Abstract
Aims
Several observational studies indicated that atrial fibrillation might aggravate other cardiovascular diseases apart from ischaemic stroke. However, it remains to be determined whether these associations reveal independent causation. Using Mendelian randomization (MR), we systematically investigated how genetically predicted atrial fibrillation affected other cardiovascular diseases and cardiac death.
Methods and results
Summary‐level data for atrial fibrillation and other cardiovascular diseases were obtained from public genome‐wide association study data. The random inverse‐variance weighted method was treated as the primary analysis. Sensitivity analyses (including weighted median, MR‐Egger, and multivariable MR methods) were also performed. Atrial fibrillation was significantly associated with higher risks of heart failure [odds ratio (OR): 1.24; 95% confidence interval (CI): 1.19–1.28; P < 0.001], ischaemic stroke (OR: 1.21; 95% CI: 1.17–1.25; P < 0.001), transient ischaemic attack (OR: 1.10; 95% CI: 1.05–1.15; P < 0.001), peripheral artery diseases (OR: 1.09; 95% CI: 1.03–1.15; P = 0.002), cardiac death (OR: 1.08; 95% CI: 1.02–1.15; P = 0.008), and hypertension (OR: 1.06; 95% CI: 1.01–1.11; P = 0.010), without effects on coronary heart disease or pulmonary embolism. Associations for heart failure and ischaemic stroke remained robust to the sensitivity analyses. MR‐Egger method (P > 0.05) and funnel plot yielded no indication of directional pleiotropy. The leave‐one‐out analysis suggested that the causal associations were not driven by individual single nucleotide polymorphism.
Conclusions
This comprehensive MR analysis verified the causal associations between atrial fibrillation and high risks of heart failure, ischaemic stroke, transient ischaemic attack, peripheral artery diseases, cardiac death, and hypertension. Interventions to reduce cardiovascular diseases beyond ischaemic stroke are warranted in patients with atrial fibrillation.
Keywords: Mendelian randomization, Atrial fibrillation, Coronary heart disease, Hypertension, Heart failure, Ischaemic stroke, Pulmonary embolism, Peripheral artery diseases
Introduction
Atrial fibrillation is the most frequent arrhythmia associated with increased risks of major adverse cardiovascular events. 1 According to the results of the Rotterdam Study, the lifetime risk of developing atrial fibrillation at the age of 55 years in men and women was 23.8% and 22.2%, respectively, and the prevalence of atrial fibrillation increased with age. 2 Current management of complications associated with atrial fibrillation has mainly focused on preventing stroke. 3 However, in addition to ischaemic stroke, the associations between atrial fibrillation and other cardiovascular diseases such as coronary heart disease, hypertension, heart failure, transient ischaemic attack, pulmonary embolism, peripheral artery disease, and cardiac death have been documented. 4 , 5 , 6 For example, it is speculated that the thrombi from the right atrium to pulmonary artery circulation may be simulated in the same manner as the thrombi from the left atrium to cerebral circulation, resulting in the occurrence of pulmonary embolism. 7 In a meta‐analysis of 15 cohort studies, atrial fibrillation may increase the risks of myocardial infarction [relative risk (RR): 1.54; 95% confidence interval (CI): 1.26–1.85], all‐cause mortality (RR: 1.95; 95% CI: 1.50–2.54), and heart failure (RR: 4.62; 95% CI: 3.13–6.83). 8 Moreover, in patients with atrial fibrillation, cardiac death represented as high as 46% of total mortality, whereas non‐haemorrhagic stroke and systemic embolism accounted for only 5.7%. 9 Therefore, it is of great significance to investigate the causal associations between atrial fibrillation and other cardiovascular diseases besides ischaemic stroke. However, results from observational studies are difficult to interpret the causality because of the influence of potential confounding factors. Some shared risk factors of atrial fibrillation and other cardiovascular diseases may reflect a common pathway of underlying disease, which may lead to the issue of reverse causality.
In this situation, Mendelian randomization (MR) can provide the causal inference between atrial fibrillation and other cardiovascular diseases as genetic variants are randomly allocated at conception and not affected by confounders. 10 Moreover, MR analysis can overcome reverse causality as alleles are fixed regardless of the onset and progression of other diseases. In previous MR analysis, the causal association between atrial fibrillation and coronary heart disease, ischaemic stroke, and heart failure has been assessed. 11 However, the MR analysis did not consider the effect of smoking, body mass index, diabetes, and dyslipidaemia, which are well‐established risk factors for cardiovascular diseases. 12 Therefore, it is essential to conduct multivariable MR analysis to adjust for the above risk factors. Besides, a meta‐analysis including 104 cohort studies investigated and found positive associations between atrial fibrillation, peripheral artery disease, and cardiac death, 13 which may provide evidence for further investigating the causality. Therefore, we conducted a two‐sample MR study to systematically explore the causal associations between atrial fibrillation and eight cardiovascular diseases (including coronary heart disease, hypertension, heart failure, ischaemic stroke, transient ischaemic attack, pulmonary embolism, peripheral artery disease, and cardiac death) using the recently published genome‐wide association study (GWAS) data.
Methods
Mendelian randomization analysis has to satisfy the following three fundamental assumptions: (i) genetic variants are strongly associated with the exposure; (ii) genetic variants are not related to confounders of the exposure–outcome association; and (iii) genetic variants affect the outcome only through the exposure.
Data sources for atrial fibrillation and other cardiovascular diseases
Summary‐level data for atrial fibrillation were obtained from recent GWAS data, which compared 60 620 cases and 970 216 controls of European ancestry. The GWAS data consisted of six contributing studies, including the HUNT (The Nord‐Trøndelag Health Study), deCODE, MGI (Michigan Genomics Initiative), DiscovEHR, UK Biobank, and AFGen Consortium. 14 Genetic associations with coronary heart disease (n = 184 305) were obtained from the CARDIoGRAMplusC4D. 15 Genetic associations with hypertension (n = 218 792), cardiac death (n = 218 792), pulmonary embolism (n = 218 792), transient ischaemic attack (n = 214 634), and peripheral artery disease (n = 218 792) were obtained from the FinnGen Consortium. Genetic associations with heart failure (n = 977 323) were obtained from the HERMES Consortium (Heart Failure Molecular Epidemiology for Therapeutic Targets). 16 Genetic associations with ischaemic stroke (n = 440 328) were obtained from MEGASTROKE Consortium. 17 This MR analysis did not require ethical approval as all analysis was based on public data.
Instrumental variable selection and data harmonization
Instrumental variables were selected according to the following criteria: (i) single nucleotide polymorphisms (SNPs) were strongly associated with atrial fibrillation at the genome‐wide significance threshold (P < 5 × 10−8); (ii) SNPs in linkage disequilibrium (r 2 > 0.001) were excluded to make sure that the contribution of selected SNPs was independent; and (iii) the strength of each SNP was evaluated using the F‐statistic, with F‐statistic greater than 10 indicating a sufficiently strong instrumental variable. SNP with F‐statistic ≤10 was deleted. To ensure that the effect of an SNP on the exposure and outcome corresponded to the same allele, data harmonization was performed. If the alleles did not correspond for the same SNP, this SNP would be deleted. We tried to infer positive strand alleles, using allele frequencies for palindromes. Proxy SNPs were not used in the present MR analysis.
Statistical analysis
Inverse variance weighting (IVW) under random effects was regarded as the primary estimate as it can provide the most precise estimates. However, IVW is also sensitive to invalid instrumental variables and pleiotropic effects. Therefore, several sensitivity analyses (including weighted median, MR‐Egger, and multivariable MR) were also performed to test the consistency of the associations and detect possible directional pleiotropy. The weighted median method can provide an accurate estimate if more than 50% of the weight in the analysis comes from valid SNPs. 18 MR‐Egger is based on the assumption that the pleiotropic effects are independently distributed. 19 The intercept in MR‐Egger method is a useful indication of whether directional horizontal pleiotropy is driving the results of an MR analysis. If the P value for intercept is less than 0.05, this suggests that the genetic variants suffer from directional pleiotropy, and once this pleiotropy is considered, there is no residual evidence for a causal effect. 20 In the multivariable MR, we adjusted for smoking, body mass index, diabetes, and total cholesterol in Model 1. Smoking, body mass index, diabetes, total cholesterol, and coronary heart disease were adjusted in Model 2. A leave‐one‐out sensitivity analysis was also conducted to determine the effect of an individual SNP on the overall estimate. The heterogeneities among selected SNPs were quantified by Cochran Q statistic, with a P value <0.05 regarded as significant heterogeneity. Results were reported as odds ratio (OR) with corresponding 95% CI. To account for multiple comparisons, we also calculated the Bonferroni correction P value (P = 0.05/8 outcomes = 0.006), with P value between 0.006 and 0.05 considered as suggestive associations. All statistical analyses were conducted using the ‘TwoSampleMR’ packages in R software (Version 4.0.3, R Foundation for Statistical Computing, Vienna, Austria).
Results
Single nucleotide polymorphism selection and validation
The GWAS data included in the present MR analysis were published between 2015 and 2021 and were mainly based on the European population (Supporting Information, Table S1 ). Among the SNPs that achieved genome‐wide significance levels, all F‐statistics were greater than 10 (Supporting Information, Table S2 ).
Associations with cardiovascular diseases
Genetically instrumented atrial fibrillation was positively associated with five of the eight cardiovascular diseases, including and with decreasing magnitude of association: heart failure (OR: 1.24; 95% CI: 1.19–1.28; P < 0.001), ischaemic stroke (OR: 1.21; 95% CI: 1.17–1.25; P < 0.001), transient ischaemic attack (OR: 1.10; 95% CI: 1.05–1.15; P < 0.001), peripheral artery disease (OR: 1.09; 95% CI: 1.03–1.15; P = 0.002), and cardiac death (OR: 1.08; 95% CI: 1.02–1.15; P = 0.008). Suggestive evidence of a positive association was obtained between atrial fibrillation and hypertension (OR: 1.06; 95% CI: 1.01–1.11; P = 0.010). In contrast, no association was observed for coronary heart disease or pulmonary embolism (Figure 1 ). In sensitivity analyses (Table 1 ), weighted median, MR‐Egger, and multivariable MR analyses revealed consistent positive estimates for heart failure and ischaemic stroke. Transient ischaemic attack was increased in the multivariable MR analysis and potentially increased in the weighted median analysis. Peripheral artery disease was potentially increased in the multivariable MR analysis. However, no significant difference was observed in all sensitivity analyses for cardiac death. The positive causal association for hypertension was observed in both the weighted median and multivariable MR methods, yet the association disappeared in the MR‐Egger analysis.
Figure 1.
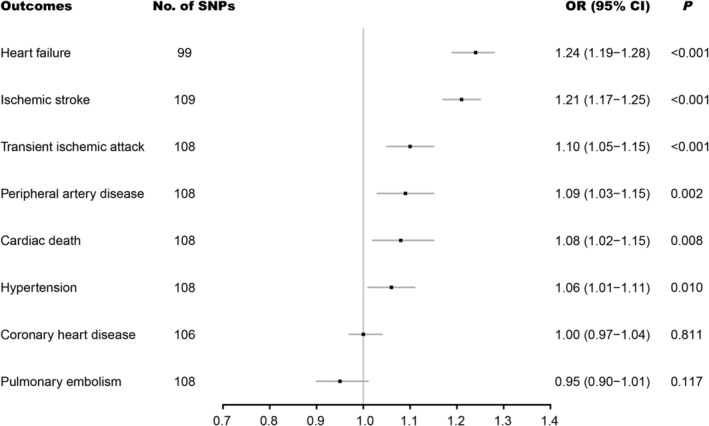
Associations of genetically predicted atrial fibrillation with eight cardiovascular diseases. CI, confidence interval; OR, odds ratio; SNP, single nucleotide polymorphism.
Table 1.
Associations between genetically predicted atrial fibrillation and cardiovascular diseases in sensitivity analyses
| Outcomes | Weighted median | MR‐Egger | Multivariable MR (Model 1 a ) | Multivariable MR (Model 2 b ) | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Coronary heart disease | 1.00 (0.95–1.05) | 0.961 | 0.95 (0.89–1.02) | 0.155 | 1.04 (0.98–1.11) | 0.148 | — | — |
| Hypertension | 1.08 (1.03–1.13) | 0.001 | 1.00 (0.92–1.09) | >0.999 | 1.10 (1.05–1.15) | <0.001 | 1.09 (1.05–1.14) | <0.001 |
| Heart failure | 1.23 (1.18–1.29) | <0.001 | 1.21 (1.13–1.30) | <0.001 | 1.29 (1.25–1.34) | <0.001 | 1.28 (1.24–1.33) | <0.001 |
| Ischaemic stroke | 1.23 (1.18–1.29) | <0.001 | 1.21 (1.14–1.29) | <0.001 | 1.22 (1.18–1.27) | <0.001 | 1.22 (1.17–1.27) | <0.001 |
| Transient ischaemic attack | 1.09 (1.00–1.18) | 0.038 | 1.02 (0.93–1.11) | 0.653 | 1.13 (1.06–1.21) | <0.001 | 1.13 (1.06–1.20) | <0.001 |
| Pulmonary embolism | 0.92 (0.82–1.03) | 0.155 | 0.94 (0.83–1.06) | 0.295 | 0.97 (0.89–1.07) | 0.562 | 0.97 (0.89–1.07) | 0.575 |
| Peripheral artery disease | 1.07 (0.98–1.18) | 0.141 | 1.07 (0.96–1.18) | 0.221 | 1.11 (1.02–1.20) | 0.011 | 1.09 (1.01–1.18) | 0.023 |
| Cardiac death | 1.04 (0.95–1.13) | 0.409 | 1.02 (0.91–1.13) | 0.766 | 1.05 (0.97–1.13) | 0.210 | 1.04 (0.97–1.12) | 0.297 |
CI, confidence interval; MR, Mendelian randomization; OR, odds ratio.
Smoking, body mass index, diabetes, and total cholesterol.
Model 1 + coronary heart disease.
The leave‐one‐out analysis suggested that the higher risks of heart failure, ischaemic stroke, transient ischaemic attack, peripheral artery disease, cardiac death, and hypertension were not driven by individual SNP (Supporting Information, Figure S1 ).
The intercept from the MR‐Egger method yielded no indication of directional pleiotropy (Table 2 ), which was also confirmed by the funnel plot in Figure 2 . Cochran's Q value implied a substantial heterogeneity in coronary heart disease, hypertension, heart failure, and ischaemic stroke. Therefore, a random‐effects model was adopted to mitigate the influence of heterogeneity.
Table 2.
Assessment of directional pleiotropy
| Outcomes | Directional pleiotropy | Heterogeneity | ||
|---|---|---|---|---|
| Intercept | P | Q | P | |
| Coronary heart disease | 0.005 | 0.07 | 202 | <0.01 |
| Hypertension | 0.006 | 0.11 | 324 | <0.01 |
| Heart failure | 0.002 | 0.45 | 223 | <0.01 |
| Ischaemic stroke | 0 | 0.94 | 192 | <0.01 |
| Transient ischaemic attack | 0.007 | 0.06 | 111 | 0.38 |
| Pulmonary embolism | 0.002 | 0.75 | 95 | 0.79 |
| Peripheral artery disease | 0.002 | 0.64 | 108 | 0.46 |
| Cardiac death | 0.006 | 0.20 | 127 | 0.09 |
Figure 2.
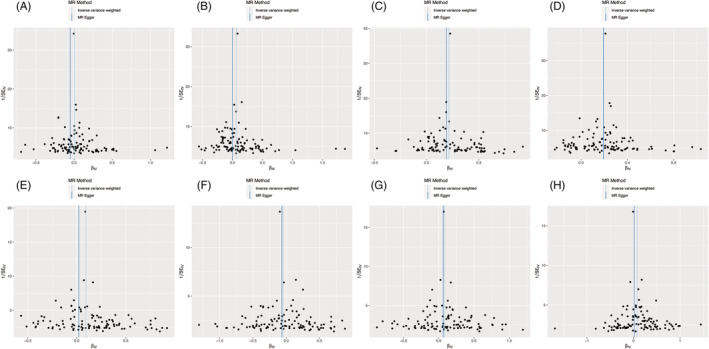
Funnel plot of the association of atrial fibrillation with eight cardiovascular diseases: (A) coronary heart disease, (B) hypertension, (C) heart failure, (D) ischaemic stroke, (E) transient ischaemic attack, (F) pulmonary embolism, (G) peripheral artery disease, and (H) cardiac death. Each black dot indicates a single nucleotide polymorphism. MR, Mendelian randomization.
Discussion
In the present MR analysis, we found causal associations between atrial fibrillation and heart failure, ischaemic stroke, transient ischaemic attack, peripheral artery disease, and cardiac death. Especially, the absolute risk increase was the highest for heart failure. No causal associations were observed for coronary heart disease or pulmonary embolism.
Adverse effects of atrial fibrillation on the development of ischaemic stroke have been demonstrated consistently in numerous studies, 21 , 22 but comparatively less attention has been paid to other complications associated with atrial fibrillation. In our MR analysis, we found that atrial fibrillation had the highest impact on heart failure. Similarly, in a cohort study prospectively enrolling 15 400 individuals with atrial fibrillation from 47 countries, the percentage of patients developing stroke or heart failure was 4% (604/15400) and 13% (1922/15400), respectively, during 1 year of follow‐up. Moreover, among the 1758 cases of death, stroke just caused 8% (148/1758) of death compared with the 30% (519/1758) caused by heart failure. 23 In a meta‐analysis conducted by Odutayo et al., 13 atrial fibrillation may increase the risks of cardiovascular death (RR: 2.03; 95% CI: 1.79–2.30), ischaemic stroke (RR: 2.33; 95% CI: 1.84–2.94), ischaemic heart disease (RR: 1.61; 95% CI: 1.38–1.87), heart failure (RR: 4.99; 95% CI: 3.04–8.22), and peripheral artery disease (RR: 1.31; 95% CI: 1.19–1.45). The highest absolute risk increase was also observed for heart failure. By applying MR analysis, our results provided directly causal evidence that more aggressive support and attention are required for not only ischaemic stroke but also heart failure prevention for populations with atrial fibrillation. Similarly, the bidirectional MR analysis performed by Kwok and Schooling revealed that atrial fibrillation was causally associated with higher risk of heart failure and vice versa. 11
The underlying reasons for the highest absolute risk increase in heart failure may be explained by the following mechanisms. First, the atrial contraction contributes about 20–25% of the total left ventricular stroke volume in normal sinus rhythm. However, in the context of atrial fibrillation, the atrial contraction was lost, and corresponding stroke volume was decreased, which can lead to heart failure. 24 Second, ventricular contraction during atrial fibrillation was also irregular, resulting in variable left ventricular filling and end‐diastolic volume. Therefore, the myocardial contractibility and total cardiac output were decreased. 25 Third, atrial fibrillation is often accompanied by a high ventricular rate. 5 The filling and release of calcium from the sarcoplasmic reticulum were also affected due to shorter cycle lengths. A positive correlation between high ventricular rate and severe left ventricular systolic dysfunction was also observed. 26 Fourth, atrial fibrillation may lead to the activation of several neurohumoral pathways, such as the renin–angiotensin–aldosterone system and adrenergic system. During shorter periods, angiotensin and aldosterone can result in vasoconstriction, fluid retention, and increased blood pressure. However, during longer periods, angiotensin and aldosterone can result in cardiomyocyte hypertrophy, apoptosis, and adverse structural remodelling in the atrial and ventricular wall, which can promote the development of systolic and diastolic left ventricular dysfunction. 27 Similarly, although the sympathetic stimulation during atrial fibrillation can contribute to increased contractility and heart rate in the short term, it may lead to heart failure in the long time. Fifth, the aforementioned haemodynamic alterations and overactivated regulatory mechanisms may result in the permanent structural change of the atrial and ventricular myocardium. The cardiac magnetic resonance imaging in patients with atrial fibrillation revealed increased levels of diffuse interstitial ventricular fibrosis, which may be the reason for increased left ventricular dysfunction. 28
In our MR analysis, we also found the causal effect of atrial fibrillation on cardiac death and peripheral artery disease, which is concordant with a meta‐analysis including 104 cohort studies and 9 686 513 participants where atrial fibrillation was associated with increased risks of cardiovascular death (RR: 2.03; 95% CI: 1.79–2.30) and peripheral artery disease (RR: 1.31; 95% CI: 1.19–1.45). 13 According to the data from the United States, cardiovascular death related to atrial fibrillation was increased with time going on, from 18.0 per 100 000 in 2011 to 22.3 per 100 000 in 2018. 29 In an analysis of the RE‐LY (Randomized Evaluation of Long‐Term Anticoagulant Therapy) trial involving patients with atrial fibrillation, cardiac death (including sudden cardiac death and progressive heart failure) accounted for 37.35% of all death. In contrast, stroke or peripheral embolism‐related death accounted for only 7.00% of all death. 30 In a post hoc analysis of the Systolic Hypertension in the Elderly Program database, cardiovascular death was also significantly higher in the atrial fibrillation group after 4.7 years [hazard ratio (HR): 2.39; 95% CI: 1.05–5.43] and 14.3 years of follow‐up (HR: 2.21; 95% CI: 1.54–3.17). 6 Therefore, the causal effect of atrial fibrillation on cardiac death should arise our attention in view of the growing prevalence of cardiovascular death related to atrial fibrillation. As to peripheral artery disease, the ARAPACIS (Ankle‐brachial Index Prevalence Assessment: Collaborative Italian Study) study revealed that the prevalence of ankle–brachial index ≤0.90 in patients with atrial fibrillation was 21% (428/2027 patients), which indicated that atrial fibrillation might be complicated with systemic atherosclerosis. 4 Correspondingly, the CHADS2 score has been refined by the CHA2DS2‐VASc score, which incorporates vascular diseases such as symptomatic peripheral artery disease. 31 The mechanisms linking peripheral artery disease and atrial fibrillation are not completely understood. It is plausible that the effect of atrial fibrillation on peripheral artery disease reflects an underlying state with increased levels of inflammation and platelet‐mediated thrombosis, 32 , 33 which may increase the predisposition for the development of peripheral artery disease. Meanwhile, further research is needed to elucidate these underlying mechanisms.
Taken together, our results provided directly causal evidence that more aggressive support and attention are required for not only ischaemic stroke but also heart failure, cardiac death, and peripheral artery disease prevention for populations with atrial fibrillation. The American Heart Association Scientific Statement pinpointed four pillars of atrial fibrillation management, including lifestyle modification, stroke prevention, rate control, and rhythm control. 34 Reversible risk factors for atrial fibrillation include obesity, hypertension, diabetes mellitus, obstructive sleep apnoea syndrome, alcohol consumption, and tobacco use. 35 Positive effects have been demonstrated in patients administered with risk factors such as weight, blood pressure, lipid, and obstructive sleep apnoea syndrome. 3 Although ischaemic strokes and transient ischaemic attacks prevention have improved dramatically with the availability of direct‐acting anticoagulants in patients with atrial fibrillation, 36 real‐world studies showed that just 44.9% of patients with atrial fibrillation are taking oral anticoagulants, and 23.8% of patients did not receive any antithrombotic therapy. 37 Medication adherence is a cornerstone of the management of atrial fibrillation, and good adherence can confer a significant decrease in death. 38 Novel oral anticoagulants reduced stroke‐related mortality, whereas little reduction was observed in heart failure or sudden cardiac death‐related mortality. 30 Therefore, patients with atrial fibrillation should be treated with either rhythm control or rate control. The beneficial effects of rhythm control have been confirmed in the recently published EAST‐AFNET trial (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial). 39 With the maturing of the catheter ablation techniques for the treatment of atrial fibrillation, catheter ablation may be a better choice than antiarrhythmic drugs for rhythm control. 40
Limitations
First, our analysis was based on people primarily of European ancestry, which might limit the generalization of our results to other populations. However, considering that population stratification might also affect MR estimates, the European origin makes population stratification bias unlikely to influence our results. Another limitation is the potential effect of pleiotropy on the results. However, we detected no indication of directional pleiotropy in the MR‐Egger analysis and funnel plots, and the multivariable MR analysis yielded similar results. Third, substantial heterogeneity was observed in coronary heart disease, hypertension, heart failure, and ischaemic stroke. Therefore, a random‐effects model was adopted to mitigate the influence of heterogeneity on results. Fourth, we only observed significant association for cardiac death in the IVW method, yet the difference disappeared in the sensitivity analyses. Considering there was no evidence of directional pleiotropy or heterogeneity for cardiac death, IVW method can be regarded as the primary estimate as it can provide the most precise estimates. Meanwhile, further MR analysis with larger sample size is needed to validate or refute our findings.
Conclusions
The present MR study verified the causal associations between atrial fibrillation and heart failure, ischaemic stroke, transient ischaemic attack, peripheral artery disease, and cardiac death. The absolute risk increase was the highest for heart failure. In light of the harmful effects of atrial fibrillation on other cardiovascular diseases besides ischaemic stroke, prevention through multiple perspectives to address reversible risk factors and optimize clinical treatment may be beneficial in delaying disease progression and improving survival. Meanwhile, considering the lack of difference in cardiac death in sensitivity analyses, further MR analysis with larger sample size is warranted to confirm or refute our findings.
Conflict of interest
None declared.
Funding
This work was supported by the National Key Research and Development Program of China (2017YFC1700503), CAMS Innovation Fund for Medical Sciences (2016‐I2M‐1‐009), and the National Science and Technology Program during the Twelfth Five‐year Plan Period (2011BAI11B02).
Supporting information
Table S1. Baseline characteristics of atrial fibrillation and other cardiovascular diseases.
Table S2. Single nucleotide polymorphisms used as instrumental variables in the Mendelian randomization analyses.
Figure S1. Leave‐one‐out sensitivity analysis of the association of atrial fibrillation with eight cardiovascular diseases.
A, coronary heart disease; B, hypertension; C, heart failure; D, ischemic stroke; E, transient ischemic attack; F, pulmonary embolism; G, peripheral artery disease; H, cardiac death.
Hu, M. , Tan, J. , Yang, J. , Gao, X. , and Yang, Y. (2023) Use of Mendelian randomization to evaluate the effect of atrial fibrillation on cardiovascular diseases and cardiac death. ESC Heart Failure, 10: 628–636. 10.1002/ehf2.14237.
References
- 1. Dzeshka MS, Shantsila A, Shantsila E, Lip GYH. Atrial fibrillation and hypertension. Hypertension. 2017; 70: 854–861. [DOI] [PubMed] [Google Scholar]
- 2. Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006; 27: 949–953. [DOI] [PubMed] [Google Scholar]
- 3. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): the Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021; 42: 373–498. [DOI] [PubMed] [Google Scholar]
- 4. Violi F, Daví G, Hiatt W, Lip GY, Corazza GR, Perticone F, Proietti M, Pignatelli P, Vestri AR, Basili S. Prevalence of peripheral artery disease by abnormal ankle‐brachial index in atrial fibrillation: implications for risk and therapy. J Am Coll Cardiol. 2013; 62: 2255–2256. [DOI] [PubMed] [Google Scholar]
- 5. Verhaert DVM, Brunner‐La Rocca HP, van Veldhuisen DJ, Vernooy K. The bidirectional interaction between atrial fibrillation and heart failure: consequences for the management of both diseases. Europace. 2021; 23: ii40–ii45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vagaonescu TD, Wilson AC, Kostis JB. Atrial fibrillation and isolated systolic hypertension: the Systolic Hypertension in the Elderly Program and Systolic Hypertension in the Elderly Program‐Extension study. Hypertension. 2008; 51: 1552–1556. [DOI] [PubMed] [Google Scholar]
- 7. Yetkin E, Cuglan B, Turhan H, Ozturk S, Yetkin O. Ignored identity of age‐dependent increase in pulmonary embolism: atrial fibrillation. Chest. 2019; 156: 1271–1272. [DOI] [PubMed] [Google Scholar]
- 8. Ruddox V, Sandven I, Munkhaugen J, Skattebu J, Edvardsen T, Otterstad JE. Atrial fibrillation and the risk for myocardial infarction, all‐cause mortality and heart failure: a systematic review and meta‐analysis. Eur J Prev Cardiol. 2017; 24: 1555–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gómez‐Outes A, Lagunar‐Ruíz J, Terleira‐Fernández AI, Calvo‐Rojas G, Suárez‐Gea ML, Vargas‐Castrillón E. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2016; 68: 2508–2521. [DOI] [PubMed] [Google Scholar]
- 10. Yuan S, Larsson SC. An atlas on risk factors for type 2 diabetes: a wide‐angled Mendelian randomisation study. Diabetologia. 2020; 63: 2359–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kwok MK, Schooling CM. Mendelian randomization study on atrial fibrillation and cardiovascular disease subtypes. Sci Rep. 2021; 11: 18682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheng S, Claggett B, Correia AW, Shah AM, Gupta DK, Skali H, Ni H, Rosamond WD, Heiss G, Folsom AR, Coresh J, Solomon SD. Temporal trends in the population attributable risk for cardiovascular disease: the Atherosclerosis Risk in Communities Study. Circulation. 2014; 130: 820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta‐analysis. BMJ. 2016; 354: i4482. [DOI] [PubMed] [Google Scholar]
- 14. Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, Herron TJ, McCarthy S, Schmidt EM, Sveinbjornsson G, Surakka I, Mathis MR, Yamazaki M, Crawford RD, Gabrielsen ME, Skogholt AH, Holmen OL, Lin M, Wolford BN, Dey R, Dalen H, Sulem P, Chung JH, Backman JD, Arnar DO, Thorsteinsdottir U, Baras A, O'Dushlaine C, Holst AG, Wen X, Hornsby W, Dewey FE, Boehnke M, Kheterpal S, Mukherjee B, Lee S, Kang HM, Holm H, Kitzman J, Shavit JA, Jalife J, Brummett CM, Teslovich TM, Carey DJ, Gudbjartsson DF, Stefansson K, Abecasis GR, Hveem K, Willer CJ. Biobank‐driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. 2018; 50: 1234–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, Saleheen D, Kyriakou T, Nelson CP, Hopewell JC, Webb TR, Zeng L, Dehghan A, Alver M, Armasu SM, Auro K, Bjonnes A, Chasman DI, Chen S, Ford I, Franceschini N, Gieger C, Grace C, Gustafsson S, Huang J, Hwang SJ, Kim YK, Kleber ME, Lau KW, Lu X, Lu Y, Lyytikäinen LP, Mihailov E, Morrison AC, Pervjakova N, Qu L, Rose LM, Salfati E, Saxena R, Scholz M, Smith AV, Tikkanen E, Uitterlinden A, Yang X, Zhang W, Zhao W, de Andrade M, de Vries PS, van Zuydam NR, Anand SS, Bertram L, Beutner F, Dedoussis G, Frossard P, Gauguier D, Goodall AH, Gottesman O, Haber M, Han BG, Huang J, Jalilzadeh S, Kessler T, König IR, Lannfelt L, Lieb W, Lind L, Lindgren CM, Lokki ML, Magnusson PK, Mallick NH, Mehra N, Meitinger T, Memon FU, Morris AP, Nieminen MS, Pedersen NL, Peters A, Rallidis LS, Rasheed A, Samuel M, Shah SH, Sinisalo J, Stirrups KE, Trompet S, Wang L, Zaman KS, Ardissino D, Boerwinkle E, Borecki IB, Bottinger EP, Buring JE, Chambers JC, Collins R, Cupples LA, Danesh J, Demuth I, Elosua R, Epstein SE, Esko T, Feitosa MF, Franco OH, Franzosi MG, Granger CB, Gu D, Gudnason V, Hall AS, Hamsten A, Harris TB, Hazen SL, Hengstenberg C, Hofman A, Ingelsson E, Iribarren C, Jukema JW, Karhunen PJ, Kim BJ, Kooner JS, Kullo IJ, Lehtimäki T, Loos RJF, Melander O, Metspalu A, März W, Palmer CN, Perola M, Quertermous T, Rader DJ, Ridker PM, Ripatti S, Roberts R, Salomaa V, Sanghera DK, Schwartz SM, Seedorf U, Stewart AF, Stott DJ, Thiery J, Zalloua PA, O'Donnell CJ, Reilly MP, Assimes TL, Thompson JR, Erdmann J, Clarke R, Watkins H, Kathiresan S, McPherson R, Deloukas P, Schunkert H, Samani NJ, Farrall M. A comprehensive 1000 Genomes‐based genome‐wide association meta‐analysis of coronary artery disease. Nat Genet. 2015; 47: 1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shah S, Henry A, Roselli C, Lin H, Sveinbjörnsson G, Fatemifar G, Hedman ÅK, Wilk JB, Morley MP, Chaffin MD, Helgadottir A, Verweij N, Dehghan A, Almgren P, Andersson C, Aragam KG, Ärnlöv J, Backman JD, Biggs ML, Bloom HL, Brandimarto J, Brown MR, Buckbinder L, Carey DJ, Chasman DI, Chen X, Chen X, Chung J, Chutkow W, Cook JP, Delgado GE, Denaxas S, Doney AS, Dörr M, Dudley SC, Dunn ME, Engström G, Esko T, Felix SB, Finan C, Ford I, Ghanbari M, Ghasemi S, Giedraitis V, Giulianini F, Gottdiener JS, Gross S, Guðbjartsson DF, Gutmann R, Haggerty CM, van der Harst P, Hyde CL, Ingelsson E, Jukema JW, Kavousi M, Khaw KT, Kleber ME, Køber L, Koekemoer A, Langenberg C, Lind L, Lindgren CM, London B, Lotta LA, Lovering RC, Luan J, Magnusson P, Mahajan A, Margulies KB, März W, Melander O, Mordi IR, Morgan T, Morris AD, Morris AP, Morrison AC, Nagle MW, Nelson CP, Niessner A, Niiranen T, O'Donoghue ML, Owens AT, Palmer CNA, Parry HM, Perola M, Portilla‐Fernandez E, Psaty BM, Rice KM, Ridker PM, Romaine SPR, Rotter JI, Salo P, Salomaa V, van Setten J, Shalaby AA, Smelser DT, Smith NL, Stender S, Stott DJ, Svensson P, Tammesoo ML, Taylor KD, Teder‐Laving M, Teumer A, Thorgeirsson G, Thorsteinsdottir U, Torp‐Pedersen C, Trompet S, Tyl B, Uitterlinden AG, Veluchamy A, Völker U, Voors AA, Wang X, Wareham NJ, Waterworth D, Weeke PE, Weiss R, Wiggins KL, Xing H, Yerges‐Armstrong LM, Yu B, Zannad F, Zhao JH, Hemingway H, Samani NJ, McMurray JJV, Yang J, Visscher PM, Newton‐Cheh C, Malarstig A, Holm H, Lubitz SA, Sattar N, Holmes MV, Cappola TP, Asselbergs FW, Hingorani AD, Kuchenbaecker K, Ellinor PT, Lang CC, Stefansson K, Smith JG, Vasan RS, Swerdlow DI, Lumbers RT. Genome‐wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat Commun. 2020; 11: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, Rutten‐Jacobs L, Giese AK, van der Laan SW, Gretarsdottir S, Anderson CD, Chong M, Adams HHH, Ago T, Almgren P, Amouyel P, Ay H, Bartz TM, Benavente OR, Bevan S, Boncoraglio GB, Brown RD Jr, Butterworth AS, Carrera C, Carty CL, Chasman DI, Chen WM, Cole JW, Correa A, Cotlarciuc I, Cruchaga C, Danesh J, de Bakker PIW, DeStefano AL, den Hoed M, Duan Q, Engelter ST, Falcone GJ, Gottesman RF, Grewal RP, Gudnason V, Gustafsson S, Haessler J, Harris TB, Hassan A, Havulinna AS, Heckbert SR, Holliday EG, Howard G, Hsu FC, Hyacinth HI, Ikram MA, Ingelsson E, Irvin MR, Jian X, Jiménez‐Conde J, Johnson JA, Jukema JW, Kanai M, Keene KL, Kissela BM, Kleindorfer DO, Kooperberg C, Kubo M, Lange LA, Langefeld CD, Langenberg C, Launer LJ, Lee JM, Lemmens R, Leys D, Lewis CM, Lin WY, Lindgren AG, Lorentzen E, Magnusson PK, Maguire J, Manichaikul A, McArdle PF, Meschia JF, Mitchell BD, Mosley TH, Nalls MA, Ninomiya T, O'Donnell MJ, Psaty BM, Pulit SL, Rannikmäe K, Reiner AP, Rexrode KM, Rice K, Rich SS, Ridker PM, Rost NS, Rothwell PM, Rotter JI, Rundek T, Sacco RL, Sakaue S, Sale MM, Salomaa V, Sapkota BR, Schmidt R, Schmidt CO, Schminke U, Sharma P, Slowik A, Sudlow CLM, Tanislav C, Tatlisumak T, Taylor KD, Thijs VNS, Thorleifsson G, Thorsteinsdottir U, Tiedt S, Trompet S, Tzourio C, van Duijn CM, Walters M, Wareham NJ, Wassertheil‐Smoller S, Wilson JG, Wiggins KL, Yang Q, Yusuf S, Bis JC, Pastinen T, Ruusalepp A, Schadt EE, Koplev S, Björkegren JLM, Codoni V, Civelek M, Smith NL, Trégouët DA, Christophersen IE, Roselli C, Lubitz SA, Ellinor PT, Tai ES, Kooner JS, Kato N, He J, van der Harst P, Elliott P, Chambers JC, Takeuchi F, Johnson AD, Sanghera DK, Melander O, Jern C, Strbian D, Fernandez‐Cadenas I, Longstreth WT Jr, Rolfs A, Hata J, Woo D, Rosand J, Pare G, Hopewell JC, Saleheen D, Stefansson K, Worrall BB, Kittner SJ, Seshadri S, Fornage M, Markus HS, Howson JMM, Kamatani Y, Debette S, Dichgans M. Multiancestry genome‐wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018; 50: 524–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology. 2017; 28: 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015; 44: 512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR‐Egger method. Eur J Epidemiol. 2017; 32: 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gage BF. Stroke prediction rules in atrial fibrillation. J Am Coll Cardiol. 2018; 71: 133–134. [DOI] [PubMed] [Google Scholar]
- 22. Freedman B, Potpara TS, Lip GY. Stroke prevention in atrial fibrillation. Lancet. 2016; 388: 806–817. [DOI] [PubMed] [Google Scholar]
- 23. Healey JS, Oldgren J, Ezekowitz M, Zhu J, Pais P, Wang J, Commerford P, Jansky P, Avezum A, Sigamani A, Damasceno A, Reilly P, Grinvalds A, Nakamya J, Aje A, Almahmeed W, Moriarty A, Wallentin L, Yusuf S, Connolly SJ. Occurrence of death and stroke in patients in 47 countries 1 year after presenting with atrial fibrillation: a cohort study. Lancet. 2016; 388: 1161–1169. [DOI] [PubMed] [Google Scholar]
- 24. Zakeri R, Moulay G, Chai Q, Ogut O, Hussain S, Takahama H, Lu T, Wang XL, Linke WA, Lee HC, Redfield MM. Left atrial remodeling and atrioventricular coupling in a canine model of early heart failure with preserved ejection fraction. Circ Heart Fail. 2016; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clark DM, Plumb VJ, Epstein AE, Kay GN. Hemodynamic effects of an irregular sequence of ventricular cycle lengths during atrial fibrillation. J Am Coll Cardiol. 1997; 30: 1039–1045. [DOI] [PubMed] [Google Scholar]
- 26. Hendrick DA, Smith AC, Kratz JM, Crawford FA, Spinale FG. The pig as a model of tachycardia and dilated cardiomyopathy. Lab Anim Sci. 1990; 40: 495–501. [PubMed] [Google Scholar]
- 27. Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol. 2017; 14: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ling LH, Kistler PM, Ellims AH, Iles LM, Lee G, Hughes GL, Kalman JM, Kaye DM, Taylor AJ. Diffuse ventricular fibrosis in atrial fibrillation: noninvasive evaluation and relationships with aging and systolic dysfunction. J Am Coll Cardiol. 2012; 60: 2402–2408. [DOI] [PubMed] [Google Scholar]
- 29. Tanaka Y, Shah NS, Passman R, Greenland P, Lloyd‐Jones DM, Khan SS. Trends in cardiovascular mortality related to atrial fibrillation in the United States, 2011 to 2018. J Am Heart Assoc. 2021; 10: e020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marijon E, Le Heuzey JY, Connolly S, Yang S, Pogue J, Brueckmann M, Eikelboom J, Themeles E, Ezekowitz M, Wallentin L, Yusuf S. Causes of death and influencing factors in patients with atrial fibrillation: a competing‐risk analysis from the randomized evaluation of long‐term anticoagulant therapy study. Circulation. 2013; 128: 2192–2201. [DOI] [PubMed] [Google Scholar]
- 31. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010; 137: 263–272. [DOI] [PubMed] [Google Scholar]
- 32. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004; 110: 738–743. [DOI] [PubMed] [Google Scholar]
- 33. Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet. 2009; 373: 155–166. [DOI] [PubMed] [Google Scholar]
- 34. Chung MK, Eckhardt LL, Chen LY, Ahmed HM, Gopinathannair R, Joglar JA, Noseworthy PA, Pack QR, Sanders P, Trulock KM. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020; 141: e750–e772. [DOI] [PubMed] [Google Scholar]
- 35. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang NY, Tsao CW. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. 2021; 143: e254–e743. [DOI] [PubMed] [Google Scholar]
- 36. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014; 383: 955–962. [DOI] [PubMed] [Google Scholar]
- 37. Hsu JC, Maddox TM, Kennedy KF, Katz DF, Marzec LN, Lubitz SA, Gehi AK, Turakhia MP, Marcus GM. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: insights from the NCDR PINNACLE registry. JAMA Cardiol. 2016; 1: 55–62. [DOI] [PubMed] [Google Scholar]
- 38. Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, Stricker B, Mendis S, Hofman A, Mant J, Franco OH. Adherence to cardiovascular therapy: a meta‐analysis of prevalence and clinical consequences. Eur Heart J. 2013; 34: 2940–2948. [DOI] [PubMed] [Google Scholar]
- 39. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, Hamann F, Heidbüchel H, Hindricks G, Kautzner J, Kuck KH, Mont L, Ng GA, Rekosz J, Schoen N, Schotten U, Suling A, Taggeselle J, Themistoclakis S, Vettorazzi E, Vardas P, Wegscheider K, Willems S, Crijns H, Breithardt G. Early rhythm‐control therapy in patients with atrial fibrillation. N Engl J Med. 2020; 383: 1305–1316. [DOI] [PubMed] [Google Scholar]
- 40. Wazni OM, Dandamudi G, Sood N, Hoyt R, Tyler J, Durrani S, Niebauer M, Makati K, Halperin B, Gauri A, Morales G, Shao M, Cerkvenik J, Kaplon RE, Nissen SE. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021; 384: 316–324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics of atrial fibrillation and other cardiovascular diseases.
Table S2. Single nucleotide polymorphisms used as instrumental variables in the Mendelian randomization analyses.
Figure S1. Leave‐one‐out sensitivity analysis of the association of atrial fibrillation with eight cardiovascular diseases.
A, coronary heart disease; B, hypertension; C, heart failure; D, ischemic stroke; E, transient ischemic attack; F, pulmonary embolism; G, peripheral artery disease; H, cardiac death.


