Abstract
Background
Sleep disturbance is common and problematic among both patients with cancer and their sleep partner caregivers. Although 70% of the general adult population sleep in the same bed with a significant other, as do adult cancer patients and their spousal/partner caregivers, and one's sleep affect the partner's sleep, existing psychobehavioral interventions have targeted patients' and caregivers' sleep problems independently.
Methods
We developed a new sleep intervention, My Sleep Our Sleep (MSOS), for both adult patients with cancer and their sleep-partner caregivers together. This protocol is to test the feasibility and acceptability as well as to provide preliminary efficacy of the MSOS intervention, which is a dyadic intervention designed to reduce sleep disturbance and improving sleep quality of both adult cancer patients and their sleep-partner caregivers (dyads). The intervention will be delivered weekly for 4 weeks. Questionnaire and daily sleep logs will be collected at baseline (T1) and one-week after conclusion of the intervention (T2). Satisfaction with the intervention will be assessed weekly for 4 weeks.
Results
We estimate 43 dyads be enrolled (43 patients and 43 sleep-partner caregivers). We expect >75% of eligible and screened dyads will enroll within the enrollment period, >80% of enrolled dyads will complete the intervention, and >80% of participants will report satisfaction across all acceptability measures. We also expect MSOS will reveal a small-to-medium effect on sleep efficiency (primary outcome), overall sleep disturbance, subjective sleep quality, and insomnia severity (secondary outcomes).
Conclusions
Results will inform the feasibility and acceptability of conducting a dyadic sleep intervention, and provide preliminary efficacy data to guide further refinement of the intervention content and procedure for adult patients with cancer and their sleep-partner caregivers.
Trial registration
Keywords: Sleep disturbance, Adult cancer, Patient-caregiver dyad, Sleep intervention protocol
1. Introduction
Over 16.9 million people in the United States have a history of at least one cancer diagnosis, and over 1.9 million new cancer cases are estimated each year [1]. Among this population, sleep disturbance, which includes difficulties falling asleep and difficulties staying asleep due to frequent and prolonged nighttime awakenings that result in poor sleep efficiency [[2], [3], [4]], is a prominent concern. Any form of sleep disturbance has been reported by 65%–95% [5,6] of patients with cancer, which is notably higher than that of age-matched individuals who have not had cancer (14%–30% [5,7,8]: and the adult U.S. general population (35%: 9). Sleep disturbance in patients with cancer has been viewed as a treatment-related symptom along with other cytokine-induced sickness behaviors, including fatigue, depression, and pain [[10], [11], [12]]. Sleep disturbance contributes to cancer patients’ impaired quality of life and disease-related outcomes, including disease progression and poorer overall survival [4,13,14].
Sleep disturbance is also commonly reported among cancer patients' family caregivers: 63%–90% [15,16]. Such prevalence is higher than that reported by caregivers of individuals with chronic disease such as cardiovascular disease [17], Parkinson's disease [18], and dementia [19]. Sleep disturbance in caregivers has been viewed due to sharing the cancer-related stress with the patients. Sleep disturbance in cancer caregivers is also associated with poor quality of life and adverse health outcomes [20,21].
Sleep disturbance that is common and problematic among both patients with cancer and their caregivers has been studied and targeted in patients [22,23] and caregivers [24] independently. For example, the efficacy of the modified Cognitive Behavioral Therapy for Insomnia (CBT-I) has been tested solely for adult patients with cancer [12,22,25,26]. In parallel, 21 sleep intervention studies solely targeted unpaid caregivers, which used various intervention approaches including cognitive behavioral, massage, psychoeducational, exercise, and palliative care [27]. Of those, 13 studies were for caregivers of patients with cancer. Overall, although low quality, evidence suggested that interventions improved sleep quality compared with control [27]. Only one intervention study, to date, tested the efficacy of sleep intervention for caregivers of adult patients with cancer. This intervention included stimulus control, relaxation, cognitive therapy, and sleep hygiene elements and was found to be effective in improving sleep quality and decreasing depressive symptoms in caregivers [20]. In addition, sleep intervention tailored from CBT-I has been suggested as an effective way to reduce sleep disturbance among family cancer caregivers [24].
Approximately, 70% of the general adult population sleep in the same bed with a significant other [9], as do adult patients with cancer and their spousal caregivers/intimate partners. As one's sleep can affect the partner's sleep, taking the interpersonal approach into consideration by targeting both patients with cancer and their sleep partnered caregivers for mitigating their sleep disturbance would yield optimal outcomes of improved sleep health and general quality of life for both sleep partners [28,29]. Such approach may be a critical first step to advance understanding and treating sleep, a shared health behavior in these highly vulnerable populations touched by cancer. Yet, such interpersonal approach thus far has been applied only to healthy young-to-middle-aged adults [[30], [31], [32], [33], [34], [35], [36]], patients with insomnia [37,38], or parents with newborn babies [39,40], whose sources of sleep disturbance exclusively differ from those in cancer patient-caregiver dyads.
Thus, the proposed dyadic sleep intervention includes not only the components of CBT-I but also close relationship components associated with a diagnosis of cancer in the family and caring for someone with cancer. We report here the newly developed study protocol that is registered on ClinicalTrials.gov (NCT04712604) and approved by the University of Miami Institutional Review Board for improving sleep health of adult patients with cancer and their sleep-partner caregivers.
2. Methods
2.1. Study design
This study describes the development of the My Sleep Our Sleep (MSOS) intervention, which is a dyadic sleep intervention designed to improve sleep health of both adult patients with cancer and their sleep-partner caregivers. As a first step, the MSOS will be tested in a single arm study design, delivered weekly for 4 weeks. This study aims to test the feasibility and acceptability of the MSOS intervention for both adult patients with cancer and their sleep-partner caregivers. This study also aims to provide preliminary efficacy of the MSOS intervention in reducing sleep disturbance and improving sleep quality for both adult patients with cancer and their sleep-partner caregivers.
2.2. Recruitment
Forty-three dyads (86 persons) will be recruited from the University of Miami Sylvester Comprehensive Cancer Center clinics in South Florida. Patients with a diagnosis of a gastrointestinal cancer (anus, colon, esophagus, gallbladder, large and small intestine, liver, pancreas, rectum, stomach, other biliary or digestive organs) in the past 5 years will be identified using medical records. Patients who agreed to allow investigators to contact them for research purposes will be prioritized. The project coordinator will call patients via telephone to screen them for study eligibility. Eligible patients will nominate their sleep-partner caregivers, who will also be screened for study eligibility by the project coordinator. After the dyads’ eligibility is ascertained and both meet the eligibility criteria, the project coordinator will schedule the study sessions.
2.3. Eligibility (see Table 1)
Table 1.
Inclusion and exclusion criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| For Patients | For both Patients and Caregivers |
|
|
|
|
| For Caregivers |
|
|
|
| For both Patients and Caregivers |
|
|
|
| |
| |
|
Eligibility, inclusion criteria for patients will be [1] having a diagnosis of stage I to IV of a gastrointestinal (GI) cancer (anus, colon, esophagus, gallbladder, large and small intestine, liver, pancreas, rectum, stomach, other biliary or digestive organs) in the past 5 years at the time of enrollment and [2] having a consistent sleep partner who shares a bed with the patient most of the time (≥6 out of 7 nights per week) when either partner is not traveling and who has been the sleep partner for more than 5 years. GI cancer is chosen as it affects both genders equally. Eligibility criterion for caregivers will be being a sleep partner of the patient. Additional eligibility criteria for both patients and caregivers will be [1] having at least mild-to-moderate sleep disturbance (Pittsburgh Sleep Quality Index: PSQI ≥5 [2,41]: willing to change sub-optimal sleep habits [3], 18 years or older [4], able to speak/read English at the 5th grade level, and [5] > 4 weeks after surgery, if any, prior to enrollment because surgery affects sleep.
2.3.1. Exclusion criteria
Exclusion criteria for both patients and caregivers will be [1] having had a diagnosis of psychosis, major depressive disorder, or bipolar disorder that is not currently treated [2]; having had substance or alcohol dependency, or active suicidality in the past year [3]; currently have narcolepsy or restless leg syndrome [4]; both patients and caregivers have an extreme chronotype, or do shift work to have no overlap in sleep schedule between patients and caregivers [5]; plan trans-meridian travel during the period of data collection blocks; and [6] have hearing or visual impairment, dementia, or cognitive dysfunction.
2.4. Informed consent
This study is approved by the University of Miami Institutional Review Boards. The protocol is registered with ClinicalTrials.gov (NCT04712604). The project coordinator will explain to each participant the purpose and procedures, the risks and benefits, the terms of confidentiality, and the compensation of the study. Participants will have the opportunity to carefully review the written consent form presented on a web-based Research Electronic Data Capture (REDCap) application that is a Health Insurance Portability and Accountability Act (HIPAA) complaint platform and ask questions prior to signing. Signed consent, approved by the University of Miami Institutional Review Boards, will be obtained from each participant. Each participant's informed consent form will be placed in his or her respective file. Participants will also have the opportunity to keep a copy of their signed consent form.
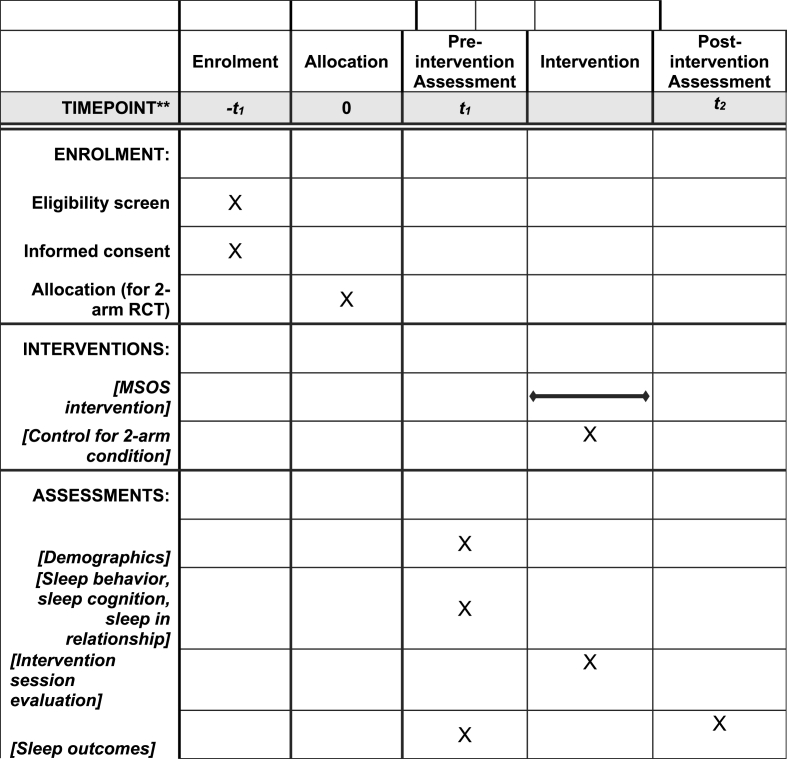
2.5. Study procedure (Fig. 1)
Fig. 1.
Schedule of enrolment, interventions, and assessments.
Patients will be identified by the diagnosis of a GI cancer and diagnosis date, using medical records at oncology clinics. Participants will sign an informed consent form individually on a web-based REDCap application before providing any study data. Participants (patient and caregiver as a unit) will participate in the study together; the data are collected simultaneously from both members of the dyad individually.
Participants will complete the pre-intervention assessment (T1) that includes a questionnaire to be completed once and daily sleep measures for 7 days on a web-based Qualtrics application. This study will employ a single arm study design as a first step. The intervention will be delivered via a HIPAA-compliant Zoom video platform once a week to both patients and caregivers together for 4 weeks. Participants will complete an intervention satisfaction survey immediately after the end of each session on a web-based Qualtrics application. Project coordinator manages the intervention satisfaction survey, so that participants will be informed that the interventionist is blind to the survey data. The post-intervention assessment (T2) includes a questionnaire that is completed once and daily sleep measures for 7 days on a web-based Qualtrics application, which begins 7 days after final intervention session (see Fig. 2). In both T1 and T2 questionnaires, participants will be asked to list medical conditions [42] and medications taken to manage each condition. The interventionist will review the list of medications and note its potential effects on participants’ sleep quality to incorporate the information into the intervention session. The interventionist also asks the type and date of cancer treatment received at each intervention session to incorporate it into the intervention content.
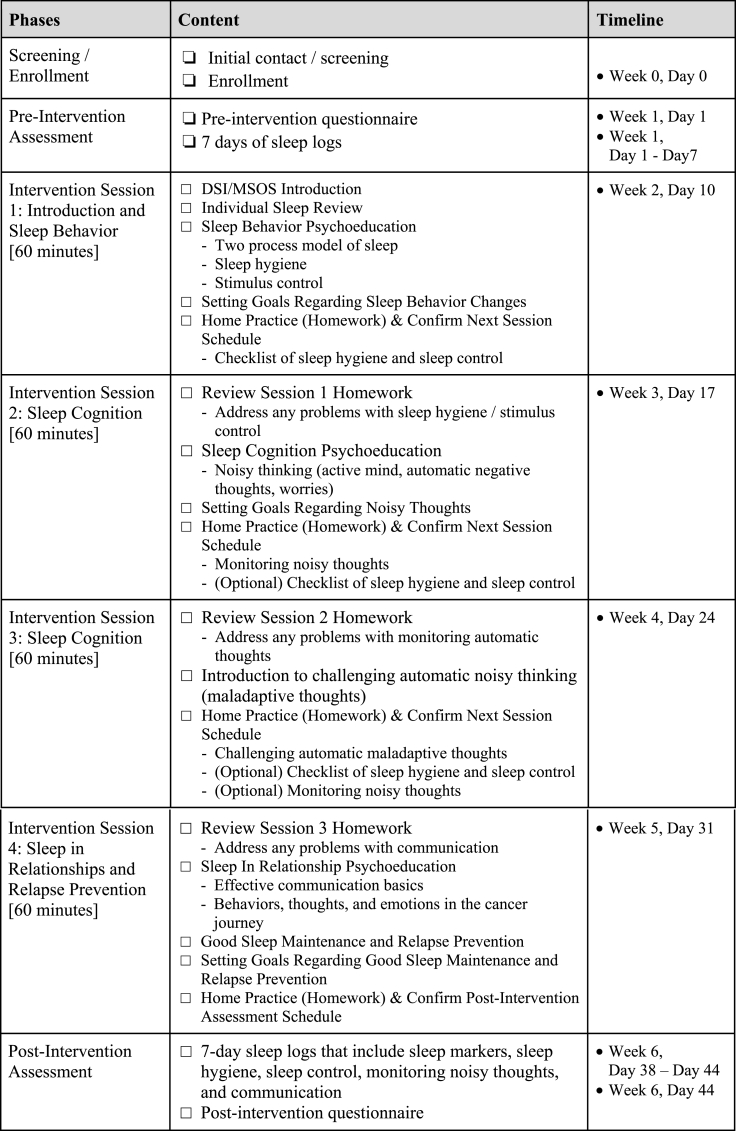
Fig. 2.
MSOS intervention content and timeline.
2.6. Intervention
The My Sleep Our Sleep (MSOS) intervention is adapted some components of Cognitive Behavioral Therapy for Insomnia (CBT-I), the gold standard intervention endorsed by the American Academy of Sleep Medicine for treating sleep disturbance among the general population, for patients with GI cancer and their sleep partner caregivers as a dyad. The MSOS intervention will help participants (i.e., sleep partners) increase sleep hygiene and stimulus control behaviors and reduce dysfunctional beliefs about sleep in order to reduce sleep disturbance and improve sleep quality. The fundamental behavioral (sleep hygiene and stimulus control) and cognitive (monitoring and managing maladaptive thoughts about sleep) components of CBT-I are adapted in the MSOS intervention. The MSOS, however, relaxes sleep restriction therapy (SRT) component of CBT-I to accommodate sleepiness or drowsiness from cancer treatment and the side effects as well as to reflecting the finding that most cancer patients and caregivers sleep less than 7 h, which does not require sleep restriction [43,44]. However, if participants sleep for more than 9 h, sleep restriction will be recommended. Furthermore, the MSOS incorporates cancer-related experiences and thoughts that contribute to disturbed sleep, targets both sleep partners, and discusses the aspects of partners’ close relationship that underlie their cancer-related experiences together as well as their shared sleep.
The intervention will consist of four 1-h weekly sessions delivered by a Master's level interventionist who has been trained in Clinical Psychology, Behavioral Medicine, Social Work, or related field. The competency of the interventionist in the behavioral and cognitive principles of sleep modification, cancer treatment and symptom management, communication in close relationships will be ensured through minimum of three didactic training sessions and two full sets of intervention sessions with mock participants role playing. In addition, the intervention fidelity will be assessed by the intervention sessions of the first 12 dyads (50%) being reviewed by a research scientist who specializes psycho-oncology or a licensed clinical psychologist. Additional training will be performed with the interventionists if they do not meet ≥90% of the protocol adherence checklist. The MSOS is delivered via HIPAA-compliant video platform to the patient and caregiver simultaneously. Topics covered in the weekly sessions (see Fig. 2) will include psychoeducation on sleep processes and behavioral components of sleep, including sleep hygiene and stimulus control (Session 1: Sleep behavior), psychoeducation on cancer-related cognitions that contribute to sleep disturbance (Session 2: Sleep cognition), how to identify and challenge unhelpful automatic thoughts (Session 3: Sleep cognition), and psychoeducation on sleeping well together in the context of sharing a close relationship and addressing relapse prevention (Session 4: Sleep in relationship and relapse prevention).
Session 1 introduces the intervention and focuses on providing psychoeducation about the two-process model of sleep, sleep hygiene, and stimulus control. Each partner's current habits for sleep hygiene and stimulus control are reviewed, and goals for relevant behavioral changes are collaboratively discussed and negotiated.
During Session 2, progress with behavior changes for sleep hygiene and stimulus control is reviewed and barriers adhering to behavior changes are addressed. Session 2 also focuses on providing psychoeducation on the connections between thoughts, emotions, and behaviors, as well as identifying and discussing automatic thoughts that are cancer-related and sleep specific that contribute to each partner's sleep disturbance. In addition to practicing behavior changes for sleep hygiene and stimulus control, partners practice monitoring their automatic thoughts that contribute to their sleep disturbance.
Session 3 focuses on providing psychoeducation on challenging unhelpful automatic thoughts, which involves identifying the unhelpful thinking style and reframing the automatic thought to produce a more balanced alternative thought. In addition to practicing behavior changes for sleep hygiene and stimulus control as well as monitoring their maladaptive thoughts, partners practice challenging their automatic thoughts contributing to their sleep disturbance.
Session 4 focuses on discussing aspects of the close relationship and shared cancer experiences that also contribute to the couples’ sleep problems. Psychoeducation on effective communication, including self-disclosure, partner responsiveness, and relationship engagement is provided. Behaviors, thoughts, and emotions throughout the cancer journey, such as fear of recurrence, cancer prognosis, caregiving stress, etc., are collaboratively discussed. Psychoeducation on maintaining changed healthy sleep habits and relapse prevention is also discussed.
The order of the session content can be tailored for individual dyads based on the information obtained from pre-intervention questionnaire and daily sleep measures. For example, a dyad whose member scores less than 13, indicating distressed relationship on the 4-item Dyadic Adjustment Scale [45] that ranges 0 to 21 (higher scores indicate more satisfactory relationship), the topic of sleep in the relationship that is the content of session 4 can be discussed in the first session after the general introduction of the MSOS intervention. In other words, the psychoeducation on effective communication and general aspects of the close relationship and shared cancer experiences, which contribute to their sleep problems can be discussed in the first session and its progress can be monitored throughout the remaining sessions. On the other hand, for a dyad whose member does not have any problems with maladaptive sleep-related cognition (≤5 on the Dysfunctional Beliefs and Attitudes about Sleep (DBAS [46]: nor have noticeable problems in their relationship but has several behaviors that contribute to severe sleep disturbance, the topic of sleep behaviors can be discussed in sessions 1 and 2 to 3, whereas sleep cognition, relationship, and relapse prevention can be discussed sessions 3 and/or 4. Interventionist creates the tailored intervention planner reflecting each dyad's information collected from pre-intervention assessment prior to the first session and modifies the subsequent session planner, if necessary, after each intervention session ends.
2.7. Outcome measures
2.7.1. Feasibility measures
The enrollment rate will determine the feasibility of the MSOS intervention. The feasibility criteria will be met if 75% of eligible dyads enroll within the 12-month enrollment period, if 80% of enrolled dyads complete the intervention (one week after the last intervention session and assessment), and no adverse events are reported.
2.7.2. Acceptability measures
Participants’ evaluation of the intervention content and mode will determine the acceptability of the MSOS intervention. Participants will evaluate the intervention content after each session by completing brief questions on a 5-point Likert scale (1: strongly disagree, 5: strongly agree). Acceptability questions are regarding the extent to which the session being engaging, easy to understand, comprehensive, useful, relevant, motivating sleep behavior changes, and motivating sleep cognition changes, and helping to prepare for making sleep-related changes. The delivery mode of the intervention will be assessed at the post-intervention session via open-ended questions to each participant. Specifically, participants will provide their opinions on the frequency (weekly), delivery mode (Zoom vs. in-person or telephone), and interaction mode with interventionist (live vs. non-interactive or animated interactions). The acceptability criteria will be met if 80% of participants report satisfaction across all acceptability measures.
2.7.3. Sleep cognition measures
Dysfunctional beliefs about sleep. Dysfunctional beliefs and attitudes about sleep will be assessed pre- and post-intervention using the 16-item Dysfunctional Beliefs and Attitudes about Sleep (DBAS: [46], on an 11-point Likert type format (0: strongly disagree, 10: strongly agree)). The total and subscale (5-item consequence, 6-item worry, 2-item sleep expectations, and 3-item sleep medication) scores of the DBAS will serve to tailor the cognition module of the dyadic sleep intervention for individual participants.
2.7.4. Interpersonal relationship measures
Relationship styles will be assessed using the 14-item Measures of Attachment Quality (MAQ: [47]) that assesses three adult attachment orientations: security, anxiety, and avoidance. Relationship satisfaction will be assessed using the 4-item Dyadic Adjustment Scale (DAS: [45]). The MAQ and DAS scores will serve to tailor the relationship module of the dyadic sleep intervention.
2.7.5. Sleep behavior measures
Participants will complete a sleep diary each morning for 7 consecutive days using a modified consensus sleep diary [48]. The sleep diary includes entries for bedtime, sleep onset, number and duration of awakenings, sleep offset, out-of-bed time, naps, physical activity, and caffeine or alcohol intake. The sleep diary data collected during the pre-intervention block served to tailor the behavioral module of the dyadic sleep intervention. Sleep efficiency derived from the sleep diary will also serve as a study outcome.
2.7.6. Efficacy measures
Sleep efficiency. The sleep efficiency will be derived from daily sleep diary (see 2.7.5). Sleep efficiency is calculated by the total time spent for sleeping divided by the total time spent in bed per day. Average sleep efficiency scores across 7 days pre- and post-intervention blocks will served as a primary outcome.
Global sleep disturbance and subjective sleep quality. The overall sleep disturbance and subjective sleep quality will be assessed using the 19-item Pittsburgh Sleep Quality Index (PSQI) at baseline and post-intervention [41]. Higher scores of overall sleep disturbance and subjective sleep quality indicate greater sleep disturbance and poorer sleep quality. The global sleep disturbance score will serve as an eligibility criterion. Both the global sleep disturbance and subjective sleep quality scores will serve as secondary outcomes.
Insomnia severity. The severity of insomnia symptoms will be assessed using the Insomnia Severity Index (ISI: [49]) at pre- and post-intervention. ISI total scores 0–7 indicate absence of insomnia, 8–14 indicate sub-threshold levels of insomnia, 15–21 indicate moderate levels of clinical insomnia, and 22–28 indicate severe levels of clinical insomnia. The insomnia severity score will serve as a secondary outcome.
2.8. Statistical considerations
2.8.1. Statistical analysis
Demographic characteristics of the sample, means and standard deviations or percentages of study variables will be reported. Differences in demographics and study variables between patients and caregivers at pre- and post-intervention will be tested using paired t-tests. Feasibility will be supported when enrollment rate is ≥ 75% and retention rate at intervention completion is ≥ 80%. Acceptability will be supported when ratings on the 8 satisfaction domains are ≥4.0 (out of 5: ≥80% satisfaction). Preliminary efficacy will be tested with changes in study variables from pre-intervention (T1) to post-intervention (T2) using paired t-tests. Cohen's d will also be reported for information regarding the effect size [50]. Statistical significance will be set at a 2-tailed p-value <.05.
2.8.2. Sample size and power
To detect a small-to-medium effect of the MSOS intervention (f = 0.18: [50]), on 4 sleep indices (sleep efficiency, overall sleep disturbance, subjective sleep quality, and insomnia severity) that are correlated at .5 with each other, with 80% power, and two-tailed alpha at .05, we will need 34 dyads (68 persons: 80% of 43 dyads enrolled) at the completion of the study.
2.9. Data and safety monitoring considerations
The informed consent, all assessment measures, and intervention modules have been reviewed and approved by the University of Miami Institutional Review Boards. In addition, the study will be closely monitored by the Sylvester Comprehensive Cancer Center Data and Safety Monitoring Committee (DSMC) in accordance to the Cancer Center's Data and Safety Monitoring Plan (DSMP). In its oversight capacity, the DSMC bears responsibility for suspending or terminating this study. DSMC oversight of the conduct of this trial includes ongoing review of adverse event data, and periodic review of the study's aims. In addition, the DSMC will review reports from all audits, site visits, or study reviews pertaining to this study and take appropriate action.
2.10. Study timeline
All information provided regarding the research, as well as all information collected and documented during the course of the study will be regarded as confidential. The financial disclosure information has been completed prior to study participation from the principal investigator and co-investigators who are involved in the research study. Participant recruitment and data collection have begun March 2021. A total of 10 dyads (20 persons) has been recruited by January 2022. Data collection will resume after securing funding. Results will be published per agreements with the funding agency and per institutional guidelines. Once accepted for publication, we will register the study protocol and make the data available on Open Science Forum.
3. Discussion
This study is designed to investigate the feasibility, acceptability, and preliminary efficacy of the 4-week My Sleep Our Sleep (MSOS) intervention for both adult patients with cancer and their sleep-partner caregivers simultaneously. It will also provide data on the effects of this intervention on modifying sleep hygiene and stimulus control behaviors, reducing dysfunctional beliefs about sleep, reducing sleep disturbance, and improving sleep quality in sleep-partners.
We learned several lessons while developing the protocol. Recruitment to the MSOS intervention will involve contacting a list of patients who are identified to meet the eligibility requirements for the study and have agreed to allow investigators to contact them for research purposes. Recruiting patients who are already willing to participate in research may facilitate recruitment and assist investigators in meeting their recruitment goals. However, it may also limit the sample to those who are motivated to engage in an intervention and/or study that assesses the efficacy of the interventions. In addition, a rapid screening tool for sleep problems available in oncology clinics will help identify eligible patients. The proposed recruitment method facilitates the testing of this novel intervention. However, it should also be taken into consideration that patients who are recently diagnosed, diagnosed with advanced stage, or currently on active cancer treatment may be less likely to agree to participate in the study.
Additionally, data collection using web-based applications (as opposed to paper-and-pencil) is likely to improve the data integrity. The web-based Qualtrics surveys, for example, are designed to send participants daily sleep diary at their wake time and a reminder a few hours later if they have yet to complete the daily sleep diary. Both questionnaires and daily sleep diaries are also designed to flag missing or invalid responses and prompt participants to correct their mistake prior to continuing the survey. The benefits of having real-time data quality assurance will decrease random missingness and increase the validity of the data. Although the ownership of smartphone or tablets among older adults and individuals with lower income has ever increased dramatically in recent years [51], some may still be reluctant to web-based assessment and intervention delivery, which would limit the generalizability of the proposed methodology. Readily available in-person assessment and intervention sessions, lending means to participate in the study (e.g., smart phone or tablet, internet service), or providing educations to improve literacy in technology may help addressing health equity concerns pertaining to the resources that are required to participate in the study.
Study participants will highly favor flexibility in scheduling the pre- and post-assessment sessions as well as intervention sessions in the evening and weekends, because many adult patients with cancer and their family caregivers/partners wish to continue carrying out their existing social roles (e.g., maintaining their employment, caring for children or grandchildren, taking care of an aging parent). However, it may also require additional study personnel cost. Clear communication about the study expectations and allowing flexible working hours and days for study staff will be necessary.
Finally, some participants may prefer shorter (e.g., 30 min) duration per session with larger number of sessions (e.g., 8 30-min, as opposed to 4 60-min sessions). Other participants may request extended or additional sessions for certain module as well as additional practice time between sessions. Assessing certain cancer- or cancer-treatment-related symptoms that affect the patients' sleep is a must at the pre-intervention assessment to be incorporated throughout the intervention sessions. The interventionist should be cognizant of cancer- and treatment-related symptom management strategies. Furthermore, the interventionist may have to make efforts to maintain the engagement of the intervention for any member in a dyad who has fewer sleep problems or achieves the desired sleep health sooner than the other member. The interventionist may also focus facilitating collaborative relationship between the sleep partners to enhance each other's sleep health.
The results of this study will inform the feasibility and acceptability of conducting a dyadic sleep intervention. Results will also guide further refinement of the MSOS intervention content and procedure, which efficacy will be tested in randomized control trials for adult patients with cancer and their sleep-partner caregivers.
Funding
The writing of this manuscript was supported by National Institute of Nursing Research (R01NR016838) to YK. NINR has not involved in writing of this manuscript.
Author contributions
Youngmee Kim: Conceptualization, Methodology, Writing-Original draft preparation, Writing-Reviewing and Editing. Resource Provision. Amanda Ting: Methodology, Writing-Original draft preparation, Writing-Reviewing and Editing. Jennifer Steel: Methodology, Writing-Original draft preparation, Writing-Reviewing and Editing. Thomas Tsai: Methodology, Writing-Original draft preparation, Writing-Reviewing and Editing.
Declaration of competing interest
None of the authors have conflict of interest associated with this publication and no financial conflict of interest to disclose.
Acknowledgements
The authors extend their appreciation to Drs. Alberto Ramos and William Wohlgemuth for their inputs to earlier phase of the investigation. The first author dedicates this research to the memory of Heekyoung Kim and Charles S. Carver.
Data availability
No data was used for the research described in the article.
References
- 1.American Cancer Society . American Cancer Society; Atlanta: 2022. Cancer Facts & Figures 2022. [cited 2022 6/20] [Google Scholar]
- 2.Dean G.E., Redeker N.S., Wang Y.J., Rogers A.E., Dickerson S.S., Steinbrenner L.M., et al. Sleep, mood, and quality of life in patients receiving treatment for lung cancer. Oncol. Nurs. Forum. 2013;40(5):441–451. doi: 10.1188/13.ONF.441-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotronoulas G., Wengstrom Y., Kearney N. Sleep patterns and sleep-impairing factors of persons providing informal care for people with cancer A critical review of the literature. Cancer Nurs. 2013;36(1):E1–E15. doi: 10.1097/NCC.0b013e3182456c38. [DOI] [PubMed] [Google Scholar]
- 4.Palesh O., Aldridge-Gerry A., Zeitzer J.M., Koopman C., Neri E., Giese-Davis J., et al. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep. 2014;37(5):837–842. doi: 10.5665/sleep.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gooneratne N.S., Dean G.E., Rogers A.E., Nkwuo J.E., Coyne J.C., Kaiser L.R. Sleep and quality of life in long-term lung cancer survivors. Lung Cancer. 2007;58(3):403–410. doi: 10.1016/j.lungcan.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otte J.L., Carpenter J.S., Manchanda S., Rand K.L., Skaar T.C., Weaver M., et al. Systematic review of sleep disorders in cancer patients: can the prevalence of sleep disorders be ascertained? Cancer Med-Us. 2015;4(2):183–200. doi: 10.1002/cam4.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson K.O., Getto C.J., Mendoza T.R., Palmer S.N., Wang X.S., Reyes-Gibby C.C., et al. Fatigue and sleep disturbance in patients with cancer, patients with clinical depression, and community-dwelling adults. J. Pain Symptom Manag. 2003;25(4):307–318. doi: 10.1016/s0885-3924(02)00682-6. [DOI] [PubMed] [Google Scholar]
- 8.Palesh O.G., Roscoe J.A., Mustian K.M., Roth T., Savard J., Ancoli-Israel S., et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: university of Rochester cancer center-community clinical oncology program. J. Clin. Oncol. 2010;28(2):292–298. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Sleep Foundation. Bedroom Poll; 2012. 2012. [Google Scholar]
- 10.Berger A.M. Update on the state of the science: seep-wake disturbances an adult patients with cancer. Oncol. Nurs. Forum. 2009;36(4):401. doi: 10.1188/09.ONF.E165-E177. [DOI] [PubMed] [Google Scholar]
- 11.Harrington C.B., Hansen J.A., Moskowitz M., Todd B.L., Feuerstein M. It's not over when it's over: long-term symptoms in cancer survivors--a systematic review. Int. J. Psychiatr. Med. 2010;40(2):163–181. doi: 10.2190/PM.40.2.c. [DOI] [PubMed] [Google Scholar]
- 12.Palesh O., Peppone L., Innominato P.F., Janelsins M., Jeong M., Sprod L., et al. Prevalence, putative mechanisms, and current management of sleep problems during chemotherapy for cancer. Nat. Sci. Sleep. 2012;4:151–162. doi: 10.2147/NSS.S18895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ancoli-Israel S., Liu L., Rissling M., Natarajan L., Neikrug A.B., Palmer B.W., et al. Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: a 1-year longitudinal study. Support. Care Cancer. 2014;22(9):2535–2545. doi: 10.1007/s00520-014-2204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steel J.L., Terhorst L., Collins K.P., Geller D.A., Vodovotz Y., Kim J., et al. Prospective analyses of cytokine mediation of sleep and survival in the context of advanced cancer. Psychosom. Med. 2018;80(5):483–491. doi: 10.1097/PSY.0000000000000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibbins J., McCoubrie R., Kendrick A.H., Senior-Smith G., Davies A.N., Hanks G.W. Sleep-wake disturbances in patients with advanced cancer and their family carers. J. Pain Symptom Manag. 2009;38(6):860–870. doi: 10.1016/j.jpainsymman.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 16.Morris B.A., Thorndike F.P., Ritterband L.M., Glozier N., Dunn J., Chambers S.K. Sleep disturbance in cancer patients and caregivers who contact telephone-based help services. Support. Care Cancer. 2015;23(4):1113–1120. doi: 10.1007/s00520-014-2458-y. [DOI] [PubMed] [Google Scholar]
- 17.McCurry S.M., Logsdon R.G., Teri L., Vitiello M.V. Sleep disturbances in caregivers of persons with dementia: contributing factors and treatment implications. Sleep Med. Rev. 2007;11(2):143–153. doi: 10.1016/j.smrv.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Happe S., Berger K., Investigators F.S. The association between caregiver burden and sleep disturbances in partners of patients with Parkinson's disease. Age Ageing. 2002;31(5):349–354. doi: 10.1093/ageing/31.5.349. [DOI] [PubMed] [Google Scholar]
- 19.Peng H.L., Chang Y.P. Sleep disturbance in family caregivers of individuals with dementia: a review of the literature. Perspect. Psychiatr. Care. 2013;49(2):135–146. doi: 10.1111/ppc.12005. [DOI] [PubMed] [Google Scholar]
- 20.Carter P.A. A brief behavioral sleep intervention for family caregivers of persons with cancer. Cancer Nurs. 2006;29(2):95–103. doi: 10.1097/00002820-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Dhruva A., Lee K., Paul S.M., West C., Dunn L., Dodd M., et al. Sleep-wake circadian activity rhythms and fatigue in family caregivers of oncology patients. Cancer Nurs. 2012;35(1):70–81. doi: 10.1097/NCC.0b013e3182194a25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson J.A., Rash J.A., Campbell T.S., Savard J., Gehrman P.R., Perlis M., et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med. Rev. 2016;27:20–28. doi: 10.1016/j.smrv.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Zhou E.S., Partridge A.H., Syrjala K.L., Michaud A.L., Recklitis C.J. Evaluation and treatment of insomnia in adult cancer survivorship programs. J. Cancer Surviv. 2017;11(1):74–79. doi: 10.1007/s11764-016-0564-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaffer K.M., Garland S.N., Mao J.J., Applebaum A.J. Insomnia among cancer caregivers: a proposal for tailored cognitive behavioral therapy. J. Psychother. Integrat. 2018;28(3):275–291. doi: 10.1037/int0000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savard J., Ivers H., Savard M.H., Morin C.M., Caplette-Gingras A., Bouchard S., et al. Efficacy of a stepped care approach to deliver cognitive-behavioral therapy for insomnia in cancer patients: a noninferiority randomized controlled trial. Sleep. 2021;44(11) doi: 10.1093/sleep/zsab166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palesh O., Solomon N., Hofmeister E., Jo B., Shen H., Cassidy-Eagle E., et al. A novel approach to management of sleep-associated problems in patients with breast cancer (MOSAIC) during chemotherapy : a pilot study. Sleep. 2020;43(10) doi: 10.1093/sleep/zsaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper C.J., Owen P.J., Sprajcer M., Crowther M.E., Craige E.A., Ferguson S.A., et al. Interventions to improve sleep in caregivers: a systematic review and meta-analysis. Sleep Med. Rev. 2022;64 doi: 10.1016/j.smrv.2022.101658. [DOI] [PubMed] [Google Scholar]
- 28.Chen Q., Terhorst L., Geller D.A., Marsh W., Antoni M., Dew M.A., et al. Trajectories and predictors of stress and depressive symptoms in spousal and intimate partner cancer caregivers. J. Psychosoc. Oncol. 2020;38:527–542. doi: 10.1080/07347332.2020.1752879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunn H.E., Buysse D.J., Hasler B.P., Begley A., Troxel W.M. Sleep concordance in couples is associated with relationship characteristics. Sleep. 2015;38(6):933–939. doi: 10.5665/sleep.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasler B.P., Troxel W.M. Couples' nighttime sleep efficiency and concordance: evidence for bidirectional associations with daytime relationship functioning. Psychosom. Med. 2010;72(8):794–801. doi: 10.1097/PSY.0b013e3181ecd08a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Troxel W.M., Robles T.F., Hall M., Buysse D.J. Marital quality and the marital bed: examining the covariation between relationship quality and sleep. Sleep Med. Rev. 2007;11(5):389–404. doi: 10.1016/j.smrv.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kane H.S., Slatcher R.B., Reynolds B.M., Repetti R.L., Robles T.F. Daily self-disclosure and sleep in couples. Health Psychol. 2014;33(8):813–822. doi: 10.1037/hea0000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segrin C., Burke T.J. Loneliness and sleep quality: dyadic effects and stress effects. Behav. Sleep Med. 2015;13(3):241–254. doi: 10.1080/15402002.2013.860897. [DOI] [PubMed] [Google Scholar]
- 34.Gunn H.E., Lee S., Eberhardt K.R., Buxton O.M., Troxel W.M. Nightly sleep-wake concordance and daily marital interactions. Sleep Health. 2021;7(2):266–272. doi: 10.1016/j.sleh.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunn H.E., Buysse D.J., Matthews K.A., Kline C.E., Cribbet M.R., Troxel W.M. Sleep-wake concordance in couples is inversely associated with cardiovascular disease risk markers. Sleep. 2017;40(1) doi: 10.1093/sleep/zsw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walters E.M., Phillips A.J.K., Boardman J.M., Norton P.J., Drummond S.P.A. Vulnerability and resistance to sleep disruption by a partner: a study of bed-sharing couples. Sleep Health. 2020;6(4):506–512. doi: 10.1016/j.sleh.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Walters E.M., Phillips A.J.K., Mellor A., Hamill K., Jenkins M.M., Norton P.J., et al. Sleep and wake are shared and transmitted between individuals with insomnia and their bed-sharing partners. Sleep. 2020;43(1) doi: 10.1093/sleep/zsz206. [DOI] [PubMed] [Google Scholar]
- 38.Mellor A., Hamill K., Jenkins M.M., Baucom D.H., Norton P.J., Drummond S.P.A. Partner-assisted cognitive behavioural therapy for insomnia versus cognitive behavioural therapy for insomnia: a randomised controlled trial. Trials. 2019;20 doi: 10.1186/s13063-019-3334-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feinberg M.E., Jones D.E., Hostetler M.L., Roettger M.E., Paul I.M., Ehrenthal D.B. Couple-focused prevention at the transition to parenthood, a randomized trial: effects on coparenting, parenting, family violence, and parent and child adjustment. Prev. Sci. 2016;17(6):751–764. doi: 10.1007/s11121-016-0674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadeh A., Mindell J.A., Owens J. Why care about sleep of infants and their parents? Sleep Med. Rev. 2011;15(5):335–337. doi: 10.1016/j.smrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Buysse D.J., Reynolds C.F., Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh sleep quality Index - a new instrument for psychiatric practice and research. Psychiatr. Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 42.Kim Y., Carver C.S., Cannady R.S., Shaffer K.M. Self-reported medical morbidity among informal caregivers of chronic illness: the case of cancer. Qual. Life Res. 2013;22(6):1265–1272. doi: 10.1007/s11136-012-0255-y. [DOI] [PubMed] [Google Scholar]
- 43.Maltby K.F., Sanderson C.R., Lobb E.A., Phillips J.L. Sleep disturbances in caregivers of patients with advanced cancer: a systematic review. Palliat. Support Care. 2017;15(1):125–140. doi: 10.1017/S1478951516001024. [DOI] [PubMed] [Google Scholar]
- 44.Chen Q., Terhorst L., Lowery-Allison A., Cheng H., Tsung A., Layshock M., et al. Sleep problems in advanced cancer patients and their caregivers: who is disturbing whom? J. Behav. Med. 2020;43(4):614–622. doi: 10.1007/s10865-019-00088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sabourin S., Valois P., Lussier Y. Development and validation of a brief version of the Dyadic Adjustment SCale with a nonparametric item analysis model. Psychol. Assess. 2005;17(1):15–27. doi: 10.1037/1040-3590.17.1.15. [DOI] [PubMed] [Google Scholar]
- 46.Morin C.M., Vallieres A., Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16) Sleep. 2007;30(11):1547–1554. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carver C.S. Adult attachment and personality: converging evidence and a new measure. Pers. Soc. Psychol. Bull. 1997;23(8):865–883. [Google Scholar]
- 48.Carney C.E., Buysse D.J., Ancoli-Israel S., Edinger J.D., Krystal A.D., Lichstein K.L., et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morin C.M., Belleville G., Belanger L., Ivers H. The insomnia severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen J. second ed. Routledge; New York: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 51.Perrin A. Pew Research Center; 2021. Mobile Technology and Home Broadband 2021.https://www.pewresearch.org/internet/2021/06/03/mobile-technology-and-home-broadband-2021/ [Available from: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.