Abstract
MPT53 is a secreted protein of Mycobacterium tuberculosis. Southern transfer and hybridization showed mpt53 to be conserved in the M. tuberculosis complex and to have homology with DNA from Mycobacterium avium and other nontuberculous mycobacteria. However, anti-MPT53 polyclonal antibodies detected no antigen in the culture filtrates of M. avium and other nontuberculous mycobacteria. MPT53 of M. tuberculosis induced strong, tuberculosis-specific antibody responses in guinea pigs but induced no delayed-type hypersensitivity. Involvement in immune responses during human tuberculosis was very modest.
Proteins secreted into the extracellular environment by Mycobacterium tuberculosis are usually targets of immune responses in the infected host. Thus, the filtrate of M. tuberculosis cultures has constituted an important source of antigens that induce protective immunity and immune responses having diagnostic value (reviewed in references 1, 6, and 20). In the 1980s and early 1990s S. Nagai and his collaborators purified several proteins from culture filtrates of M. tuberculosis and Mycobacterium bovis bacillus Calmette-Guérin (BCG). These proteins were termed MPT (for M. tuberculosis) or MPB (for M. bovis BCG) followed by a number indicating the relative mobility during nondenaturing polyacrylamide gel electrophoresis (13). Of the MPT and MPB proteins known to be secreted (18), many elicit immune responses that are specific for the M. tuberculosis complex and therefore of diagnostic value (for example, MPT64 [2], MPB70 [7], and MPT63 [12]). Others confer protective immunity (MPT44, MPT59, and MPT45, i.e., the members of the Ag85 complex [8, 17]). While all other genes encoding MPT and MPB proteins were identified and sequenced in the pregenome era, the mpt53 gene was identified only recently (19) from the analysis of the NH2-terminal sequence of the purified protein (13) and from the genome sequence of M. tuberculosis (mpt53 is Rv2878c) (5). MPT53 is a 15-kDa protein (13) that induces antibody responses in tuberculous cattle (19). In the present work, we characterized the specificity of MPT53 for the M. tuberculosis complex and the involvement of MPT53 in immune responses to tuberculosis (TB) in guinea pigs and in humans.
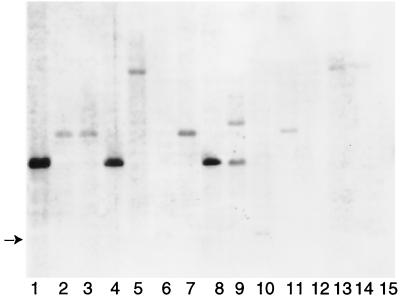
The distribution of mpt53 among tuberculous and nontuberculous mycobacteria was determined by Southern transfer and hybridization. The gene was present in the DNA of members of the M. tuberculosis complex (Fig. 1, lanes 1, 4, and 8, and data not shown). Analysis of 50 clinical isolates of M. tuberculosis identified one isolate bearing a chromosomal deletion encompassing mpt53 (data not shown), indicating a relative gene instability. We next investigated the distribution of mpt53 among nontuberculous mycobacteria. Analysis of the partial M. avium genome sequence (http://www.tigr.org/tdb/) by BLAST homology programs indicated that M. avium contains a homolog of mpt53 (≈80% identity with nucleotide and amino acid sequences corresponding to the extracellular MPT53 protein of M. tuberculosis) (data not shown). Accordingly, mpt53 DNA hybridized weakly with DNAs extracted from reference strains and clinical isolates of M. avium (Fig. 1, lanes 2 and 3). mpt53 DNA also gave weak hybridization signals with DNAs extracted from M. intracellulare, M. fortuitum, M. haemophilum, M. kansasii, M. malmoense, M. marinum, M. scrofulaceum, and M. ulcerans; it failed to hybridize with DNAs from M. phlei and M. xenopi (Fig. 1). Thus, mpt53 homologs are broadly distributed among nontuberculous mycobacteria.
FIG. 1.
Genomic analysis of mpt53 in tuberculous and nontuberculous mycobacteria. Tuberculous and nontuberculous mycobacteria were from the Public Health Research Institute TB Center (PHRI-TB), the American Type Culture Collection (ATCC), and the Trudeau Mycobacteria Collection (TMC). Methods for culturing mycobacteria, isolating DNA, and performing Southern transfer and hybridization analyses were described elsewhere (4, 16). DNA was digested with PvuII and electrophoresed on 1% agarose gels. The separated digestion products were transferred to nylon membranes and hybridized with a nonradioactively labeled 507-bp DNA fragment internal to the mpt53 gene. After chemiluminescence detection, a single band of ≈3.5 kb was detected with DNA of the M. tuberculosis complex. Hybridization signals obtained with nontuberculous mycobacteria were invariably of lower intensity than those obtained with tuberculous mycobacteria. Results are shown for 3 strains of tuberculous mycobacteria and 12 strains of nontuberculous mycobacteria. Lanes: 1, M. tuberculosis H37Rv; 2, M. avium ATCC 25291; 3, M. avium M-64 (clinical isolate); 4, M. africanum TMC 5122; 5, M. fortuitum ATCC 1530; 6, M. haemophilum ATCC 29548; 7, M. intracellulare ATCC 13950; 8, M. bovis PHRI-TB 5022 (clinical isolate); 9, M. kansasii ATCC 12478; 10, M. malmoense ATCC 29571; 11, M. marinum ATCC 1218; 12, M. phlei ATCC 11758; 13, M. scrofulaceum ATCC 1302; 14, M. ulcerans ATCC 1615; 15, M. xenopi ATCC 19250. The arrow on the left indicates the mobility of a very weak band in lanes 6 and 10. Additional members of the M. tuberculosis complex tested for presence of mpt53 were three reference strains of M. bovis (TMC 410, TMC 401, and TMC 407), four strains of M. bovis BCG (Pasteur TMC 1011, Montreal TMC 1012, Connaught TMC 1030, and Japan ATCC 35737), M. microti TMC 1619, and 50 clinical isolates of M. tuberculosis in the strain collection of the PHRI-TB Center (data not shown).
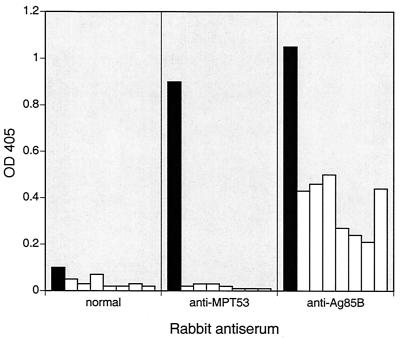
To estimate levels of secreted MPT53 and its homologs, we probed culture filtrates of tuberculous and nontuberculous mycobacteria by enzyme-linked immunosorbent assay (ELISA) using a rabbit antiserum raised against MPT53 purified from M. tuberculosis culture filtrates. MPT53 was detected in the culture filtrates of M. tuberculosis H37Rv (and nine clinical isolates [data not shown]) but not in filtrates obtained from nontuberculous mycobacteria (Fig. 2). Furthermore, binding to MPT53 of anti-MPT53 rabbit antiserum was significantly inhibited in competitive ELISA by tuberculin purified protein derivative (PPD) of M. bovis but not by PPD of M. avium (data not shown). Taken together, these results indicate that there is little, if any, secretion or synthesis of MPT53 homologs in M. avium and other nontuberculous mycobacteria during growth in vitro.
FIG. 2.
Detection of MPT53 antigen in culture filtrates of mycobacteria. Culture filtrates of tuberculous and nontuberculous mycobacterial species were tested by ELISA with rabbit hyperimmune sera. Filtrates were obtained from mycobacterial cultures grown to stationary phase in Middlebrook 7H9 supplemented with 10% (vol/vol) albumin-dextrose-catalase and 0.05% (vol/vol) Tween 80 (9). ELISA was performed in polystyrene microtiter plates. The culture filtrate coating concentration was 5 μg/ml. Rabbit antisera were used at a dilution of 1:300. Immunodetection was performed with goat anti-rabbit IgG antibodies conjugated with alkaline phosphatase (Sigma) and an alkaline phosphatase substrate kit (Bio-Rad). Optical density at 405 nm (OD405) was measured. Three sets of parallel experiments were performed: with normal rabbit serum, with rabbit anti-MPT53 antiserum, and with rabbit anti-Ag85B antiserum (positive control; Ag85B is an antigen common to tuberculous and nontuberculous mycobacteria [14]). Solid bars, M. tuberculosis H37Rv; open bars, nontuberculous mycobacteria (from left to right, M. avium, M. fortuitum, M. kansasii, M. malmoense, M. marinum, M. scrofulaceum, and M. ulcerans).
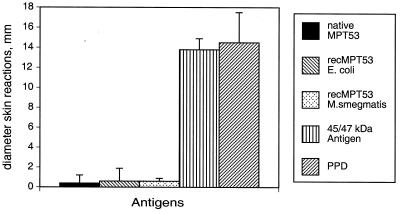
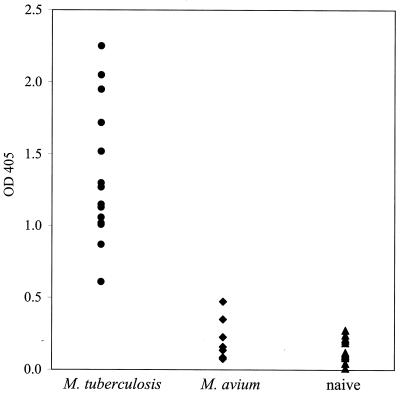
To investigate the immunogenicity of MPT53 during experimental and human TB, the mpt53 gene was cloned into the pQE30 (Qiagen) expression vector of Escherichia coli, and the recombinant protein was purified by using a three-step chromatography protocol detailed elsewhere (3). Purified protein was >95% homogeneous, as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by staining of gels with Coomassie blue (data not shown). Immunogenicity of MPT53 during experimental TB was assessed in tuberculous guinea pigs by measuring delayed-type hypersensitivity (DTH) and serum immunoglobulin G (IgG) antibodies to MPT53. Animals were aerosol infected with M. tuberculosis and skin tested 6 to 8 weeks after infection. The animals reacted to positive sensitization controls (PPD and the purified 45/47-kDa antigen of M. tuberculosis), but no DTH response was elicited by recombinant MPT53 purified from E. coli (Fig. 3). Some antigens of M. tuberculosis require posttranslational modifications, such as glycosylation, to express DTH activity (15). Since the state of MPT53 in M. tuberculosis cells is not known, we also measured DTH responses in tuberculous guinea pigs to MPT53 protein purified from mycobacteria (native MPT53 from M. tuberculosis culture filtrates [13] and M. tuberculosis protein purified from recombinant M. smegmatis [19]). Both protein preparations gave negative skin test results (Fig. 3). We concluded that tuberculous guinea pigs exhibit no DTH responses to MPT53. We next examined antibody responses to MPT53 in tuberculous guinea pigs. Since antigen-specific IgG antibody levels are too low at 8 to 9 weeks postinfection for detection by ELISA (our unpublished observations), anti-MPT53 IgG antibodies were measured in tuberculous guinea pigs 15 weeks after low-dose infection by aerosol. These animals exhibited high levels of anti-MPT53 antibodies in serum (Fig. 4). In contrast, sera from seven animals sensitized with M. avium failed to react with MPT53 (Fig. 4), suggesting that the mpt53 homolog of M. avium may not be expressed. The possibility cannot be excluded, however, that some MPT53-like protein is present in M. avium but does not elicit a measurable antibody production. In conclusion, the above results indicate that the antibody response elicited by MPT53 in guinea pigs is specific for TB.
FIG. 3.
DTH responses to MPT53 in tuberculous guinea pigs. Two groups of four to six outbred Hartley female guinea pigs (weighing ≈300 g) were used for skin testing. One group was infected with M. tuberculosis H37Rv by aerosol with a dose of ≈100 CFU per animal. A second, control group was mock sensitized by intradermal injection of phosphate-buffered saline. Six to 8 weeks after sensitization, animals were injected intradermally with 1 to 2 μg of each preparation of purified MPT53 protein in 0.1 ml of phosphate-buffered saline. As sensitization controls, 1 μg of purified recombinant 45/47-kDa antigen (10) and 1 μg of M. tuberculosis PPD were used. Skin reactions (diameter of erythema and induration, in millimeters) were measured 24 h after antigen injection. Results obtained in tuberculous guinea pigs with recombinant (rec) MPT53 purified from M. smegmatis were normalized against the saline controls, since this protein preparation gave 1.5- to 3-mm background reactions in saline-treated control animals.
FIG. 4.
Antibody responses to MPT53 in guinea pigs. Guinea pig sera were obtained from animals aerosol infected with 10 to 15 CFU of M. tuberculosis H37Rv 15 weeks after infection, animals sensitized by intradermal injection of 107 cells of M. avium 8 weeks after sensitization, and naive control animals injected intradermally with 0.1 ml of phosphate-buffered saline. ELISA was performed in polystyrene microtiter plates. The coating concentration of recombinant MPT53 purified from E. coli was 0.25 μg/ml. Sera were used at a dilution of 1:50. Immunodetection was performed with rabbit anti-guinea pig IgG antibodies conjugated with alkaline phosphatase (Sigma) and an alkaline phosphatase substrate kit (Bio-Rad). Optical density at 405 nm (OD405) was measured. Each data point represents one animal. Sera obtained from M. avium-sensitized animals all reacted strongly with M. tuberculosis culture filtrates (data not shown), indicating that the animals had mounted an antibody response to infection at the time of testing.
We next evaluated immune responses to MPT53 in human TB. Two sets of experiments were conducted. In the first experiment, we used sera from 167 patients having pulmonary TB and 75 control sera (from 16 patients having non-TB mycobacterioses, 9 patients having pulmonary diseases other than TB, and 50 healthy blood donors). Detection of serum antibodies against MPT53 was conducted by a nitrocellulose membrane-based assay devised in our laboratory for serological evaluation of antigens (11). Only a small proportion of TB patients (3 of 167; <2%) had serum IgG antibodies against MPT53 (data not shown). None of the control sera reacted with the antigen. In a second set of experiments, we investigated T-cell responses to MPT53 during latent TB. MPT53 was tested for the ability to induce in vitro secretion of gamma interferon by peripheral blood mononuclear cells obtained from two asymptomatic, tuberculin skin test (TST) reactors and two TST-negative controls. No gamma interferon was secreted by peripheral blood mononuclear cells from TST reactors and negative controls in response to stimulation with MPT53 (data not shown). Taken together, these results suggest minimal, if any, involvement of MPT53 in immune responses to human TB.
The finding that MPT53 elicits strong antibody responses in experimental TB but not during human disease parallels observations made in bovine TB studies. Experimentally infected cattle mount strong antibody responses to this antigen (19; K. P. Lyashchenko, J. M. Pollock, H. G. Wiker, M. Harboe, and M. L. Gennaro, Third International Conference on Mycobacterium bovis, 13 to 16 August 2000, Cambridge, United Kingdom, abstr., p. 65). However, none of the naturally infected cattle had detectable anti-MPT53 antibodies in serum (19). Perhaps anti-MPT53 IgG antibodies are detectable in serum only transiently during early stages of natural infection or only when the infecting dose is higher than that usually associated with natural infection.
In summary, we found that mpt53 homologs are present in M. avium and other nontuberculous mycobacteria but that the level of extracellular protein under the tested conditions of growth in vitro is below that needed for detection with a specific polyclonal antibody. The lack of detectable anti-MPT53 serum antibodies in M. avium-sensitized guinea pigs lends support to the idea that the mpt53 homolog of M. avium may not even be expressed during infection. MPT53 induces a vigorous antibody response and no DTH in tuberculous guinea pigs. We also obtained negative evidence of cell-mediated immune responses to MPT53 in latent TB in humans. Finally, MPT53 elicits very modest antibody responses in human TB (this work) and in bovine TB (19).
Acknowledgments
We thank Alan Roberts for aerosol infection of guinea pigs, Elisa French and Julia Granowski for excellent animal care at the Painter Center at Colorado State University, David McMurray for sera of guinea pigs infected with M. tuberculosis H37Rv, Yuk Ming Liu for assistance with the cytokine secretion experiment, Alex Ravikovitch at the Public Health Research Institute TB Center for assistance in preparing Fig. 1, and Karl Drlica for comments on the manuscript.
This work was supported by NIH grant AI-36989 (to M.L.G.) and by NIH/NIAID contract NO1-AI-75320, “Tuberculosis Research Materials and Vaccine Testing.”
Footnotes
Report no. 80 of the Public Health Research Institute TB Center.
REFERENCES
- 1.Andersen Å B, Brennan P. Proteins and antigens of Mycobacterium tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C.: ASM Press; 1994. pp. 307–327. [Google Scholar]
- 2.Andersen Å B, Kjungqvist L, Hasløv K, Bentzon M W. MPT64 possesses “tuberculosis-complex”-specific B- and T-cell epitopes. Scand J Immunol. 1991;34:365–372. doi: 10.1111/j.1365-3083.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- 3.Colangeli R, Heijbel A, Williams A, Manca C, Chan J, Lyashchenko K, Gennaro M L. Three-step purification of lipopolysaccharide-free, polyhistidine-tagged recombinant antigens of Mycobacterium tuberculosis. J Chromatogr B. 1998;714:223–235. doi: 10.1016/s0378-4347(98)00094-2. [DOI] [PubMed] [Google Scholar]
- 4.Colangeli R, Spencer J S, Bifani P, Williams A, Lyashchenko K, Keen M A, Hill P J, Belisle J, Gennaro M L. MTSA-10, the product of the Rv3874 gene of Mycobacterium tuberculosis, elicits tuberculosis-specific, delayed-type hypersensitivity in guinea pigs. Infect Immun. 2000;68:990–993. doi: 10.1128/iai.68.2.990-993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin H, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 6.Cooper A M, Flynn J L. The protective immune response to Mycobacterium tuberculosis. Curr Opin Immunol. 1995;7:512–516. doi: 10.1016/0952-7915(95)80096-4. [DOI] [PubMed] [Google Scholar]
- 7.Harboe M, Nagai S, Patarroyo M E, Torres M, Ramirez C, Cruz N. Properties of proteins MPB64, MPB70, and MPB80 of Mycobacterium bovis BCG. Infect Immun. 1986;52:293–302. doi: 10.1128/iai.52.1.293-302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D'Souza C, Drowart A, Lozes E, Vandenbussche P, Vooren J-P V, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs W R, Jr, Kalpana G V, Cirillo J D, Pascopella L, Snapper S B, Udani R A, Jones W, Barletta R G, Bloom B R. Genetic systems for mycobacteria. Methods Enzymol. 1991;204:537–555. doi: 10.1016/0076-6879(91)04027-l. [DOI] [PubMed] [Google Scholar]
- 10.Laqueyrerie A, Militzer P, Romain F, Eiglmeier K, Cole S, Marchal G. Cloning, sequencing, and expression of the apa gene coding for the Mycobacterium tuberculosis 45/47-kilodalton secreted antigen complex. Infect Immun. 1995;63:4003–4010. doi: 10.1128/iai.63.10.4003-4010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyashchenko K P, Singh M, Colangeli R, Gennaro M L. A multi-antigen print immunoassay for the development of serological diagnosis of infectious diseases. J Immunol Methods. 2000;242:91–100. doi: 10.1016/s0022-1759(00)00241-6. [DOI] [PubMed] [Google Scholar]
- 12.Manca C, Lyashchenko K, Wiker H G, Usai D, Colangeli R, Gennaro M L. Molecular cloning, purification, and serological characterization of MPT63, a novel antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1997;65:16–23. doi: 10.1128/iai.65.1.16-23.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagai S, Wiker H G, Harboe M, Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991;59:372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohara N, Ohara-Wada N, Kitaura H, Nishiyama T, Matsumoto S, Yamada T. Analysis of genes encoding the antigen 85 complex and MPT51 from Mycobacterium avium. Infect Immun. 1997;65:3680–3685. doi: 10.1128/iai.65.9.3680-3685.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romain F, Horn C, Pescher P, Namane A, Riviere M, Puzo G, Barzu O, Marchal G. Deglycosylation of the 45/47-kilodalton antigen complex of Mycobacterium tuberculosis decreases its capacity to elicit in vivo or in vitro cellular immune responses. Infect Immun. 1999;67:5567–5572. doi: 10.1128/iai.67.11.5567-5572.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Embden J D, Cave M D, Crawford J T, Dale J W, Eisenach K D, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiker H G, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiker H G, Harboe M, Nagai S. A localization index for distinction between extracellular and intracellular antigens of Mycobacterium tuberculosis. J Gen Microbiol. 1991;137:875–884. doi: 10.1099/00221287-137-4-875. [DOI] [PubMed] [Google Scholar]
- 19.Wiker H G, Michell S L, Hewinson R G, Spierings E, Nagai S, Harboe M. Cloning, expression and significance of MPT53 for identification of secreted proteins of Mycobacterium tuberculosis. Microb Pathog. 1999;26:207–219. doi: 10.1006/mpat.1998.0267. [DOI] [PubMed] [Google Scholar]
- 20.Young D B, Kaufmann S H E, Hermans P W M, Thole J E R. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992;6:133–145. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]