Abstract
Aims
Limited data are available on the outcomes of cryoballoon ablation (CBA)‐based pulmonary vein isolation (PVI) for atrial fibrillation (AF) in patients with heart failure (HF) with preserved ejection fraction (HFpEF) and mildly reduced ejection fraction (HFmrEF). The present study aimed to evaluate the safety and effectiveness of CBA in such patients.
Methods and results
Consecutive patients with AF referred for CBA‐based PVI from two highly experienced electrophysiology centres were included in this retrospective study. Of 651 patients undergoing CBA, 471 cases were divided into four groups: No HF (n = 255), HFpEF (n = 101), HFmrEF (n = 78), and HF with reduced ejection fraction (n = 37). Similar early recurrence of atrial arrhythmia was found among groups (16.2% vs. 15.4% vs. 14.9% vs. 12.2%, P = 0.798), and no significant difference of long‐term sinus rhythm (SR) maintenance was identified among the HFmrEF, HFpEF, and No HF groups (71.8% vs. 75.2% vs. 79.6%, P = 0.334). CBA is safe for patients with HFmrEF and HFpEF with similar complications compared with the No HF group (3.8% vs. 4.0% vs. 3.1%, P = 0.814). The reassessment of cardiac function after CBA showed that patients with HF indicated beneficial outcomes. Left atrial diameter (LAD) and left ventricular ejection fraction were significantly improved in the HFmrEF group. There were 41.6% of patients in the HFpEF group who were completely relieved from HF. LAD and New York Heart Association (NYHA) were associated with recurrence in the HFpEF and HFmrEF groups, and the maintenance of SR was an independent predictor of NYHA improvement for all HF groups.
Conclusions
Patients with HFmrEF and HFpEF could benefit from CBA with high SR maintenance and significant HF improvement.
Keywords: Atrial fibrillation, Cryoballoon ablation, Heart failure with preserved ejection fraction, Heart failure with mildly reduced ejection fraction, Safety and effectiveness
Introduction
According to the international guidelines, heart failure (HF) is classified into three subtypes: HF with reduced left ventricular ejection fraction (LVEF) (HFrEF; EF < 40%), HF with preserved LVEF (HFpEF; EF > 50%), and HF with mildly reduced LVEF (HFmrEF; EF 40–49%). 1 Atrial fibrillation (AF) and HF frequently coexist. 2 AF is the most common arrhythmia in patients with HF, affecting approximately 33% of patients, and HF predisposes AF and vice versa. 3 , 4 , 5 Hitherto, the most effective management for patients with AF and HF is yet to be clarified. 6
Catheter ablation (CA) is a well‐established option for AF, which mainly includes radiofrequency ablation (RFA) and second‐generation cryoballoon ablation (CBA). 7 Several studies have shown that RFA reduces mortality and hospitalization for AF patients with HFrEF. 8 , 9 Although data are sparse, some studies demonstrated that RFA is safe and effective for AF patients with HFpEF. 10 , 11 In contrast to RFA, data from CBA for patients with HF are limited, especially for patients with HFpEF and HFmrEF 12 ; however, CBA has some potential advantages for HF patients, such as no additional fluid burden caused by irrigation catheter, shorter procedure time, and fewer complications. Therefore, a study evaluating the CBA procedure characteristics and outcomes in patients with HFpEF and HFmrEF is necessary.
This multicentre retrospective study was performed to evaluate CBA procedural characteristics and long‐term outcomes in patients with HFmrEF and HFpEF, and for the first time, factors of HF remission and recurrence of AF in patients with HFmrEF and HFpEF after CBA are predicted.
Methods
Data source
Data of all patients obtained from two experienced electrophysiology (EP) centres by three operators in China (Zhongshan Hospital Affiliated Fudan University and Hangzhou First People's Hospital) from July 2018 to August 2021 were analysed retrospectively.
The inclusion criteria included (i) patients with drug‐resistant paroxysmal AF (PAF) or persistent AF (PerAF) who underwent CBA‐based pulmonary vein isolation (PVI) for the first time; (ii) HF diagnosed by cardiologists at each institution plus a documented history of HF. HF was defined as having symptoms of HF [New York Heart Association (NYHA) Class II–IV], a history of hospitalization for HF, and elevated levels of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) (>125 pg/mL). Transthoracic echocardiography (TTE) was performed prior to the procedure and HF was stratified by LVEF as HFrEF (<40%), HFmrEF (40–49%), and HFpEF (≥50%) LVEF 1 ; (iii) all available patients completed the follow‐up. The exclusion criteria included (i) redo ablation; (ii) additional RFA required after CBA; (iii) a lack of TTE baseline data; and (iv) unable to complete the corresponding follow‐up.
Data were abstracted by two investigators initially blinded to the hypotheses. Random samples were cross‐checked to verify the accuracy of data abstraction. All patients provided written informed consent. The study was approved by the local ethics board and performed in accordance with the ethical standards of the Declaration of Helsinki and its later amendments.
Pre‐procedural management
The pre‐procedural management has been described previously. 13 Briefly, transoesophageal echocardiography (TEE) and TTE were performed prior to the procedure to rule out left atrial (LA) thrombus and assess LVEF and LA diameter (LAD). Vitamin K antagonists (VKAs) were administered uninterruptedly to a target international normalized ratio (INR) of 2.0–2.5 at the time of the procedure. In patients treated with novel oral anticoagulants (NOACs), the drug was discontinued ≤24 h prior to the procedure.
Cryoballoon ablation procedure
The choice of ablation technique was based on the discretion of the operating electrophysiologists, and there were no significant differences in patient characteristics for choosing CBA or RFA. In this retrospective study, we only included patients who received CBA. Similar procedure protocol and sheaths were applied for all patients. All procedures were performed under deep sedation using fentanyl and propofol. A total of four sheaths were used for procedures [an 8.5 Fr sheath was inserted from the right femoral vein for transseptal puncture, the long sheath was removed after transseptal puncture, and a 15 Fr steerable sheath (FlexCath, Medtronic, Minneapolis, MN, USA) was inserted from the original route; two 6 Fr short sheaths were inserted from the left femoral vein for placement of the quadripolar catheter and coronary sinus catheter]. Then, a CB‐2 (Arctic Front Advance, Medtronic) was introduced into the sheath, inflated, and advanced to the ostium of each pulmonary vein (PV). The PV occlusion was assessed by venous angiography. Optimal vessel occlusion was achieved when selective contrast injection showed total contrast retention without backflow to the atrium. After the occlusion was documented, ablation was performed with at least two applications per vein, each for 150–180 s. The target temperature for cryo‐applications was −45°C to −55°C, and the application would be terminated if the temperature exceeds −55°C. If the temperature could reach −40°C within 60 s, the application time was 150 s; otherwise, the application would be last for 180 s. However, in case of an inadequate temperature drop (not reaching −36°C at 60 s), the application was also terminated. The PV activity was recorded using the circular Achieve catheter (Achieve, Medtronic) at a proximal site in the ostium prior to ablation in each vein. During the ablation of right PVs, a quadripolar catheter was inserted in the superior vena cava to monitor phrenic nerve palsy (PNP) by pacing the right phrenic nerve with a 1500 ms cycle and a 20 mA output. The freezing cycle was terminated immediately after a loss of capture or the strength of the right hemidiaphragmatic contractions was attenuated. No additional RFAs were applied. The Achieve catheter was reintroduced, and the bidirectional block was checked with a waiting period of 20 min after the last application. After isolation, if the AF did not convert to sinus rhythm (SR), external electrical cardioversion (ECv) was performed. Heparin was administered after a transseptal puncture to maintain an activated clotting time of ≥300 s. 13 All sheaths were removed after procedure, and compression haemostasis was routinely used, followed by bandages and gauze.
Post‐procedural management
Following ablation, all patients underwent TTE to rule out pericardial effusion. Low‐molecular‐weight heparin was administered to patients on VKAs and an INR < 2.0 until a therapeutic INR of 2–3 was achieved. NOACs were re‐initiated 6 h after ablation. Anticoagulation was recommended for at least 3 months and thereafter according to the individual CHA2DS2‐VASc scores. Previously, anti‐arrhythmic drugs were continued for 3 months after ablation. All HF patients were treated with optimal medical treatment according to the latest guidelines. 12
Follow‐up
The patients were scheduled for follow‐up visits with a 12‐lead electrocardiogram (ECG) and 24 h Holter monitoring at 1, 3, 6, and 12 months after CBA and thereafter every 6 months. The HF parameters, including TTE and NYHA, were compared in each group at the 12 month follow‐up. Experienced technicians blinded to the clinical data performed TTE and recorded the parameters, LAD and LVEF. Additional telephonic interviews were conducted regularly. In the case of symptoms suggesting recurrent arrhythmia, additional visits were recommended.
Endpoints
The primary endpoint was atrial arrhythmia recurrence after CBA. The secondary endpoints were early recurrence of atrial arrhythmia (ERAA), in‐hospital adverse events, and NYHA functional classification improvement at the 12 month follow‐up. Procedural characteristics included procedural time and fluoroscopy time. The in‐hospital adverse events included access site complications that included access site bleeding, groin hematoma, groin pseudoaneurysm, and groin arteriovenous fistula, acute HF (AHF), atrioesophageal fistula, PV stenosis, pericardial tamponade, acute stroke or transient ischaemic attack (TIA), and in‐hospital death. 14 ERAA in the hospital is defined as any documented episode of AF or atrial tachycardia lasting for >30 s after ablation during hospitalization. 13 Atrial arrhythmia recurrence is defined as any symptomatic or asymptomatic atrial arrhythmia lasting >30 s after completion of the blanking period (3 months) after CBA. 15
Statistical analysis
Continuous data were expressed as mean ± standard deviation (M ± SD) for normally distributed variables or as the median (25th, 75th percentiles) for non‐normally distributed variables and compared using a Student's t‐test or Mann–Whitney U test, respectively. Categorical variables were compared using a χ 2 test or Fisher's exact test. The paired test was used to compare pre‐CBA and 12 month post‐CBA variables (NYHA, LVEF, and LAD) in each group. If the comparison of multiple groups (n ≥ 3) suggested significant differences (P < 0.05), further multiple comparisons would be performed to explore the source of the differences. Recurrence‐free survival was estimated using the Kaplan–Meier analysis. The differences in recurrence‐free survival were analysed using the log‐rank test. In order to identify the independent predictors of recurrence and 1 year recovery from HF, univariate analysis was performed first, whereas multivariate analysis was performed with variables that were significant in the univariate analysis (P ≤ 0.05). Because AF recurrence is caused by various factors, multiple models were used for multivariate analysis: In Model 1, not only significant univariate variables but also other important risk factors for AF were included; in Model 2, only significant univariate variables were included. The Cox regression model was utilized to predict the recurrence and logistic regression model to predict the 1 year recovery from HF after CBA. A value of P < 0.05 was considered significant. Statistical analysis was carried out using R Version 4.0.3 (www.r‐project.org) and GraphPad Prism 6 (GraphPad Software, Inc.).
Results
Patient characteristics
Data (age > 85 years, haemodialysis, severe stroke, cancer, redo ablation, combined with RFA during the procedure, and losing follow‐up) of 180/651 patients were excluded. Finally, 471 inpatients were subjected to chart review, procedure notes and logs, clinic notes, and follow‐up data. The detailed process is illustrated in Figure 1 . Subsequently, 37, 78, 101, and 255 patients comprised the HFrEF, HFmrEF, HFpEF, and No HF groups, respectively. No significant difference was detected among the groups in the baseline characteristics. Additionally, HF groups were more likely to have a history of diabetes, prior TIA/stroke, and chronic kidney disease. The detailed baseline characteristics are summarized in Table 1 .
Figure 1.
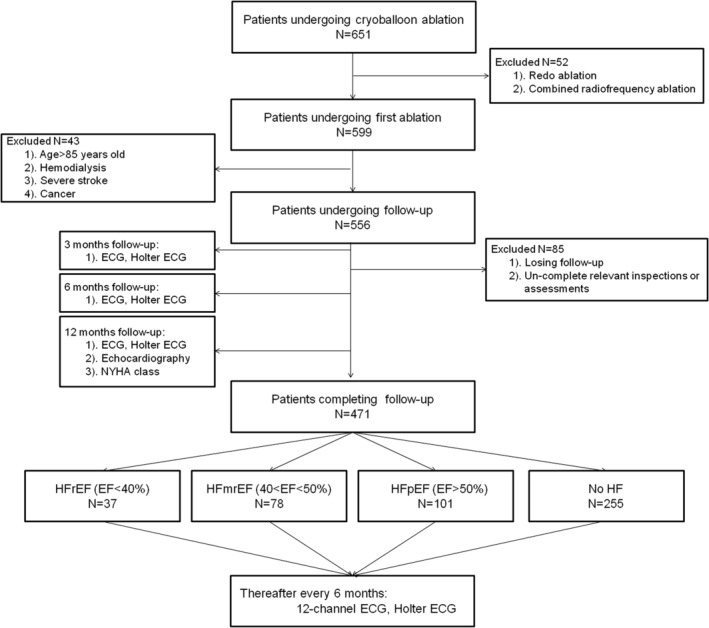
Flow chart illustrating the study population. ECG, electrocardiogram; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; NYHA, New York Heart Association.
Table 1.
Baseline characteristics of the study patients
| Variable | HFrEF | HFmrEF | HFpEF | No HF | P‐value |
|---|---|---|---|---|---|
| Patients (n) | 37 | 78 | 101 | 255 | |
| Age (years) | 68 [62, 75] | 69 [65, 75] | 67 [55, 77] | 64 [53, 76] | 0.062 |
| Male, n (%) | 23 (62.2%) | 48 (61.5%) | 60 (59.4%) | 152 (59.6%) | 0.981 |
| Persistent AF, n (%) | 9 (24.3%) | 17 (21.8%) | 27 (26.7%) | 47 (18.4%) | 0.355 |
| BMI (kg/m2) | 23 [19.5, 25.5] | 24.3 [19.7, 27] | 23.1 [20.2, 26] | 22.6 [20.1, 25.8] | 0.291 |
| Duration of AF (months) | 13 [10, 17] | 11 [8, 15] | 11 [11, 15] | 10 [8, 14] | 0.092 |
| LVEF (%) | 35 [33, 37] | 45 [41, 48] a | 57 [53, 62] a , b | 60 [58, 63] a , b , c | <0.001 |
| LAD (mm) | 46.9 [43.7, 47.8] | 44.5 [42, 48] | 42.5 [38.7, 45.9] a | 43 [39, 45.8] a , b | <0.001 |
| CHA2DS2‐VASc score | 3 [2, 4] | 3 [2, 3] | 2 [2, 3] a | 2 [1, 3] a , b | <0.001 |
| HAS‐BLED score | 2 [1, 2] | 2 [1, 2] | 2 [1, 2] a | 1 [1, 1] a , b | <0.001 |
| NYHA class | 2.73 ± 0.73 | 2.50 ± 0.70 | 2.24 ± 0.47 a | 1.52 ± 0.69 a , b , c | <0.001 |
| NT‐proBNP (pg/mL) | 2265.9 ± 1559.7* | 2100 ± 1218.6* | 1821 ± 970.3* | 54.3 ± 22.6 | <0.001 |
| Follow‐up time (months) | 21.9 ± 7.3 | 21.8 ± 6.8 | 23.7 ± 7.0 | 23.4 ± 6.0 | 0.147 |
| Medical history | |||||
| CAD, n (%) | 15 (40.5) | 28 (35.9) | 30 (29.7) | 81 (31.8) | 0.591 |
| Hypertension, n (%) | 24 (64.9) | 46 (59) | 67 (66.3) | 109 (42.7) c | <0.001 |
| Diabetes, n (%) | 13 (35.1) | 25 (31.6) | 28 (27.7) | 50 (19.6) | 0.037 |
| Prior TIA/stroke, n (%) | 3 (8.1) | 6 (7.7) | 3 (3) | 1 (0.4) a , b | 0.006 |
| CKD, n (%) | 11 (29.7) | 18 (23.1) | 18 (17.8) | 19 (7.5) a , b , c | <0.001 |
| Medications before ablation | |||||
| ACE‐I or ARB, n (%) | 26 (70.2) | 48 (61.5) | 55 (54.5) | 84 (32.9) a , b , c | <0.001 |
| Aldosterone antagonist | 5 (13.5) | 9 (11.5) | 11 (10.9) | 12 (4.7) | 0.049 |
| Diuretics, n (%) | 20 (54.1) | 35 (44.9) | 23 (22.8) a , b | 11 (4.3) a , b , c | <0.001 |
| Nitrates, n (%) | 6 (16.2) | 11 (14.1) | 16 (15.8) | 0 | 0.936 |
| Digoxin, n (%) | 7 (18.9) | 10 (12.8) | 12 (11.9) | 0 | 0.551 |
| Beta‐blocker, n (%) | 24 (64.9) | 44 (56.4) | 45 (44.6) | 98 (38.4) a , b | 0.002 |
| Anticoagulation, n (%) | 31 (83.8) | 64 (82.1) | 76 (75.2) | 139 (54.9) a , b , c | <0.001 |
| Amiodarone, n (%) | 6 (16.2) | 11 (14.1) | 19 (18.8) | 25 (9.8) | 0.124 |
| Propafenone, n (%) | 1 (2) | 2 (2.6) | 3 (3) | 12 (4.7) | 0.752 |
Abbreviations: ACE‐I, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; TIA, transient ischaemic attack.
Continuous data are summarized as n (%), mean ± standard deviation, or median [25th, 75th percentiles].
P < 0.05 vs. HFrEF.
P < 0.05 vs. HFmrEF.
P < 0.05 vs. HFpEF.
P = 0.265: HFrEF vs. HFmrEF vs. HFpEF.
Procedural characteristics
Ablation characteristics are summarized in Figure 2 . The procedural time was longer in the HFmrEF (119 ± 13.5 min, P < 0.001) and HFpEF (116.6 ± 15.3 min, P = 0.007) groups relative to the No HF group (111.7 ± 14.8 min) (Figure 2 A ). A prolonged fluoroscopy time was recorded in the HF groups compared with the No HF group (16.5 ± 3.4 min) and increased in the following order: No HF < HFpEF (17.8 ± 3.7 min) < HFmrEF (18.5 ± 3.2 min) < HFrEF (19.7 ± 4.1 min) (Figure 2 B ). All the groups showed a high rate of PVI without significant difference (P = 0.503) (Figure 2 C ). More times of applications per PV were identified in the HFrEF group (2.30 ± 0.35) and no significant difference was observed between the HFmrEF and HFpEF groups (2.23 ± 0.29 vs. 2.19 ± 0.26, P = 0.873) (Figure 2 D ).
Figure 2.
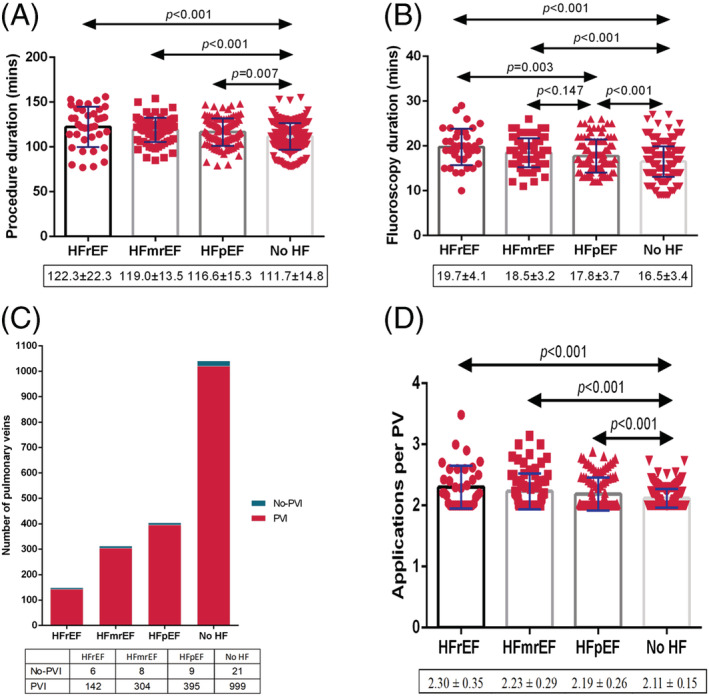
Procedural characteristics. (A) Procedure time, (B) fluoroscopy time, (C) percentage of PVI, and (D) applications per PV. HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; PV, pulmonary vein; PVI, pulmonary vein isolation.
Primary and secondary endpoints
After a mean follow‐up of 23.1 ± 6.5 (range: 12–39) months, patients with HFmrEF and HFpEF presented similar recurrence compared with the No HF groups (28.2% vs. 23.8% vs. 20.4%, P = 0.334) (Figure 3 A ); however, high recurrence rate was detected in the HFrEF group compared with the No HF group (35.1% vs. 20.4%, P = 0.044) (Figure 3 A ). The Kaplan–Meier curve analysis revealed significant differences among these groups (log‐rank P = 0.033) (Figure 4 ).
Figure 3.
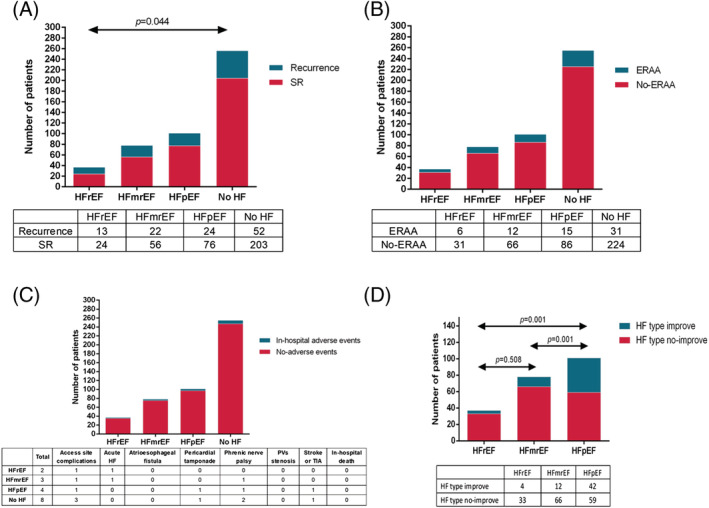
Primary and secondary endpoints. (A) Long‐term recurrence, (B) ERAA, (C) in‐hospital adverse events, and (D) HF classification improvement 1 year after cryoballoon ablation. ERAA, early recurrence of atrial arrhythmia; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; PVs, pulmonary veins; SR, sinus rhythm; TIA, transient ischaemic attack.
Figure 4.
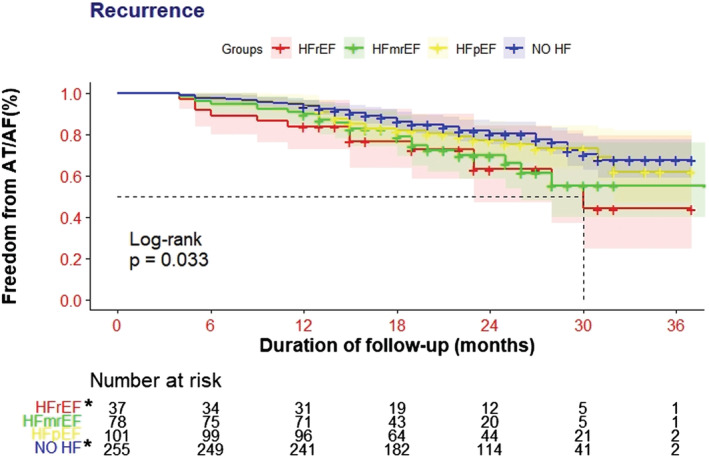
Kaplan–Meier curves for freedom from atrial arrhythmia recurrence. AT/AF, atrial tachyarrhythmia/atrial fibrillation; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction. *Groups with the most significant differences.
Until discharge, a similar ERAA rate and in‐hospital adverse events were observed among the HFrEF, HFmrEF, HFpEF, and No HF groups (16.2% vs. 15.4% vs. 14.9% vs. 12.2%, P = 0.798; 5.4% vs. 3.8% vs. 4.0% vs. 3.1%, P = 0.778) (Figure 3 B,C ). The HF symptoms were significantly relieved, as shown by the improvement in NYHA for all HF groups, and percentage of improved patients was similar in each group (Figure 5 A,B ). The LAD was significantly improved at 1 year after CBA in the HFmrEF group, with a 66.7% of patients showing shortened LAD, which was significantly better than the other groups (Figure 5 C,D ). CBA significantly improved LVEF in AF patients with HFrEF and HFmrEF, especially for the HFmrEF group; 55.1% of patients showed an improvement in the LVEF at varying degrees (Figure 5 E,F ), and all of them maintained SR at 12 month follow‐up. Notably, the reassessment of different subtypes of HF criteria after 12 months showed an improvement in the HF classifications, especially for the HFpEF group; 42 (41.6%) patients were completely relieved from HF (P = 0.001) (Figure 3 D ).
Figure 5.
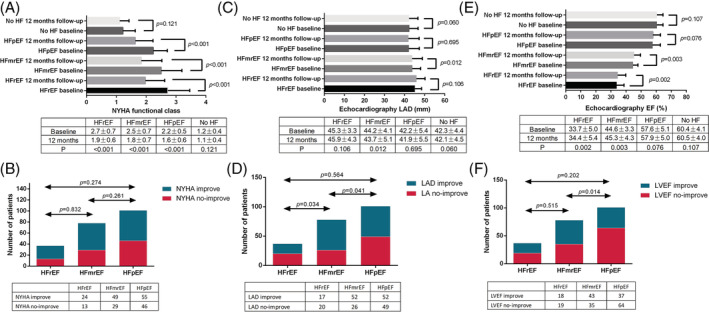
Reassessment of NYHA, LAD, and LVEF 1 year after cryoballoon ablation. (A, B) NYHA, (C, D) LAD, and (E, F) LVEF. EF, ejection fraction; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LA, left atrial; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Predictors of recurrence in patients with HF
To identify the independent predictors of recurrence, we performed univariate and multivariate Cox regression analyses. For the HFmrEF group, the univariate analysis identified baseline LVEF, LAD, NYHA, NT‐proBNP, ERAA, and diabetes maybe as predictors of recurrence. And then, the multivariate analysis in Model 1 showed that LAD and diabetes may be more associated with recurrence [hazard ratio (HR): 1.25, 95% confidence interval (CI): 1.04–1.52, P = 0.020, and HR: 3.88, 95% CI: 1.12–7.32, P = 0.029, respectively; Table 2 ]; however, in Model 2, only LAD remained significantly associated with recurrence (HR: 1.26, 95% CI: 1.02–1.55, P = 0.029; Table 2 ). For the HFpEF group, baseline LAD, NYHA, NT‐proBNP, PerAF, AF duration, and ERAA were identified as predictors of recurrence by univariate analysis, but only NYHA was found significantly associated with recurrence in both Model 1 and Model 2 (HR: 4.97, 95% CI: 1.67–15.87, P = 0.003, and HR: 5.29, 95% CI: 1.23–22.70, P = 0.019, respectively; Table 2 ).
Table 2.
Identification of predictors of recurrence
| Variables | HFmrEF | HFpEF | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||||||||
| Model 1 | Model 2 | Model 1 | Model 2 | |||||||||||||||
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Age | 1.08 | 0.99–1.16 | 0.07 | 1.01 | 0.97–1.05 | 0.667 | ||||||||||||
| Sex | 1.13 | 0.41–3.14 | 0.81 | 1.36 | 0.41–4.53 | 0.618 | 0.75 | 0.29–1.90 | 0.550 | 1.18 | 0.38–3.65 | 0.773 | ||||||
| BMI | 1.09 | 0.96–1.24 | 0.19 | 1.07 | 0.95–1.22 | 0.256 | ||||||||||||
| CHA2DS2‐VASc score | 1.74 | 0.62–3.28 | 0.102 | 1.34 | 0.87–2.08 | 0.185 | 0.71 | 0.38–1.29 | 0.359 | 0.84 | 0.48–1.49 | 0.559 | ||||||
| Baseline LVEF | 0.84 | 0.72–0.99 | 0.033 | 0.96 | 0.78–1.19 | 0.737 | 0.87 | 0.70–1.08 | 0.205 | 0.92 | 0.84–1.02 | 0.107 | ||||||
| Baseline LAD | 1.32 | 1.10–1.57 | 0.002 | 1.25 | 1.04–1.52 | 0.020 | 1.26 | 1.02–1.55 | 0.029 | 1.20 | 1.07–1.35 | 0.001 | 1.15 | 0.92–1.43 | 0.221 | 1.05 | 0.87–1.23 | 0.653 |
| Persistent AF | 2.98 | 0.97–9.18 | 0.06 | 0.91 | 0.21–3.93 | 0.903 | 5.82 | 2.14–15.82 | 0.001 | 2.46 | 0.95–6.33 | 0.063 | 1.99 | 0.54–7.39 | 0.303 | |||
| AF duration | 1.08 | 0.97–1.20 | 0.15 | 1.21 | 0.94–1.31 | 0.091 | 1.17 | 1.07–1.28 | 0.001 | 1.02 | 0.88–1.18 | 0.834 | 1.01 | 0.86–1.20 | 0.873 | |||
| Baseline NYHA class | 3.14 | 1.50–6.56 | 0.002 | 1.54 | 0.72–3.29 | 0.269 | 1.90 | 0.76–4.76 | 0.369 | 10.9 | 3.76–32.04 | <0.001 | 4.97 | 1.67–15.87 | 0.003 | 5.29 | 1.23–22.70 | 0.025 |
| NT‐proBNP | 1.00 | 1.00–1.01 | 0.024 | 1.00 | 0.99–1.00 | 0.461 | 1.00 | 1.00–1.01 | 0.656 | 1.00 | 1.00–1.01 | 0.003 | 1.00 | 0.99–1.00 | 0.618 | 1.00 | 1.00–1.01 | 0.359 |
| ERAA | 4.76 | 1.32–17.19 | 0.017 | 2.66 | 0.51–14.02 | 0.248 | 0.74 | 0.13–4.37 | 0.741 | 0.20 | 0.06–0.63 | 0.006 | 0.76 | 0.24–2.37 | 0.64 | 1.68 | 0.35–8.01 | 0.515 |
| CAD | 0.78 | 0.27–2.22 | 0.64 | 0.25 | 0.07–0.91 | 0.036 | 0.26 | 0.08–1.04 | 0.053 | 0.25 | 0.05–1.18 | 0.080 | ||||||
| Hypertension | 0.46 | 0.17–1.26 | 0.13 | 3.085 | 0.96–9.93 | 0.059 | ||||||||||||
| Diabetes | 4.33 | 2.12–12.94 | 0.037 | 3.88 | 1.12–7.32 | 0.041 | 2.71 | 0.81–9.07 | 0.105 | 1.43 | 0.53–3.83 | 0.483 | ||||||
| Stroke | 2.79 | 0.52–15.03 | 0.23 | 0.145 | 0.01–1.67 | 0.122 | ||||||||||||
| CKD | 1.38 | 0.44–4.28 | 0.68 | 0.99 | 0.29–3.35 | 0.980 | ||||||||||||
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; CKD, chronic kidney disease; ERAA, early recurrence of atrial arrhythmia; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HR, hazard ratio; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association.
Boldface denotes significant P‐values.
Predictors of recovery from HF
Univariate and multivariate logistic regression analyses were performed to estimate the recovery from HF. AF freedom within 1 year after CBA was identified as a significant predictor of remission of all HF groups (HFmrEF: HR: 0.018, 95% CI: 0.001–0.26, P = 0.003, and HFpEF: HR: 0.22, 95% CI: 0.068–0.71, P = 0.012; Table 3 ).
Table 3.
Identification of predictors of NYHA improvement 1 year after cryoballoon ablation
| Variables | HFmrEF | HFpEF | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Age | 1.00 | 0.94–1.07 | 0.915 | 1.05 | 1.01–1.09 | 0.016 | 1.04 | 0.99–1.09 | 0.073 | |||
| Sex | 0.97 | 0.38–2.48 | 0.941 | 0.58 | 0.26–1.30 | 0.184 | ||||||
| BMI | 0.98 | 0.86–1.11 | 0.802 | 0.99 | 0.89–1.10 | 0.868 | ||||||
| Baseline LVEF | 1.12 | 0.97–1.29 | 0.127 | 1.02 | 0.94–1.09 | 0.672 | ||||||
| Baseline LAD | 0.84 | 0.79–0.99 | 0.049 | 0.72 | 0.58–0.89 | 0.004 | 0.98 | 0.91–1.05 | 0.506 | |||
| Persistent AF | 1.56 | 0.49–4.98 | 0.456 | 0.604 | 0.25–1.48 | 0.271 | ||||||
| AF duration | 0.98 | 0.89–1.08 | 0.680 | 0.99 | 0.93–1.07 | 0.911 | ||||||
| Baseline NYHA class | 2.820 | 1.21–6.57 | 0.016 | 52.26 | 5.83–468.7 | <0.001 | 1.67 | 0.69–4.05 | 0.257 | |||
| Baseline NT‐proBNP | 1.00 | 0.99–1.01 | 0.509 | 1.00 | 1.00–1.01 | 0.846 | ||||||
| ERAA | 0.80 | 0.23–2.79 | 0.727 | 0.34 | 0.11–1.09 | 0.070 | ||||||
| CAD | 2.36 | 0.85–6.55 | 0.100 | 2.1 | 0.87–5.10 | 0.101 | ||||||
| Hypertension | 0.31 | 0.11–0.85 | 0.022 | 5.69 | 0.97–30.78 | 0.055 | 0.40 | 0.16–0.96 | 0.041 | 1.65 | 0.58–4.67 | 0.35 |
| Diabetes | 0.51 | 0.19–1.36 | 0.177 | 0.61 | 0.25–1.45 | 0.261 | ||||||
| Stroke | 3.18 | 0.35–28.68 | 0.302 | 1.63 | 0.14–18.57 | 0.694 | ||||||
| CKD | 1.733 | 0.55–5.49 | 0.350 | 1.38 | 1.25–17.44 | 0.022 | 0.22 | 0.048–1.04 | 0.056 | |||
| AF freedom | 0.277 | 0.10–0.78 | 0.014 | 0.018 | 0.001–0.26 | 0.003 | 0.235 | 0.08–0.64 | 0.004 | 0.22 | 0.068–0.71 | 0.012 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; CKD, chronic kidney disease; ERAA, early recurrence of atrial arrhythmia; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HR, hazard ratio; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association.
Boldface denotes significant P‐values.
Discussion
This study demonstrated the following key findings. First, the CBA is safe and effective for patients with HFmrEF and HFpEF with similar recurrence and adverse events compared with the No HF group. Second, both objective and subjective functional improvements (LVEF, LAD, NYHA, and HF subtypes reassessment) were confirmed at 1 year after CBA, especially for the HFmrEF group. Finally, baseline LVEF, LAD, and NYHA were independent predictors of recurrence for HFrEF, HFmrEF, and HFpEF, respectively. The freedom from AF recurrence was an independent predictor of recovery from HF, irrespective of the subtypes.
Previous studies have shown that RFA is effective for patients with HFrEF or HFpEF. 8 , 9 , 12 , 16 , 17 , 18 , 19 , 20 The CASTLE‐AF (Catheter Ablation vs. Standard Conventional Therapy in Patients with Left Ventricular Dysfunction and Atrial Fibrillation) described a reduction in the composite endpoint of death and HF hospitalizations, from 44.6% in the medical group to 28.5% in the RFA group. 9 The STALL AF‐HFpEF (STudy using invAsive haemodynamic measurements foLLowing catheter ablation for AF and early HFpEF) showed that RFA improves haemodynamic parameters, BNP, and HF symptoms for the patients with HFpEF. 11 CBA has potential advantages for HF patients, such as no additional fluid burden caused by irrigation catheter, short procedure time, and fewer complications. 13 Heeger et al. included 551 patients with HFrEF for CBA and found that CBA was effective with significantly improved NYHA and LVEF at 12 month follow‐up. 21 Recently, Rattka et al. identified 35 patients with HFpEF for CBA and showed that CBA improves patients' symptoms and decreases the number of hospitalizations. 12 However, due to sample limitations, it is difficult to prove the conclusion of this study. Therefore, there is still a lack of long‐term follow‐up data of CBA in patients with HF, especially for patients with HFmrEF and HFpEF. In order to verify the efficacy of CBA in patients with HFpEF and HFmrEF, we enrolled a large number of patients in the study. Moreover, to the best of our knowledge, this is the first study systematically analysing CBA in HFmrEF patients, independently.
Procedural characteristics
In the current study, longer procedure, and fluoroscopy time and more applications per PV were found in the HFmrEF and HFpEF groups when compared with the No HF group. Patients with HF are always accompanied by anatomical structure changes in LA, which might necessitate additional applications to achieve PVI. These, in turn, may lead to prolonged procedure and fluoroscopy time.
Sinus rhythm maintenance of cryoballoon ablation in patients with HF with mildly reduced and preserved ejection fraction
Previous studies have confirmed that the recurrence in patients with HFrEF is higher than that of patients without HF. 22 Moreover, compared with HFpEF, Cha et al. showed that patients with diastolic dysfunction were more likely to maintain SR at 1 year relative than those with systolic dysfunction. 23 Conversely, Black‐Maier et al. conducted a retrospective study of patients with HF for RFA and found similar recurrence between HFpEF and HFrEF groups. 10 A limitation for this study was that patients enrolled were based on the clinical diagnosis of HF rather than echocardiographic diastolic. Another study by Yamauchi et al. also suggested similar results with Black‐Maier et al. 24 However, in this study, authors included HFmrEF and HFrEF into one group (HFmrEF + HFrEF), and lower recurrence in patients with HFmrEF may reduce the total recurrence of the HFmrEF + HFrEF group.
In the current study, the ERAA was similar among different groups and in line with previous studies. 25 After a mean follow‐up of 23.1 ± 6.5 (range: 12–39) months, the long‐term recurrence of patients with HFmrEF and HFpEF was similar to No HF patients. A significantly higher recurrence was found in the HFrEF group (35.1%), which contributes to the statistical difference in the Kaplan–Meier curve analysis among groups (P = 0.033). HF results in elevated left ventricular (LV) end‐diastolic pressure, which increases LA filling pressures. This, in turn, increases atrial wall stress, consequently affecting the renin‐angiotensin system, calcium handling, profibrotic, and proinflammatory pathways, all of which promote electrical and structural remodelling. 26 , 27 , 28 However, the unique haemodynamics of each HF subtype has differing effects on LA structure and function. The eccentric remodelling of the LA occurs to a larger extent in HFrEF, with a greater overall increase in LAD and volume. A greater pulsatility and maximal pressure in HFpEF lead to increased LA stiffness relative to HFrEF. 29 HFmrEF is in the transitional stage of HFrEF and HFpEF, which has diastolic dysfunction but a lesser degree of LA stiffness than that of HFpEF and a tendency of systolic dysfunction but less than that of HFrEF. Therefore, the underlying substrate driving AF could be varied in patients with different HF subtypes, which might lead to the difference in recurrence after CBA. In the current study, recurrence of HFmrEF is between HFrEF and HFpEF, and the difference is not significant with HFpEF. Additionally, the recurrence is closely related to the deterioration of the atrial matrix. The high rate of SR maintenance in the HFmrEF and HFpEF groups was similar to the No HF group, indicating that CBA may delay the deterioration of the atrial matrix of patients with HFpEF and HFmrEF.
Safety of cryoballoon ablation in patients with HF with mildly reduced and preserved ejection fraction
CA has the potential to harm subjects by procedural complications. The CABANA (Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation) trial consists of 6.6% of patients with HF who suffered procedural adverse events. 8 In the CASTLE‐AF trial, there was seven (3.9%) patients who suffered procedural adverse events compared with patients without HF, as reported previously. 9 Jayanna et al. did not find any difference in peri‐procedural complications between HF and No HF groups. 18 Moreover, Aldaas et al. found that patients with HFrEF and HFpEF suffered similar complications as patients without HF. 19 Additionally, limited data determined that CBA was safe for HFpEF and HFrEF with low complication (HFrEF: 4/50, 8%, and control: 3/50, 6%; P = 0.695). 21 In the current series of patients, the rate of complications was low, which was in line with previous studies (2–7.5%). 7 , 30 , 31 , 32 No in‐hospital death occurred in all patients. No significant differences were observed among the groups for complications. Typically, HF would not increase CBA procedural complications regardless of HF subtypes. Two patients experienced AHF after CBA, although the symptoms were relieved after drug treatment. Also, for patients with stable HF before procedure, the procedure is a major inducement of AHF, especially in patients with reduced LVEF.
All‐cause death after cryoballoon ablation in patients with HF with mildly reduced and preserved ejection fraction
The CABANA trial showed that the RFA had a 43% relative reduction in all‐cause mortality for patients with HF compared with drug therapy over a median follow‐up of 48.5 months. 8 After a median follow‐up of 37.8 months, for patients with HFrEF, the CASTLE‐AF trial showed that significantly fewer patients in the RFA group died from any cause (HR: 0.53, 95% CI: 0.32–0.86, P = 0.01). 9 Seven all‐cause deaths occurred after RFA during the median follow‐up of 31 months, and no significant difference was found among HFrEF, HFmrEF, and HFpEF groups (P = 0.15). 33 The all‐cause deaths were lower in the current study after the median follow‐up of 23.1 months (1.06%, 5/471, HFrEF: 3, HFmrEF: 1, No HF: 1). In the HFrEF group, two patients died of AHF and cardiac shock, and the other died of recurrent stroke. All three patients had AF recurrence during follow‐up. The failure to maintain SR may be one of the leading causes of death. One patient in the HFmrEF group died of diabetes complications. This patient also experienced AF recurrence. Previous studies demonstrated that diabetes increases AF recurrence after CA. Hence, the treatment of comorbidities is crucial as it directly affects the overall outcomes.
HF improvement and left atrial reversing after cryoballoon ablation in patients with HF with mildly reduced and preserved ejection fraction
The follow‐up data of the CABANA trial showed that RFA improves the quality of life (QOL) for HF patients relative to drug therapy. 8 Rattka et al. also demonstrated the improvement of NYHA, LV reverse remodelling, intraventricular septal thickness, and LV mass index (LVMI) 1 year after RFA. 12 Similar results were found in other studies. 14 , 24 , 34 In another study by Heeger et al., wherein CBA was performed for the patients with HF, LVEF improved from a median of 37% to 55% after 12 months of follow‐up (P < 0.0001). 21 The SR maintained by CBA may be a critical factor for improving the outcomes. 11 , 20 In the current study, NYHA was improved significantly in the HF groups. Additionally, we found that LVEF was significantly improved in the patients with HFmrEF and HFrEF. In the HFmrEF group, 43 patients with improved LVEF maintained SR at 12 month follow‐up. Although the recurrence was similar, LAD was significantly shortened in the HFmrEF group compared with the HFpEF group. In other words, CBA could reverse the LA remodelling of patients with HFmrEF significantly, which could be attributed to the special pathophysiology of HFmrEF. The reassessment of HF subtypes after 12 months showed that more than half of the patients in the HFpEF group recovered from HF. AF could aggravate HF not only objectively but also subjectively affecting the patient's main complaint. Therefore, maintaining SR improves the HF symptoms and affects the patient's main complaint, which is represented by improved NYHA. 35 Compared with the No HF group, HFpEF is mainly manifested by poor NYHA. Hence, if NYHA of HFpEF is significantly improved at the time of reassessment, it may be assessed as a recovery of HF. For other HF subtypes, even if NYHA is significantly improved, the improvement of LVEF does not meet the criteria of another subtype and cannot be judged as the recovery of HF subtypes. Therefore, compared with other HF subtypes, HFpEF is likely to show recovery of HF subtypes.
Predictors of recurrence and recovery of HF
For the general population, several factors were used to predict the recurrence after CA, including AF type (paroxysmal or persistent), AF duration, gender, and LAD. For the patients with AF and HF, Jayanna et al. found that LA enlargement, PerAF, and a high CHA2DS2‐VASc score are independent risk factors for recurrence. 18 Yamauchi et al. found that decreased BNP level and freedom from recurrence were independent predictors of HF remission. 24 After multivariate regression, Rattka et al. also found that the absence of atrial tachyarrhythmia (AT) recurrence was an independent predictor of recovery from HFpEF. 12 Currently, there are no studies on the predictors of AF recurrence and HF remission after CBA in patients with HFpEF and HFmrEF. In this study, univariate analysis was performed first, followed by multivariate analysis through Model 1 and Model 2. For patients with HFmrEF, the multivariate analysis found that LAD was significantly associated with AF recurrence in both Model 1 and Model 2. It was found that CBA could reverse the LA remodelling in our study (mainly manifested as significantly shortened LAD), and more than two‐thirds of patients in the HFmrEF group experienced LAD shortening after CBA, which was superior to other variables, so for patients with HFmrEF, LAD shortening may contribute more to maintaining SR. In addition, the shortening of LAD in the HFmrEF group was more significant than that in the HFpEF group, which may be due to the relatively severe but better reversibility of LA remodelling in the HFmrEF group compared with the HFpEF group, and the reversibility of LA remodelling is important for the maintenance of SR. Therefore, LAD was more important in patients with HFmrEF compared with the HFpEF, whereas for patients with HFpEF, who are away not accompanied by severe cardiac remodelling, NYHA deterioration is an important distinction between patients with HFpEF and those without HF. NYHA reflects the HF dynamic changes of HFpEF and may predict the recurrence of these patients after CBA. The multivariate analysis of patients with HFpEF showed that NYHA was significantly associated with recurrence after CBA in Model 1 and Model 2. In the current study, more than half of the patients in the HFpEF group experienced significant improvement of NYHA, and the proportion was much higher than that in the HFmrEF group, which made NYHA more useful in predicting the recurrence of AF in the patients with HFpEF. Maintaining SR is a primary indicator to evaluate the effectiveness of AF treatment. Some studies showed that maintaining SR can alleviate the HF symptoms, improve LVEF, and even reverse cardiac remodelling for patients with AF and HF. In the current study, we found that regardless of the HF subtypes, maintaining SR is a major predictor of improvement in the NYHA reassessment, which was consistent with the results of previous studies. 14 , 19 , 24
Therefore, the CBA procedure parameters, the follow‐up data, prognosis and predictors of cardiac function improvement, and recurrence in patients with HFpEF and HFmrEF differ from those without HF or HFrEF, especially those with HFmrEF, which has always been combined with other subgroups for analysis in previous studies. Thus, we suggested that patients with HFmrEF should be studied as an independent group in future studies.
Study limitations
Nevertheless, the present study has several limitations. Firstly, this was a retrospective, observational study, and the number of patients may not be sufficient to reach a definite conclusion. Hence, randomized controlled trials (RCTs) are warranted for verification of the reproducibility and generalizability of the findings. Secondly, AF recurrence might be underestimated because it was not a continuous telemetry monitoring, except for some patients with implantable pacemakers, implantable cardioverter defibrillator (ICD) or cardiac resynchronization therapy with defibrillator (CRTD). Thirdly, although the same CBA procedure protocol was applied for all patients, the details of the procedure obtained from two EP centres were not identical, which might affect the outcome.
Conclusions
Patients with HFmrEF or HFpEF with AF can benefit significantly from CBA, and its safety and effectiveness are not inferior to those without HF. Successful CBA can significantly improve the HF symptoms and parameters, especially for patients with HFmrEF, which can be reversed with LA remodelling after CBA. LAD and NYHA are independent predictors of the recurrence in the HFpEF and HFmrEF groups, respectively, whereas SR maintenance is an independent predictor of NYHA improvement irrespective of the HF subtypes. However, large RCTs are required to examine the long‐term outcomes of CBA in HFmrEF and HFpEF.
Conflict of interest
The authors declare no potential conflict of interest.
Funding
This work was supported by the Science and Technology Commission of Shanghai Municipality, Grant No. 21S31906903.
Chen, C. , Cheng, K. , Gao, X. , Zou, T. , Pang, Y. , Ling, Y. , Xu, Y. , Xu, Y. , Chen, Q. , Zhu, W. , and Ge, J. (2023) Cryoballoon ablation for atrial fibrillation in patients with heart failure with mildly reduced and preserved ejection fraction. ESC Heart Failure, 10: 518–531. 10.1002/ehf2.14212.
Chaofeng Chen and Kuan Cheng contributed equally to this work.
Contributor Information
Qingxing Chen, Email: chenqxsci@163.com.
Wenqing Zhu, Email: zhuwqsci@163.com.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 2. Parkash R, Wells G, Rouleau J, Talajic M, Essebag V, Skanes A, Wilton SB, Verma A, Healey JS, Tang AS. A randomized ablation‐based atrial fibrillation rhythm control versus rate control trial in patients with heart failure and high burden atrial fibrillation: the RAFT‐AF trial rationale and design. Am Heart J 2021; 234: 90–100. [DOI] [PubMed] [Google Scholar]
- 3. Kelly JP, DeVore AD, Wu J, Hammill BG, Sharma A, Cooper LB, Felker GM, Piccini JP, Allen LA, Heidenreich PA, Peterson ED, Yancy CW, Fonarow GC, Hernandez AF. Rhythm control versus rate control in patients with atrial fibrillation and heart failure with preserved ejection fraction: insights from get with the guidelines‐heart failure. J Am Heart Assoc 2019; 8: e011560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol 2016; 68: 2217–2228. [DOI] [PubMed] [Google Scholar]
- 5. Sartipy U, Dahlström U, Fu M, Lund LH. Atrial fibrillation in heart failure with preserved, mid‐range, and reduced ejection fraction. JACC Heart failure 2017; 5: 565–574. [DOI] [PubMed] [Google Scholar]
- 6. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation 2019; 140: e125–e151. [DOI] [PubMed] [Google Scholar]
- 7. Kuck KH, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KR, Elvan A, Arentz T, Bestehorn K, Pocock SJ, Albenque JP, Tondo C. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016; 374: 2235–2245. [DOI] [PubMed] [Google Scholar]
- 8. Packer DL, Piccini JP, Monahan KH, Al‐Khalidi HR, Silverstein AP, Noseworthy PA, Poole JE, Bahnson TD, Lee KL, Mark DB, CABANA Investigators . Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation 2021; 143: 1377–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H, Christ H, Vogt J, Bänsch D, CASTLE‐AF Investigators . Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018; 378: 417–427. [DOI] [PubMed] [Google Scholar]
- 10. Black‐Maier E, Ren X, Steinberg BA, Green CL, Barnett AS, Rosa NS, Al‐Khatib SM, Atwater BD, Daubert JP, Frazier‐Mills C, Grant AO, Hegland DD, Jackson KP, Jackson LR, Koontz JI, Lewis RK, Sun AY, Thomas KL, Bahnson TD, Piccini JP. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Heart Rhythm 2018; 15: 651–657. [DOI] [PubMed] [Google Scholar]
- 11. Sugumar H, Nanayakkara S, Vizi D, Wright L, Chieng D, Leet A, Mariani JA, Voskoboinik A, Prabhu S, Taylor AJ, Kalman JM, Kistler PM, Kaye DM, Ling LH. A prospective STudy using invAsive haemodynamic measurements foLLowing catheter ablation for AF and early HFpEF: STALL AF‐HFpEF. Eur J Heart Fail 2021; 23: 785–796. [DOI] [PubMed] [Google Scholar]
- 12. Rattka M, Pott A, Kühberger A, Weinmann K, Scharnbeck D, Stephan T, Baumhardt M, Bothner C, Iturbe Orbe M, Rottbauer W, Dahme T. Restoration of sinus rhythm by pulmonary vein isolation improves heart failure with preserved ejection fraction in atrial fibrillation patients. Europace 2020; 22: 1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen CF, Liu MJ, Jin CL, Gao XF, Liu XH, Xu YZ. Costs and long‐term outcomes following pulmonary vein isolation for atrial fibrillation in elderly patients using second‐generation cryoballoon vs. open‐irrigated radiofrequency in China. J Interv Card Electrophysiol 2020; 59: 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rattka M, Kühberger A, Pott A, Stephan T, Weinmann K, Baumhardt M, Aktolga D, Teumer Y, Bothner C, Scharnbeck D, Rottbauer W, Dahme T. Catheter ablation for atrial fibrillation in HFpEF patients—a propensity‐score‐matched analysis. J Cardiovasc Electrophysiol 2021; 32: 2357–2367. [DOI] [PubMed] [Google Scholar]
- 15. Su WW, Reddy VY, Bhasin K, Champagne J, Sangrigoli RM, Braegelmann KM, Kueffer FJ, Novak P, Gupta SK, Yamane T, Calkins H, STOP Persistent AF Investigators . Cryoballoon ablation of pulmonary veins for persistent atrial fibrillation: results from the multicenter STOP Persistent AF trial. Heart Rhythm 2020; 17: 1841–1847. [DOI] [PubMed] [Google Scholar]
- 16. Elkaryoni A, Al Badarin F, Spertus JA, Kennedy KF, Wimmer AP. Comparison of the effect of catheter ablation for atrial fibrillation on all‐cause hospitalization in patients with versus without heart failure (from the nationwide readmission database). Am J Cardiol 2020; 125: 392–398. [DOI] [PubMed] [Google Scholar]
- 17. Ichijo S, Miyazaki S, Kusa S, Nakamura H, Hachiya H, Kajiyama T, Iesaka Y. Impact of catheter ablation of atrial fibrillation on long‐term clinical outcomes in patients with heart failure. J Cardiol 2018; 72: 240–246. [DOI] [PubMed] [Google Scholar]
- 18. Jayanna MB, Mohsen A, Inampudi C, Alvarez P, Giudici MC, Briasoulis A. Procedural outcomes of patients with heart failure undergoing catheter ablation of atrial fibrillation. Am J Ther 2019; 26: e333–e338. [DOI] [PubMed] [Google Scholar]
- 19. Aldaas OM, Malladi CL, Mylavarapu PS, Lupercio F, Darden D, Han FT, Hoffmayer KS, Krummen D, Ho G, Raissi F, Feld GK, Hsu JC. Comparison of outcomes after ablation of atrial fibrillation in patients with heart failure with preserved versus reduced ejection fraction. Am J Cardiol 2020; 136: 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Machino‐Ohtsuka T, Seo Y, Ishizu T, Sugano A, Atsumi A, Yamamoto M, Kawamura R, Machino T, Kuroki K, Yamasaki H, Igarashi M, Sekiguchi Y, Aonuma K. Efficacy, safety, and outcomes of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol 2013; 62: 1857–1865. [DOI] [PubMed] [Google Scholar]
- 21. Heeger CH, Abdin A, Mathew S, Reissmann B, Yalin K, Liosis S, Fink T, Proietti R, Eitel C, Vogler J, Lemeš C, Maurer T, Rillig A, Meyer‐Saraei R, Graf T, Wohlmuth P, Goldmann B, Ouyang F, Kuck KH, Metzner A, Tilz RR. Efficacy and safety of cryoballoon ablation in patients with heart failure and reduced left ventricular ejection fraction—a multicenter study. Circ J 2019; 83: 1653–1659. [DOI] [PubMed] [Google Scholar]
- 22. Providência R, de Asmundis C, Chun J, Chierchia G, Defaye P, Anselme F, Creta A, Lambiase PD, Schmidt B, Chen S, Hunter RJ, Combes S, Honarbakhsh S, Combes N, Sousa MJ, Jebberi Z, Albenque JP, Boveda S. Catheter ablation of atrial fibrillation in patients with heart failure with reduced ejection fraction: real world experience from six European centers. J Cardiovasc Electrophysiol 2019; 30: 1270–1277. [DOI] [PubMed] [Google Scholar]
- 23. Cha YM, Wokhlu A, Asirvatham SJ, Shen WK, Friedman PA, Munger TM, Oh JK, Monahan KH, Haroldson JM, Hodge DO, Herges RM, Hammill SC, Packer DL. Success of ablation for atrial fibrillation in isolated left ventricular diastolic dysfunction: a comparison to systolic dysfunction and normal ventricular function. Circ Arrhythm Electrophysiol 2011; 4: 724–732. [DOI] [PubMed] [Google Scholar]
- 24. Yamauchi R, Morishima I, Okumura K, Kanzaki Y, Morita Y, Takagi K, Nagai H, Watanabe N, Furui K, Yoshioka N, Miyazawa H, Shimojo K, Imaoka T, Sakamoto G, Murohara T. Catheter ablation for non‐paroxysmal atrial fibrillation accompanied by heart failure with preserved ejection fraction: feasibility and benefits in functions and B‐type natriuretic peptide. Europace 2021; 23: 1252–1261. [DOI] [PubMed] [Google Scholar]
- 25. Andrade JG, Khairy P, Verma A, Guerra PG, Dubuc M, Rivard L, Deyell MW, Mondesert B, Thibault B, Talajic M, Roy D, Macle L. Early recurrence of atrial tachyarrhythmias following radiofrequency catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol 2012; 35: 106–116. [DOI] [PubMed] [Google Scholar]
- 26. Mayyas F, Niebauer M, Zurick A, Barnard J, Gillinov AM, Chung MK, Van Wagoner DR. Association of left atrial endothelin‐1 with atrial rhythm, size, and fibrosis in patients with structural heart disease. Circ Arrhythm Electrophysiol 2010; 3: 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Jong AM, Maass AH, Oberdorf‐Maass SU, Van Veldhuisen DJ, Van Gilst WH, Van Gelder IC. Mechanisms of atrial structural changes caused by stretch occurring before and during early atrial fibrillation. Cardiovasc Res 2011; 89: 754–765. [DOI] [PubMed] [Google Scholar]
- 28. Ling LH, Kistler PM, Kalman JM, Schilling RJ, Hunter RJ. Comorbidity of atrial fibrillation and heart failure. Nat Rev Cardiol 2016; 13: 131–147. [DOI] [PubMed] [Google Scholar]
- 29. Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail 2015; 8: 295–303. [DOI] [PubMed] [Google Scholar]
- 30. Mugnai G, de Asmundis C, Ciconte G, Irfan G, Saitoh Y, Velagic V, Ströker E, Wauters K, Hünük B, Brugada P, Chierchia GB. Incidence and characteristics of complications in the setting of second‐generation cryoballoon ablation: a large single‐center study of 500 consecutive patients. Heart Rhythm 2015; 12: 1476–1482. [DOI] [PubMed] [Google Scholar]
- 31. Rottner L, Fink T, Heeger CH, Schlüter M, Goldmann B, Lemes C, Maurer T, Reißmann B, Rexha E, Riedl J, Santoro F, Wohlmuth P, Mathew S, Sohns C, Ouyang F, Kuck KH, Metzner A. Is less more? Impact of different ablation protocols on periprocedural complications in second‐generation cryoballoon based pulmonary vein isolation. Europace 2018; 20: 1459–1467. [DOI] [PubMed] [Google Scholar]
- 32. Koektuerk B, Turan CH, Yorgun H, Keskin K, Schoett M, Dahmen A, Gorr E, Yang A, Hoppe C, Horlitz M, Turan RG. The total incidence of complications and the impact of an anticoagulation regime on adverse events after cryoballoon ablation of atrial fibrillation: a single‐center study of 409 patients. Cardiovasc Ther 2016; 34: 144–151. [DOI] [PubMed] [Google Scholar]
- 33. Yazaki K, Ejima K, Kataoka S, Higuchi S, Kanai M, Yagishita D, Shoda M, Hagiwara N. Prognostic significance of post‐procedural left ventricular ejection fraction following atrial fibrillation ablation in patients with systolic dysfunction. Circ Rep 2020; 2: 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vecchio N, Ripa L, Orosco A, Tomas L, Mondragón I, Acosta A, Talavera L, Rivera S, Albina G, Diez M, Scazzuso F. Atrial fibrillation in heart failure patients with preserved or reduced ejection fraction. Prognostic significance of rhythm control strategy with catheter ablation. J Atr Fibrillation 2019; 11: 2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferre‐Vallverdu M, Ligero C, Vidal‐Perez R, Martinez‐Rubio A, Vinolas X, Alegret JM. Improvement in atrial fibrillation‐related symptoms after cardioversion: role of NYHA functional class and maintenance of sinus rhythm. Clin Interv Aging 2021; 16: 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]


