Abstract
Aims
To determine the incidence of hyperkalaemia in patients with heart failure with reduced ejection fraction (HFrEF) during up‐titration of guideline‐directed medical therapy (GDMT) in real‐world settings.
Methods
A retrospective review of medical records of all patients hospitalized for newly onset HFrEF at Sahlgrenska University Hospital, Sweden, between 1 January 2016 and 31 December 2019. Based on mineralocorticoid receptor antagonist (MRA) treatment within the first 6 months, patients were divided into four groups: (i) never received MRA, (ii) needed MRA dose reduction, (iii) needed discontinuation of MRA, and (iv) stable MRA treatment. Potassium levels were assessed at baseline and has the highest potassium level during the 6 months of up‐titration.
Results
Of 3456 patients hospitalized for heart failure, 630 (18%) were eligible (68.4% men, 66.8 years, mean EF of 29.4%). After up‐titration of GDMT 48.4% of patients received MRAs. Patients without MRA treatment were older (P < 0.0001), had lower EF (P = 0.022), had higher NTproBNP (P = 0.017), had lower eGFR (P = 0.001), and were more often treated with angiotensin receptor inhibitors/angiotensin receptor blockers/angiotensin receptor neprilysin inhibitors (all P < 0.0001). In overall study population, hyperkalaemia increased from 5.9 to 24.4% after 6 months of up‐titration of GDMT (P < 0.0001). Among four groups, the incidence of hyperkalaemia throughout up‐titration of GDMT increased from 6.8 to 54.5% in patients with dose reduction of MRA, from 8.8 to 50.9% in those with discontinuation of MRA, from 5 to 10% in patients with stable MRA treatment, and from 6 to 28% in patients who were MRA naive (all P < 0.0001). In the MRA‐naive group, normokalaemia/hypokalaemia occurred in 87.5% at baseline, and after 6 months of up‐titration of GDMT, normokalaemia/hypokalaemia remained in 47.8%, whereas mild, moderate, and severe hyperkalaemia occurred in 22.4%, 5.7%, and 0.9%, respectively.
Conclusions
Hyperkalaemia increased significantly during up‐titration of GDMT but with varying magnitudes in different clinical phenotypes, which might explain why physicians refrain from prescribing MRAs to patients with HFrEF.
Keywords: Hyperkalaemia, Heart failure, Epidemiology, Treatment, Mineralocorticoid receptor antagonists
Introduction
Remarkable progress in treating patients with HFrEF has been achieved during the past few decades. Although the ability to improve the outcome never has been better than today, the 5‐year survival rate after hospitalization with HFrEF still remains as low as 25%. 1 One of the underlying causes is the suboptimal clinical implementation of guideline‐directed medical therapy (GDMT). It is well known that treatment with angiotensin receptor inhibitors (ACEis), angiotensin receptor blockers (ARBs), angiotensin receptor neprilysin inhibitors (ARNIs), and beta‐blockers (BB) reduce mortality and morbidity in patients with HFrEF. 2 , 3 , 4 , 5 , 6 , 7 The effect of the MRAs on reducing mortality and hospitalizations due to HF is also well documented. 8 , 9
Despite the proven benefits of MRAs for patients with HFrEF, there are substantial disparities between the recommendations in the guidelines and implementation of MRAs in clinical practice, both in the United States and in Europe. 10 , 11 , 12 , 13 Observational data from the United States note the under‐use of MRAs in 50% of eligible patients over almost one decade. Even when prescribed, the doses were often below the target doses and without obvious improvement over time. 10 , 13 If prescribed, MRAs were often discontinued due to adverse events as hyperkalaemia and impaired renal function. 11 , 14
Hyperkalaemia and worsening renal function have been reported as potential significant causes of the under‐use of MRAs. 2 , 15 Yet, the reported incidence of hyperkalaemia varied between 0.9 and 23%. 16 In a Swedish study, the under‐use of MRAs was not associated with elevated potassium levels but with impaired renal function, despite the range of the creatinine clearance was 30–59.9 mL/min, where MRAs are not contraindicated, and non‐specialist care recovered HF and no use of other HF therapy. 12 Another study showed that MRAs were more likely to be discontinued in patients with chronic kidney dysfunction. These authors reported an event with moderate or severe hyperkalaemia in 9.3% of patients with HF, which was also the cause for discontinuation of MRAs in 47% of the cases. 14 Essentially, the reported incidence of hyperkalaemia is far too low in relation to the percentage of undertreated patients and varies extensively in different reports. Consequently, the fraction of patients at risk for hyperkalaemia most likely presents a substantial subgroup that is still not well described and may only be estimated at an individual level. Thus, data on the total burden of hyperkalaemia in real‐life settings are insufficient to explain the under‐use of MRA.
This study aims to determine the incidence and magnitude of documented hyperkalaemia and the potential risk of hyperkalaemia in patients with HFrEF at baseline and during optimal up‐titration of GDMT in a real‐world HF population.
Methods
Patient selection
All patients 18–85 years of age at baseline, hospitalized for newly onset HFrEF, with an ejection fraction (EF) less than 40% in the period between 1 January 2016 and 31 December 2019, at one of three affiliations at Sahlgrenska University Hospital, Gothenburg, Sweden, were identified retrospectively and consecutively included from the hospital discharge registry. Baseline refers to the time when diagnosed with HFrEF, which in this study is the date of discharge from hospitalization when a patient receives a diagnosis of HFrEF. Eligible patients were identified using the International Classification of Disease (ICD)‐10 codes I50.0–I50.9 as principal diagnosis at discharge. These patients formed the hospital cohort.
Data collection and validation
All patient‐related data at Sahlgrenska University Hospital have been stored electronically since the beginning of the 21st century. A well‐trained physician carefully evaluated medical records for each patient with an HF diagnosis to differentiate patients with newly onset HFrEF with EF < 40%, regardless of the genesis of HFrEF, from other patients with HF, including results from different examinations (e.g. cardiac ultrasound, laboratory results and written documentation) from the beginning of the 21st century. In questionable cases, medical records were discussed with three experienced cardiologists. All data, including co‐morbidities (e.g. diabetes mellitus, ischaemic heart disease, atrial fibrillation/flutter, and cancer), were collected. Diagnostic tests and medical and device treatment at baseline or before baseline were also collected. Patients who died, underwent heart transplantation, and received a long‐term mechanical assist device or ongoing permanent dialysis within 6 months, as well as patients with incomplete medical records (e.g. referral to other follow‐up sites), were excluded.
Up‐titration of GDMT and MRA use
All newly diagnosed HFrEF must go through maximum up‐titration of ACEi/ARB/ARNI, BB, and MRA as recommended by the European Society of Cardiology (ESC) guidelines within 6 months from diagnosis (baseline) at a nurse‐based specialized heart failure (HF) outpatient clinic. 17 After 6 months, medical records were evaluated if treatment with MRA was initiated and maximal dose achieved. Assessments were also done to determine whether treatment with MRA needed adjustment (e.g. dose reduction or discontinuation), as well as documented reasons for these actions. Patients were divided into four prespecified subgroups (see the ‘Group division based on MRA use’).
Definition of hyperkalaemia, borderline hyperkalaemia, and expected risk of hyperkalaemia
The potassium values in the study were analysed either in serum or plasma. Hyperkalaemia was defined as K ≥ 5.0 mmol/L and normokalaemia as K < 4.8 mmol/L. The potassium levels were documented at baseline, as the last recorded potassium level before hospital discharge of the index hospitalization when patients were diagnosed with HFrEF. The levels of potassium were also recorded as the highest potassium documented within 6 months after baseline. Hyperkalaemia was presented at different levels: K ≥ 6.0, K ≥ 5.5, and K ≥ 5.0 mmol/L. Borderline hyperkalaemia was defined as K 4.8–4.9 mmol/L, mild hyperkalaemia as K 5.0–5.4, moderate hyperkalaemia as K 5.5–5.9, and severe hyperkalaemia as K ≥ 6.0. Hypokalaemia was defined as K < 3.5. The expected risk of hyperkalaemia included an overall assessment based on previous studies. 18 In addition to borderline hyperkalaemia, K 4.8–4.9 mmol/L, presence of diabetes mellitus, and eGFR < 45 mL/min were taken into account, and together with HFrEF, confer patients at higher risk of developing hyperkalaemia.
Physicians' reasoning for under‐use of MRA
Physician's reasoning for the under‐use of MRAs was collected from medical records.
Group division based on MRA use
The study population was divided into four groups: patients who never received MRA treatment within 6 months after baseline; patients who received MRA but needed dose reduction within 6 months after baseline; patients who received MRA, but treatment was discontinued within 6 months after baseline; and patients who received MRA treatment within 6 months after baseline, without any dose reduction.
Exploratory analysis
An exploratory analysis of all‐cause mortality was performed in patients from the hospital cohort who were not treated with MRA compared with matched (age, sex, co‐morbidities) patients treated with MRA from the Swedish Heart Failure Registry (SwedeHF), here defined as controls (Table S1). The SwedeHF is a nationwide HF registry implemented in 2003. 12 The annual reports, protocol, and registration form are available at http://www.swedehf.se. Individual written consent is not required for registration in national registries, but patients can withdraw their consent at any time. With the unique 12‐digit personal identification number that all Swedish permanent residents receive, the patient cohort from SwedeHF was linked to other registers. Data on co‐morbidities were obtained from the Swedish National Patient Registry, mandatory in Sweden since 1987 and having full coverage throughout Sweden. Diagnoses are registered according to the International Classification of Diseases (ICD), and for this study, the tenth revision (ICD‐10) was used. Mortality data were obtained from the Cause Specific Death Registry (http://www.socialstyrelsen.se). The establishment of the registry and analysis of the data were approved by a multisite Ethics Committee (2012/285‐31, 2013/302‐32, 2017/510‐32). The registry and this study comply with the 1964 Declaration of Helsinki and its later amendments. 19
Statistical analysis
The Pearson chi‐square test was used to assess differences between categorical variables, the Mann–Whitney test for continuous variables with respect to two treatment groups, and Kruskal–Wallis test with respect to four treatment groups. Following the visual review of the variable distribution and as indicated by the Kolmogorov–Smirnov test, normal distribution could not be assumed for the analysed continuous variables why the non‐parametric tests were chosen. Each patient with HFrEF in the hospital cohort without MRA treatment after 6 months from inclusion was matched with one patient with HF from the SwedeHF using propensity score matching analysis with 1:1 nearest neighbour matching. Variables used in developing the propensity score included age, sex, height, weight, systolic blood pressure, LVEF, creatinine, atrial fibrillation, diabetes mellitus, and treatment with ACEi/ARB and BB. Cox regression analysis was calculated to assess mortality risks within 1 year in patients without MRA treatment in the hospital cohort compared with matched controls from the SwedeHF. Finally, a univariate Cox regression model was applied to estimate the effect of mild and moderate hyperkalaemia on survival. Statistical analyses were performed using SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL, USA). Figures and tables were created in Microsoft Office Word. All P values < 0.05 (two‐sided) were considered statistically significant.
Results
Baseline data
Some 3456 unique medical records of patients who received HF diagnosis were identified from the local hospital discharge registry. Exclusion criteria were EF ≥ 40%, incomplete data, on permanent dialysis, with chronic HF, who died at baseline or within 6 months from baseline, were heart transplanted at baseline or within 6 months from baseline, received long‐term mechanical assist device within 6 months after HFrEF diagnosis, or where data in the medical records were incomplete or not available (see the flowchart in Figure 1 ). The final population included 630 patients (68.4% men), with a median (IQR) age of 69 (59, 76) years and a mean (±SD) EF of 29.4 (6.8) %.
Figure 1.
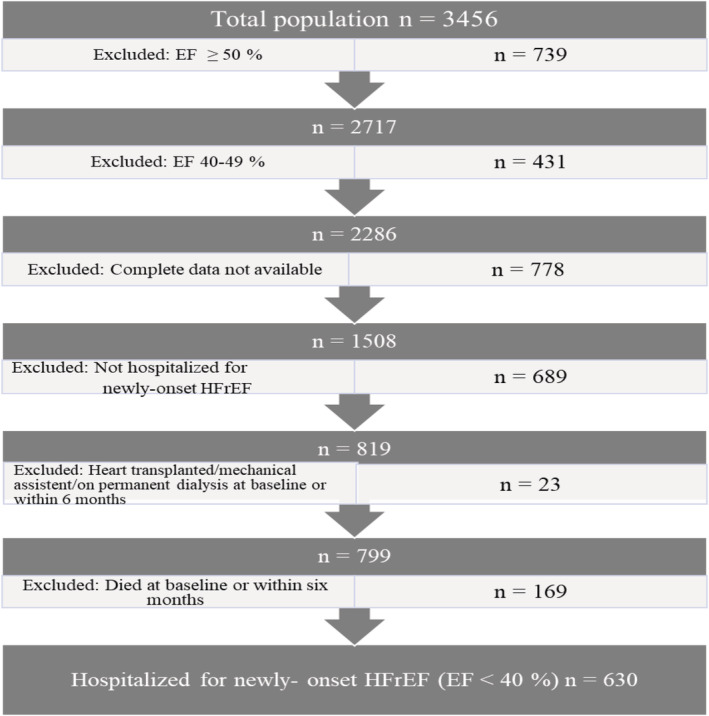
Flowchart illustrating the selection procedure of the study population. EF, ejection fraction; HFrEF, heart failure with reduced ejection fraction.
After up‐titration of GDMT for 6 months, 48.4% of patients received MRA treatment. Out of the total study population, 268 patients (42.5%) of the study population never received MRA treatment within 6 months after baseline (group 1), 44 patients (7%) of the study population received MRA but needed dose reduction within 6 months after baseline (group 2), 57 patients (9%) of the study population received MRA but treatment was discontinued within 6 months after baseline (group 3), and 261 patients (41.4%) of the study population received MRA treatment within 6 months after baseline, without any dose reduction (group 4). Depending on the initial dose, some patients in group 4 could have the same dosing as those in group 2.
As shown in Table 1 , patients who were not on treatment with MRA were older (P < 0.0001), were more likely to be female (P = 0.002), had lower EF (P = 0.022) and higher NTproBNP (P = 0.017), had lower eGFR (P = 0.001), had more co‐morbidities, and were more often treated with ACE/ARB (P < 0.0001) or ARNI (P < 0.0001) within 6 months from baseline.
Table 1.
Characteristics of patients at baseline or before baseline categorized by treatment with or without MRA 6 months after established HFrEF diagnosis
| Total | MRA | Non‐MRA | P value | |
|---|---|---|---|---|
| n = 630 | n = 305 | n = 325 | ||
| Age (IQR) | 69 (59, 76) | 66 (56, 74) | 71 (63,78) | <0.0001 |
| Male, n (%) | 431 (68.4) | 227 (74.4) | 204 (62.8) | 0.002 |
| EF (mean ± SD) | 29.4 (6.8) | 30.6 (6.4) | 28.0 (7.0) | 0.022 |
| Systolic BP (mmHg) (IQR) | 123 (110, 136) | 123 (110, 135) | 123 (110, 137) | 0.694 |
| Diastolic BP (mmHg) (IQR) | 74 (66, 84) | 75 (69, 86) | 72 (65, 82) | 0.017 |
| Heart rate (bpm) (IQR) | 78 (66, 94) | 77 (65, 93) | 79 (68, 94) | 0.268 |
| NTproBNP (IQR) | 3840 (1810, 7520) | 3530 (1743, 6633) | 4310 (1820, 8545) | 0.041 |
| eGFR, (IQR) | 63 (51, 75) | 67 (56, 76) | 58 (44, 74) | <0.0001 |
| Co‐morbidities | Total | MRA | Non‐MRA | P value |
|---|---|---|---|---|
| n = 630 | n = 305 | n = 325 | ||
| Hypertension, n (%) | 309 (49) | 147 (48.2) | 162 (49.8) | 0.809 |
| Diabetes mellitus, n (%) | 141 (22.4) | 67 (22) | 74 (22.8) | 0.436 |
| IHD, n (%) | 237 (37.6) | 110 (36.1) | 127 (39.1) | 0.720 |
| VHD, n (%) | 33 (5.2) | 15 (4.9) | 18 (5.6) | 0.510 |
| AF, n (%) | 277 (44) | 130 (42.6) | 147 (45.2) | 0.001 |
| COPD, n (%) | 54 (8.6) | 27 (8.9) | 27 (8.3) | 0.012 |
| eGFR <30, n (%) | 21 (3.4) | 3 (1) | 18 (5.6) | 0.266 |
| Cancer, n (%) | 93 (14.8) | 34 (11.1) | 59 (18.3) | 0.761 |
| Stroke, n (%) | 99 (15.8) | 43 (14.1) | 56 (17.3) | 0.004 |
| Medical treatment | Total | MRA | Non‐MRA | P value |
|---|---|---|---|---|
| n = 630 | n = 305 | n = 325 | ||
| ACEi/ARB, n (%) | 502 (79.7) | 246 (80.7) | 256 (78.8) | 0.0001 |
| BB, n (%) | 597 (94.8) | 297 (97.4) | 300 (92.3) | 0.136 |
| ARNI, n (%) | 68 (10.8) | 49 (16.1) | 19 (5.8) | 0.0001 |
| SGLT2, n (%) | 21 (3.3) | 14 (4.6) | 7 (2.2) | 0.136 |
ACEi, angiotensin‐converting‐enzyme inhibitors; AF, atrial fibrillation; ARB, angiotensin II‐receptor antagonist; ARNI, angiotensin receptor neprilysin inhibitor; BB, beta‐blockers; CHD, congenital heart disease; COPD, chronic obstructive heart disease; eGFR, estimated glomerular filtration rate; IHD, ischaemic heart disease; IQR, interquartile range; NTproBNP, N‐terminal prohormone of brain natriuretic peptide; SGLT2i, sodium/glucose cotransporter 2 inhibitors; SD, standard deviation; VHD, valve heart disease.
Values are expressed as numbers (percentages) unless otherwise stated.
Goal achievement of up‐titration of GDMT in subgroups
After initiation/up‐titration of GDMT, patients from the four subgroups (patients naive, needed dose reduction, discontinuation, and stable on treatment with MRA) achieved the treatment goals: BB were used in approximately 92–98% of the cases (P = 0.025), ACEi/ARB (P = 0.861) in 77–82% but varying between subgroups, and ARNI in 10.4% (P = 0.001). Patients who tolerated MRA treatment well (group 4) achieved the highest treatment rates: BB were used in 98% of the cases, ACEi/ARB in 82%, and ARNI in 17%. In contrast, naive patients to MRA treatment (group 1) achieved the lowest treatment rates: BB were used in 92% of the cases, ACEi/ARB in 79%, and ARNI in 4%. However, this patient group had more often patients of older age, with a higher prevalence of hypotension and lower eGFR compared with group 1 (Table 2 ).
Table 2.
Up‐titration of guideline‐directed medical therapy (GDMT) during the first 6 months by patterns of treatment with MRA
| Group 1 | Group 2 | Group 3 | Group 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Patients never received MRA n = 268 |
Patients needed dose reduction of MRA n = 44 |
Patients needed discontinuation of MRA n = 57 |
Patients with unchanged MRA dose n = 261 |
|||||||
| Up‐titrations of GDMT, n (%) | Before baseline | 6 months from baseline | Before baseline | 6 months from baseline | Before baseline | 6 months from baseline | Before baseline | 6 months from baseline | P values before baseline* | P values 6 months from baseline* |
| ACEi/ARB, n (%) | 77 (32.2) | 211 (79) | 14 (32.6) | 34 (77.3) | 23 (42.6) | 45 (80.4) | 86 (39.1) | 212 (81.5) | 0.303 | 0.861 |
| BB, n (%) | 83 (34.9) | 246 (91.8) | 20 (46.5) | 42 (95.5) | 20 (37.7) | 54 (94.7) | 83 (37.7) | 255 (97.7) | 0.536 | 0.025 |
| ARNI, n (%) | 12 (4.5) | 6 (13.6) | 6 (10.5) | 43 (16.5) | 0.001 | |||||
| SGLT2, n (%) | 5 (1.9) | 1 (2.3) | 2 (3.5) | 12 (4.6) | 0.565 | |||||
| Clinical phenotype | Baseline | 6 months from baseline | Baseline | 6 months from baseline | Baseline | 6 months from baseline | Baseline | 6 months from baseline | P values baseline* | P values 6 months from baseline* |
|---|---|---|---|---|---|---|---|---|---|---|
| Systolic BP (mmHg) (IQR) | 124 (110, 137) | 130 (114, 140) | 123 (109, 132) | 114 (102, 124) | 120 (111, 138) | 119 (98, 140) | 123 (110, 137) | 126 (111, 139) | 0.737 | 0.0006 |
| Diastolic BP (mmHg) (IQR) | 73 (65, 82) | 72 (64, 80) | 71 (66, 80) | 71 (61, 78) | 70 (65, 80) | 68 (61, 80) | 76 (65, 93) | 80 (70, 90) | 0.010 | <0.0001 |
| Hypotension, n (%) a , b | 5 (1.9) | 3 (1.4) | 0 (0) | 4 (10) | 2 (3.5) | 3 (5.5) | 2 (0.8) | 2 (1) | 0.320 | 0.002 |
| eGFR (IQR) | 59 (45, 75) | 56 (39, 67) | 61 (48, 71) | 53 (36, 65) | 58 (42, 71) | 41 (31, 55) | 68 (57, 78) | 68 (59, 79) | <0.0001 | <0.0001 |
| eGFR <30 mL/min, n (%) c | 24 (9) | 12 (4.5) | 0 | 6 (13.6) | 7 (12.3) | 7 (12.3) | 3 (1.1) | 3 (1.1) | 0.004 | <0.001 |
ACEi, angiotensin‐converting‐enzyme inhibitors; ARB, angiotensin II receptor antagonists; ARNI, angiotensin receptor neprilysin inhibitor; BB, beta‐blockers; BP, blood pressure; IQR, interquartile range; NTproBNP, N‐terminal prohormone of brain natriuretic peptide; SD, standard deviation; SGLT2i, sodium/glucose cotransporter 2 inhibitors.
Values are expressed as numbers (percentages) unless otherwise stated. Group 1: MRA‐naive patients. Group 2: Patients needed dose reduction of MRA. Group 3: Patients needed discontinuation of MRA. Group 4: Unchanged treatment with MRA.
Hypotension is defined as systolic BP < 90 mmHg.
Values are missing in 111 cases.
Values missing for eGFR 6 months from baseline in 265 cases.
The rate and magnitude of incident hyperkalaemia: Overall and between subgroups
Hyperkalaemia occurred in 37 patients (5.9) at baseline and 154 patients (24.4) within 6 months after the initiation/up‐titration of GDMT. However, the increase in the incidence of hyperkalaemia differed greatly among the subgroups. In group 1 (42.5% of the study population), the observed increase of incident hyperkalaemia was 467% (from 6% at baseline to 28%, 6 months later), in group 2 (7.0% of the study population) 801% (from 6.8% at baseline to 54.5% 6 months later), in group 3 (9.0% of the study population) 578% (from 8.8% at baseline to 50.9%, 6 months later), and in group 4 (41.4% of the study population) 200% (from 5.0% at baseline to 10%, 6 months later) (all P > 0.0001). In contrast to groups 2–4, 6% of patients naive to MRA treatment (group 1) had hyperkalaemia registered at baseline. For patients in group 1, despite no treatment with MRA, hyperkalaemia increased by 467% (central illustration). The incidence of borderline hyperkalaemia in group 1 increased by 204%, from 7.5% to 15.3%. In the total study population, there was a 2.7‐fold increased risk of hyperkalaemia within 6 months after the up‐titration of GDMT (Table 3 ).
Table 3.
Potassium levels before baseline and during up‐titrations of GDMT at 6 months by subgroup
| Group 1 | Group 2 | Group 3 | Group 4 |
Total group n = 630 |
P values* | |
|---|---|---|---|---|---|---|
|
Patients never received MRA n = 268 |
Patients needed dose reduction of MRA n = 44 |
Patients needed discontinuation of MRA n = 57 |
Patients with unchanged MRA dose n = 261 |
|||
| At baseline | ||||||
| K ≥ 6.0 mmol, n (%) | 1 (0.4) | 1 (2.3) | 0 | 0 | 2 (0.3) | 0.098 |
| K ≥ 5.5 mmol/L, n (%) | 1 (0.4) | 1 (2.3) | 1 (1.8) | 0 | 3 (0.5) | 0.102 |
| K ≥ 5.0 mmol/L, n (%) | 16 (16.1) | 3 (6.8) | 5 (8.8) | 13 (5) | 37 (5.9) | 0.723 |
| K ≥ 4.8 mmol/L, n (%) | 36 (13.7) | 8 (18.2) | 10 (17.5) | 40 (15.3) | 94 (14.9) | 0.806 |
| K 4.8–4.9 mmol/L and diabetes mellitus and eGFR < 45 mL/min/1.73 m2, n (%) | 2 (0.8) | 0 | 0 | 0 | 2 (0.3) | 0.428 |
| Group 1 | Group 2 | Group 3 | Group 4 |
Total group n = 630 |
P values* | |
|---|---|---|---|---|---|---|
|
Patients never received MRA n = 268 |
Patients needed dose reduction of MRA n = 44 |
Patients needed discontinuation of MRA n = 57 |
Patients with unchanged MRA dose n = 261 |
|||
| During 6 months of follow‐up | ||||||
| Highest K during initiation/up‐titration of GDMT mean (SD), minimum, maximum | 4.8 (0.5), 3.7, 7.6* | 5.0 (0.5), 3.7, 6.4 | 5.1 (0.8), 3.6, 7.5 | 4.4 (0.5), 3.0, 5.7 | ||
| K ≥ 6.0 mmol, n (%) | 4 (1.5) | 3 (6.8) | 6 (1) | 0 | 13 (2.1) | <0.001 |
| K ≥ 5.5 mmol/L, n (%) | 18 (7.3) | 6 (13.6) | 13 (23.2) | 3 (1.2) | 40 (6.8) | <0.001 |
| K ≥ 5.0 mmol/L, n (%) | 75 (28) | 24 (54.5) | 29 (50.9) | 26 (10.7) | 154 (26) | <0.001 |
| K ≥ 4.8 mmol/L, n (%) | 116 (46.8) | 31 (70.5) | 36 (64.3) | 48 (19.7) | 231 (39) | <0.001 |
| K 4.8–4.9 mmol/L and diabetes mellitus and eGFR < 45 mL/min/1.73 m2, n (%)a | 2 (0.7) | 0 | 0 | 3 (1.1) | 5 (0.8) | 0.732 |
eGFR, estimated glomerular filtration rate; SD, standard deviation.
Values are expressed as numbers (percentages) unless otherwise stated.
Values missing for eGFR during 6 months of follow‐up in 265 cases.
P values for the whole group.
Central illustration. Prevalence of documented hyperkalaemia and borderline hyperkalaemia and patients at potential risk of hyperkalaemia in the total study population at baseline and 6 months after established HFrEF diagnosis.
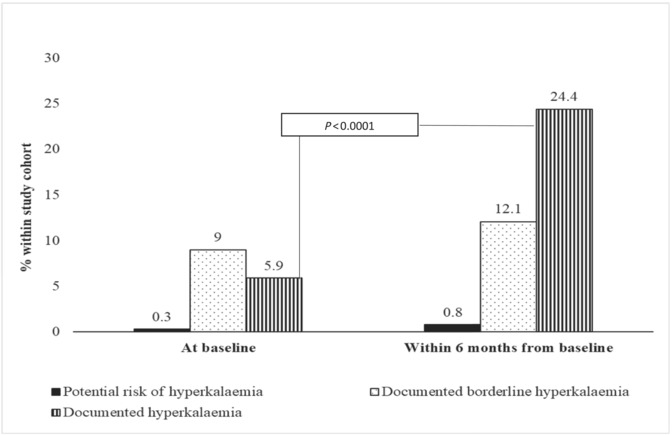
Of the total population, 85.1% had hypokalaemia/normokalaemia at baseline; within 6 months from baseline, 70.9% in the group with MRA with an unchanged dose still had hypokalaemia/normokalaemia. In contrast, only 47.8% had hypokalaemia/normokalaemia in the MRA‐naive group, 27.3% in the group with the reduced MRA dose, and 33.3% in the group that had MRA discontinued (P < 0.001) (Figure 2 ).
Figure 2.
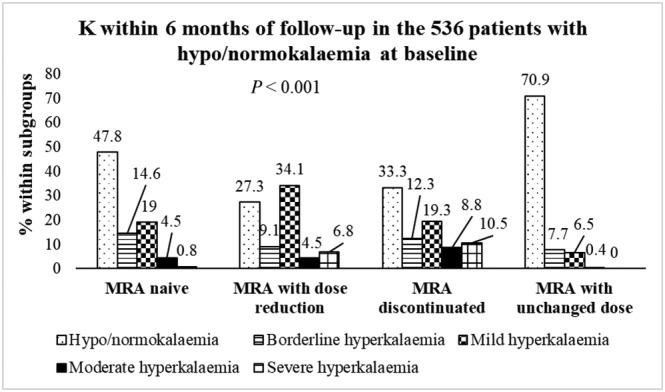
K within 6 months from baseline in the 536 patients with hypokalaemia/normokalaemia at baseline.
Potassium binders were not used in clinical practice at the three hospitals affiliated with Sahlgrenska during the study period (2016–2019). Only one patient had potassium binders three times a week 6 months after being diagnosed with HFrEF.
In the MRA‐naive group 87.5% had normokalaemia/hypokalaemia at baseline; of these patients, 16.7% had borderline, 22.4% mild, 5.7% moderate, and only 0.9% severe hyperkalaemia during the 6‐month follow‐up. Of patients who needed a dose reduction of MRA, 81.8% had normokalaemia/hypokalaemia at baseline. Of these patients, 15.9% had borderline, 40.9% mild, 6.8% K moderate, and 6.8% severe hyperkalaemia at the 6‐month follow‐up. In the group that had MRA discontinued, 84% had normokalaemia/hypokalaemia at baseline. Of these patients, 12.5% had borderline, 28.6% mild, 12.5% moderate, and 10.7% severe hyperkalaemia during the 6‐month follow‐up. For patients with an unchanged MRA dose, 86.2% had normokalaemia/hypokalaemia at baseline. Of these, 9.1% had borderline, 9.5% mild, and 1.2% moderate hyperkalaemia during the 6‐month follow‐up. No patients had severe hyperkalaemia (P < 0.001 for potassium values within 6 months and subgroups) (P = 0.099 for values of potassium at baseline and within subgroups) (Figure 3 A–D ).
Figure 3.
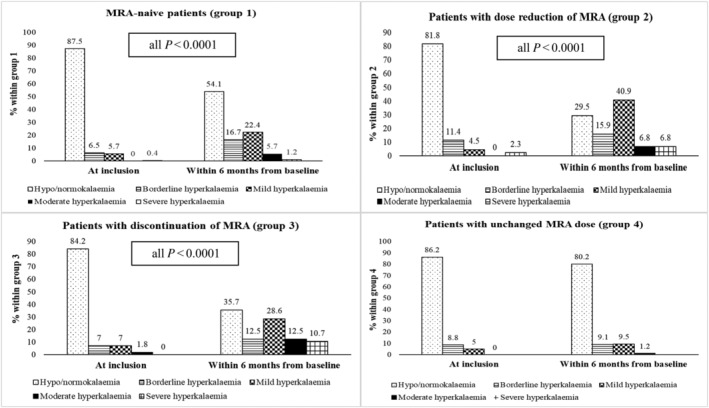
Prevalence of documented hyperkalaemia at baseline and within 6 months from baseline within the four subgroups. (A) MRA‐naive patients (group 1). (B) Patients with dose reduction of MRA (group 2). (C) Patients with discontinuation (group 3). (D) Patients with unchanged MRA dose (group 4).
Of the 5.9% that had hyperkalaemia at baseline, 56.8% had no MRA treatment within the 6‐month follow‐up, whereas 43.2% had treatment with MRA (P = 0.486) (Figure 4 ).
Figure 4.
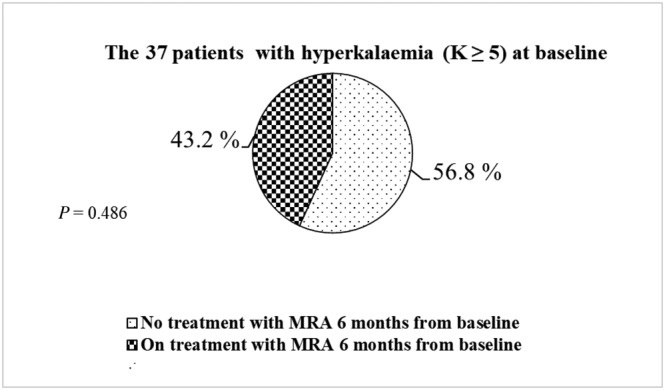
Patients with hyperkalaemia (K ≥ 5) at baseline and prescription of MRA within 6 months from baseline.
When the study was conducted in the autumn in 2020, which is a mean observation time of 13.4 (±SD 29.12) months, 209 (80.1%) patients in the MRA‐naive group still did not have treatment with MRA, as well as 12 (27.3%) of those patients that needed MRA dose reduction, 45 (78.9%) in the group that had MRA discontinued, and 40 (15.3%) patients with unchanged dose of MRA that did not have treatment with MRA in the autumn in 2020 (P < 0.001).
Physicians reasoning about MRA use
As a reason for refraining from MRA treatment, hyperkalaemia was documented in the medical records by the attending physician in only 1.5% of the cases. In those patients who needed a dose reduction or termination of MRA treatment, physicians documented hyperkalaemia as a cause of their action in 13.6% and 40.4% of the cases, respectively. The investigator interpreted hyperkalaemia as a reason of dose reduction of MRA in 46.3% of the cases and as the reason for discontinuation of MRA in 42.1% of the cases. In 8.2% of the cases, the investigator interpreted hyperkalaemia as a reason for not prescribing MRA, whereas recovered EF was interpreted to be the reason in 8.1% and waiting for up‐titration of ACEi/ARB/ARNI and BB in 10% of the cases. The investigator could not see any reason for not prescribing MRAs in 27.6% of the cases, and in 18.7% of the cases, the investigator interpreted it to be of less common reasons such as patient's wish, missed information, and no follow‐up within 6 months.
Exploratory analysis
Patients without MRA treatment from the hospital cohort were compared with those with incident HF from SwedeHF, registered within 6 months from HF diagnosis and matched by age and sex. The exploratory analysis showed that the use of MRA was not associated with a higher risk for 1‐year all‐cause mortality within 1 year after baseline (Figure 5 ).
Figure 5.
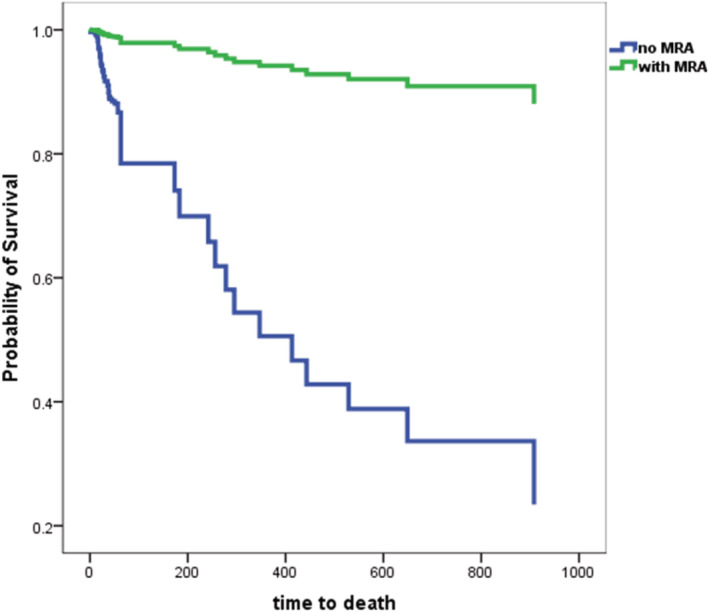
Exploratory analysis of all‐cause mortality between non‐MRA of the current hospital cohort and the matched MRA group from the Swedish Heart Failure Registry.
During the observation period, 14.2% of patients died in the group that never received MRA, 27.2% in the group that needed a dose reduction of MRA, and 17.5% in the group that needed to cease treatment with MRA. Also, 9.6% of the patients with an unchanged dose of MRA died during the follow‐up period (P = 0.009).
During the mean observation period of 13.4 (±SD 29.12) months, a Cox regression analysis showed that patients with mild hyperkalaemia had an HR of 1.206 (CI 95% 0.914–1.590) and patients with moderate hyperkalaemia had an HR of 0.859 (CI 95% 0.271–2.729) regarding mortality risk.
Discussions
Our study showed a significant increase in hyperkalaemia during subsequent up‐titration of HF therapy for documented and expected risk of hyperkalaemia.
Incidence of hyperkalaemia and its magnitude in general
The 2021 ESC guidelines for HF recommend using MRA as a part of first‐line therapy for patients with HFrEF. 17 However, recent studies have demonstrated suboptimal use of MRA treatment. Approximately 40–56% of patients with HFrEF are not treated with MRA worldwide. 12 Underlying causes of the under‐use of MRA are multifactorial but are likely mainly driven by the risk of hyperkalaemia. The reported incidence of hyperkalaemia varies from 0.9 to 23%. 16 In patients treated with MRAS in randomized HF trials, the reported overall rate of hyperkalaemia was 18% over 27 months in the PARADIGM‐HF trial, 7 19% over 24 months in the RALES trial, 20 and 11% over 21 months in the EMPHASIS‐HF trials. 8 Moreover, results showed that under‐use of MRA was not associated with elevated potassium levels. Also, available data about reported hyperkalaemia in real‐life settings are insufficient to explain the widespread under‐use of MRA. Accordingly, it is reasonable to assume that many clinicians do not prescribe MRA in real‐world clinical practice because of the anticipated or expected risk of hyperkalaemia based on their empirical clinical judgement. The documented occurrence of hyperkalaemia alone does not entirely explain the widespread under‐use of MRA in clinical practice. For the same reason, further specification of the expected risk at the individual patient level may provide additional data to better understand the underlying causes of the under‐use of MRAs.
By enrolling all patients with newly diagnosed HFrEF consecutively and all maximally up‐titrated at the physicians' discretion, we demonstrated that despite up‐titration of GDMT for 6 months, less than half of the patients (48.4%) remained on treatment with MRA, which is in line with previous studies. 12 However, our results on patients with hyperkalaemia differ from those previously reported. 16 Hyperkalaemia was low at baseline (5.9%) but dramatically increased to 24.4% after up‐titration.
Incidence of hyperkalaemia and its magnitude per subgroup with a focus on MRA‐naive patients
The incidence of hyperkalaemia and its magnitude varied considerably in our subgroups. Hyperkalaemia was present in 5.9% of patients at baseline but increased to 24.4% (e.g. 414%) after the up‐titration of GDMT in patients naive to MRAs. In patients who were given a dose reduction of MRAs, the incidence of hyperkalaemia increased by 801%. When MRAs had to be discontinued, the incidence of hyperkalaemia increased by 578%. Finally, hyperkalaemia increased by 200% in patients with unchanged MRA therapy. Indeed, up‐titration successfully achieved its treatment goals with BB and ACEi/ARB or ARNI as recommended by ESC guidelines. For example, in those patients who were MRA‐naive, BB were used in 92% of the cases, ACEi/ARB in 79%, and ARNI in 4%. Of note, these patients were older (P < 0.0001) and had lower EF (P = 0.022), higher NTproBNP (P = 0.017), lower eGFR (P = 0.001), and more co‐morbidities than patients in the other groups. In the total study population, there was a 2.7‐fold increased risk of hyperkalaemia within 6 months after the initiation/up‐titration of GDMT. Our overall findings suggest the possibility that physicians refrain from prescribing MRA because of the anticipated risk of hyperkalaemia. Considering that hyperkalaemia can cause life‐threatening heart rhythm or cardiac arrhythmias, suggestion seems plausible. However, our data do not match previous findings showing that under‐use of MRA was not associated with elevated potassium. 11 A possible explanation for this discrepancy may be that, in the previous study, 12 registry data about changes in potassium levels during up‐titration of GDMT were not available. In contrast, changes in potassium levels during up‐titration were essential in our study.
Our results provide new insight into understanding the under‐use of MRAs
Our study has added new knowledge about the underlying cause of under‐use of MRAs by differing from previous studies in the following aspects: (i) As stated above, we ensured that all patients followed up‐titration procedures to know not only the time‐dependent development of incident hyperkalaemia but also its magnitude during up‐titration. This manipulation is essential as many HF medications affect potassium levels. We observed more than a fourfold increase in incident hyperkalaemia during up‐titration. However, in a previous study, hyperkalaemia was often reported either on one occasion or cumulatively. (ii) Our study has shown that the overall incidence of hyperkalaemia (5.9% at baseline and 24.4% after up‐titration) increased as did different clinical phenotypes, which differed considerably. This issue is clinically important given that patients from our HF population were generally older with different co‐morbidities, probably congruent with different tolerabilities.(iii) In this study, we have shown for the first time the attributable relevance of the potential risk of hyperkalaemia in addition to documented hyperkalaemia. This observation is crucial as documented hyperkalaemia cannot provide a complete picture of the burden of hyperkalaemia. Consequently, this may explain why physicians in clinical practice do not prescribe MRAs when they consider that the risk of developing hyperkalaemia is high based on their clinical experience. However, scientific evidence for this decision to prescribe MRAs is incomplete. Our study also provides data on borderline hyperkalaemia during up‐titration to illustrate changes in potassium levels. (iv) Our study cohort allows us to explore individual patient data representative of real‐world clinical settings. Therefore, differences from previous studies that included patients in randomized clinical trials and registries are expected.
Our exploratory analysis showed that patients without MRA treatment were at a higher risk for 1‐year all‐cause mortality. However, because this is an observational study, only conclusions about associations and not effects are warranted. Despite using a matched cohort from the SwedeHF registry, it is not suitable to draw any conclusions about the effectiveness of MRAs. During the observation period, mild or moderate hyperkalaemia did not affect HFrEF patients' survival, a finding congruent with the data from BIOSTAT‐CHF that identified hyperkalaemia as a risk marker. 11
Limitations
Like all natural retrospective studies, we had to accept that some data would be lost, which we dealt with according to prespecified principles. Because we identified eligible patients by using the ICD‐10 codes I50.0–I50.9 for discharge diagnosis, patients that were hospitalized with newly onset HFrEF but who had, for example, ischaemic heart disease or atrial flutter as the principal diagnosis and HF as the secondary diagnosis were missed. However, those patients those patients were identified whether hospitalized again within 6 months from baseline and HF was defined as the principal diagnosis. Another limitation is that only patients hospitalized for newly onset HFrEF were included, but the sickest patients who benefit the most from MRAs and have the biggest challenge regarding hyperkalaemia were included in the study. The use of ARNI was suboptimal even though we included ARNI treatment in our analysis. The study period was defined from 1 January 2016 because ARNI was formally introduced in Sweden in 2016. In accordance with the previous ESC guidelines from 2016, ARNI as a medical choice came after ACEi/ARB, BB, and MRAs. Besides, early initiation of treatment with ARNI was not common as the studies on the safety of ARNI were first published in 2019. 21 , 22 Thus, a small number of patients on ARNI were expected. Another limitation is the small sample size in two of the MRA subgroups; thus, comparing the four subgroups should be done with some caution. According to previous ESC guidelines, all patients with HFrEF should achieve GDMT within 6 months after being diagnosed. Despite this, the short time frame of 6 months in our study to achieve GDMT was shown to be too short with only 48.4% treated with MRA 6 months after established HFrEF diagnosis. A sizable proportion of MRA‐naive patients might have received MRAs 12 months after baseline as physicians might have prioritized ACEi/ARB/ARNI and BB up‐titration over initiation of MRAs during the first 6 months of follow‐up. Thus, it turned out that 48.6% of the study population were still not on treatment with MRA in the autumn in 2020 when the study was conducted (P < 0.001).
Clinical implications
Despite compelling evidence for the clinical benefit of MRAs and strong guideline‐based directives for MRA use, undertreatment of MRAs remains high in patients with HFrEF. One concern is the risk of hyperkalaemia, which is enhanced by the up‐titration of inhibitors of the renin–angiotensin–aldosterone system and moderated by the up‐titration of ARNI. However, previously reported data on hyperkalaemia are not only lacking but also unclear whether the data refer to before or after the up‐titration of HF medication. Most importantly, the potential risk of hyperkalaemia is not taken into consideration. Our results seem to provide comprehensive information about incident hyperkalaemia and its magnitude before and after up‐titration per clinical phenotype, as well as the potential risk of hyperkalaemia in addition to that documented in patient medical records. Our data should help clinicians estimate the ‘real and potential’ risk of hyperkalaemia and, most importantly, provide tailored therapy to HF patients by treating them with GDMT while preventing hyperkalaemia, either by increasing the use of ARNI and SGLT2 inhibitors, which may moderate the risk of hyperkalaemia, or by adding a potassium binder in those with a higher risk of hyperkalaemia.
Conclusions
Incident hyperkalaemia increased significantly during subsequent up‐titration of HF therapy for documented and expected risk of hyperkalaemia but with varying magnitudes in different clinical phenotypes. This observation in conjunction with greatly increased borderline hyperkalaemia during up‐titration might explain why many physicians are reluctant to prescribe MRAs to patients with HFrEF.
Clinical perspective
Despite compelling evidence for the clinical benefit of MRAs in patients with symptomatic HFrEF, MRA treatment remains under‐used. It is unclear whether previously reported incident hyperkalaemia refers to the condition before or after up‐titration of HF medication. Another issue of concern is ignoring the potential risk of hyperkalaemia. In this study, we reported incident hyperkalaemia and its magnitude before and after up‐titration and the potential risk of hyperkalaemia. Such information will help clinicians better estimate hyperkalaemia's actual and potential risks.
Translational outlook
During initiation/up‐titration with GDMT, both documented hyperkalaemia and the expected risk of hyperkalaemia increased, which might explain why clinicians are unwilling to prescribe MRAs. Accordingly, accurately estimating the magnitude of hyperkalaemia enables clinicians to provide tailored therapy either by initiating SLGT2 inhibitors to ameliorate hyperkalaemia or adding potassium binders when necessary.
Conflict of interest
Josefin Henrysson: No conflicts of interest. Erik Thunström: Unrelated to present work: personal fees from Pfizer and Resmed. Xiaojing Chen: No conflicts of interest. Michael Fu: Related to present work: grant to author's institution from Vifor Pharma. Unrelated to present work: personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Vifor Fresenius. Carmen Basic: Unrelated to present work: personal fees from Boehringer Ingelheim and Vifor Pharma.
Funding
Vifor Pharma partly funded this study.
Supporting information
Table S1. Clinical characteristics at baseline of patients with heart failure with reduced ejection fraction in the non‐MRA treated group at Sahlgrenska University Hospital and matched controls that were in the MRA‐treated group from the Swedish Heart Failure Registry.
Acknowledgement
We would like to thank Aldina Pivodic, a professional statistician, for her expert contribution to this manuscript.
Henrysson, J. , Thunström, E. , Chen, X. , Fu, M. , and Basic, C. (2023) Hyperkalaemia as a cause of undertreatment with mineralocorticoid receptor antagonists in heart failure. ESC Heart Failure, 10: 66–79. 10.1002/ehf2.14137.
References
- 1. Murphy SP, Ibrahim NE, Januzzi JL Jr. Heart failure with reduced ejection fraction: A review. JAMA. 2020; 324: 488–504 Erratum in: JAMA. 2020;324:2107. [DOI] [PubMed] [Google Scholar]
- 2. Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am J Cardiol. 1988; 62: 60A–66A. [DOI] [PubMed] [Google Scholar]
- 3. Garg R, Yusuf S. Overview of randomized trials of angiotensin‐converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA. 1995; 273: 1450–1456 Erratum in: JAMA 1995;274:462. [PubMed] [Google Scholar]
- 4. Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K, CHARM investigators and committees . Effects of candesartan in patients with chronic heart failure and reduced left‐ventricular systolic function intolerant to angiotensin‐converting‐enzyme inhibitors: The CHARM‐Alternative trial. Lancet. 2003; 362: 772–776. [DOI] [PubMed] [Google Scholar]
- 5. The cardiac insufficiency bisoprolol study II (CIBIS‐II): A randomised trial. Lancet. 1999; 353: 9–13. [PubMed] [Google Scholar]
- 6. Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, Wikstrand J, El Allaf D, Vítovec J, Aldershvile J, Halinen M, Dietz R, Neuhaus KL, Jánosi A, Thorgeirsson G, Dunselman PH, Gullestad L, Kuch J, Herlitz J, Rickenbacher P, Ball S, Gottlieb S, Deedwania P. Effects of controlled‐release metoprolol on total mortality, hospitalizations, and well‐being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT‐HF). MERIT‐HF Study Group. JAMA. 2000; 283: 1295–1302. [DOI] [PubMed] [Google Scholar]
- 7. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 8. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, EMPHASIS‐HF Study Group . Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011; 364: 11–21.21073363 [Google Scholar]
- 9. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999; 341: 709–717. [DOI] [PubMed] [Google Scholar]
- 10. Thorvaldsen T, Benson L, Dahlström U, Edner M, Lund LH. Use of evidence‐based therapy and survival in heart failure in Sweden 2003‐2012. Eur J Heart Fail. 2016; 18: 503–511 Epub 2016 Feb 11. [DOI] [PubMed] [Google Scholar]
- 11. Ferreira JP, Rossignol P, Machu JL, Sharma A, Girerd N, Anker SD, Cleland JG, Dickstein K, Filippatos G, Hillege HL, Lang CC, Ter Maaten JM, Metra M, Ng L, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zwinderman AH, Voors A, Zannad F. Mineralocorticoid receptor antagonist pattern of use in heart failure with reduced ejection fraction: Findings from BIOSTAT‐CHF. Eur J Heart Fail. 2017; 19: 1284–1293. [DOI] [PubMed] [Google Scholar]
- 12. Savarese G, Carrero JJ, Pitt B, Anker SD, Rosano GMC, Dahlström U, Lund LH. Factors associated with underuse of mineralocorticoid receptor antagonists in heart failure with reduced ejection fraction: An analysis of 11 215 patients from the Swedish heart failure registry. Eur J Heart Fail. 2018; 20: 1326–1334. [DOI] [PubMed] [Google Scholar]
- 13. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF, Fonarow GC. Medical therapy for heart failure with reduced ejection fraction: The CHAMP‐HF registry. J Am Coll Cardiol. 2018; 72: 351–366. [DOI] [PubMed] [Google Scholar]
- 14. Trevisan M, de Deco P, Xu H, Evans M, Lindholm B, Bellocco R, Barany P, Jernberg T, Lund LH, Carrero JJ. Incidence, predictors and clinical management of hyperkalaemia in new users of mineralocorticoid receptor antagonists. Eur J Heart Fail. 2018; 20: 1217–1226 Epub 2018 Apr 18. Erratum in: Eur J Heart Fail. 2019; 21:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martens P, Kooij J, Maessen L, Dauw J, Dupont M, Mullens W. The importance of developing hyperkalaemia in heart failure during long‐term follow‐up. Acta Cardiol. 2020; 8: 1–9. [DOI] [PubMed] [Google Scholar]
- 16. Damman K, Tang WH, Felker GM, Lassus J, Zannad F, Krum H, McMurray JJ. Current evidence on treatment of patients with chronic systolic heart failure and renal insufficiency: Practical considerations from published data. J Am Coll Cardiol. 2014; 63: 853–871. [DOI] [PubMed] [Google Scholar]
- 17. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members; Document Reviewers . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 18. Thomsen RW, Nicolaisen SK, Hasvold P, Sanchez RG, Pedersen L, Adelborg K, Egstrup K, Egfjord M, Sørensen HT. Elevated potassium levels in patients with chronic kidney disease: Occurrence, risk factors and clinical outcomes‐A Danish population‐based cohort study. Nephrol Dial Transplant. 2018; 33: 1610–1620. [DOI] [PubMed] [Google Scholar]
- 19. World Medical Association . World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 20. Kulbertus H. L'étude clinique du mois. L'étude RALES (randomized aldactone evaluation study) [Study of the month. The RALES study (randomized aldactone evaluation study)]. Rev Med Liege. 1999; 54: 770–772 French. [PubMed] [Google Scholar]
- 21. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E, PIONEER‐HF Investigators . Angiotensin‐neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019; 380: 539–548 Epub 2018 Nov 11. Erratum in: N Engl J Med. 2019; 380:1090. [DOI] [PubMed] [Google Scholar]
- 22. Wachter R, Senni M, Belohlavek J, Straburzynska‐Migaj E, Witte KK, Kobalava Z, Fonseca C, Goncalvesova E, Cavusoglu Y, Fernandez A, Chaaban S, Bøhmer E, Pouleur AC, Mueller C, Tribouilloy C, Lonn E, Buraiki JAL, Gniot J, Mozheiko M, Lelonek M, Noè A, Schwende H, Bao W, Butylin D, Pascual‐Figal D, TRANSITION Investigators . Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: Primary results of the randomised TRANSITION study. Eur J Heart Fail. 2019; 21: 998–1007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical characteristics at baseline of patients with heart failure with reduced ejection fraction in the non‐MRA treated group at Sahlgrenska University Hospital and matched controls that were in the MRA‐treated group from the Swedish Heart Failure Registry.


