Abstract
Aims
There are no previous studies focusing on collaborative follow‐ups between hospitals and clinics for patients discharged after acute heart failure (AHF) in Japan. The purpose of this study was to determine the status of collaboration between hospitals and clinics for patients with AHF in Japan and to compare patient characteristics and clinical outcomes using a large Japanese observational database.
Methods and results
Of 4056 consecutive patients hospitalized for AHF in the Kyoto Congestive Heart Failure registry, we analysed 2862 patients discharged to go home, who were divided into 1674 patients (58.5%) followed up at hospitals with index hospitalization (hospital follow‐up group) and 1188 (41.5%) followed up in a collaborative fashion with clinics or other general hospitals (collaborative follow‐up group). The primary outcome was a composite of all‐cause death or heart failure (HF) hospitalization within 1 year after discharge. Previous hospitalization for HF and length of hospital stay longer than 15 days were associated with hospital follow‐up. Conversely, ≥80 years of age, hypertension, and cognitive dysfunction were associated with collaborative follow‐up. The cumulative 1‐year incidence of the primary outcome, all cause death, and cardiovascular death were similar between the hospital and collaborative follow‐up groups (31.6% vs. 29.6%, P = 0.51, 13.1% vs, 13.9%, P = 0.35, 8.4% vs. 8.2%, P = 0.96). Even after adjusting for confounders, the difference in risk for patients in the hospital follow‐up group relative to those in the collaborative follow‐up group remained insignificant for the primary outcome, all‐cause death, and cardiovascular death (HR: 1.11, 95% CI: 0.97–1.27, P = 0.14, HR: 1.10, 95% CI: 0.91–1.33, P = 0.33, HR: 0.96, 95% CI: 0.87–1.05, P = 0.33). The cumulative 1‐year incidence of HF hospitalization was higher in the hospital follow‐up group than in the collaborative follow‐up group (25.5% vs. 21.3%, P = 0.02). The risk of HF hospitalization was higher in the hospital follow‐up group than in the collaborative follow‐up group (HR: 1.19, 95% CI: 1.01–1.39, P = 0.04).
Conclusions
In patients hospitalized for AHF, 41.5% received collaborative follow‐up after discharge. The risk of HF hospitalization was higher in the hospital follow‐up group than in the collaborative follow‐up, although risk of the primary outcome, all‐cause death, and cardiovascular death were similar between groups.
Keywords: Post discharge follow‐up, Heart failure, Collaborative follow‐up, Transitional care, Clinical outcome
Introduction
The widening gap between healthy life expectancy and average life expectancy for patients with heart failure (HF) increases the demand for medical and long‐term care and makes it more difficult for them to continue living in their community. Management of HF requires a multifaceted approach, and in Western countries, multidisciplinary HF disease management has been practised since the 1990s. 1 In Europe and the USA, appropriate transitional care has been reported to improve quality of life and outcomes. 2 , 3 Seamless transitional care in collaboration with primary care physicians is expected to become the standard multidisciplinary approach and to improve HF disease management. 4 , 5 For example, it is reported that about 85% of primary care physicians in Switzerland and 78.3% of family physicians in Ontario are collaborating with cardiologists in the management of HF. 6 , 7
In response to this situation, the Japanese government is promoting the establishment of ‘community‐centered medical care’ in which medical care, nursing care, and welfare are provided in an integrated manner (‘Integrated Community Care System’) to enable people to continue living in their familiar communities, in contrast to ‘hospital‐centered medical care’. 8 , 9 Despite government policies, collaboration between the communities and the hospitals is still insufficient. The costs of healthcare are covered by the social insurance system, which allows patients to receive high‐quality healthcare at relatively low cost, anytime and anywhere. 9 In addition, there is no distinction in Japan between primary care and secondary care, which provide inpatient and specialized outpatient care, respectively, and there is no gatekeeping system. 9 Patients can go directly to secondary medical facilities even for minor symptoms. Many patients discharged from acute care hospitals seek follow‐up care by going to the outpatient departments of acute care hospitals, at the discretion of the attending physician or at the patient's request. In Japan, the treatment pathway and order of treatment in collaborations between hospitals and clinics are similar to that in Europe and the USA, as specialists are closely involved. However, the division of roles in other care areas is not clearly defined, and an enforceable collaboration model between hospitals and clinics has not yet been established.
In the present study, we defined collaborative follow‐up as follows: (i) Both the hospital and clinic follow up the patients regularly, but the hospital conducts follow‐ups less frequently; (ii) only the clinic follows up the patients regularly, and the hospital follows up when requested by the clinic. It is important for transitional care that the collaborators share information and co‐operate with each other to ensure a seamless follow‐up. We hypothesized that collaborative follow‐up between hospitals with index hospitalization and clinics would improve outcomes compared with follow‐up at hospitals with index hospitalization only. Therefore, to provide helpful information for collaboration between hospitals and clinics for patients with HF in Japan, we conducted this study using a large Japanese observational database of patients with AHF, with the following objectives: first, to determine the status of collaboration between hospitals and clinics for patients with AHF in Japan and to compare patient characteristics and clinical outcomes; and second, as a subanalysis, to compare patient characteristics and clinical outcomes of patients aged ≥65years at different follow‐up points. The reason 65 was set as the cut‐off is that this is the age of the elderly as defined by the WHO, 10 and the age at which Japan's social insurance system, the long‐term care insurance system, applies to the elderly. 11 The results of this study are expected to increase awareness of current policies and help establish a transitional care system for patients with HF that is appropriate for the Japanese medical and social systems.
Methods
Study design
This retrospective, multicentre, cohort study included patients with data in a multicentre registry, the Kyoto Congestive Heart Failure (KCHF) registry. KCHF is a physician‐initiated, prospective, observational, multicentre cohort study that enrolled consecutive patients who were hospitalized for AHF between 1 October 2014 and 31 March 2016. 12
Setting and population
Patients were admitted to 19 rural and urban facilities with different numbers of beds, including a secondary emergency hospital that accepts critically ill patients, including those requiring hospitalization and surgery, on a 24‐h basis, and a tertiary emergency hospital that provides more advanced emergency care. The details of the KCHF study design and patient enrolment are described in detail elsewhere. 12 , 13 , 14 Briefly, we enrolled consecutive patients with AHF who were admitted to participating hospitals, diagnosed according to the modified Framingham criteria, and who received intravenous diuretics, vasodilators, and inotropic drugs specific for HF within 24 h of admission. Clinical follow‐up data at 1 year were collected by the attending physician or research collaborator at the participating hospitals, who confirmed survival by phone or in writing, and events such as hospitalizations during the 1 year were collected from the medical records. In this study, we investigated differences in baseline characteristics and clinical outcomes of patients with AHF who were discharged home and only followed up at the hospital for index AHF hospitalization (hospital follow‐up group) and those who were followed up at clinics or general hospitals in collaboration with the hospitals used at the index AHF hospitalization (collaborative follow‐up group). We defined collaborative follow‐up as follows: (i) Both the hospital and clinic follow up the patients regularly, but the hospital conducts follow‐ups less frequently; (ii) only the clinic follows up the patients regularly, and the hospital follows up when requested by the clinic (Table S1 ). The physicians in the hospital follow‐up group were cardiologists, whereas the clinic physicians in the collaborative follow‐up group and the physicians in other hospitals are often general internists, some of whom may be cardiologists. Of the 4056 patients enrolled in the KCHF registry, those who died during the index hospitalization, had no information on their post‐discharge residence type, were transferred to other hospitals, were discharged to nursing homes, or had no information on their follow‐up destination after discharge were excluded. Consequently, the present study included 2862 patients (Figure 1 ).
Figure 1.
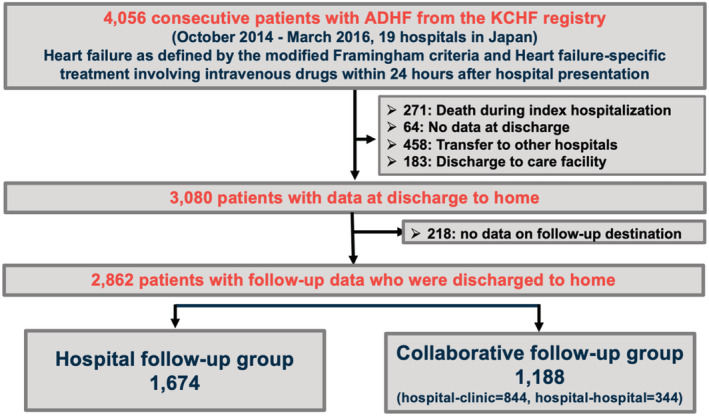
Study flowchart.
Ethics
The study conformed to the principles outlined in the Declaration of Helsinki. The study protocol was approved by the ethical committees of the Kyoto University Hospital (local identifier: E2311) and each participating hospital. A waiver for written informed consent from each patient was granted by the Institutional Review Boards of Kyoto University and each participating centre. This study also met the conditions of the Japanese ethical guidelines for medical and health research involving human subjects. 15 We disclosed the details of the present study to the patients using an opt‐out method, and the notice clearly informed patients of their right to refuse enrolment.
Definitions
Based on the four levels of living independence used in Japanese long‐term care insurance, we classified the functional levels of the patients into four levels: ambulatory (including those who use aids, such as sticks), wheelchair‐ridden outdoors only, wheelchair‐ridden both indoors and outdoors, and bedridden. 13 Functional decline during index AHF hospitalization was defined as a decline in at least one activity level from admission to discharge. 16 In‐hospital worsening of HF was defined as the requirement for additional administration of intravenous HF drugs, haemodialysis, or mechanical circulatory or respiratory support after the patient's condition had improved and at least 24 h after initiation of therapy. 12 , 13 Cognitive dysfunction was subjectively judged by the attending physician. Detailed definitions of the baseline clinical characteristics have been provided in the Supporting Information.
Outcome measures
The primary outcome measure was a composite of all‐cause death or HF hospitalization within 1 year after discharge. The secondary outcome measures were the individual components of the primary composite endpoint and cardiovascular death. HF hospitalization was defined as hospitalization due to worsening of HF that required intravenous drug therapy. 12 Cardiovascular death was defined as HF death, sudden cardiac death, vascular death, and other cardiac deaths.
Statistical analysis
Categorical variables are presented as numbers with percentages and were compared using the χ2 test. Continuous variables are expressed as the mean with standard deviation, or the median with interquartile range (IQR), and were compared using Student's t‐test when normally distributed or the Wilcoxon rank‐sum test when not normally distributed.
We explored the factors independently associated with hospital follow‐up using the multivariable logistic regression models. We included those potential candidate factors that had P values < 0.1 in the univariate analysis.
We used the Kaplan–Meier method to estimate the cumulative 1‐year incidence of the outcome measures and assessed the differences using a log‐rank test. The risk of the collaborative follow‐up group relative to the hospital follow‐up group for all the outcome measures are presented as hazard ratio (HR) with 95% confidence interval (CI) using multivariable Cox proportional hazards models. We incorporated the following 23 clinically relevant risk‐adjusted variables in the multivariable Cox models as listed in Table 1 , and as reported previously 13 , 14 : 80 years and older, female, body mass index (BMI) ≤ 22 kg/m2, acute coronary syndrome, previous history of hospitalizations for HF, atrial fibrillation or flutter, hypertension, diabetes mellitus, chronic lung disease, previous myocardial infarction, previous stroke, current smoking, living alone, walking ability, systolic blood pressure <90 mmHg at admission, heart rate <60 beats/min at admission, patients with HF with reduced ejection fraction (HFrEF), anaemia, estimated glomerular filtration rate <30 mL/min/1.73 m2 at admission, blood albumin levels <3.0 g/dL at admission, blood sodium levels <135 mEq/L at admission, β‐blockers with HFrEF at discharge, and ACE‐I or ARB with HFrEF at discharge.
Table 1.
Patient characteristics, laboratory test results, and medications
| Variables | Hospital follow‐up (N = 1674, 58.5%) | Collaborative follow‐up (N = 1188, 41.5%) | P value | Number of patients analysed |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age (years) | 76 (67–83) | 81 (74–86) | <0.001 | 2862 |
| Age ≧80 years a , b | 641 (38.3) | 657 (55.3) | <0.001 | 2862 |
| Women a , b | 650 (38.8) | 525 (44.2) | 0.004 | 2862 |
| BMI (kg/m2) | 22.9 (20.5–25.6) | 22.4 (20.0–25.3) | 0.004 | 2780 |
| BMI ≦22 kg/m2 a , b | 654 (40.0) | 527 (46.1) | 0.002 | 2780 |
| Causes of heart failure | <0.001 | 2862 | ||
| Acute coronary syndrome a | 94(5.6) | 63 (5.3) | 0.72 | 2862 |
| Coronary artery disease other than acute coronary syndrome | 451 (26.9) | 352 (29.6) | 0.11 | 2862 |
| Cardiomyopathy b | 316 (18.9) | 155 (13.1) | <0.001 | 2862 |
| Hypertensive heart disease b | 368 (22.0) | 323 (27.2) | 0.001 | |
| Valvular disease | 316 (18.9) | 196 (16.5) | 0.10 | |
| Others | 129 (7.7) | 99 (8.3) | 0.91 | |
| Medical history | ||||
| Heart failure hospitalization a , b | 664 (39.7) | 360 (30.3) | <0.001 | 2862 |
| Atrial fibrillation or flutter b | 695 (41.5) | 508 (42.8) | 0.51 | 2862 |
| Hypertension a , b | 1164 (69.5) | 909 (76.5) | <0.001 | 2862 |
| Diabetes mellitus a | 652 (39.0) | 462 (38.9) | 1.00 | 2862 |
| Chronic lung disease a | 215 (12.8) | 163 (13.7) | 0.50 | 2862 |
| Myocardial infarction a | 386 (23.1) | 299 (25.2) | 0.20 | 2862 |
| Stroke a , b | 230 (13.7) | 204 (17.2) | 0.013 | 2862 |
| Cognitive dysfunction b | 151 (9.0) | 207 (17.4) | <0.001 | 2862 |
| Current smoking a | 253 (15.3) | 156 (13.3) | 0.13 | 2828 |
| Social background | ||||
| Living alone a | 368 (22.0) | 250 (21.0) | 0.58 | 2862 |
| Living with a spouse | 790(47.2) | 577(48.6) | 0.47 | 2862 |
| Living with a child b | 581(34.7) | 512(43.1) | <0.001 | 2861 |
| Living with a sibling b | 41(2.5) | 45(3.8) | 0.045 | 2861 |
| Living with parents | 79(4.7) | 48(4.0) | 0.41 | 2861 |
| Living with grandchildren b | 65(3.9) | 71(6.0) | 0.01 | 2861 |
| Living with non‐relatives b | 49(2.9) | 52(4.4) | 0.04 | 2860 |
| Public assistance | 97 (5.8) | 63 (5.3) | 0.62 | 2862 |
| Long‐term care insurance for aged 65 and over | 342 (57.9) | 387 (65.7) | 0.006 | 1180 |
| Functional status before admission | ||||
| Physical activity | ||||
| Ambulatory state a | 1469 (88.5) | 954 (81.0) | <0.001 | 2838 |
| Vital signs and symptoms on presentation | ||||
| Systolic BP < 90 mmHg a | 45 (2.7) | 26 (2.2) | 0.46 | 2855 |
| HR < 60 beats/min a , b | 97 (5.8) | 89 (7.6) | 0.08 | 2844 |
| BT ≧ 37.5°C b | 75 (4.7) | 71 (6.2) | 0.08 | 2731 |
| NYHA class IV | 738 (44.2) | 559 (47.2) | 0.12 | 2852 |
| Test on admission | ||||
| LVEF | 0.005 | 2855 | ||
| HFrEF (LVEF < 40%) a , b | 695 (41.7) | 424 (35.7) | 0.001 | 2855 |
| HFmrEF (40% ≤ LVEF <50%) | 305 (18.3) | 230 (19.4) | 0.47 | 2855 |
| HFpEF (LVEF ≥ 50%) b | 668 (40.1) | 533 (44.9) | 0.01 | 2855 |
| Haemoglobin (g/dL) | 11.9 ± 2.43 | 11.6 ± 2.29 | 0.002 | 2855 |
| Anaemia (men <13 g/dL, women<12 g/dL) a , b | 1028 (61.6) | 772 (65.0) | 0.07 | 2855 |
| BNP (pg/mL) | 700.9 (386.2–1210.9) | 692.8 (378.9–1219.7) | 0.96 | 2537 |
| NT‐proBNP (pg/mL) | 4958 (2512.5–11042.5) | 4920 (2514.8–10921.3) | 0.99 | 501 |
| BNP > 695.7 (pg/mL) or NT‐proBNP >4958 (pg/mL) | 820 (49.9) | 583 (49.5) | 0.85 | 2823 |
| Creatinine (mg/dL) | 1.1(0.83–1.6) | 1.09 (0.82–1.58) | 0.92 | 2857 |
| eGFR (mL/min/1.73 m2) | 46.0 (30.4–62.9) | 45.0 (29.8–59.7) | 0.048 | 2857 |
| eGFR <30 mL/min/1.73 m2 a | 407 (24.4) | 300 (25.2) | 0.60 | |
| Albumin (g/dL) | 3.53 ± 0.48 | 3.53 ± 0.48 | 0.81 | 2772 |
| Albumin <3.0 g/dL a | 178 (11.0) | 129 (11.2) | 0.90 | |
| Sodium (mEq/L) | 139.2 ± 4.08 | 139.4 ± 3.98 | 0.12 | 2851 |
| Sodium <135 mEq/L a | 191 (11.5) | 120 (10.1) | 0.27 | |
| Test at discharge | ||||
| Haemoglobin (g/dL) | 11.8 ± 2.26 | 11.5 ± 2.13 | 0.004 | 2800 |
| Anaemia (men <13 g/dL, women <12 g/dL) | 1088 (66.3) | 798 (68.9) | 0.15 | 2800 |
| BNP (pg/mL) | 257.0 (131.4–498.6) | 271.6 (139.0–516.0) | 0.46 | 1830 |
| NT‐proBNP (pg/mL) | 1875 (712–4054) | 1794 (754.6–4058.3) | 0.96 | 333 |
| BNP > 263.7 (pg/mL) or NT‐proBNP >1825 (pg/mL) | 607 (49.5) | 414 (51.3) | 0.44 | 2034 |
| Creatinine (mg/dL) | 1.12 (0.86–1.57) | 1.14 (0.88–1.6) | 0.37 | 2821 |
| eGFR (mL/min/1.73 m2) | 44.7 (29.9–60.9) | 42.3 (29.4–57) | 0.003 | 2821 |
| eGFR <30 mL/min/1.73 m2 | 414 (25.1) | 300 (25.7) | 0.73 | 2821 |
| Albumin (g/dL) | 3.45 ± 0.48 | 3.37 ± 0.46 | <0.001 | 2496 |
| Albumin <3.0 g/dL | 223 (15.4) | 183 (17.5) | 0.15 | 2496 |
| Sodium (mEq/L) | 138.4 ± 3.48 | 138.6 ± 3.46 | 0.16 | 2813 |
| Sodium <135 mEq/L | 194 (11.8) | 137 (11.8) | 1.00 | 2813 |
| Symptoms at discharge | ||||
| Dyspnoea on exercise | 422 (26.0) | 293 (25.3) | 0.72 | 2781 |
| Fatigue b | 241 (15.8) | 138 (12.1) | 0.007 | 2660 |
| Loss of appetite | 150 (9.4) | 95 (8.2) | 0.31 | 2753 |
| NYHA class III or IV | 75 (4.6) | 43 (3.7) | 0.26 | 2796 |
| Events during hospitalization | ||||
| Worsening heart failure | 263 (15.7) | 195 (16.4) | 0.64 | 2862 |
| An increase in serum creatinine levels ≥0.3 mg/dL | 542 (32.8) | 401 (34.2) | 0.47 | 2825 |
| Stroke | 14 (0.8) | 10 (0.8) | 1.00 | 2862 |
| Infection | 140 (8.4) | 83 (7.0) | 0.18 | 2862 |
| Condition at discharge | ||||
| Functional status | ||||
| Ambulatory b | 1456 (87.5) | 939 (79.7) | <0.001 | 2842 |
| Functional decline b | 90 (5.5) | 97 (8.3) | 0.003 | 2818 |
| Medications at discharge | ||||
| β‐blocker in HFrEF a | 572 (82.3) | 339 (81.4) | 0.34 | 1119 |
| ACE‐I or ARB in HFrEF a | 486 (69.9) | 281 (66.3) | 0.21 | 1119 |
| Implantable devices at discharge | ||||
| CRT‐D or CRT‐P b | 56 (3.4) | 24 (2.0) | 0.03 | 2862 |
| ICD | 38 (2.3) | 24 (2.0) | 0.65 | 2862 |
| Length of hospital stay (days) | 16 (11–23) | 15 (10–21) | <0.001 | 2862 |
| Length of hospital stay >15 days b | 854 (51.0) | 533 (44.9) | 0.001 | 2862 |
ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, brain‐type natriuretic peptide; BP, blood pressure; BT, body temperature; CRT‐D, Defibrillator with cardiac resynchronization therapy; CRT‐P, Cardiac resynchronization therapy‐ pacemaker; eGFR, estimated glomerular filtration rate; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; ICD Implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal‐proBNP; NYHA, New York Heart Association.
Continuous variables are presented as mean ± standard deviation or median with interquartile range. Categorical variables are presented as number (percentage).
Risk‐adjusting variables selected for the multivariable Cox proportional hazards models.
Potential factors association with hospital follow‐up selected in the multivariable logistic regression models.
To determine the extent to which the percentage of collaborated follow‐up varied by facility, we calculated the percentage of collaborated follow‐up for each facility participating in this study. In the subanalysis, a comparison of patient background factors and a survival analysis were also performed in the group of patients aged ≥65 years.
All statistical analyses were performed by two investigators (K. Washida, T. Kato) and a statistician (T. Morimoto) using JMP Pro V.15.2.0. Two‐tailed P values < 0.05 were considered statistically significant.
Results
Baseline clinical characteristics
Of the 2862 patients registered, 1674 (58.5%) were in the hospital follow‐up group, and 1188 (41.5%) were in the collaborative follow‐up group. Collaborative follow‐up was performed at a clinic for 844 patients and at general hospital for 344 patients. The median duration of the index hospital stay was 15 (IQR: 11–22) days in the overall cohort; 16 (IQR: 11–23) days in the hospital follow‐up group; and 15 (IQR: 10–21) days in the collaborative follow‐up group (P < 0.001). Patients in the collaborative follow‐up group were older, were more often women, were less likely to be able to walk independently, and had a higher proportion of BMI ≤ 22 kg/m2 compared with patients in the hospital follow‐up group. Regarding the social background data, there were no between‐group differences in the proportions of people living alone or on public assistance. However, the people living with the patients differed between groups. Patients in the collaborative follow‐up group were more likely to live with younger generations (children or grandchildren) than those in the hospital follow‐up group. The aetiology of HF differed by group. Patients in the collaborative follow‐up group were more likely to have coronary artery disease and hypertensive heart disease and less likely to have cardiomyopathy and valvular disease than those in the hospital follow‐up group. In the classification of HF based on left ventricular ejection fraction, the collaborative follow‐up group had fewer patients with HFrEF and more patients with HF with preserved ejection fraction (HFpEF) than the hospital follow‐up group. Fewer patients in the collaborative follow‐up group had defibrillator with cardiac resynchronization therapy (CRT‐D) or a cardiac resynchronization therapy‐pacemaker (CRT‐P) implanted than in the hospital follow‐up group. The collaborative follow‐up group had less residual fatigue at discharge than the hospital follow‐up group had, but there were no differences in the rates of residual dyspnoea on exertion, residual loss of appetite, or New York Heart Association (NYHA) classifications III and IV. Patients in the collaborative follow‐up group had a higher prevalence of hypertension and cognitive dysfunction and a lower prevalence of history of hospitalization for HF than those in the hospital follow‐up group. At admission, patients in the collaborative follow‐up group had lower haemoglobin levels and estimated glomerular filtration rates (eGFR) than those in the hospital follow‐up group. The levels of brain natriuretic peptide (BNP), N‐terminal pro BNP (NT‐proBNP), creatinine, albumin, and sodium on admission were not different between the two groups (Table 1 ).
In‐hospital events and the status at discharge
There were no differences in the incidences of in‐hospital worsening of HF, stroke, or infection, or frequency of increases in serum creatinine levels >0.3 mg/dL between the groups (Table 1 ). Patients in the collaborative follow‐up group had lower haemoglobin, eGFR, and serum albumin levels at discharge and had residual fatigue less often than those in the hospital follow‐up group. There were no significant differences in serum BNP and NT‐pro BNP levels at discharge between the two groups (Table 1 ).
Factors related to hospital follow‐up
We performed a logistic regression analysis with 23 adjustment factors to identify factors associated with hospital follow‐up. Previous hospitalization for HF was found to be most closely associated with hospital follow‐up, followed by length of hospital stay longer than 15 days. Conversely, ≥80 years of age, hypertension, and cognitive dysfunction were found to be associated with collaborative follow‐up (Table 2 ).
Table 2.
Factors independently associated with hospital follow‐up
| Variables | Adjusted OR | 95% CI | P value |
|---|---|---|---|
| Age ≥80 years | 0.53 | (0.44–0.65) | <0.001 |
| Women | 1.03 | (0.86–1.24) | 0.71 |
| BMI ≤ 22 kg/m2 | 0.88 | (0.74–1.05) | 0.14 |
| Cardiomyopathy as cause of heart failure | 1.11 | (0.87–1.42) | 0.46 |
| Hypertensive heart disease as cause of heart failure | 1.00 | (0.82–1.24) | 0.93 |
| Previous hospitalization for heart failure | 1.69 | (1.41–2.03) | <0.001 |
| Hypertension | 0.76 | (0.62–0.93) | 0.01 |
| Onset stroke during hospitalization | 0.69 | (0.26–1.83) | 0.46 |
| Cognitive dysfunction | 0.65 | (0.50–0.86) | 0.002 |
| Living with a child before admission | 0.90 | (0.75–1.08) | 0.25 |
| Living with a sibling before admission | 0.70 | (0.40–1.24) | 0.22 |
| Living with a grandchildren before admission | 0.83 | (0.54–1.29) | 0.41 |
| Living with a non‐relatives before admission | 1.02 | (0.61–1.72) | 0.93 |
| HR < 60 beats/min on presentation | 0.91 | (0.65–1.27) | 0.58 |
| BT ≧ 37.5°C on presentation | 0.84 | (0.58–1.22) | 0.36 |
| HFrEF (LVEF <40%) | 0.98 | (0.77–1.26) | 0.89 |
| HFpEF (LVEF ≥50%) | 0.99 | (0.78–1.26) | 0.96 |
| Anaemia on admission | 1.00 | (0.83–1.21) | 0.96 |
| Residual fatigue at discharge | 1.26 | (0.98–1.62) | 0.07 |
| Residual ambulatory function at discharge | 1.24 | (0.91–1.69) | 0.18 |
| Functional decline | 0.90 | (0.58–1.38) | 0.62 |
| CRT‐P or CRT‐D implantation | 1.03 | (0.58–1.82) | 0.92 |
| Length of hospital stay >15 days | 1.31 | (1.11–1.55) | 0.002 |
BMI, body mass index; BP, blood pressure; BT, body temperature; CI, confidence interval; CRT‐D, defibrillator with cardiac resynchronization therapy; CRT‐P; cardiac resynchronization therapy‐pacemaker; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; LVEF, left ventricular ejection fraction; OR, odds ratio.
We explored the factors independently associated with hospital follow‐up in the multivariable logistic regression models. We included those potential candidate factors that had P value < 0.1 in the univariate analysis.
Prevalence of hospital follow‐up and collaborative follow‐up among the participating centres
The prevalence of hospital follow‐up varied from 32.6 to 92.3%, depending on the hospital facility, across the centres participating in this study (Figure 2 ). In terms of the age quartile, the prevalence of collaborative follow‐up incrementally increased as the patients became older (Figure 3 ).
Figure 2.
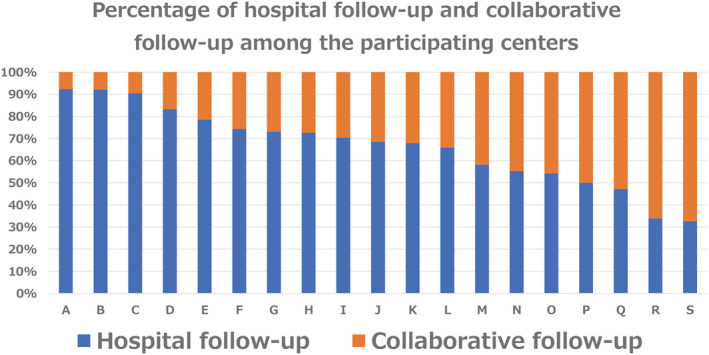
Prevalence of hospital follow‐up and collaborative follow‐up among the participating centres.
Figure 3.
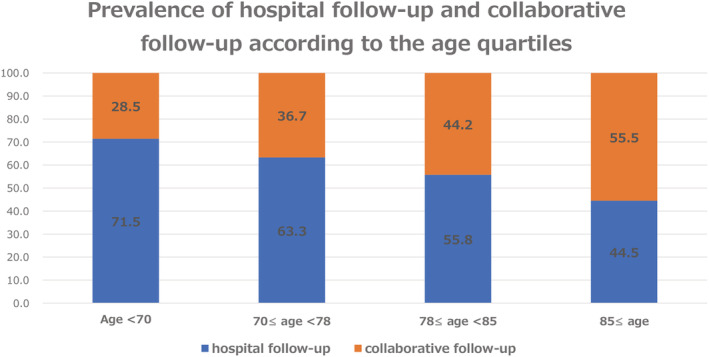
Prevalence of hospital follow‐up and collaborative follow‐up according to the age quartiles.
Long‐term outcomes: Hospital follow‐up group vs. collaborative follow‐up group
The follow‐up rate at 1 year was 95.6%. The cumulative 1‐year incidence of the primary outcome measure (a composite of all‐cause death or HF hospitalization) was similar between the hospital and collaborative follow‐up groups (31.6% vs. 29.6%, P = 0.51) (Figure 4 A ). Even after adjusting for confounders, the difference in risk for patients in the hospital follow‐up group relative to those in the collaborative follow‐up group remained insignificant for the primary outcome measure (Table 3 ). The cumulative 1‐year incidence of all‐cause death was similar between the two groups (13.1% vs. 13.9%, P = 0.35) (Figure 4 B ). After adjusting for confounders, the difference in risk of all‐cause death for patients in the hospital follow‐up group relative to those in the collaborative follow‐up group remained similar (Table 3 ). The cumulative 1‐year incidence of cardiovascular death was similar between the two groups (8.4% vs. 8.2%, P = 0.33) (Figure 4 C ). After adjusting for confounders, the risk of cardiovascular death for patients in the hospital follow‐up group relative to those in the collaborative follow‐up group remained similar (Table 3 ). The cumulative 1‐year incidence of HF hospitalization was higher in the hospital follow‐up group than in the collaborative follow‐up group (25.5% vs. 21.3%, P = 0.02) (Figure 4 D ). After adjusting for confounders, the risk of HF hospitalization in patients in the hospital follow‐up group relative to those in the collaborative follow‐up group remained higher (Table 3 ).
Figure 4.
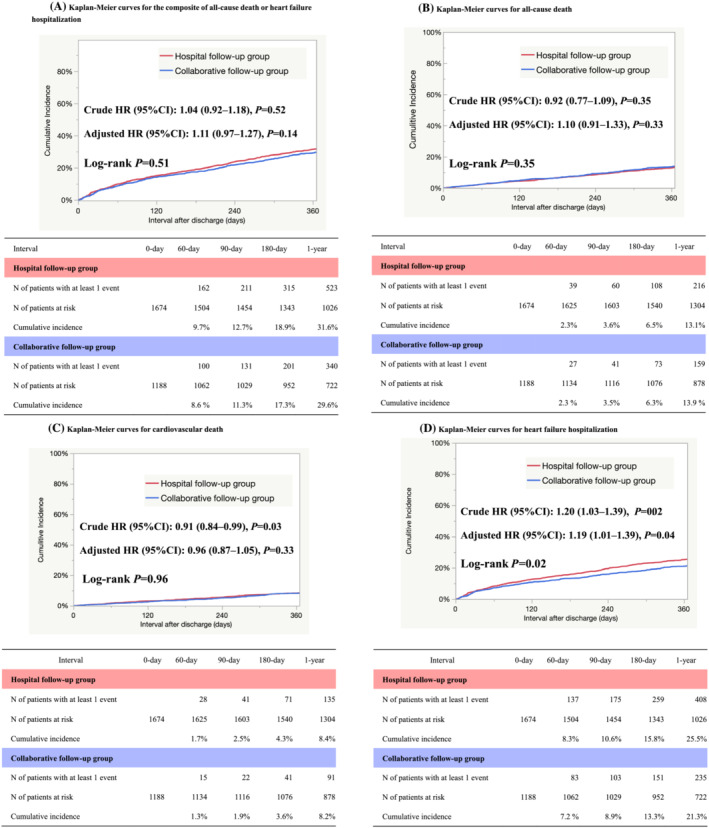
(A) Kaplan–Meier curves for the composite of all‐cause death or heart failure hospitalization. (B) Kaplan–Meier curves for all‐cause death. (C) Kaplan–Meier curves for cardiovascular death. (D) Kaplan–Meier curves for heart failure hospitalization.
Table 3.
Clinical outcomes
| Hospital follow‐up | Collaborative follow‐up | Unadjusted hazard ratio (95% CI) | P value | Adjusted hazard ratio (95% CI) | P value | |
|---|---|---|---|---|---|---|
| N of patients with event/N of patients at risk [cumulative 1‐year incidence (%)] | N of patients with event/N of patients at risk [cumulative 1‐year incidence (%)] | |||||
| A composite of all‐cause death or heart failure hospitalization | 523/1674(31.6%) | 340/1188 (29.6%) | 1.04 (0.92–1.18) | 0.52 | 1.11 (0.97–1.27) | 0.14 |
| All‐cause death | 216/1674 (13.1%) | 159/1188 (13.9%) | 0.92 (0.77–1.09) | 0.35 | 1.10 (0.91–1.33) | 0.33 |
| Cardiovascular death | 135/1674 (8.4%) | 91/1188 (8.2%) | 0.91 (0.84–0.99) | 0.03 | 0.96 (0.87–1.05) | 0.33 |
| Heart failure hospitalization | 408/1674 (25.5%) | 235/1188 (21.3%) | 1.20 (1.03–1.39) | 0.02 | 1.19 (1.01–1.39) | 0.04 |
CI, confidence interval
Subanalysis of patients aged ≥65 years
The proportions of patients ≥65 years of age in the hospital and collaborative follow‐up groups were comparable with that in the overall study population: 1350 (55.8%) patients and 1071 (44.2%) patients, respectively. The use of long‐term care insurance services was higher in the collaborative follow‐up group than in the hospital follow‐up group when limited to those ≥65 years of. In patients ≥65 years of age, there were no differences in BMI, stroke history, HFrEF and HFpEF distribution, haemoglobin levels at admission and discharge, or eGFR between the groups and the overall study population. Other differences in the baseline characteristics were consistent with the overall study population (Table S2 ).
The cumulative 1‐year incidence of the primary outcome measure (a composite of all‐cause death or HF hospitalization) was higher in the hospital follow‐up group than in the collaborative follow‐up group (35.4% vs. 30.6%, P = 0.04) (Figure S1 ). The cumulative 1‐year incidence of all‐cause death was similar between the 2 groups (15.3% vs. 14.7%, P = 0.96) (Figure S1 ). The cumulative 1‐year incidence of HF hospitalization was higher in the hospital follow‐up group than in the collaborative follow‐up group (28.5% vs. 21.8%, P < 0.001) (Figure S1 ).
Among patients ≥65 years of age, after adjusting for confounders, the risk of the primary outcome measure and HF hospitalization in patients in the hospital follow‐up group relative to those in the collaborative follow‐up group remained higher (Figure S1 ). After adjusting for confounders, the risk of all‐cause death in patients in the hospital follow‐up group relative to those in the collaborative follow‐up group remained similar (Figure S1 ).
Discussion
The main findings of the present study are as follows: (i) Among patients with AHF who were discharged to go home, 41.5% underwent collaborative follow‐up with a clinic or general hospital. (ii) Compared with the hospital follow‐up group, more patients in the collaborative follow‐up group were over 80 years of age, had hypertension complications, and had cognitive dysfunction complications. Conversely, patients receiving hospital follow‐up were characterized by previous HF hospitalization and a longer hospital stay. (iii) The risk of primary outcome measure and all‐cause death in patients in the hospital follow‐up group relative to those in the collaborative follow‐up group remained similar. (iv) The risk of HF hospitalization in patients in the hospital follow‐up group relative to those in the collaborative follow‐up group remained higher.
This is the first large‐scale clinical study to clarify follow‐up status after home discharge in patients hospitalized for AHF, focusing on the collaborative follow‐up between the hospital at index hospitalization and a clinic or general hospital in Japan. In this cohort study, only 41.5% of patients underwent collaborative follow‐up. The results of this study are largely consistent with a survey of hospital cardiologists that reported that 42% of cardiologists collaborate with general practitioners for the treatment and management of patients with HF after discharge from the hospital, 17 although the prevalence of collaborative follow‐up varied widely across facilities (Figure 2 ). Our study showed that the collaborative follow‐up rate for Japanese patients with acute heart failure (AHF) is lower than that reported overseas. 6 , 7 Japan is a country without a well‐developed primary care system, and hospitals with index hospitalization also have outpatient functions, and it has been taken for granted that patients receive follow‐up care at the hospital of index hospitalization after discharge. The attending physician at admission decides whether to conduct collaborative follow‐up after discharge, taking into consideration the policy of the hospital with index hospitalization, the number of hospitals and clinics in the residential area, and the patient's wishes and condition. Although the length of hospital stay for patients with AHF in Japan is longer than that in Western countries, 13 , 18 the hospitalization rate within 30 days after discharge is 4.6–5.5%, which is lower than that in Western countries. 19 , 20 This may be one of the reasons why post‐discharge follow‐up style has not been discussed in Japan, unlike in Western countries where transitional care has been recommended to reduce 30‐day readmission. 21 This study also revealed large differences in the rates of collaborative follow‐up among facilities. Patient characteristics associated with hospital follow‐up alone do not explain the differences between facilities. Many factors not examined in this study may contribute to differences between facilities, including hospital location, the number of clinics in the patient's area of residence, and the relationship between the hospital and the clinic.
This paper highlights two key points: (i) The risk of primary outcome, all‐cause death, and cardiovascular death were unexpectedly similar between hospital and collaborative follow‐up; (ii) the risk of HF hospitalization in hospital follow‐up is higher compared with collaborative follow‐up, as expected. As for the first point, our results differed from those of two U.S. reports, which showed that collaborative care by cardiologists and general internists reduced the risk of 1‐year mortality compared with follow‐up by general internists alone. 22 , 23 Unlike the U.S. report, there are several possible reasons why the risk of all‐cause death and cardiovascular death was not reduced in the clinic follow‐up group. One is the difference in patients' backgrounds between the two groups in the present study. The patients in the collaborative follow‐up group tended to be older, to have cognitive dysfunction, and to have higher rates of co‐morbidities than those in the hospital follow‐up group. Moreover, these factors have been reported to be independent prognostic determinants of HF. 24 Although we adjusted for these factors in our analysis, we could not rule out the possibility that important clinical differences not included in the database, or other unmeasured confounding factors may have influenced mortality more than the collaborated follow‐up intervention. The second reason is that the main causes of death differ between Japan and other countries. 25 , 26 , 27 The proportion of non‐cardiovascular deaths is higher in Japan than it is in other countries, 25 , 26 , 27 which might hamper the beneficial effect of collaboration with hospitals and clinics, possibly due to differences in demographics and healthcare delivery systems. In addition, the risk of all‐cause mortality and cardiovascular death was not different between hospital follow‐up and collaborative follow‐up.
In contrast, the risk of hospitalization for heart failure was lower in the collaborative follow‐up group than in the hospital follow‐up group. As the frequency of monitoring and timely engagement with healthcare providers reportedly contributes to improved outcomes, 28 , 29 , 30 the follow‐up structure of collaboration may have influenced the outcomes of HF patients. In Japan, a family doctor at a clinic treats a patient with HF more frequently, and, if hospitalization is deemed necessary, the patient is referred to an inpatient index hospital that provides advanced secondary care to prevent hospitalization for HF. The other reason may be the differences in the threshold of hospitalization between the collaborative follow‐up and the hospital follow‐up. In the case of an outpatient clinic at a secondary care facility that is an inpatient facility, when a patient with heart failure is considered to need hospitalization, the hospitalization can be coordinated immediately. On the other hand, in the case of a clinic, when a patient with HF is considered to need hospitalization, a referral must first be made to an inpatient index hospital that provides secondary care. In the case of clinic care, if the patient's HF worsens, the clinic may respond by increasing the frequency of home visits so that hospitalization can be avoided as much as possible. If patients are cognitively impaired or at high risk for delirium, care at home is more likely to be continued, rather than hospitalization. These results may involve multiple factors, including patient characteristics, hospital/clinic characteristics, and the interventions performed there, and thus require further study.
Although Japan has its own healthcare system and the rate of hospitalization for heart failure is lower than that in Western countries, 18 , 19 it was found that in Japan, as in other countries, collaborated follow‐up has the potential to reduce the risk of hospitalization for HF. This also indicates that collaborative follow‐up between hospitals and clinics after discharge of AHF patients may be used as a quality indicator of medical care. 31 , 32 , 33 Although further studies are needed to determine which patients and what type of collaborative follow‐up improves prognosis, our data suggested that clinic–hospital collaboration for patients with HF in Japan can be implemented as transitional care to improve the prognosis of patients with AHF.
Limitations
This study has several limitations. First, ‘general practitioners’ are a relatively new specialty in Japan. 34 As such, primary care services are primarily provided by physicians in clinics, who have little to no background in general or family medicine. Instead, they tend to be certified as specialists in some subspecialty. Their role should not be regarded as equivalent to that of primary care physicians in other countries. We were unable to obtain detailed information on the specialties of the doctors and how they collaborated in delivering post‐hospital care. Some patients in the hospital follow‐up group may have had a family physician, which may have led to misclassification of the patient's group. Second, there was no information about interventions other than those provided by the hospital or clinic after discharge (such as interventions by visiting nurses or participation in outpatient cardiac rehabilitation). Third, this study included only patients discharged home and excluded patients who were transferred or institutionalized. There is no uniform standard for determining inpatient transfers or institutionalizations, and the patients excluded from this study may have biased the study and may have influenced the results. Fourth, information on the type of post‐discharge follow‐up for patients with AHF was only examined at the time of discharge, and detailed information on whether the type of follow‐up has changed since then was not available. Fifth, there was no information on the healthcare systems, such as the function of the hospitals and the number of hospitals and clinics in the areas of the facilities participating in the study or in the areas the patients' place of residence. Accessible medical facilities are an important condition for patients to choose a hospital or clinic, and geographical environmental factors may have affected the collaboration rate, which in turn may have affected clinical outcomes.
Conclusions
In patients hospitalized for AHF, 41.5% underwent collaborative follow‐up after discharge. The risk of HF hospitalization in patients in the hospital follow‐up group was higher than that in the collaborative follow‐up, although there was no difference in primary outcome measures between the two groups.
Conflict of interest
None declared.
Funding
This study was supported by grant 18059186 from the Japan Agency for Medical Research and Development (Drs T. Kato, Kuwahara, and Ozasa). The founder had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
IRB information
The study was approved by the institutional review boards of Kyoto University Graduate School of Medicine (approval number: E2311), Shiga General Hospital (approval number: 20141120‐01), Tenri Hospital (approval number: 640), Kobe City Medical Center General Hospital (approval number: 14094), Hyogo Prefectural Amagasaki General Medical Center (approval number: Rinri 26‐32), National Hospital Organization Kyoto Medical Center (approval number: 14‐080), Mitsubishi Kyoto Hospital (approved 11/12/2014), Okamoto Memorial Hospital (approval number: 201503), Japanese Red Cross Otsu Hospital (approval number: 318), Hikone Municipal Hospital (approval number: 26‐17), Japanese Red Cross Osaka Hospital (approval number: 392), Shimabara Hospital (approval number: E2311), Kishiwada City Hospital (approval number: 12), Kansai Electric Power Hospital (approval number: 26‐59), Shizuoka General Hospital (approval number: Rin14‐11‐47), Kurashiki Central Hospital (approval number: 1719), Kokura Memorial Hospital (approval number: 14111202), Kitano Hospital (approval number: P14‐11‐012), and Japanese Red Cross Wakayama Medical Center (approval number: 328).
Supporting information
Table S1. Follow‐up style details.
Table S2. Patient characteristics, laboratory test results, and medications in patients with ≥65years of age.
Figure S1. Kaplan–Meier curve for a composite of all‐cause death or heart failure hospitalization, all‐cause death, and heart failure hospitalization compared between hospital follow‐up group and collaborative follow‐up group in patients ≥65years of age.
Figure S1‐A. Kaplan–Meier curves for the composite of all‐cause death or heart failure hospitalization.
Figure S1‐B. Kaplan–Meier curves for all‐cause death.
Figure S1‐C. Kaplan–Meier curves for heart failure rehospitalization.
Acknowledgements
We sincerely appreciate the staff of the KCHF registry and the other members of the participating centres.
Washida, K. , Kato, T. , Ozasa, N. , Morimoto, T. , Yaku, H. , Inuzuka, Y. , Tamaki, Y. , Seko, Y. , Yamamoto, E. , Yoshikawa, Y. , Shiba, M. , Kitai, T. , Yamashita, Y. , Taniguchi, R. , Iguchi, M. , Nagao, K. , Kawase, Y. , Nishimoto, Y. , Kuragaichi, T. , Hotta, K. , Morinaga, T. , Toyofuku, M. , Furukawa, Y. , Ando, K. , Kadota, K. , Sato, Y. , Kuwahara, K. , and Kimura, T. (2023) A comparison between hospital follow‐up and collaborative follow‐up in patients with acute heart failure. ESC Heart Failure, 10: 353–365. 10.1002/ehf2.14200.
References
- 1. Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995; 333: 1190–1195. [DOI] [PubMed] [Google Scholar]
- 2. Feltner C, Jones CD, Cené CW, Zheng ZJ, Sueta CA, Emmanuel JL. Transitional care interventions to prevent readmissions for persons with heart failure: A systematic review and meta‐analysis. Ann Intern Med. 2014; 160: 774–784. [DOI] [PubMed] [Google Scholar]
- 3. Albert NM, Barnason S, Deswal A, Hernandez A, Kociol R, Lee E, Paul S, Ryan CJ, White‐Williams C. Transitions of care in heart failure: A scientific statement from the American Heart Association. Circ Heart Fail. 2015; 8: 384–409. [DOI] [PubMed] [Google Scholar]
- 4. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JG. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 5. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2022; 145: e895–e1032. [DOI] [PubMed] [Google Scholar]
- 6. Muntwyler J, Follath F. Management of heart failure in Switzerland. Eur J Heart Fail. 2000; 2: 113–115. [DOI] [PubMed] [Google Scholar]
- 7. Boa Sorte Silva NC, Pulford RW, Lee DS, Petrella RJ. Heart failure management insights from primary care physicians and allied health care providers in southwestern Ontario. BMC Fam Pract. 2020; 21: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuwabara M, Mori M, Komoto S. Japanese national plan for promotion of measures against cerebrovascular and cardiovascular disease. Circulation. 2021; 143: 1929–1931. [DOI] [PubMed] [Google Scholar]
- 9. Arai H, Ouchi Y, Toba K, Endo T, Shimokado K, Tsubota K, Matsuo S, Mori H, Yumura W, Yokode M, Rakugi H, Ohshima S. Japan as the front‐runner of super‐aged societies: Perspectives from medicine and medical care in Japan. Geriatr Gerontol Int. 2015; 15: 673–687. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization . Men ageing and health: Achieving health across the life span. 2001. http://apps.who.int/iris/bitstream/handle/10665/66941/WHO_NMH_NPH_01.2.pdf?sequence=1 (Accessed August 15, 2022) [Google Scholar]
- 11. Health and Welfare Bureau for the Elderly Ministry of Health, Labour and Welfare . Long‐term care insurance system of Japan. 2016. https://www.mhlw.go.jp/english/policy/care‐welfare/care‐welfare‐elderly/dl/ltcisj_e.pdf. (Accessed August 15, 2022)
- 12. Yamamoto E, Kato T, Ozasa N, Yaku H, Inuzuka Y, Tamaki Y, Kitai T, Morimoto T, Taniguchi R, Iguchi M, Kato M, Takahashi M, Jinnai T, Ikeda T, Nagao K, Kawai T, Komasa A, Nishikawa R, Kawase Y, Morinaga T, Kawashima T, Motohashi Y, Kawato M, Toyofuku M, Sato Y, Kuwahara K, Shioi T, Kimura T, KCHF study investigators . Kyoto congestive heart failure (KCHF) study: Rationale and design. ESC Heart Fail. 2017; 4: 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yaku H, Ozasa N, Morimoto T, Inuzuka Y, Tamaki Y, Yamamoto E, Yoshikawa Y, Kitai T, Taniguchi R, Iguchi M, Kato M, Takahashi M, Jinnai T, Ikeda T, Nagao K, Kawai T, Komasa A, Nishikawa R, Kawase Y, Morinaga T, Su K, Kawato M, Sasaki K, Toyofuku M, Furukawa Y, Nakagawa Y, Ando K, Kadota K, Shizuta S, Ono K, Sato Y, Kuwahara K, Kato T, Kimura T, KCHF Study Investigators . Demographics, management, and in‐hospital outcome of hospitalized acute heart failure syndrome patients in contemporary real clinical practice in Japan ‐ observations from the prospective, multicenter Kyoto congestive heart failure (KCHF) registry. Circ J. 2018; 82: 2811–2819. [DOI] [PubMed] [Google Scholar]
- 14. Yaku H, Kato T, Morimoto T, Inuzuka Y, Tamaki Y, Ozasa N, Yamamoto E, Yoshikawa Y, Kitai T, Taniguchi R, Iguchi M, Kato M, Takahashi M, Jinnai T, Ikeda T, Nagao K, Kawai T, Komasa A, Nishikawa R, Kawase Y, Morinaga T, Toyofuku M, Seko Y, Furukawa Y, Nakagawa Y, Ando K, Kadota K, Shizuta S, Ono K, Sato Y, Kuwahara K, Kimura T, KCHF Study Investigators . Association of mineralocorticoid receptor antagonist use with all‐cause mortality and hospital readmission in older adults with acute decompensated heart failure. JAMA Netw Open. 2019; 2: e195892–e195814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ministry of Education, Culture, Sports, Science and Technology; Ministry of Health, Labour and Welfare . Japan's ethical guidelines for epidemiologic research. http://www.lifescience.mext.go.jp/files/pdf/n796_01.pdf (Accessed December 20, 2021)
- 16. Yaku H, Kato T, Morimoto T, Inuzuka Y, Tamaki Y, Ozasa N, Yamamoto E, Yoshikawa Y, Kitai T, Kato M, Ikeda T, Furukawa Y, Nakagawa Y, Sato Y, Kuwahara K, Kimura T. Risk factors and clinical outcomes of functional decline during hospitalisation in very old patients with acute decompensated heart failure: An observational study. BMJ Open. 2020; 10: e032674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kinugasa Y, Saitoh M, Ikegame T, Ikarashi A, Kadota K, Kamiya K, Kohsaka S, Mizuno A, Miyajima I, Nakane E, Nei A, Shibata T, Yokoyama H, Yumikura S, Yumino D, Watanabe N, Isobe M, Research Team for the Provision of Heart Failure Care Centered on General Practitioners in the Community . Differences in priorities for heart failure management between cardiologists and general practitioners in Japan. Circ J. 2021; 85: 1565–1574. [DOI] [PubMed] [Google Scholar]
- 18. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 19. Shiraishi Y, Kohsaka S, Sato N, Takano T, Kitai T, Yoshikawa T, Matsue Y. 9‐year trend in the management of acute heart failure in Japan: A report from the national consortium of acute heart failure registries. J Am Heart Assoc. 2018; 7: e008687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dharmarajan K, Wang Y, Lin Z, Normand SLT, Ross JS, Horwitz LI, Desai NR, Suter LG, Drye EE, Bernheim SM, Krumholz HM. Association of changing hospital readmission rates with mortality rates after hospital discharge. JAMA. 2017; 318: 270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Y, Fu MR, Luo B, Li M, Zheng H, Fang J. The effectiveness of transitional care interventions on health care utilization in patients discharged from the hospital with heart failure: A systematic review and meta‐analysis. J Am Med Dir Assoc. 2021; 22: 621–629. [DOI] [PubMed] [Google Scholar]
- 22. Ezekowitz JA, Walraven CV, McAlister FA, Armstrong PW, Kaul P. Impact of specialist follow‐up in outpatients with congestive heart failure. CMAJ. 2005; 172: 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Indridason OS, Coffman CJ, Oddone EZ. Is specialty care associated with improved survival of patients with congestive heart failure? Am Heart J. 2003; 145: 300–309. [DOI] [PubMed] [Google Scholar]
- 24. Murad K, Goff DC Jr, Morgan TM, Burke GL, Bartz TM, Kizer JR, Chaudhry SI, Gottdiener JS, Kitzman DW. Burden of comorbidities and functional and cognitive impairments in elderly patients at the initial diagnosis of heart failure and their impact on total mortality: The cardiovascular health study. JACC Heart Fail. 2015; 3: 542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization . The top 10 causes of death. 2020. https://www.who.int/news‐room/fact‐sheets/detail/the‐top‐10‐causes‐of‐death (Accessed August 15, 2022)
- 26. Ministry of Health, Labour and Welfare . Annual health, labour and welfare report 2021; overview of the system and the basic statistics. https://www.mhlw.go.jp/english/wp/wp‐hw14/dl/01e.pdf. (Accessed August 15, 2022)
- 27. Bando M, Miyatake N, Kataoka H, Kinoshita H, Tanaka N, Suzuki H, Katayama A. Changes and variations in death due to senility in Japan. Healthcare (Basel). 2020; 8: 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yaffe RD, Stitt A, Lee JJ, Mohamed S, Lee DS. Assessing risk and preventing 30‐day readmissions in decompensated heart failure: Opportunity to intervene? Curr Heart Fail Rep. 2015; 12: 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feltner C, Jones CD, Cene CW, Zheng ZJ, Sueta CA, Coker‐Schwimmer EJL, Arvanitis M, Lohr KN, Middleton JC, Jonas DE. Transitional care interventions to prevent readmissions for persons with heart failure. Ann Intern Med. 2014; 160: 774–784. [DOI] [PubMed] [Google Scholar]
- 30. Raat W, Smeets M, Janssens S, Vaes B. Impact of primary care involvement and setting on multidisciplinary heart failure management: A systematic review and meta‐analysis. ESC Heart Failure. 2021; 8: 802–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Pieper K, Sun JL, Yancy CW, Young JB, OPTIMIZE‐HF Investigators and Hospitals . Influence of a performance‐improvement initiative on quality of care for patients hospitalized with heart failure; results of the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE‐HF). Arch Intern Med. 2007; 167: 1493–1502. [DOI] [PubMed] [Google Scholar]
- 32. Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Pieper K, Sun JL, Yancy C, Young JB, OPTIMIZE‐HF Investigators and Hospitals . Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA. 2007; 297: 61–70. [DOI] [PubMed] [Google Scholar]
- 33. DeVore AD, Granger BB, Fonarow GC, Al‐Khalidi HR, Albert NM, Lewis EF, Butler J, Piña IL, Allen LA, Yancy CW, Cooper LB, Felker GM, Kaltenbach LA, McRae AT, Lanfear DE, Harrison RW, Disch M, Ariely D, Miller JM, Granger CB, Hernandez AF. Effect of a hospital and postdischarge quality improvement intervention on clinical outcomes and quality of care for patients with heart failure with reduced ejection fraction: The CONNECT‐HF randomized clinical trial. JAMA. 2021; 326: 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yoshida S, Matsumoto M, Kashima S, Koike S, Tazuma S, Maeda T. Geographical distribution of family physicians in Japan: A nationwide cross‐sectional study. BMC Fam Pract. 2019; 20: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Follow‐up style details.
Table S2. Patient characteristics, laboratory test results, and medications in patients with ≥65years of age.
Figure S1. Kaplan–Meier curve for a composite of all‐cause death or heart failure hospitalization, all‐cause death, and heart failure hospitalization compared between hospital follow‐up group and collaborative follow‐up group in patients ≥65years of age.
Figure S1‐A. Kaplan–Meier curves for the composite of all‐cause death or heart failure hospitalization.
Figure S1‐B. Kaplan–Meier curves for all‐cause death.
Figure S1‐C. Kaplan–Meier curves for heart failure rehospitalization.


