Abstract
Aims
Cellular communication network factor 1 (CCN1) is an independent predictor of MACE after ACS and elevated levels correlated with infarct size after STEMI. We compared the prognostic accuracy of baseline levels of CCN1, NT‐proBNP, hsTnT, and ST2 and changes in levels over time to predict the development of structural and functional alterations typical of LV remodelling.
Methods
Serial 3‐T cMRI scans were performed to determine LVEF, LVEDV, LVESV, infarct size, and relative infarct size, which were correlated with serial measurements of the four biomarkers. The prognostic significance of these biomarkers was assessed by multiple logistic regression analysis by examining their performance in predicting dichotomized cardiac MRI values 12 months after STEMI based on their median. For each biomarker three models were created using baseline (BL), the Δ value (BL to 6 months), and the two values together as predictors. All models were adjusted for age and renal function. Receiver operator curves were plotted with area under the curve (AUC) to discriminate the prognostic accuracy of individual biomarkers for MRI‐based structural or functional changes.
Results
A total of 44 predominantly male patients (88.6%) from the ETiCS (Etiology, Titre‐Course, and Survival) study were identified at a mean age of 55.5 ± 11.5 (SD) years treated by successful percutaneous coronary intervention (97.7%) at a rate of 95.5% stent implantation within a median pain‐to‐balloon time of 260 min (IQR 124–591). Biomarkers hsTnT and ST2 were identified as strong predictors (AUC > 0.7) of LVEDV and LVEF. BL measurement to predict LVEF [hsTnT: AUC 0.870 (95% CI: 0.756–0.983), ST2: AUC 0.763 (95% CI: 0.615–0.911)] and the Δ value BL‐6M [hsTnT: AUC 0.870 (95% CI: 0.756–0.983), ST2: AUC 0.809 (95% CI: 0.679–0.939)] showed a high prognostic value without a significant difference for the comparison of the BL model vs. the Δ‐value model (BL‐6M) for hsTnT (P = 1) and ST2 (P = 0.304). The combined model that included baseline and Δ value as predictors was not able to improve the ability to predict LVEF [hsTnT: AUC 0.891 (0.791–0.992), P = 0.444; ST2: AUC 0.778 (0.638–0.918), P = 0.799]. Baseline levels of CCN1 were closely associated with LVEDV at 12 months [AUC 0.708 (95% CI: 0.551–0.865)] and infarct size [AUC 0.703 (95% CI: 0.534–0.872)].
Conclusions
Baseline biomarker levels of hsTnT and ST2 were the strongest predictors of LVEF and LVEDV at 12 months after STEMI. The association of CCN1 with LVEDV and infarct size warrants further study into the underlying pathophysiology of this novel biomarker.
Keywords: Biomarkers, Cardiac magnetic resonance imaging, ST‐elevation myocardial infarction, Left ventricular remodelling, Heart failure
Introduction
Inflammation and fibrotic turnover are characteristic features of infarct healing after acute myocardial infarction that promote structural changes typical of adverse left ventricular (LV) remodelling and subsequent heart failure. 1 , 2 The structural changes in LV volumes and function can be assessed by cardiac magnetic resonance imaging (cMRI) and circulating biomarkers. 3 The observation of reverse remodelling and myocardial recovery in heart failure suggests that early recognition of such alterations may enable implementation of therapeutic measures to attenuate or even reverse severe heart failure. 4 , 5 Circulating biomarkers that reflect underlying pathology may serve as a useful tool to assess alterations in LV volumes and function and thus enable the use of personalized precision medicine in heart failure patients. 6
Cellular communication network factor 1 (CCN1, previously denoted cysteine‐rich angiogenic inducer 61, Cyr61) is a secreted matricellular protein involved in inflammation and angiogenesis. 7 , 8 , 9 , 10 , 11 CCN1 is expressed in cardiomyocytes from patients with ischaemic cardiomyopathy and promotes the transition of fibroblasts to a senescent phenotype, limiting cardiac fibrosis. 8 , 12 We recently identified circulating CCN1 as an independent predictor of all‐cause mortality and the composite of all‐cause mortality and recurrent myocardial infarction in patients with an acute coronary syndrome (ACS). 13 , 14 In patients with ST‐elevation myocardial infarction (STEMI), CCN1 levels were associated with infarct size measured enzymatically and LV dysfunction in the acute phase. 15 Further biomarkers commonly used in diagnosis and risk stratification of heart failure are N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), high‐sensitivity troponin (hsTnT), and suppression of tumorigenicity 2 (ST2), a member of the interleukin (IL)‐1 receptor family. 16 In the present study, circulating levels of CCN1, NT‐proBNP, hsTnT, and ST2 were serially measured in order to evaluate which of these biomarkers and what points in time best reflect morphologic changes detected by cMRI that are typical of LV remodelling in patients after an acute STEMI.
Methods
Patient population
The investigator‐initiated, prospective, multicentre diagnostic ETiCS (Etiology, Titre‐Course, and Survival) study recruited patients with acute myocarditis or acute myocardial infarction between 2010 and 2013. 17 In the present single‐centre substudy of ETiCS, patients presenting to the University Hospital of Würzburg with an acute STEMI with serial serum samples and serial 3‐T cMRI scans available during a 12‐month follow‐up period were included.
Imaging was serially performed to determine LV ejection fraction (LVEF), LV end‐systolic stroke volume (LVESV), LV end‐diastolic volume (LVEDV), stroke volume, LV mass, infarct size, and relative infarct size defined as percentage of infarct size per systolic LV mass at Days 1–4 (Scan 1), Days 7–9 (Scan 2), and 12 months (Scan 3). Procedural details for this substudy, including cMRI, were previously reported. 18
Serum samples were available at baseline [single measurement at a median time interval of 1 (IQR 1, 2) day post‐PCI (min 0, max 3 days)] and at 6 and 12 months to measure concentrations of biomarkers. The study complies with the Declaration of Helsinki, and informed consent was obtained from all individuals as approved by the local ethics committee.
Biomarkers
Concentrations of CCN1 in serum were measured in stored, thawed duplicates of single serum aliquots using an immunoassay (Quantikine Immunoassay, R&D Systems, Minneapolis, MN, USA). The inter‐assay and intra‐assay coefficients of variation of CCN1 were 5.47% and 2.2%, respectively, as reported by the manufacturer. Soluble ST2 was measured using the Presage ST2 Assay (Critical Diagnostics, USA). NT‐proBNP and hsTnT (both from Roche Diagnostics, Mannheim, Germany) were measured in serum aliquots on a Cobas e 602 reader (Roche Diagnostics, Mannheim, Germany) with assay characteristics as reported by the manufacturer.
Statistical analyses
Dichotomized values from cMRI scans were correlated with serial measurements of biomarker concentrations (continuous variable). The prognostic significance of these biomarkers was assessed by multiple logistic regression analysis by examining their performance in predicting dichotomized cMRI values 12 months after STEMI based on their median. For each biomarker, three models were created using baseline (BL), the Δ value from BL to 6 months (BL‐6M), or both values together as predictors. P < 0.05 was considered statistically significant. All models were adjusted for age and renal function represented by Modification of Renal Diet (MDRD)‐based estimated glomerular filtration rate. Receiver operator curves (ROC) were plotted with area under the curve (AUC) to discriminate the prognostic accuracy of individual biomarkers for MRI‐based structural or functional changes.
All statistical analyses were carried out using the statistical software R (v4.1.1, R Foundation for Statistical Computation, Vienna, Austria).
Results
Baseline characteristics
A total of 44 patients from the ETiCS study who survived the first year after STEMI had serum samples available from baseline, 6 months, and 12 months together with three serial cMRI scans performed (Scans 1–3; Figure 1 ). The mean age was 55.5 ± 11.5 (standard deviation) years with a male preponderance (88.6%) and a prevalence of known coronary artery disease in a fifth of patients. Successful percutaneous coronary intervention was performed in 97.7% of patients at a rate of 95.5% stent implantation within a median pain‐to‐balloon time of 260 (IQR 124–591) min. Baseline characteristics are shown in Table 1 .
Figure 1.
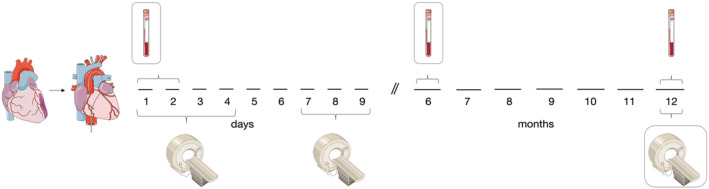
Timeline of data collection. Blood draws are illustrated by red tubes; cMRI scans are shown by grey appliances.
Table 1.
Baseline characteristics
| Median [IQR] or n (%) | Unit | Data | |
|---|---|---|---|
| Age | 55.5 [49–64.25] | Years | 44/44 |
| Sex | Male 39 (88.6), female 5 (11.4) | n (%) | 44/44 |
| BMI | 26.84 [24.27–29.29] | kg/m2 | 44/44 |
| Systolic BP | 120 [110–130.5] | mmHg | 44/44 |
| Diastolic BP | 72.5 [63–78.5] | mmHg | 44/44 |
| Co‐morbidities | |||
| Hypertension | 17 (38.6) | n (%) | 44/44 |
| Diabetes mellitus | 7 (15.9) | n (%) | 44/44 |
| Dyslipidaemia | 7 (15.9) | n (%) | 44/44 |
| Smoker | Current 17 (38.6), previous 10 (22.7) | n (%) | 44/44 |
| CAD | 9 (20.5) | n (%) | 44/44 |
| Previous MI | 0 (0) | n (%) | 44/44 |
| Heart failure | 1 (0.02) | n (%) | 44/44 |
| PCI | 44 (100) | n (%) | 44/44 |
| Pain‐to‐balloon time | 260 [123.8–591] | Min | 44/44 |
| Successful intervention | 43 (97.7) | n (%) | 44/44 |
| Stent implantation | 42 (95.5) | n (%) | 44/44 |
| 1‐vessel disease | 26 (59.1) | n (%) | 44/44 |
| 2‐vessel disease | 14 (31.8) | n (%) | 44/44 |
| 3‐vessel disease | 4 (9.1) | n (%) | 44/44 |
| Infarct‐related artery | |||
| RCA | 15 (34.1) | n (%) | 44/44 |
| LAD | 19 (43.2) | n (%) | 44/44 |
| LCX | 10 (22.7) | n (%) | 44/44 |
| LMCA | 0 (0) | n (%) | 44/44 |
| Clinical chemistry (baseline) | |||
| Creatinine in serum | 0.9 [0.8–0.98] | mg/dL | 44/44 |
| eGFR (MDRD) peak value (d1–9) | 91 [80.5–104] | mL/min/1.73 m2 | 43/44 |
| CK‐MB | 59.0 [45.18–138.6] | U/L | 33/44 |
| CK‐MB peak value (d1–9) | 57.95 [43–135.48] | U/L | 33/44 |
| Leucocytes | 9.2 [8.25–10.8] | n*1000/μL | 43/44 |
| C‐reactive protein | 1.07 [0.52–4.06] | mg/dL | 42/44 |
| cMRI parameters (12 months) | |||
| LVEF | 52.8 [47.2–58.52] | % | 44/44 |
| LVEDV | 174.75 [150.78–190.95] | mL | 44/44 |
| LVESV | 77.3 [61.85–97.2] | mL | 44/44 |
| Infarct size | 9.7 [7.3–16.1] | g | 44/44 |
| Stroke volume | 90.95 [79.98–98.03] | mL | 44/44 |
| Biomarker (baseline) | |||
| hsTnT | 1969 [883.22–3032] | pg/mL | 44/44 |
| ST2 | 33.47 [26.56–49.95] | ng/mL | 44/44 |
| NT‐proBNP | 885.5 [668.25–1476.75] | pg/mL | 44/44 |
| CCN1 | 152.85 [116.88–190.02] | pg/mL | 44/44 |
Abbreviations: BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CCN1, cellular communication network factor 1; CK‐MB, creatine phosphokinase‐MB; eGFR, estimated glomerular filtration rate; hsTnT, high‐sensitivity troponin T; LAD, left anterior descending artery; LCX, left circumflex artery; LMCA, left main coronary artery; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; MDRD, modified renal diet; MI, myocardial infarction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PCI, percutaneous coronary intervention; RCA, right coronary artery; ST2, suppression of tumorigenicity 2.
Successful intervention was defined as restituted TIMI‐3 flow post‐PCI.
Differential time course of biomarkers
Serial measurements of biomarkers showed remarkable homogeneity for ST2, NT‐proBNP, and hsTnT with a pronounced and persistent decline in concentration at 6 and 12 months after STEMI compared with baseline levels. Conversely, the concentration of CCN1 increased from baseline to 6 months, reaching a plateau thereafter with no further increase from 6 to 12 months (Figure 2 ).
Figure 2.
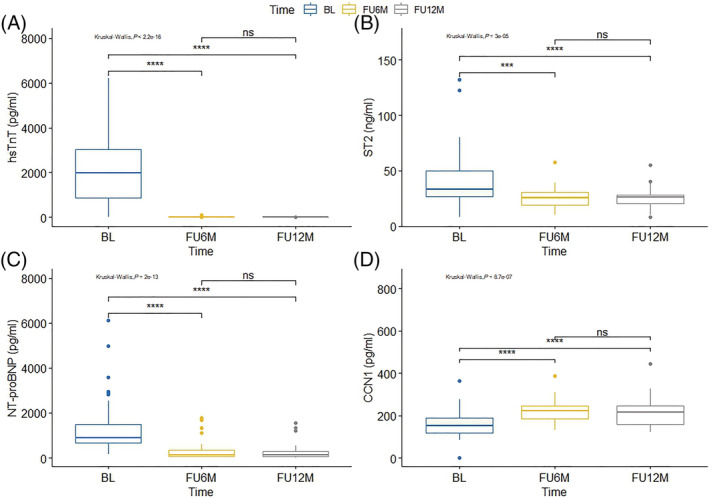
Differential time course of biomarkers in the first 12 months after STEMI. Boxplot illustration showing median with the first and third quartiles. CCN1, cellular communication network factor 1; hsTnT, high‐sensitivity troponin T; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; ST2, suppression of tumorigenicity 2. ****P < 0.001.
Prognostic value of biomarkers at baseline to predict cMRI‐based structural or functional changes
A consistent pattern of strong correlations with cMRI parameters at 12 months (P < 0.001) was found only for baseline hsTnT: LVEF (rho = −0.71), LVESV (rho = 0.61), and infarct size (rho = 0.66). Multivariable analysis comprising hsTnT, ST2, NT‐proBNP, CCN1, age, and eGFR identified as independent predictors of cMRI parameters at 12 months (above or below the median; P < 0.05) a consistent pattern for baseline hsTnT to predict LVEF odds ratio (OR) 0.13 (95% CI 0.03, 0.49); infarct size OR 3.26 (95% CI 1.18, 8.99), relative infarct size OR 3.46 (95% CI 1.22, 9.79), LVESV OR 4.74 (95% CI 1.65, 13.57), and LVEDV OR 2.95 (95% CI 1.26, 6.91), whereas baseline CCN1 was singularly associated with LVEDV OR 0.39 (95% CI 0.16, 0.92). Baseline ST2 was associated with LVEF OR 0.31 (95% CI 0.11, 0.84) as well as LVESV OR 3.17 (95% CI 1.13, 8.91) and LVEDV OR 3.01 (1.08, 8.37).
Comparison of prognostic value of serial changes of biomarkers vs. baseline to predict MR‐based structural or functional changes
ROC analysis via multiple logistic regression models of the levels of individual biomarkers at baseline and 6 months and the delta change baseline vs. 6 months (Δ value BL‐6M) was performed to predict changes in cMRI‐based structural or functional parameters at 12 months compared with baseline. Considering AUC values >0.7 as a clinically meaningful cut‐off, the highest AUC to predict LVEF was found for hsTnT at baseline (0.870, 95% CI 0.756–0.983), which was identical to the AUC of the hsTnT Δ value BL‐6M (0.870, 95% CI 0.756–0.983, P = 1 for comparison) and was not improved when the two approaches were combined (0.891, 95% CI 0.791–0.992, P = 0.444 for comparison baseline vs. combined and P = 0.444 for comparison of Δ value BL‐6M vs. combined) (Figure 3 A, scatter plot Figure 5 A , calibration plot Figure S6A ). ST2 levels at baseline showed a clinically meaningful association with LVEF (0.763, 95% CI 0.615–0.911) but a numerically higher association for the ST2 Δ value BL‐6M (0.809, 95% CI 0.679–0.939, P = 0.304 for comparison) and the combination of the two (0.778, 95% CI 0.638–0.918, P = 0.799 for comparison baseline vs. combined and P = 0.234 for comparison of Δ value BL‐6M vs. combined) (Figure 3 B ). NT‐proBNP and CCN1 were not associated with LVEF (Figure 3 C,D ). Scatter plots are provided in Figure S7A .
Figure 3.
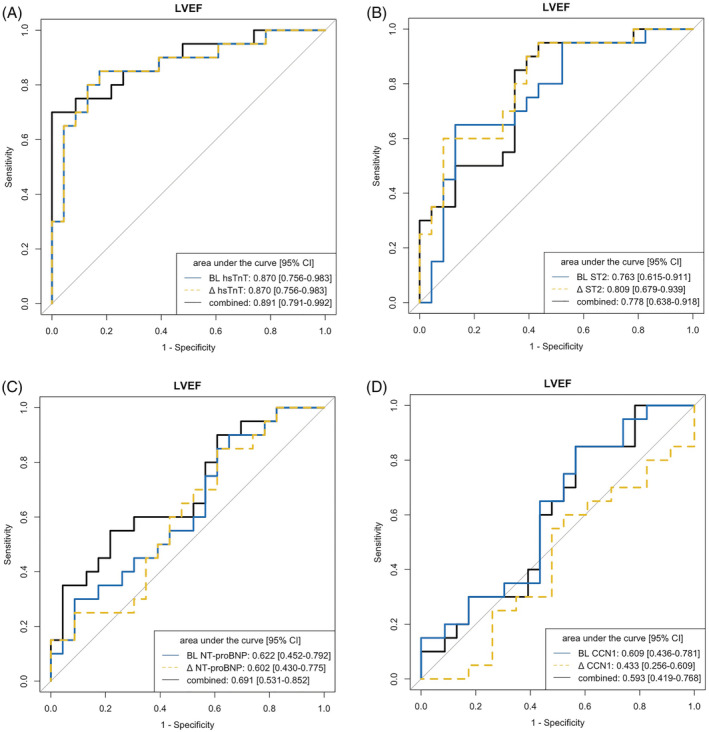
ROC analysis of individual biomarkers at baseline and 6 months to predict left ventricular ejection fraction (LVEF) at 12 months. (A) hsTnT, high‐sensitivity troponin T. (B) ST2, suppression of tumorigenicity 2. (C) NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide. (D) CCN1, cellular communication network factor 1.
Figure 5.
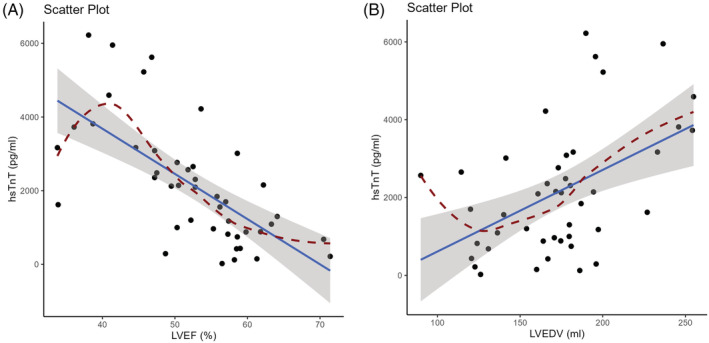
Scatter plot of association between the cardiac biomarker high‐sensitivity troponin T (hsTnT) at baseline and cMRI parameters at 12 months. (A) LVEF, left ventricular ejection fraction. (B) LVEDV, left ventricular end‐diastolic volume.
For the prediction of LVEDV at 12 months, baseline hsTnT had the highest AUC (0.753, 95% CI 0.604–0.903), which was identical to the hsTnT Δ value BL‐6M (0.753, 95% CI 0.604–0.903, P = 1 for comparison) and similar to the value when the two approaches were combined (0.771, 95% CI 0.624–0.918, P = 0.599 for comparison baseline vs. combined and P = 0.599 for comparison of Δ value BL‐6M vs. combined) (Figure 4A , scatter plot Figure 5 B , calibration plot Figure S6B ). For ST2, the highest association with LVEDV was for the Δ value BL‐6M (0.781, 95% CI 0.641–0.921), which was the same as when the baseline and Δ values were combined (0.771, 95% CI 0.629–0.912, P = 0.426 for comparison); the AUC for ST2 at baseline was numerically lower (0.745, 95% CI 0.589–0.901, P = 0.685 for comparison baseline vs. combined and P = 0.512 for comparison of Δ value BL‐6M vs. baseline) (Figure 4 B ). For LVEDV, there were no clinically meaningful associations for NT‐proBNP (Figure 4 C ). For CCN1, the highest association with LVEDV was for the baseline value (0.708, 95% CI 0.551–0.903), which was the same as when the baseline and Δ values were combined (0.729, 95% CI 0.578–0.881, P = 0.520 for comparison). The CCN1 Δ value BL‐6M did not show clinically relevant predictive power for LVEDV (Figure 4 D ). Scatter plots are provided in Figure S7B .
Figure 4.
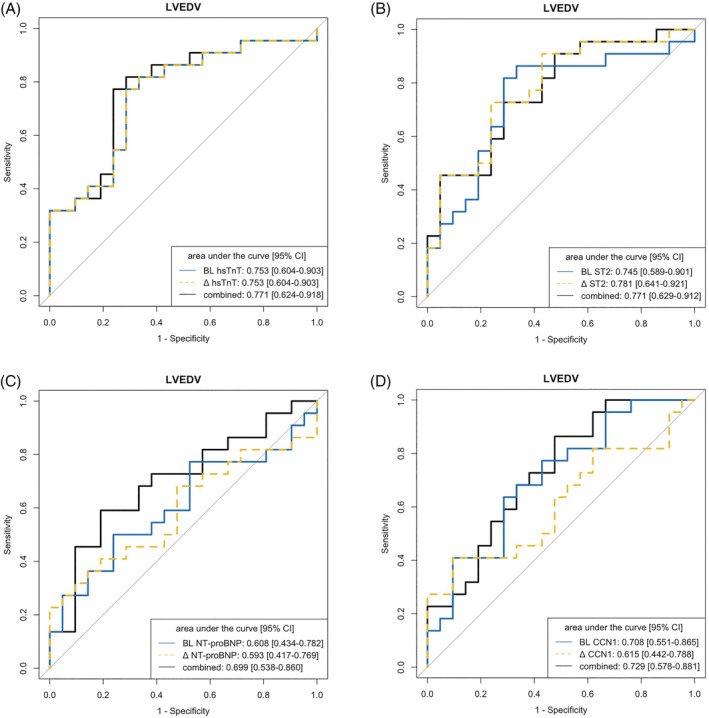
ROC analysis of individual biomarkers at baseline and 6 months to predict left ventricular end‐diastolic volume (LVEDV) at 12 months. (A) hsTnT, high‐sensitivity troponin T. (B) ST2, suppression of tumorigenicity 2. (C) NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide. (D) CCN1, cellular communication network factor 1.
Among the four biomarkers evaluated, only hsTnT and ST2 showed multiple associations (AUC >0.7) with infarct size (Figure S1A,B ), relative infarct size ( Figure S2A,B ) and LVESV ( Figure S3A,B ), but there was no difference in AUC between baseline and the Δ value BL‐6M. None of the four biomarkers were able to predict stroke volume ( Figure S4 ).
Baseline levels of CCN1 were closely associated with infarct size (Figure S1D ) besides LVEDV (Figure 4 D ). In light of the differential time course of the four biomarkers after STEMI in the first year, with a rise and subsequent plateau phase after 6 months observed only for CCN1, a separate ROC analysis was performed. CCN1 levels at 6 months could not predict cMRI‐based changes at 12 months at AUC levels >0.7 ( Figure S5 ).
Discussion
In this multimarker substudy of the ETiCS study, we identified baseline levels of hsTnT and ST2 as strong predictors of changes in LVEF and LVEDV at 12 months after STEMI.
The ETiCS study provides several time points for measurement of biomarkers that can be correlated with serial cMRI scans after STEMI, 17 , 18 enabling comparison of serial changes in biomarker levels with cMRI‐based structural and functional changes typical of remodelling. In our study, hsTnT measured at baseline after STEMI showed the strongest association with long‐term LV remodelling (in particular LVEF, but also LVEDV, infarct size, and relative infarct size), corroborating previous data. 19 , 20 , 21 Of note, individual time points early after STEMI—in particular at Day 4—were shown to adequately predict infarct size. 22 The present study extends these data to 6 months, demonstrating that baseline levels provide as much information as does the change in biomarker levels between baseline and 6 months.
Of note, a recent study in 374 STEMI patients with serial cMRI scans performed at 6 days and 6 months after reperfusion and assessment of adverse clinical events showed that a single cMRI scan at baseline is enough to serve as a surrogate marker for clinical endpoints ascertained during a 6‐year follow‐up. In comparison with exclusive use of LVEDV, the combination of LVEDV and LVEF determined by a cMRI scan at baseline improved the predictive value regarding adverse outcome. Conversely, evaluation of LVEDV and LVEF by cMRI scan at 6 months did not add further prognostic value to the early cMRI. 23 In our study, a single biomarker measurement (especially hsTnT) performed early after reperfusion in STEMI patients provides valid information on LVEF and LVEDV, obviating the need for further biomarker measurements and serial cMRI scans after STEMI. Our study extends the data on associations of cMRI‐based LVEF and LVEDV with adverse outcome reported by Rodríguez‐Palomares, 23 suggesting that a single biomarker measurement early after reperfusion may be enough to discern patients at high risk for adverse events. Future studies are warranted to assess whether biomarker‐guided therapies addressing the underlying pathophysiology can change cMRI‐based structural and functional parameters and ultimately adverse events to inform treatment decisions. Our data showing the ability of ST2 to predict deterioration of LVEF determined late after STEMI are in line with previous data. 24 The lack of an association of NT‐proBNP with long‐term LV volume indices is likely attributable to the timing of biomarker measurements, as a later time point may be more appropriate. 19
This is the first study to describe the time course of the novel circulating biomarker CCN1 during the first year in STEMI patients. Several aspects delineated from the current study suggest a distinct underlying pathophysiology for CCN1. First, the kinetics characterized by an increase in CCN1 levels that reaches a plateau at 6 months after the index STEMI clearly distinguish it from the established cardiovascular biomarkers hsTnT, ST2, and NT‐proBNP, which show a decrease with persisting low levels after 6 months. Second, CCN1 levels measured at baseline after STEMI showed a clinically meaningful association (c‐statistics >0.7) with LVEDV and infarct size but not LVEF as determined by cMRI at 12 months. LV remodelling has a complex pathophysiology, with some but not all cMRI parameters being reflected by individual biomarkers. The reason for this may be biological (distinct pathophysiology not reflected by the biomarker) or numerical (small patient number to detect weak associations of a biomarker with cMRI‐based pathology). Data from our study may serve as a starting point to conduct a power calculation for a future, larger study. These findings warrant further study into the underlying pathophysiology of CCN1 after STEMI to explain the previously observed association of elevated CCN1 levels with all‐cause mortality and recurrent MI. 13 , 14 Our current data put previously published data into perspective, as cMRI provides better assessment of infarct‐related injury and subsequent LV remodelling than creatine kinase levels measured shortly after STEMI. 15 Beyond a mere association, it appears rather that elevated CCN1 levels in STEMI patients represent different strata of patients with larger or smaller infarcts in the early phase, which, however, might have only a minor impact on LV remodelling in the ensuing later stages after STEMI.
Limitations
This substudy of the ETiCS study analysed a rather small number of patients. However, the strength of the study lies in the well‐characterized patients and in the serial measurements of biomarkers and serial cMRI scans performed. The distinct time points of sampling on Days 0–3 (baseline) after STEMI are noteworthy, and we cannot exclude some level of confounding here. However, this represents the timeframe of a plateau, at least for troponin T, and our data on hsTnT are line with published data. 22
Conclusion
After STEMI, baseline hsTnT is the one of four biomarkers analysed that was most closely correlated with heart failure‐related changes in structural and functional LV pathology at 12 months. The novel biomarker CCN1 was closely related to LVEDV and infarct size, warranting further study into the underlying pathophysiology.
Conflict of interest
The authors report no conflicts of interest pertinent to the manuscript.
Funding
ETiCS received public funding from the Bundesministerium für Bildung und Forschung (BMBF; MolDiag: 01ES0816, 01ES01901, and 01ES01902; Comprehensive Heart Failure Center: 01EO1004). S.F. and A.F. were supported by the CRC1525 (project number 453989101). The current analysis was supported by a grant to R.K. and T.K from the Kerckhoff Research Foundation.
Supporting information
APPENDIX S1. Use of serial changes in biomarkers versus baseline levels to predict left ventricular remodelling after STEMI.
Acknowledgements
We are grateful to the members of the ETiCS study team for conducting the study and for providing samples for the current analysis and to the biomarker team at the Franz Groedel Institute, Bad Nauheim. Figure 1 contains items from https://smart.servier.com/?s=heart. In addition, we thank Elizabeth Martinson, PhD, of the KHFI Editorial Office for her editorial assistance.
Open Access funding enabled and organized by Projekt DEAL.
Klingenberg, R. , Holtkamp, F. , Grün, D. , Frey, A. , Jahns, V. , Jahns, R. , Gassenmaier, T. , Hamm, C. W. , Frantz, S. , and Keller, T. (2023) Use of serial changes in biomarkers vs. baseline levels to predict left ventricular remodelling after STEMI. ESC Heart Failure, 10: 432–441. 10.1002/ehf2.14204.
References
- 1. Frantz S, Bauersachs J, Ertl G. Post‐infarct remodelling: Contribution of wound healing and inflammation. Cardiovasc Res. 2009; 81: 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Westman PC, Lipinski MJ, Luger D, Waksman R, Bonow RO, Wu E, Epstein SE. Inflammation as a driver of adverse left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol. 2016; 67: 2050–2060. [DOI] [PubMed] [Google Scholar]
- 3. Aimo A, Gaggin HK, Barison A, Emdin M, Januzzi JL Jr. Imaging, biomarker, and clinical predictors of cardiac remodeling in heart failure with reduced ejection fraction. JACC Heart Fail. 2019; 7: 782–794. [DOI] [PubMed] [Google Scholar]
- 4. Andreadou I, Cabrera‐Fuentes HA, Devaux Y, Frangogiannis NG, Frantz S, Guzik T, Liehn EA, Gomes CPC, Schulz R, Hausenloy DJ. Immune cells as targets for cardioprotection: New players and novel therapeutic opportunities. Cardiovasc Res. 2019; 115: 1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim GH, Uriel N, Burkhoff D. Reverse remodelling and myocardial recovery in heart failure. Nat Rev Cardiol. 2018; 15: 83–96. [DOI] [PubMed] [Google Scholar]
- 6. Bayes‐Genis A, Voors AA, Zannad F, Januzzi JL, Mark Richards A, Díez J. Transitioning from usual care to biomarker‐based personalized and precision medicine in heart failure: Call for action. Eur Heart J. 2018; 39: 2793–2799. [DOI] [PubMed] [Google Scholar]
- 7. Emre Y, Imhof BA. Matricellular protein CCN1/CYR61: A new player in inflammation and leukocyte trafficking. Semin Immunopathol. 2014; 36: 253–259. [DOI] [PubMed] [Google Scholar]
- 8. Hilfiker‐Kleiner D, Kaminski K, Kaminska A, Fuchs M, Klein G, Podewski E, Grote K, Kiian I, Wollert KC, Hilfiker A, Drexler H. Regulation of proangiogenic factor CCN1 in cardiac muscle: Impact of ischemia, pressure overload, and neurohumoral activation. Circulation. 2004; 109: 2227–2233. [DOI] [PubMed] [Google Scholar]
- 9. Hinkel R, Trenkwalder T, Petersen B, Husada W, Gesenhues F, Lee S, Hannappel E, Bock‐Marquette I, Theisen D, Leitner L, Boekstegers P, Cierniewski C, Müller OJ, le Noble F, Adams RH, Weinl C, Nordheim A, Reichart B, Weber C, Olson E, Posern G, Deindl E, Niemann H, Kupatt C. MRTF‐A controls vessel growth and maturation by increasing the expression of CCN1 and CCN2. Nat Commun. 2014; 5: 3970. [DOI] [PubMed] [Google Scholar]
- 10. Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010; 12: 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim KH, Won JH, Cheng N, Lau LF. The matricellular protein CCN1 in tissue injury repair. J Cell Commun Signal. 2018; 12: 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meyer K, Hodwin B, Ramanujam D, Engelhardt S, Sarikas A. Essential role for premature senescence of myofibroblasts in myocardial fibrosis. J Am Coll Cardiol. 2016; 67: 2018–2028. [DOI] [PubMed] [Google Scholar]
- 13. Klingenberg R, Aghlmandi S, Liebetrau C, Räber L, Gencer B, Nanchen D, Carballo D, Akhmedov A, Montecucco F, Zoller S, Brokopp C, Heg D, Jüni P, Marti Soler H, Marques‐Vidal PM, Vollenweider P, Dörr O, Rodondi N, Mach F, Windecker S, Landmesser U, von Eckardstein A, Hamm CW, Matter CM, Lüscher TF. Cysteine‐rich angiogenic inducer 61 (Cyr61): A novel soluble biomarker of acute myocardial injury improves risk stratification after acute coronary syndromes. Eur Heart J. 2017; 38: 3493–3502. [DOI] [PubMed] [Google Scholar]
- 14. Klingenberg R, Aghlmandi S, Räber L, Akhmedov A, Gencer B, Carballo D, Nanchen D, Bucher HC, Rodondi N, Mach F, Windecker S, Landmesser U, von Eckardstein A, Hamm CW, Lüscher TF, Matter CM. Cysteine‐rich angiogenic inducer 61 improves prognostic accuracy of GRACE (global registry of acute coronary events) 2.0 risk score in patients with acute coronary syndromes. J Am Heart Assoc. 2021; 10: e020488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahendiran T, Klingenberg R, Nanchen D, Gencer B, Meier D, Räber L, Carballo D, Matter CM, Lüscher TF, Mach F, Rodondi N, Muller O, Fournier S. CCN family member 1 (CCN1) is an early marker of infarct size and left ventricular dysfunction in STEMI patients. Atherosclerosis. 2021; 335: 77–83. [DOI] [PubMed] [Google Scholar]
- 16. Aimo A, Januzzi JL Jr, Vergaro G, Richards AM, Lam CSP, Latini R, Anand IS, Cohn JN, Ueland T, Gullestad L, Aukrust P, Brunner‐la Rocca HP, Bayes‐Genis A, Lupón J, Boer RA, Takeishi Y, Egstrup M, Gustafsson I, Gaggin HK, Eggers KM, Huber K, Gamble GD, Ling LH, Leong KTG, Yeo PSD, Ong HY, Jaufeerally F, Ng TP, Troughton R, Doughty RN, Passino C, Emdin M. Circulating levels and prognostic value of soluble ST2 in heart failure are less influenced by age than N‐terminal pro‐B‐type natriuretic peptide and high‐sensitivity troponin T. Eur J Heart Fail. 2020; 22: 2078–2088. [DOI] [PubMed] [Google Scholar]
- 17. Deubner N, Berliner D, Schlipp A, Gelbrich G, Caforio ALP, Felix SB, Fu M, Katus H, Angermann CE, Lohse MJ, Ertl G, Störk S, Jahns R, on behalf of the ETiCS‐Study Group . Cardiac beta1‐adrenoceptor autoantibodies in human heart disease: Rationale and design of the etiology, titre‐course, and survival (ETiCS) study. Eur J Heart Fail. 2010; 12: 753–762. [DOI] [PubMed] [Google Scholar]
- 18. Frey A, Gassenmaier T, Hofmann U, Schmitt D, Fette G, Marx A, Herterich S, Boivin‐Jahns V, Ertl G, Bley T, Frantz S, Jahns R, Störk S. Coagulation factor XIII activity predicts left ventricular remodelling after acute myocardial infarction. ESC Heart Fail. 2020; 7: 2354–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hendriks T, Hartman MHT, Vlaar PJJ, Prakken NHJ, van der Ende YMY, Lexis CPH, van Veldhuisen DJ, van der Horst ICC, Lipsic E, Nijveldt R, van der Harst P. Predictors of left ventricular remodeling after ST‐elevation myocardial infarction. Int J Cardiovasc Imaging. 2017; 33: 1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mohammad MA, Koul S, Lønborg JT, Nepper‐Christensen L, Høfsten DE, Ahtarovski KA, Bang LE, Helqvist S, Kyhl K, Køber L, Kelbæk H, Vejlstrup N, Holmvang L, Schoos MM, Göransson C, Engstrøm T, Erlinge D. Usefulness of high sensitivity troponin T to predict long‐term left ventricular dysfunction after ST‐elevation myocardial infarction. Am J Cardiol. 2020; 134: 8–13. [DOI] [PubMed] [Google Scholar]
- 21. Reinstadler SJ, Feistritzer HJ, Klug G, Mair J, Tu AMD, Kofler M, Henninger B, Franz WM, Metzler B. High‐sensitivity troponin T for prediction of left ventricular function and infarct size one year following ST‐elevation myocardial infarction. Int J Cardiol. 2016; 202: 188–193. [DOI] [PubMed] [Google Scholar]
- 22. Giannitsis E, Steen H, Kurz K, Ivandic B, Simon AC, Futterer S, Schild C, Isfort P, Jaffe AS, Katus HA. Cardiac magnetic resonance imaging study for quantification of infarct size comparing directly serial versus single time‐point measurements of cardiac troponin T. J Am Coll Cardiol. 2008; 51: 307–314. [DOI] [PubMed] [Google Scholar]
- 23. Rodriguez‐Palomares JF, Gavara J, Ferreira‐González I, Valente F, Rios C, Rodríguez‐García J, Bonanad C, García del Blanco B, Miñana G, Mutuberria M, Nuñez J, Barrabés J, Evangelista A, Bodí V, García‐Dorado D. Prognostic value of initial left ventricular remodeling in patients with reperfused STEMI. JACC Cardiovasc Imaging. 2019; 12: 2445–2456. [DOI] [PubMed] [Google Scholar]
- 24. Miñana G, Núñez J, Bayés‐Genís A, Revuelta‐López E, Ríos‐Navarro C, Núñez E, Chorro FJ, López‐Lereu MP, Monmeneu JV, Lupón J, Bodí V. ST2 and left ventricular remodeling after ST‐segment elevation myocardial infarction: A cardiac magnetic resonance study. Int J Cardiol. 2018; 270: 336–342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1. Use of serial changes in biomarkers versus baseline levels to predict left ventricular remodelling after STEMI.


