Abstract
Aims
Heart failure (HF) impairs all aspects of health‐related quality of life (HRQoL), but little is known about the effect of developing HF on HRQoL over time. We aimed to report changes in HRQoL over a 13‐year period.
Methods and results
HRQoL was measured in the Echocardiographic Heart of England Screening (ECHOES) study and the ECHOES‐X follow‐up study (N = 1618) using the SF‐36 questionnaire (Version 1). Mixed modelling compared changes in HRQoL across diagnostic groups, adjusting for potential predictors and design variables. Patients who had developed HF with reduced ejection fraction (HFrEF) or HF with preserved ejection fraction (HFpEF) at rescreening had significantly greater reduction in physical functioning (PF) and role physical (RP) scores compared with those without HF; adjusted mean difference in PF: HFrEF −16.1, [95% confidence interval (CI) −22.2 to −10.1]; HFpEF −14.6, (95% CI −21.2 to −8.1); in RP: HFrEF −20.7, (95% CI −31.8 to −9.7); HFpEF −19.3, (95% CI −31.0 to −7.6). Changes in HRQoL of those with a HF diagnosis at baseline and rescreen, with exception of role emotion, were similar to those without HF but started from a much lower baseline score.
Conclusions
People with a new diagnosis of HF at rescreening had a significant reduction in HRQoL. Conversely, for those with HF detected on initial screening, little change was observed in HRQoL scores on rescreening. Further research is required to understand the development of HF over time and to test interventions designed to prevent decline in HRQoL, potentially through earlier diagnosis and treatment optimization.
Keywords: Heart failure, Primary care, Quality of life, SF‐36
Background
Heart failure (HF) is a common and complex clinical syndrome affecting over 40 million people globally and associated with significant mortality, morbidity, and costs. 1 , 2 Symptoms of HF such as shortness of breath, fatigue, and ankle swelling can significantly impact an individuals' health‐related quality of life (HRQoL). Despite new and effective treatments, people with HF suffer lower HRQoL compared with the general population, with physical health burden greater than that observed in other chronic conditions. 3 Worse HRQoL is also an independent predictor of hospitalization and death in those with preserved and reduced ejection fraction and in mild to severe symptomatic HF. 4
The Short‐Form 36 (SF‐36) and the Minnesota Living with Heart Failure Questionnaire (MLHFQ) are, by far, the most commonly used general and HF‐specific HRQoL tools evaluated in chronic HF research. 5 , 6 The generic EQ‐5D and condition‐specific Kansas City Cardiomyopathy Questionnaire (KCCQ) increasingly being used in European randomized controlled trials in HF. 7
A meta‐analysis of 14 studies (2034 patients), utilizing the SF‐36, reported reduced physical HRQoL in people with a diagnosis of HF compared with the US population norms (mean = 50, SD = 10), with overall pooled means for the physical component score of 33.1 [95% confidence interval (CI) 31.9–34.7] and mental component score of 50.6 (95% CI 43.8–57.4). 6 Among individual dimensions, physical functioning was identified as having the lowest mean scores (40.5) and social functioning with the highest (64.8). 6
The majority of studies of HRQoL in HF report on short term changes in HRQoL after diagnosis. A systematic review of HRQoL reporting in clinical trials found nearly half of all trials measured HRQoL over a period of 3 months or less. 8 Changes in HRQoL over short periods following HF diagnosis have also been explored in observational research; however, there are a lack of data exploring long‐term changes in HRQoL and more specifically a lack of data associated with changes in health status.
We aimed to examine the long term changes in QoL of participants recruited to a large UK community screening study for HF and its rescreening study over a decade later. 9 , 10 These linked studies enable evaluation of changes in HRQoL of individuals with long‐term HF and newly diagnosed HF and those where HF was not identified by screening.
Methods
Data source and study population
Full details of the ECHOES and ECHOES‐X studies are described elsewhere. 9 , 10 In brief, the Echocardiographic Heart of England Screening (ECHOES) study recruited 6,162 patients, aged 45 years and over, from 16 general practices in the West Midlands region of the UK between 1995 and 1999. Patients were randomly sampled from the general population and from three subgroups identified from general practice registers [pre‐existing diagnosis of HF; on prescribed diuretics; high risk of HF (history of previous myocardial infarction, angina, hypertension, or diabetes)]. The study estimated the community prevalence of HF and left ventricular systolic dysfunction and evaluated the quality of life of participants, via the SF‐36 health status questionnaire (version 1). 3 A longitudinal follow‐up of the ECHOES cohort, the ECHOES‐X study, took place over a decade later (2008 to 2011) with 1618 patients rescreened (47% of eligible survivors). During clinic visits, participants were assessed by clinical history and examination, 12‐lead electrocardiogram, and echocardiography, with participants self‐completing the SF‐36. Natriuretic peptide levels were measured from blood samples obtained from a random 10% sample of the ECHOES cohort and all of ECHOES‐X participants.
HF diagnosis
Echocardiography was carried out by a cardiologist or British Society of Echocardiography‐accredited echocardiographer during the clinic visit. Clinical assessment of HF was made by a cardiologist, general practitioner with an interest in cardiovascular disease, or a trained research nurse. HF was defined in accordance with European Society of Cardiology chronic HF guidelines as patients who were symptomatic (shortness of breath, fatigue, ankle oedema) with objective evidence of cardiac dysfunction (left ventricular ejection fraction <40%, or atrial fibrillation or moderate to severe valve disease, or any combination). 11 In addition, participants in ECHOES‐X with reduced ejection fraction (< = 50%) were classified as HFrEF, whereas those with preserved ejection fraction (EF > 50%) and evidence of diastolic dysfunction (E:e′ > 13 or E:e′ 8–13) with LV hypertrophy (IVS > 1.2 cm) or LA enlargement [>4 cm (males); >3.8 cm (females)], significant valve disease or arrhythmia diagnosed as HF with preserved ejection fraction (HFpEF). Adjudication by an expert panel of three clinicians took place where diagnosis was unclear. Natriuretic peptide level was not accounted for in the diagnosis, due to test performance of NP being an objective of ECHOES‐X.
Quality of life questionnaire
The SF‐36 is one of the most widely used and validated generic HRQoL tools, measuring eight domains over a 4‐week recall period [physical functioning (PF); role limitations due to physical problems (RP); role limitations due to emotional problems (RE); mental health (MH); energy and vitality (V); bodily pain (BP); and general health perception (GHP)] and two summary scores [physical component score (PCS) and mental component score (MCS)]. Each domain and summary component score is measured on a 0–100 scale, with a score of 100 indicating the best quality of life. Questionnaire responses were coded and transformed into scales following authors' instructions and summary component scores standardized to UK norms. 3 , 12 Minimum clinically important differences (MCID) in SF‐36 scores (version 2), for persons with HF, are reported to vary by domain from 15 units for PF to 25 units for SF. 13 The MCID for PCS and MCS have not been reported in this clinical group, but a general MCID of between 3 and 5 units has been suggested by Samsa et al. 14
Statistical analysis
Participants were divided into four health status groups based on original and rescreening diagnoses: no HF at baseline and follow‐up (No HF); no HF at baseline and HFrEF at follow‐up (new HFrEF); no HF at baseline and HFpEF at follow‐up (new HFpEF); and HF at baseline and follow‐up (previous HF). Baseline characteristics are presented for participants in each of these four groups with statistical comparisons made using multinomial logistic regression, new HFrEF and new HFpEF groups being combined due to low numbers. A Kruskal–Wallis test was used to compare the median length of follow‐up between the health status groups. Linear mixed modelling analysis was used to compare the effect of HF diagnosis on the change in HRQoL scores over the follow‐up period. Analyses were firstly adjusted for baseline HRQoL score, age, sex, and study design variables and secondly additionally adjusted for factors previously shown to be associated with change in HRQoL. 15 For each of the eight SF‐36 domains and summary scores, the change in HRQoL score was included as the dependent variable and health status as an independent variable with HRQoL score at baseline, sex, age at baseline, and sample cohort as covariates and general practice included as a random effect. Further adjustment included baseline recorded: body mass index category (<25, 25–30, 30 + kg/m2), arthritis, diabetes, and depression as additional covariates. 15 Comparisons of adjusted mean change in scores were made between the different HF status groups and those with no HF. To allow for multiple testing, the Dunnett–Hui adjustment was applied to confidence intervals and P values of differences in adjusted mean scores between HF diagnostic groups and those with no HF. Bootstrapped resampling (1000 replications) was performed as a sensitivity analysis to test the robustness of results to non‐normality of scores.
Two‐sided P values < 0.05 were considered statistically significant. Statistical analysis was performed on complete cases using SAS version 9.4 and Stata version 15.
Results
The median time between screenings was 13.0 years (interquartile range 12.0–14.2 years). Forty‐four per cent of those screened in ECHOES, including 378 (84.2%) of those with HF, had died during this period, and, of those surviving, 1618 (47%) were re‐screened in ECHOES‐X (Figure 1 ).
Figure 1.
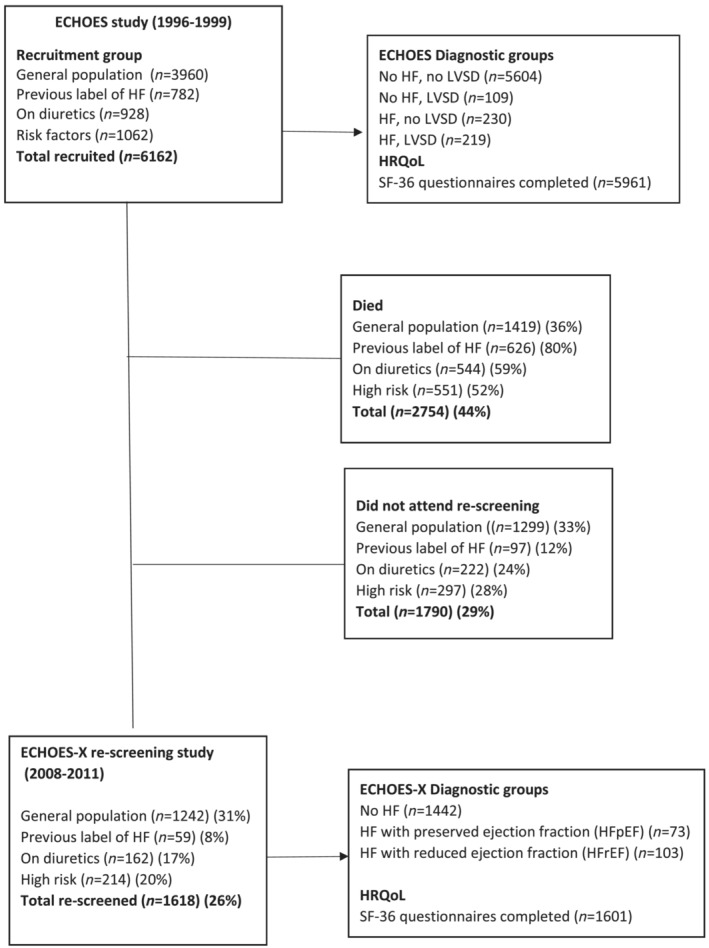
Flow diagram of numbers of patients screened and HRQoL questionnaires completed in ECHOES and ECHOES‐X.
Of those screened, SF‐36 questionnaire completion rates were high with 5961 (96.8%) completed at baseline, 1601 (99.6%) completed at follow‐up and 1596 (98.6%) completed at both screenings. Completion rates varied within individual dimensions, with physical functioning the most completed, ranging from 99.5% at baseline to 97.7% at follow‐up, and role emotional the least complete from 98.1% at baseline to 90.5% at follow‐up. Of those completing both baseline and follow‐up questionnaires, 26 individuals were diagnosed with HF at the baseline screening (previous HF), and a further 150 individuals were identified with HF at rescreening. Eighty‐two of these new 150 cases having heart failure with reduced ejection fraction (new HFrEF) and 68 having heart failure with preserved ejection fraction (new HFpEF). The remaining 1420 participants did not have an HF diagnosis at either screening study (no HF). No difference in length of follow‐up was observed between these four health status groups (P = 0.21).
Those that attended the follow‐up screening were younger (57.8 vs. 64.1 years) and had fewer co‐morbidities than those in the overall screened population at baseline (Table S1 ). They were also more likely to have better baseline physical and mental health (mean PCS 49.2 vs. 45.6; mean MCS 53.5 vs. 51.5).
Baseline characteristics differed between HF status groups; however, few were independently statistically significant when considered concurrently in a multinomial logistic model (Table 1 ). Those with newly diagnosed HF at rescreening were older and more likely to be male; have a history of MI; have shortness of breath; or be taking diuretics, angiotensin‐converting enzyme inhibitors, or angiotensin receptor blockers at baseline, compared with those without HF.
Table 1.
Baseline characteristics of participants completing baseline and follow‐up SF‐36 questionnaire, by health status
| Baseline characteristic | Health status | |||||
|---|---|---|---|---|---|---|
| No HF | New HFpEF | New HFrEF | Previous HF | Total | P value a | |
| Sample size | 1420 | 68 | 82 | 26 | 1596 | |
| Age (years) mean (SD) | 57.0 (7.9) | 65.4 (6.5) | 63.1 (7.7) | 63.0 (5.6) | 57.8 (8.1) | <0.0001 |
| Male (%) | 690 (48.6) | 31 (45.6) | 59 (72.0) | 14 (53.9) | 794 (49.7) | 0.04 |
| Ethnicity: white | 1387 (97.7) | 67 (98.5) | 81 (98.8) | 26 (100) | 1561 (97.8) | 0.96 |
| Body mass index (kg/m2) mean (SD) | 26.3 (4.7) | 27.2 (5.3) | 27.9 (5.2) | 28.0 (7.3) | 26.4 (4.8) | 0.41 |
| Medical history | ||||||
| Hypertension | 373 (26.3) | 39 (57.4) | 33 (40.2) | 13 (50.0) | 458 (28.7) | 0.23 |
| Myocardial infarction | 70 (4.9) | 5 (7.4) | 23 (28.1) | 10 (38.5) | 108 (6.8) | 0.04 |
| Angina | 107 (7.5) | 10 (14.7) | 25 (30.5) | 14 (53.9) | 156 (9.8) | 0.99 |
| Diabetes | 61 (4.3) | 3 (4.4) | 4 (4.9) | 3 (11.5) | 71 (4.5) | 0.54 |
| Arthritis | 87 (6.1) | 11 (16.2) | 9 (10.9) | 6 (23.1) | 113 (7.1) | 0.03 |
| Depression | 26 (1.8) | 3 (4.4) | 0 (0) | 1 (3.9) | 30 (1.9) | 0.82 |
| NYHA class | ||||||
| I | 1284 (90.4) | 48 (70.6) | 61 (74.4) | 0 (0.0) | 1393 (87.3) | 0.85 |
| II | 122 (8.6) | 19 (27.9) | 20 (24.4) | 18 (69.2) | 179 (11.2) | |
| III | 4 (0.3) | 1 (1.5) | 1 (1.2) | 4 (15.4) | 10 (0.6) | |
| IV | 10 (0.7) | 0 (0.0) | 0 (0.0) | 4 (15.4) | 14 (0.9) | |
| Symptoms | ||||||
| Shortness of breath | 218 (15.4) | 25 (36.8) | 32 (39.0) | 26 (100.0) | 301 (18.9) | 0.04 |
| Fatigue | 383 (27.0) | 27 (39.7) | 38 (46.4) | 21 (80.8) | 469 (29.4) | 0.12 |
| Ankle oedema | 305 (21.5) | 28 (41.2) | 23 (28.1) | 10 (38.5) | 366 (23.0) | 0.06 |
| Medications | ||||||
| Diuretics only | 105 (7.4) | 12 (17.7) | 17 (20.7) | 8 (30.8) | 142 (8.9) | <0.0001 |
| BBL only | 99 (7.0) | 6 (8.8) | 9 (11.0) | 3 (11.5) | 117 (7.3) | |
| ACE or ARB | 41 (2.9) | 4 (5.9) | 3 (3.7) | 0 (0.0) | 48 (3.0) | |
| Combination | 88 (6.2) | 21 (30.9) | 18 (22.0) | 10 (38.5) | 137 (8.6) | |
| NTproBNP pg/mL median [IQR], n | 63.0 [31.0–113.7], 131 | 127.1 [67.2–169.3], 6 | 128.4 [66.8–232.6], 6 | 83 [80.0–96.0], 4 | 68.8 [34.2–119], 147 | 0.65 b |
| Original diagnosis (ECHOES) | ||||||
| HF and LVSD | 0 (0) | 0 (0) | 0 (0) | 13 (50.0) | 13 (0.8) | ‐ |
| HF and no LVSD | 0 (0) | 0 (0) | 0 (0) | 13 (50.0) | 13 (0.8) | ‐ |
| No HF and LVSD | 6 (0.4) | 1 (1.5) | 6 (7.3) | 0 (0) | 13 (0.8) | ‐ |
| No HF and no LVSD | 1414(99.6) | 67 (98.5) | 76 (92.7) | 0 (0) | 1557 (97.6) | ‐ |
HF, heart failure; LVSD, left ventricular systolic dysfunction; NYHA class, New York Heart Association functional classification system, I = no symptoms and limitations in normal physical activity; II mild limitations; III marked limitations: IV severe limitations.
P values obtained from a multinomial logistic model, excluding original diagnosis and NT‐proBNP.
Including NT‐proBNP.
Figure 2 illustrates that at baseline, the No HF, new HFrEF, and new HFpEF groups had similar mean scores (above 60), with No HF having the highest scores, ranging from 66.6 (V) to 88.4 (SF). Lowest scores were in the previous HF group with mean scores between 20.2 (RP) and 65.5 (MH). With the exception of GHP and MH, scores declined over time across all health status groups. The largest reduction was observed in mean RP scores of the new HFpEF and new HFrEF groups.
Figure 2.
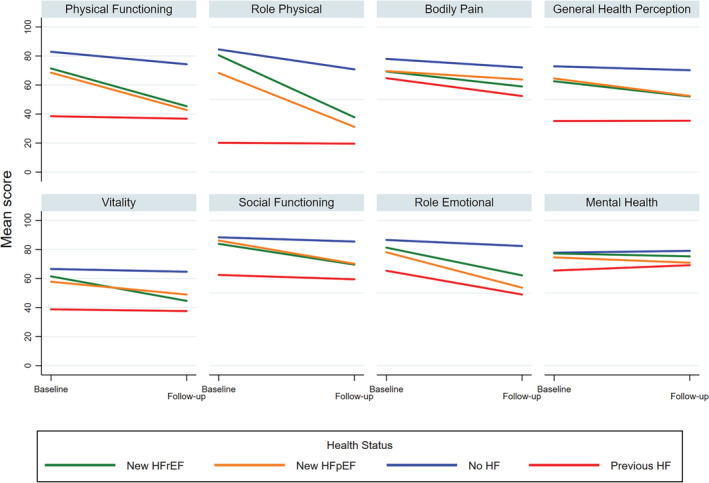
Baseline and follow‐up SF‐36 scores, by domain and health status.
Similar results were observed for the partially and fully adjusted mixed models (Table 2 ). Comparisons of fully adjusted mean change scores between each HF status group and those without HF are shown in Figure 3 . Heterogeneity between the group comparisons was observed, with both new HFrEF and new HFpEF groups having a greater reduction in scores over the follow‐up period than those without HF, whereas adjusted mean score reductions in the previous HF and no HF groups were similar except for the role emotional domain. Persons with newly diagnosed HFrEF had significantly larger reductions in scores than those without HF for all dimensions except BP and MH, with large clinical differences for PF [−16.1 (95% CI −22.2 to −10.1)] and RP [−20.7 (95% CI −31.8 to −9.7)]. Significant differences were also identified between persons with new HFpEF and those without HF for all dimensions except BP, with PF and RP again showing the largest differences of −14.6 (95% CI −21.2 to −8.1) and −19.3 (95% CI −31.0 to −7.6), respectively. A significant difference in RE change scores was identified between those with previous HF and those without HF (−21.7 (95% CI −43.4 to −0.04), P = 0.049). No other differences were observed between these two groups, although it should be noted that change scores for those with previous HF were calculated from a much lower baseline score.
Table 2.
Mean change in SF‐36 scores, by domain
| SF‐36 domain | Diagnosis at baseline | Diagnosis at follow‐up | Baseline score | Follow‐up score | Change score | Comparison with No HF group | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Adjusted a mean difference (95% CI) | Adjusted a , b P value | Fully adjusted c mean difference (95% CI) | Fully adjusted P value b , c | |||
| Physical functioning | ||||||||||||
| No HF | HFrEF | 71.4 (23.3) | 82 | 45.4 (27.2) | 82 | −26.0 (25.8) | 82 | −16.6 (−22.6 to −10.5) | <0.0001 | −16.1 (−22.2 to −10.1) | <0.0001 | |
| HFpEF | 68.5 (25.7) | 68 | 42.8 (25.7) | 68 | −25.5 (24.5) | 68 | −14.8 (−21.3 to −8.2) | <0.0001 | −14.6 (−21.2 to −8.1) | <0.0001 | ||
| No HF | 82.9 (19.7) | 1434 | 74.3 (26.6) | 1404 | −8.7 (22.7) | 1397 | ||||||
| HF | HF | 38.5 (26.3) | 26 | 36.8 (27.9) | 26 | −1.7 (32.4) | 26 | 0.04 (−11.0 to 11.0) | 0.99 | 0.7 (−10.3 to 11.8) | 0.99 | |
| Role physical | ||||||||||||
| No HF | HFrEF | 80.5 (35.2) | 81 | 37.8 (42.2) | 72 | −41.8 (41.9) | 71 | −21.7 (−32.8 to −10.6) | <0.0001 | −20.7 (−31.8 to −9.7) | <0.0001 | |
| HFpEF | 68.3 (41.6) | 67 | 31.2 (42.6) | 65 | −34.1 (51.3) | 63 | −19.0 (−30.8 to −7.3) | 0.0003 | −19.3 (−31.0 to −7.6) | 0.0003 | ||
| No HF | 84.5 (31.1) | 1426 | 70.8 (40.7) | 1363 | −13.9 (41.1) | 1353 | ||||||
| HF | HF | 20.2 (33.2) | 26 | 19.6 (36.1) | 23 | −1.1 (52.5) | 23 | −10.8 (−33.9 to 9.4) | 0.49 | −12.5 (−32.8 to 7.6) | 0.36 | |
| Bodily pain | ||||||||||||
| No HF | HFrEF | 69.3 (25.0) | 81 | 59.0 (28.7) | 77 | −11.1 (30.3) | 76 | −5.0 (−11.9 to 1.9) | 0.23 | −4.7 (−11.7 to 2.2) | 0.28 | |
| HFpEF | 69.6 (27.2) | 68 | 63.8 (29.3) | 63 | −4.6 (30.1) | 62 | 2.4 (−5.2 to 9.9) | 0.83 | 3.6 (−4.0 to 11.2) | 0.58 | ||
| No HF | 78.0 (24.2) | 1419 | 72.1 (26.3) | 1350 | −6.1 (27.3) | 1330 | ||||||
| HF | HF | 64.7 (28.0) | 26 | 52.4 (30.6) | 23 | −10.9 (32.6) | 25 | −2.7 (−14.9 to 9.4) | 0.93 | −2.4 (−14.7 to 9.9) | 0.95 | |
| General health perception | ||||||||||||
| No HF | HFrEF | 62.6 (21.7) | 82 | 52.1 (23.0) | 76 | −10.9 (26.2) | 76 | −10.1 (−14.8 to −5.3) | <0.0001 | −10.0 (−14.8 to −5.3) | <0.0001 | |
| HFpEF | 64.5 (19.8) | 68 | 52.5 (20.5) | 65 | −11.4 (17.3) | 64 | −10.1 (−15.2 to −5.0) | <0.0001 | −10.0 (−15.2 to −4.9) | <0.0001 | ||
| No HF | 72.9 (18.4) | 1426 | 70.2 (19.5) | 1347 | −2.8 (17.4) | 1335 | ||||||
| HF | HF | 35.2 (20.2) | 26 | 35.4 (13.7) | 21 | 1.1 (18.8) | 21 | −6.3 (−15.6 to 3.0) | 0.28 | −6.1 (−15.6 to 3.3) | 0.32 | |
| Vitality | ||||||||||||
| No HF | HFrEF | 61.5 (21.0) | 81 | 44.6 (21.7) | 77 | −16.0 (21.5) | 76 | −12.8 (−17.6 to −8.1) | <0.0001 | −12.8 (−17.6 to −8.0) | <0.0001 | |
| HFpEF | 57.8 (22.5) | 68 | 49.0 (19.7) | 67 | −8.6 (20.9) | 66 | −6.2 (−11.3 to −1.2) | 0.012 | −6.0 (−11.1 to −0.9) | 0.014 | ||
| No HF | 66.6 (20.4) | 1431 | 64.7 (20.1) | 1392 | −1.9 (18.9) | 1383 | ||||||
| HF | HF | 38.8 (15.6) | 26 | 37.6 (21.2) | 26 | −1.3 (21.6) | 26 | −5.7 (−14.1 to 2.6) | 0.27 | −4.3 (−12.7 to 4.1) | 0.53 | |
| Social functioning | ||||||||||||
| No HF | HFrEF | 83.9 (24.4) | 80 | 69.6 (31.7) | 78 | −13.3 (32.0) | 76 | −8.6 (−15.0 to −2.2) | 0.004 | −7.9 (−14.3 to −1.6) | 0.009 | |
| HFpEF | 86.2 (20.3) | 67 | 70.2 (29.4) | 65 | −15.5 (29.2) | 63 | −9.1 (−16.0 to −2.1) | 0.006 | −8.7 (−15.6 to −1.7) | 0.009 | ||
| No HF | 88.4 (20.6) | 1424 | 85.5 (22.7) | 1358 | −3.0 (24.5) | 1342 | ||||||
| HF | HF | 62.5 (30.4) | 26 | 59.5 (33.5) | 25 | −2.0 (43.4) | 25 | −8.3 (−19.7 to 3.0) | 0.22 | −9.2 (−20.6 to 2.3) | 0.16 | |
| Role emotional | ||||||||||||
| No HF | HFrEF | 81.3 (33.9) | 81 | 62.2 (45.3) | 67 | −18.7 (49.7) | 66 | −11.5 (−22.1 to −0.9) | 0.028 | −11.1 (−21.6 to −0.5) | 0.037 | |
| HFpEF | 78.1 (37.2) | 64 | 53.7 (47.1) | 59 | −26.7 (49.0) | 55 | −18.0 (−29.5 to −6.5) | 0.0006 | −18.3 (−29.8 to −6.7) | 0.0005 | ||
| No HF | 86.6 (29.0) | 1417 | 82.4 (34.1) | 1322 | −4.5 (39.0) | 1304 | ||||||
| HF | HF | 65.4 (41.6) | 26 | 49.0 (50.2) | 17 | −21.6 (66.6) | 17 | −19.3 (−40.5 to 1.9) | 0.09 | −21.7 (−43.4 to −0.04) | 0.049 | |
| Mental health | ||||||||||||
| No HF | HFrEF | 77.3 (15.9) | 81 | 75.3 (17.9) | 76 | −2.1 (18.4) | 75 | −2.6 (−6.9 to 1.8) | 0.41 | −2.7 (−7.0 to 1.6) | 0.36 | |
| HFpEF | 74.6 (19.0) | 68 | 70.9 (20.0) | 65 | −4.7 (17.0) | 64 | −5.9 (−10.5 to −1.3) | 0.007 | −5.6 (−10.2 to −0.9) | 0.012 | ||
| No HF | 77.8 (16.6) | 1432 | 79.1 (16.5) | 1391 | 1.2 (16.9) | 1383 | ||||||
| HF | HF | 65.5 (21.4) | 26 | 69.2 (16.3) | 26 | 3.6 (26.7) | 26 | −2.6 (−10.1 to 4.9) | 0.79 | −2.9 (−10.5 to 4.7) | 0.73 | |
Adjusted by baseline score, age, sex, cohort, and practice (random effect).
Includes Dunnett–Hsu adjustment; improvement is indicated by a positive change.
Adjusted by baseline score, age, sex, body mass index, diabetes, arthritis, depression, cohort, and practice (random effect).
Figure 3.
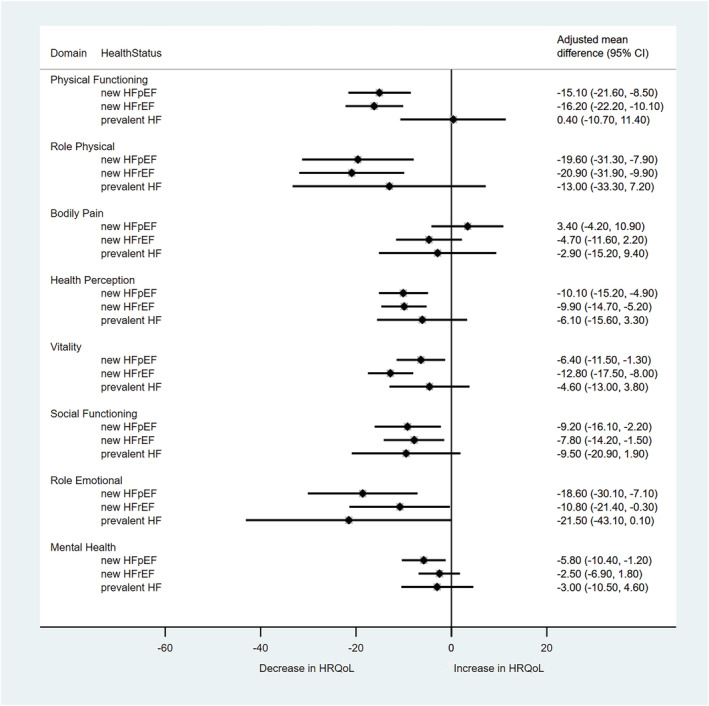
Comparison of change in HRQoL between HF and no HF groups, by SF‐36 domain. Comparison group is no HF; Mean change is adjusted by baseline score, age, body mass index, diabetes, arthritis, depression, original cohort, and general practice.
Clinically significant differences between the groups were also observed in the comparisons of PCS and MCS scores (Table S2 and Figure S1 ). Those with new HFrEF or new HFpEF had significantly greater reduction in PCS change scores than those without HF, with mean adjusted differences of −4.5 (95% CI −7.2 to −1.9) and −3.5 (95% CI −6.4 to −0.6), respectively. MCS differences occurred between persons with new HFpEF and those without HF [mean difference −4.7 (95% CI −8.2 to −1.2)] and between previous HF and no HF [mean difference −11.4 (95% CI −18.0 to −4.8)]. The latter difference concurring with the differences observed for the RE but not MH domain. Results from the bootstrapped sensitivity analysis were similar to the primary analysis (data not shown).
Discussion
This is the first study to report long‐term changes in HRQoL prior to diagnosis with HF. Those who developed HFrEF, or HFpEF, had significantly greater reduction in physical functioning and role physical scores compared with those who did not develop HF over the average 13 years between screenings. Individuals with HF at first screening had the lowest baseline HRQoL scores; however, their change in scores over time was similar to those without HF, across all aspects of HRQoL, except mental health, where there was some evidence of a greater decline.
Strengths and weaknesses
ECHOES is one of the largest well‐phenotyped HF screening studies in the world, providing a unique opportunity to follow HRQoL alongside HF disease progression. Although the original ECHOES cohort reflected the ethnic mix of European community populations, black Africans were under‐represented in the follow‐up ECHOES‐X. The prevalence of HF in minority communities, however, has been shown to be similar to the general population in England. 16
The numbers of individuals in the HF‐related health status groups were small; however, clinically significant differences were observed for PF, RP, and RE domain comparisons. In addition, MCID estimates are based on version 2 of the SF‐36, where the RP and RE domains relate to questions with more reporting options, and therefore, the MCID for these domains are likely to be conservative.
The analysis presented classifies participants into groups based on the HF diagnosis at each screening; the timing of when the HF diagnosis was established within the follow‐up period, however, is unknown. Both screening and rescreening of the ECHOES cohort took place before newer HF treatments, such as angiotensin receptor‐neprilysin inhibitors and sodium glucose cotransporter 2 inhibitors, became available which in clinical trials improved both HRQoL and survival for people living with HF. 17 , 18
Mixed modelling methodology was chosen to evaluate differences in average change in scores over time. This method allows for demographic adjustments and clustering of patients within general practices. Analysis based on change scores rather than follow‐up scores was chosen because the former has been found to be less biased in observational studies. 19 Additional adjustment for baseline scores was included to reduce the potential effects of regression to the mean. The distribution of model residuals was slightly skewed for some domains; however, the bootstrapping sensitivity analysis confirmed the robustness of the results.
HRQoL scores were adjusted for factors previously shown to be associated with temporal change in HRQoL. These include most factors associated with new HF identified by the multinomial logistic modelling. Other potential confounders such as chronic kidney disease and anaemia were not recorded.
Only 26% of those with SF‐36 at baseline were rescreened and hence have long‐term follow‐up data available. Furthermore, those followed up were younger and had less co‐morbidities than those that had died or declined to take part in ECHOES‐X. The mortality rate of the cohort was similar to long‐term trends of UK general practice data. 20 Forty‐four per cent of the cohort died between screenings, including the majority of those with HF diagnosed at baseline. The assumption of data missing at random is unlikely; therefore, sensitivity analysis, imputing lower QoL scores for those who had died/missing, within the framework of controlled multiple imputation methodology was attempted. Unfortunately, the low number of cases in the newly diagnosed HFpEF group caused quasi‐separation of health status groups, producing invalid imputation values (not reported). The complete case analysis presented should therefore be considered hypothesis generating, and more data are required to confirm our results.
Comparison with existing literature
The reduction in HRQoL of newly diagnosed HF at rescreening is expected because symptoms are likely to be present for some time before HF is confirmed. HF is more frequently diagnosed at an acute stage on emergency hospital settings, 21 where physical aspects of HRQoL has been shown to be impaired. 22 Diagnosis can be missed or delayed in primary care due to its commonality of symptoms with other conditions or by patients frequently mistaking symptoms of HF such as breathlessness for the normal ageing process or an existing long‐term condition. 23
The similarity of HRQoL scores at follow‐up in those with newly diagnosed HFpEF and HFrEF in our data [PCS mean (SD): 36.9 (10.7) vs. 37.5 (9.7); MCS mean (SD): 49.5(13.2) vs. 46.8 (13.9)], although not directly comparable, are consistent with findings by Austin et al., who demonstrated, after an 8‐year follow‐up of patients hospitalized with acute HF, the KQQC scores of survivors did not vary by systolic function [mean (SD): HFrEF 77 (21) vs. HFpEF 76 (25)]. 24
Full medication history was unavailable for the ECHOES cohort; nevertheless, patients diagnosed with HF at the initial screening were likely to have been treated early on in the follow‐up period with ACE inhibitors or ARBs, of which there is little evidence of benefit to HRQoL. 25 More recent treatments have been shown to improve HRQoL of patients with HF and hence may explain the more stable scores in those diagnosed with HF at initial screening. The positive impact on QoL of newer treatments for HFrEF has recently been demonstrated by the EMPEROR‐Reduced 26 and DAPA‐HF 27 trials, where treatment with sodium glucose transporter‐2 inhibitors was associated with improvements in health status as measured by the KCCQ. A higher odds of clinical improvement (≥5 points) in KCCQ‐Overall Summary Score with empagliflozin compared to placebo at 12‐month follow‐up [OR 1.16 (1.01–1.35)] and dapagliflozin at 8‐month follow‐up [OR 1.15 (1.08–1.23)]. 26 , 27 Greater improvement in KCCQ has also been shown in people with HFpEF treated with empagliflozin at 12 months [OR 1.16 (1.04–1.29)] and with spironolactone (mean difference 1.86 at 36 months, P = 0.02). 28 , 29 In addition, an individual patient data meta‐analysis of nine trials of exercise training interventions (3000 participants), reported an improvement in standardized mean scores of the MLHFQ, KCCQ, or Chronic Heart Failure Questionnaire of 0.2 (95% CI 0.03–0.37), compared with a standardized mean improvement of 0.3 in MCS in our participants with prior HF. 30
Policy and practice
The utility of patient‐reported outcome measures (PROMs) in cardiovascular trials has been debated for decades. A recent review by von Haehling et al., however, identified the lack of adequately powered studies designed to improve functional capacity and measure long‐term changes in HRQoL. 25 Their absence is partly due to concerns regarding additional clinician and patient time involved in their administration and by the previous lack of an internationally endorsed HRQoL measurement tool. The focus has instead been on measuring ‘hard’ clinical outcomes such as survival and HF hospitalization. For many patients with HF, the quality of daily living may be more important than the length of life. 31 In a recent James Lind Alliance Priority Setting Partnership, the number one research priority set by patients, carers, and clinicians was ‘identification of treatments that have the biggest impact on QoL of people with advanced heart failure’. 32
In more recent studies, improvements in KCCQ clinical and total summary scores have been reported, alongside clinical outcomes, including the large HFrEF trials of sacubitril/valsartan, 33 dapagliflozin, 27 and empagliflozin. 26 Within‐study comparisons of generic and disease‐specific HRQoL tools are also being undertaken. 34
HRQoL tools should be reliable, validated in an HF population, responsive to a change in health status, widely available, acceptable to patients, and inexpensive to use. A core outcome set for HF was published in 2020 by the International Consortium for Health Outcomes Measurement (ICHOM), following a comprehensive structured review by a working party of clinical HF experts, researchers, and patient representatives. 35 A set of 17 measures were defined to facilitate international comparisons in treatment and research. The short form of the KCCQ (KCCQ‐12) was the recommended tool for assessment of patient‐reported HRQoL, due to its superior sensitivity and ease of use. 35 The publication of this standardized outcome set should hopefully impact HF research and increase HRQoL assessment. One study piloting an implementation of the core set was identified to date. 36
Furthermore, a multinational registry, designed to collate HF characteristics and items that impact on the clinical course of HF, including HRQoL, is planned to follow 23 047 participants over a period of 5 years. 37 This registry goes someway to address the information gap, but more long‐term data are needed.
Our study highlights the burden of HF development on physical functioning, regardless of systolic function. However, despite evidence from trials of effective treatments and interventions demonstrating short‐term improvements in HRQoL, there is a lack of evidence regarding long term benefits for people with HF.
Quality of life is important to patients and therefore trials should follow international guidelines and assess the impact of therapies on HRQoL, as well as survival. 38 Research proposals should include the use and comparison of different HRQoL assessment tools, including generic and disease‐specific questionnaires, with consideration given to items in the ICHOM core outcome set. In addition, more research is required to explore the development of HF and to evaluate the impact of treatments on long‐term changes in HRQoL.
Conclusion
People with a new screen‐detected diagnosis of HF (HFrEF and HFpEF) at rescreening had a significant reduction in HRQoL as measured with the SF‐36. Conversely, for those with HF detected on initial screening who survived for more than a decade, all aspects of quality of life as measured with the SF‐36, except emotional role limitation, remained the same, albeit from a low baseline. Further research is required to understand the development of HF over time and to test interventions designed to prevent decline in HRQoL, potentially through earlier diagnosis and treatment optimization.
Conflict of interest
CJT reports personal fees and non‐financial support from Roche outside the submitted work. AKR and FDRH have nothing to disclose.
Funding
AKR is supported by the NIHR Oxford Biomedical Research Centre. FDRH acknowledges support from the NIHR Applied Research Collaborative (ARC) Oxford and Thames Valley and the NIHR Oxford BRC. CJT is funded by a NIHR Academic Clinical Lectureship. This work is part of the NIHR Community Healthcare Medtech and In Vitro Diagnostics Cooperative (MIC) long‐term conditions theme led by CJT and FDRH. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Supporting information
Table S1. Baseline characteristics of participants who completed the SF‐36 at baseline and follow‐up, by cohort.
Table S2. Mean change in SF‐36 Physical and Mental Component scores.
Figure S1. Comparison of change in health‐related quality of life between HF and no HF groups, by SF‐36 physical and mental component scores.
Acknowledgements
We acknowledge Dr Russell Davis and Rachel Iles for their contribution to the ECHOES studies.
Roalfe, A. K. , Taylor, C. J. , and Hobbs, F. D. R. (2023) Long term changes in health‐related quality of life for people with heart failure: the ECHOES study. ESC Heart Failure, 10: 211–222. 10.1002/ehf2.14182.
Contributor Information
Andrea K. Roalfe, Email: andrea.roalfe@phc.ox.ac.uk.
F.D. Richard Hobbs, Email: richard.hobbs@phc.ox.ac.uk.
References
- 1. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the global Burden of disease study 2015. Lancet. 2016; 388: 1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roger VL. Epidemiology of heart failure. Circ Res. 2013; 113: 646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hobbs FDR, Kenkre JE, Roalfe AK, Davis RC, Hare R, Davies MK. Impact of heart failure and left ventricular systolic dysfunction on quality of life. A cross‐sectional study comparing common chronic cardiac and medical disorders and a representative adult population. Eur Heart J. 2002; 23: 1867–1876. [DOI] [PubMed] [Google Scholar]
- 4. Johansson I, Joseph P, Balasubramanian K, McMurray JJV, Lund LH, Ezekowitz JA, Kamath D, Alhabib K, Bayes‐Genis A, Budaj A, Dans ALL, Dzudie A, Probstfield JL, Fox KAA, Karaye KM, Makubi A, Fukakusa B, Teo K, Temizhan A, Wittlinger T, Maggioni AP, Lanas F, Lopez‐Jaramillo P, Silva‐Cardoso J, Sliwa K, Dokainish H, Grinvalds A, McCready T, Yusuf S, the G‐CHF Investigators . Health‐related quality of life and mortality in heart failure: The global congestive heart failure study of 23 000 patients from 40 countries. Circulation. 2021; 143: 2129–2142. [DOI] [PubMed] [Google Scholar]
- 5. Mackintosh A, Gibbons E, Fitzpatrick R. A Structured Review of Patient‐Reported Outcome Measures for People with Heart Failure: An Update 2009. Oxford, United Kingdom: University of Oxford; 2009. [Google Scholar]
- 6. Moradi M, Daneshi F, Behzadmehr R, Rafiemanesh H, Bouya S, Raeisi M. Quality of life of chronic heart failure patients: A systematic review and meta‐analysis. Heart Fail Rev. 2020; 25: 993–1006. [DOI] [PubMed] [Google Scholar]
- 7. Eliya Y, Averbuch T, Le N, Xie F, Thabane L, Mamas MA, Van Spall HGC. Temporal trends and factors associated with the inclusion of patient‐reported outcomes in heart failure randomized controlled trials: A systematic review. J Am Heart Assoc. 2021; 10: e022353. Epub 2021 Oct 23. PMID: 34689608; PMCID: PMC8751837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang S, Davidson PM, Newton PJ, Krum H, Salamonson Y, Macdonald P. What is the methodological and reporting quality of health related quality of life in chronic heart failure clinical trials? Int J Cardiol. 2013; 164: 133–140. [DOI] [PubMed] [Google Scholar]
- 9. Davies MK, Hobbs FDR, Davis RC, Kenkre JE, Roalfe AK, Hare R, Wosornu D, Lancashire RJ. Prevalence of left‐ventricular systolic dysfunction and heart failure in the echocardiographic heart of England screening study: A population based study. Lancet. 2001; 358: 439–444. [DOI] [PubMed] [Google Scholar]
- 10. Taylor CJ, Roalfe AK, Tait L, Davies RC, Iles R, Derit M, Hobbs FR. Observational longitudinal cohort study to determine progression to heart failure in a screened community population: The echocardiographic heart of England screening extension (ECHOES‐X) study. BMJ Open; 4: e005256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The task force on heart failure of the European Society of Cardiology . Guidelines for the diagnosis of heart failure. Eur Heart J. 1995; 16: 741–751. [PubMed] [Google Scholar]
- 12. Ware JE Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF‐36 health profile and summary measures: Summary of results from the medical outcomes study. Med Care. 1995; 33: AS264–AS279. [PubMed] [Google Scholar]
- 13. Wyrwich KW, Tierney WM, Babu AN, Kroenke K, Wolinsky FD. A comparison of clinically important differences in health‐related quality of life for patients with chronic lung disease, asthma or heart disease. Health Serv Res. 2005; 40: 557–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures: A general approach with illustration to the health utilities index mark II. Pharmacoeconomics. 1999; 15: 141–155. [DOI] [PubMed] [Google Scholar]
- 15. Dale CE, Bowling A, Adamson J, Kuper H, Amuzu A, Ebrahim S, Casas JP, Nüesch E. Predictors of patterns of change in health‐related quality of life in older women over 7 years: Evidence from a prospective cohort study. Age Ageing. 2013; 42: 312–318. Epub 2013 Mar 28. [DOI] [PubMed] [Google Scholar]
- 16. Gill PS, Calvert M, Davis R, Davies MK, Freemantle N, Lip G. Prevalence of heart failure and atrial fibrillation in minority ethnic subjects: The ethnic‐echocardiographic heart of England screening study (E‐ECHOES). PLoS ONE. 2011; 6: e26710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sacubitril valsartan for treating symptomatic chronic heart failure with reduced ejection fraction. Technical appraisal guidance. NICE 2021. https://www.nice.org.uk/Guidance/TA388. Accessed 14 July 2022.
- 18. Dapagliflozin for treating chronic heart failure with reduced ejection fraction. Technical appraisal guidance. NICE. 2021. https://www.nice.org.uk/Guidance/TA679. Accessed 14 July 2022.
- 19. Van Breukelen GJ. ANCOVA versus change from baseline: More power in randomized studies, more bias in nonrandomized studies [corrected]. J Clin Epidemiol. 2006; 59: 920–925. [DOI] [PubMed] [Google Scholar]
- 20. Taylor CJ, Ordonez‐Mena JM, Roalfe AK, Lay‐Flurrie S, Jones NR, Marshall T, Hobbs FR. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000‐2017: Population based cohort study. BMJ. 2019; 364: 1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bottle A, Kim D, Aylin P, Cowie M, Majeed A, Hayhoe B. Routes to diagnosis of heart failure: Observational study using linked data in England. Heart. 2018; 104: 600–605. [DOI] [PubMed] [Google Scholar]
- 22. Hoekstra T, Jaarsma T, van Veldhuisen DJ, Hillege HL, Sanderman R, Lesman‐Leegte I. Quality of life and survival in patients with heart failure. EJHF. 2013; 15: 94–102. [DOI] [PubMed] [Google Scholar]
- 23. Taylor CJ, Hartshorne‐Evans N, Satchithananda D, Hobbs FR. FASTer diagnosis: Time to BEAT heart failure. BJGP Open. 2021; 5: BJGPO.2021.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Austin BA, Wang Y, Smith GL, Vaccarine V, Krumholz HM, McNamara RL. Systolic function as a predictor of mortality and quality of life in long‐term survivors with heart failure. Clin Cardiol. 2008; 31: 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. von Haehling S, Arzt M, Doehner W, Edelmann F, Evertz R, Ebner N, Herrmann‐Lingen C, Garfias Macedo T, Koziolek M, Noutsias M, Schulze PC, Wachter R, Hasenfuß G, Laufs U. Improving exercise capacity and quality of life using non‐invasive heart failure treatments: Evidence from clinical trials. Eur J Heart Fail. 2021; 23: 92–113. [DOI] [PubMed] [Google Scholar]
- 26. Butler J, Anker SD, Filippatos G, Khan MS, Ferreira JP, Pocock SJ, Giannetti N, Januzzi JL, Piña IL, Lam CSP, Ponikowski P, Sattar N, Verma S, Brueckmann M, Jamal W, Vedin O, Peil B, Zeller C, Zannad F, Packer M, the EMPEROR‐Reduced Trial Committees and Investigators . Empagliflozin and health‐related quality of life outcomes in patients with heart failure with reduced ejection fraction: The EMPEROR‐reduced trial. Eur Heart J. 2021; 42: 1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, DeMets DL, Inzucchi SE, Køber L, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Bengtsson O, Lindholm D, Niklasson A, Sjöstrand M, Langkilde AM, McMurray JJV. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: Results from the DAPA‐HF trial. Circulation. 2020; 141: 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Butler J, Filippatos G, Jamal Siddiqi T, Brueckmann M, Böhm M, Chopra VK, Pedro Ferreira J, Januzzi JL, Kaul S, Piña IL, Ponikowski P, Shah SJ, Senni M, Vedin O, Verma S, Peil B, Pocock SJ, Zannad F, Packer M, Anker SD. Empagliflozin, health status, and quality of life in patients with heart failure and preserved ejection fraction: The EMPEROR‐preserved trial. Circulation. 2021; 145: 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lewis EF, Kim HY, Claggett B, Spertus J, Heitner JF, Assmann SF, Kenwood CT, Solomon SD, Desai AS, Fang JC, McKinlay S, Pitt BA, Pfeffer MA, TOPCAT Investigators . TOPCAT investigators. Impact of spironolactone on longitudinal changes in health‐related quality of life in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2016; 9: e001937. PMID: 26962133. [DOI] [PubMed] [Google Scholar]
- 30. Taylor RS, Walker S, Ciani O, Warren F, Smart NA, Piepoli M, Davos CH. Exercise‐based cardiac rehabilitation for chronic heart failure: The EXTRAMATCH II individual participant data meta‐analysis. Health Technol Assess. 2019; 23: 1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kraai IH, Vermeulen KM, Luttik ML, Hoekstra T, Jaarsma T, Hillege HL. Preferences of heart failure patients in daily clinical practice: Quality of life or longevity? Eur J Heart Fail. 2013; 15: 1113–1121. [DOI] [PubMed] [Google Scholar]
- 32. Taylor CJ, Huntley AL, Burden J, Gadoud A, Gronlund T, Jones NR, Wicks E, McKelvie S, Byatt K, Lehman R, King A, Mumford B, Feder G, Mant J, Hobbs R, Johnson R. Research priorities in advanced heart failure: James Lind alliance priority setting partnership. Open Heart. 2020; 7: e001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lewis EF, Claggett BL, McMurray JJV, Packer M, Lefkowitz MP, Rouleau JL, Liu J, Shi VC, Zile MR, Desai AS, Solomon SD, Swedberg K. Health‐related quality of life outcomes in PARADIGM‐HF. Circulation: Heart Fail. 2017; 10: e003430. [DOI] [PubMed] [Google Scholar]
- 34. Ravera A, Santema BT, Sama IE, Meyer S, Lombardi CM, Carubelli V, Ferreira JP, Lang CC, Dickstein K, Anker SD, Samani NJ, Zannad F, van Veldhuisen DJ, Teerlink JR, Metra M, Voors AA. Quality of life in men and women with heart failure: Association with outcome, and comparison between the Kansas City cardiomyopathy questionnaire and the EuroQol 5 dimensions questionnaire. Eur J Heart Fail. 2021; 23: 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Burns DJP, Arora J, Okunade O, Beltrame JF, Bernardez‐Pereira S, Crespo‐Leiro MG, Filippatos GS, Hardman S, Hoes AW, Hutchison S, Jessup M, Kinsella T, Knapton M, Lam CSP, Masoudi FA, McIntyre H, Mindham R, Morgan L, Otterspoor L, Parker V, Persson HE, Pinnock C, Reid CM, Riley J, Stevenson LW, McDonagh TA. International consortium for health outcomes measurement (ICHOM): Standardized patient‐centered outcomes measurement set for heart failure patients. JACC Heart Fail. 2020; 8: 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aerts H, Kalra D, Sáez C, Ramírez‐Anguita JM, Mayer MA, Garcia‐Gomez JM, Durà‐Hernández M, Thienpont G, Coorevits P. Quality of hospital electronic health record (EHR) data based on the international consortium for health outcomes measurement (ICHOM) in heart failure: Pilot data quality assessment study. JMIR Med Inform. 2021; 9: e27842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Joseph P, Dokainish H, McCready T, Budaj A, Roy A, Ertl G, Gomez‐Mesa JE, Leong D, Ezekowitz J, Hage C, Lanas F, Maggioni AP, Sliwa K, Zhu J, Rouleau J, Balasubramanian K, Yusuf S, G‐CHF Investigators . A multinational registry to study the characteristics and outcomes of heart failure patients: The global congestive heart failure (G‐CHF) registry. Am Heart J. 2020; 227: 56–63. [DOI] [PubMed] [Google Scholar]
- 38. AHA/ACC/HFSA guideline for the Management of Heart Failure: A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2022; Apr 1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics of participants who completed the SF‐36 at baseline and follow‐up, by cohort.
Table S2. Mean change in SF‐36 Physical and Mental Component scores.
Figure S1. Comparison of change in health‐related quality of life between HF and no HF groups, by SF‐36 physical and mental component scores.


