Abstract
Aims
Wild‐type transthyretin cardiac amyloidosis (ATTRwt) is an infiltrative cardiomyopathy with a poor prognosis. The condition is associated with carpal tunnel syndrome (CTS), which often precedes the ATTRwt diagnosis by several years. The aim of the study was (i) to screen patients with a recent history of CTS for ATTRwt using red flags, (ii) to determine whether patients with screened ATTRwt had less advanced disease compared with patients with clinical ATTRwt, and (iii) to assess the sensitivity and specificity of known red flags in ATTRwt.
Methods and results
Patients aged ≥60 years at the time of CTS surgery were invited for screening. Red flags were defined as elevated biomarker levels of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) or cardiac troponin, an electrocardiogram pattern associated with ATTRwt, left ventricular hypertrophy (LVH), and impaired longitudinal strain with apical sparring. All patients with a red flag were referred for a diagnostic scintigraphy. Patients with ATTRwt diagnosed by screening were compared with patients with clinical ATTRwt (n = 51) matched by age, gender, and CTS surgery. Among the 120 enrolled subjects (mean age 74.5 years, 90% male), the suspicion of ATTR was raised in 67 (55.8%), and 10 (8.3%) were diagnosed with ATTRwt. Patients identified with ATTRwt were predominantly asymptomatic and had mildly elevated NT‐proBNP, mildly increased LVH, preserved left ventricular ejection fraction, and systolic longitudinal function, which differed significantly from clinical ATTRwt controls (P < 0.001).
Conclusions
The study found an ATTRwt prevalence of 8.3% in a population of age and gender‐selected patients with a recent history of CTS. The identified patients with ATTRwt had less structural and functional cardiac involvement than clinical ATTRwt controls.
Keywords: Transthyretin cardiac amyloidosis, Carpal tunnel syndrome, Screening, Echocardiography
Introduction
Transthyretin cardiac amyloidosis (ATTR) is a progressive cardiomyopathy in which amyloid fibrils accumulate in the myocardial interstitial space, which leads to a restrictive heart failure (HF) condition. The most common form is wild‐type transthyretin cardiac amyloidosis (ATTRwt), which increases in prevalence with age and predominantly affects men. 1 Carpal tunnel syndrome (CTS) is the most common peripheral neuropathy worldwide and is closely associated with ATTRwt due to amyloid deposition in the tenosynovial tissue of the wrist. 2 , 3 Of note, the CTS symptoms caused by amyloid deposition often precede the symptoms and diagnosis of ATTRwt by 5–9 years, indicating that screening for ATTRwt around the time of CTS surgery may identify patients at an early disease stage. 4 Early diagnosis is particularly important because it increases the yield of novel medical therapies such as tafamidis, a transthyretin stabilizer that inhibits protein dissociation and thereby amyloid formation. 5 , 6 The beneficial effects of tafamidis are most pronounced in patients with mild ATTR disease, which underlines the importance of early disease detection. 7 Consequently, prospective screening studies of ATTR risk populations are warranted, but such studies have so far been limited by undefined screening for multiple types of amyloidosis, resulting in screening populations of too young an age and/or too high a proportion of females. 8 , 9 , 10 , 11 , 12
ATTRwt is associated with several clinical, biochemical, and echocardiographic characteristics such as elevated biomarker levels of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) and cardiac troponin, 13 , 14 , 15 Electrocardiogram (ECG) patterns low voltage, pseudoinfarction, and atrioventricular abnormalities, 14 echocardiographic signs of left ventricular wall thickening, and impaired longitudinal strain with apical sparring. 14 , 16 Although these so‐called ‘red flags’ are obvious candidates to utilize for ATTR screening, their sensitivity and specificity have yet to be fully investigated. 17
The primary aim of this study was to examine the prevalence of ATTRwt in an aged (≥60 years) and predominantly male population with idiopathic CTS release surgery within the past 1–6 years. The secondary aim was to characterize the cardiac disease stage of patients with ATTRwt identified by screening compared with clinically diagnosed patients with symptomatic ATTRwt matched by gender, age, and previous CTS surgery.
Methods
Study population and design
Patients aged ≥60 years at surgery for idiopathic CTS from 2014 to 2018 at the orthopaedic departments at the Regional Hospital West Jutland and Aarhus University Hospital, Denmark, received a written invitation for participation in a screening study for ATTR. Patient status was checked with the Danish Civil Registry to ensure that all participants were alive when receiving a study invitation. First‐time non‐responders to invitation were re‐invited after 2 months. The screening was performed at the Department of Cardiology, Aarhus University Hospital, Denmark, and included a physical examination, biomarker analysis, ECG, and transthoracic echocardiography (TTE).
Patients were invited at a rate of one female for every nine males. 5 , 18 , 19 Exclusion criteria were CTS release surgery by non‐idiopathic indication and established diagnosis of ATTR or light chain amyloidosis.
Participants who met one or more of the following red flag screening criteria for ATTR were referred for a 99mTc‐DPD (technetium‐99m 3,3‐diphosphono‐1,2‐propanodicarboxylic acid) scintigraphy 20 :
-
ECG
-
1.
Presence of low voltage in limb or precordial leads. Low voltage was defined as a QRS amplitude of <5 mm in the limb leads and/or <10 mm in the precordial leads.
-
2.
A pseudoinfarction pattern. A pseudoinfarction pattern was defined as a profound rS pattern in V1–V3 without corresponding segmental dyskinesis of the left ventricle (LV) by TTE.
-
3.
Atrioventricular block. First‐degree atrioventricular block was defined as a PR interval > 220 ms. Second‐degree atrioventricular block was defined as intermittent non‐conducted P waves either with or without prolongation of the PR interval. Third‐degree atrioventricular block was defined as complete dissociation of the P waves and QRS complexes.
-
1.
-
Biochemistry
-
4
Abnormal plasma NT‐proBNP > 300 ng/L.
-
5
Elevated cardiac troponin I (TnI) or T (TnT) plasma levels (TnI > 45 ng/L and TnT > 14 ng/L).
-
4
-
TTE
6. Left ventricular hypertrophy (LVH) and end‐diastolic interventricular septum (IVS) ≥ 12 mm.
7. A relative apical sparring (RAS) pattern on global longitudinal strain (GLS) plot analysis.
8. A relative apical sparring ratio (RASr) ≥ 1.5.
A positive 99mTc‐DPD scintigraphy with Perugini Grade 2 or 3 was considered diagnostic for ATTR, and these patients were subsequently genetically tested for variant transthyretin cardiac amyloidosis (ATTRv). Patients with Perugini Grade 1 were evaluated with further investigations including 123I‐metaiodobenzylguanidine scintigraphy (MIBG), endomyocardial biopsy with mass spectrometry, and/or cardiac magnetic resonance imaging. ATTR was considered excluded when the Perugini grade was 0. 21
Light chain amyloidosis was excluded by normal serum kappa/light chain ratio and normal serum/urine immunofixation analysis. Patients with positive 99mTc‐DPD scintigraphy and an abnormal serum kappa/lambda light chain ratio and/or the presence of a monoclonal spike band on immunofixation analysis were referred for confirmatory diagnosis by endomyocardial biopsy with mass spectrometry.
The CTS‐screened patients with confirmed ATTRwt were compared with a control group of patients with clinical ATTRwt (n = 51) and signs of HF diagnosed from 2017 to 2020. Patient groups were compared using data from their time of screening and diagnosis, respectively. Controls were matched by gender, age, and a history of CTS surgery. The diagnosis of these patients with clinically diagnosed ATTRwt was confirmed by 99mTc‐DPD scintigraphy (n = 29), endomyocardial biopsy (n = 11), or both (n = 11). All controls were genetically tested to rule out ATTRv.
The disease stage definitions of the National Amyloidosis Centre (NAC), London, were used to assess disease severity: Stage 1, estimated glomerular filtration rate (eGFR) ≥ 45 mL/min/1.73 m2 and NT‐proBNP < 3000 ng/L; Stage 3, eGFR < 45 mL/min/1.73 m2 and NT‐proBNP ≥ 3000 ng/L; and stage 2, any patient who did not meet Stage 1 or 3 criteria. 5
The study was approved by the Committee of Scientific Ethics of the Central Denmark Region (ID: 1‐10‐72‐330‐18). All participants provided written informed consent. The study was conducted in accordance with the Helsinki Declaration. The study was registered with clinicaltrials.org (ID: NCT03996382).
Biomarker analysis
Blood samples were drawn from all participants and analysed for the following: TnT or TnI, NT‐proBNP, plasma sodium, plasma potassium, plasma creatinine, eGFR, haemoglobin, leucocytes, thrombocytes, kappa and lambda light chains, and serum/urine immunofixation.
Transthoracic echocardiography
Examinations were performed using Vivid E95 or E9 (GE Healthcare, Horten, Norway) with a 3.5 MHz transducer and a 4D probe. The examinations were analysed and stored using EchoPAC software.
Left ventricular ejection fraction (LVEF) and left atrial volume were calculated from the apical views using Simpson's biplane method with left atrial volume indexed to body surface area. Trans‐mitral flow was assessed by measurement of E, A, E/A ratio, E deceleration time, and E/e′.
Left ventricular GLS (LV‐GLS) was measured by frame‐by‐frame tracking of speckle patterns in standard 2D cine loops. The speckle area of interest was adjusted manually to achieve optimal results. Segments of unacceptably low tracking quality were excluded. LV‐GLS was measured at peak values during systole with a 17 myocardial segment model. Higher negative values equal higher levels of strain. If LV‐GLS was not reported due to unacceptable tracking quality in two or more segments, an average of the acceptable segments was calculated by hand and used as a measure of the projection. Finally, LV‐GLS was determined as the average of all three projections.
RAS was both assessed as a visual pattern on the GLS plot and calculated as RASr in which the mean strain of the five apical segments is divided by the mean of the 12 middle and basal segments. A RASr ≥ 1.5 was considered abnormal. 22
99mTc‐DPD scintigraphy
Patients were administered 750 MBq of 99mTc‐DPD intravenously and imaged 3 h later on either a Siemens Symbia T16 SPECT/CT (Single Photon Emission Tomography/Computed Tomography) or General Electric Discovery 670 SPECT/CT. Anterior/posterior images from the lower neck to below the diaphragm were acquired to 600 kCts using low‐energy high‐resolution collimators and were immediately followed by a SPECT/CT of the heart. Radiation dose from the entire procedure was 6 mSv per patient.
Intensity of myocardial uptake on the planar 99mTc‐DPD scan was categorized as 0–3 according to the grading system described by Perugini et al. 21
Statistical analysis
Normality of data was assessed using histograms and Q–Q plots. Normally distributed variables are presented as mean and standard deviation (±SD), and non‐normally distributed variables are presented as median and interquartile range (IQR). Categorical and dichotomous variables are presented as numbers (n) and percentages (%). Groups were compared using the Student's t‐test, the Mann–Whitney U test, the Kruskal–Wallis test, the one‐way ANOVA test, and the χ 2 test depending on data and the number of groups analysed.
The sensitivity and specificity of red flags were assessed using receiver‐operating characteristic (ROC) curves. ROC curves were compared using the area under the curve (AUC).
All analyses used two‐sided P‐values. A P‐value of <0.05 was considered statistically significant. Statistical analyses were performed using STATA 16 software (StataCorp LP, College Station, Texas). Figures were created using GraphPad PRISM 9.3.1.
Results
Baseline characteristics
From 1 March 2019 to 1 November 2020, 369 patients with a history of idiopathic CTS surgery were invited for screening. Among these, 128 (34.7%) responded, and 120 (32.5%) accepted to participate (Figure 1 ). The mean age at screening was 74.5 years, and 109 (90.8%) were male. The median time from surgery to screening was 4.4 years (IQR 2.9–5.7), and 59 (49.6%) had received bilateral CTS release surgery. There was no significant difference in proportion of bilateral CTS surgery among patient groups.
Figure 1.
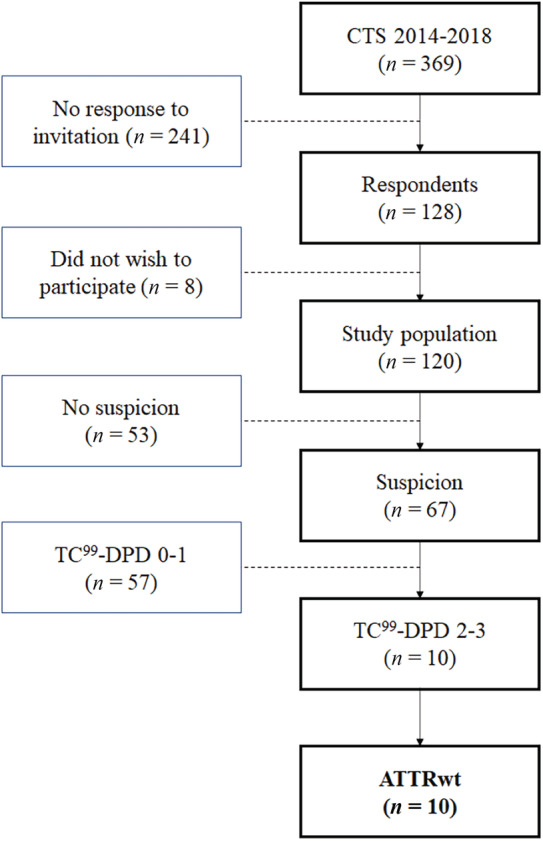
Flowchart of the study population. ATTRwt, wild‐type transthyretin cardiac amyloidosis; CTS, carpal tunnel syndrome; DPD, technetium‐99m 3,3‐diphosphono‐1,2‐propanodicarboxylic acid scintigraphy.
Based on examination findings, patients were grouped by presence or absence of ATTR red flags as patients with (n = 67) or without (n = 53) suspicion of ATTR. Depending on the result of the 99mTc‐DPD scintigraphy, patients were further divided into ATTR not confirmed (n = 57) or ATTR confirmed (n = 10). The characteristics of the study population and its three groups are shown in Table 1 . The mean systolic and diastolic blood pressures were 132 (±17) and 80 (±10) mmHg, respectively, in the total population; the mean heart rate was 69 (±12) with no differences between the three groups. Furthermore, no difference between the three groups was noted regarding treatment with diuretics (P = 0.09), anticoagulation therapy (P = 0.054), use of angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers (P = 0.77), or dihydropyridine calcium channel blockers (P = 0.48).
Table 1.
Clinical and echocardiographic characteristics of the total cohort and red flag ATTR suspicion groups
| Total study population (n = 120) | No suspicion of ATTR (n = 53) | Suspicion, ATTR not confirmed (n = 57) | Suspicion, ATTR confirmed (n = 10) | P‐values | |
|---|---|---|---|---|---|
| Clinical | |||||
| Age at screening, years | 74.5 ± 6.1 | 72.4 ± 4.9 | 75.4 ± 5.8 | 81.2 ± 7.6 | 0.00* |
| Male | 109 (90.8) | 47 (88.7) | 53 (92.98) | 9 (90.0) | 0.73 |
| Hypertension | 65 (54.2) | 29 (54.7) | 30 (52.6) | 6 (60.0) | 0.91 |
| Diabetes | 15 (12.5) | 5 (9.4) | 9 (15.8) | 1 (10.0) | 0.58 |
| IHD | 18 (15.0) | 5 (9.4) | 11 (19.3) | 2 (20.0) | 0.32 |
| Atrial fibrillation | 11 (9.2) | 1 (1.9) | 8 (14.0) | 2 (20.0) | 0.04* |
| Low voltage | 9 (7.5) | 0 (0) | 6 (10.5) | 3 (30.0) | 0.00* , a |
| Bilateral CTS | 59 (49.6) | 27 (51.9) | 26 (45.6) | 6 (60.0) | 0.64 |
| Years since surgery | 4.4 [2.9–5.7] | 3.9 [2.9–5.6] | 4.7 [2.9–5.7] | 4.2 [2.9–5.4] | 0.93 |
| Laboratory | |||||
| Creatinine, μmol/L | 75 [65–85] | 71.0 [65–81] | 78 [69–88] | 79.5 [65–89] | 0.65 |
| eGFR, mL/min/1.73 m2 | 86 [76–90] | 88 [81–90] | 83 [74–90] | 78.5 [67–90] | 0.02* |
| Elevated troponin T or I | 19 (15.8) | 1 (1.9) | 9 (15.8) | 6 (60.0) | 0.00* |
| NT‐proBNP, ng/L | 115 [64–313] | 101 [50–142] | 174 [72–516] | 742 [383–1157] | 0.00* |
| Echocardiography | |||||
| IVS, mm | 11.0 ± 2.8 | 9.9 ± 2.4 | 11.5 ± 2.7 | 14.0 ± 3.0 | 0.00* |
| PW, mm | 8.9 ± 2.2 | 8.5 ± 1.8 | 8.8 ± 2.3 | 11.1 ± 2.6 | 0.00* |
| EDD, mm | 49.7 ± 6.4 | 49.9 ± 6.4 | 49.5 ± 6.7 | 50.1 ± 5.1 | 0.92 |
| ESD, mm | 36.6 ± 7.6 | 37.4 ± 7.6 | 35.8 ± 7.8 | 36.9 ± 6.1 | 0.54 |
| LVEF, % | 56.7 ± 7.2 | 56.2 ± 5.97 | 57.3 ± 7.7 | 55.6 ± 9.7 | 0.63 |
| GLS, % | −17.3 ± 2.7 | −17.5 ± 2.3 | −17.4 ± 2.9 | −15.5 ± 2.98 | 0.09 |
| RAS pattern | 43 (35.8) | 0 (0.0) | 34 (59.6) | 9 (90.0) | 0.02* , a |
| RASr | 1.26 [1.1–1.5] | 1.12 [1.05–1.25] | 1.4 [1.2–1.6] | 1.8 [1.3–2.3] | 0.00* |
| E/A ratio | 0.94 [0.755–1.14] | 0.89 [0.74–1.02] | 0.94 [0.75–1.22] | 1.23 [0.99–2.1] | 0.43* |
| E/e′ | 7.8 [6.0–10.0] | 6.7 [5.6–9.0] | 8.0 [6.2–10.6] | 10.2 [8.7–12.5] | 0.00* |
| LAVi, mL/m2 | 34.9 ± 13.7 | 29.3 ± 9.8 | 37.6 ± 12.1 | 46 ± 17.2 | 0.00* |
| TAPSE, mm | 22.9 ± 5.3 | 23.2 ± 5.9 | 22.4 ± 4.6 | 23.8 ± 6.1 | 0.63 |
| S′ | 13.0 ± 3.5 | 12.9 ± 3.0 | 12.7 ± 3.5 | 15.2 ± 5.2 | 0.14 |
| TRG, mmHg | 19.1 ± 8.4 | 18.7 ± 8.1 | 18.4 ± 8.1 | 24.8 ± 10.0 | 0.08 |
ATTR, transthyretin cardiac amyloidosis; CTS, carpal tunnel syndrome; EDD, end‐diastolic diameter; eGFR, estimated glomerular filtration rate; ESD, end‐systolic diameter; GLS, global longitudinal strain; IHD, ischaemic heart disease; IVS, interventricular septum; LAVi, left atrial volume index; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PW, posterior wall; RAS, relative apical sparring; RASr, relative apical sparring ratio; TAPSE, tricuspid annular plane systolic excursion; TRG, tricuspid return gradient.
Summary statistics are presented as median [interquartile range 25–75] or mean ± SD for continuous variables and as numbers (%) for categorical data.
Comparison of a red flag is performed between the two groups with values above 0, not all three.
P‐value < 0.05.
Red flags
In 67 patients (55.8%), a red flag screening criterion was met, and these patients were referred for a 99mTc‐DPD scintigraphy. Among patients referred for scintigraphy, 53 (79%) had Perugini Grade 0, 4 (6%) had Perugini Grade 1, and 10 (14.9%) had Perugini Grades 2–3. Patients with Perugini Grade 1 were referred for an MIBG scan with normal findings in three patients. One patient demonstrated signs of impaired cardiac sympathetic innervation, which might indicate ATTR involvement. Supplementary endomyocardial biopsies were taken and stained for cardiac amyloid in all four patients without providing any signs of cardiac amyloid deposition.
The prevalence of ATTRwt in the total study population was 8.3%. All patients with ATTR confirmed by 99mTc‐DPD scintigraphy had genetic testing performed, and no one had mutations compatible with ATTRv.
All patients with ATTRwt met two or more red flag criteria. The mean number of red flag criteria met was significantly higher in the ATTRwt group than those with a negative scintigraphy (4.0 ± 2.3 vs. 2.29 ± 0.98, P = 0.021).
ROC analysis of red flags showed the following AUCs: RAS pattern 0.71 [confidence interval (CI): 0.55–0.86], NT‐proBNP 0.78 (CI: 0.65–0.91), elevated cardiac troponin 0.72 (CI: 0.53–0.91), IVS ≥ 12 mm 0.68 (CI: 0.49–0.88), RASr ≥ 1.5 0.69 (0.51–0.87), and ECG 0.60 (CI: 0.39–0.80). An analysis of the number of red flags was performed with an AUC of 0.873 (CI: 0.77–0.97).
The sensitivity and specificity of the red flags were as follows: RAS pattern had a sensitivity of 90% and specificity of 51%, NT‐proBNP ≥ 300 ng/L had a sensitivity of 80% and a specificity of 70%, elevated cardiac troponin had a sensitivity of 60% and a specificity of 84%, IVS ≥ 12 mm had a sensitivity of 70% and a specificity of 34%, RASr ≥ 1.5 had a sensitivity of 60% and a specificity of 68%, and ECG had a sensitivity of 30% and a specificity of 89%. At three red flags, analysis showed a sensitivity of 90% and a specificity of 70%.
A graphic presentation of the ROC plots and AUCs is displayed in Figure 2 .
Figure 2.
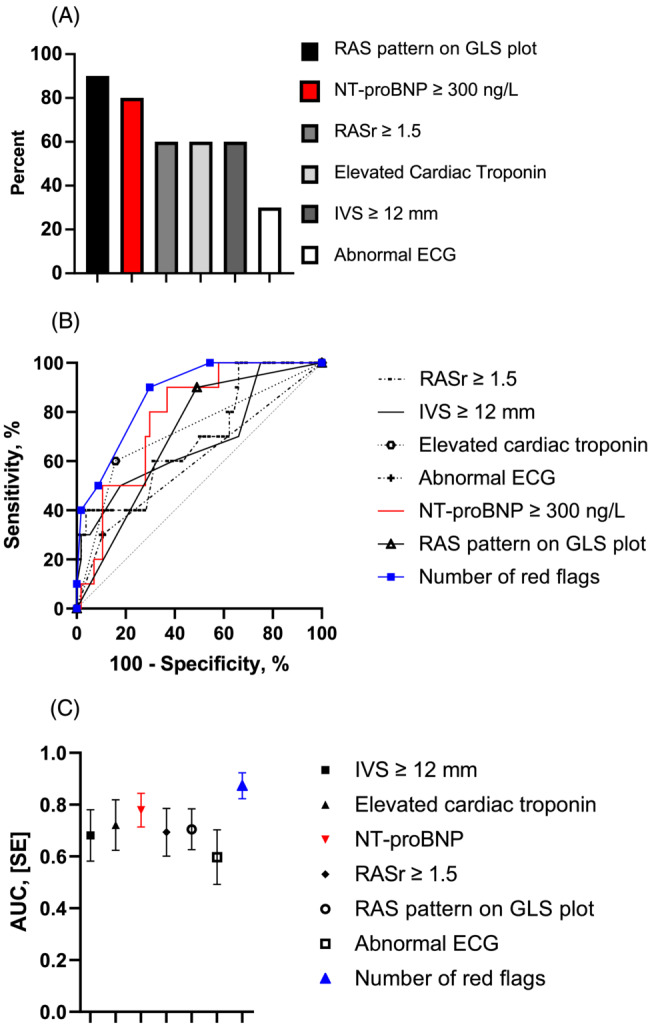
Sensitivity and specificity of red flags: (A) Bar chart, (B) receiver‐operating characteristic curves, and (C) area under the curve (AUC). ECG, electrocardiogram; GLS, global longitudinal strain; IVS, interventricular septum; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; RAS, relative apical sparring; RASr, relative apical sparring ratio.
Clinical characteristics and echocardiography
The clinical, biomarker, and echocardiographic characteristics of the study population and its groups are shown in Table 1 . Patients with confirmed ATTRwt were generally older (P < 0.001) and were more likely to have low voltage on ECG (P = 0.002). Patients with diagnosed ATTRwt had a lower eGFR than the other groups, though within the normal range (78.5 mL/min/1.73 m2 vs. 88 and 83 mL/min/1.73 m2, P = 0.02). ATTRwt participants were also more likely to present with elevated biomarker levels of cardiac troponin (60% vs. 7.5% and 15.8%, P < 0.001) and abnormal levels of NT‐proBNP (742 ng/L vs. 101 and 174 ng/L, P < 0.001) compared with the other groups.
On echocardiography, patients with ATTRwt had significantly more LVH of both the IVS and posterior wall compared with the other groups (P < 0.001). The LV systolic function was comparable, as LVEF and LV‐GLS did not differ significantly between groups. Of notice, LVEF was preserved in all groups, whereas LV‐GLS was borderline reduced in the ATTRwt group. LV‐GLS plot analysis showed a significantly higher presence of RAS patterns in the ATTRwt group than in the ATTR not confirmed group (90% vs. 59.6%, P = 0.018), and the ATTRwt group also had a significantly higher RASr (1.71 vs. 1.14 and 1.39, P < 0.001) than the other groups.
A detailed overview of the patients diagnosed with ATTRwt with respect to individual clinical characteristics, red flags, diagnostics procedures, and results is presented in Tables 2 and 3 .
Table 2.
Diagnostic characteristics of patients with confirmed ATTRwt
| Patient | Amyloid type | Age, years | Gender (M/F) | Years since CTS | History of spinal stenosis | Kappa, mg/L | Lambda, mg/L | K/L ratio | Monoclonal spike band | DPD Perugini grade | Myocardial biopsy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | ATTRwt | 71 | M | 5.6 | No | 26.3 | 15.1 | 1.75 | No | 3 | No |
| #2 | ATTRwt | 71 | M | 5.4 | No | 13.3 | 15.7 | 0.85 | No | 3 | No |
| #3 | ATTRwt | 90 | M | 4.7 | No | 12.7 | 12.1 | 1.05 | No | 2 | Yes |
| #4 | ATTRwt | 87 | M | 4.7 | No | 23.3 | 19.9 | 1.17 | No | 2 | No |
| #5 | ATTRwt | 69 | M | 3.7 | No | 22.5 | 18.0 | 1.25 | No | 3 | Yes |
| #6 | ATTRwt | 83 | M | 2.9 | No | 27.7 | 11.2 | 2.47 | No | 3 | Yes |
| #7 | ATTRwt | 84 | M | 2.9 | No | 35.2 | 30.0 | 1.17 | Yes | 3 | Yes |
| #8 | ATTRwt | 87 | F | 2.7 | Yes | 22.3 | 14.4 | 1.55 | No | 3 | No |
| #9 | ATTRwt | 85 | M | 6.5 | No | 20.0 | 18.6 | 1.08 | No | 3 | Yes |
| #10 | ATTRwt | 79 | M | 2.9 | No | 45.8 | 6.6 | 7.0 | Yes | 2 | Yes |
ATTRwt, wild‐type transthyretin cardiac amyloidosis; CTS, carpal tunnel syndrome; DPD, technetium‐99m 3,3‐diphosphono‐1,2‐propanodicarboxylic acid scintigraphy.
Table 3.
Clinical, biomarker, ECG, and echocardiographic characteristics of patients with confirmed ATTRwt
| Patient | NYHA class | Elevated cardiac troponin | NT‐proBNP, ng/L | eGFR, mL/min/1.73 m2 | NAC stage | IVS, mm | PW, mm | LVEF, % | GLS, % | RAS pattern | RASr | Pseudoinfarction | Low voltage | AV conduction disorder | Number of red flags |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | I | Yes | 383 | 88 | I | 17 | 14 | 56 | −13.9 | Yes | 1.94 | No | Yes | No | 6 |
| #2 | II | Yes | 403 | >90 | I | 11 | 10 | 53 | −16.4 | Yes | 1.40 | No | No | No | 3 |
| #3 | I | Yes | 5303 | 50 | II | 13 | 11 | 49 | −11.5 | Yes | 2.05 | No | No | No | 5 |
| #4 | I | No | 1080 | 67 | I | 14 | 9 | 40 | −11.9 | Yes | 1.33 | No | Yes | No | 4 |
| #5 | I | Yes | 399 | >90 | I | 11 | 9 | 58 | −18.8 | No | 1.30 | No | No | No | 2 |
| #6 | II | No | 1752 | 79 | I | 18 | 16 | 76 | −15.9 | Yes | 2.48 | Yes | Yes | No | 6 |
| #7 | I | Yes | 284 | 74 | I | 11 | 8 | 56 | −18.6 | Yes | 1.50 | No | No | No | 3 |
| #8 | II | No | 1149 | 43 | II | 14 | 12 | 63 | −16.6 | Yes | 1.31 | No | No | No | 3 |
| #9 | I | Yes | 1157 | 78 | I | 19 | 13 | 47 | −12.2 | Yes | 2.29 | No | No | No | 5 |
| #10 | I | No | 96 | >90 | I | 12 | 8 | 58 | −19.4 | Yes | 1.50 | No | No | No | 3 |
ATTRwt, wild‐type transthyretin cardiac amyloidosis; AV, atrioventricular; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; GLS, global longitudinal strain; IVS, interventricular septum; LVEF, left ventricular ejection fraction; NAC, National Amyloidosis Centre; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PW, posterior wall; RAS, relative apical sparring; RASr, relative apical sparring ratio.
Patients with ATTRwt diagnosed by screening compared with patients with clinical ATTRwt
In Table 4 , the clinical characteristics, disease stage, and echocardiographic parameters of the patients with screened ATTRwt were compared with a matched control group of contemporary patients with ATTRwt (n = 51) diagnosed by routine clinical practice.
Table 4.
Comparison of characteristics between CTS‐screened patients with ATTRwt versus clinically diagnosed ATTRwt
| CTS‐screened ATTRwt (n = 10) | Matched clinical ATTRwt (n = 51) | P‐values | |
|---|---|---|---|
| Clinical | |||
| Age at diagnosis, years | 81.2 ± 7.6 | 79.7 ± 5.3 | 0.46 |
| Male | 9 (90) | 46 (90.4) | 0.97 |
| Hypertension | 6 (60.0) | 37 (72.5) | 0.43 |
| IHD | 2 (20.0) | 12 (23.5) | 0.81 |
| Atrial fibrillation | 2 (20.0) | 21 (40.3) | 0.24 |
| Pacemaker status | 0 (0.0) | 15 (29.4) | 0.04* |
| NYHA class (I/II/III–IV) | 7/3/0 (70/30/0) | 7/35/9 (13.5/69.2/17.3) | 0.00* |
| NAC stage (I/II/III) | 8/2/0 (80/20/0) | 22/17/12 (43.1/33.3/23.5) | 0.09 |
| Medications | |||
| Loop diuretics | 1 (10.0) | 38 (73.1) | 0.00* |
| ACE/ARB | 5 (50.0) | 31 (60.8) | 0.53 |
| Anticoagulants | 1 (10.0) | 32 (62.7) | 0.00* |
| Laboratory | |||
| Creatinine, μmol/L | 79.5 [65–89] | 98.1 [83–138] | 0.01* |
| eGFR, mL/min/1.73 m2 | 78.5 [67–90] | 60.0 [44–80] | 0.04* |
| Elevated troponin T or I | 6 (60.0) | 41 (80.4) | 0.15 |
| NT‐proBNP, ng/L | 742 [383–1157] | 2523 [980–4990] | 0.00* |
| Echocardiography | |||
| IVS, mm | 14.0 ± 3.0 | 17.3 ± 2.9 | 0.00* |
| PW, mm | 11.0 ± 2.6 | 14.1 ± 2.8 | 0.01* |
| LVEF, % | 55.6 ± 9.7 | 44.2 ± 10.5 | 0.02* |
| GLS, % | −15.5 ± 2.98 | −11.0 ± 3.1 | 0.01* |
| RASr | 1.8 [1.3–2.3] | 2.7 [2.2–3.5] | 0.01* |
| E/A ratio | 1.2 [0.99–2.1] | 1.5 [0.9–2.5] | 0.16 |
| E/e′ | 10.2 [8.7–12.5] | 12.3 [9.0–15.6] | 0.30 |
| TAPSE, mm | 23.8 ± 6.1 | 17.1 ± 4.2 | 0.00* |
ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; ATTRwt, wild‐type transthyretin cardiac amyloidosis; CTS, carpal tunnel syndrome; eGFR, estimated glomerular filtration rate; GLS, global longitudinal strain; IHD, ischaemic heart disease; IVS, interventricular septum; LVEF, left ventricular ejection fraction; NAC, National Amyloidosis Centre; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PW, posterior wall; RASr, relative apical sparring ratio; TAPSE, tricuspid annular plane systolic excursion.
Summary statistics are presented as median [interquartile range 25–75] or mean ± SD for continuous variables and as numbers (%) for categorical data.
P < 0.05.
None of the CTS‐screened patients with ATTRwt had a pacemaker device implanted prior to diagnosis in contrast to the clinically diagnosed ATTRwt group (0% vs. 29.4%, P < 0.04). A lower NAC disease stage was observed in the screened ATTRwt group than among the matched ATTRwt controls, but the difference was non‐significant (P = 0.09). The renal function estimated by eGFR was preserved in patients with screened ATTRwt compared with the clinical ATTRwt control group (78.5 mL/min/1.73 m2 vs. 60.0 mL/min/1.73 m2, P = 0.04), and significantly lower levels of NT‐proBNP were noted (742 ng/L vs. 2523 ng/L, P = 0.00). HF treatment differed as the CTS‐screened patients with ATTRwt were significantly less likely to receive medical treatment with loop diuretics (10% vs. 73%, P = 0.00) or anticoagulants (10% vs. 62.7%, P = 0.00) than the clinically diagnosed ATTRwt group. The LV structural changes differed significantly as the screened ATTRwt group demonstrated less pronounced LVH than the clinically diagnosed group (IVS: 14.0 mm vs. 17.3 mm, P = 0.00 and posterior wall: 11.0 mm vs. 14.1 mm, P = 0.01). The LV function of the screened ATTRwt patients was generally less affected than the clinical ATTRwt patients with respect to both LVEF (55.6% vs. 44.2%, P = 0.02) and LV‐GLS (−15.5% vs. −11.0%, P = 0.01). There was no significant difference in diastolic function between the screened patients with ATTRwt and the patients with clinical ATTRwt with respect to E/e′ ratio (10.2 vs. 12.3, P = 0.30) and E/A ratio (1.2 vs. 1.5, P = 0.16). Right ventricular systolic function seemed preserved in the screened ATTRwt group in contrast to patients with clinically diagnosed ATTRwt as evaluated by tricuspid annular plane systolic excursion (23.8 mm vs. 17.1 mm, P < 0.00) (Figure 3 ).
Figure 3.
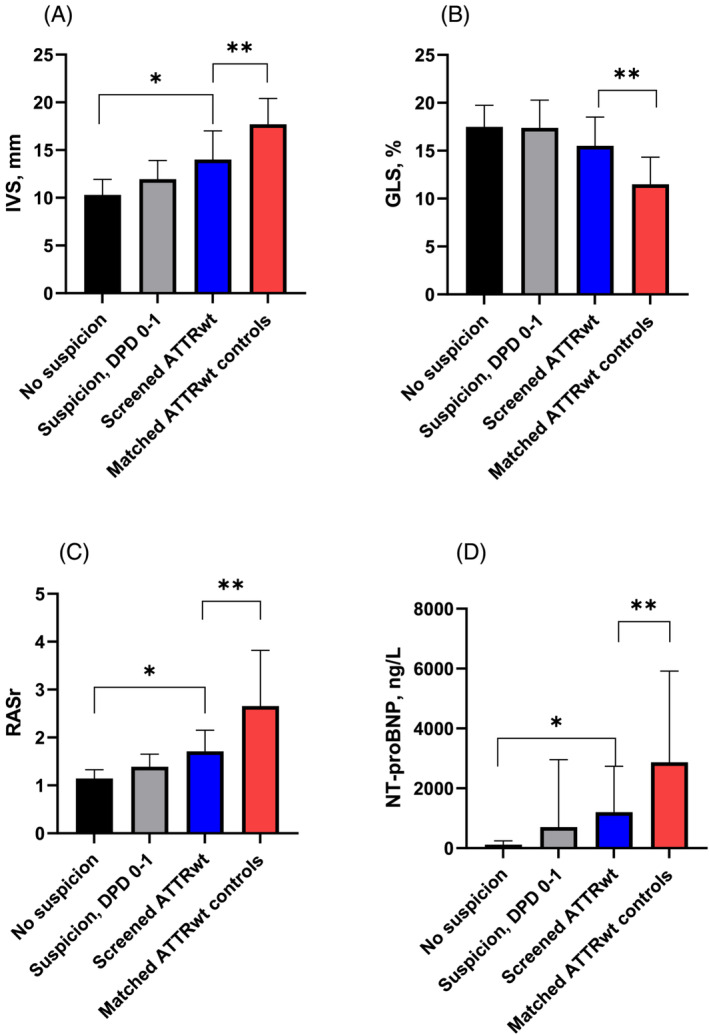
Comparison of notable red flags in the study population and the matched ATTRwt controls. (A) IVS, (B) GLS, (C) RASr, and (D) NT‐proBNP. ATTRwt, wild‐type transthyretin cardiac amyloidosis; DPD, technetium‐99m 3,3‐diphosphono‐1,2‐propanodicarboxylic acid scintigraphy; GLS, global longitudinal strain; IVS, interventricular septum; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; RASr, relative apical sparring ratio. *Significant difference between ‘suspicion, confirmed ATTRwt’, ‘no suspicion’, and ‘suspicion, not confirmed’. **Significant difference between ‘matched clinical ATTRwt’ and ‘suspicion, confirmed ATTRwt’.
Discussion
In the present study, we found an ATTRwt prevalence of 8.3% in patients aged ≥60 years with a recent history of surgery for idiopathic CTS. Patients identified through screening were all ATTRwt, were predominantly asymptomatic, had a mildly increased LV wall thickness, had a mildly elevated NT‐proBNP, and frequently presented with LV apical sparring patterns on strain echocardiography. Compared with patients with clinical ATTRwt matched by age, gender, and CTS surgery, the screened patients with ATTRwt demonstrated significantly less affected structural and functional echocardiographic parameters.
The association between CTS and ATTR is well established and might be utilized to reduce the diagnostic delay observed in the condition, thereby achieving diagnosis at a stage of less advanced disease. 4 Recent studies have indicated that screening of patients undergoing surgery for idiopathic CTS may allow for earlier diagnosis of amyloid cardiomyopathy. 3 , 23 , 24 , 25 An important aspect is the temporal relationship between CTS and development of ATTR cardiomyopathy, which has recently been investigated in a retrospective study demonstrating that the probability of diagnosing ATTRwt was highest 5–9 years after CTS surgery. 4 However, no data were presented relating to the degree of structural and functional cardiac involvement in the patients with ATTR. Screening patients aged ≥60 years at the time of CTS five or more years after surgery may yield better results in terms of prevalence of ATTRwt but naturally also increases the risk of finding patients when their disease is no longer in the pre‐clinical stage. Further research is needed to specify the optimal time of screening for ATTRwt in patients with recent CTS.
The risk of developing ATTRwt cardiomyopathy in patients operated for CTS increases greatly after the sixth decade. 4 Therefore, we predominantly screened males aged ≥60 years at the time of CTS surgery in the period 1 to 6 years after surgery. The suspicion of ATTR was raised by the presence of red flags following evaluation of ECG, biomarkers, and echocardiography as suggested by expert consensus and position statements on the diagnosis of cardiac amyloidosis (CA). 9 , 26 The suspicion of ATTR was raised for 56% of the patients undergoing screening who then had 99mTc‐DPD scintigraphy performed. Scintigraphy results demonstrated an ATTR prevalence of 8.3%. These numbers are lower than Tanskanen et al. 27 who found 25% of their autopsied study population to have cardiac amyloid deposits. A few things should be noted, namely, that the mean age of the study population in Tanskanen et al. was very advanced (mean 92.5, range 85–106) and that 78% of their population had only a mild degree of deposition, which we do not know how it correlates to the bone tracer uptake on a 99mTc‐DPD scintigraphy.
The presence of three red flags showed a sensitivity of 90% and a specificity of 70%. Several parameters seem to be of importance including age, gender, optimal timing of the cardiac screening, and investigations used to raise suspicion of CA. A consensus screening method for ATTR has yet to be established.
In the present study, we used red flags associated with ATTRwt such as ECG findings, elevated serum troponin levels, increased NT‐proBNP levels, and echocardiographic findings. 9 , 13 , 26 As expected, the ECG findings were not sensitive of ATTRwt. Thus, findings such as low voltage, atrioventricular conduction disorders, and pseudoinfarction patterns were recognized in only 40% of the patients. The biomarkers were more sensitive than ECG findings especially NT‐proBNP, which was elevated in 80% of the patients diagnosed with ATTRwt. The frequently observed elevation of NT‐proBNP could potentially be used as a screening tool to raise the suspicion of ATTR cardiomyopathy in patients with previous CTS surgery. Of notice, 30% of the patients diagnosed with ATTRwt did not demonstrate signs of LVH indicating mild ATTRwt cardiomyopathy. Strain echocardiography seems to be a central parameter in the evaluation of potential ATTR as a RAS pattern was present in nearly all patients (90%) and the calculation of a RASr ≥ 1.5 was noted for 60% of the patients. 9 , 16 , 28 , 29 As expected, no single parameter was consistently abnormal in all the patients with ATTRwt; and thus, the combination of the different red flags is essential even though elevation of serum NT‐proBNP and the presence of a RAS pattern were the most frequently occurring and therefore the most important.
To document that the patients with ATTRwt identified by the screening were genuinely diagnosed in an early disease stage, they were compared with matched contemporary patients with ATTRwt diagnosed through routine clinical practice. We demonstrated that screened ATTRwt patients were mainly asymptomatic, had a lower tendency for treatment with loop diuretics, had no pacemaker devices implanted, had lower levels of NT‐proBNP, and had preserved eGFR. In addition, the LV wall thickness was only mildly increased, and the LV systolic function was less affected in patients with screened ATTRwt than in patients diagnosed from routine clinical practice. The establishment of early‐stage ATTRwt with less cardiac involvement seems possible in a subset of patients with recent CTS surgery, which may be of benefit when initiating medical therapy to slow disease progression and improve prognosis. Our red flag screening approach using ECG, biomarkers analysis, and strain echocardiography is generally widely accessible, safe, and may be performed at relatively low costs. Supplementary bone scintigraphy was indicated in approximately half of all screened patients. The presented screening algorithm may possibly be adjusted or simplified depending on local practices and findings from similar future studies.
Study limitations
One obvious limitation is that only a third of the invited patients with CTS responded to our repeated written invitations for cardiac screening for potential CA. This may potentially affect the reported prevalence of ATTRwt because people with symptoms of cardiac disease such as dyspnoea would probably be more likely to participate in the study. Among the 10 patients with confirmed ATTRwt in this study, symptoms of heart disease were generally uncommon with 70% citing no limitations to physical activity.
For clinical purposes, the selection of patients scheduled for cardiac examination could be guided by the tenosynovial detection of transthyretin amyloid or by elevated biomarker levels of NT‐proBNP. In the present study, we focused on ATTRwt as the most prevalent form of amyloid cardiomyopathy, and the age and gender criteria were predefined accordingly. It seems more difficult to determine the optimal age and gender criteria with respect to ATTRv. The genetic ATTR mutations differ substantially with respect to both age, phenotype, and development of CTS. Furthermore, both ethnicity and geographic factors influence the prevalence of the specific ATTR mutations. 26
Another limitation of the study is the gender distribution, which favoured male gender at a 9:1 ratio. To optimize clinical screening for ATTR in patients with CTS, decisions regarding screening of female patients must be made. Females are generally less likely to develop ATTRwt and often do so at a more advanced age than males. This study found one female patient with ATTRwt aged 87 years at the time of diagnosis. Possibly, the screening age of female patients with CTS should be set higher than that of males, thereby potentially increasing the yield of the screening.
Our study did not specify separate NT‐proBNP criteria for patients with atrial fibrillation. Patients with atrial fibrillation will often present with elevated levels of NT‐proBNP, which may have created some false positives in our screening algorithm. In our study, 14% of the referred patients with Perugini Grade 0 had atrial fibrillation, suggesting a modest impact on our findings.
We elected to use bone tracer scintigraphy as the diagnostic assessment in our study due to its lower waiting time at our hospital. Because the scintigraphy includes a small dose of radiation, increasing lifetime cancer risk, cardiac magnetic resonance imaging could have been used as a radiation free alternative. Because the scintigraphy used at our facility increased lifetime cancer risk by only 0.025 percentage points, we considered it an acceptable risk.
Conclusions
This study found an ATTRwt prevalence of 8.3% in patients aged 60 years or older with a recent history of idiopathic CTS. This methodological approach identified patients with ATTRwt who were predominantly asymptomatic, presented with mildly elevated biomarkers, and had a normal to mildly increased LV wall thickness, preserved LVEF, and an only slightly impaired LV longitudinal systolic function. The patients with ATTRwt diagnosed by screening demonstrated significantly less cardiac structural change and functional impairment than patients with ATTRwt diagnosed by routine clinical practice, which is indicative of an early disease stage. These findings have important clinical implications as pre‐clinical ATTRwt detection is a valuable tool to initiate timely treatment and halt disease progression effectively.
Conflict of interest
The authors declare no competing financial interests.
Funding
None.
Acknowledgement
The authors would like to acknowledge the work of sonographer Lene Lindencrone Konrad.
Ladefoged, B. , Clemmensen, T. , Dybro, A. , Hartig‐Andreasen, C. , Kirkeby, L. , Gormsen, L. C. , Bomholt, P. , Gillmore, J. , and Poulsen, S. H. (2023) Identification of wild‐type transthyretin cardiac amyloidosis in patients with carpal tunnel syndrome surgery (CACTuS). ESC Heart Failure, 10: 234–244. 10.1002/ehf2.14173.
References
- 1. Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2019; 73: 2872–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sekijima Y, Uchiyama S, Tojo K, Sano K, Shimizu Y, Imaeda T, Hoshii Y, Kato H, Ikeda SI. High prevalence of wild‐type transthyretin deposition in patients with idiopathic carpal tunnel syndrome: a common cause of carpal tunnel syndrome in the elderly. Hum Pathol. 2011; 42: 1785–1791. [DOI] [PubMed] [Google Scholar]
- 3. Sugiura K, Kozuki H, Ueba H, Kubo T, Ochi Y, Baba Y, Miyagawa K, Noguchi T, Hirota T, Yamasaki N, Wada N, Nakashima J, Murakami I, Ikeuchi M, Kitaoka H. Tenosynovial and cardiac transthyretin amyloidosis in Japanese patients undergoing carpal tunnel release. Circ Rep. 2021; 3: 338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Milandri A, Farioli A, Gagliardi C, Longhi S, Salvi F, Curti S, Foffi S, Caponetti AG, Lorenzini M, Ferlini A, Rimessi P, Mattioli S, Violante FS, Rapezzi C. Carpal tunnel syndrome in cardiac amyloidosis: implications for early diagnosis and prognostic role across the spectrum of aetiologies. Eur J Heart Fail. 2020; 22: 507–515. [DOI] [PubMed] [Google Scholar]
- 5. Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez‐Naharro A, Quarta CC, Rezk T, Whelan CJ, Gonzalez‐Lopez E, Lane T, Gilbertson JA, Rowczenio D, Petrie A, Hawkins PN. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018; 39: 2799–2806. [DOI] [PubMed] [Google Scholar]
- 6. Bishop E, Brown EE, Fajardo J, Barouch LA, Judge DP, Halushka MK. Seven factors predict a delayed diagnosis of cardiac amyloidosis. Amyloid. 2018; 25: 174–179. [DOI] [PubMed] [Google Scholar]
- 7. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington‐Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018; 379: 1007–1016. [DOI] [PubMed] [Google Scholar]
- 8. Zegri‐Reiriz I, de Haro‐del Moral FJ, Dominguez F, Salas C, de la Cuadra P, Plaza A, Krsnik I, Gonzalez‐Lopez E, Garcia‐Pavia P. Prevalence of cardiac amyloidosis in patients with carpal tunnel syndrome. J Cardiovasc Transl Res. 2019; 12: 507–513. [DOI] [PubMed] [Google Scholar]
- 9. Garcia‐Pavia P, Rapezzi C, Adler Y, Arad M, Basso C, Brucato A, Burazor I, Caforio ALP, Damy T, Eriksson U, Fontana M, Gillmore JD, Gonzalez‐Lopez E, Grogan M, Heymans S, Imazio M, Kindermann I, Kristen AV, Maurer MS, Merlini G, Pantazis A, Pankuweit S, Rigopoulos AG, Linhart A. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2021; 42: 1554–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vianello PF, la Malfa G, Tini G, Mazzola V, Miceli A, Santolini E, Briano S, Porto I, Canepa M. Prevalence of transthyretin amyloid cardiomyopathy in male patients who underwent bilateral carpal tunnel surgery: the ACTUAL study. Int J Cardiol. 2021; 329: 144–147. [DOI] [PubMed] [Google Scholar]
- 11. Porcari A, Pagura L, Longo F, Sfriso E, Barbati G, Murena L, Longo E, Ramella V, Arnež ZM, Rapezzi C, Merlo M, Sinagra G. Prognostic significance of unexplained left ventricular hypertrophy in patients undergoing carpal tunnel surgery. ESC Heart Fail. 2022; 9: 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sood RF, Kamenko S, McCreary E, Sather BK, Schmitt M, Peterson SL, Lipira AB. Diagnosing systemic amyloidosis presenting as carpal tunnel syndrome: a risk nomogram to guide biopsy at time of carpal tunnel release. J Bone Joint Surg Am. 2021; 103: 1284–1294. [DOI] [PubMed] [Google Scholar]
- 13. Takashio S, Yamamuro M, Izumiya Y, Hirakawa K, Marume K, Yamamoto M, Ueda M, Yamashita T, Ishibashi‐Ueda H, Yasuda S, Ogawa H, Ando Y, Anzai T, Tsujita K. Diagnostic utility of cardiac troponin T level in patients with cardiac amyloidosis. ESC Heart Fail. 2018; 5: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pinney JH, Whelan CJ, Petrie A, Dungu J, Banypersad SM, Sattianayagam P, Wechalekar A, Gibbs SDJ, Venner CP, Wassef N, McCarthy CA, Gilbertson JA, Rowczenio D, Hawkins PN, Gillmore JD, Lachmann HJ. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013; 2: e000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lehrke S, Steen H, Kristen AV, Merten C, Lossnitzer D, Dengler TJ, Katus HA, Giannitsis E. Serum levels of NT‐proBNP as surrogate for cardiac amyloid burden: new evidence from gadolinium‐enhanced cardiac magnetic resonance imaging in patients with amyloidosis. Amyloid. 2009; 16: 187–195. [DOI] [PubMed] [Google Scholar]
- 16. Phelan D, Collier P, Thavendiranathan P, Popović ZB, Hanna M, Plana JC, Marwick TH, Thomas JD. Relative apical sparing of longitudinal strain using two‐dimensional speckle‐tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012; 98: 1442–1448. [DOI] [PubMed] [Google Scholar]
- 17. Bay K, Gustafsson F, Maiborg M, Bagger‐Bahnsen A, Strand AM, Pilgaard T, Poulsen SH. Suspicion, screening, and diagnosis of wild‐type transthyretin amyloid cardiomyopathy: a systematic literature review. ESC Heart Fail. 2022; 9: 1524–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. González‐López E, Gagliardi C, Dominguez F, Quarta CC, de Haro‐del Moral FJ, Milandri A, Salas C, Cinelli M, Cobo‐Marcos M, Lorenzini M, Lara‐Pezzi E, Foffi S, Alonso‐Pulpon L, Rapezzi C, Garcia‐Pavia P. Clinical characteristics of wild‐type transthyretin cardiac amyloidosis: disproving myths. Eur Heart J. 2017; 38: 1895–1904. [DOI] [PubMed] [Google Scholar]
- 19. Shah RJ, Pan S. Sex and the protein: evaluating the role of sex in the diagnosis, presentation, and clinical outcomes in cardiac amyloidosis. Int J Cardiol. 2022; 355: 28–29. [DOI] [PubMed] [Google Scholar]
- 20. Kitaoka H, Izumi C, Izumiya Y, Inomata T, Ueda M, Kubo T, Koyama J, Sano M, Sekijima Y, Tahara N, Tsukada N, Tsujita K, Tsutsui H, Tomita T, Amano M, Endo J, Okada A, Oda S, Takashio S, Baba Y, Misumi Y, Yazaki M, Anzai T, Ando Y, Isobe M, Kimura T, Fukuda K, the Japanese Circulation Society Joint Working Group . JCS 2020 guideline on diagnosis and treatment of cardiac amyloidosis. Circ J. 2020; 84: 1610–1671. [DOI] [PubMed] [Google Scholar]
- 21. Perugini E, Guidalotti PL, Salvi F, Cooke RMT, Pettinato C, Riva L, Leone O, Farsad M, Ciliberti P, Bacchi‐Reggiani L, Fallani F, Branzi A, Rapezzi C. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc‐3,3‐diphosphono‐1,2‐propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005; 46: 1076–1084. [DOI] [PubMed] [Google Scholar]
- 22. Clemmensen TS, Eiskjær H, Mikkelsen F, Granstam SO, Flachskampf FA, Sørensen J, Poulsen SH. Left ventricular pressure‐strain‐derived myocardial work at rest and during exercise in patients with cardiac amyloidosis. J Am Soc Echocardiogr. 2020; 33: 573–582. [DOI] [PubMed] [Google Scholar]
- 23. Sperry BW, Reyes BA, Ikram A, Donnelly JP, Phelan D, Jaber WA, Shapiro D, Evans PJ, Maschke S, Kilpatrick SE, Tan CD, Rodriguez ER, Monteiro C, Tang WHW, Kelly JW, Seitz WH Jr, Hanna M. Tenosynovial and cardiac amyloidosis in patients undergoing carpal tunnel release. J Am Coll Cardiol. 2018; 72: 2040–2050. [DOI] [PubMed] [Google Scholar]
- 24. Aus dem Siepen F, Hein S, Prestel S, Baumgärtner C, Schönland S, Hegenbart U, Röcken C, Katus HA, Kristen AV. Carpal tunnel syndrome and spinal canal stenosis: harbingers of transthyretin amyloid cardiomyopathy? Clin Res Cardiol. 2019; 108: 1324–1330. [DOI] [PubMed] [Google Scholar]
- 25. Nakagawa M, Sekijima Y, Yazaki M, Tojo K, Yoshinaga T, Doden T, Koyama J, Yanagisawa S, Ikeda SI. Carpal tunnel syndrome: a common initial symptom of systemic wild‐type ATTR (ATTRwt) amyloidosis. Amyloid. 2016; 23: 58–63. [DOI] [PubMed] [Google Scholar]
- 26. Maurer MS, Bokhari S, Damy T, Dorbala S, Drachman BM, Fontana M, Grogan M, Kristen AV, Lousada I, Nativi‐Nicolau J, Cristina Quarta C, Rapezzi C, Ruberg FL, Witteles R, Merlini G. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail. 2019; 12: e006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanskanen M, Peuralinna T, Polvikoski T, Notkola IL, Sulkava R, Hardy J, Singleton A, Kiuru‐Enari S, Paetau A, Tienari PJ, Myllykangas L. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2‐macroglobulin and tau: a population‐based autopsy study. Ann Med. 2008; 40: 232–239. [DOI] [PubMed] [Google Scholar]
- 28. Boldrini M, Cappelli F, Chacko L, Restrepo‐Cordoba MA, Lopez‐Sainz A, Giannoni A, Aimo A, Baggiano A, Martinez‐Naharro A, Whelan C, Quarta C, Passino C, Castiglione V, Chubuchnyi V, Spini V, Taddei C, Vergaro G, Petrie A, Ruiz‐Guerrero L, Moñivas V, Mingo‐Santos S, Mirelis JG, Dominguez F, Gonzalez‐Lopez E, Perlini S, Pontone G, Gillmore J, Hawkins PN, Garcia‐Pavia P, Emdin M, Fontana M. Multiparametric echocardiography scores for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2020; 13: 909–920. [DOI] [PubMed] [Google Scholar]
- 29. Chacko L, Martone R, Bandera F, Lane T, Martinez‐Naharro A, Boldrini M, Rezk T, Whelan C, Quarta C, Rowczenio D, Gilbertson JA, Wongwarawipat T, Lachmann H, Wechalekar A, Sachchithanantham S, Mahmood S, Marcucci R, Knight D, Hutt D, Moon J, Petrie A, Cappelli F, Guazzi M, Hawkins PN, Gillmore JD, Fontana M. Echocardiographic phenotype and prognosis in transthyretin cardiac amyloidosis. Eur Heart J. 2020; 41: 1439–1447. [DOI] [PubMed] [Google Scholar]


