Abstract
Introduction:
Previous studies have suggested that frailty among older adults with cancer is associated with a variety of negative outcomes, including greater chemotherapy toxicity and worse survival. However, results often do not include patient-reported outcomes, such as quality of life (QOL). The objective of this study was to evaluate frailty prior to receipt of moderately- or highly-emetogenic chemotherapy and acute changes in QOL in patients at least 65 years of age. It was hypothesized that frail patients would report greater declines in QOL.
Materials and Methods:
Participants completed questionnaires before receiving their first infusion and again five days later. A 59-item deficit accumulation index score was created at baseline using a modified Rockwood frailty index. QOL was assessed using the Functional Assessment of Cancer Therapy-General (FACT-G). The relationship between baseline frailty and QOL was evaluated using a dichotomized deficit accumulation index (frail vs. robust) in repeated measures ANOVA.
Results:
Study participants (n = 151) had a mean age of 72 (SD = 4.5) and 62% were female. Nearly half (42%) were frail at baseline. Frail participants reported worse QOL at baseline compared to robust participants. Frail patients reported smaller declines in overall and physical (p < 0.0001) and emotional (p = 0.006) QOL from baseline to five days after receiving chemotherapy. At five days, frail participants reported better emotional and physical QOL compared to robust participants.
Discussion:
Contrary to expectations, frail patients reported smaller declines in QOL compared to robust patients using a deficit accumulation index. These results can be used to help educate frail patients on what to expect during treatment.
Keywords: Aging, Accumulated deficits, Frailty, Older patients, cancer, Quality of life
1. Introduction
Approximately 60% of all new cancer diagnoses in the United States occur in adults age 65 and older [1], with the number of older adults with cancer projected to increase to 70% by 2030 [2]. Older adults are more likely to experience frailty, or “the state of vulnerability to poor homeostasis resolution following a stressor event that develops from a cumulative decline across multiple physiological systems.” [3] Given that the number of patients with cancer age 65 and older is increasing, and aging is the greatest risk factor for a majority of chronic diseases, including certain cancers [4], frailty has become a common consideration in oncology care and patient management [5].
Prior studies have demonstrated that frail older adults experience worse quality of life (QOL) [6]. While the association is understudied in patients with cancer, previous research has suggested patients with cancer who present as frail pretreatment experience worse QOL [7,8]. Thus, it is important to understand whether older patients with cancer and frailty are at risk for reduced QOL to inform supportive care interventions. Frailty and biological aging are considered to be drivers of disease [9,10] and frail older adults have diminished capacity to compensate for stressors compared to robust people of the same chronological age [10]. Organizations including American Society of Clinical Oncology and the International Society of Geriatric Oncology have recommended utilizing geriatric assessments to identify vulnerabilities among older patients with cancer and guide treatment decision making [11–14]. Patient-reported toxicities early in the course of treatment are highly predictive of treatment discontinuations and dose reductions, thus are important to evaluate [15]. Frailty prior to a cancer diagnosis has previously been associated with worse acute outcomes (e.g., mortality, post-operative treatment complications, and poor tolerance to chemotherapy), and long-term outcomes (e.g., increased hospitalizations, long-term care admission, and declines in cognition and functional status) [16]. However, there is limited data on the association of frailty with patient reported outcomes (PROs) such as QOL in older patients with cancer during active treatment. The dearth of research on frailty and PROs among patients with cancer is surprising, especially in light of the fact that PROs provide patient-centered and complementary information to physician-rated toxicities and functional status [17]. PROs such as QOL are increasingly considered to be an important part of clinical care [18].
The goal of this study was to evaluate the association between frailty and change in QOL in older adults starting moderately- or highly-emetogenic chemotherapy. We hypothesized that frail patients would report greater declines in QOL from baseline to five days after receiving their first infusion of chemotherapy, when patients typically report feeling their worst. The results of this study are intended to guide future research to inform cancer care and treatment decisions for older survivors and educate patients about what to expect during treatment.
2. Materials and Methods
2.1. Participants
Participants were recruited between April 2018 and December 2020 as part of an ongoing study of gastrointestinal (GI) side-effects during chemotherapy. To be included in the parent study, participants had to have completed study measures and be: (a) capable of speaking and reading standard English or Spanish, (b) diagnosed with a solid tumor, (c) moderately- or highly-emetogenic chemotherapy-naïve, (d) scheduled to receive moderately- or highly-emetogenic intravenous standard-dose chemotherapy as defined by the most recent version of NCCN antiemesis guidelines [19], and (e) able to provide informed consent. Additionally, for the current study, participants were required to be ≥65 years of age and completed the Global Assessment Tool (GAT) and Functional Assessment of Cancer Therapy QOL measures. The study was IRB approved (Identifier: 20180593) and conducted at five US sites: City of Hope, Moffitt Cancer Center, Ohio State University, Rutgers University, and University of Colorado Denver.
3. Measures
3.1. Demographic and Clinical Characteristics
Sociodemographic variables were obtained via patient self-report prior to chemotherapy and included date of birth, sex, ethnicity, race, marital status, income, and education. Clinical data were collected via medical chart review and included concurrent radiation (yes/no), cancer type (GI vs. non-GI cancer), and cancer stage (stage I, II, III vs. IV/extensive).
3.2. Frailty
From baseline assessments, a modified Rockwood deficit accumulation index score [9,20–22] was created using 59 items measuring instrumental activities of daily living [23], functional status (physical, emotional, social, and general health) [24], fatigue [25], self-reported weight, self-reported comorbidities, and clinical laboratory values (Supplemental Table 1). Data were analyzed at study completion and were not shared with the clinical team. Consistent with prior literature, scores were tabulated for participants who had no >10% missing data [21,26]. Scores were calculated by summing all non-missing items (scored as 0 = no deficit or 1 = deficit) and dividing by the total number of non-missing items to create an overall frailty index score [27]. The frailty index was dichotomized as ≤0.21 (robust) vs. > 0.21 (frail) as in previous studies [28,29].
3.3. Quality of Life
Participants completed the 27-item Functional Assessment of Cancer Therapy (FACT-G) measure at baseline (day one) and again the first five days after receiving chemotherapy. The FACT-G is a comprehensive compilation of questions that measures health-related QOL in patients with cancer [25]. In addition to an overall summary score, this measure provides four subdomains of QOL: physical, social, emotional, and functional well-being. Each item is ranked on a 5-point Likert scale ranging from 0 (not at all) to 4 (very much). Higher scores indicate better QOL.
3.4. Statistical Analysis
Descriptive statistics (means, standard deviations, frequencies, and percentages) were generated for demographic and clinical variables. Means, 95% confidence interval (CI) and independent sample t-tests were used to compare baseline QOL and five-day QOL between frail and robust participants. Repeated measures ANOVA models were used to determine the association of baseline frailty with change in QOL. Covariates in the repeated measures ANOVA models were chosen a priori based on variables known or hypothesized to be associated with QOL and frailty and were age, sex, cancer stage, and cancer type [30]. In accordance with previous literature, clinically meaningful differences, or meaningful changes in QOL scores, were computed as a 2- to 3-point difference on the FACT-G subdomain scores and 4- to 7-point difference on the overall score [31–33]. Chi square tests were conducted to determine if frail participants were less likely to receive highly emetogenic as compared to moderately emetogenic chemotherapy. Analyses were conducted using SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA). P-values <0.05 were considered statistically significant.
4. Results
The analytic sample size included 151 participants (see Fig. 1). Participant characteristics are presented in Table 1. On average, participants were 72 years old (SD = 4.5). Most participants were non-Hispanic (97%), White (92%), and female (62%). Regarding clinical characteristics, most participants did not receive concurrent radiation, were stage I-III, and were diagnosed with a cancer other than GI. Forty-two percent of participants were classified as frail and 58% as robust. There were no significant differences between frailty status and demographic or clinical characteristics at baseline.
Fig. 1.
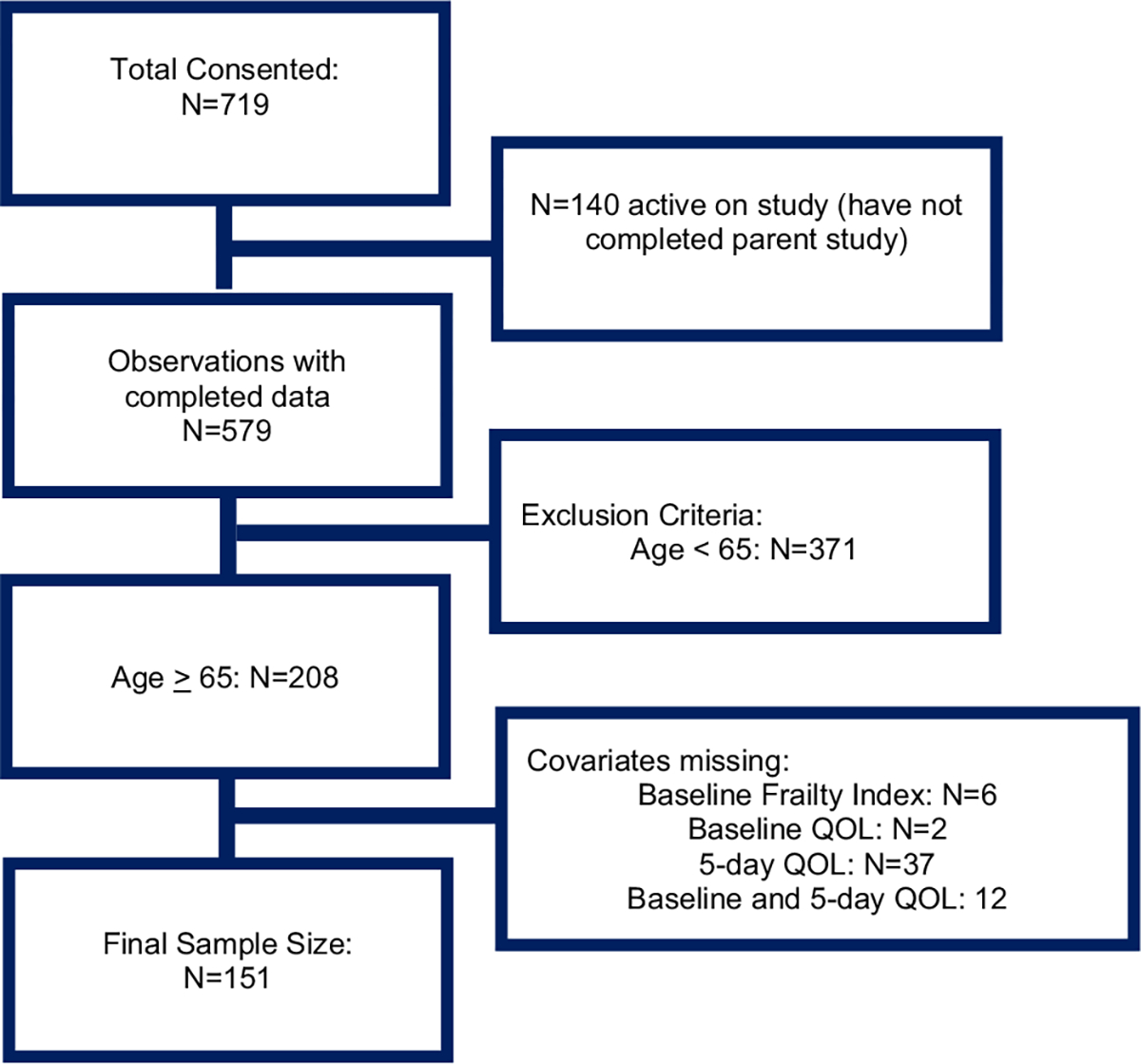
Consort Diagram for Frailty and Quality of Life in Older Patients with Cancer.
Table 1.
Participant characteristics.
| All participants |
Frail (%) | Robust (%) | X2 p-value vs. frail robust | |
|---|---|---|---|---|
| N = 151 (%) | ||||
|
| ||||
| Age: Mean ± SD [range], years | 72.0 ± 4.5 [65.0–85.0] | 73.0 + 4.5 [65.0–85.0] | 71.3 + 4.7 [65.1–84.8] | 0.51 |
| Sex: n (%) | 0.50 | |||
| Female | 94 (62.2) | 37 (58.7) | 57 (64.8) | |
| Male | 57 (37.8) | 26 (41.3) | 31 (35.2) | |
| Ethnicity: n (%) | 0.18 | |||
| Non-Hispanic | 147 (97.3) | 61 (96.8) | 86 (97.7) | |
| Hispanic | 4 (2.7) | 2 (3.2) | 2 (2.3) | |
| Race: n (%) | 0.57 | |||
| White | 139 (92.0) | 57 (90.5) | 82 (93.2) | |
| Other | 12 (8.0) | 6 (9.5) _ | 6 (6.8) | |
| Marital Status: n (%) | 0.58 | |||
| Married/Domestic Partner | 112 (74.2) | 46 (73.0) | 66 (75.0) | |
| Not married | 39 (25.8) | 17 (27.0) | 22 (25.0) | |
| Incomea: n (%) | 0.79 | |||
| >40 k | 83 (76.9) | 40 (80.0) | 43 (74.1) | |
| <40 k | 68 (23.1) | 10 (20.0) | 15 (25.9) | |
| College graduate: n (%) | 0.64 | |||
| Yes | 73 (48.3) | 30 (47.6) | 43 (48.9) | |
| No | 78 (51.7) | 33 (52.4) | 45 (51.1) | |
| Cancer Stage: n (%) | 0.55 | |||
| Stage I, II, III | 114 (75.5) | 45 (71.4) | 69 (78.4) | |
| Stage IV or extensive | 37 (24.5) | 18 (28.6) | 19 (21.6) | |
| Treatment | 0.73 | |||
| Modality: n (%) | ||||
| Concurrent radiation | 31 (20.5) | 14 (22.2) | 17 (19.3) | |
| No concurrent | 120 (79.5) | 49 (77.8) | 71 (80.7) | |
| radiation Cancer Type: n (%) | 0.84 | |||
| GI cancer | 39 (25.8) | 16 (25.4) | 23 (26.1) | |
| Non-GI cancer | 112 (74.2) | 47 (74.6) | 65 (73.9) | |
| Chemotherapy: n (%) | ||||
| Highly emetogenic | 97 (64.2) | 44 (69.8) | 53 (60.2) | 0.09 |
| Moderately emetogenic | 54 (35.8) | 19 (30.2) | 35 (39.8) | |
| Frailty Index: n (%) | ||||
| Robust ≤0.21 | 88 (58.3) | - | - | |
| Frail >0.21 | 63 (41.7) | - | - | |
SD: standard deviation; GI: gastrointestinal.
n = 108
Analysis of cross-sectional baseline data indicated that frail participants reported significantly worse QOL compared to robust participants (p < 0.001). In repeated measures ANOVA (Table 2), controlling for age, sex, cancer stage, and cancer type, frail participants reported smaller declines in overall and physical QOL (p < 0.001), and emotional QOL (p = 0.0006) from baseline to five days after receiving chemotherapy (Fig. 2). Analysis of cross-sectional data at five days indicated that participants in the frail group reported better emotional (p = 0.04) and physical (p = 0.001) QOL compared to the robust group (Fig. 2). At baseline, robust participants reported higher overall QOL (4-point clinically meaningful difference) compared to frail participants (Table 3). In contrast, at five days, frail participants reported a higher physical QOL (3-point clinically meaningful difference) compared to robust participants.
Table 2.
Repeated measures ANOVA- Baseline frailty status and changes in quality of life over time.
| Total Quality of Life | |||||
|---|---|---|---|---|---|
|
| |||||
| DF | Type III SS | Mean Square | F-Value | P-Value | |
|
| |||||
| Time | 1 | 711.14 | 711.14 | 8.22 | 0.004 |
| Time*age | 1 | 119.61 | 119.61 | 1.38 | 0.24 |
| Time*stage | 1 | 370.34 | 370.34 | 4.28 | 0.04 |
| Time*sex | 1 | 258.50 | 258.50 | 2.99 | 0.08 |
| Time*cancer type | 1 | 17.78 | 17.78 | 0.21 | 0.65 |
| Time*frailty | 1 | 3277.31 | 3277.31 | 37.9 | <0.001 |
| Social Quality of Life | |||||
| Time | 1 | 0.03 | 0.03 | 0.01 | 0.93 |
| Time*age | 1 | 0.16 | 0.16 | 0.03 | 0.86 |
| Time*stage | 1 | 1.23 | 1.23 | 0.23 | 0.63 |
| Time*sex | 1 | 0.84 | 0.84 | 0.16 | 0.69 |
| Time*cancer type | 1 | 42.73 | 42.73 | 8.02 | 0.01 |
| Time*frailty | 1 | 5.51 | 5.51 | 1.03 | 0.31 |
| Emotional Quality of Life | |||||
| Time | 1 | 93.63 | 93.63 | 4.47 | 0.03 |
| Time*age | 1 | 11.30 | 11.30 | 0.54 | 0.46 |
| Time*stage | 1 | 34.70 | 34.70 | 1.66 | 0.20 |
| Time*sex | 1 | 20.68 | 20.68 | 0.99 | 0.32 |
| Time*cancer type | 1 | 212.78 | 212.78 | 10.15 | 0.001 |
| Time*frailty | 1 | 258.64 | 258.64 | 12.34 | 0.001 |
| Functional Quality of Life | |||||
| Time | 1 | 30.35 | 30.35 | 1.98 | 0.16 |
| Time*age | 1 | 14.38 | 14.38 | 0.94 | 0.33 |
| Time*stage | 1 | 16.06 | 16.06 | 1.05 | 0.30 |
| Time*sex | 1 | 1.35 | 1.35 | 0.09 | 0.76 |
| Time*cancer type | 1 | 41.81 | 41.81 | 2.72 | 0.10 |
| Time*frailty | 1 | 48.15 | 48.15 | 3.14 | 0.07 |
| Physical Quality of Life | |||||
| Time | 1 | 127.60 | 127.60 | 3.40 | 0.06 |
| Time*age | 1 | 17.52 | 17.52 | 0.47 | 0.49 |
| Time*stage | 1 | 67.79 | 67.79 | 1.81 | 0.18 |
| Time*sex | 1 | 3.06 | 3.06 | 0.08 | 0.77 |
| Time*cancer type | 1 | 210.10 | 210.10 | 5.59 | 0.01 |
| Time*frailty | 1 | 1337.62 | 1337.62 | 35.62 | <0.001 |
Fig. 2.
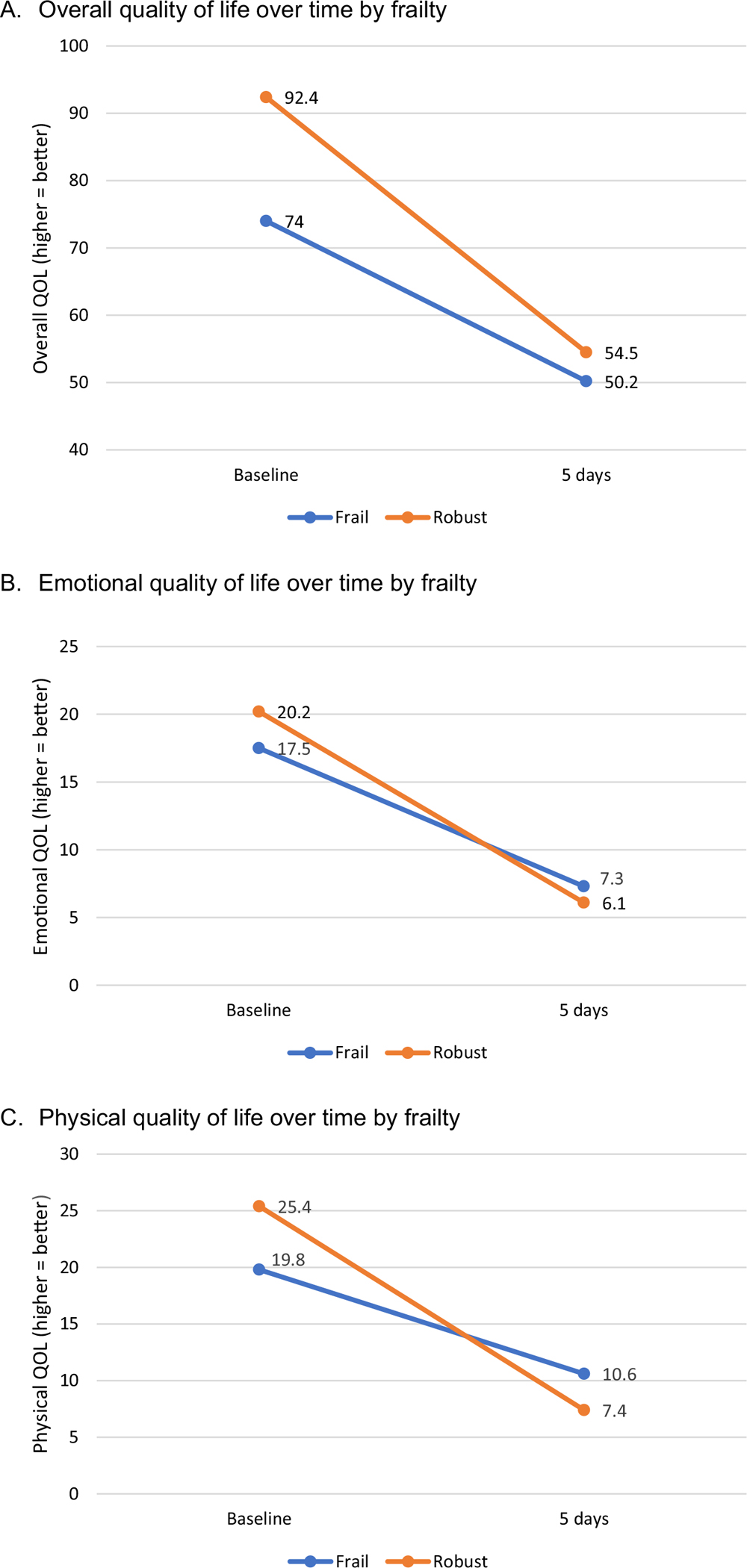
Change in Baseline vs. 5-day Quality of Life (QOL) in Frail vs. Robust Patients with Cancer age ≥ 65.
Table 3.
Mean and 95% CI – Frailty status and quality of life over time.
| Baseline | |||||
|---|---|---|---|---|---|
|
| |||||
| QOL Outcome | Minimum | Maximum | Median | Mean | 95% CI |
|
| |||||
| Robust Group | |||||
| Total | 45.0 | 108.0 | 93.0 | 92.4 | (90.3–94.5) |
| Emotional | 11.0 | 24.0 | 21.0 | 20.2 | (19.6–20.8) |
| Physical | 10.0 | 28.0 | 27.0 | 25.4 | (24.8–26.1) |
| Social | 15.0 | 28.0 | 24.0 | 23.8 | (23.1–24.4) |
| Functional | 2.0 | 28.0 | 24.0 | 22.9 | (22.0–23.9) |
| Frail Group | |||||
| Total | 26.0 | 103.0 | 77.0 | 74.0 | (69.8–78.2) |
| Emotional | 2.0 | 24.0 | 18.0 | 17.5 | (16.3–18.7) |
| Physical | 7.0 | 28.0 | 21.0 | 19.8 | (18.3–21.4) |
| Social | 2.0 | 28.0 | 22.5 | 21.3 | (20.0–22.5) |
| Functional Day Five | 2.0 | 28.0 | 15.0 | 15.4 | (13.8–16.9) |
| Robust Group | |||||
| Total | 36.0 | 72.0 | 56.0 | 54.5 | (53.1–55.9) |
| Emotional | 0 | 16.0 | 6.0 | 6.0 | (5.4–6.6) |
| Physical | 0 | 21.0 | 6.0 | 7.4 | (6.2–8.6) |
| Social | 11.0 | 28.0 | 24.0 | 22.7 | (21.9–23.5) |
| Functional | 6.0 | 28.0 | 18.0 | 18.3 | (16.9–19.6) |
| Frail Group | |||||
| Total | 32.0 | 68.0 | 51.0 | 50.2 | (48.0–52.5) |
| Emotional | 0 | 22.0 | 7.0 | 7.3 | (6.2–8.3) |
| Physical | 2.0 | 24.0 | 10.0 | 10.5 | (9.0–12.0) |
| Social | 2.0 | 28.0 | 20.0 | 19.7 | (18.4–20.9) |
| Functional | 2.0 | 28.0 | 12.0 | 12.7 | (11.1–14.3) |
CI: confidence interval; QOL: quality of life.
5. Discussion
The findings of this study add to the cancer and aging literature by describing that frail patients, defined by a deficit accumulation index, demonstrate worse QOL prior to chemotherapy but smaller decreases in QOL than robust patients among older adults receiving moderately- or highly-emetogenic intravenous standard-dose chemotherapy. Nearly half of older participants were classified in the deficit accumulation “frail” category at baseline, suggesting frailty is a concern for many older patients with cancer and should be considered in the cancer treatment decision-making process. In post-hoc testing, we had hypothesized frail participants would be less likely to receive highly emetogenic chemotherapy; however, chi-square tests did not detect a significant difference. In dichotomized findings, frail patients reported smaller declines in overall, emotional, and physical QOL from baseline to five days after receiving chemotherapy. While robust participants reported a greater general decline in overall QOL, robust participants still reported a clinically meaningful higher overall QOL at five days as compared to frail participants. Contrary to our hypothesis, self-reported QOL declines, specifically emotional and physical, were more evident among the robust participants from baseline to the first five days after receiving chemotherapy. This may be due to cognitive changes in internal standards used to evaluate QOL (i.e., response shift) [34], suggesting older patients with cancer who are frail have mentally adjusted to frailty, and thus have lower QOL expectations during treatment as compared to robust patients who experience greater QOL declines. These findings are also consistent with PROs at the end of life among patients with cancer receiving palliative chemotherapy [35]. Patients with the worst function had less negative impact to QOL than those with better function [35].
The study focuses on the first chemotherapy infusion, as previous studies suggest that these initial patient-reported toxicities are highest in the week following chemotherapy, and then gradually decrease until the next infusion [36,37]. Frailty was assessed using a deficit accumulation model, a proxy measure of biological aging [9,21,38,39], from data that can be readily obtained via patient self-report (e.g., instrumental activities of daily living) and extracted from the electronic medical record (e.g., clinical lab values) [40]. Deficit accumulation indices more readily facilitate clinic-based screening and retrospective data analysis compared to phenotype-based frailty models which require time-intensive [41] and/or specialized measures [42] of sarcopenia, muscle strength, and physical performance. Therefore, calculating a frailty index score for older patients with cancer prior to treatment using PRO and EMR data may be useful in identifying frail individuals and informing individualized care pathways and management algorithms.
Previous studies assessing frailty and quality of life outcomes in patients with cancer are mixed. In studies conducted in head and neck [8,43], prostate [44], and older patients with cancer [45], quality of life was lower for frail patients as compared to fit patients during and following treatment. However, in a colorectal cancer study, frail patients reported improvements in quality of life three-months post- surgery [46]. Future longitudinal studies are encouraged to further explore clinically meaningful differences in frailty, measured by the deficit accumulation index, and QOL outcomes throughout the cancer trajectory (i.e., diagnosis through survivorship).
Frail patients with cancer are at an increased risk of postoperative complications, treatment intolerance, disease progression, and death [5]. In a systematic review examining frailty in older patients with cancer, more 50% of patients reported pre-frailty or frailty and these patients were at an increased risk of chemotherapy intolerance, post-operative complications, and mortality [47]. However, frailty is often not well documented [5]. As frailty becomes increasingly recognized as an important component of health care and health outcomes in patients with cancer [5], the use of deficit accumulation indices in clinic, using a composite score of readily available EMR data may be useful in documenting frailty and providing patient education on treatment expectations. In a large, prospective study of older adult patients with breast cancer compared to non-cancer controls, Mandelblatt and colleagues found that most survivors and controls, when using a deficit accumulation index, remained robust and only a small proportion of each group reported increasing deficits over 36 months [9]. However, survivors reported an clinically meaningful increase in deficit accumulation compared to controls [9]. In another study, Lachmann and colleagues reported that frailty measured by a self-reported deficit accumulation index in participants with severe diseases (i.e., heart attack or cancer six months prior) was multidimensional and associated with declines in a variety of health-related domains (nutritional status, physical activity, cognition, etc.) in the European Prospective Investigation into Cancer and Nutrition (EPIC) dataset [48]. In a study by Magnuson et al., longitudinal decline in cognition and objective measures of attention and memory were associated with increased frailty, using a modified frailty score, during treatment and up to 6 months posttreatment in older patients with breast cancer [49]. These studies, coupled with our findings, suggest that frailty is highly prevalent in older patients with cancer and that sensitive and specific measures of frailty, such as a deficit accumulation index, may be easily implemented into research [9].
Overall, this study was the first to examine frailty and QOL changes in older patients with cancer before receiving their first infusion and again five days later using self-reported and electronic medical data (i.e., clinical lab values). Despite these strengths, limitations are noted. This was a secondary, unplanned analysis of an ongoing study. Our study included a highly heterogenous population of mixed cancer types, cancers other than colon, esophagus, gall bladder, liver, pancreas, rectal/anal, and stomach were considered non-GI cancers (e.g., breast, endometrium, lung, ovary). Future studies should employ methods to include a more homogenous population. Our study was comprised of research volunteers with a mean age of 72 years who may have been healthier and thus more willing to participate in research activities and provide consent. Due to participant age, we evaluated for a potential “floor effect” in which participants may have a skewed distribution of scores toward the lower limit, however, after evaluating for skewness, results suggested the data were fairly symmetrical. Our study population consisted of predominately non-Hispanic White females with an income more than $40,000; thus, our findings may not be generalizable to other patient populations. For this repeated measure analysis, participants were included if they completed day one and day five QOL data, however no significant difference between participants missing QOL data was observed (p = 0.26). The most notable limitation of this study was the short time frame. The FACT-G was keyed to the past week, covering the entire period of study participation, consistent with previous uses of the FACT-G. However, as patient-reported outcomes typically reflect severity and recency, we have reason to believe it adequately captures post-chemotherapy quality of life [50]. While we were able to detect clinically meaningful differences in QOL within the first five days after receiving chemotherapy, further research exploring QOL changes from diagnosis through treatment completion and survivorship are warranted. Unfortunately, we were unable to determine if frail patients were given monotherapy or upfront dose reductions with their first cycle of chemotherapy which may influence toxicity and impact acute QOL. Furthermore, only baseline frailty data was available therefore were unable to determine if chemotherapy affected robust patients’ frailty at day five such that they joined the frail group at day five.
Deficit accumulation indices throughout the cancer trajectory should be considered in future studies as it is likely, as a result of treatment, patients with cancer become more frail over time thus contributing to future toxicities including functional, physical, and psychological challenges [9]. Future studies examining frailty risk in older patients with cancer prior to cancer therapy to assess treatment adherence and health outcomes are critical for the development of individualized care pathways and management algorithms.
6. Conclusion
Overall, our findings indicate that frailty, defined by the deficit accumulation index, is associated with change in QOL during chemotherapy in older patients with cancer. Nearly half of the study participants were classified as “frail” at baseline, suggesting frailty is a concern for older patients with cancer even before the initiation of cancer treatment. From baseline to five days after receiving chemotherapy, older patients with cancer reported worsening overall, emotional, and physical QOL, though declines were smaller for frail patients. Further supportive care interventions addressing frailty and health-related outcomes including symptoms, sleep, QOL, disease progression, and mortality over time are warranted to inform care decisions.
Supplementary Material
Acknowledgements
This work was supported by the National Cancer Institute R01CA219389 (PI: Jim), and T32CA090314 (MPIs: Brandon/Vadaparampil). This work has been supported in part by the Biostatistics and Bioinformatics Shared Resource at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center (P30-CA076292).
Footnotes
Declaration of Competing Interest
Dr. Jim is a consultant for Janssen Scientific Affairs and Merck. She has grant funding from Kite Pharma. Dr. Li reports research funding to his institution from AstraZeneca and Brooklyn ImmunoTherapeutics. He serves as a consultant and has received honoraria from Adagene, Advanced Accelerator Applications, Bayer Healthcare, Coherus BioSciences, Eisai, Exelixis, Genentech, Ipsen Biopharmaceuticals, Lexicon Pharmaceuticals, Merck, MiNA Therapeutics, QED Therapeutics, Servier, Sun Pharma, Taiho Pharmaceutical, and TerSera Therapeutics. Dr. Brownstein served as an ad-hoc grant reviewer in 2020 for the American Cancer Society, for which she received sponsored travel during the review meeting and a stipend of $300. She also received $500 from the Statistical Consulting Section of the American Statistical Association for Best Paper Award at the 2019 Joint Statistical Meetings. Dr. Brownstein serves as a member of the Scientific Review Committee at Moffitt Cancer Center.
Ethics approval
The study was IRB approved (Identifier: 20180593) and conducted at five US sites: 1) City of Hope, 2) Moffitt Cancer Center, 3) Ohio State University, 4) Rutgers University, and 5) University of Colorado Denver.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgo.2022.08.010.
Availability of data and materials
Data is available upon reasonable request from the corresponding author.
References
- [1].Cinar D, Tas D. Cancer in the elderly. North Clin Istanb 2015;2(1):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 2009;27(17):2758–65. [DOI] [PubMed] [Google Scholar]
- [3].Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ethun CG, Bilen MA, Jani AB, Maithel SK, Ogan K, Master VA. Frailty and cancer: implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J Clin 2017;67(5):362–77. [DOI] [PubMed] [Google Scholar]
- [6].Crocker TF, Brown L, Clegg A, et al. Quality of life is substantially worse for community-dwelling older people living with frailty: systematic review and meta-analysis. Qual Life Res 2019;28(8):2041–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Williams GR, Deal AM, Sanoff HK, et al. Frailty and health-related quality of life in older women with breast cancer. Support Care Cancer 2019;27(7):2693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].de Vries J, Bras L, Sidorenkov G, et al. Frailty is associated with decline in health-related quality of life of patients treated for head and neck cancer. Oral Oncol 2020;111:105020. [DOI] [PubMed] [Google Scholar]
- [9].Mandelblatt JS, Zhou X, Small BJ, et al. Deficit accumulation frailty trajectories of older breast Cancer survivors and non-Cancer controls: the thinking and living with Cancer study. J Natl Cancer Inst 2021;113(8):1053–64. 10.1093/jnci/djab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hodes RJ, Sierra F, Austad SN, et al. Disease drivers of aging. Ann N Y Acad Sci 2016;1386(1):45–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32(24):2595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and Management of Vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol 2018;36(22):2326–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendationsdagger. Ann Oncol 2015;26(2):288–300. [DOI] [PubMed] [Google Scholar]
- [14].Verduzco-Aguirre HC, Navarrete-Reyes AP, Chavarri-Guerra Y, Avila-Funes JA, Soto-Perez-de-Celis E. The effect of a geriatric oncology clinic on treatment decisions in Mexican older adults with Cancer. J Am Geriatr Soc 2019;67(5):992–7. [DOI] [PubMed] [Google Scholar]
- [15].Flannery MA, Culakova E, Canin BE, Peppone L, Ramsdale E, Mohile SG. Understanding treatment tolerability in older adults with Cancer. J Clin Oncol 2021;39(19):2150–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ness KK, Wogksch MD. Frailty and aging in cancer survivors. Transl Res 2020;221:65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Atherton PJ, Watkins-Bruner DW, Gotay C, et al. The complementary nature of patient-reported outcomes and adverse event reporting in cooperative group oncology clinical trials: a pooled analysis (NCCTG N0591). J Pain Symptom Manage 2015;50(4):470–479 e479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bullinger M, Quitmann J. Quality of life as patient-reported outcomes: principles of assessment. Dialogues Clin Neurosci 2014;16(2):137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology Antiemesis. 2020. (Accessed July 16, 2021). chrome-extension://oemmndcbldboiebfnladdacbdfmadadm/https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf.
- [20].Cohen HJ, Smith D, Sun CL, et al. Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer. 2016;122(24):3865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rockwood K, Howlett SE. Fifteen years of progress in understanding frailty and health in aging. BMC Med 2018;16(1):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kirkhus L, Saltyte Benth J, Rostoft S, et al. Geriatric assessment is superior to oncologists’ clinical judgement in identifying frailty. Br J Cancer 2017;117(4):470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of Adl: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–9. [DOI] [PubMed] [Google Scholar]
- [24].Ware J Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34(3):220–33. [DOI] [PubMed] [Google Scholar]
- [25].Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the functional assessment of Cancer therapy (FACT) measurement system. J Pain Symptom Manage 1997;13(2):63–74. [DOI] [PubMed] [Google Scholar]
- [26].Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007;62(7):722–7. [DOI] [PubMed] [Google Scholar]
- [28].Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ. 2011;183(8):E487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Geessink N, Schoon Y, van Goor H, Olde Rikkert M, Melis R, consortium T-M.. Frailty and quality of life among older people with and without a cancer diagnosis: findings from TOPICS-MDS. PLoS One 2017;12(12):e0189648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Webster K, Cella D, Yost K. The functional assessment of chronic illness therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes 2003;1:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the functional assessment of Cancer therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage 2002;24(6):547–61. [DOI] [PubMed] [Google Scholar]
- [33].Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences: the FACIT experience. Eval Health Prof 2005;28(2):172–91. [DOI] [PubMed] [Google Scholar]
- [34].Chen PY, Yang CM. Consequences of ignoring the response-shift and measure non-invariant items in sleep studies: an empirical data based simulation of the treatment effect of CBT-I on dysfunctional sleep beliefs. Sleep Med 2020;74:99–108. [DOI] [PubMed] [Google Scholar]
- [35].Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol 2015;1(6):778–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jim HS, Jacobsen PB, Phillips KM, Wenham RM, Roberts W, Small BJ. Lagged relationships among sleep disturbance, fatigue, and depressed mood during chemotherapy. Health Psychol 2013;32(7):768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jim HS, Small B, Faul LA, Franzen J, Apte S, Jacobsen PB. Fatigue, depression, sleep, and activity during chemotherapy: daily and intraday variation and relationships among symptom changes. Ann Behav Med 2011;42(3):321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rockwood K, Howlett SE. Age-related deficit accumulation and the diseases of ageing. Mech Ageing Dev 2019;180:107–16. [DOI] [PubMed] [Google Scholar]
- [39].Rockwood K Conceptual models of frailty: accumulation of deficits. Can J Cardiol 2016;32(9):1046–50. [DOI] [PubMed] [Google Scholar]
- [40].Levit LA, Kaltenbaugh MW, Magnuson A, et al. Challenges and opportunities to developing a frailty index using electronic health record data. J Geriatr Oncol. 2021;12(5):851–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56(3):M146–56. [DOI] [PubMed] [Google Scholar]
- [42].Lewis EG, Coles S, Howorth K, et al. The prevalence and characteristics of frailty by frailty phenotype in rural Tanzania. BMC Geriatr 2018;18(1):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pottel L, Lycke M, Boterberg T, et al. Serial comprehensive geriatric assessment in elderly head and neck cancer patients undergoing curative radiotherapy identifies evolution of multidimensional health problems and is indicative of quality of life. Eur J Cancer Care (Engl) 2014;23(3):401–12. [DOI] [PubMed] [Google Scholar]
- [44].Goineau A, Campion L, d’Aillieres B, et al. Comprehensive geriatric assessment and quality of life after localized prostate cancer radiotherapy in elderly patients. PLoS One. 2018;13(4):e0194173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kirkhus L, Saltyte Benth J, Gronberg BH, et al. Frailty identified by geriatric assessment is associated with poor functioning, high symptom burden and increased risk of physical decline in older cancer patients: prospective observational study. Palliat Med 2019;33(3):312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ronning B, Wyller TB, Nesbakken A, et al. Quality of life in older and frail patients after surgery for colorectal cancer-a follow-up study. J Geriatr Oncol 2016;7(3):195–200. [DOI] [PubMed] [Google Scholar]
- [47].Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol 2015;26(6):1091–101. [DOI] [PubMed] [Google Scholar]
- [48].Lachmann R, Stelmach-Mardas M, Bergmann MM, et al. The accumulation of deficits approach to describe frailty. PLoS One. 2019;14(10):e0223449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Magnuson A, Lei L, Gilmore N, et al. Longitudinal relationship between frailty and cognition in patients 50 years and older with breast Cancer. J Am Geriatr Soc 2019;67(5):928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Stull DE, Leidy NK, Parasuraman B, Chassany O. Optimal recall periods for patient-reported outcomes: challenges and potential solutions. Curr Med Res Opin 2009;25(4):929–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon reasonable request from the corresponding author.


