Abstract
The intracellular protozoan parasite Cryptosporidium parvum accumulates host cell actin at the interface between the parasite and the host cell cytoplasm. Here we show that the actin polymerizing proteins Arp2/3, vasodilator-stimulated phosphoprotein (VASP), and neural Wiskott Aldrich syndrome protein (N-WASP) are present at this interface and that host cell actin polymerization is necessary for parasite infection.
The obligate intracellular protozoan parasite Cryptosporidium parvum causes a diarrheal illness in infected hosts (2, 6). The severity of the illness depends largely on the immune status of the host. Consequently, immunosuppressed individuals, such as people with AIDS and malnourished children in developing countries, can develop a life-threatening illness (14). Unfortunately, the pathogenesis is poorly understood and there is no curative therapy (3).
One possible explanation for the apparent drug resistance manifested by Cryptosporidium lies in the unique nature of the host-parasite interface (15). As the parasite invades the host intestinal epithelial cell, a junctional complex is formed at the host-parasite interface that separates the parasite from the host cell cytoplasm. This junctional complex appears as an electron-dense band with an adjacent filamentous network in transmission electron micrographs (18, 23, 31). We and others have recently shown that actin and the actin-binding protein, α-actinin, are associated with this junctional complex (Fig. 1F and I) (9, 13), but other components remain unknown.
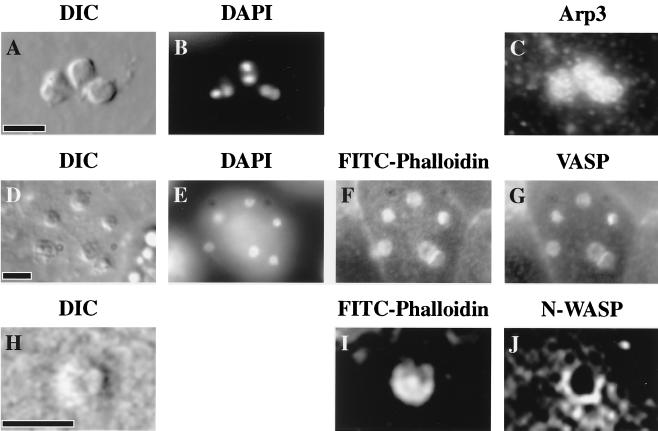
FIG. 1.
Indirect immunofluorescence microscopy of Arp3, VASP, and N-WASP in Cryptosporidium-infected cell lines. Parasites are identified by differential interference contrast (DIC) microscopy (A, D, H) and DAPI staining of parasite nuclei (B and E). The f-actin accumulation at these sites of infection is identified by fluorescein isothiocyanate-phalloidin staining (F and I). Indirect immunofluorescence microscopy of Cryptosporidium-infected cell lines reveals localization of Arp3 (C) and VASP (G) at all sites of infection. Indirect immunofluorescence confocal microscopy of the host-parasite interface in a cell line transfected with Myc-tagged N-WASP reveals a circular staining pattern at the periphery of the junctional complex (J). Scale bars = 5 μm.
Utilization of host cell actin is a common theme in microbial pathogenesis and has been observed in infections by Listeria monocytogenes, Escherichia coli, Salmonella enterica serovar Typhimurium, Shigella flexneri, and vaccinia virus (10). In enteropathogenic E. coli infections, filamentous actin (f-actin) forms the scaffolding for a host cell protuberance to which the bacteria attach (11). In Salmonella and Shigella infections, the actin microfilaments direct the engulfment of the bacteria by the host cell (4, 12). Listeria and vaccinia virus nucleate host cell actin on their surfaces to propel themselves through the host cell cytoplasm (5, 7, 30).
A recent study has identified several host cell proteins that are necessary for the actin-based motility of Listeria and Shigella by reconstitution with purified proteins (19). The proteins essential for actin polymerization include the Arp2/3 complex, actin, two actin binding proteins (cofilin and capping protein), and, for Shigella, neural Wiskott Aldrich syndrome protein (N-WASP). Another protein, vasodilator-stimulated phosphoprotein (VASP), accelerates actin polymerization but is not essential.
To test the hypothesis that the actin accumulation seen in Cryptosporidium-infected cells represented active actin polymerization rather than passive accumulation of f-actin at sites of infection, we utilized indirect immunofluorescence microscopy to identify proteins involved in actin polymerization at sites of invasion. We experimentally infected a human intestinal cell line, HCT-8 (ATCC CCL-244), with the Iowa isolate of C. parvum oocysts (Pleasant Hill Farm, Troy, Idaho) (9). Indirect immunofluorescence of infected cell lines using antibodies against the Arp3 protein of the Arp2/3 complex (a gift of Matt Welch, Berkeley, Calif.) (20, 32) revealed localization of this protein at every site of Cryptosporidium invasion (Fig. 1C). In addition, indirect immunofluorescence using an antibody against VASP (1:500; Transduction Laboratories, Venture, Conn.) showed similar localization at sites of infection (Fig. 1G). In both cases, the staining pattern did not change when the parasite was removed from the monolayer, using a method previously described (9), indicating localization to the host-parasite junctional complex. Attempts to localize N-WASP at sites of infection using indirect immunofluorescence yielded equivocal results, so a Myc-tagged human N-WASP protein was utilized for localization experiments. HCT-8 cells were transfected with Myc-N-WASP (bovine N-WASP in the pRK5-Myc vector [17]) and, after 16 h, infected with Cryptosporidium. These experiments revealed a circular staining patterns at sites of infection (Fig. 1J). The different staining patterns for N-WASP and Arp3 at sites of infection may be due to the Arp2/3 complex remaining attached to the actin network, while N-WASP acts transiently on the junctional complex (1, 29). Taken together these results indicate that host cell Arp2/3 complex, VASP, and N-WASP are present at sites of invasion (at all stages from early trophozoites to meronts), implying that Cryptosporidium infection induces host actin polymerization.
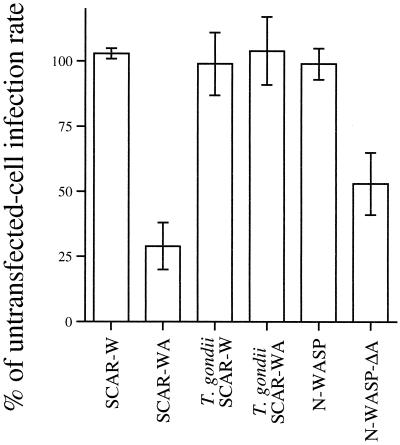
To test the hypothesis that host cell actin polymerization is necessary for Cryptosporidium infection, we utilized a fragment of the protein Scar1 (Scar-WA) (21), which is known to inhibit actin polymerization in other systems, such as Listeria actin tail formation, phagocytosis, and lamellipodia formation (21, 24, 25). This C-terminal fragment of Scar1 contains the Arp2/3 complex binding and activating domain (22). It inhibits specific actin polymerization in the host cell by binding and activating Arp2/3 throughout the cytoplasm, making it unavailable for focal processes at the cell surface. We transfected a plasmid containing Myc-tagged Scar-WA into HCT-8 cells using Novafector (VennNova, LLC., Pompano Beach, Fla.) and then infected them 16 h later with Cryptosporidium as previously described (9). Cell lines were fixed and stained with an anti-Myc antiserum (1:100; Zymed, San Francisco, Calif.), fluorescein isothiocyanate-phalloidin (5 μM/ml; Sigma) and 4′,6′-diamidino-2-phenylindole (DAPI) (10 μM/ml; Sigma) 8 h after infection. We compared the infection rate of transfected cells with the infection rate of all adjacent untransfected cells. Results are expressed as a percentage of the untransfected control infection rate. As shown in Fig. 2, inhibition of actin polymerization by Scar-WA reduced the Cryptosporidium infection rate by 71%. This effect was specific to the Scar-WA protein, since a related protein lacking the Arp2/3 binding domain (Scar-W) (21), which does not inhibit actin polymerization, had minimal effect on Cryptosporidium infection (Fig. 2). To rule out the possibility that Scar-WA expression nonspecifically damaged the host cell, making it unsuitable for parasitic invasion, we also infected Scar-WA-transfected cells with Toxoplasma gondii (obtained from Vern Carruthers, Johns Hopkins School of Public Health, Baltimore, Md.). Since T. gondii invades cells independent of host cell actin polymerization, we anticipated that Scar-WA would have no effect on T. gondii infection (8). As predicted, the infection rates of transfected and untransfected cells by T. gondii were nearly identical, arguing for a specific inhibition of Cryptosporidium infection by Scar-WA. Taken together, these data strongly suggest that host cell actin polymerization is necessary for Cryptosporidium infection.
FIG. 2.
Inhibition of Cryptosporidium infection in cell lines transfected with inhibitors of host actin polymerization (Scar-WA [n = 297 transfected cells counted] and N-WASP-ΔA [n = 130]). Results are given as a percentage of the infection rate seen in untransfected cells in the same well. Control transfections include Scar-W (n = 242) and N-WASP (n = 325), which do not interfere with actin polymerization. Scar-WA (n = 178) and Scar-W (n = 184) expression does not inhibit T. gondii infection (which is independent of host cell actin), ruling out nonspecific inhibition of parasite invasion. Data are means of internal parasites ± standard deviations (error bars) for three separate experiments.
In order to test the hypothesis that host cell N-WASP is also necessary for Cryptosporidium infection, we infected cells that had been transfected with a dominant negative form of N-WASP (N-WASP-ΔA), which contains a deletion of the C-terminal acidic domain that is necessary for Arp2/3 binding. This was produced by PCR, using primers corresponding to bases 1 to 15 and 1438 to 1453 of the bovine N-WASP sequence. The resultant fragment (corresponding to amino acids 1 to 484 of the N-WASP protein) was cloned into the pRK5-Myc vector for mammalian expression. In experimental E. coli infections (16), such mutant N-WASP is recruited to sites of E. coli attachment but blocks actin polymerization since the necessary Arp2/3 complex cannot be bound. As in the previously described experiments with the Scar-WA construct, we transfected N-WASP-ΔA into HCT-8 cells, infected them with Cryptosporidium, and compared the infection rate of transfected cells to that of untransfected cells. As shown in Fig. 2, transfection with N-WASP-ΔA inhibited infection by 47%, relative to untransfected cells. Controls transfected with wild-type N-WASP showed no significant inhibition of infection. These results suggest that Cryptosporidium utilizes host cell N-WASP to recruit Arp2/3 and polymerize host cell actin during infection and that this process is necessary for Cryptosporidium infection. It is known that the small GTPase Cdc42 and the polyphosphoinositide phosphatidylinositol 4,5-bis-phosphate can activate N-WASP and recruit it to sites of infection (26, 27, 28). Future studies will examine the role of such signaling molecules in Cryptosporidium infection.
Acknowledgments
We thank Axel Puls of the Laboratory for Molecular Cell Biology, University College, London, England, for producing the Myc-N-WASP. We are indebted to the following individuals for many helpful discussions, technical assistance, and critical reagents: Vern Carruthers, Paul Englund, Doug Murphy, and Cindy Sears.
REFERENCES
- 1.Blanchoin L, Amann K J, Higgs H N, Marchand J-B, Kaiser D A, Pollard T D. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature. 2000;404:1007–1013. doi: 10.1038/35010008. [DOI] [PubMed] [Google Scholar]
- 2.Clark D P. New insights into human cryptosporidiosis. Clin Microbiol Rev. 1999;12:554–563. doi: 10.1128/cmr.12.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark D P, Sears C L. The pathogenesis of cryptosporidiosis. Parasitol Today. 1996;12:221–225. doi: 10.1016/0169-4758(96)10018-1. [DOI] [PubMed] [Google Scholar]
- 4.Clerc P, Sansonetti P J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987;55:2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cudmore S, Cossart P, Griffiths G, Way M. Actin-based motility of vaccinia virus. Nature. 1995;378:636–638. doi: 10.1038/378636a0. [DOI] [PubMed] [Google Scholar]
- 6.Current W L, Garcia L S. Cryptosporidiosis. Clin Microbiol Rev. 1991;4:325–358. doi: 10.1128/cmr.4.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabiri G A, Sanger J M, Portnoy D A, Southwick F S. Listeria monocytogenes moves rapidly through the host-cell cytoplasm by inducing directional actin assembly. Proc Natl Acad Sci USA. 1990;87:6068–6072. doi: 10.1073/pnas.87.16.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobrowolski J M, Sibley L D. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell. 1996;34:933–939. doi: 10.1016/s0092-8674(00)81071-5. [DOI] [PubMed] [Google Scholar]
- 9.Elliott D A, Clark D P. Cryptosporidium parvum induces host cell actin accumulation at the host-parasite interface. Infect Immun. 2000;68:2315–2322. doi: 10.1128/iai.68.4.2315-2322.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finlay B B, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 11.Finlay B B, Rosenshine I, Donnenberg M S, Kaper J B. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect Immun. 1992;60:2541–2543. doi: 10.1128/iai.60.6.2541-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finlay B B, Ruschkowski S, Dedhar S. Cytoskeletal rearrangements accompanying Salmonella entry into epithelial cells. J Cell Sci. 1991;99:283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- 13.Forney J R, DeWald D B, Yang S, Speer C A, Healey M C. A role for host phosphoinositide 3-kinase and cytoskeletal remodeling during Cryptosporidium parvum infection. Infect Immun. 1999;67:844–852. doi: 10.1128/iai.67.2.844-852.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffiths J K. Human cryptosporidiosis: epidemiology, transmission, clinical disease, treatment, and diagnosis. Adv Parasitol. 1998;40:37–85. doi: 10.1016/s0065-308x(08)60117-7. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths J K, Balakrishnan R, Widmer G, Tzipori S. Paromomycin and geneticin inhibit intracellular Cryptosporidium parvum without trafficking through the host cell cytoplasm: implications for drug delivery. Infect Immun. 1998;66:3874–3883. doi: 10.1128/iai.66.8.3874-3883.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalman D, Weiner O D, Goosney D L, Sedat J W, Finlay B B, Abo A, Bishop J M. Enteropathogenic E. coli acts through WASP and Arp2/3 complex to form actin pedestals. Nat Cell Biol. 1999;1:389–391. doi: 10.1038/14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamarche N, Tapon N, Stowers L, Burbelo P D, Aspenström P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- 18.Lefkowitch J H, Krumholz S, Feng-Chen K-C, Griffin P, Despommier D, Brasitus T A. Cryptosporidiosis of the human small intestine: a light and electron microscopic study. Hum Pathol. 1984;15:746–752. doi: 10.1016/s0046-8177(84)80165-3. [DOI] [PubMed] [Google Scholar]
- 19.Loisel T P, Boujemaa R, Pantoloni D, Carlier M-F. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature. 1999;401:613–616. doi: 10.1038/44183. [DOI] [PubMed] [Google Scholar]
- 20.Machesky L M, Reeves E, Wientjes F, Mattheyse F J, Grogan A, Totty N F, Burlingame A L, Hsuan J J, Segal A W. Mammalian actin-related protein 2/3 complex localizes to regions of lamellipodial protrusion and is composed of evolutionarily conserved proteins. J Biochem. 1997;328:105–112. doi: 10.1042/bj3280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machesky L M, Insall R H. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr Biol. 1998;8:1347–1356. doi: 10.1016/s0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 22.Marchand J-B, Kaiser D A, Pollard T D, Higgs H N. Interaction of WASP/Scar proteins with actin and vertebrate Arp2/3 complex. Nat Cell Biol. 2001;3:76–82. doi: 10.1038/35050590. [DOI] [PubMed] [Google Scholar]
- 23.Marcial M A, Madara J L. Cryptosporidium: cellular localization, structural analysis of absorptive cell-parasite membrane-membrane interactions in guinea pigs, and suggestion of protozoan transport by M cells. Gastroenterology. 1986;90:583–594. doi: 10.1016/0016-5085(86)91112-1. [DOI] [PubMed] [Google Scholar]
- 24.May R C, Caron E, Hall A, Machesky L M. Involvement of the Arp2/3 complex in phagocytosis mediated by FcγR or CR3. Nat Cell Biol. 2000;2:246–248. doi: 10.1038/35008673. [DOI] [PubMed] [Google Scholar]
- 25.May R C, Hall M E, Higgs H N, Pollard T D, Chakraborty T, Wehland J, Machesky L M, Sechi A S. The Arp2/3 complex is essential for the actin-based motility of Listeria monocytogenes. Curr Biol. 1999;9:759–762. doi: 10.1016/s0960-9822(99)80337-6. [DOI] [PubMed] [Google Scholar]
- 26.Prehoda K E, Scott J A, Mullins R D, Lim W A. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science. 2000;290:801–806. doi: 10.1126/science.290.5492.801. [DOI] [PubMed] [Google Scholar]
- 27.Rohatgi R, Ho H, Kirschner M W. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4,5-bisphosphate. J Cell Biol. 2000;150:1299–1309. doi: 10.1083/jcb.150.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner M W. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- 29.Svitkina T M, Borisy G G. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J Cell Biol. 1999;145:1009–1026. doi: 10.1083/jcb.145.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vetterling J M, Takeuchi A, Madden P A. Ultrastructure of Cryptosporidium wrairi from the guinea pig. J Protozool. 1971;18:248–260. doi: 10.1111/j.1550-7408.1971.tb03316.x. [DOI] [PubMed] [Google Scholar]
- 32.Welch M D, DePace A H, Verma S, Iwamatsu A, Mitchison T J. The human Arp2/3 complex is composed of evolutionarily conserved subunits and is localized to cellular regions of dynamic actin filament assembly. J Cell Biol. 1997;138:375–384. doi: 10.1083/jcb.138.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]