Abstract
Heart failure is a complex disease with a poor prognosis. A number of widely used prognostic tools have limitations, so efforts to identify novel predictive markers and measures are important. As a metabolomics tool, amino acid profiling has shown promise in predicting heart failure prognosis; however, the evidence has not yet been sufficiently evaluated. We describe the utilization of amino acids in the healthy heart and in heart failure before reviewing the literature on amino acid profiling for prognostic prediction. We expertly interpret the findings and provide suggestions for future research to advance the understanding of the prognostic potential of amino acid profiling in heart failure. Our analysis revealed correlations between amino acid biomarkers and traditional prognostic factors, the additional prognostic value of amino acid biomarkers over traditional prognostic factors, and the successful use of amino acid biomarkers to distinguish heart failure aetiology. Although certain amino acid biomarkers have demonstrated additional prognostic value over traditional measures, such as New York Heart Association functional class, these measures are deeply rooted in clinical practice; thus, amino acid biomarkers may be best placed as additional prognostic tools to improve current risk stratification rather than as surrogate tools. Once the metabolic profiles of different heart failure aetiologies have been clearly delineated, the amino acid biomarkers with the most value in prognostic prediction should be determined. Amino acid profiling could be useful to evaluate the pathophysiology and metabolic status of different heart failure cohorts, distinguish heart failure aetiologies, and improve risk stratification and prognostic prediction.
Keywords: Amino acid profiling, Heart failure, Metabolomics, Prognosis, Risk stratification
Introduction
Heart failure is a global public health concern. Worldwide, the number of patients with heart failure increased from 33.5 million in 1990 to 64.3 million in 2017. 1 In addition to its increasing prevalence, heart failure is associated with poor survival. In 2019, a large study of 55 959 patients with heart failure showed a survival rate of 75.9% at 1 year, 45.5% at 5 years, 24.5% at 10 years, and 12.7% at 15 years. 2 As well as being associated with a poor prognosis, heart failure is complicated by the large number of possible aetiologies, including myocardial infarction, myocarditis, and cardiomyopathy. Other cardiovascular‐related insults, such as hypertension, diabetes mellitus, atrial fibrillation, valvular disease, and coronary artery disease, can also lead to heart failure. These variable aetiologies mean that there is substantial heterogeneity in the clinical progression and prognosis of patients with heart failure. 3 As such, efforts to improve the clinical management of patients with heart failure, including diagnosis, prognostic prediction, and treatment, are continuously evolving.
There are a large number of widely used prognostic prediction factors for heart failure, such as New York Heart Association functional class, 4 prior heart failure hospitalization, 5 brain natriuretic peptide concentration, 6 resting cardiac output, 7 and cardiac power index, 8 among others. 9 , 10 Comprehensive risk‐scoring tools for heart failure are also widely used, including Get With the Guidelines‐Heart Failure, 11 , 12 , 13 the Emergency Heart Failure Mortality Risk Grade, 14 the Multiple Estimation of Risk based on the Emergency department Spanish Score in patients with Acute Heart Failure score, 15 the Heart Failure Survival Score, 16 and the AHEAD score 17 , 18 for acute heart failure; and the Seattle Heart Failure Model 19 , 20 and the Meta‐Analysis Global Group in Chronic Heart Failure risk score 21 , 22 for chronic heart failure. Other indices are used to assess the nutritional status of patients with heart failure 23 , 24 , 25 and are calculated based on body weight, albumin concentration, cholesterol concentration, and lymphocyte count. In addition, the Global Leadership Initiative on Malnutrition, which is a new framework of nutritional indicators, can also be used in patients with heart failure due to the presence of systemic inflammation. 26 However, some of these prognostic methods have demonstrated important limitations; for example, New York Heart Association functional class has high inter‐operator variability. 27 Moreover, according to a published study, prior heart failure hospitalization does not predict 180 day mortality. 5 In addition, general risk stratification tools, such as the Acute Physiology and Chronic Health Evaluation II and the Sequential Organ Failure Assessment, 28 , 29 which can be used to assess the severity of disease and prognosis of patients admitted to the intensive care unit, including patients with acute heart failure, have high inter‐operator variability. In one analysis, only 48% of Sequential Organ Failure Assessment scores were fully in agreement with gold‐standard assessment by two expert examiners due to inconsistency among clinicians. 30 Thus, despite the availability of validated tools and measurement indices, prognostic accuracy for patients with heart failure remains poor, and there is still scope for improvement.
Given the limitations of these predictive measures, efforts to identify novel biomarkers that could be incorporated into existing risk stratification tools to improve prognostic prediction and inform patient outcomes could hold the key to future progress. For example, researchers have shown that the discriminatory ability of the Get With the Guidelines‐Heart Failure tool was improved by addition of brain natriuretic peptide concentration. 12
Metabolomics is a relatively new and rapidly expanding research tool that can be used to measure metabolites, such as amino acids, in biological samples, such as plasma, saliva, urine, and tissues. As a metabolomics tool, amino acid profiling using high‐resolution mass spectroscopy or nuclear magnetic resonance spectroscopy is useful to study disease pathogenesis, 31 identify novel biomarkers of prognosis, and identify possible therapeutic targets. 32 It has been utilized to study a number of diseases and conditions, such as diabetes mellitus, 33 , 34 coronary artery disease, 35 neurodegenerative diseases, 36 cancer, 37 and functional limitation in the elderly. 38 Moreover, amino acid profiling is receiving increasing research interest in cardiovascular diseases. 39 However, until recently, the usefulness of amino acid profiling in heart failure has received less attention. Amino acids are not routinely measured during blood tests in patients with heart failure, and none of the existing heart failure risk scores include amino acid biomarkers. However, when considering the pathophysiology of heart failure, it appears that amino acids might be useful as prognostic biomarkers because heart failure is accompanied by chronic inflammation, which promotes catabolism, resulting in sarcopenia and cachexia. In recent years, a number of studies on amino acid profiling in heart failure have been published, showing evidence that amino acid profiling has the potential to become a novel prognostic tool. The evidence on amino acid profiling in heart failure has been reviewed in the context of diagnosis 40 and treatment, 40 , 41 but its use in predicting heart failure prognosis has not yet been evaluated in detail.
In this study, we aim to review the usefulness of amino acid profiling to predict the prognosis of patients with heart failure, particularly as a tool that can be incorporated into existing heart failure risk scores to predict prognosis. We will introduce the role of amino acids in the heart and describe their utilization in both the healthy heart and in heart failure. Next, we will review the literature on amino acid profiling for prognostic prediction in heart failure and provide an expert interpretation of the findings. Finally, we will suggest directions for future research to advance the understanding of the prognostic potential of amino acid profiling in heart failure.
Importance of amino acids in the failing heart
Under physiological conditions, approximately 70–90% of cardiac adenosine triphosphate is produced by fatty acid oxidation to fuel cardiac contraction. The remaining energy is produced by glucose and lactate oxidation, and to a lesser degree, ketone body and amino acid metabolism. 42 , 43 In the failing heart, the metabolism of these substrates changes dramatically, from primary reliance on fatty acids for energy production to primary reliance on glucose metabolism. 44 , 45 Thus, amino acids are not readily utilized as substrates to generate energy in the form of adenosine triphosphate; instead, they are primarily used for protein synthesis and turnover. 46 , 47 , 48 It follows that in heart failure, the increase in anabolic activity increases the utilization of amino acids. 49
Branched‐chain amino acids, including leucine, isoleucine, and valine, are associated with protein metabolism in the heart and skeletal muscles 50 and may function as regulators of cell signaling 51 while aromatic amino acids, including phenylalanine and tyrosine, are associated with hepatic metabolism. 52 In fact, a wide range of amino acids are produced from protein metabolism and tissue degradation, and these amino acids can be measured to inform the state of metabolism in various diseases, including heart failure (Figure 1 ). Adding to the complexity of amino acid metabolism, there are complex interactions between the heart and other organs, such as the kidneys, 53 blood vessels, 54 liver, 55 and skeletal muscle. 56 , 57 For example, patients with heart failure are in a hypercatabolic state that causes amino acids to be released from muscles. 58 Moreover, a previous study observed a change in plasma branched‐chain amino acid levels in myocardial infarction, which was thought to be affected by enhanced branched‐chain amino acid metabolism in skeletal muscle. 59 Thus, changes in amino acid concentrations may reflect interactions between the heart and other organs.
Figure 1.
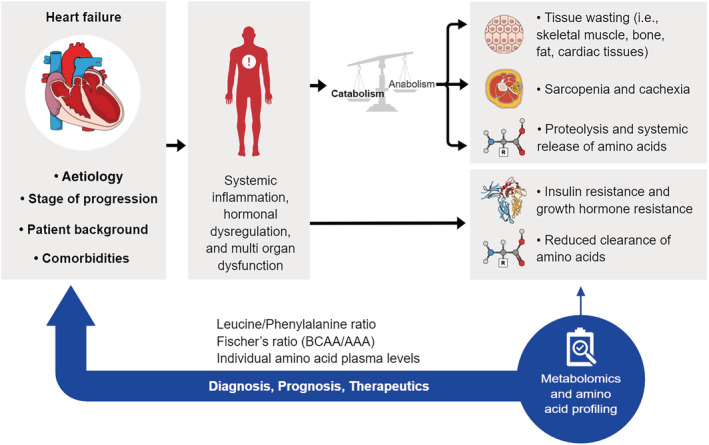
Schematic illustrating the relationship between heart failure and amino acid plasma levels. Heart failure is a complex syndrome characterized by its aetiology (e.g. coronary heart disease, valvular heart disease, and dilated cardiomyopathy), stage of progression, patient background (e.g. sex and age), and comorbidities (e.g. hypertension, atrial fibrillation, and chronic kidney disease). Heart failure leads to a state of systemic inflammation, hormonal dysregulation, and multi‐organ dysfunction that in turn causes a systemic imbalance between a catabolic and anabolic state favouring global catabolism. As a result, atrophy of multiple tissues as well as insulin and growth hormone resistance lead to sarcopenia, cachexia, protein degradation, and release of amino acid from tissues into the systemic circulation. This is further exacerbated by reduced kidney and hepatic clearance of amino acids due to tissue hypoperfusion. Given the central phenomenon of tissue breakdown in heart failure and subsequent changes to systemic amino acid levels, metabolomics and amino acid profiling hold great potential as diagnostic, prognostic, and therapeutic tools in heart failure. Specifically, the plasma leucine/phenylalanine ratio, Fischer's ratio (ratio of branched‐chain amino acid to aromatic amino acid levels), and levels of individual amino acids have been shown to have prognostic value in heart failure.
Prior studies have demonstrated that an increase in plasma branched‐chain amino acids is a risk factor for cardiac events in patients with ischaemic heart disease, which is one of the most common causes of heart failure. 60 , 61 Furthermore, several reports have described changes in both branched‐chain amino acid metabolism 50 , 62 and aromatic amino acid metabolism 63 as well as in the ratio of branched‐chain amino acids to aromatic amino acids (Fischer's ratio), in heart failure. Building on these observations that show changes in amino acid concentrations in heart failure, recent studies have suggested that these amino acid biomarkers could be useful to predict heart failure prognosis. The evidence on the use of amino acid profiling in predicting heart failure prognosis will be reviewed herein.
Amino acid profiling to predict heart failure prognosis
Correlations between amino acid biomarkers and traditional prognostic factors in heart failure
Given the interactions between the heart and other organs, systemic factors, including oxidative stress, nitric oxide, and inflammation, are closely correlated with cardiac function and prognosis in heart failure. 64 , 65 Recent studies have demonstrated correlations between systemic factors/cardiac function and different amino acid biomarkers in patients with heart failure (Table 1 ; reference values provided in Supporting information, Table S1 ), suggesting that amino acid biomarkers could also be useful predictors of prognosis.
Table 1.
Summary of recent studies examining the prognostic value of amino acids, ratios, and metabolites associated with heart failure
| Publication | Study design | Population | No. of patients | Biomarkers examined | Key results |
|---|---|---|---|---|---|
| Hakuno et al., 2015 | Case–control study | Stable HF of NYHA class >II, BNP > 40 pg/mL, LVEF <45% vs. asymptomatic population with normal BNP | HF: n = 38; control: n = 33 | 41 AAs | Multivariate correlations:
|
| Du et al., 2018 | Observational study | STEMI with acute HF | n = 138 | 26 AAs |
|
| Wang et al., 2018 | Observational study | Acute/decompensated HF | Acute/decompensated HF: n = 599; control: n = 94 | Leucine, phenylalanine |
|
| Wang et al., 2019 | Observational study | HF of NYHA Class ≤III; prior hospitalization for acute/decompensated HF and discharged >1 month prior to enrolment; aged 20–85 years | n = 890 | Histidine, ornithine, phenylalanine |
|
| Chen et al., 2020 | Observational study | HF with reduced LVEF <40% (HFrEF), 40–49% (HFmrEF), or ≥50% (HFpEF); NT‐proBNP >900 pg/mL; critical status (APACHE II > 15); ICU stay >48 h; aged >20 years | HF: n = 115; control: n = 37 | Phenylalanine |
|
| Hiraiwa et al., 2020 | Retrospective cohort study | Worsening HF | n = 157 | Fischer's ratio |
|
| Kimura et al., 2020 | Observational study | NIDCM (reduced LVEF <50%) and LV dilation, in the absence of CAD and VHD | n = 39 | BCAA/total AA ratio |
|
| Kouzu et al., 2021 | Retrospective observational study | Patients admitted to hospital for HF diagnosis and management | n = 301 | 3‐methylhistidine, beta‐alanine, valine, hydroxyproline, tryptophan |
|
| Liu et al., 2021 | Case–control study | HF | HF: n = 96; control: n = 97 | 23 AAs |
|
AA, amino acid; AF, atrial fibrillation; BCAA, branched‐chain amino acid; BMI, body mass index; BNP, brain natriuretic peptide; BP, blood pressure; CKD, chronic kidney disease; CRP, C‐reactive protein; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FR, Fischer's ratio; HF, heart failure; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICU, intensive care unit; IL‐8, interleukin‐8; IL‐10, interleukin‐10; IVC, inferior vena cava; LVEDVi, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; NIDCM, non‐ischaemic dilated cardiomyopathy; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; and STEMI, ST‐segment elevation myocardial infarction.
Hakuno et al. 39 analysed the plasma concentrations of 41 amino acids in 38 patients with stable heart failure (New York Heart Association functional Class ≥2). The authors found that Fischer's ratio and five amino acids, namely, monoethanolamine, methionine, tyrosine, 1‐methylhistidine, and histidine, correlated significantly with brain natriuretic peptide concentration, left ventricular ejection fraction, left ventricular end‐diastolic volume index, inferior vena cava diameter, ratio of early diastolic velocity of the mitral inflow to mitral annulus, and brain natriuretic peptide concentration, respectively. In another study, Hiraiwa et al. 63 showed that patients with worsening heart failure with a low Fischer's ratio had more heart failure hospitalizations and more cardiac events than patients with a high Fischer's ratio. Moreover, Hiraiwa et al. 63 observed a negative correlation between Fischer's ratio and the concentrations of aspartate transaminase and alkaline phosphatase as indicators of liver function, as well as significant positive correlations between Fischer's ratio and cholinesterase concentration, albumin concentration, and geriatric nutritional risk index, which are all traditional prognostic predictors in heart failure. The correlation between Fischer's ratio and geriatric nutritional risk index could suggest that a change in Fischer's ratio in heart failure reflects a change in nutritional status. 63
Chen et al. 66 examined the correlation of phenylalanine with inflammation and immune cytokines in 115 patients with New York Heart Association functional class IV heart failure on the basis that phenylalanine metabolism is functionally attenuated by inflammation. 67 , 68 The data showed that an increased phenylalanine concentration in the plasma predicted mortality in patients with New York Heart Association functional Class IV heart failure and correlated with higher Acute Physiology and Chronic Health Evaluation II and Sequential Organ Failure Assessment scores, inflammation, changes in cytokine concentrations, and malnutrition. Kimura et al. 69 evaluated plasma branched‐chain amino acids in patients with non‐ischaemic dilated cardiomyopathy as one cause of heart failure. The authors showed that the branched‐chain amino acid/total amino acid ratio was positively correlated with body mass index, total cholesterol, and left ventricular ejection fraction, and negatively correlated with total bilirubin and brain natriuretic peptide concentration. Moreover, patients with a low branched‐chain amino acid/total amino acid ratio had a poorer prognosis than those with a higher ratio, and a low ratio was a predictor of composite cardiac events in patients with non‐ischaemic dilated cardiomyopathy.
The above evidence demonstrates the presence of correlations between traditional prognostic markers of heart failure and certain amino acids, suggesting that amino acids may also have prognostic value. Although a number of amino acids have demonstrated correlations with traditional prognostic factors, their relative predictive value has not yet been determined, and the amino acid biomarkers with the greatest prognostic potential in heart failure are yet to be determined. Thus, given that this field is in its infancy, identifying which amino acids (either individually or in combination) produce the most accurate prognostic score will be important in the future.
Amino acid biomarkers demonstrate additional value over traditional prognostic factors in heart failure
In addition to examining the correlations between amino acid biomarkers and traditional prognostic factors of heart failure, some recent studies have also examined whether amino acid profiling has additional value over traditional prognostic factors.
In their multivariate analysis, Chen et al. 66 showed that phenylalanine predicted mortality over 1 year in critically ill patients with heart failure, independent of several traditional measures, such as Acute Physiology and Chronic Health Evaluation II and Sequential Organ Failure Assessment scores, C‐reactive protein, cholesterol, and atrial fibrillation. Although the correlation was only relevant for patients with acute/decompensated heart failure in whom the Acute Physiology and Chronic Health Evaluation II and Sequential Organ Failure Assessment scores were used to predict disease severity and prognosis while in the intensive care unit, the observation that phenylalanine predicted 1 year mortality independent of the general Acute Physiology and Chronic Health Evaluation II and Sequential Organ Failure Assessment scores suggests that it could provide more information on longer term risk in selected patient populations. In another study, Wang et al. 70 demonstrated that a leucine concentration of ≥145 μM and phenylalanine concentration of ≥88.9 μM, as well as a leucine concentration of <81.2 μM, were significant predictors of prognosis in patients with acute/decompensated heart failure, even after adjusting for other risk factors, such as age, sex, brain natriuretic peptide, diabetes mellitus, chronic kidney disease, and hypertension. These observations suggest that leucine and/or phenylalanine may provide additional prognostic information over traditional risk factors in patients with acute/decompensated heart failure; thus, the usefulness of these amino acid biomarkers in other types of heart failure should also be evaluated.
In their 2019 study, Wang et al. 71 measured histidine, ornithine, and phenylalanine in 890 patients with heart failure, 387 of whom underwent cardiopulmonary exercise testing to measure metabolic equivalents. In this study, metabolic equivalents were used to define functional class. The authors showed that the concordance between metabolic equivalents functional class and New York Heart Association functional class was only 47%. In fact, the histidine, ornithine, and phenylalanine score was more effective than New York Heart Association functional class for distinguishing metabolic equivalents functional Class II and III patients from metabolic equivalents functional Class I patients. Moreover, a histidine, ornithine, and phenylalanine score of ≥8.8 was a powerful predictor of a poor prognosis (death/heart failure re‐hospitalization), independent of other risk factors, including brain natriuretic peptide, albumin, and age, in both heart failure with reduced ejection fraction and heart failure with preserved ejection fraction, and brain natriuretic peptide, body mass index, and age in heart failure with mid‐range ejection fraction. New York Heart Association functional class is a subjective classification based on clinical presentation and varies by clinician 72 ; however, it is still very commonly used in clinical practice. Although it cannot be simply compared with the New York Heart Association functional classification, as they differ in terms of the information they provide, the histidine, ornithine, and phenylalanine score based on cardiopulmonary exercise testing could provide an accurate and objective means to classify patients with heart failure based on exercise‐induced metabolism, which could reflect skeletal muscle metabolism and hepatic reserve. Thus, prognostic scores based on exercise‐induced amino acid metabolism may be rare, but they are worthy of further exploration.
In another study, Du et al. 60 measured 26 amino acids using mass spectrometry in patients with acute heart failure. In a multivariate analysis, branched‐chain amino acid concentrations were independent predictors of cardiovascular events in patients with acute heart failure, and the prognostic value of these markers was better than that of N‐terminal pro‐brain natriuretic peptide concentration. Furthermore, the authors showed that the combination of branched‐chain amino acids and N‐terminal pro‐brain natriuretic peptide had stronger prognostic value than N‐terminal pro‐brain natriuretic peptide alone, further supporting the hypothesis that amino acid biomarkers could provide additional prognostic value in heart failure.
Hiraiwa et al. 63 suggested that the ability of a low Fischer's ratio to predict cardiac events in heart failure was independent of liver function tests and geriatric nutritional risk index. The observation that Fischer's ratio was correlated with measurements of liver function and the geriatric nutritional risk index, but could still independently determine the prognosis of patients with heart failure, suggests that some aspects of liver function and nutritional status may not be expressed in patients with heart failure. Moreover, it could suggest that amino acids have important effects on the heart that are separate from the liver and skeletal muscle and that influence prognosis; however, the exact role of Fischer's ratio in determining heart failure prognosis remains to be determined.
Kouzu et al. 73 measured amino acids in 301 patients with stabilized heart failure. They showed that a high 3‐methylhistidine concentration and low beta‐alanine and valine concentrations were independently associated with adverse events, defined as all‐cause death and unscheduled readmission due to worsening heart failure, and event‐free survival rates were lower. Elaborating on this, the authors showed that inclusion of both high 3‐methylhistidine and low beta‐alanine or low valine in the adjustment model with N‐terminal pro‐brain natriuretic peptide improved the accuracy of event prediction after discharge, suggesting that the evaluation of these amino acids provided additive value in prognostic prediction.
All of these studies demonstrate that some combinations of amino acid biomarkers and traditional prognostic factors have additional prognostic value over traditional factors alone. We also suggest that the combinations of leucine and N‐terminal pro‐brain natriuretic peptide, leucine/phenylalanine ratio and N‐terminal pro‐brain natriuretic peptide, and Fischer's ratio and N‐terminal pro‐brain natriuretic peptide are worthy of further investigation in terms of whether they provide additional prognostic value over each individual parameter alone.
Amino acid biomarkers to distinguish heart failure aetiology and pathophysiology
Previously, clinical trials evaluated patients with heart failure of different aetiologies collectively. However, a recent study by Liu et al. 74 emphasized the importance of distinguishing metabolic differences between patients with different heart failure aetiologies. In that study, the authors attempted to identify metabolic profile differences among coronary heart disease, dilated cardiomyopathy, and valvular heart disease. Their results showed that arginine, glutamine, and hydroxytetradecanoyl‐carnitine could effectively distinguish heart failure caused by dilated cardiomyopathy from heart failure caused by coronary heart disease. Moreover, hydroxytetradecanoyl‐carnitine and aspartic acid could distinguish heart failure caused by valvular heart disease from heart failure caused by coronary heart disease.
Notably, both the studies by Liu et al. 74 and Hakuno et al. 39 were case–control analyses in patients with heart failure; however, they demonstrated conflicting results in terms of the direction of change in amino acids. For example, Liu et al. 74 reported that the phenylalanine concentration increased in coronary heart disease and dilated cardiomyopathy, but decreased in valvular heart disease, whereas Hakuno et al. 39 demonstrated an increase in phenylalanine concentration in the general heart failure cohort. This disparity may be explained by heterogeneity among the patient populations evaluated. In the study by Liu et al., 74 patients had either New York Heart Association III or IV heart failure, while in the study by Hakuno et al., 39 patients with New York Heart Association II were also included. Differences in other patient characteristics, such as age, sex, comorbidities, treatment, and pathophysiology may also influence the balance between catabolism and anabolism and may determine the direction of change in amino acids. Thus, it will also be important to determine the relative importance of amino acid biomarkers according to heart failure pathophysiology, such as in patients with acute/decompensated vs. chronic/stable disease, and to understand whether amino acid biomarkers demonstrate prognostic value in the short and/or long term in these different cohorts. However, to date, no studies have compared the amino acid profiles of patients with acute/decompensated vs. chronic/stable heart failure. In the future, it will be important to consider all such variables when examining changes in amino acids within the wider clinical population classified as having heart failure.
The study by Liu et al. 74 is particularly valuable as it illustrates the importance of examining the metabolic profiles of different heart failure aetiologies. Although the chronic inflammatory nature and cardiac insufficiency of heart failure are present irrespective of aetiology and stage, the metabolic profiles of patients with heart failure differ depending on the aetiology and stage of heart failure. Thus, examining the metabolic differences between cohorts could help to produce more personalized and precise prognostic prediction tools, which may have the potential to improve outcomes for patients.
Future perspectives
Although a number of studies on amino acid profiling in patients with heart failure have been published recently, this area of research is still in its infancy. This review has revealed a number of research avenues that would help to advance the knowledge of amino acid profiling in heart failure in the future.
First, amino acid tracing would be useful to identify which organs circulating amino acids originate from, and whether they act as signalling molecules between organs. 75 This could help researchers and clinicians to better understand disease pathogenesis and progression and, thus, to predict prognosis. Along this line, it would also be useful to identify cardiac‐specific amino acids to examine metabolic changes in the heart that could influence cardiac function in heart failure.
Second, the amino acid biomarkers that best predict prognosis should be identified. Fischer's ratio appears to be particularly popular and has demonstrated correlations with BNP, which is commonly measured in patients with heart failure as a prognostic predictor. 76 However, the amino acids that would provide the most benefit in terms of prognostic prediction in heart failure are yet to be clarified.
Third, although certain amino acid biomarkers, such as histidine, ornithine, and phenylalanine, have shown benefit over traditional prognostic measures, such as New York Heart Association functional class, the traditional measures are deeply rooted in clinical practice. Thus, in future studies, it will be important to examine whether amino acid biomarkers are best placed as additional prognostic tools to improve current risk stratification rather than as surrogate tools. Notably, none of the existing heart failure risk scores include amino acid biomarkers. However, given that heart failure is accompanied by chronic inflammation, which promotes catabolism and subsequent sarcopenia and cachexia, amino acid biomarkers may have the potential to improve the prognostic power of existing risk tools.
Fourth, the clinical significance and interpretation of amino acid biomarkers may depend on the aetiology and pathophysiology of heart failure; thus, once the metabolic profiles of different types of heart failure and their associated physiological processes have been more clearly delineated, deciding which biomarkers should be assessed during both short‐term and long‐term prognostic prediction in the clinic will be important. A suitable balance should be achieved between ensuring the adequacy of amino acid measurements and minimizing the cost/labour of obtaining these measurements.
Finally, if amino acid profiling is implemented in the clinic, it will be important to consider the influence of diet and supplementation on plasma amino acid concentrations. Plasma amino acid concentrations increase with protein intake. 77 Schmidt et al. 78 showed that the concentrations of methionine, tryptophan, and tyrosine were higher in fish‐eaters and vegetarians than meat‐eaters and vegans. In fact, all 18 dietary amino acids differed by diet group, with amino acid concentrations being up to 47% lower in vegans than in meat‐eaters. Moreover, Alcock et al. 79 demonstrated that dairy protein intake increases plasma leucine concentration. As such, when performing amino acid profiling, it would be important to consider this variability and to be able to distinguish with certainty whether changes in amino acid concentrations are associated with heart failure or whether they are due to other influencing factors, such as diet. One way to achieve this would be for patients to attend regular clinic visits to establish baseline concentrations for amino acids of interest considering their dietary intake, such that subsequent changes in serum amino acid concentrations can be better attributed to the progression of heart failure.
Conclusions
Amino acid profiling has received substantial interest in the areas of cardiovascular disease diagnosis, prognosis, and treatment, with numerous studies published on heart failure in recent years. Several studies have demonstrated correlations between certain amino acid biomarkers and traditional prognostic factors in patients with heart failure. Moreover, some amino acid biomarkers have also demonstrated additional value over traditional prognostic factors for heart failure. However, many traditional factors are deeply rooted in clinical practice. Therefore, amino acid biomarkers may be best placed as additional prognostic tools to improve risk stratification rather than as surrogate tools. Amino acid profiling could be useful to distinguish heart failure aetiologies and to evaluate the pathophysiology and metabolic status of different heart failure cohorts, making it useful for more personalized prognostic prediction. In clinical practice, it will be important to determine which amino acids should be incorporated into existing prognostic models to improve current risk stratification and to ensure the use of amino acid profiling is justified in terms of cost and convenience.
Conflict of interest
Takahiro Okumura received research grants from Ono Pharmaceutical, Amgen Astellas BioPharma, Pfizer Japan, Alnylam Japan, and Alexion (not in connection with the submitted work) as well as honoraria from Ono Pharmaceutical, Otsuka Pharmaceutical, Novartis Pharma, and AstraZeneca. Toyoaki Murohara received lecture fees from Bayer Pharmaceutical, Daiichi‐Sankyo, Dainippon Sumitomo Pharma, Kowa, MSD, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Novartis Pharma, Pfizer Japan, Sanofi‐Aventis, and Takeda Pharmaceutical. Toyoaki Murohara also received an unrestricted research grant from the Department of Cardiology of Nagoya University Graduate School of Medicine, as well as honoraria from Astellas Pharma, Daiichi‐Sankyo, Dainippon Sumitomo Pharma, Kowa, MSD, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Novartis Pharma, Otsuka Pharma, Pfizer Japan, Sanofi‐Aventis, Takeda Pharmaceutical, and Teijin Pharma. All other authors declare that they have no relationships with industry relevant to the contents of this paper.
Funding
This work was supported in part by a research grant from the Nagoya University Medical Association.
Supporting information
Table S1. Reference ranges for amino acids, ratios, and metabolites associated with heart failure in healthy patients.
Acknowledgements
The authors would like to thank Ryota Morimoto, MD, PhD; Toru Kondo, MD, PhD; Shingo Kazama, MD; Yuki Kimura, MD; Ryota Ito, MD; Gaku Sakamoto, MD; and Yuichiro Koyama, MD, for their useful discussion and/or helpful comments on the manuscript. The authors would like to thank Emily Woodhouse, PhD, of Edanz Pharma for providing medical writing services during the development of this manuscript.
Hiraiwa, H. , Okumura, T. , and Murohara, T. (2023) Amino acid profiling to predict prognosis in patients with heart failure: an expert review. ESC Heart Failure, 10: 32–43. 10.1002/ehf2.14222.
Institution where the work was performed: Department of Cardiology, Nagoya University Graduate School of Medicine, Nagoya, Japan.
References
- 1. Bragazzi NL, Zhong W, Shu J, Much AA, Lotan D, Grupper A, Younis A, Dai H. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur J Prev Cardiol 2021; 28: 1682–1690. [DOI] [PubMed] [Google Scholar]
- 2. Taylor CJ, Ordóñez‐Mena JM, Roalfe AK, Lay‐Flurrie S, Jones NR, Marshall T, Hobbs FDR. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000–2017: population based cohort study. Br Med J 2019; 364: I223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pecini R, Møller DV, Torp‐Pederson C, Hassager C, Køber L. Heart failure etiology impacts survival of patients with heart failure. Int J Cardiol 2011; 149: 211–215. [DOI] [PubMed] [Google Scholar]
- 4. Ahmed A. A propensity matched study of New York Heart Association class and natural history end points in heart failure. Am J Cardiol 2007; 99: 549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blumer V, Mentz RJ, Sun J‐L, Butler J, Metra M, Voors AA, Hernandez AF, O'Connor CM, Greene SJ. Prognostic role of prior heart failure hospitalization among patients hospitalized for worsening chronic heart failure. Circ Heart Fail 2021; 14: e007871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kociol RD, Horton JR, Fonarow GC, Reyes EM, Shaw LK, O'Connor CM, Felker GM, Hernandez AF. Admission, discharge, or change in B‐type natriuretic peptide and long‐term outcomes: data from Organized Program To Initiate lifesaving treatMent in hospitalIZEd patients with heart failure (OPTIMIZE‐HF) linked to Medicare claims. Circ Heart Fail 2011; 4: 628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yildiz O, Aslan G, Demirozu ZT, Yenigun CD, Yazicioglu N. Evaluation of resting cardiac power output as a prognostic factor in patients with advanced heart failure. Am J Cardiol 2017; 120: 973–979. [DOI] [PubMed] [Google Scholar]
- 8. Morimoto R, Mizutani T, Araki T, Oishi H, Kimura Y, Kazama S, Shibata N, Kuwayama T, Hiraiwa H, Kondo T, Furusawa K, Okumura T, Murohara T. Prognostic value of resting cardiac power index depends on mean arterial pressure in dilated cardiomyopathy. ESC Heart Fail 2021; 8: 3206–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hiraiwa H, Okumura T, Sawamura A, Sugiura Y, Kondo T, Watanabe N, Aoki S, Ichii T, Kitagawa K, Kano N, Fukaya K, Furusawa K, Morimoto R, Takeshita K, Bando YK, Murohara T. The Selvester QRS score as a predictor of cardiac events in nonischemic dilated cardiomyopathy. J Cardiol 2018; 71: 284–290. [DOI] [PubMed] [Google Scholar]
- 10. Lim NK, Lee SE, Lee HY, Cho HJ, Choe WK, Kim H, Choi JO, Jeon E‐S, Kim M‐S, Kim J‐J, Hwang K‐K, Chae SC, Baek SH, Kang S‐M, Choi D‐J, Yoo B‐S, Kim KH, Cho M‐C, Oh B‐H, Park H‐Y. Risk prediction for 30‐day heart failure‐specific readmission or death after discharge: data from the Korean Acute Heart Failure (KorAHF) registry. J Cardiol 2019; 73: 108–113. [DOI] [PubMed] [Google Scholar]
- 11. Peterson PN, Rumsfeld JS, Liang L, Albert NM, Hernandez AF, Peterson ED, Fonarow GC, Masoudi FA. American Heart Association get with the guidelines‐heart failure program. A validated risk score for in‐hospital mortality in patients with heart failure from the American Heart Association get with the guidelines program. Circ Cardiovasc Qual Outcomes 2010; 3: 25–32. [DOI] [PubMed] [Google Scholar]
- 12. Shiraishi Y, Kohsaka S, Abe T, Mizuno A, Goda A, Izumi Y, Yagawa M, Akita K, Sawano M, Inohara T, Takei M, Kohno T, Higuchi S, Yamazoe M, Mahara K, Fukuda K, Yoshikawa T, West Tokyo Heart Failure Registry Investigators . Validation of the get with the guideline‐heart failure risk score in Japanese patients and the potential improvement of its discrimination ability by the inclusion of B‐type natriuretic peptide level. Am Heart J 2016; 171: 33–39. [DOI] [PubMed] [Google Scholar]
- 13. Suzuki S, Yoshihisa A, Kanno Y, Watanabe S, Abe S, Sato T, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Ishida T, Takeishi Y. Clinical significance of get with the guidelines–heart failure risk score in patients with chronic heart failure after hospitalization. J Am Heart Assoc 2018; 7: e008316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee DS, Stitt A, Austin PC, Stukel TA, Schull MJ, Chong A, Newton GE, Lee JS, Tu JV. Prediction of heart failure mortality in emergent care: a cohort study. Ann Intern Med 2012; 156: 767–775. [DOI] [PubMed] [Google Scholar]
- 15. Miró Ò, Rossello X, Gil V, Martín‐Sánchez FJ, Llorens P, Herrero‐Puente P, Jacob J, Bueno H, Pocock SJ, ICA‐SEMES Research Group . Predicting 30‐day mortality for patients with acute heart failure in the emergency department: a cohort study. Ann Intern Med 2017; 167: 698–705. [DOI] [PubMed] [Google Scholar]
- 16. Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation 1997; 95: 2660–2667. [DOI] [PubMed] [Google Scholar]
- 17. Spinar J, Jarkovsky J, Spinarova L, Mebazaa A, Gayat E, Vitovec J, Linhart A, Widimsky P, Miklik R, Zeman K, Belohlavek J, Malek F, Felsoci M, Kettner J, Ostadal P, Cihalik C, Vaclavik J, Taborksy M, Dusek L, Littnerova S, Parenica J. AHEAD score—long‐term risk classification in acute heart failure. Int J Cardiol 2016; 202: 21–26. [DOI] [PubMed] [Google Scholar]
- 18. Chen YJ, Sung SH, Cheng HM, Huang WM, Wu CL, Huang CJ, Hsu P‐F, Yeh J‐S, Guo C‐Y, Yu W‐C, Chen C‐H. Performance of AHEAD score in an Asian cohort of acute heart failure with either preserved or reduced left ventricular systolic function. J Am Heart Assoc 2017; 6: e004297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole‐Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation 2006; 113: 1424–1433. [DOI] [PubMed] [Google Scholar]
- 20. Mozaffarian D, Anker SD, Anand I, Linker DT, Sullivan MD, Cleland JG, Carson PE, Maggioni AP, Mann DL, Pitt B, Poole‐Wilson PA, Levy WC. Prediction of mode of death in heart failure: the Seattle Heart Failure Model. Circulation 2007; 116: 392–398. [DOI] [PubMed] [Google Scholar]
- 21. Sartipy U, Dahlstrom U, Edner M, Lund LH. Predicting survival in heart failure: validation of the MAGGIC heart failure risk score in 51,043 patients from the Swedish heart failure registry. Eur J Heart Fail 2014; 16: 173–179. [DOI] [PubMed] [Google Scholar]
- 22. Pocock SJ, Ariti CA, McMurray JJV, Maggioni A, Køber L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN, Meta‐analysis global Group in Chronic Heart Failure . Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 2012; 34: 1404–1413. [DOI] [PubMed] [Google Scholar]
- 23. Cheng YL, Sung SH, Cheng HM, Hsu PF, Guo CY, Yu WC, Chen CH. Prognostic nutritional index and the risk of mortality in patients with acute heart failure. J Am Heart Assoc 2017; 6: e004876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kinugasa Y, Kato M, Sugihara S, Hirai M, Yamada K, Yanagihara K, Yamamoto K. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J 2013; 77: 705–711. [DOI] [PubMed] [Google Scholar]
- 25. Formiga F, Chivite D, Corbella X. Utility of the controlling nutritional status (CONUT) score in patients admitted due to acute heart failure. Int J Cardiol 2017; 235: 203. [DOI] [PubMed] [Google Scholar]
- 26. Ito R, Hiraiwa H, Araki T, Mizutani T, Kazama S, Kimura Y, Oishi H, Kuwayama T, Kondo T, Morimoto R, Okumura T, Murohara T. Prognostic value of malnutrition evaluated using the global leadership initiative on malnutrition criteria and its association with psoas muscle volume in non‐ischemic dilated cardiomyopathy. Heart Vessels 2022. [DOI] [PubMed] [Google Scholar]
- 27. Raphael C, Briscoe C, Davies J, Whinnett ZA, Manisty C, Sutton R, Mayet J, Francis DP. Limitations of the New York Heart Association functional classification system and self‐reported walking distances in chronic heart failure. Heart 2007; 93: 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818–829. [PubMed] [Google Scholar]
- 29. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis‐related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis‐Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22: 707–710. [DOI] [PubMed] [Google Scholar]
- 30. Tallgren M, Bäcklund M, Hynninen M. Accuracy of Sequential Organ Failure Assessment (SOFA) scoring in clinical practice. Acta Anaesthesiol Scand 2009; 53: 39–45. [DOI] [PubMed] [Google Scholar]
- 31. McGarrah RW, Crown SB, Zhang GF, Shah SH, Newgard CB. Cardiovascular metabolomics. Circ Res 2018; 122: 1238–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Griffin JL, Atherton H, Shockcor J, Atzori L. Metabolomics as a tool for cardiac research. Nat Rev Cardiol 2011; 8: 630–643. [DOI] [PubMed] [Google Scholar]
- 33. Cheng S, Rhee EP, Larson MG, Lewis GD, McCabe EL, Shen D, Palma MJ, Roberts LD, Dejam A, Souza AL, Deik AA, Magnusson M, Fox CS, O'Donnell CJ, Vasan RS, Melander O, Clish CB, Gerszten RE, Wang TJ. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012; 125: 2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O'Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med 2011; 17: 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, Dungan J, Newby LK, Hauser ER, Ginsburg GS, Newgard CB, Kraus WE. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet 2010; 3: 207–214. [DOI] [PubMed] [Google Scholar]
- 36. Socha E, Koba M, Kośliński P. Amino acid profiling as a method of discovering biomarkers for diagnosis of neurodegenerative diseases. Amino Acids 2019; 51: 367–371. [DOI] [PubMed] [Google Scholar]
- 37. Miyagi Y, Higashiyama M, Gochi A, Akaike M, Ishikawa T, Miura T, Saruki N, Bando E, Kimura H, Imamura F, Moriyama M, Ikeda I, Chiba A, Oshita F, Imaizumi A, Yamamoto H, Miyano H, Horimoto K, Tochikubo O, Mitsushima T, Yamakado M, Okamoto N. Plasma free amino acid profiling of five types of cancer patients and its application for early detection. PLoS ONE 2011; 6: e24143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lustgarten MS, Price LL, Chale A, Phillips EM, Fielding RA. Branched chain amino acids are associated with muscle mass in functionally limited older adults. J Gerontol A Biol Sci Med Sci 2014; 69: 717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hakuno D, Hamba Y, Toya T, Adachi T. Plasma amino acid profiling identifies specific amino acid associations with cardiovascular function in patients with systolic heart failure. PLoS ONE 2015; 10: e0117325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Müller J, Bertsch T, Volke J, Schmid A, Klingbell R, Metodiev Y, Karaca B, Kim S‐H, Lindner S, Schupp T, Kittel M, Poschet G, Akin I, Behnes M. Narrative review of metabolomics in cardiovascular disease. J Thorac Dis 2021; 13: 2532–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Karwi QG, Lopaschuk GD. Branched‐chain amino acid metabolism in the failing heart. Cardiovasc Drugs Ther 2022. 10.1007/s10557-022-07320-4 [DOI] [PubMed] [Google Scholar]
- 42. Gertz EW, Wisneski JA, Stanley WC, Neese RA. Myocardial substrate utilization during exercise in humans. Dual carbon‐labeled carbohydrate isotope experiments. J Clin Invest 1988; 82: 2017–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wisneski JA, Gertz EW, Neese RA, Gruenke LD, Craig JC. Dual carbon‐labeled isotope experiments using D‐[6‐14C] glucose and L‐[1,2,3‐13C3] lactate: a new approach for investigating human myocardial metabolism during ischemia. J Am Coll Cardiol 1985; 5: 1138–1146. [DOI] [PubMed] [Google Scholar]
- 44. Fukushima A, Milner K, Gupta A, Lopaschuk GD. Myocardial energy substrate metabolism in heart failure: from pathways to therapeutic targets. Curr Pharm Des 2015; 21: 3654–3664. [DOI] [PubMed] [Google Scholar]
- 45. Wende AR, Brahma MK, McGinnis GR, Young ME. Metabolic origins of heart failure. JACC Basic Transl Sci 2017; 2: 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barrio JR, Egbert JE, Henze E, Schelbert HR, Baumgartner FJ. L‐[4‐11C]aspartic acid: enzymatic synthesis, myocardial uptake, and metabolism. J Med Chem 1982; 25: 93–96. [DOI] [PubMed] [Google Scholar]
- 47. Morgan HE, Earl DC, Broadus A, Wolpert EB, Giger KE, Jefferson LS. Regulation of protein synthesis in heart muscle. I. Effect of amino acid levels on protein synthesis. J Biol Chem 1971; 246: 2152–2162. [PubMed] [Google Scholar]
- 48. Young LH, McNulty PH, Morgan C, Deckelbaum LI, Zaret BL, Barrett EJ. Myocardial protein turnover in patients with coronary artery disease. Effect of branched chain amino acid infusion. J Clin Invest 1991; 87: 554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Neubauer S, Horn M, Kramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K. Myocardial phosphocreatine‐to‐ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 1997; 96: 2190–2196. [DOI] [PubMed] [Google Scholar]
- 50. Huang Y, Zhou M, Sun H, Wang Y. Branched‐chain amino acid metabolism in heart disease: An epiphenomenon or a real culprit? Cardiovasc Res 2011; 90: 220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lopaschuk GD, Ussher JR. Evolving concepts of myocardial energy metabolism. Circ Res 2016; 119: 1173–1176. [DOI] [PubMed] [Google Scholar]
- 52. Dejong CHC, van de Poll MCG, Soeters PB, Jalan R, Olde Damink SWM. Aromatic amino acid metabolism during liver failure. J Nutr 2007; 137: 1579S–1585S. [DOI] [PubMed] [Google Scholar]
- 53. Givertz MM, Postmus D, Hillege HL, Mansoor GA, Massie BM, Davison BA, Ponikowski P, Metra M, Teerlink JR, Cleland JG, Dittrich HC, O'Connor CM, Cotter G, Voors AA. Renal function trajectories and clinical outcomes in acute heart failure. Circ Heart Fail 2014; 7: 59–67. [DOI] [PubMed] [Google Scholar]
- 54. Indik JH, Goldman S, Gaballa MA. Oxidative stress contributes to vascular endothelial dysfunction in heart failure. Am J Physiol Heart Circ Physiol 2001; 281: H1767–H1770. [DOI] [PubMed] [Google Scholar]
- 55. Samsky MD, Patel CB, DeWald TA, Smith AD, Felker M, Rogers JG, Hernandez AF. Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol 2013; 61: 2397–2405. [DOI] [PubMed] [Google Scholar]
- 56. Drexler H, Riede U, Münzel T, König H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation 1992; 85: 1751–1759. [DOI] [PubMed] [Google Scholar]
- 57. Okita K, Kinugawa S, Tsutsui H. Exercise intolerance in chronic heart failure—Skeletal muscle dysfunction and potential therapies. Circ J 2013; 77: 293–300. [DOI] [PubMed] [Google Scholar]
- 58. Pasini E, Aquilani R, Dioguardi FS. Amino acids: chemistry and metabolism in normal and hypercatabolic states. Am J Cardiol 2004; 93: 3–5. [DOI] [PubMed] [Google Scholar]
- 59. Wang W, Zhang F, Xia Y, Zhao S, Yan W, Wang H, Lee Y, Li C, Zhang L, Lian K, Gao E, Cheng H, Tao L. Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol 2016; 311: H1160–H1169. [DOI] [PubMed] [Google Scholar]
- 60. Du X, Li Y, Wang Y, You H, Hui P, Zheng Y, Du J. Increased branched‐chain amino acid levels are associated with long‐term adverse cardiovascular events in patients with STEMI and acute heart failure. Life Sci 2018; 209: 167–172. [DOI] [PubMed] [Google Scholar]
- 61. Ruiz‐Canela M, Toledo E, Clish CB, Hruby A, Liang L, Salas‐Salvadó J, Razquin C, Corella D, Estruch R, Ros E, Fitó M, Gómez‐Gracia E, Arós F, Fiol M, Lapetra J, Serra‐Majem L, Martínez‐González MA, Hu FB. Plasma branched‐chain amino acids and incident cardiovascular disease in the PREDIMED trial. Clin Chem 2016; 62: 582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sun H, Wang Y. Branched chain amino acid metabolic reprogramming in heart failure. Biochim Biophys Acta 2016; 1862: 2270–2275. [DOI] [PubMed] [Google Scholar]
- 63. Hiraiwa H, Okumura T, Kondo T, Kato T, Kazama S, Ishihara T, Iwata E, Shimojo M, Kondo S, Aoki S, Kanzaki Y, Tanimura D, Sano H, Awaji Y, Yamada S, Murohara T. Usefulness of the plasma branched‐chain amino acid/aromatic amino acid ratio for predicting future cardiac events in patients with heart failure. J Cardiol 2020; 75: 689–696. [DOI] [PubMed] [Google Scholar]
- 64. Kalantar‐Zadeh K, Anker SD. Nutritional and anti‐inflammatory interventions in chronic heart failure. Am J Cardiol 2008; 101: S89–S103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA. Oxidant stress from nitric oxide synthase‐3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest 2005; 115: 1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen WS, Wang CH, Cheng CW, Liu MH, Chu CM, Wu HP, Huang P‐C, Lin Y‐T, Ko T, Chen W‐H, Wang H‐J, Lee S‐C, Liang C‐Y. Elevated plasma phenylalanine predicts mortality in critical patients with heart failure. ESC Heart Fail 2020; 7: 2884–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Murr C, Grammer TB, Meinitzer A, Kleber ME, März W, Fuchs D. Immune activation and inflammation in patients with cardiovascular disease are associated with higher phenylalanine to tyrosine ratios: the Ludwigshafen risk and cardiovascular health study. J Amino Acids 2014; 2014: 783730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Neurauter G, Schröcksnadel K, Scholl‐Bürgi S, Sperner‐Unterweger B, Schubert C, Ledochowski M, Fuchs D. Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr Drug Metab 2008; 9: 622–627. [DOI] [PubMed] [Google Scholar]
- 69. Kimura Y, Okumura T, Kazama S, Shibata N, Oishi H, Arao Y, Kuwayama T, Kato H, Yamaguchi S, Hiraiwa H, Kondo T, Morimoto R, Murohara T. Usefulness of plasma branched‐chain amino acid analysis in predicting outcomes of patients with nonischemic dilated cardiomyopathy. Int Heart J 2020; 61: 739–747. [DOI] [PubMed] [Google Scholar]
- 70. Wang CH, Cheng ML, Liu MH. Simplified plasma essential amino acid‐based profiling provides metabolic information and prognostic value additive to traditional risk factors in heart failure. Amino Acids 2018; 50: 1739–1748. [DOI] [PubMed] [Google Scholar]
- 71. Wang CH, Cheng ML, Liu MH, Fu TC. Amino acid‐based metabolic profile provides functional assessment and prognostic value for heart failure outpatients. Dis Markers 2019; 2019: 8632726–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. The Criteria Committee of the New York Heart Association . Diseases of the Heart and Blood Vessels: Nomenclature and Criteria for Diagnosis, 6th ed. Boston: Little, Brown and Co; 1964. [Google Scholar]
- 73. Kouzu H, Katano S, Yano T, Ohori K, Nagaoka R, Inoue T, Takamura Y, Ishigo T, Watanabe A, Koyama M, Nagano N, Fujito T, Nishikawa R, Ohwada W, Miura T. Plasma amino acid profiling improves predictive accuracy to adverse events in patients with heart failure. ESC Heart Fail 2021; 8: 5045–5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu C, Li R, Liu Y, Li Z, Sun Y, Yin P, Huang R. Characteristics of blood metabolic profile in coronary heart disease, dilated cardiomyopathy and valvular heart disease induced heart failure. Front Cardiovasc Med 2021; 7: 622236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Naemat A, Elsheikha HM, Boitor RA, Notingher I. Tracing amino acid exchange during host‐pathogen interaction by combined stable‐isotope time‐resolved Raman spectral imaging. Sci Rep 2016; 6: 20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T, Matsui T, Kinoshita M. Effect of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. J Am Coll Cardiol 2001; 37: 1228–1233. [DOI] [PubMed] [Google Scholar]
- 77. Yoshii N, Sato K, Ogasawara R, Nishimura Y, Shinohara Y, Fujita S. Effect of mixed meal and leucine intake on plasma amino acid concentrations in young men. Nutrients 2018; 10: 1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schmidt JA, Rinaldi S, Scalbert A, Ferrari P, Achaintre D, Gunter MJ, Appleby PN, Key TJ, Travis RC. Plasma concentrations and intakes of amino acids in male meat‐eaters, fish‐eaters, vegetarians and vegans: a cross‐sectional analysis in the EPIC‐Oxford cohort. Eur J Clin Nutr 2016; 70: 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Alcock RD, Shaw GC, Tee N, Burke LM. Plasma amino acid concentrations after the ingestion of dairy and collagen proteins, in healthy active males. Front Nutr 2019; 6: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Reference ranges for amino acids, ratios, and metabolites associated with heart failure in healthy patients.


