Summary
Background
The evidence of radiofrequency ablation (RFA) following transarterial chemoembolisation (TACE) combined with sorafenib for intermediate-stage recurrent hepatocellular carcinoma (RHCC) is limited. Patient responses to this treatment vary because of the heterogeneous nature of RHCC, making it important to identify patients who are most likely to benefit from this combination therapy. The aim of this study was to evaluate the efficacy of RFA following TACE and sorafenib for the intermediate-stage RHCC.
Methods
This retrospective, multicentre, cohort study included 363 patients with intermediate-stage RHCC underwent TACE combined with sorafenib (TACE-sorafenib group) or RFA following TACE and sorafenib (TACE-sorafenib + RFA group) between January 01, 2009 to December 31, 2015 from four institutions in China. Overall survival (OS), progression-free survival (PFS) and efficacy of patients were compared between the two groups by propensity score–matching (PSM).
Findings
The 1-, 3-, and 5-year OS rates were 97.7%, 83.7%, 54.7% in TACE-sorafenib + RFA group, and 93.3%, 57.0%, 32.7% in TACE-sorafenib group. The 1-, 2-, and 3-year PFS rates were 85.3%, 58.0%, 26.9% in TACE-sorafenib + RFA group, and 55.3%, 30.7%, 15.3% in TACE-sorafenib group. Compared with the TACE-sorafenib group, the TACE-sorafenib + RFA group had significantly longer OS (HR, 0.54; 95%CI, 0.40–0.73; P < 0.001) and PFS (HR, 0.52; 95% CI, 0.41–0.66; P < 0.001). Subgroup analysis was conducted to precisely screen out the beneficial population from RFA treatment.
Interpretation
Our findings suggest that addition of RFA following TACE and sorafenib combination was superior to TACE combined with sorafenib for intermediate-stage RHCC, resulting in longer OS and PFS. Patients who had good response to TACE and achieved downstaging successfully could not benefit from the RFA therapy.
Funding
This research was funded by National Natural Science Foundation of China (No. 81627803), Chen Xiao-Ping Science and Technology Development Fund (CXPJJH1200009-06).
Keywords: Recurrent hepatocellular carcinoma, Intermediate-stage, Transarterial chemoembolisation, Sorafenib, Radiofrequency ablation
Research in context.
Evidence before this study
The evidence of radiofrequency ablation (RFA) following transarterial chemoembolization (TACE) combined with sorafenib for intermediate-stage recurrent hepatocellular carcinoma (RHCC) is limited. We screened MEDLINE, Web of Science for relevant articles on November 1, 2022, without language or date restrictions using the terms (“radiofrequency ablation following transarterial chemoembolization combined with sorafenib” OR “TACE in combination with sorafenib and ablation”) AND (“recurrent hepatocellular carcinoma” OR “recurrent liver cancer”). There was no study to evaluate the efficacy of RFA following TACE combined with sorafenib for the intermediate-stage RHCC.
Added value of this study
We found that addition of RFA following TACE and sorafenib combination was associated longer progression-free survival and overall survival than TACE and sorafenib combination for intermediate-stage RHCC. In addition, patients who showed good response to TACE and achieved downstaging successfully could not benefit from the RFA therapy.
Implications of all the available evidence
Our research provides new evidence that TACE-sorafenib + RFA treatment significantly improved the overall survival (OS) and progression-free survival (PFS) of intermediate-stage RHCC patients compared with those who received TACE-sorafenib treatment. It provides a strong reference for clinical treatment.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver and the fourth leading cause of cancer-related death worldwide.1 In China, HCC is mainly caused by chronic hepatitis B virus (HBV) infection.2 Hepatectomy is the first-choice treatment for patients with early-stage HCC. However, more than 50% of patients experienced recurrence within 5 years after hepatectomy, and approximately 30% of patients with recurrent hepatocellular carcinoma (RHCC) are diagnosed at intermediate-stage.3 Barcelona Clinic Liver Cancer stage B (BCLC B) was the intermediate-stage which consists of patients burdened multinodular HCCs without extrahepatic metastasis and preserved liver function.4,5 This definition of BCLC B is rather broad and includes a heterogeneous patient population, and poses unique challenges for therapeutic management which is different from the early and advanced stages.6 Therapies for RHCC are almost the same as those for primary HCC in recurrent guidelines.7 The recommended treatment modality for HCC at BCLC B stage is transarterial chemoembolisation (TACE).8 However, according to the heterogeneity of the intermediate population, patients are best served when the treatment decision is individualised and taken within a multidisciplinary team. For instance, TACE as a non-radical treatment, achieves complete radiological necrosis in about 20% of cases. For patients not achieving complete necrosis, in this case other treatments such as ablation and system therapy could be taken into consideration.
Previous studies have shown that combination therapy of TACE with sorafenib could achieve better survival outcomes than TACE only for primary HCC at BCLC B stage.9,10 The embolism of TACE induced the ischemic changes which led to the upregulation of vascular endothelial growth factor (VEGF).11 Sorafenib is an oral multi-kinase inhibitor, which could inhibit VEGF expression and tumour angiogenesis.12 Many researchers have reported the favorable safety and efficacy of sorafenib combined with TACE.10,13,14 Peng et al. showed that TACE plus sorafenib obtained obvious longer overall survival (OS) and progression-free survival (PFS) than TACE only for the intermediate-stage RHCC in patients with microvascular invasion positive at primary resection.15
Although TACE combined with sorafenib could obtain favorable controlling effect of intermediate-stage RHCC, the ablation could be applied for this population with limited tumours. Previous studies have shown that TACE combined thermal ablation provided better tumour control and longer survival than TACE alone in patients with primary intermediate-stage HCC.16, 17, 18 In fact, ablation in combination with TACE has been proposed for inoperative intermediate-stage HCC with a diameter of 3–7 cm in the Chinese guidelines.19,20 While for intermediate-stage RHCC, ablation was more flexible applied due to the smaller tumour sise compared with primary HCC. Wang et al. proved that TACE combined with radiofrequency ablation (RFA) was a superior strategy to TACE alone in intermediate-stage RHCC, and the OS and PFS were longer than TACE.21 Although Wang et al. proved that RFA was effective for intermediate-stage RHCC, there still needs further exploration of TACE plus sorafenib and RFA on intermediate-stage RHCC and more related risk factors such as initial hepatectomy stage data (microvascular invasion [MVI], tumour number, tumour differentiation), recurrent stage data (tumour sise, tumour number), recurrence patterns (early or late recurrence). Thus, the aim of this multicentre study is to evaluate the efficacy of RFA following TACE and sorafenib for the intermediate-stage RHCC and identifies the relevant risk factors of prognosis.
Methods
Patients and study design
This retrospective multicentre cohort study was conducted in patients who had RHCC with intermediate-stage between January 01, 2009 to December 31, 2015 in Chinese PLA General Hospital, Hunan Provincial People's Hospital, Sun Yat-sen University Cancer Centre, The First Affiliated Hospital, Sun Yat-Sen University. The study was centrally approved by the ethics committee of these four centres and was conducted according to the guidelines of the Declaration of Helsinki.22 Informed consent was waived because this study was retrospective.
Eligibility criteria included clinical diagnosis of RHCC based on a history of partial hepatectomy for primary HCC. Patients who met the following criteria were enrolled: (1) 18–70 years; (2) RHCC diagnosed by imaging studies (triphasic computed tomography [CT] and/or magnetic resonance imaging [MRI]) showing both early enhancement and delayed decreased enhancement, in accordance with the American Association for the Study of Liver Diseases Practice Guideline for Management of HCC.23; (3) intermediate-stage RHCC (two to three lesions which at least one was >3 cm in sise or more than three tumours); (4) the tumour number was no more than six, and the maximum tumour diameter was ≤5 cm; (5) absence of extrahepatic metastasis or macrovascular invasion; (6) received sorafenib more than 3 months; (7) Child–Pugh class A or B; (8) TACE as initial treatment after tumour recurrence and showed no tumour progression after TACE. The excluding criteria were as follows: (1) under 18 years or over 70 years of age; (2) primary intermediate-stage HCC; (3) RHCC after radical thermal ablation; (4) RHCC with more than six tumours; (5) tumour progression after TACE; (6) incomplete information about prognostic variables; (7) patients stopped sorafenib administration within 3 months; (8) lost follow-up information within 3 months.
We collected data on the baseline demographic characteristics of each patient. Initial hepatectomy stage data included initial maximum tumour sise, tumour differentiation, MVI, tumour capsule, resection margin, BCLC stage of primary HCC. RHCC information included the number of RHCC tumours, tumour sise of RHCC, early and late stage of RHCC (recurrence was divided into early (≤2 years) and late recurrence (>2 years)24), and cirrhosis (pathological results of initial resection, or diminished sise of liver lobe, compensatory enlargement of caudate lobe of liver, and splenomegaly on imaging). We used the Albumin-Bilirubin (ALBI) grade to evaluate liver function.25
TACE procedure
TACE procedure was performed by radiologists with more than 5 years of experience in interventional therapy for RHCC. The procedure was a super-selective microcatheter inserted into the artery supplying each tumour. Then a combination of lipiodol (5–15 ml), lobaplatin (30–50 mg), and Pirarubicin (30–50 mg) was infused into each tumour. We defined technical success as complete embolisation of the tumour-feeding artery resulting in no tumour staining observed by angiogram at the end of procedure. TACE procedures were repeated when the clinician judged in demand after 3 experienced clinician's discussion. The evaluation of tumour response to TACE was according to modified Response Evaluation Criteria in Solid Tumours (mRECIST) criteria one month after TACE by contrast enhanced CT or MRI.26 Tumour response was concluded complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD).
Assessment of downstaging
One month after first TACE procedure, contrast-enhanced CT scan or MRI was performed. When assessing tumour response to TACE, the contrast-enhanced portions of viable tumours was evaluated by experienced roentgenologist. Intra-tumoural arterial enhancement was considered as viable tumour tissue. Tumour area that showed complete absence of contrast uptake can be assumed to represent necrotic tissue according the mRICIST criteria.26,27 Successful downstaging was defined as reduction in viable tumour sise or number to meet the Milan criteria. Namely the viable tumour diameter was ≤3 cm (viable tumour number was ≤3) or viable tumour diameter was ≤5 cm (viable tumour number was single).
Sorafenib administration
Sorafenib were given at an initial dosage of 400 mg twice daily without additional systemic therapies. Sorafenib was administered 4–7 days after first TACE treatment, and patients received continual sorafenib. Drug-related complications were recorded. Sorafenib dose reduction was based on the presence of toxicity. If grade 3 or 4 hematologic toxicity, skin toxicity, gastrointestinal toxicity, hypertension, or hepatic dysfunction defined by National Cancer Institute Common Terminology Criteria for Adverse Events occurred,28 and a dose adjustment (400 mg once daily) was required until the adverse events were alleviated or eliminated. After dose adjustment, if grade 3 or 4 adverse events continued, sorafenib treatment was halted until the adverse effects were alleviated or until they disappeared.
RFA procedure
RFA followed TACE within 6–8 weeks and sorafenib continued. RFA at each institution was performed by a physician with experience in at least 100 prior cases of hepatic RFA. Percutaneous RFA was performed using the cool-tip RFA system to achieve a single or multiple overlapping ablations with a goal to cover an area larger than the entire lesion plus an ablative margin of 0.5 cm or more. Contrast-enhanced ultrasound was used for tumour visualisation. Complete ablation was defined as no area of enhancement was seen within or at the periphery of the ablated zone. If imaging studies showed radiological features of residual tumour that suggested incomplete ablation in contrast enhanced ultrasound or CT/MRI, an additional session of percutaneous RFA with the intention of complete ablation was performed again. The RFA was considered curative when all the tumours were completely ablated. Whether or not a patient with intermediate-stage RHCC receives RFA depends on the severity of the disease, physical condition, financial status, and the physician's recommendation (after multidisciplinary discussion).
Follow-up
The first follow-up was performed 4 weeks after first TACE treatment, and then every 8–12 weeks until death or dropout. At each follow-up visit, alpha-fetoprotein (AFP) and liver function tests and abdominal contrast enhanced CT or MRI was performed. The primary endpoint for the study was OS, and the secondary endpoint was PFS. OS was defined as the time from accepting TACE to death or last follow-up, and PFS was defined as the time from accepting the date of TACE to disease progression or last follow-up. The follow-up period for this study was terminated on December 30, 2021.
Statistical analysis
Propensity score-matching (PSM) analysis was used to reduce the effect of selection bias and potential confounding between the two groups. Propensity scores were estimated using a multivariate logistic regression model, by inserting the following variables: initial hepatectomy stage data (tumour diameter, tumour capsule, BCLC stage, MVI, cirrhosis); recurrent stage data (sex, RHCC diameter, number of RHCC, recurrent stage). Patients were matched 1:1 using the nearest neighbor method with a caliber of 0.1 (Supplementary Fig. S1), and this matching process has been described in a previous study.29
To evaluate difference between the two groups, the Pearson χ2 test and Fisher's exact test were used to compare categorical variables. The survival curves of OS and PFS were constructed according to the Kaplan–Meier method with the log-rank test, and the OS and PFS rates were determined using a life table using the z test. All statistical tests were 2 sides, and P < 0.05 was considered significant. The statistical analyses were performed using the Statistical Package for the Social Science (SPSS) software (version 22.0, SPSS Inc., Chicago, IL, USA) for Windows and R software for Windows (Version 3.6.4 http://www.r-project.org).
Role of the funding source
The funding sources had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication.
Results
Baseline characteristics of the patients
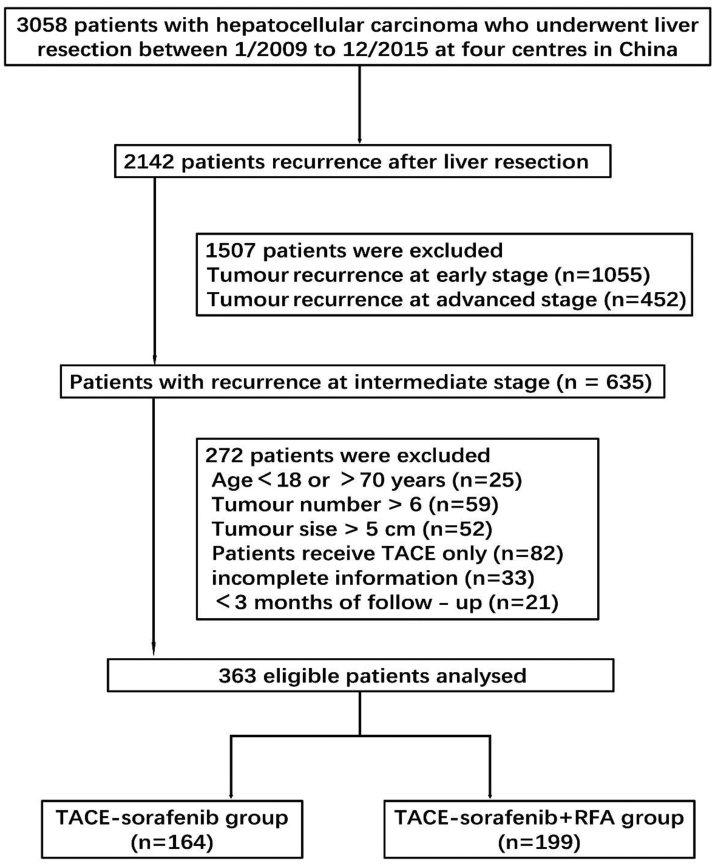
A total of 3058 patients with HCC accepted curative resection from January 2009 to December 2015 in four centres. 2142 patients had recurrence and total of 363 patients were enrolled in this study at last. 164 patients received TACE combined with sorafenib (TACE-sorafenib group) and 199 patients received RFA following TACE combined with sorafenib (TACE-sorafenib + RFA group). The patient selection criterion was shown in Fig. 1. Propensity score–matching (1:1 matching) analysis generated two new cohorts of 150 and 150 patients in the TACE-sorafenib and TACE-sorafenib + RFA groups, respectively, and the characteristics of the two groups were balanced, with the standardised mean difference less than 10% for all baseline variables (Supplementary Fig. S2). Patients in the entire cohort, the median duration of follow-up was 42.2 months (range, 10.0–134.0 months), compared to the TACE-sorafenib group, the TACE-sorafenib + RFA group showed more patients with complete tumour capsule (73.4% vs. 61.0%) and cirrhosis (54.3% vs. 41.5%), male proportion (92.5% vs. 84.8%), RHCC number ≤3 (48.2% vs. 31.1%) and late recurrence proportion (57.8% vs. 31.7%). After PSM, there were no significant difference between the 2 groups. The demographic data, etiology of liver disease, and tumour characteristics of patients in the entire cohort and in the matched cohort are summarised in Table 1.
Fig. 1.
Flow chart of patient selection.
Table 1.
Baseline characteristics of patients who underwent transarterial chemoembolization (TACE)-sorafenib or TACE-sorafenib + radiofrequency ablation (RFA) treatment for intermediate-stage recurrent hepatocellular carcinoma (RHCC).
|
Variable |
Entire cohort |
Propensity score-matched cohort (1:1 ratio) |
||||
|---|---|---|---|---|---|---|
| TACE-sorafenib (n = 164) | TACE-sorafenib + RFA (n = 199) | P value | TACE-sorafenib (n = 150) | TACE-sorafenib + RFA (n = 150) | P value | |
| Initial hepatectomy stage data | ||||||
| Surgical margin, cm | ||||||
| ≤1 | 85 (51.8) | 110 (55.3) | 0.512 | 79 (52.7) | 83 (55.3) | 0.643 |
| >1 | 79 (48.2) | 89 (44.7) | 71 (47.3) | 67 (44.7) | ||
| Tumour diameter, cm | ||||||
| ≤5 | 30 (18.3) | 30 (15.1) | 0.124 | 30 (20.0) | 26 (17.3) | 0.255 |
| >5, <10 | 85 (51.8) | 124 (62.3) | 75 (50.0) | 89 (59.3) | ||
| ≥10 | 49 (29.9) | 45 (22.6) | 45 (30.0) | 35 (23.4) | ||
| BCLC stage | ||||||
| A | 107 (65.2) | 114 (57.3) | 0.122 | 98 (65.3) | 89 (59.3) | 0.284 |
| B | 57 (34.8) | 85 (42.7) | 52 (34.7) | 61 (40.7) | ||
| Tumour capsule | ||||||
| Incomplete | 64 (39.0) | 53 (26.6) | 0.012 | 54 (36.0) | 49 (32.7) | 0.543 |
| Complete | 100 (61.0) | 146 (73.4) | 96 (64.0) | 101 (67.3) | ||
| MVI | ||||||
| Negative | 104 (63.4) | 134 (67.3) | 0.434 | 100 (66.7) | 103 (68.7) | 0.711 |
| Positive | 60 (36.6) | 65 (32.7) | 50 (33.3) | 47 (31.3) | ||
| Tumour differentiation | ||||||
| I-II | 137 (83.5) | 160 (80.4) | 0.441 | 104 (69.3) | 106 (70.7) | 0.801 |
| III-IV | 27 (16.5) | 39 (19.6) | 46 (30.7) | 44 (29.3) | ||
| Cirrhosis | ||||||
| Negative | 96 (58.5) | 91 (45.7) | 0.015 | 84 (56.0) | 78 (52.0) | 0.487 |
| Positive | 68 (41.5) | 108 (54.3) | 66 (44.0) | 72 (48.0) | ||
| Recurrent stage data | ||||||
| Age, years | ||||||
| <60 | 127 (77.4) | 158 (79.4) | 0.651 | 116 (77.3) | 121 (80.7) | 0.478 |
| ≥60 | 37 (22.6) | 41 (20.6) | 34 (22.7) | 29 (19.3) | ||
| Sex | ||||||
| Male | 139 (84.8) | 184 (92.5) | 0.020 | 130 (86.7) | 135 (90.0) | 0.369 |
| Female | 25 (15.2) | 15 (7.5) | 20 (13.3) | 15 (10.0) | ||
| Anti-virus therapy | ||||||
| No | 48 (29.3) | 53 (26.6) | 0.577 | 45 (30.0) | 43 (28.7) | 0.800 |
| Yes | 116 (70.7) | 146 (73.4) | 105 (70.0) | 107 (71.3) | ||
| AFP, ng/mL | ||||||
| <400 | 94 (57.3) | 119 (59.8) | 0.633 | 87 (58.0) | 90 (60.0) | 0.725 |
| ≥400 | 70 (42.7) | 80 (40.2) | 63 (42.0) | 60 (40.0) | ||
| RHCC diameter, cm | ||||||
| ≤3 | 68 (41.5) | 103 (51.8) | 0.051 | 67 (44.7) | 70 (46.7) | 0.728 |
| >3 | 96 (58.5) | 96 (49.2) | 83 (55.3) | 80 (53.3) | ||
| RHCC number | ||||||
| ≤3 | 51 (31.1) | 96 (48.2) | 0.001 | 51 (34.0) | 53 (35.3) | 0.808 |
| 4–6 | 113 (68.9) | 103 (51.8) | 99 (66.0) | 97 (64.7) | ||
| ALBI grade | ||||||
| Grade 1 | 109 (66.5) | 125 (62.8) | 0.470 | 97 (64.7) | 94 (62.7) | 0.719 |
| Grade 2 | 55 (33.5) | 74 (37.2) | 53 (35.3) | 56 (37.3) | ||
| Recurrent stage | ||||||
| Early | 112 (68.3) | 84 (42.2) | 0.040 | 98 (65.3) | 89 (59.3) | 0.284 |
| Late | 52 (31.7) | 115 (57.8) | 52 (34.7) | 61 (40.7) | ||
Data are n (%).
TACE, transarterial chemoembolization; RFA, radiofrequency ablation; BCLC, Barcelona Clinic Liver Cancer; MVI, microvascular invasion; AFP, alpha-fetoprotein; RHCC, recurrent hepatocellular carcinoma; ALBI, albumin-bilirubin.
Overall survival analysis between TACE-sorafenib and TACE-sorafenib + RFA groups
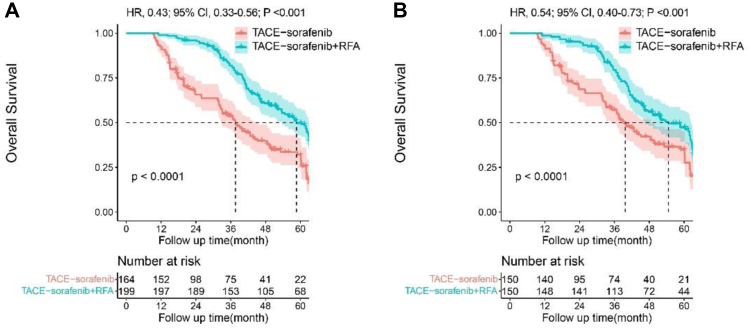
During the follow-up period, 238 (65.6%) patients died in the entire cohort. Of the patients who had died, 110 (67.1%) patients in the TACE-sorafenib group and 128 (64.3%) patients in the TACE-sorafenib + RFA group, respectively. Patients in the entire cohort, the median OS was 37.6 ± 2.8 months (95% confidence interval [CI], 32.1–43.2) in the TACE-sorafenib group, and 58.7 ± 3.1 months (95% CI, 52.7–64.7) in the TACE-sorafenib + RFA group, The 1-, 3-, and 5-year OS were 99.0%, 81.9%, 49.7% in TACE-sorafenib + RFA group, and 92.7%, 53.5%, 30.1% in TACE-sorafenib group. After propensity matching, the 1-, 3-, and 5-year OS were 98.7%, 79.5%, 46.2% in TACE-sorafenib + RFA group, and 93.3%, 57.0%, 32.7% in TACE-sorafenib group. The TACE-sorafenib + RFA group had significantly longer OS than the TACE-sorafenib group both in the entire cohort (Fig. 2A, hazard ratio [HR], 0.43; 95%CI, 0.33–0.56; P < 0.001) and in the matched cohort (Fig. 2B, HR, 0.54; 95%CI, 0.40–0.73; P < 0.001).
Fig. 2.
Kaplan–Meier analysis of overall survival (OS). Data in the entire cohort (2A) and in the propensity score-matched cohort (2B) in patients with intermediate-stage recurrent hepatocellular carcinoma (RHCC).
Univariate analysis of OS and PFS were presented in Supplementary Table S1. Multivariate analysis revealed recurrent tumour diameter > 3 cm (HR, 1.54; 95% CI, 1.12–2.11; P = 0.007), recurrent tumour number >3 (HR, 2.57; 95% CI, 1.81–3.63; P < 0.001), early recurrent stage (HR, 1.81; 95% CI, 1.28–2.56; P = 0.001), TACE-sorafenib treatment (HR, 2.41; 95% CI, 1.77–3.26; P < 0.001); primary tumour diameter ≥10 cm (HR, 1.92; 95% CI, 1.18–3.12; P = 0.009), primary HCC at BCLC B stage (HR, 1.45; 95% CI, 1.05–2.00; P = 0.022), MVI positive (HR, 1.54; 95% CI, 1.14–2.09; P = 0.022) were risk factors related with poorer OS (Table 2).
Table 2.
Multivariate analysis of prognostic factors for overall survival (OS) and progression-free survival (PFS) in patients with intermediate-stage recurrent hepatocellular carcinoma (RHCC) after propensity score matching (PSM).
| Variable Comparison | Overall survival |
Progression-free survival |
|||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Recurrent stage data | |||||
| Tumour diameter. cm | ≤3 vs. >3 | 1.54 (1.12–2.11) | 0.007 | 1.89 (1.46–2.44) | <0.001 |
| Tumour number | ≤3 vs. >3 | 2.57 (1.81–3.63) | <0.001 | 2.80 (2.21–3.69) | <0.001 |
| Recurrent stage | Late vs. Early | 1.81 (1.28–2.56) | 0.001 | 1.66 (1.27–2.16) | <0.001 |
| Types of treatment | TACE-sorafenib + RFA vs. TACE-sorafenib | 2.41 (1.77–3.26) | <0.001 | 2.71 (2.10–3.50) | <0.001 |
| Initial hepatectomy stage data | |||||
| Tumour diameter, cm | ≤5 | Reference | |||
| >5, <10 | 1.68 (0.89–2.58) | 0.074 | |||
| BCLC stage | ≥10 | 1.92 (1.18–3.12) | 0.009 | ||
| BCLC stage | A vs. B | 1.45 (1.05–2.00) | 0.022 | 1.44 (1.11–1.86) | 0.006 |
| MVI | Negative vs. Positive | 1.54 (1.14–2.09) | 0.005 | 1.24 (0.94–1.63) | 0.127 |
HR, hazard ratio; CI, confidence interval; TACE, transarterial chemoembolization; RFA, radiofrequency ablation; BCLC, Barcelona Clinic Liver Cancer; MVI, microvascular invasion.
Progression-free survival analysis between TACE-sorafenib and TACE-sorafenib + RFA groups
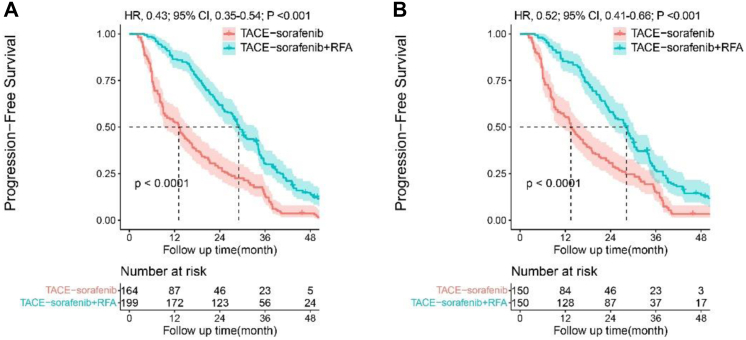
Patients in the entire cohort, the median PFS was 13.1 ± 1.7 months (95% CI, 9.8–16.4) in TACE-sorafenib group, and 29.1 ± 1.0 months (95% CI, 27.1–31.0) in TACE-sorafenib + RFA group. The 1-, 2-, and 3-year PFS rates were 52.4%, 28.0%, 12.8% in TACE-sorafenib group, and 86.4%, 61.8%, 30.5% in TACE-sorafenib + RFA group (Fig. 3A). After propensity matching, the 1-, 2-, and 3-year PFS rates were 55.3%, 30.7%, 15.3% in TACE-sorafenib group, and 85.3%, 58.0%, 26.9% in TACE-sorafenib + RFA group, respectively (Fig. 3B). The TACE-sorafenib + RFA group had significantly longer PFS than the TACE-sorafenib group both in the entire cohort (HR, 0.43; 95% CI, 0.35–0.54; P < 0.001) and in the matched cohort (HR, 0.52; 95% CI, 0.41–0.66; P < 0.001).
Fig. 3.
Kaplan–Meier analysis of progression-free survival (PFS). Data in the entire cohort (3A) and in the propensity score-matched cohort (3B) in patients with intermediate-stage recurrent hepatocellular carcinoma (RHCC).
Multivariate analysis revealed that recurrent tumour diameter >3 cm (HR, 1.89; 95% CI, 1.46–2.44; P < 0.001), recurrent tumour number >3 (HR, 2.80; 95% CI, 2.21–3.69; P < 0.001), early recurrent stage (HR, 1.66; 95% CI, 1.27–2.16; P < 0.001), TACE-sorafenib treatment (HR, 2.71; 95% CI, 2.10–3.50; P < 0.001); primary HCC at BCLC B stage (HR, 1.44; 95% CI, 1.11–1.86; P = 0.006) were risk factors related with poorer PFS (Table 2).
Downstaging outcomes and survival analysis between TACE-sorafenib and TACE-sorafenib + RFA groups
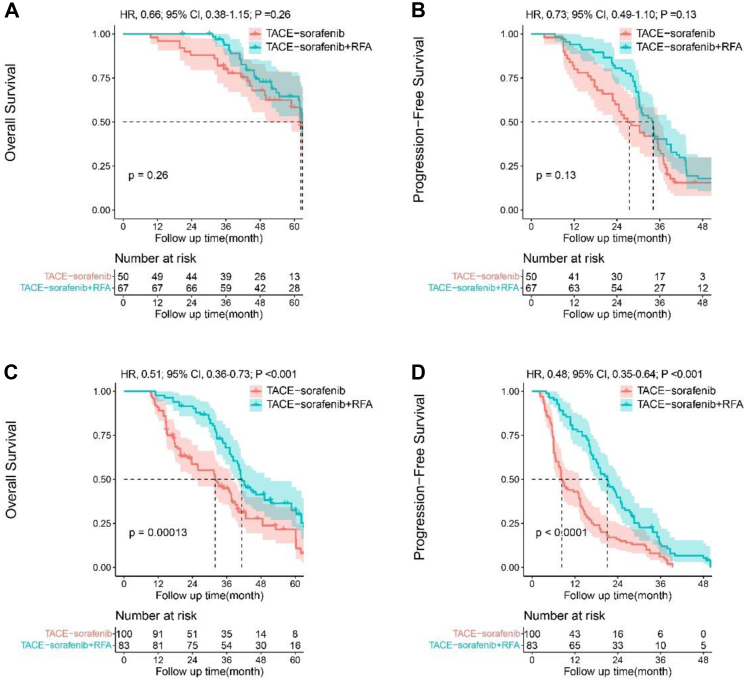
In order to clarify the beneficial populations from RFA, we further analysed tumour response to TACE in the matched cohort. Some patients in the two groups showed good response to TACE (PR and CR) and achieved downstaging, while other patients may only show partial response without downstaging. As shown in Table 3, 50 patients (33.3%) in TACE-sorafenib group and 67 patients (44.7%) patients in TACE-sorafenib + RFA group achieved downstaging. The rates of TACE downstaging were obviously higher in TACE-sorafenib + RFA group than TACE-sorafenib group. For patients who achieved downstaging, no difference was observed with OS (Fig. 4A, HR, 0.66; 95% CI, 0.38–1.15; P = 0.261) and PFS (Fig. 4B, HR, 0.73; 95% CI, 0.49–1.10; P = 0.130) between TACE-sorafenib and TACE-sorafenib + RFA groups. On the contrary, 100 patients (66.7%) in TACE-sorafenib group and 83 patients (55.3%) in TACE-sorafenib + RFA group did not achieve downstaging. For patients who did not achieve downstaging, OS (Fig. 4C) and PFS (Fig. 4D) were obviously different between the two groups. The TACE-sorafenib + RFA group had significantly longer OS (HR, 0.51; 95% CI, 0.36–0.73; P < 0.001) and PFS (HR, 0.48; 95% CI, 0.35–0.64; P < 0.001) than the TACE-sorafenib group.
Table 3.
Outcome of intermediate stage recurrent hepatocellular carcinoma (RHCC) response to transarterial chemoembolization (TACE) with modified Response Evaluation Criteria in Solid Tumours (mRECIST) evaluation.
| Main outcome | TACE-sorafenib N = 150 |
TACE-sorafenib + RFA N = 150 |
P value |
|---|---|---|---|
| Tumour response | 0.058 | ||
| CR | 16 (10.7) | 24 (16.0) | |
| PR | 86 (57.3) | 95 (63.3) | |
| SD | 48 (32.0) | 31 (20.7) | |
| ORR | 102 (68.0) | 119 (79.3) | 0.026 |
| TACE downstaging | 50 (33.3) | 67 (44.7) | 0.044 |
TACE, transarterial chemoembolization; RFA, radiofrequency ablation; CR, complete response; PR, partial response; SD, stable disease; ORR, objective response rate.
Fig. 4.
Kaplan–Meier survival curves for patients with different response to transarterial chemoembolisation (TACE). (4A) Overall survival (OS) and (4B) progression-free survival (PFS) of patients achieved TACE downstaging between TACE-sorafenib and TACE-sorafenib + RFA groups (4C) Overall survival (OS) and (4D) progression-free survival (PFS) of patients didn't achieve TACE downstaging between TACE-sorafenib and TACE-sorafenib + RFA groups.
Treatment-related adverse events between TACE-sorafenib and TACE-sorafenib + RFA groups
All of 363 patients in cohort received at least 3 months of sorafenib. Grades of treatment-related and drug-related adverse events from TACE-sorafenib and TACE-sorafenib + RFA treatment were recorded (Supplementary Table S2). No unexpected adverse events or treatment-related deaths occurred. Median duration of sorafenib in TACE-sorafenib group was 12.2 months (range, 5.0–23.0 months) and 13.5 months (range, 5.0–24.0 months) in TACE-sorafenib + RFA group. For the grade 1–2 of adverse events, patients alleviated after accepting symptomatic treatment or dose reduction. For the 3–4 grade level, patients were temporary stopped the sorafenib until the adverse effects were alleviated, and low dose of sorafenib were continued if possible after recovery.
Discussion
In the present study, we analysed 363 consecutive patients who were treated with TACE-sorafenib (n = 164) or TACE-sorafenib + RFA (n = 199) for intermediate-stage of RHCC. Results from our study suggested that TACE-sorafenib + RFA treatment significantly improved the OS and PFS of intermediate-stage RHCC patients compared with those who received TACE-sorafenib treatment, which was consistently observed in unadjusted, propensity score-matched, and competing risk analyses. In addition, we also found that patients who showed good response to TACE and achieved downstaging successfully could not benefit from the RFA therapy.
The 5-year recurrence rate of HCC after curative hepatectomy is about 70%, which is the main cause of cancer-related death.30,31 Intermediate-stage of RHCC counts 30% of all recurrence, as the primary intermediate-stage HCC, TACE is still the first-line choice.32,33 Previous studies have shown that TACE combined sorafenib showed longer survival than TACE alone for intermediate-stage RHCC.15 While, for patients with limited tumours after recurrence, RFA could provide complete ablation which certainly prolong the survival and slow disease progression.21 The reasons of TACE combined ablation superior to TACE are that TACE could reduce the heat-sink effect of subsequent ablation by obstructing the tumour-feeding flow and the hyperthermia in the tumour area induced by ablation could strengthen the effect of TACE.34 Although local combination therapy has achieved certain efficacy in primary intermediate-stage HCC, it still needs clinical evidence that TACE combined sorafenib and RFA could provide higher survival benefit than TACE combined sorafenib for intermediate-stage RHCC after hepatectomy. To bridge the gap, we conduct this multicentre study.
In our study, patients in the matched cohort, the 1-, 3-, and 5-year OS rates were 98.7%, 79.5%, 46.2% in TACE-sorafenib + RFA group, and 93.3%, 57.0%, 32.7% in TACE-sorafenib group. Compared with the TACE-sorafenib group, the TACE-sorafenib + RFA group had significantly longer OS. Peng et al. illustrated that the 1-, 3-, and 5-year OS of TACE-sorafenib on intermediate-stage RHCC were 77.1%, 49.3%, 38.9%.15 The OS rates of TACE-sorafenib group in our study was higher than the Peng's results, and the reason may be that patients in our study had less tumour number. Wang et al. demonstrated that the 1-, 3- and 5-year OS rates in TACE-RFA on intermediate-stage RHCC were 81.2%, 52.4%, 41.6%.21 The OS rates of TACE-sorafenib + RFA group in our study was longer than Wang's research due to the benefit of sorafenib.
In the present study, our results suggested that TACE-sorafenib + RFA was superior to TACE-sorafenib with respect to both OS and PFS in patients with intermediate-stage RHCC. The underlying reasons for these results might be complicated, but it may be explained by the following aspects. First, through the TACE procedure, tumour number and tumour location were identified, which made the subsequent RFA performed easily and clearly under CT guidance, and tumours were ablated after RFA which certainly provided survival benefit. Second, TACE could also help to control and detect recurrent tumours which might be too small to be distinguished in CT or MRI, providing important information for further local combination therapy and targeted therapy. Third, the effectiveness of sorafenib on HCC has been demonstrated in the SHARP trial,35 thus, the sorafenib acted as effective strategy from controlling state to preventing state after RFA. Studies had proved that TACE-sorafenib was superior to TACE or sorafenib alone on primary and recurrent intermediate-stage HCC.36,37 As was expected, addition of RFA for patients accepting TACE-sorafenib was an effective strategy and should be considered when patients experienced intermediate-stage RHCC with limited tumours.
Interestingly, our study showed that not all patients need RFA. When patients showed good response to TACE, RFA may not be needed. A series of studies have explored downstaging with TACE before liver transplantation, and the results showed that the survival outcome for downstaging were comparable to those initially met the Milan criteria for patients receiving liver transplantation.38,39 Chen et al. have demonstrated that thermal ablation following TACE downstaging outside Milan criteria obtained comparable survival with thermal ablation of HCC within Milan criteria.40,41 Currently, there is no literature about the use of TACE as downstaging tool for intermediate-stage RHCC. In this multicentre study, we found that patients who showed good response to TACE and achieved downstaging successfully could not benefit from the RFA treatment. There were several possible explanations for this result. First, tumours showed good response to TACE and achieved downstaging successfully, and tumour downstaging was thought to be associated with the less biologically aggressive nature of RHCC.42 Second, a good response to TACE, the residual tumour aggression was decreased after TACE downstaging, and added the susceptibility to sorafenib.37 Thus, sorafenib could plenarily control the residual tumours from progression, so addition of RFA was not prerequisite.37 Third, the reprogramming of the microenvironment after TACE, macrophages and natural killer were recruited to tumour, and sorafenib could enhance the function of macrophages and cytotoxic NK cells.43 Therefore, our study revealed that different response to TACE may represent a strategy to select patients for different treatment.
Additionally, it is well known that prior to the immunotherapy, treatment of advanced HCC was dominated by antiangiogenic tyrosine kinase inhibitors, mainly sorafenib.44 The demonstration of the efficacy of programmed death-1 (PD-1) and programmed cell death-Ligand 1 (PD-L1) inhibitor, showed durable responses, manageable safety, and promising survival in HCC therapy.45 The IMbrave150 trial, combination of atezolizumab and bevacizumab, showed obviously better prognosis both in OS and PFS than sorafenib.46 This combination therapy replacing the sorafenib, was approved as first-line treatment in advanced HCC by Food and Drug Administration (FDA).46,47 As the emergence of new multi-kinase inhibitors, immunotherapy, and the combination of the two, sorafenib was not the first choice for HCC patients worldwide.48,49 However, we believe that our study could be applied not only for the sorafenib, but also for other multi-kinase inhibitors as Lenvatinib, and atezolizumab and bevacizumab.
This study has several limitations. First, this study was retrospectively and carried out without randomisation which may have resulted in selection bias, but we tried to minimise such limitation by propensity score matching. Second, although we have carefully selected patients with several clinical characteristics, the influence of measured and unmeasured confounders on the outcome of patients is inevitable. For example, heterogeneous RFA modalities and doctor's experience, and their combinations may have some influence on the results. Third, although we have included data from multiple centres, the number of patients included in the analysis is limited, there may be some influence on the results due to insufficient sample sise, future prospective and large-scale multicentre study needed to verify our founding, which could be as a guideline to treat the intermediate-stage RHCC after thermal ablation.
In conclusion, our study indicated that addition of RFA following TACE and sorafenib combination was associated with longer progression-free survival and overall survival than TACE and sorafenib combination for intermediate-stage RHCC. In addition, patients who showed good response to TACE and achieved downstaging successfully could not benefit from the RFA therapy.
Contributors
WXH, ZQF, MXH and LP were responsible for conception and design. WXH, ZQF, DWB, LW, CMS, XXY, LH and LSQ provided statistical analysis. All authors participated in the data collection. WXH, ZQF were involved in drafting the manuscript, and MXH, LP, DWB, CMS and LSQ were involved in reviewing the manuscript. ZQF, LP and MXH have accessed and verified the data. LP is responsible for the decision to submit the manuscript.
Data sharing statement
Data available from the authors upon reasonable request and with permission of four hospitals authority in China.
Declaration of interests
The authors declare no conflicts of interest.
Acknowledgments
This research was funded by National Natural Science Foundation of China (No. 81627803), Chen Xiao-Ping Science and Technology Development Fund (CXPJJH1200009-06).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2022.101816.
Contributor Information
Ping Liang, Email: liangping301@hotmail.com.
Xian-Hai Mao, Email: maoxh2022@hunnu.edu.cn.
Qun-Fang Zhou, Email: zhouqun988509@163.com.
Appendix A. Supplementary data
References
- 1.Yang J.D., Hainaut P., Gores G.J., Amadou A., Plymoth A., Roberts L.R. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu X., Qin S. Immune checkpoint inhibitors in hepatocellular carcinoma: opportunities and challenges. Oncologist. 2019;24(Suppl 1):3–10. doi: 10.1634/theoncologist.2019-IO-S1-s01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reig M., Cabibbo G. Antiviral therapy in the palliative setting of HCC (BCLC-B and -C) J Hepatol. 2021;74(5):1225–1233. doi: 10.1016/j.jhep.2021.01.046. [DOI] [PubMed] [Google Scholar]
- 4.Llovet J.M., Brú C., Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 5.Tsilimigras D.I., Bagante F., Sahara K., et al. Prognosis after resection of Barcelona clinic liver cancer (BCLC) stage 0, A, and B hepatocellular carcinoma: a comprehensive assessment of the current BCLC classification. Ann Surg Oncol. 2019;26(11):3693–3700. doi: 10.1245/s10434-019-07580-9. [DOI] [PubMed] [Google Scholar]
- 6.Bolondi L., Burroughs A., Dufour J.F., et al. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32(4):348–359. doi: 10.1055/s-0032-1329906. [DOI] [PubMed] [Google Scholar]
- 7.Marrero J.A., Kulik L.M., Sirlin C.B., et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 8.Sieghart W., Hucke F., Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol. 2015;62(5):1187–1195. doi: 10.1016/j.jhep.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Varghese J., Kedarisetty C., Venkataraman J., et al. Combination of TACE and sorafenib improves outcomes in BCLC stages B/C of hepatocellular carcinoma: a single centre experience. Ann Hepatol. 2017;16(2):247–254. doi: 10.5604/16652681.1231583. [DOI] [PubMed] [Google Scholar]
- 10.Chao Y., Chung Y.H., Han G., et al. The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in Asian patients with hepatocellular carcinoma: final results of the START trial. Int J Cancer. 2015;136(6):1458–1467. doi: 10.1002/ijc.29126. [DOI] [PubMed] [Google Scholar]
- 11.Couri T., Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int. 2019;13(2):125–137. doi: 10.1007/s12072-018-9919-1. [DOI] [PubMed] [Google Scholar]
- 12.Llovet J.M., Montal R., Sia D., Finn R.S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15(10):599–616. doi: 10.1038/s41571-018-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosgrove D.P., Reyes D.K., Pawlik T.M., Feng A.L., Kamel I.R., Geschwind J.F. Open-label single-arm phase II trial of sorafenib therapy with drug-eluting bead transarterial chemoembolization in patients with unresectable hepatocellular carcinoma: clinical results. Radiology. 2015;277(2):594–603. doi: 10.1148/radiol.2015142481. [DOI] [PubMed] [Google Scholar]
- 14.Park J.W., Koh Y.H., Kim H.B., et al. Phase II study of concurrent transarterial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatol. 2012;56(6):1336–1342. doi: 10.1016/j.jhep.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Peng Z., Chen S., Xiao H., et al. Microvascular invasion as a predictor of response to treatment with sorafenib and transarterial chemoembolization for recurrent intermediate-stage hepatocellular carcinoma. Radiology. 2019;292(1):237–247. doi: 10.1148/radiol.2019181818. [DOI] [PubMed] [Google Scholar]
- 16.Zhang R., Shen L., Zhao L., Guan Z., Chen Q., Li W. Combined transarterial chemoembolization and microwave ablation versus transarterial chemoembolization in BCLC stage B hepatocellular carcinoma. Diagn Interv Radiol. 2018;24(4):219–224. doi: 10.5152/dir.2018.17528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren Y., Cao Y., Ma H., et al. Improved clinical outcome using transarterial chemoembolization combined with radiofrequency ablation for patients in Barcelona clinic liver cancer stage A or B hepatocellular carcinoma regardless of tumor tumor: results of a single-center retrospective case control study. BMC Cancer. 2019;19(1):983. doi: 10.1186/s12885-019-6237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin X., Zhang L., Wang Y.H., et al. Transcatheter arterial chemoembolization combined with radiofrequency ablation delays tumor progression and prolongs overall survival in patients with intermediate (BCLC B) hepatocellular carcinoma. BMC Cancer. 2014;14:849. doi: 10.1186/1471-2407-14-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan T., Mu L.W., Wu C., et al. Comparison of combined transcatheter arterial chemoembolization and CT-guided radiofrequency ablation with surgical resection in patients with hepatocellular carcinoma within the up-to-seven criteria: a multicenter case-matched study. J Cancer. 2017;8(17):3506–3513. doi: 10.7150/jca.19964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie D.Y., Ren Z.G., Zhou J., Fan J., Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463. doi: 10.21037/hbsn-20-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C., Liao Y., Qiu J., et al. Transcatheter arterial chemoembolization alone or combined with ablation for recurrent intermediate-stage hepatocellular carcinoma: a propensity score matching study. J Cancer Res Clin Oncol. 2020;146(10):2669–2680. doi: 10.1007/s00432-020-03254-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 23.Bruix J., Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S., Shin J., Kim D.Y., Choi G.H., Kim M.J., Choi J.Y. Radiomics on gadoxetic acid-enhanced magnetic resonance imaging for prediction of postoperative early and late recurrence of single hepatocellular carcinoma. Clin Cancer Res. 2019;25(13):3847–3855. doi: 10.1158/1078-0432.CCR-18-2861. an official journal of the American Association for Cancer Research. [DOI] [PubMed] [Google Scholar]
- 25.Johnson P.J., Berhane S., Kagebayashi C., et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi: 10.1200/JCO.2014.57.9151. official journal of the American Society of Clinical Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lencioni R., Llovet J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Llovet J.M., Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020;72(2):288–306. doi: 10.1016/j.jhep.2019.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trask P.C., Dueck A.C., Piault E., Campbell A. Patient-reported outcomes version of the common Terminology criteria for adverse events: methods for item selection in industry-sponsored oncology clinical trials. Clin Trials. 2018;15(6):616–623. doi: 10.1177/1740774518799985. [DOI] [PubMed] [Google Scholar]
- 29.Wang X.H., Hu Z.L., Fu Y.Z., et al. Tenofovir vs. entecavir on prognosis of hepatitis B virus-related hepatocellular carcinoma after curative resection. J Gastroenterol. 2022;57(3):185–198. doi: 10.1007/s00535-022-01855-x. [DOI] [PubMed] [Google Scholar]
- 30.Poon R.T., Fan S.T., Lo C.M., et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234(1):63–70. doi: 10.1097/00000658-200107000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X.F., Xing H., Han J., et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg. 2019;154(3):209–217. doi: 10.1001/jamasurg.2018.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabrizian P., Jibara G., Shrager B., Schwartz M., Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261(5):947–955. doi: 10.1097/SLA.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 33.Han K., Kim J.H. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona clinic liver cancer staging system. World J Gastroenterol. 2015;21(36):10327–10335. doi: 10.3748/wjg.v21.i36.10327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng Z.W., Zhang Y.J., Liang H.H., Lin X.J., Guo R.P., Chen M.S. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012;262(2):689–700. doi: 10.1148/radiol.11110637. [DOI] [PubMed] [Google Scholar]
- 35.Llovet J.M., Ricci S., Mazzaferro V., et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 36.Dufour J.F., Bargellini I., De Maria N., De Simone P., Goulis I., Marinho R.T. Intermediate hepatocellular carcinoma: current treatments and future perspectives. Ann Oncol. 2013;24(Suppl 2):24–29. doi: 10.1093/annonc/mdt054. official journal of the European Society for Medical Oncology. [DOI] [PubMed] [Google Scholar]
- 37.Kudo M., Ueshima K., Ikeda M., et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–1501. doi: 10.1136/gutjnl-2019-318934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parikh N.D., Waljee A.K., Singal A.G. Downstaging hepatocellular carcinoma: a systematic review and pooled analysis. Liver Transplant. 2015;21(9):1142–1152. doi: 10.1002/lt.24169. official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. [DOI] [PubMed] [Google Scholar]
- 39.Chapman W.C., Majella Doyle M.B., Stuart J.E., et al. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248(4):617–625. doi: 10.1097/SLA.0b013e31818a07d4. [DOI] [PubMed] [Google Scholar]
- 40.Shi F., Wu M., Lian S.S., et al. Radiofrequency ablation following downstaging of hepatocellular carcinoma by using transarterial chemoembolization: long-term outcomes. Radiology. 2019;293(3):707–715. doi: 10.1148/radiol.2019181991. [DOI] [PubMed] [Google Scholar]
- 41.Shi F., Lian S., Mai Q., et al. Microwave ablation after downstaging of hepatocellular carcinoma: outcome was similar to tumor within Milan criteria. Eur Radiol. 2020;30(5):2454–2462. doi: 10.1007/s00330-019-06604-y. [DOI] [PubMed] [Google Scholar]
- 42.Yao F.Y., Mehta N., Flemming J., et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61(6):1968–1977. doi: 10.1002/hep.27752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hage C., Hoves S., Strauss L., et al. Sorafenib induces pyroptosis in macrophages and triggers natural killer cell-mediated cytotoxicity against hepatocellular carcinoma. Hepatology. 2019;70(4):1280–1297. doi: 10.1002/hep.30666. [DOI] [PubMed] [Google Scholar]
- 44.Kudo M., Finn R.S., Qin S., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 45.Pinter M., Scheiner B., Peck-Radosavljevic M. Immunotherapy for advanced hepatocellular carcinoma: a focus on special subgroups. Gut. 2021;70(1):204–214. doi: 10.1136/gutjnl-2020-321702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finn R.S., Qin S., Ikeda M., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 47.Gordan J.D., Kennedy E.B., Abou-Alfa G.K., et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38(36):4317–4345. doi: 10.1200/JCO.20.02672. official journal of the American Society of Clinical Oncology. [DOI] [PubMed] [Google Scholar]
- 48.Qin S., Bi F., Gu S., et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled phase II-III trial. J Clin Oncol. 2021;39(27):3002–3011. doi: 10.1200/JCO.21.00163. official journal of the American Society of Clinical Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valery M., Cervantes B., Samaha R., et al. Immunotherapy and hepatocellular cancer: where are we now? Cancers (Basel) 2022;14(18) doi: 10.3390/cancers14184523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.