contributor Information
Xiaolin Cui, Email: stevencui@cuhk.edu.cn.
Jinying Zhang, Email: jyzhang@zzu.edu.cn.
Junnan Tang, Email: fcctangjn@zzu.edu.cn.
References
- 1. Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta‐analysis. Eur Heart J. 2014; 35: 455–469. [DOI] [PubMed] [Google Scholar]
- 2. Patel RB, Mehta R, Redfield MM, Borlaug BA, Hernandez AF, Shah SJ, Dubin RF. Renal dysfunction in heart failure with preserved ejection fraction: insights from the RELAX trial. J Card Fail. 2020; 26: 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Givertz MM, Postmus D, Hillege HL, Mansoor GA, Massie BM, Davison BA, Ponikowski P, Metra M, Teerlink JR, Cleland JGF, Dittrich HC, O’Connor CM, Cotter G, Voors AA. Renal function trajectories and clinical outcomes in acute heart failure. Circ Heart Fail. 2014; 7: 59–67. [DOI] [PubMed] [Google Scholar]
- 4. Bagshaw SM, Cruz DN, Aspromonte N, Daliento L, Ronco F, Sheinfeld G, Anker SD, Anand I, Bellomo R, Berl T, Bobek I, Davenport A, Haapio M, Hillege H, House A, Katz N, Maisel A, Mankad S, McCullough P, Mebazaa A, Palazzuoli A, Ponikowski P, Shaw A, Soni S, Vescovo G, Zamperetti N, Zanco P, Ronco C, for the Acute Dialysis Quality Initiative (ADQI) Consensus Group . Epidemiology of cardio‐renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant. 2010; 25: 1406–1416. [DOI] [PubMed] [Google Scholar]
- 5. House AA, Anand I, Bellomo R, Cruz D, Bobek I, Anker SD, Aspromonte N, Bagshaw S, Berl T, Daliento L, Davenport A, Haapio M, Hillege H, McCullough P, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P, Ronco C, for the Acute Dialysis Quality Initiative (ADQI) consensus group . Definition and classification of cardio‐renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant. 2010; 25: 1416–1420. [DOI] [PubMed] [Google Scholar]
- 6. Hanna A, Frangogiannis NG. Inflammatory cytokines and chemokines as therapeutic targets in heart failure. Cardiovasc Drugs Ther. 2020; 34: 849–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010; 10: 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang BL, Tian L, Gao XH, Ma XL, Wu J, Zhang CY, Zhou Y, Guo W, Yang XR. Dynamic change of the systemic immune inflammation index predicts the prognosis of patients with hepatocellular carcinoma after curative resection. Clin Chem Lab Med. 2016; 54: 1963–1969. [DOI] [PubMed] [Google Scholar]
- 9. Mozos I, Malainer C, Horbańczuk J, Gug C, Stoian D, Luca CT, Atanasov AG. Inflammatory markers for arterial stiffness in cardiovascular diseases. Front Immunol. 2017; 8: 1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group , de Boer RA, Christian Schulze P, Abdelhamid M, Aboyans V, Adamopoulos S, Anker SD, Arbelo E, Asteggiano R, Bauersachs J, Bayes‐Genis A, Borger MA, Budts W, Cikes M, Damman K, Delgado V, Dendale P, Dilaveris P, Drexel H, Ezekowitz J, Falk V, Fauchier L, Filippatos G, Fraser A, Frey N, Gale CP, Gustafsson F, Harris J, Iung B, Janssens S, Jessup M, Konradi A, Kotecha D, Lambrinou E, Lancellotti P, Landmesser U, Leclercq C, Lewis BS, Leyva F, Linhart A, Løchen ML, Lund LH, Mancini D, Masip J, Milicic D, Mueller C, Nef H, Nielsen JC, Neubeck L, Noutsias M, Petersen SE, Sonia Petronio A, Ponikowski P, Prescott E, Rakisheva A, Richter DJ, Schlyakhto E, Seferovic P, Senni M, Sitges M, Sousa‐Uva M, Tocchetti CG, Touyz RM, Tschoepe C, Waltenberger J, Adamo M, Baumbach A, Böhm M, Burri H, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gardner RS, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Piepoli MF, Price S, Rosano GMC, Ruschitzka F, Skibelund AK. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 11. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, ESC Scientific Document Group , de Backer G, Heagerty AM, Agewall S, Bochud M, Borghi C, Boutouyrie P, Brguljan J, Bueno H, Caiani EG, Carlberg B, Chapman N, Cífková R, Cleland JGF, Collet JP, Coman IM, de Leeuw PW, Delgado V, Dendale P, Diener HC, Dorobantu M, Fagard R, Farsang C, Ferrini M, Graham IM, Grassi G, Haller H, Hobbs FDR, Jelakovic B, Jennings C, Katus HA, Kroon AA, Leclercq C, Lovic D, Lurbe E, Manolis AJ, McDonagh TA, Messerli F, Muiesan ML, Nixdorff U, Olsen MH, Parati G, Perk J, Piepoli MF, Polonia J, Ponikowski P, Richter DJ, Rimoldi SF, Roffi M, Sattar N, Seferovic PM, Simpson IA, Sousa‐Uva M, Stanton AV, van de Borne P, Vardas P, Volpe M, Wassmann S, Windecker S, Zamorano JL, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet JP, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh TA, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Sousa‐Uva M, Zamorano JL, Tsioufis C, Lurbe E, Kreutz R, Bochud M, Rosei EA, Jelakovic B, Azizi M, Januszewics A, Kahan T, Polonia J, van de Borne P, Williams B, Borghi C, Mancia G, Parati G, Clement DL, Coca A, Manolis A, Lovic D, Benkhedda S, Zelveian P, Siostrzonek P, Najafov R, Pavlova O, de Pauw M, Dizdarevic‐Hudic L, Raev D, Karpettas N, Linhart A, Olsen MH, Shaker AF, Viigimaa M, Metsärinne K, Vavlukis M, Halimi JM, Pagava Z, Schunkert H, Thomopoulos C, Páll D, Andersen K, Shechter M, Mercuro G, Bajraktari G, Romanova T, Trušinskis K, Saade GA, Sakalyte G, Noppe S, DeMarco DC, Caraus A, Wittekoek J, Aksnes TA, Jankowski P, Polonia J, Vinereanu D, Baranova EI, Foscoli M, Dikic AD, Filipova S, Fras Z, Bertomeu‐Martínez V, Carlberg B, Burkard T, Sdiri W, Aydogdu S, Sirenko Y, Brady A, Weber T, Lazareva I, Backer TD, Sokolovic S, Jelakovic B, Widimsky J, Viigimaa M, Pörsti I, Denolle T, Krämer BK, Stergiou GS, Parati G, Trušinskis K, Miglinas M, Gerdts E, Tykarski A, de Carvalho Rodrigues M, Dorobantu M, Chazova I, Lovic D, Filipova S, Brguljan J, Segura J, Gottsäter A, Pechère‐Bertschi A, Erdine S, Sirenko Y, Brady A. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018; 39: 3021–3104. [DOI] [PubMed] [Google Scholar]
- 12. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa‐Uva M, Valensi P, Wheeler DC, ESC Scientific Document Group , Piepoli MF, Birkeland KI, Adamopoulos S, Ajjan R, Avogaro A, Baigent C, Brodmann M, Bueno H, Ceconi C, Chioncel O, Coats A, Collet JP, Collins P, Cosyns B, di Mario C, Fisher M, Fitzsimons D, Halvorsen S, Hansen D, Hoes A, Holt RIG, Home P, Katus HA, Khunti K, Komajda M, Lambrinou E, Landmesser U, Lewis BS, Linde C, Lorusso R, Mach F, Mueller C, Neumann FJ, Persson F, Petersen SE, Petronio AS, Richter DJ, Rosano GMC, Rossing P, Rydén L, Shlyakhto E, Simpson IA, Touyz RM, Wijns W, Wilhelm M, Williams B, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa‐Uva M, Valensi P, Wheeler DC, Windecker S, Aboyans V, Baigent C, Collet JP, Dean V, Delgado V, Fitzsimons D, Gale CP, Grobbee DE, Halvorsen S, Hindricks G, Iung B, Jüni P, Katus HA, Landmesser U, Leclercq C, Lettino M, Lewis BS, Merkely B, Mueller C, Petersen SE, Petronio AS, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Sousa‐Uva M, Touyz RM, Zelveian PH, Scherr D, Jahangirov T, Lazareva I, Shivalkar B, Naser N, Gruev I, Milicic D, Petrou PM, Linhart A, Hildebrandt P, Hasan‐Ali H, Marandi T, Lehto S, Mansourati J, Kurashvili R, Siasos G, Lengyel C, Thrainsdottir IS, Aronson D, di Lenarda A, Raissova A, Ibrahimi P, Abilova S, Trusinskis K, Saade G, Benlamin H, Petrulioniene Z, Banu C, Magri CJ, David L, Boskovic A, Alami M, Liem AH, Bosevski M, Svingen GFT, Janion M, Gavina C, Vinereanu D, Nedogoda S, Mancini T, Ilic MD, Fabryova L, Fras Z, Jiménez‐Navarro MF, Norhammar A, Lehmann R, Mourali MS, Ural D, Nesukay E, Chowdhury TA. 2019 ESC Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020; 41: 255–323. [DOI] [PubMed] [Google Scholar]
- 13. van Deursen VM, Urso R, Laroche C, Damman K, Dahlström U, Tavazzi L, Maggioni AP, Voors AA. Co‐morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail. 2014; 16: 103–111. [DOI] [PubMed] [Google Scholar]
- 14. Whitman IR, Feldman HI, Deo R. CKD and sudden cardiac death: epidemiology, mechanisms, and therapeutic approaches. J Am Soc Nephrol. 2012; 23: 1929–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rangaswami J, Bhalla V, Blair J, Chang TI, Costa S, Lentine KL, Lerma EV, Mezue K, Molitch M, Mullens W, Ronco C, Tang WHW, McCullough P, American Heart Association Council on the Kidney in Cardiovascular Disease and Council on Clinical Cardiology . Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2019; 139: e840–e878. [DOI] [PubMed] [Google Scholar]
- 16. Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WHW. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009; 53: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009; 53: 582–588. [DOI] [PubMed] [Google Scholar]
- 18. Murata A, Kasai T, Matsue Y, Matsumoto H, Yatsu S, Kato T, Suda S, Hiki M, Takagi A, Daida H. Relationship between blood urea nitrogen‐to‐creatinine ratio at hospital admission and long‐term mortality in patients with acute decompensated heart failure. Heart Vessels. 2018; 33: 877–885. [DOI] [PubMed] [Google Scholar]
- 19. Ellison DH, Felker GM. Diuretic treatment in heart failure. N Engl J Med. 2017; 377: 1964–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oppermann M, Hansen PB, Castrop H, Schnermann J. Vasodilatation of afferent arterioles and paradoxical increase of renal vascular resistance by furosemide in mice. Am J Physiol Renal Physiol. 2007; 293: F279–F287. [DOI] [PubMed] [Google Scholar]
- 21. Machowska A, Carrero JJ, Lindholm B, Stenvinkel P. Therapeutics targeting persistent inflammation in chronic kidney disease. Transl Res. 2016; 167: 204–213. [DOI] [PubMed] [Google Scholar]
- 22. von Haehling S, Schefold JC, Lainscak M, Doehner W, Anker SD. Inflammatory biomarkers in heart failure revisited: much more than innocent bystanders. Heart Fail Clin. 2009; 5: 549–560. [DOI] [PubMed] [Google Scholar]
- 23. Colombo PC, Ganda A, Lin J, Onat D, Harxhi A, Iyasere JE, Uriel N, Cotter G. Inflammatory activation: cardiac, renal, and cardio‐renal interactions in patients with the cardiorenal syndrome. Heart Fail Rev. 2012; 17: 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schefold JC, Zeden JP, Fotopoulou C, von Haehling S, Pschowski R, Hasper D, Volk HD, Schuett C, Reinke P. Increased indoleamine 2,3‐dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: a possible link between chronic inflammation and uraemic symptoms. Nephrol Dial Transplant. 2009; 24: 1901–1908. [DOI] [PubMed] [Google Scholar]
- 25. Hoffmann J, Shmeleva EV, Boag SE, Fiser K, Bagnall A, Murali S, Dimmick I, Pircher H, Martin‐Ruiz C, Egred M, Keavney B, von Zglinicki T, Das R, Todryk S, Spyridopoulos I. Myocardial ischemia and reperfusion leads to transient CD8 immune deficiency and accelerated immunosenescence in CMV‐seropositive patients. Circ Res. 2015; 116: 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res. 2014; 114: 266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Münzel T, Gori T, Keaney JF Jr, Maack C, Daiber A. Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur Heart J. 2015; 36: 2555–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J, Fan J. Systemic immune‐inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014; 20: 6212–6222. [DOI] [PubMed] [Google Scholar]
- 29. Zhang H, Shang X, Ren P, Gong L, Ahmed A, Ma Z, Ma R, Wu X, Xiao X, Jiang H, Tang P, Yu Z. The predictive value of a preoperative systemic immune‐inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol. 2019; 234: 1794–1802. [DOI] [PubMed] [Google Scholar]
- 30. Yan X, Li G. Preoperative systemic immune‐inflammation index predicts prognosis and guides clinical treatment in patients with non‐small cell lung cancer. Biosci Rep. 2020; 40: BSR20200352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang X, Hu H, Zhang W, Shao Z. Prognostic value of prognostic nutritional index and systemic immune‐inflammation index in patients with osteosarcoma. J Cell Physiol. 2019; 234: 18408–18414. [DOI] [PubMed] [Google Scholar]
- 32. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen J, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ, JUPITER Study Group . Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. N Engl J Med. 2008; 359: 2195–2207. [DOI] [PubMed] [Google Scholar]
- 33. Wang Y, Sano S, Oshima K, Sano M, Watanabe Y, Katanasaka Y, Yura Y, Jung C, Anzai A, Swirski FK, Gokce N, Walsh K. Wnt5a‐mediated neutrophil recruitment has an obligatory role in pressure overload‐induced cardiac dysfunction. Circulation. 2019; 140: 487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Valiente‐Alandi I, Potter SJ, Salvador AM, Schafer AE, Schips T, Carrillo‐Salinas F, Gibson AM, Nieman ML, Perkins C, Sargent MA, Huo J, Lorenz JN, DeFalco T, Molkentin JD, Alcaide P, Blaxall BC. Inhibiting fibronectin attenuates fibrosis and improves cardiac function in a model of heart failure. Circulation. 2018; 138: 1236–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida‐Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017; 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- 36. Jensen LJ, Kistorp C, Bjerre M, Raymond I, Flyvbjerg A. Plasma calprotectin levels reflect disease severity in patients with chronic heart failure. Eur J Prev Cardiol. 2012; 19: 999–1004. [DOI] [PubMed] [Google Scholar]
- 37. Ndumele CE, Coresh J, Lazo M, Hoogeveen RC, Blumenthal RS, Folsom AR, Selvin E, Ballantyne CM, Nambi V. Obesity, subclinical myocardial injury, and incident heart failure. JACC Heart Fail. 2014; 2: 600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Müller I, Vogl T, Kühl U, Krannich A, Banks A, Trippel T, Noutsias M, Maisel AS, Linthout S, Tschöpe C. Serum alarmin S100A8/S100A9 levels and its potential role as biomarker in myocarditis. ESC Heart Fail. 2020; 7: 1442–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nakazawa D, Kumar SV, Marschner J, Desai J, Holderied A, Rath L, Kraft F, Lei Y, Fukasawa Y, Moeckel GW, Angelotti ML, Liapis H, Anders HJ. Histones and neutrophil extracellular traps enhance tubular necrosis and remote organ injury in ischemic AKI. J Am Soc Nephrol. 2017; 28: 1753–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ling S, Xu JW. NETosis as a pathogenic factor for heart failure. Oxid Med Cell Longev. 2021; 2021: 6687096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004; 303: 1532–1535. [DOI] [PubMed] [Google Scholar]
- 42. Reichlin T, Socrates T, Egli P, Potocki M, Breidthardt T, Arenja N, Meissner J, Noveanu M, Reiter M, Twerenbold R, Schaub N, Buser A, Mueller C. Use of myeloperoxidase for risk stratification in acute heart failure. Clin Chem. 2010; 56: 944–951. [DOI] [PubMed] [Google Scholar]
- 43. Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, Nash GB, Chavakis T, Albelda SM, Rainger GE, Meda P, Imhof BA, Nourshargh S. The junctional adhesion molecule JAM‐C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol. 2011; 12: 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Colom B, Bodkin JV, Beyrau M, Woodfin A, Ody C, Rourke C, Chavakis T, Brohi K, Imhof BA, Nourshargh S. Leukotriene B4‐neutrophil elastase axis drives neutrophil reverse transendothelial cell migration in vivo. Immunity. 2015; 42: 1075–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Robertson AL, Holmes GR, Bojarczuk AN, Burgon J, Loynes CA, Chimen M, Sawtell AK, Hamza B, Willson J, Walmsley SR, Anderson SR, Coles MC, Farrow SN, Solari R, Jones S, Prince LR, Irimia D, Rainger GE, Kadirkamanathan V, Whyte MK, Renshaw SA. A zebrafish compound screen reveals modulation of neutrophil reverse migration as an anti‐inflammatory mechanism. Sci Transl Med. 2014; 6: 225ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mehta J, Mehta P. Platelet function studies in heart disease. VI. Enhanced platelet aggregate formation activity in congestive heart failure: inhibition by sodium nitroprusside. Circulation. 1979; 60: 497–503. [DOI] [PubMed] [Google Scholar]
- 47. Li B, Lu J, Peng DZ, Zhang XY, You Z. Elevated platelet distribution width predicts poor prognosis in hilar cholangiocarcinoma. Medicine (Baltimore). 2020; 99: e19400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Soydinc S, Turkbeyler IH, Pehlivan Y, Soylu G, Goktepe MF, Bilici M, Zengin O, Kisacik B, Onat AM. Mean platelet volume seems to be a valuable marker in patients with systemic sclerosis. Inflammation. 2014; 37: 100–106. [DOI] [PubMed] [Google Scholar]
- 49. Chandrashekar L, Rajappa M, Revathy G, Sundar I, Munisamy M, Ananthanarayanan PH, Thappa DM, Basu D. Is enhanced platelet activation the missing link leading to increased cardiovascular risk in psoriasis? Clin Chim Acta. 2015; 446: 181–185. [DOI] [PubMed] [Google Scholar]
- 50. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC [published correction appears in Eur Heart J. 2016 Dec 30;]. Eur Heart J. 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 51. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride P, McMurray J, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation , American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 52. Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990; 323: 236–241. [DOI] [PubMed] [Google Scholar]
- 53. Ferrari R, Bachetti T, Confortini R, Opasich C, Febo O, Corti A, Cassani G, Visioli O. Tumor necrosis factor soluble receptors in patients with various degrees of congestive heart failure. Circulation. 1995; 92: 1479–1486. [DOI] [PubMed] [Google Scholar]
- 54. Rechciński T, Jasińska A, Foryś J, Krzemińska‐Pakuła M, Wierzbowska‐Drabik K, Plewka M, Peruga JZ, Kasprzak JD. Prognostic value of platelet indices after acute myocardial infarction treated with primary percutaneous coronary intervention. Cardiol J. 2013; 20: 491–498. [DOI] [PubMed] [Google Scholar]
- 55. Larsen SB, Grove EL, Hvas AM, Kristensen SD. Platelet turnover in stable coronary artery disease—influence of thrombopoietin and low‐grade inflammation. PLoS ONE. 2014; 9: e85566. Published 2014 Jan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hu CP, Du Y, Zhu Y, Shi C, Qin Z, Zhao YX. Platelet distribution width on admission predicts in‐stent restenosis in patients with coronary artery disease and type 2 diabetes mellitus treated with percutaneous coronary intervention. Chin Med J (Engl). 2018; 131: 757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xie JX, Alderson H, Ritchie J, Kalra PA, Xie Y, Ren K, Nguyen H, Chen T, Brewster P, Gupta R, Dworkin LD, Malhotra D, Cooper CJ, Tian J, Haller ST. Circulating CD40 and sCD40L predict changes in renal function in subjects with chronic kidney disease. Sci Rep. 2017; 7: 7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stach K, Karb S, Akin I, Borggrefe M, Krämer B, Kälsch T, Kälsch AI. Elevation of platelet and monocyte activity markers of atherosclerosis in haemodialysis patients compared to peritoneal dialysis patients. Mediators Inflamm. 2017; 2017: 8506072–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Targher G, Dauriz M, Tavazzi L, Temporelli PL, Lucci D, Urso R, Lecchi G, Bellanti G, Merlo M, Rossi A, Maggioni AP, IN‐HF Outcome Investigators . Prognostic impact of in‐hospital hyperglycemia in hospitalized patients with acute heart failure: Results of the IN‐HF (Italian Network on Heart Failure) Outcome registry. Int J Cardiol. 2016; 203: 587–593. [DOI] [PubMed] [Google Scholar]
- 60. Núñez J, Núñez E, Miñana G, Sanchis J, Bodí V, Rumiz E, Palau P, Olivares M, Merlos P, Bonanad C, Mainar L, Llàcer A. Effectiveness of the relative lymphocyte count to predict one‐year mortality in patients with acute heart failure. Am J Cardiol. 2011; 107: 1034–1039. [DOI] [PubMed] [Google Scholar]


