Abstract
Aims
The long‐term outcome in patients with heart failure (HF) after hospitalization may vary substantially depending on their age and left ventricular ejection fraction (LVEF). We aimed to assess the relative rates of cardiovascular death (CVD) and non‐CVD based on the age and how the rates differ under the updated LVEF classification system.
Methods and results
Consecutively registered hospitalized patients with HF (N = 3558; 39.7% women with a mean age of 73.9 ± 13.3 years) were followed for a median of 2 (interquartile range, 0.8–3.1) years. The CVDs and non‐CVDs were evaluated based on age [young (<65 years), older (65–84 years), and very old (≥85 years)] and LVEF classification [HF with preserved EF (HFpEF; LVEF ≥50%) and non‐HFpEF (LVEF <50%)]. The adverse clinical events were adjudicated independently by a central committee. Overall, 1505 (42.3%) had HFpEF [young: n = 182 (12.1%), older: n = 894 (59.4%), very old: n = 429 (28.5%)], and 2053 (57.7%) had non‐HFpEF [young: n = 575 (28.0%), older: n = 1159 (56.5%), very old: n = 319 (15.5%)]. During the follow‐up, the crude incidence of all‐cause death was higher in non‐HFpEF than in HFpEF across all age groups (non‐HFpEF vs. HFpEF, young: 10.4% vs. 5.5%, log‐rank P = 0.10; older: 26.6% vs. 20.9%, log‐rank P = 0.002; very old: 36.7% vs. 31.7%, log‐rank P = 0.043). CVDs accounted for more than half of all deaths in non‐HFpEF (young 65.0%, older 64.2%, and very old 55.6%), whereas the proportion of CVDs remained less than half in HFpEF (young 50.0%, older 41.2%, very old 38.2%). HF readmission was associated with subsequent all‐cause death in non‐HFpEF [hazard ratio (HR): 1.72, 95% confidence interval (CI): 1.41–2.09, P < 0.001], but not in HFpEF (HR: 1.12, 95% CI: 0.87–1.43, P = 0.39).
Conclusions
The probability of a non‐CVD increases in both LVEF categories with advancing age, but that it is greater in the HFpEF category. The findings indicate that mitigating CV‐related outcomes alone may be insufficient for treating HF in older population, particularly in the HFpEF category.
Keywords: Heart failure, Left ventricular ejection fraction, Mode of death, Older patients
Introduction
The incidence of heart failure (HF) has increased substantially over the last decade. HF is a serious healthcare concern given its high morbidity and mortality, particularly in countries with rapidly ageing populations. 1 , 2 There have been notable advances achieved in HF management, and numerous clinical trials have demonstrated that neurohormonal agents contribute to reduced mortality in patients with HF with reduced ejection fraction [HFrEF; left ventricular ejection fraction (LVEF) ≤ 40%]. 3 , 4 , 5 More recently, novel agents, such as angiotensin receptor‐neprilysin inhibitor or sodium‐coupled glucose transporter 2 inhibitor, have demonstrated clinical benefits in patients with HF with reduced and mildly reduced ejection fraction (HFmrEF, LVEF 41–49%) and with preserved ejection fraction (HFpEF; LVEF ≥50%). 6 , 7 , 8
The beneficial effect of these HF‐specific therapies is primarily attributed to fewer adverse cardiovascular (CV) events. 3 , 4 , 5 , 6 , 7 , 8 However, patients with HF remain at risk of both CV and non‐CV events, and the relative proportion of each may vary based on their age and HF phenotype. 9 The number of patients with HF with non‐cardiac multi‐co‐morbidities has been increasing in recent years, 10 driven by the burden of older patients and those with preserved LVEF. 10 , 11 Nonetheless, to date, data on the relative mode of death among patients with HF based on age and LVEF‐based classification are limited.
In order to facilitate both patient‐level decision‐making and population‐level strategies for reducing morbidity among patients with HF, it is crucial to understand the mode of death in real‐world patients with HF equipped with a broad range of symptoms and complications. This study used a multicentre registry of patients discharged after HF admission to elucidate the interrelationship between HF readmission, mode of death, age, and LVEF.
Methods
This study was part of the West Tokyo Heart Failure Registry (WET‐HF). In brief, the WET‐HF is a multicentre, prospective registry including data from consecutively hospitalized patients with acute HF at six institutions within the Tokyo metropolitan area from 2006 to 2017. 12 , 13 A central study committee adjudicated the mode of death to ensure accuracy. Initially, all deaths were reviewed by the investigators and divided into two groups: those requiring adjudication or those with a clearly defined mode of death. Then, central committee members reviewed the abstracted records and adjudicated the modes of death.
Before the launch of this registry, information on the present study's objectives, social significance, and an abstract were provided for clinical trial registration to the University Hospital Medical Information Network of Japan (UMIN000001171). The institutional review boards at each site approved the study protocol, and the research followed the Declaration of Helsinki. Written or oral informed consent was obtained from each patient before the study.
Patient data and selection
Participants aged ≥20 years with acute HF were diagnosed based on the Framingham criteria and physical examinations including electrocardiography, echocardiography, and laboratory tests analysed by experienced cardiologists at each institution. 3 , 4 , 5 , 14 Patients who refused to participate in the study or presented with concurrent HF and acute coronary syndrome were excluded from registration. Patient data were entered into an electronic data capture system with a robust data query engine and system validations for data quality. In addition, the principal investigators verified the quality of the reporting at least once a year, and periodic queries were conducted to ensure quality.
Of 4000 consecutively registered patients, we excluded patients who died during the index hospitalization [n = 164 (4.1%)], those who were not followed up [n = 244 (6.1%)], and those without LVEF data [n = 34 (0.8%)]. The remaining 3558 patients were included and grouped by LVEF status [HFpEF, LVEF ≥50% and non‐HFpEF (HFmrEF and HFrEF), LVEF <50%], which was based on the universal classification of HF [5]. Finally, for each LVEF category, we further categorized the participants into three age groups: <65 years (young), 65–84 years (older), and ≥85 years (very old) (Figure S1 ). The definition of very old was based on previous epidemiology studies. 10 Board‐certified physicians or physiology technicians assessed LVEF on echocardiography using the modified Simpson's method during the index hospitalization after the HF signs and symptoms were stabilized.
Variables and outcomes
Ischaemic aetiology was defined as a history of myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, or at least one major epicardial coronary artery with ≥75% stenosis. The New York Heart Association (NYHA) functional class was evaluated at discharge by the treating cardiologists at each institution. Cardiogenic shock was defined as systolic blood pressure ≤90 mmHg or inotropes use according to a previous study. 15 Regarding the co‐morbidities, anaemia was defined according to the World Health Organization criteria (haemoglobin at discharge <13 g/dL for men and <12 g/dL for women). Chronic kidney disease was defined as estimated glomerular filtration rate (eGFR) at discharge <60 mL/min/1.73 m2. The eGFR was calculated using the Modification of Diet in Renal Disease Equation for Japanese Patients, proposed by the Japanese Society of Nephrology. 16 We assessed nutritional status via the Geriatric Nutritional Risk Index (GNRI). 17
HF death (HFD), sudden cardiac death (SCD), and other cardiovascular deaths (CVDs), including acute coronary syndrome, acute aortic syndrome, intracranial haemorrhage, and stroke, were considered CVDs. SCD was defined as unexpected and otherwise unexplained death in a previously stable patient or death from documented or presumed cardiac arrhythmia without a clear non‐CV cause in a previously stable patient within 24 h from the onset. 18 Non‐CVDs were all other causes of death. Treating physicians at each participating hospital identified HF readmissions according to standard definitions. 12
Statistical analyses
We compared the patient characteristics among the three age groups stratified by LVEF. Parametric and non‐parametric variables and their respective differences were assessed using a one‐way analysis of variance and the Kruskal–Wallis test. Significant differences between the independent categorical variables were assessed using the chi‐squared test.
The incidence of all‐cause death was estimated using the Kaplan–Meier survival function and compared among the different age groups stratified by LVEF using the log‐rank test. Furthermore, considering the long‐time interval in this study, we divided the participants into those enrolled from 2006 to 2012 (N = 1291, 36.2%) and those enrolled from 2013 to 2017 (N = 2267, 63.8%). We subsequently compared both crude incidence of all‐cause death and readmission among them.
The cumulative incidence of each mode of death was estimated, accounting for competing risks; CVD (including HFD, SCD, and other‐CVD) and non‐CVD were considered competing events. The incidence of each mode of death stratified by age and LVEF categories were compared using the log‐rank test. The CVD proportion was compared among the three age groups using the Cochran–Armitage test for trends. For sensitivity analyses, we evaluated the CVD proportion among patients with HFrEF, HFmrEF, and HFpEF for each age group. We also re‐categorized the participants using different LVEF cut‐offs (55% and 60%) and assessed the CVD proportions.
Cox proportional hazards models were used to evaluate risk factors for all‐cause death for each LVEF category. Covariates included in the multivariable model were as follows: age, sex, LVEF, diabetes, atrial fibrillation, previous stroke, hyperuricemia, chronic obstructive pulmonary disease (COPD), history of smoking, N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) level at admission, HF readmission, the NYHA functional class at discharge, and levels of systolic blood pressure, haemoglobin, eGFR, and GNRI at discharge. When analysing patients with non‐HFpEF, we also added the following covariates in the model: the prescription of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers and β‐blockers at discharge and implantation of implantable cardioverter‐defibrillators or cardiac resynchronization therapy devices. In addition, we assessed the interaction between HF readmission and age for all‐cause death. Multivariate Fine–Gray competing risk models were used to explore the association between covariates and CVDs or non‐CVDs.
P‐values of <0.05 were considered statistically significant. All statistical analyses were performed using SPSS version 24.0 (IBM Corporation, Armonk, NY, USA) and R software (version 3.6.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
Of the 3558 discharged patients with HF included in the present analysis, 1505 (42.3%) were HFpEF patients [young: n = 182 (12.1%), older: n = 894 (59.4%), very old: n = 429 (28.5%)], and 2053 (57.7%) were non‐HFpEF (HFmrEF and HFrEF) patients [young: n = 575 (28.0%), older: n = 1159 (56.5%), very old: n = 319 (15.5%); Figure S1 ]. The distribution of LVEF is shown in Figure 1 . Across the LVEF categories, older patients were more female and had poor renal function and nutrition status and a higher number of co‐morbidities. Among patients with non‐HFpEF, older patients were less likely to receive β‐blockers, angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers, and mineralocorticoid receptor antagonists (Table 1 ).
Figure 1.
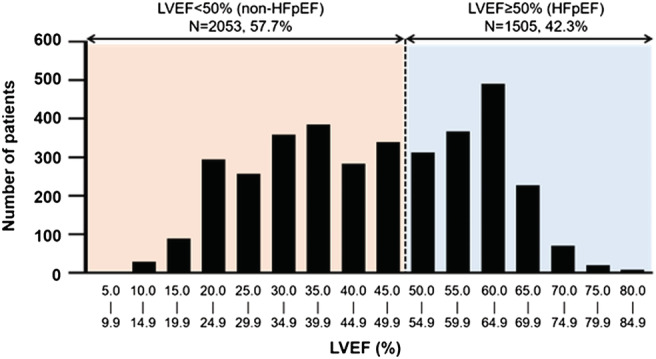
Distributions of LVEF.
Table 1.
Comparison of patient characteristics
| Non‐HFpEF (LVEF <50%) | HFpEF (LVEF ≥50%) | |||||||
|---|---|---|---|---|---|---|---|---|
|
Young N = 575 |
Older N = 1159 |
Very old N = 319 |
P‐value |
Young N = 182 |
Older N = 894 |
Very old N = 429 |
P‐value | |
| Age, years | 56 (48–61) | 76 (71–80) | 88 (86–91) | ‐ | 57 (50–61) | 77 (73–81) | 88 (86–91) | ‐ |
| Female, % | 19.0 | 32.4 | 48.3 | <0.001 | 37.4 | 50.8 | 59.2 | <0.001 |
| BMI, kg/m2 | 23.5 ± 4.82 | 21.4 ± 3.61 | 19.9 ± 3.09 | <0.001 | 23.5 ± 4.98 | 21.8 ± 3.97 | 20.8 ± 3.58 | <0.001 |
| Prior HF admission, % | 28.1 | 33.7 | 33.2 | 0.054 | 16.0 | 28.1 | 29.4 | <0.001 |
| History of smoking, % | 56.7 | 45.6 | 31.7 | <0.001 | 52.8 | 39.0 | 28.5 | <0.001 |
| Cause of HF, % | ||||||||
| Ischaemic | 24.2 | 41.2 | 48.6 | <0.001 | 12.6 | 18.1 | 15.6 | 0.018 |
| Dilated | 42.3 | 18.6 | 8.2 | 2.7 | 1.3 | 0.2 | ||
| Valvular | 6.6 | 16.1 | 26.3 | 36.8 | 42.1 | 42.2 | ||
| Hypertensive | 5.7 | 4.8 | 3.8 | 8.8 | 6.0 | 8.6 | ||
| Hypertrophic | 2.1 | 2.8 | 1.9 | 8.8 | 5.5 | 3.3 | ||
| Others | 19.1 | 16.3 | 11.3 | 30.2 | 26.9 | 30.0 | ||
| Echocardiography | ||||||||
| LVDD, cm | 6.06 ± 0.97 | 5.61 ± 0.89 | 5.16 ± 0.83 | <0.001 | 4.91 ± 0.82 | 4.66 ± 0.83 | 4.35 ± 0.72 | <0.001 |
| LVDS, cm | 5.17 ± 1.05 | 4.65 ± 1.00 | 4.20 ± 0.88 | <0.001 | 3.24 ± 0.69 | 3.16 ± 0.67 | 2.92 ± 0.59 | <0.001 |
| LAD, cm | 4.50 ± 0.80 | 4.49 ± 0.94 | 4.20 ± 0.76 | <0.001 | 4.46 ± 1.13 | 4.58 ± 1.00 | 4.45 ± 0.89 | 0.09 |
| LVEF, % | 30.9 ± 9.45 | 34.0 ± 9.01 | 36.1 ± 8.41 | <0.001 | 60.1 ± 6.55 | 59.9 ± 6.41 | 59.6 ± 5.88 | 0.49 |
| Co‐morbidities, % | ||||||||
| Atrial fibrillation | 33.8 | 45.1 | 43.4 | <0.001 | 37.9 | 57.6 | 61.3 | <0.001 |
| Hypertension | 56.1 | 67.9 | 72.1 | <0.001 | 51.1 | 68.2 | 71.1 | <0.001 |
| Diabetes | 35.3 | 40.4 | 28.5 | <0.001 | 25.8 | 33.6 | 24.2 | <0.01 |
| Dyslipidaemia | 36.4 | 45.2 | 35.8 | <0.001 | 32.4 | 38.9 | 20.4 | 0.006 |
| Hyperuricemia | 51.3 | 43.1 | 46.4 | 0.006 | 38.5 | 38.5 | 37.5 | 0.94 |
| Previous stroke | 8.5 | 14.8 | 13.2 | <0.001 | 9.3 | 13.6 | 16.5 | 0.06 |
| COPD | 2.3 | 4.9 | 5.3 | <0.001 | 1.1 | 6.1 | 6.3 | 0.008 |
| Anaemia | 30.1 | 60.1 | 76.8 | <0.001 | 47.3 | 68.3 | 77.2 | <0.001 |
| SAS | 3.1 | 2.2 | 0.6 | 0.049 | 3.8 | 1.3 | 0.5 | 0.005 |
| CKD | 47.8 | 73.3 | 83.7 | <0.001 | 39.0 | 67.3 | 79.0 | <0.001 |
| Dialysis | 3.8 | 3.2 | 0.6 | <0.001 | 5.5 | 3.5 | 0.9 | 0.002 |
| Number of co‐morbidities | 3.04 ± 1.66 | 3.96 ± 1.52 | 4.06 ± 1.37 | <0.001 | 2.86 ± 1.71 | 3.93 ± 1.54 | 4.04 ± 1.32 | <0.001 |
| ≥3 comorbidities | 60.2 | 81.4 | 87.8 | <0.001 | 57.1 | 82.3 | 88.1 | <0.001 |
| Status on admission, % | ||||||||
| Cardiogenic shock | 17.1 | 18.1 | 14.1 | 0.033 | 14.5 | 10.2 | 9.5 | 0.18 |
| SBP, mmHg | 136 ± 35.1 | 137 ± 32.1 | 141 ± 29.7 | 0.12 | 143 ± 40.5 | 144 ± 34.0 | 146 ± 30.7 | 0.46 |
| NT‐proBNP, pg/mL | 3371 (1872–5572) | 4084 (2190–8064) | 5574 (3201–9417) | <0.001 | 1493 (707–3337) | 2063 (1132–3890) | 2811 (1475–4727) | <0.001 |
| In‐hospital treatment | ||||||||
| Inotropic use | 14.4 | 16.4 | 12.3 | 0.163 | 12.2 | 8.1 | 8.3 | 0.21 |
| IABP | 2.8 | 2.3 | 1.3 | 0.33 | 1.7 | 1.5 | 0.5 | 0.25 |
| VA‐ECMO | 1.4 | 0.3 | 0 | 0.008 | 1.7 | 0.01 | 0.5 | 0.010 |
| Status at discharge | ||||||||
| SBP, mmHg | 107 ± 17.8 | 110 ± 17.3 | 112 ± 15.5 | <0.001 | 111 ± 16.7 | 116 ± 18.8 | 117 ± 16.9 | <0.001 |
| HR, bpm | 72.0 (64.0–82.0) | 70.0 (62.0–80.0) | 70.0 (62.0–80.0) | 0.002 | 70.5 (61.0–80.0) | 70.0 (60.0–76.0) | 70.0 (61.0–77.0) | 0.25 |
| Hb, g/dL | 14.0 (12.2–15.6) | 12.1 (10.6–13.5) | 11.1 (10.2–12.4) | <0.001 | 12.7 (11.2–14.3) | 11.5 (10.1–12.8) | 11.1 (10.0–12.2) | <0.001 |
| eGFR, mL/min/1.73m2 | 61.4 (45.5–74.6) | 48.0 (32.9–61.3) | 40.8 (31.8–53.7) | <0.001 | 65.4 (49.2–79.8) | 50.9 (34.4–63.6) | 44.7 (32.0–74.6) | <0.001 |
| NT‐proBNP, pg/mL | 878 (429–1854) | 1463 (819–3074) | 1994 (1606–4171) | <0.001 | 548 (262–1291) | 954 (451–11 880) | 1994 (1066–4171) | <0.001 |
| NYHA classification, % | ||||||||
| I | 15.5 | 14.6 | 8.5 | <0.001 | 24.6 | 17.7 | 15.6 | <0.001 |
| II | 74.1 | 65.7 | 60.1 | 66.5 | 60.4 | 52.7 | ||
| III | 9.3 | 18.3 | 29.7 | 6.1 | 20.9 | 29.8 | ||
| IV | 1.1 | 1.5 | 1.6 | 2.8 | 1.0 | 1.9 | ||
| GNRI | 99.4 (90.9–107) | 92.0 (84.0–100) | 87.7 (79.1–94.2) | <0.001 | 97.9 (92.0–105) | 92.7 (83.5–100) | 87.6 (79.6–95.9) | <0.001 |
| Pacemaker | 1.6 | 7.5 | 15.0 | <0.001 | 3.8 | 10.5 | 18.4 | <0.001 |
| ICD/CRT | 7.7 | 10.1 | 2.2 | <0.001 | 3.8 | 1.6 | 1.2 | 0.06 |
| Medications at discharge, % | ||||||||
| β‐blocker | 91.5 | 86.1 | 72.0 | <0.001 | 64.3 | 68.8 | 57.8 | <0.001 |
| ACE‐I or ARB | 76.7 | 64.8 | 60.5 | <0.001 | 59.9 | 58.4 | 58.0 | 0.61 |
| MRA | 48.0 | 39.4 | 33.5 | <0.001 | 30.2 | 27.4 | 22.6 | 0.16 |
| Loop diuretic | 73.2 | 77.4 | 81.5 | 0.04 | 63.2 | 72.6 | 80.9 | <0.001 |
| Tolvaptan | 4.3 | 5.4 | 4.1 | <0.001 | 3.8 | 4.3 | 6.1 | 0.04 |
| Anticoagulant | 47.8 | 56.7 | 39.3 | <0.001 | 47.8 | 62.6 | 53.7 | <0.001 |
ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstruction pulmonary disease; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; GNRI, geriatric nutritional risk index; Hb, haemoglobin; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; IABP, intra‐aortic balloon pumping; ICD, implantable cardioverter‐defibrillator; LAD, left atrial dimension; LVDD, left ventricular end‐diastolic diameter; LVDS, left ventricular end‐systolic diameter, LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; SAS, sleep apnoea syndrome; SBP, systolic blood pressure; VA‐ECMO, veno‐arterial extracorporeal membrane oxygenation.
Values are mean ± standard deviation, median (interquartile range), or per cent.
Clinical outcomes after discharge
The crude incidence of all‐cause death and HF readmission was similar between the non‐HFpEF and HFpEF patients (all‐cause death: 23.6% vs. 22.1%, long‐rank P = 0.60, HF readmission: 31.2% vs. 31.2%, log‐rank P = 0.80) during the median follow‐up period of 2.0 [interquartile range, 0.8–3.1] years. Regarding temporal trends, mean age increased in patients enrolled in 2013–2017 than those in 2006–2012: non‐HFpEF, 72.0 ± 13.5 years vs. 70.1 ± 14.5 years, P = 0.004; HFpEF, 77.9 ± 11.6 years vs. 76.7 ± 11.3 years vs., P = 0.07, respectively. In non‐HFpEF category, both the 2‐year crude incidence of all‐cause death and HF readmission did not show any significant differences between patients enrolled in 2006–2012 (N = 819) and those enrolled in 2013–2017 (N = 1234): all‐cause death; 18.4% vs. 15.6%, log‐rank P = 0.90 (Figure S2 ), HF readmission; 30.6% vs. 25.4%, log‐rank P = 0.60, respectively. On the contrary, in HFpEF category, these incidences were significantly higher in patients enrolled in 2006–2012 (N = 472) compared with those enrolled in 2013–2017 (N = 1033): all‐cause death; 14.2% vs. 16.5%, log‐rank P = 0.002 (Figure S3 ), HF readmission; 23.1% vs. 28.2%, log‐rank P < 0.001, respectively.
When subdivided by predefined age groups, the cumulative mortality rate was higher among non‐HFpEF patients than HFpEF patients across all age groups (non‐HFpEF vs. HFpEF, young: 10.4% vs. 5.5%, log‐rank P = 0.10; older: 26.6% vs. 20.9%, log‐rank P = 0.002; very old: 36.7% vs. 31.7%, log‐rank P = 0.043) (Figure 2 ).
Figure 2.
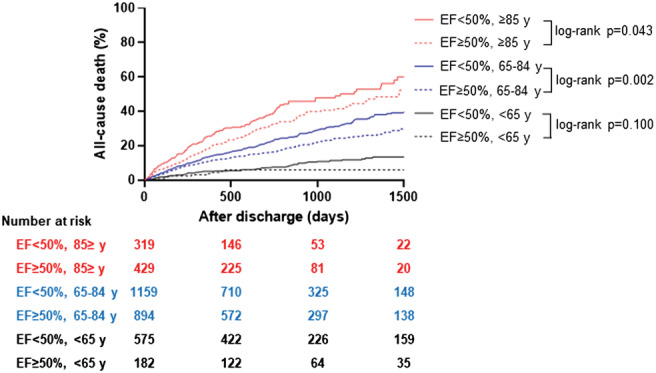
Kaplan–Meier survival curves for the cumulative incidence of all‐cause death.
CV vs. non‐CV mortality
The crude CV and non‐CV mortality increased with age, regardless of the LVEF category (Figure 3 and Table 2 ). The proportion of non‐CVD gradually increased among older age groups (P for trend = 0.003). The trend was particularly prominent among HFpEF patients. Non‐CVDs outnumbered CVDs as the primary cause of death in patients with HFpEF (the proportion of CVDs; young: 50.0%, older: 41.2%, very old: 38.2%), whereas CVDs consistently accounted for more than half of all‐cause deaths across all age groups in non‐HFpEF patients (young: 65.0%, older: 64.3%, very old: 58.6%). Furthermore, when non‐HFpEF patients were divided into HFmrEF and HFrEF subgroup, the proportion of CVDs was higher in HFrEF (young: 68.8%, older: 65.0%, very old: 57.5%) than in HFmrEF (young: 50.0%, old: 62.2%, very old: 41.2%) across all age groups (Figure 4 ).
Figure 3.
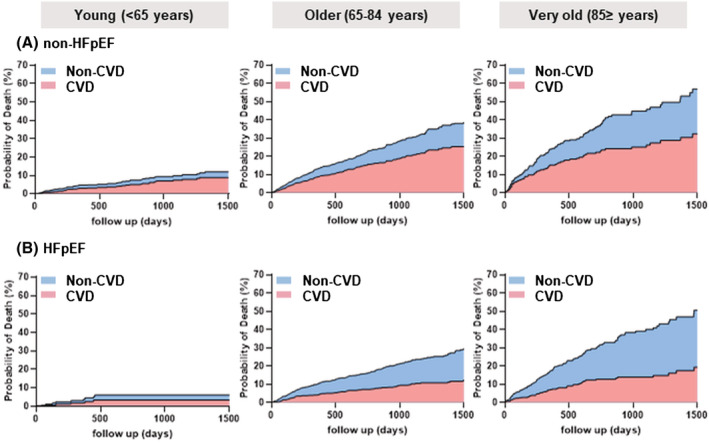
Incidence of CVD and non‐CVD. (A) Non‐HFpEF and (B) HFpEF. The cumulative incidence was estimated by the Fine–Gray model.
Table 2.
Comparison of clinical outcomes
| Mode of death | Non‐HFpEF (LVEF <50%) | HFpEF (LVEF ≥50%) | ||||||
|---|---|---|---|---|---|---|---|---|
|
Young N = 575 |
Older N = 1159 |
Very old N = 319 |
P‐value |
Young N = 182 |
Older N = 894 |
Very old N = 429 |
P‐value | |
| All‐cause death | 60 (10.4) | 308 (26.6) | 117 (36.7) | <0.001 | 10 (5.5) | 187 (20.9) | 136 (31.7) | <0.001 |
| CVD | 39 (6.8) | 198 (17.1) | 65 (20.4) | <0.001 | 5 (2.7) | 77 (8.6) | 52 (12.1) | <0.001 |
| HFD | 18 (3.1) | 104 (9.0) | 44 (13.8) | <0.001 | 2 (1.1) | 40 (4.5) | 29 (6.8) | <0.001 |
| SCD | 15 (2.6) | 75 (6.5) | 15 (4.7) | 0.001 | 3 (1.6) | 23 (2.6) | 14 (3.3) | 0.55 |
| Other‐CVD | 6 (1.0) | 19 (1.6) | 6 (1.9) | 0.52 | 0 | 14 (1.6) | 9 (2.1) | 0.14 |
| Non‐CVD | 21 (3.7) | 110 (9.5) | 52 (16.3) | <0.001 | 5 (2.7) | 110 (12.3) | 84 (19.6) | <0.001 |
| Infection | 2 (0.3) | 28 (2.4) | 13 (4.1) | <0.001 | 2 (1.1) | 27 (3.0) | 19 (4.4) | 0.09 |
| Malignancy | 3 (0.5) | 26 (2.2) | 12 (3.8) | <0.001 | 0 | 27 (3.0) | 13 (3.0) | 0.03 |
| Fatal bleeding | 2 (0.3) | 4 (0.3) | 1 (0.3) | >0.99 | 1 (0.5) | 3 (0.3) | 4 (0.9) | 0.27 |
| Digestive disease | 0 | 2 (0.2) | 2 (0.6) | 0.14 | 1 (0.5) | 4 (0.4) | 3 (0.7) | 0.78 |
| Respiratory failure | 1 (0.2) | 9 (0.8) | 0 | 0.14 | 0 | 13 (1.5) | 4 (0.9) | 0.27 |
| Renal failure | 0 | 3 (0.3) | 2 (0.6) | 0.17 | 0 | 3 (0.3) | 7 (1.6) | 0.02 |
| Others | 8 (1.4) | 32 (2.8) | 16 (5.0) | <0.001 | 1 (0.5) | 29 (3.2) | 30 (7.0) | <0.001 |
| Unknown | 5 (0.9) | 6 (0.5) | 6 (1.9) | 0.06 | 0 | 4 (0.4) | 4 (0.9) | 0.38 |
| HF readmission | 96 (16.7) | 345 (29.8) | 319 (38.6) | <0.001 | 33 (18.1) | 232 (26.0) | 135 (31.5) | 0.002 |
CVD, cardiovascular death; HFD, heart failure death; SCD, sudden cardiac death.
Values are n (%).
Figure 4.
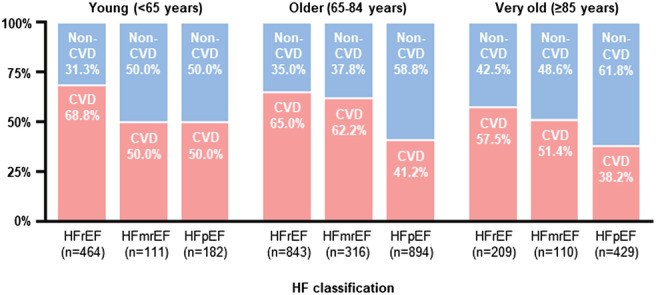
Proportion of CVD stratified by age and LVEF categories.
Table 2 and Figure S4 detail the cause‐specific mortalities among the age groups. HFD and SCD were the leading and second leading causes of CVDs across all age groups, regardless of the LVEF status. HFD occurred more frequently with increasing age, and the incidence was substantially higher in patients with non‐HFpEF than in those with HFpEF of the same age (Figure S5 ). The 2‐year crude incidences of SCD in non‐HFpEF and HFpEF categories were 5.1% and 2.7%, respectively. Further, the incidence of SCD in the non‐HFpEF category was significantly higher in the older group than that in the younger and very old groups, whereas its incidence did not differ in the HFpEF category. The other‐CVD incidence was relatively lower than SCD and HFD and was not associated with age in either LVEF category. Deaths due to infection and malignancy were the leading and second leading causes of non‐CVDs in older and very‐old groups.
Predictors of all‐cause death, CVD, and non‐CVD
HF readmission was the strongest predictor of CVD in both non‐HFpEF [subdisributional hazard ratio (sHR) 2.68, 95% confidence interval (CI) 2.09–3.45, P < 0.001] and HFpEF (sHR 1.86, 95% CI 1.30–2.65, P < 0.001) (Figure 5 A,B , respectively). On the contrary, HF readmission remained the significant predictor of all‐cause death in non‐HFpEF (HR 1.72, 95% CI 1.41–2.09, P < 0.001), but not in HFpEF (HR 1.12, 95% CI 0.90–1.65, P = 0.19) (Table 3 ). In addition, we found a significant interaction between HF readmission and age categories for all‐cause death in non‐HFpEF (interaction P < 0.001), but not in HFpEF (interaction P = 0.48).
Figure 5.
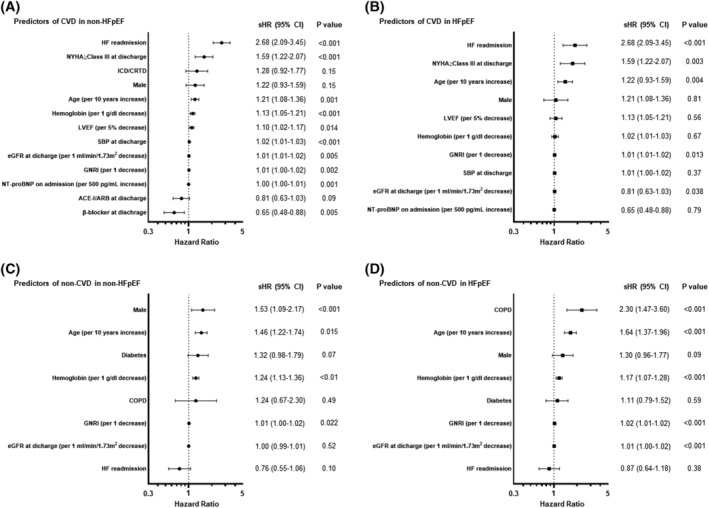
Predictors of cardiovascular and non‐cardiovascular mortality. (A) Predictors of CVD in non‐HFpEF. (B) Predictors of CVD in HFpEF. (C) Predictors of non‐CVD in non‐HFpEF. (D) Predictors of non‐CVD in HFpEF. Covariates: (i) CVD: age, sex, LVEF, NT‐proBNP level at admission, HF readmission, the NYHA functional class at discharge, and levels of SBP, haemoglobin, eGFR, and GNRI at discharge. When analysing patients with non‐HFpEF, we also added the prescription of ACE‐inhibitors/ARBs and β‐blockers at discharge as covariates in the model. (ii) Non‐CVD: age, sex, COPD, HF readmission, and levels of haemoglobin, eGFR, and GNRI at discharge.
Table 3.
Independent predictors of all‐cause death
| Variables | Non‐HFpEF | HFpEF | ||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Age (per 10 years increase) | 1.36 (1.23–1.50) | <0.001 | 1.62 (1.39–1.88) | <0.001 |
| Male (vs. female) | 1.50 (1.18–1.90) | <0.001 | 1.12 (0.85–1.47) | 0.41 |
| GNRI at discharge (per 1 decrease) | 1.01 (1.00–1.02) | <0.001 | 1.02 (1.01–1.03) | <0.001 |
| SBP at discharge (per 1 mmHg decrease) | 1.02 (1.01–1.02) | <0.001 | 1.00 (0.99–1.01) | 0.38 |
| LVEF (per 5% decrease) | 1.08 (1.02–1.14) | 0.009 | 1.08 (0.97–1.20) | 0.14 |
| NT‐proBNP at admission (per 500 pg/mL increase) | 1.00 (1.00–1.01) | <0.001 | 1.00 (0.99–1.01) | 0.20 |
| eGFR at discharge (per 1 mL/min/1.73 m2 increase) | 1.01 (1.00–1.01) | 0.011 | 1.01 (1.00–1.02) | 0.004 |
| Haemoglobin at discharge (per 1 g/dL decrease) | 1.20 (1.13–1.27) | <0.001 | 1.15 (1.07–1.24) | <0.001 |
| NYHA classification at discharge ≥III | 1.66 (1.35–2.04) | <0.001 | 1.65 (1.30–2.11) | <0.001 |
| HF readmission | 1.72 (1.41–2.09) | <0.001 | 1.12 (0.88–1.43) | 0.34 |
| Diabetes | 1.31 (1.07–1.60) | 0.009 | 1.12 (0.87–1.46) | 0.38 |
| Atrial fibrillation | 0.95 (0.78–1.16) | 0.59 | 1.12 (0.87–1.43) | 0.39 |
| Previous stroke | 0.97 (0.73–1.28) | 0.82 | 1.22 (0.90–1.65) | 0.19 |
| Hyperuricemia | 1.01 (0.83–1.23) | 0.91 | 1.17 (0.92–1.51) | 0.20 |
| COPD | 1.57 (1.05–2.33) | 0.026 | 1.59 (1.04–2.44) | 0.032 |
| History of smoking | 1.00 (0.81–1.24) | 0.97 | 1.13 (0.85–1.50) | 0.39 |
| ACE‐I/ARB (at discharge) | 0.75 (0.62–0.91) | 0.004 | ||
| β‐Blocker (at discharge) | 0.67 (0.53–0.86) | 0.002 | ||
| ICD/CRT‐D (at discharge) | 0.99 (0.73–1.34) | 0.94 | ||
ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; CI, confidence interval; COPD; chronic obstructive pulmonary disease; CVD, cardiovascular death; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, hazard ratio; ICD, implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SBP, systolic blood pressure.
Regardless of the LVEF categories, age, lower levels of GNRI, haemoglobin, eGFR, and NYHA functional class ≥III were significant predictors of both all‐cause death and CVD. On the contrary, lower LVEF and higher NT‐proBNP levels were associated with CVD in non‐HFpEF, but not in HFpEF. Regarding the non‐CVD, age, lower levels of haemoglobin, eGFR, and GNRI were significant predictors across the LVEF categories (Figure 5 C,D ). The presence of COPD was significant predictor of non‐CVD in HFpEF (sHR 2.30 95% CI 1.47–3.60, P < 0.001), but not in non‐HFpEF (sHR 1.24, 95% CI 0.67–2.30, P = 0.49).
Discussion
This study analysed cause‐specific mortality based on age and LVEF classification. Our major findings were as follows: (i) patients with non‐HFpEF had higher mortality than those with HFpEF for each age group; (ii) for patients in the non‐HFpEF category, CVDs accounted for more than half of the deaths across age groups, and the proportion of CVDs was higher than in patients with HFpEF; (iii) the proportion of CVDs gradually decreased with increasing age, regardless of the LVEF status; (iv) HF readmission was associated with subsequent all‐cause death in patients with non‐HFpEF, but not in those with HFpEF; and (v) age, lower haemoglobin level, and poor nutritional status were associated with non‐CVD across the LVEF categories.
As older patients have been traditionally recruited less often in randomized controlled trials, there remains inconsistent evidence regarding the optimal therapeutic approach. 3 , 4 , 13 In our study, although CVDs were the primary mode of death for patients with non‐HFpEF (HFmrEF and HFrEF), its proportion decreased gradually with age, probably owing to the increasing burden of non‐cardiac co‐morbidities and frailty. 19 , 20 , 21 , 22 However, older non‐HFpEF patients had more SCDs, the second leading cause of CVDs, compared with the young patients. These findings underscore the fact that even though the absolute benefit of HF therapies may be less for older patients compared with that for young patients, it is still important to apply the therapies if they can be delivered at an acceptable cost. Given the growing burden of older patients with HF, 3 , 22 , 23 our findings highlight the need for novel strategies that can assist in facilitating optimal disease‐specific pharmacotherapies.
In contrast to non‐HFpEF patients, the majority of patients with HFpEF in our study died from non‐CV causes, especially in older age groups. The proportion of non‐CVD seemed to be higher than that in previous randomized controlled trials for HFpEF, 24 , 25 although the difference can be partially explained by the fact that epidemiological studies, such as ours, enrol more older patients with non‐cardiac co‐morbidities than those in previous clinical trials. 26 Previously, a joint analysis of three large‐scale trials targeting HFpEF highlighted the heterogeneity of HFpEF by age, including HFpEF in younger patients with obesity and older patients with non‐cardiac co‐morbidities. 27 Among the patients who died, older patients with non‐cardiac co‐morbidities died more often from non‐CV causes, 3 , 27 consistent with the results of the present study. Although the therapeutic effects of novel pharmacotherapies are known to extend to patients in the higher LVEF range, 8 our findings suggest that their absolute benefit may be attenuated in older patients given the higher incidence of non‐CVD, a competing risk for therapies that focus on reducing CV mortality. The absolute benefit will be expected to become smaller given lower CVD and higher non‐CVD rates. This has important implications for the clinical and economic value of therapies that target CVD morbidity among HFpEF populations, whereas a given therapy's effectiveness may be substantially higher among HFrEF patients. Collectively, a challenge is to markedly improve the prognosis of HFpEF patients by simply applying therapeutic interventions targeting cardiac co‐morbidities.
We found an association between lower haemoglobin levels and non‐CVD, regardless of the LVEF categories, emphasizing the importance of screening for causes of anaemia. 3 This is probably related to the fact that anaemia reflects more co‐morbidities faced by patients (e.g. cancer, chronic renal failure, infections, iron deficiency, or malnutrition). 3 In addition, the post hoc analysis of the DAPA‐HF (Dapagliflozin And Prevention of Adverse‐outcomes in Heart Failure) study revealed that resolution of anaemia was better achieved with dapagliflozin than with placebo and patients with resolution had better outcomes than those without. 28 Further investigations are needed to assess its feasibility in a broader range of patients with HF. Additionally, in line with previous studies, 29 , 30 a lower GNRI value was consistently associated with the increased risk for both CVD and non‐CVD, suggesting the importance of nutritional assessments in all patients with HF. On the contrary, given the optimal management for patients with HF with malnutrition is still under discussion, there is a need to develop multimodal dietary interventions. 31
The association between HF readmission and CV mortality in both HFpEF and non‐HFpEF is consistent with previously published studies. 32 Thus, efforts targeting the prevention of readmission remain crucial, regardless of the LVEF category. Meanwhile, we found that the association between HF readmission and all‐cause mortality varied based on the LVEF category, indicating that reduction in HF readmission would be a weaker surrogate for overall benefits in HFpEF patients than in non‐HFpEF patients. For HFpEF, hospitalization due to non‐CV causes likely has a stronger association with all‐cause death given the higher risk of non‐CVD. Thus, the implementation of integrated risk management is warranted to decrease the burden of non‐CV events among HFpEF patients. For instance, the presence of chronic application of specific treatment measures is frequently challenging, driven by the fact that most HFpEF patients are older and have impaired physical status and cognitive function and frailty. 22 In this context, our findings underscore the importance of a multiple domain approach, including medical, emotional, physical function, and social environmental. 3 , 4 , 22 A recent randomized clinical trial revealed that the rehabilitation intervention improved physical function compared with usual care even in older HFpEF patients. 33 Nevertheless, further investigations are needed for optimizing the integrated care for HFpEF patients.
Our research has several limitations. First, this was a multicentre observational study. Because the treatment strategy for HF was not predetermined, the treatment varied according to the physicians and medical centres. In addition, similar to previous reports, the present study included only patients who could be followed up and whose mode of death data were obtained, potentially leading to selection bias. Second, we could not observe the temporal trends in data on the LVEF and the medications taken due to these data not being collected after discharge. Particularly, considering the effects of the LVEF trajectory on clinical outcomes, 5 we could not exclude LVEF changes that affected the modes of death. Third, we could not assess the significant factors relevant to the clinical outcomes within patients with HF, such as the prevalence of frailty, 21 , 22 sarcopenia, 3 , 22 iron deficiency, 3 , 34 and cancer. 3 This limitation would have affected the findings with the predictors of deaths in this study. Fourth, the data on the incidence of deaths due to pulmonary embolism were not available for this study. Although the incidence of pulmonary embolism in Japan was about 20% of that of the United States, 1 , 35 , 36 not including it in our analysis may have underestimated the incidence of CVD proportion. Finally, the majority of participants enrolled in this registry were Asian patients. Previous multinational studies have demonstrated substantial regional differences in clinical outcomes and prescribing patterns of guideline‐directed medical therapy. 37 , 38 Thus, racial or environmental differences should be considered when translating these findings in other countries. However, we would like to emphasize that our registry included a very large number of elderly patients with distinct phenotypes; additionally, we conducted an objective assessment of 2‐year outcomes and offered novel insights into addressing serious health concerns in a markedly increased population of elderly patients with HF.
Conclusions
The 2‐year incidence of CVD and non‐CVD varied substantially by age and LVEF status. Our findings indicate that mitigating CVDs alone is insufficient, highlighting the challenge of treating HF in the older population. The traditional clinical trials in HF have focused on CV outcomes, and caution is warranted in applying the results in real‐world scenarios, particularly in patients with HFpEF.
Conflict of interest
Dr Shiraishi is affiliated with an endowed department by Nippon Shinyaku Co., Ltd., Medtronic Japan Co., Ltd., and BIOTRONIK JAPAN Inc. and received research grants from the SECOM Science and Technology Foundation and the Uehara Memorial Foundation and honoraria from Otsuka Pharmaceutical Co., Ltd. and Ono Pharmaceutical Co., Ltd.; Dr Kohsaka received an unrestricted research grant from Daiichi Sankyo Co., Ltd., and Novartis Pharmaceutical Co., Ltd. The remaining authors have no conflict of interest to disclose. ATS receives research support from the National Heart, Lung, and Blood Institute (1K23HL151672‐01).
Funding
This work was supported by a Grant‐in‐Aid for Young Scientists (Japan Society for the Promotion of Science KAKENHI, 18K15860), a Grant‐in‐Aid for Scientific Research (23591062, 26461088, 21K08064, 17K09526, 16KK0186, and 16H05215), a Health Labour Sciences Research Grant (14528506), the Sakakibara Clinical Research Grant for Promotion of Sciences (2012–2020), and a grant from the Japan Agency for Medical Research and Development (201439013C).
Supporting information
Figure S1. Study flowchart.
Figure S2. Comparison of incidence of all‐cause death between enrolled in 2006‐2012 and 2013‐2017 in non‐HFpEF.
Figure S3. Comparison of incidence of all‐cause death between enrolled in 2006‐2012 and 2013‐2017 in HFpEF.
Figure S4. Comparison of modes of death among three age groups. (A) non‐HFpEF, (B) HFpEF.
Figure S5. Incidence of each mode of death.
Acknowledgements
The authors are grateful to the members of the WET‐HF investigators.
Nakamaru, R. , Shiraishi, Y. , Sandhu, A. T. , Heidenreich, P. A. , Shoji, S. , Kohno, T. , Takei, M. , Nagatomo, Y. , Nakano, S. , Kohsaka, S. , and Yoshikawa, T. (2023) Cardiovascular vs. non‐cardiovascular deaths after heart failure hospitalization in young, older, and very old patients. ESC Heart Failure, 10: 673–684. 10.1002/ehf2.14245.
References
- 1. Aparicio HJ, Benjamin EJ, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang N‐Y, Tsao CW, on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke Statistics‐2021 update a report from the American Heart Association. Circulation 2021; 143: e254–e743. [DOI] [PubMed] [Google Scholar]
- 2. Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, Abbasi‐Kangevari M, Abbastabar H, Abd‐Allah F, Abdelalim A, Abdollahi M. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020; 396: 1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group , de Boer RA, Christian Schulze P, Abdelhamid M, Aboyans V, Adamopoulos S, Anker SD, Arbelo E, Asteggiano R, Bauersachs J, Bayes‐Genis A, Borger MA, Budts W, Cikes M, Damman K, Delgado V, Dendale P, Dilaveris P, Drexel H, Ezekowitz J, Falk V, Fauchier L, Filippatos G, Fraser A, Frey N, Gale CP, Gustafsson F, Harris J, Iung B, Janssens S, Jessup M, Konradi A, Kotecha D, Lambrinou E, Lancellotti P, Landmesser U, Leclercq C, Lewis BS, Leyva F, Linhart A, Løchen ML, Lund LH, Mancini D, Masip J, Milicic D, Mueller C, Nef H, Nielsen JC, Neubeck L, Noutsias M, Petersen SE, Sonia Petronio A, Ponikowski P, Prescott E, Rakisheva A, Richter DJ, Schlyakhto E, Seferovic P, Senni M, Sitges M, Sousa‐Uva M, Tocchetti CG, Touyz RM, Tschoepe C, Waltenberger J, Adamo M, Baumbach A, Böhm M, Burri H, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gardner RS, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Piepoli MF, Price S, Rosano GMC, Ruschitzka F, Skibelund AK. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 4. Writing Committee , Maddox TM, Januzzi JL Jr, Allen LA, Breathett K, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Lindenfeld J, Masoudi FA, Motiwala SR, Oliveros E, Patterson JH, Walsh MN, Wasserman A, Yancy CW, Youmans QR. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2021; 77: 772–810. [DOI] [PubMed] [Google Scholar]
- 5. Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, Anker SD, Atherton J, Böhm M, Butler J, Drazner MH, Felker GM, Filippatos G, Fonarow GC, Fiuzat M, Gomez‐Mesa J–E, Heidenreich P, Imamura T, Januzzi J, Jankowska EA, Khazanie P, Kinugawa K, Lam CSP, Matsue Y, Metra M, Ohtani T, Francesco Piepoli M, Ponikowski P, Rosano GMC, Sakata Y, SeferoviĆ P, Starling RC, Teerlink JR, Vardeny O, Yamamoto K, Yancy C, Zhang J, Zieroth S. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition. J Card Fail 2021; 27: 387–413. [DOI] [PubMed] [Google Scholar]
- 6. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen D, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin‐Colet J, Cleland J, Düngen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP, PARAGON‐HF Investigators and Committees . Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019; 381: 1609–1620. [DOI] [PubMed] [Google Scholar]
- 7. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner–la Rocca HP, Choi DJ, Chopra V, Chuquiure‐Valenzuela E, Giannetti N, Gomez‐Mesa JE, Janssens S, Januzzi JL, Gonzalez‐Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021; 385: 1451–1461. [DOI] [PubMed] [Google Scholar]
- 8. Lam CSP, Solomon SD. Classification of heart failure according to ejection fraction: JACC review topic of the week. J Am Coll Cardiol 2021; 77: 3217–3225. [DOI] [PubMed] [Google Scholar]
- 9. Metra M, Mentz RJ, Chiswell K, Bloomfield DM, Cleland JGF, Cotter G, Davison BA, Dittrich HC, Fiuzat M, Givertz MM, Lazzarini V, Mansoor GA, Massie BM, Ponikowski P, Teerlink JR, Voors AA, O'Connor CM. Acute heart failure in elderly patients: worse outcomes and differential utility of standard prognostic variables. Insights from the PROTECT trial. Eur J Heart Fail 2015; 17: 109–118. [DOI] [PubMed] [Google Scholar]
- 10. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJV, Rahimi K. Temporal trends and patterns in heart failure incidence: a population‐based study of 4 million individuals. Lancet 2018; 391: 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goyal P, Sauer AJ, Rich MW. All‐cause mortality as an end point for heart failure with preserved ejection fraction: underperformance or overambitious? J Card Fail 2022; 28: 863–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kitakata H, Kohno T, Kohsaka S, Shiraishi Y, Parizo JT, Niimi N, Goda A, Nishihata Y, Heidenreich PA, Yoshikawa T. Prognostic implications of early and midrange readmissions after acute heart failure hospitalizations: a report from a Japanese multicenter registry. J Am Heart Assoc 2020; 9: e014949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Akita K, Kohno T, Kohsaka S, Shiraishi Y, Nagatomo Y, Izumi Y, Goda A, Mizuno A, Sawano M, Inohara T, Fukuda K, Yoshikawa T, West Tokyo Heart Failure Registry Investigators . Current use of guideline‐based medical therapy in elderly patients admitted with acute heart failure with reduced ejection fraction and its impact on event‐free survival. Int J Cardiol 2017; 235: 162–168. [DOI] [PubMed] [Google Scholar]
- 14. Ho KKL, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham heart study subjects. Circulation 1993; 88: 107–115. [DOI] [PubMed] [Google Scholar]
- 15. Bauer T, Zeymer U, Hochadel M, Möllmann H, Weidinger F, Zahn R, Nef HM, Hamm CW, Marco J, Gitt AK. Use and outcomes of multivessel percutaneous coronary intervention in patients with acute myocardial infarction complicated by cardiogenic shock (from the EHS‐PCI registry). Am J Cardiol 2012; 109: 941–946. [DOI] [PubMed] [Google Scholar]
- 16. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR . Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 17. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C. Geriatric nutritional risk index: a new index for evaluating at‐risk elderly medical patients. Am J Clin Nutr 2005; 82: 777–783. [DOI] [PubMed] [Google Scholar]
- 18. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, Limacher MC, Mahaffey KW, Mehran R, Nissen SE, Smith EE, Targum SL. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association task force on clinical data standards (writing committee to develop cardiovascular endpoints data standards). Circulation 2015; 28: 302–361. [DOI] [PubMed] [Google Scholar]
- 19. Loungani RS, Teerlink JR, Metra M, Allen LA, Butler J, Carson PE, Chen CW, Cotter G, Davison BA, Eapen ZJ, Filippatos GS, Gimpelewicz C, Greenberg B, Holbro T, Januzzi JL Jr, Lanfear DE, Pang PS, Piña IL, Ponikowski P, Miller AB, Voors AA, Felker GM. Cause of death in patients with acute heart failure: insights from RELAX‐AHF‐2. JACC Hear Fail 2020; 8: 999–1008. [DOI] [PubMed] [Google Scholar]
- 20. Ueda T, Kawakami R, Horii M, Sugawara Y, Matsumoto T, Okada S, Nishida T, Soeda T, Okayama S, Somekawa S, Takeda Y, Watanabe M, Kawata H, Uemura S, Saito Y. Noncardiovascular death, especially infection, is a significant cause of death in elderly patients with acutely decompensated heart failure. J Card Fail 2014; 20: 174–180. [DOI] [PubMed] [Google Scholar]
- 21. Sze S, Pellicori P, Zhang J, Weston J, Squire IB, Clark AL. Effect of frailty on treatment, hospitalisation and death in patients with chronic heart failure. Clin Res Cardiol 2021; 110: 1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gorodeski EZ, Goyal P, Hummel SL, Krishnaswami A, Goodlin SJ, Hart LL, Forman DE, Wenger NK, Kirkpatrick JN, Alexander KP, Geriatric Cardiology Section Leadership Council, American College of Cardiology . Domain management approach to heart failure in the geriatric patient: present and future. J Am Coll Cardiol 2018; 71: 1921–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shiraishi Y, Kohsaka S, Sato N, Takano T, Kitai T, Yoshikawa T, Matsue Y. 9‐year trend in the management of acute heart failure in Japan: a report from the national consortium of acute heart failure registries. J Am Heart Assoc 2018; 7: e008687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kitai T, Miyakoshi C, Morimoto T, Yaku H, Murai R, Kaji S, Furukawa Y, Inuzuka Y, Nagao K, Tamaki Y, Yamamoto E, Ozasa N, Tang WHW, Kato T, Kimura T. Mode of death among Japanese adults with heart failure with preserved, midrange, and reduced ejection fraction. JAMA Netw Open 2020; 3: e204296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vaduganathan M, Patel RB, Michel A, Shah SJ, Senni M, Gheorghiade M, Butler J. Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol 2017; 69: 556–569. [DOI] [PubMed] [Google Scholar]
- 26. Chan MMY, Lam CSP. How do patients with heart failure with preserved ejection fraction die? Eur J Heart Fail 2013; 15: 604–613. [DOI] [PubMed] [Google Scholar]
- 27. Tromp J, Shen L, Jhund PS, Anand IS, Carson PE, Desai AS, Granger CB, Komajda M, McKelvie RS, Pfeffer MA, Solomon SD, Køber L, Swedberg K, Zile MR, Pitt B, Lam CSP, McMurray JJV. Age‐related characteristics and outcomes of patients with heart failure with preserved ejection fraction. J Am Coll Cardiol 2019; 74: 601–612. [DOI] [PubMed] [Google Scholar]
- 28. Docherty KF, Curtain JP, Anand IS, Bengtsson O, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Ponikowski P, Sabatine MS, Schou M, Sjöstrand M, Solomon SD, Jhund PS, McMurray JJV, DAPA‐HF Investigators and Committees . Effect of dapagliflozin on anaemia in DAPA‐HF. Eur J Heart Fail 2021; 23: 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C, Lau CP, van Gelder IC, Hohnloser SH, Carlson M, Fain E, Nakamya J, Mairesse GH, Halytska M, Deng WQ, Israel CW, Healey JS. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation 2014; 129: 2094–2099. [DOI] [PubMed] [Google Scholar]
- 30. Minamisawa M, Seidelmann SB, Claggett B, Hegde SM, Shah AM, Desai AS, Lewis EF, Shah SJ, Sweitzer NK, Fang JC, Anand IS, O'Meara E, Rouleau JL, Pitt B, Solomon SD. Impact of malnutrition using geriatric nutritional risk index in heart failure with preserved ejection fraction. JACC Hear Fail 2019; 7: 664–675. [DOI] [PubMed] [Google Scholar]
- 31. Driggin E, Cohen LP, Gallagher D, Karmally W, Maddox T, Hummel SL, Carbone S, Maurer MS. Nutrition assessment and dietary interventions in heart failure: JACC review topic of the week. J Am Coll Cardiol 2022; 79: 1623–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bello NA, Claggett B, Desai AS, McMurray JJV, Granger CB, Yusuf S, Swedberg K, Pfeffer MA, Solomon SD. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail 2014; 7: 590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kitzman DW, Whellan DJ, Duncan P, Pastva AM, Mentz RJ, Reeves GR, Nelson MB, Chen H, Upadhya B, Reed SD, Espeland MA, Hewston LA, O'Connor CM. Physical rehabilitation for older patients hospitalized for heart failure. N Engl J Med 2021; 385: 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation 2018; 138: 80–98. [DOI] [PubMed] [Google Scholar]
- 35. White RH. The epidemiology of venous thromboembolism. Circulation 2003; 107: 4–8. [DOI] [PubMed] [Google Scholar]
- 36. Sakuma M, Nakamura M, Yamada N, Ota S, Shirato K, Nakano T, Ito M, Kobayashi T. Venous thromboembolism: deep vein thrombosis with pulmonary embolism, deep vein thrombosis alone, and pulmonary embolism alone. Circ J 2009; 73: 305–309. [DOI] [PubMed] [Google Scholar]
- 37. Teng THK, Tromp J, Tay WT, Anand I, Ouwerkerk W, Chopra V, Wander GS, Yap JJ, MacDonald M, Xu CF, Chia YM, Shimizu W, ASIAN‐HF investigators , Richards AM, Voors A, Lam CS. Prescribing patterns of evidence‐based heart failure pharmacotherapy and outcomes in the ASIAN‐HF registry: a cohort study. Lancet Glob Health 2018; 6: e1008–e1018. [DOI] [PubMed] [Google Scholar]
- 38. Tromp J, Ouwerkerk W, Teng THK, Cleland JGF, Bamadhaj S, Angermann CE, Dahlstrom U, Tay WT, Dickstein K, Ertl G, Hassanein M, Perrone SV, Ghadanfar M, Schweizer A, Obergfell A, Collins SP, Filippatos G, Lam CSP. Global disparities in prescription of guideline‐recommended drugs for heart failure with reduced ejection fraction. Eur Heart J 2022; 43: 2224–2234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study flowchart.
Figure S2. Comparison of incidence of all‐cause death between enrolled in 2006‐2012 and 2013‐2017 in non‐HFpEF.
Figure S3. Comparison of incidence of all‐cause death between enrolled in 2006‐2012 and 2013‐2017 in HFpEF.
Figure S4. Comparison of modes of death among three age groups. (A) non‐HFpEF, (B) HFpEF.
Figure S5. Incidence of each mode of death.


