Abstract
Aims
We aimed to study initiation, adherence, and long‐term persistence to beta‐blockers (BB), renin–angiotensin system inhibitors (RASi), and mineralocorticoid receptor antagonists (MRA) in a nationwide cohort of patients with heart failure (HF).
Methods
Patients aged 18–80 years in Norway with a first diagnosis of HF from 2014 until 2020 that survived ≥30 days were identified from the Norwegian Patient Registry and linked to the Norwegian Prescription Database. We collected information about BB, RASi [angiotensin‐converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARB), and angiotensin receptor‐neprilysin inhibitors (ARNI)], and MRA. Dual HF therapy was defined as taking at least two out of three drug classes, whereas triple HF therapy was defined as taking all three. Initiation (time to initiation) and persistence (time to discontinuation using a grace period of 30 days) of HF drugs was calculated by the Kaplan–Meier method, followed to outcome of interest, death, or December 2020. One‐year adherence was measured as proportion of days covered (PDC) using a cut‐off at 80%. For adherence and persistence measurements, we allowed for maximum 60 days of stockpiling and switching within drug groups. We performed sensitivity analyses to test the robustness of our findings.
Results
Out of 54 899 patients included in the cohort, 75%, 69%, and 21% initiated a BB, RASi, and MRA, respectively, whereas 13% did not receive any. Dual and triple HF therapy was prescribed to 61% and 16%, respectively. The proportion of adherent patients during the first year following initiation was 83%, 81%, 84%, and 61% for BB, RASi, ARNI, and MRA, whereas 42% and 5% were adherent to dual and triple HF therapy, respectively. From 2 to 5 years following initiation, persistence decreased from 58% to 38%, 57% to 37%, and 31% to 15% for BB, RASi, and MRA, respectively. Within the RASi group, persistence was higher for ARNI than for ACEI and ARB. There were no major changes in either initiation or adherence of the drug classes from 2014 to 2019, except for an increase in initiation and adherence of MRA.
Conclusions
We found low adherence to dual and triple HF therapies in this nationwide cohort study of newly diagnosed HF patients. Efforts are needed to increase adherence and persistence to HF therapies into clinical practice, emphasizing maintenance of multiple drug therapies in patients with such an indication.
Keywords: Heart failure, Guideline‐directed medical therapy, Adherence, Persistence, PDC
Introduction
Despite medical innovations and advancement in heart failure (HF) therapies the past decades, mortality and morbidity remain high. 1 , 2 , 3 Angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor‐neprilysin inhibitors (ARNI), beta‐blockers (BB), mineralocorticoid receptor antagonists (MRA), and sodium‐glucose co‐transporter 2 (SGLT2) inhibitors improve survival in patients with heart failure with reduced ejection fraction (HFrEF) and are the cornerstones of HF treatment as recommended by the 2021 European Society of Cardiology (ESC) guidelines. 3
Medication adherence refers to the patient's propensity to conform with the prescribed drug regimen, measured over a certain time period and reported as a percentage. 4 In contrast, medication persistence is defined as ‘the duration of time from initiation to discontinuation of therapy’, operationalized by determining how long the patient stays on medication without a specified length of permissible treatment gap. 4 This distinction allows for differentiation of ‘how well’ the patients take their medication, from ‘how long’ they take their medication. Poor drug adherence or non‐persistence in patients with HF have in several studies demonstrated increased morbidity and mortality, as well as increased healthcare costs. 5 , 6 , 7 , 8
Studies on drug adherence and persistence are important for patients, caregivers, and decision makers to understand targets for implementation of guideline‐directed medical therapy (GDMT). Interventions that improve adherence and persistence might have higher impact on HF outcomes than new drugs in the real‐world setting. However, research on real‐world patient populations is often hampered by limitations inherent in many patient registries such as lack of cross‐linking or incomplete follow‐up, or they are based on highly selected patient populations. We aimed to study initiation, adherence, and long‐term persistence to BB, renin–angiotensin system inhibitors (RASi), and MRA in patients with HF aged 18–80 using nationwide registry data with complete coverage and follow‐up.
Methods
Data sources
The study was based on data from two nationwide registries, the Norwegian Patient Registry (NPR) and the Norwegian Prescription Database (NorPD) (Figure 1 ). The NPR contains all inpatient and outpatient hospital contacts in Norway including specialists' consultations. Diagnoses are coded according to the International Classification of Diseases version 10 (ICD‐10). The NorPD is a registry containing all prescription drugs dispensed from pharmacies since 2004. The data in NorPD are registered on an individual level, except for in patients being resident in institutions. The drugs are classified according to the Anatomical Therapeutic Chemical (ATC) system. In addition, the NorPD contains information on dispensed date, quantity (i.e. number of pills) and drug strength. All pharmacies are required by law to register all dispensed prescriptions.
Figure 1.
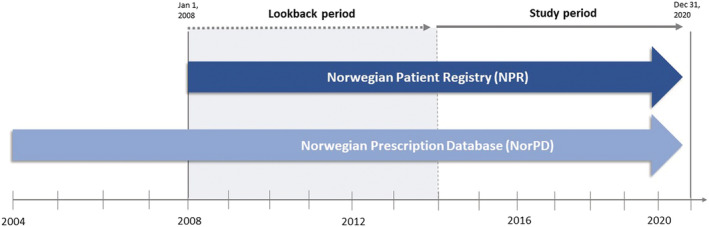
Registers included and their follow‐up period.
Patient selection and cohort creation
Data of all patients ≥18 years with at least one hospital contact for HF registered in the NPR were available in the study period from January 2014 until December 2020. A lookback period from 2008 was applied to exclude prevalent HF cases from the cohort. 9 The first HF contact in the study period was defined as the index HF event. We excluded patients ≥80 years of age at index to avoid the potential selection bias of lacking patient‐level prescription data on institutionalized elderly (Figure 2 ). Additionally, their propensity to use HF drugs might be confounded by fragility and multimorbidity. Patients that died within 30 days after the index HF event were also excluded to assess long‐term adherence and persistence. The study population from NPR was linked to NorPD on an individual level to obtain information on drug use during follow‐up. Patients were followed until the end of 2020, outcome of interest or death, whichever came first (Figure 1 ).
Figure 2.
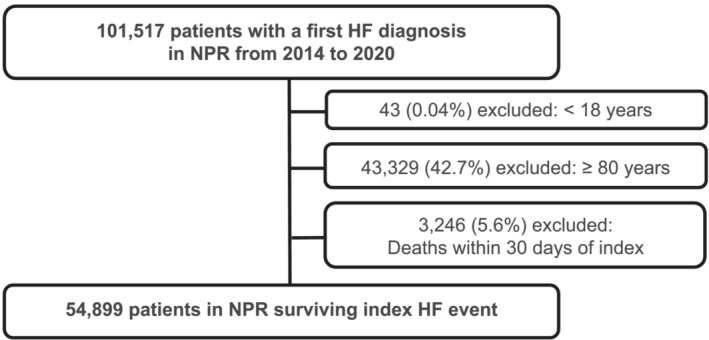
Cohort creation flow chart. HF, heart failure; NorPD, Norwegian Prescription Database; NPR, Norwegian Patient Registry.
The group of patients initiating ARNI was studied separately. Due to local reimbursement criteria, ARNI was limited to patients with LVEF ≤35% and NYHA Class II–IV, previously treated with both a BB and an ACEI/ARB or MRA. 10 Individual index date in this analysis was set to the first prescription of ARNI following the index HF event.
Definitions
HF, medications, and co‐morbidities
HF was defined as a hospital contact with ICD‐10 codes I11.0, I13.0, I13.2, I42.x, or I50.x as primary or secondary diagnosis. We collected information about pharmacological HF therapies in the following drug classes: (i) RASi, consisting of ACEI, ARB, and ARNI, (ii) BB, and (iii) MRA. Dual HF therapy was defined as taking at least two out of three drug classes (BB and RASi, RASi and MRA, or BB and MRA). Triple HF therapy was defined as taking all three drug classes (BB, RASi, and MRA). Co‐morbidities at baseline were based on registrations in NPR and/or NorPD during the lookback period. Medication at baseline was registered 180 days before index. The full list of definitions of comorbidities and medications are outlined in table 1 (Table S1 ).
Table 1.
Baseline characteristics of the study population
| Total | ||
|---|---|---|
| Patients (n) | 54 899 | |
| Age (median, IQR) | 69 (60–75) | |
| Age groups (%) | ||
| 18–64 | 35% | |
| 65–80 | 65% | |
| Women age (median, IQR) | 71 (62–76) | |
| Men age (median, IQR) | 68 (59–74) | |
| Women, n (%) | 37% | |
| ICD‐codes for HF | ||
| I50.x Congestive heart failure | 84.0% | |
| I42.x Cardiomyopathy | 15.2% | |
| I11.0 Hypertensive heart disease with (congestive) heart failure | 3.5% | |
| I13. 0 Hypertensive heart and renal disease with (congestive) heart failure | 0.2% | |
| I13.2 Hypertensive heart and renal disease with both (congestive) heart failure and renal failure | 0.1% | |
| Comorbidities | ||
| Cardiovascular | ||
| Atrial fibrillation | 40% | |
| Cerebrovascular event | 14% | |
| Hypertension | 67% | |
| Ischaemic heart disease | 48% | |
| Myocardial infarction | 26% | |
| Peripheral arterial disease | 16% | |
| Other | ||
| COPD | 24% | |
| Anaemia | 18% | |
| Cancer | 21% | |
| Chronic kidney disease | 16% | |
| Dementia | 2% | |
| Depression | 29% | |
| Diabetes mellitus | 23% | |
| Dyslipidaemia | 57% | |
| Thyroid disease | 10% | |
| Patients on HF drugs before index | ||
| ACEI | 18% | |
| ARB | 21% | |
| ARNI | 0% | |
| BB | 42% | |
| MRA | 3% | |
| Loop diuretics | 20% | |
| Other medication before index | ||
| Anticoagulant agents | 26% | |
| Platelet aggregation inhibitors | 36% | |
| Calcium channel blockers | 21% | |
| SGLT2i | 1% | |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor‐neprilysin inhibitor; BB, beta‐blocker; COPD, chronic obstructive pulmonary disorder; HF, heart failure; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; SD, standard deviation; SGLT2i, sodium‐glucose co‐transporter 2 inhibitor.
Initiation of HF therapy
We counted the days from the index HF event to the first HF prescription, dual HF therapy, and triple HF therapy within the first 365 days, censoring for death. We also performed initiation analyses on the drug‐naïve patients (patients that did not receive the drug under investigation during the lookback period), because some patients received HF drugs for other indications (i.e. hypertension).
Among the patients initiating triple HF therapy, we calculated the proportion of patients that received at least one drug prescription of loop diuretics within +/− 180 days from initiation of triple HF therapy.
Adherence and persistence
Adherence was measured as proportion of days covered (PDC), defined as number of days on medication divided by number of days under observation. This method allows for adherence measurement of patients initiating treatment only. We measured adherence for a period of 1 year starting at therapy initiation. Patients with an index HF event in 2020 or patients that died within 365 days from initiation were consequently excluded from the analysis to ensure 1 year of follow‐up. Non‐adherence to monotherapy with BB, RASi, or MRAs was defined as PDC < 80%. 11 , 12 , 13 , 14 In addition, we measured adherence to multiple HF drugs by determining the number of the observed drugs that were taken each day of the observation period and calculated PDC for having at least two out of the three drug groups on the same day (dual HF therapy), as well as all three drug groups on the same day (triple HF therapy). 15
Patients were considered persistent if they did not experience a treatment break (grace period) of more than 30 days 16 after therapy initiation. We used a grace period of 90 days a sensitivity analysis. This method allows patients to be studied until their first treatment break.
We allowed for switching between generics and within the same therapeutic drug class. Also, we allowed for switching between ACEI, ARB, and ARNI. We allowed for stockpiling so that available tablets from prior prescriptions were added to the following prescription up to a maximum of 60 days. Two exceptions from this rule were made when the dosage changed (e.g., from enalapril 10 mg to enalapril 20 mg) to avoid overestimation of persistence and adherence measures when tablets were discarded in relation to up‐titration or down‐titration. In addition, potential stockpiling was disregarded if patients shifted medication within the same therapeutic drug class (e.g., from ramipril to enalapril) or when switching between ACEI, ARB, and ARNI (e.g., shifted from ramipril to valsartan). A schematic example of the PDC method is outlined in Figure S1 .
Adherence and persistence analyses were performed on patient initiating treatment only.
Definition of daily dose
We divided the dispensed quantity (number of pills) on the expected number of doses per day for each drug (the recommended daily regimen from the agents product monograph or from clinical expert opinion) to calculate the duration of each dispensed prescription. We used one tablet a day for all drugs, except for captopril, carvedilol, valsartan, and sacubitril/valsartan (twice daily). Further details and rationale behind the selected method are outlined in Table S2 . To test the robustness of our assumptions, we performed sensitivity analyses based on a fixed prescription length. Pharmacies in Norway are entitled to deliver reimbursed prescription drugs for a maximum of 3 months. 17 We therefore assumed that a prescription lasted for 90 days when patients claimed a prescription (hereby referred to as the ‘fill frequency method’).
Statistical analysis
Descriptive statistics were applied to summarize data. For categorical variables, counts and percentages were provided. Mean or median values were presented with standard deviations (SD) or interquartile range (IQR) as applicable. The rates of initiation of drugs and time until treatment breaks (persistence) were estimated by the Kaplan–Meier method censoring for death or end of follow‐up. PDC was calculated in 10% intervals. For medication non‐adherence, we used dichotomous variables with a cut‐off at <0.8. Python version 3.X was used for data management and analysis.
Ethics
The investigation conforms with the principles outlined in the Declaration of Helsinki. The protocol was approved by the Norwegian Data Protection Authority and Regional Committee for Medical and Health Research Ethics (2017/1243).
Results
Baseline characteristics
A total of 101 517 adult patients had a first HF event between 2014 and 2020; of these, 54 899 patients were aged 18–80 years and alive after 30 days and hence included in the study cohort (Figure 2 ). The baseline characteristics are shown in Table 1 . There were an increasing number of incident HF patients in the cohort from 7299 patients in 2014 to 8474 patients in 2019, however, a decline to 7999 patients in 2020. The baseline characteristics were similar each year (Table S3 ).
A total of 2747 patients in the cohort received ARNI following the index HF event from 2016–2020. The median number of days from the index HF event to the first ARNI prescription was 267 days. At the time of ARNI initiation, 90% were treated with an ACEI/ARB, 95% with a BB, and 59% with an MRA. Most drug recipients were male (81%). The baseline characteristics in total and by each calendar year from 2016‐2020 are outlined in Table S4 .
Drug initiation
Within the first year following the index HF event, 87% of the cohort retrieved a HF drug. BB, RASi, and MRA were initiated by 75%, 69%, and 21%, respectively (Figure 3 A ). Most of the prescriptions were claimed within the first 90 days (Table S5 ). Dual HF therapy was prescribed to 61% of the patients and triple HF therapy to 16%. Among patients initiating triple therapy, 73% received loop diuretics. When performing the analyses on drug‐naïve patients only, a lower proportion received HF drugs within the first year: BB, 61%; RASi, 55%; and MRA, 19%. However, among the drug‐naïve patients, a high proportion of HF drugs was collected within the first 30 days (Figure 3 B ). When performing the analyses by calendar year, there were no major changes except from a minor reduction in initiation of BB from 2019 to 2020 and an increase in MRA and ARNI each year from 2014 to 2020 (Figure S2 ).
Figure 3.
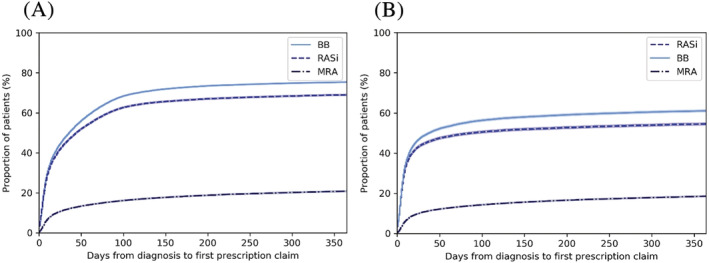
Cumulative frequency of patients who claimed at least one prescription of BB, RASi, and MRA within the first year following diagnosis (censored for death) for the (A) total patient cohort and (B) drug‐naïve patients. BB, beta‐blocker; MRA, mineralocorticoid receptor antagonist; RASi, renin–angiotensin system inhibitor.
Drug adherence
Among patients with 1 year of follow‐up, drug adherence was higher for both BB (83%, n = 33 106) and RASi (81%, n = 30 555) relative to MRA (61%, n = 9774) as shown in Figure 4 . After initiation of ARNI, 84% (n = 1464) were adherent during the first year of follow‐up. Among patients that initiated dual HF therapy (n = 26 154), 42% of the patients were adherent, whereas only 5% of the patients were adherent to triple HF therapy (n = 6181). Using the modified PDC adherence measure with fill frequency, adherence to monotherapy was lower except for ARNI (RASI 80%, ARNI 88%, BB 80%, MRA 55%) and equivalent for dual and triple HF therapy (Table S6 ). When performing the analyses by calendar year, there was no change in PDC from 2014 to 2019 for BB and RASi (Figure S3 ). The proportion of patients being adherent to MRA, however, increased from 58% in 2014 to 64% in 2019. There was a minor increase in proportion of patients on dual or triple HF therapy, from 40 to 42% and 4 to 6%, respectively. Table S6 shows the distribution of PDC in 10% intervals.
Figure 4.
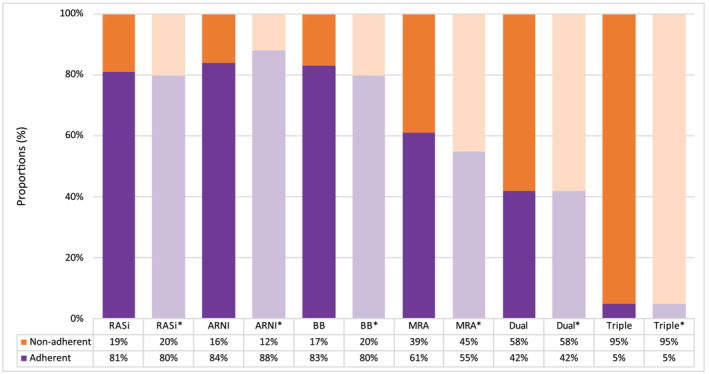
Proportion of adherent patients 1 year after therapy initiation, defined as PDC ≥ 80%. BB, beta‐blocker; MRA, mineralocorticoid receptor antagonist; PDC, proportion of days covered; RASi, renin–angiotensin system inhibitor. The asterisk (*) shows the results when using the modified PDC adherence measure with the fill frequency method.
Long‐term persistence
Among patients that initiated at least one HF drug, persistence was higher for BB and RASi than for MRA (Figure 5 ). A large reduction in persistence was seen around 3 and 6 months after the index HF event. One year after initiation, 72% of the patients who started were still on a BB, 71% on RASi, and 48% on MRA. Two years after initiation, the proportions of patients on BB, RASi, and MRA decreased to 58%, 57%, and 31%, respectively. The 5‐year persistence was 38% for BB, 37% for RASi and 15% for MRA. Sensitivity analyses showed that persistence was lower when using fill frequency and higher when applying a grace period of 90 days (Figure 5 and Table S7 ). Within the RASi groups, persistence was higher for ARNI than ACEI and ARBs, regardless of method applied (Figure 6 ).
Figure 5.
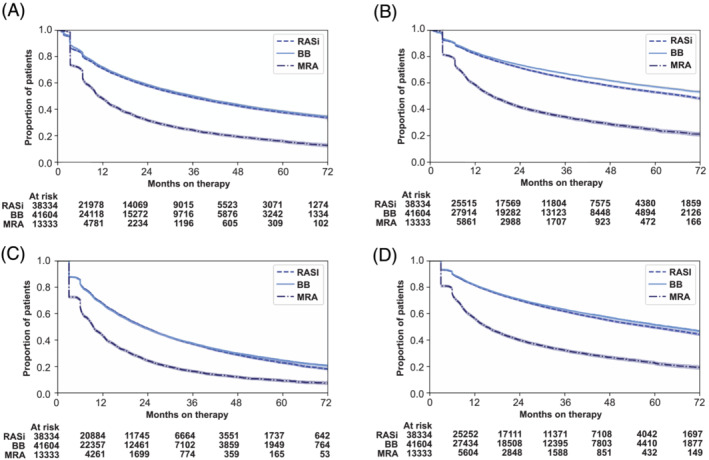
Long‐term persistence with RASi, BB, and MRA. The proportion of patients who were on treatment on each day, censored for death. BB, beta‐blocker; MRA, mineralocorticoid receptor antagonist; RASi, renin–angiotensin system inhibitor. (A) Main analysis, 30 days grace period. (B) Main analysis, 90 days grace period. (C) Fill frequency method, 30 days grace period. (D) Fill frequency method, 90 days grace period.
Figure 6.
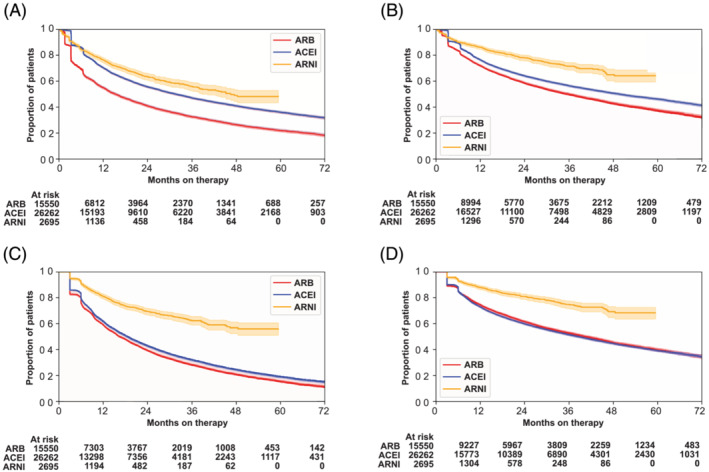
Long‐term persistence with ARB, ACEI, and ARNI. The proportion of patients who were on treatment on each day, censored for death. ACEI, angiotensin‐converting enzyme inhibitor; ARB; angiotensin II receptor blocker; ARNI, angiotensin receptor‐neprilysin inhibitor. (A) Main analysis, 30 days grace period. (B) Main analysis, 90 days grace period. (C) Fill frequency method, 30 days grace period. (D) Fill frequency method, 90 days grace period.
Discussion
In this nationwide cohort study with longitudinal follow‐up from 2014 to 2020, we addressed initiation, adherence, and long‐term persistence to BB, RASi, and MRA in a real‐world HF population. There are four main findings: (i) Within the first year following the index HF event, 61% and 16% initiated dual and triple HF therapy, respectively. (ii) During the first year following treatment initiation, 42% of patients were adherent to dual HF therapy, whereas 5% were adherent to triple HF therapy. (iii) Long‐term persistence was poor for all drug categories, particularly for MRA. Nearly half of the patients discontinued BB and RASi within 2 years from initiation. (iv) There were no major changes in either initiation or adherence of the drug classes from 2014 to 2019, except for an increase in initiation and adherence of MRA.
Strengths and limitations
The main strength of this study is the completeness and longitudinal follow‐up of nationwide individual‐level data. As the NPR covers all inpatient and outpatient hospital contacts in the country, selection bias is reduced compared to studies based on selected HF populations. The healthcare system is publicly funded for all citizens through the national tax scheme, minimizing differences in healthcare access due to socio‐economic status. The NorPD includes all prescription drugs dispensed nationally. Use of electronic prescription registries is considered reliable to estimate drug compliance 18 as they avoid recall bias, interviewer bias, and observer bias. Patients must pay a deductible for prescription drugs, increasing the likelihood that patients have the intention to take medication as prescribed.
Some important limitations that might compromise the validity of this study need to be addressed. Firstly, the definition of HF was based on ICD‐10 codes for HF, and data on phenotypes (HFrEF, HFmrEF, and HFpEF) were not available. As for numerous population‐based studies on compliance in the past, 6 , 8 , 19 , 20 , 21 we were not able to separate HFrEF from HEFpEF patients due to unavailability of ejection fraction assessments. The ICD‐10 diagnosis is provided by the treating consultant based on clinical evaluation, blood testes including natriuretic peptides, and echocardiography measurements. However, only the ICD‐codes are entered into the database and available. The HF diagnoses in NPR have not been validated. However, NPR has demonstrated high quality for several other cardiovascular diagnoses 22 , 23 and the positive predictive value for HF is generally high in comparable patient registries from other countries. 24 A validation study from Denmark found a positive predictive value of 95% for HFrEF when combining the ICD‐10 code I50 with use of RASi and BB. 25 Secondly, data from the NorPD do not include variables on the prescribed daily dose. We have described our assumptions comprehensively and transparently along with sensitivity analyses to address this limitation. Further, by using claims data, it is assumed that patients actually take the medications if dispensed. Thus, our findings represent the best possible scenario and most likely overestimated adherence and persistence measurements. We did not consider compliance to SGLT2 inhibitors because this drug class was introduced as HF treatment regardless of diabetes status after the end of the study period. Lastly, we restricted the analyses to patients <80 years of age and our results might not be valid for a patient population ≥80 years of age.
Discussion of results
The 2016 ESC HF guidelines 26 recommended treatment with RASi, BB, and MRA in maximum tolerable doses in patients with HFrEF. The recommendations were extended to include patients with HFmrEF in 2021 (Class IIb). 3 In our cohort, 61% of the patients initiated dual HF therapy, and 16% initiated triple HF therapy, of which 42% and 5% were considered adherent, respectively. These adherence numbers are low, even when considering that some of the patients in the cohort might have HFpEF.
Long‐term persistence was inadequate for all drug categories, with nearly half the patients discontinuing RASi or BB within 2 years. Persistence analyses must include a grace period reflecting the number of days allowed between prescription refills. We applied a 30‐day grace period for our main analyses based on the treatment situation, pharmacologic properties, and previous findings in the literature. 20 , 21 , 27 Within the RASi groups, persistence was higher for ARNI than ACEI and ARBs regardless of method applied. Patients initiated on ARNI are most likely a reflection of patients switching from ACEI/ARB as per current national reimbursement criteria. Our results suggest that ARNI is well tolerated as reflected by the higher persistence. Yet, persistence was not optimal even in this highly selected patient population of patients with symptomatic HFrEF.
Over the past decade, drug adherence has been studied in selected HF populations, with oftentimes conflicting results. Our results are generally aligned with international findings, even though differences in study populations, data sources, and methodology in particular make comparison between studies difficult. Data from the CHAMP‐HF registry on 3518 patients with HFrEF found that 22.1% of patients eligible for triple HF therapy received some dose of ACEI/ARB/ARNI, BB, and MRA simultaneously, whereas 1.1% received target doses. 28 A Danish study from the DenHeart Survey of 1464 HF patients discharged from a heart centre found that 68% were adherent to RASi and 68% were adherent to BB after 1 year, somewhat less than in our population. 27 On the contrary, more patients were adherent to MRAs compared with our findings. A study from the USA on claims and Medicare data from 2009 to 2012 reported that only 40% of the patients were treated with dual HF therapy, 20 consistent with our finding despite differences in data sources and populations. Studies of long‐term persistence are scarce; a Danish study from 1994 to 2007 reported higher proportions of persistent users after 5 years than we found in our study, even when applying a grace period of 90 days. 6
There are a variety of different approaches to measure medication adherence. 29 Full transparency of the methods and assumptions is important to allow for comparison and reproduction of results. The PDC‐method is the most common and conservative measurement of adherence. 30 , 31 The prescribed daily dose (PDD) is rarely included as a variable in most claims databases, 32 as is true for the NorPD. Consequently, surrogates must be used to mimic the PDD as no gold standard exists. The DDD, number of tablets per day, and ‘mean daily dose as per SmPC’ are methods used in previous studies, each with different strengths and limitations. However, many studies do not provide information on data sources or assumptions for dosage. 30 Among the parameters needed to calculate PDC, the daily dosing assumptions have the greatest influence on the results. 33 Therefore, we performed sensitivity analyses as outlined in the Methods section. When we assumed that all prescriptions lasted for 90 days to estimate the number of days covered, both persistence and adherence were lower for all drug groups. Hence, our results are conservative and particularly discouraging considering the patient population of relatively young patients surviving their first HF event. We did not censor hospital stays, as it is shown to have a minor effect on the PDC‐measures. 33
We excluded patients over 80 years of age from our cohort due to potential selection bias (lost to follow‐up). We anticipate drug adherence and persistence to be even lower in the elderly. Our results are especially discouraging given the young patient population.
Policy implications and call to action
The 2021 ESC HF guidelines do not provide a detailed practical solution to the introduction of multiple drug therapies; however, a strategy of rapid, parallel sequencing of the foundational four drug classes on low starting doses within 2–4 weeks is proposed in patients with HFrEF. 34 Among the 16% that initiated triple HF therapy in our cohort, only 5% of the patients were considered adherent to all three drug classes throughout the first year of follow‐up. The most critical time point for discontinuation was the first 6 months following therapy initiation. With this is mind, practical considerations towards implementation of the 2021 ESC HF guidelines into clinical practice are needed. We did not investigate reasons for non‐adherence of HF drugs in this study; however, several reasons have been proposed and involve features within the healthcare system, clinicians, and patients. 35 Possible organizational aspects might include restrictive pharmacotherapy policy, reimbursement issues, or drug costs; however, the medications in scope are in general low‐cost drugs reimbursed for all Norwegian patients through the general reimbursement scheme. Other organizational aspects even more pertinent might include inadequate health information technology and inaccessible or scarcity of specialized multidisciplinary HF centres. 35 , 36 HF management in Norway is mainly initiated during hospital stay or at time of discharge. A selected patient population is followed with repeated visits in specialized multidisciplinary nurse‐driven HF clinics, whereas the majority of patients are followed in primary care. Possible reasons for non‐prescription among clinicians might include knowledge or communication gaps, safety/tolerability concerns, disbelief in treatment effectiveness or trial generalizability, biases (e.g. age, gender, and ethnicity), and therapeutic inertia. Some patients in the cohort had HFpEF, who did not have evidence‐based treatment options during the study period. Possible patient reasons for non‐adherence might involve inadequate knowledge or health literacy, frailty, and co‐morbidity (especially depression or cognitive disorders affecting attention), polypharmacy, socio‐economic status (e.g. lack of social support or language barriers), patient–provider relationship, preferences, or intolerable side effects.
Increasingly complex pharmacotherapy might lead to delays in therapy initiation, or this can be regarded an opportunity to simplify the message of parallel initiation of the four pillars in low doses to both healthcare providers and patients. Wider implementation of structured multidisciplinary care, that is, GDMT clinics, is anticipated to benefit patient adherence with structured follow‐up. 36 , 37 Utilization of new technology and decision support have demonstrated potential to improve guideline compliance in clinical practice. Examples are clinical decision support systems integrated with electronic health records, 38 , 39 mobile health interventions, 40 telemedicine/telenursing, 41 and sensor implantations. 42 The barriers to implement and maintain therapy, for clinicians, patients, and healthcare systems alike, are subject to further research and identification. Further studies on strategies to facilitate the implementation of GDMT into clinical practice are warranted to improve treatment initiation, adherence, and persistence and subsequently outcomes in this vulnerable patient group. Furthermore, endeavours to distinguish HF by phenotypes in administrative health registries are warranted to permit evaluation of drug treatment of HF by phenotype, especially considering treatment strategies emerging for HFpEF.
Conclusions
Despite the well‐known risk of death and hospitalizations following new‐onset HF, we found low adherence and long‐term persistence to HF pharmacotherapy in this nationwide cohort of patients with HF. Urgent efforts are needed to improve implementation of HF drug treatment with decision support to clinicians and patients and ensure broader access to structured multidisciplinary care, particularly addressing adherence of multiple drug therapies.
Conflict of interest
KMO is a PhD candidate at the University of Oslo and an employee of Novartis Norway AS. SSL reports consultancy fees from Novartis during the conduct of the study. JH was an employee of Novartis Norway AS at the time the study was conducted. HOM has received speaker honoraria and research funding from Biogen, Pfizer, and Takeda. SH has received speaker honoraria from Novartis, Sanofi and Astra Zeneca.
Funding
This work was supported by the Research Council of Norway and Novartis Norway AS.
Supporting information
Table S1. Definitions.
Figure S1. Proportion of days covered.
Table S2. Guideline recommended treatment for heart failure and rationale for method.
Table S3. Baseline characteristics of the study population from 2014‐2020.
Table S4. Baseline characteristics of patients initiating ARNI.
Table S5. Drug initiation. Days from index HF to first prescription claim.
Figure S2. Initiation per calendar year for (A) BB, (B) RASI, (C) MRA, and (D) ARNI.
Table S6. Adherence during the first year after initiation by 10% intervals in PDC.
Figure S3. Proportion of adherent patients (PDC ≥ 0.8) from 2014 to 2019.
Table S7. Proportion of persistent patients with sensitivity analysis.
Ødegaard, K. M. , Lirhus, S. S. , Melberg, H. O. , Hallén, J. , and Halvorsen, S. (2023) Adherence and persistence to pharmacotherapy in patients with heart failure: a nationwide cohort study, 2014–2020. ESC Heart Failure, 10: 405–415. 10.1002/ehf2.14206.
References
- 1. Metra M, Teerlink JR. Heart failure. Lancet (London, England). 2017; 390: 1981–1995. [DOI] [PubMed] [Google Scholar]
- 2. Arrigo M, Jessup M, Mullens W, Reza N, Shah AM, Sliwa K, Mebazaa A. Acute heart failure. Nat Rev Dis Primers. 2020; 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022; 24: 4–131. [DOI] [PubMed] [Google Scholar]
- 4. Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: Terminology and definitions. Value Health. 2008; 11: 44–47. [DOI] [PubMed] [Google Scholar]
- 5. Bitar S, Agrinier N, Alla F, Rossignol P, Mebazaa A, Thilly N. Adherence to ESC guideline‐recommended medications over a 36‐month follow‐up period after hospitalization for heart failure: Results from the EPICAL2 cohort study. Pharmacoepidemiol Drug Saf. 2019; 28: 1489–1500. [DOI] [PubMed] [Google Scholar]
- 6. Gislason GH, Rasmussen JN, Abildstrom SZ, Schramm TK, Hansen ML, Buch P, Sørensen R, Folke F, Gadsbøll N, Rasmussen S, Køber L, Madsen M, Torp‐Pedersen C. Persistent use of evidence‐based pharmacotherapy in heart failure is associated with improved outcomes. Circulation. 2007; 116: 737–744. [DOI] [PubMed] [Google Scholar]
- 7. Hood SR, Giazzon AJ, Seamon G, Lane KA, Wang J, Eckert GJ, Tu W, Murray MD. Association between medication adherence and the outcomes of heart failure. Pharmacotherapy. 2018; 38: 539–545. [DOI] [PubMed] [Google Scholar]
- 8. Savarese G, Bodegard J, Norhammar A, Sartipy P, Thuresson M, Cowie MR, Fonarow GC, Vaduganathan M, Coats AJS. Heart failure drug titration, discontinuation, mortality and heart failure hospitalization risk: A multinational observational study (US, UK and Sweden). Eur J Heart Fail. 2021; 23: 1499–1511. [DOI] [PubMed] [Google Scholar]
- 9. Ødegaard KM, Lirhus SS, Melberg HO, Hallén J, Halvorsen S. A nationwide registry study on heart failure in Norway from 2008 to 2018: Variations in lookback period affect incidence estimates. BMC Cardiovasc Disord. 2022; 22: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Statens legemiddelverk (Norwegian Medicines Agency) . Hurtig metodevurdering sakubitril/valsartan til behandling av HFrEF. 2018. Internet. [2022‐06‐20]. Available from https://legemiddelverket.no/Documents/Offentlig%20finansiering%20og%20pris/Metodevurderinger/E/Entresto_endringer_2018.pdf (accessed on June 20, 2022).
- 11. Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. Good and poor adherence: Optimal cut‐point for adherence measures using administrative claims data. Curr Med Res Opin. 2009; 25: 2303–2310. [DOI] [PubMed] [Google Scholar]
- 12. Hansen RA, Kim MM, Song L, Tu W, Wu J, Murray MD. Comparison of methods to assess medication adherence and classify nonadherence. Ann Pharmacother. 2009; 43: 413–422. [DOI] [PubMed] [Google Scholar]
- 13. Lamb DA, Eurich DT, McAlister FA, Tsuyuki RT, Semchuk WM, Wilson TW, Blackburn DF. Changes in adherence to evidence‐based medications in the first year after initial hospitalization for heart failure: Observational cohort study from 1994 to 2003. Circ Cardiovasc Qual Outcomes. 2009; 2: 228–235. [DOI] [PubMed] [Google Scholar]
- 14. Vegter S, Nguyen NH, Visser ST, de Jong‐van den Berg LT, Postma MJ, Boersma C. Compliance, persistence, and switching patterns for ACE inhibitors and ARBs. Am J Manag Care. 2011; 17: 609–616. [PubMed] [Google Scholar]
- 15. Arnet I, Abraham I, Messerli M, Hersberger KE. A method for calculating adherence to polypharmacy from dispensing data records. Int J Clin Pharm. 2014; 36: 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greevy RA Jr, Huizinga MM, Roumie CL, Grijalva CG, Murff H, Liu X, Griffin MR. Comparisons of persistence and durability among three oral antidiabetic therapies using electronic prescription‐fill data: The impact of adherence requirements and stockpiling. Clin Pharmacol Ther. 2011; 90: 813–819. [DOI] [PubMed] [Google Scholar]
- 17. The Norwegian Health Economics Administration . HELFO. Internet [2021‐11‐10] [2021‐11‐10]. Available from: https://www.helfo.no/regelverk‐og‐takster/overordnet‐regelverk/regelverk‐for‐apotek‐og‐bandasjist (accessed on November 10, 2021).
- 18. Kronish IM, Ye S. Adherence to cardiovascular medications: Lessons learned and future directions. Prog Cardiovasc Dis. 2013; 55: 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spreafico M, Gasperoni F, Barbati G, Ieva F, Scagnetto A, Zanier L, Iorio A, Sinagra G, Di Lenarda A. Adherence to disease‐modifying therapy in patients hospitalized for HF: Findings from a community‐based study. Am J Cardiovasc Drugs. 2020; 20: 179–190. [DOI] [PubMed] [Google Scholar]
- 20. Deschaseaux C, McSharry M, Hudson E, Agrawal R, Turner SJ. Treatment initiation patterns, modifications, and medication adherence among newly diagnosed heart failure patients: A retrospective claims database analysis. J Manag Care Spec Pharm. 2016; 22: 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scalvini S, Bernocchi P, Villa S, Paganoni AM, La Rovere MT, Frigerio M. Treatment prescription, adherence, and persistence after the first hospitalization for heart failure: A population‐based retrospective study on 100785 patients. Int J Cardiol. 2021; 330: 106–111. [DOI] [PubMed] [Google Scholar]
- 22. Govatsmark RES, Janszky I, Slordahl SA, Ebbing M, Wiseth R, Grenne B, Vesterbekkmo E, Bonaa KH. Completeness and correctness of acute myocardial infarction diagnoses in a medical quality register and an administrative health register. Scand J Public Health. 2018: 1403494818803256. [DOI] [PubMed] [Google Scholar]
- 23. Varmdal T, Bakken IJ, Janszky I, Wethal T, Ellekjær H, Rohweder G, Fjærtoft H, Ebbing M, Bønaa KH. Comparison of the validity of stroke diagnoses in a medical quality register and an administrative health register. Scand J Public Health. 2016; 44: 143–149. [DOI] [PubMed] [Google Scholar]
- 24. McCormick N, Lacaille D, Bhole V, Avina‐Zubieta JA. Validity of heart failure diagnoses in administrative databases: A systematic review and meta‐analysis. PLoS ONE. 2014; 9: e104519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Madelaire C, Gustafsson F, Køber L, Torp‐Pedersen C, Andersson C, Kristensen SL, Gislason G, Schou M. Identification of patients with new‐onset heart failure and reduced ejection fraction in Danish administrative registers. Clin Epidemiol. 2020; 12: 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M , Document R . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 27. Rasmussen AA, Wiggers H, Jensen M, Berg SK, Rasmussen TB, Borregaard B, Thrysoee L, Thorup CB, Mols RE, Larsen SH, Johnsen SP. Patient‐reported outcomes and medication adherence in patients with heart failure. Eur Heart J ‐ Cardiovasc Pharmacother. 2020; 7: 287–295. [DOI] [PubMed] [Google Scholar]
- 28. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF, Fonarow GC. Medical therapy for heart failure with reduced ejection fraction: The CHAMP‐HF registry. J Am Coll Cardiol. 2018; 72: 351–366. [DOI] [PubMed] [Google Scholar]
- 29. Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006; 40: 1280–1288. [DOI] [PubMed] [Google Scholar]
- 30. Krueger K, Griese‐Mammen N, Schubert I, Kieble M, Botermann L, Laufs U, Kloft C, Schulz M. In search of a standard when analyzing medication adherence in patients with heart failure using claims data: A systematic review. Heart Fail Rev. 2018; 23: 63–71. [DOI] [PubMed] [Google Scholar]
- 31. Raebel MA, Schmittdiel J, Karter AJ, Konieczny JL, Steiner JF. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care. 2013; 51: S11–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schulz M, Krueger K, Schuessel K, Friedland K, Laufs U, Mueller WE, Ude M. Medication adherence and persistence according to different antihypertensive drug classes: A retrospective cohort study of 255,500 patients. Int J Cardiol. 2016; 220: 668–676. [DOI] [PubMed] [Google Scholar]
- 33. Ihle P, Krueger K, Schubert I, Griese‐Mammen N, Parrau N, Laufs U, Schulz M. Comparison of different strategies to measure medication adherence via claims data in patients with chronic heart failure. Clin Pharmacol Ther. 2019; 106: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Packer M, McMurray JJV. Rapid evidence‐based sequencing of foundational drugs for heart failure and a reduced ejection fraction. Eur J Heart Fail. 2021; 23: 882–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spall HGCV, Fonarow GC, Mamas MA. Underutilization of guideline‐directed medical therapy in heart failure. J Am Coll Cardiol. 2022; 79: 2214–2218. [DOI] [PubMed] [Google Scholar]
- 36. Seferović PM, Polovina M, Adlbrecht C, Bělohlávek J, Chioncel O, Goncalvesová E, Milinković I, Grupper A, Halmosi R, Kamzola G, Koskinas KC, Lopatin Y, Parkhomenko A, Põder P, Ristić AD, Šakalytė G, Trbušić M, Tundybayeva M, Vrtovec B, Yotov YT, Miličić D, Ponikowski P, Metra M, Rosano G, Coats AJS. Navigating between Scylla and Charybdis: Challenges and strategies for implementing guideline‐directed medical therapy in heart failure with reduced ejection fraction. Eur J Heart Fail. 2021; 23: 1999–2007. [DOI] [PubMed] [Google Scholar]
- 37. O'Connor CM. Guideline‐directed medical therapy clinics: A call to action for the heart failure team. JACC: Heart Failure. 2019; 7: 442–443. [DOI] [PubMed] [Google Scholar]
- 38. Karlsson LO, Nilsson S, Bång M, Nilsson L, Charitakis E, Janzon M. A clinical decision support tool for improving adherence to guidelines on anticoagulant therapy in patients with atrial fibrillation at risk of stroke: A cluster‐randomized trial in a Swedish primary care setting (the CDS‐AF study). PLoS Med. 2018; 15: e1002528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ghazi L, Yamamoto Y, Riello RJ, Coronel‐Moreno C, Martin M, O'Connor KD, Simonov M, Huang J, Olufade T, McDermott J, Dhar R, Inzucchi SE, Velazquez EJ, Wilson FP, Desai NR, Ahmad T. Electronic alerts to improve heart failure therapy in outpatient practice: A cluster randomized trial. J Am Coll Cardiol. 2022; 79: 2203–2213. [DOI] [PubMed] [Google Scholar]
- 40. Indraratna P, Biswas U, McVeigh J, Mamo A, Magdy J, Vickers D, Watkins E, Ziegl A, Liu H, Cholerton N, Li J, Holgate K, Fildes J, Gallagher R, Ferry C, Jan S, Briggs N, Schreier G, Redmond SJ, Loh E, Yu J, Lovell NH, Ooi SY. A smartphone‐based model of care to support patients with cardiac disease transitioning from hospital to the community (TeleClinical care): Pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022; 10: e32554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan BA, Winkler S, Vettorazzi E, Bruch L, Oeff M, Zugck C, Doerr G, Naegele H, Störk S, Butter C, Sechtem U, Angermann C, Gola G, Prondzinsky R, Edelmann F, Spethmann S, Schellong SM, Schulze PC, Bauersachs J, Wellge B, Schoebel C, Tajsic M, Dreger H, Anker SD, Stangl K. Efficacy of telemedical interventional management in patients with heart failure (TIM‐HF2): A randomised, controlled, parallel‐group, unmasked trial. Lancet (London, England). 2018; 392: 1047–1057. [DOI] [PubMed] [Google Scholar]
- 42. Cowie MR, Flett A, Cowburn P, Foley P, Chandrasekaran B, Loke I, Critoph C, Gardner RS, Guha K, Betts TR, Carr‐White G, Zaidi A, Lim HS, Hayward C, Patwala A, Rogers D, Pettit S, Gazzola C, Henderson J, Adamson PB. Real‐world evidence in a national health service: Results of the UK CardioMEMS HF system post‐market study. ESC Heart Fail. 2022; 9: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Definitions.
Figure S1. Proportion of days covered.
Table S2. Guideline recommended treatment for heart failure and rationale for method.
Table S3. Baseline characteristics of the study population from 2014‐2020.
Table S4. Baseline characteristics of patients initiating ARNI.
Table S5. Drug initiation. Days from index HF to first prescription claim.
Figure S2. Initiation per calendar year for (A) BB, (B) RASI, (C) MRA, and (D) ARNI.
Table S6. Adherence during the first year after initiation by 10% intervals in PDC.
Figure S3. Proportion of adherent patients (PDC ≥ 0.8) from 2014 to 2019.
Table S7. Proportion of persistent patients with sensitivity analysis.


