Abstract
Objective
To investigate whether monitoring of cerebral tissue oxygen saturation using near infrared spectroscopy in addition to routine monitoring combined with defined treatment guidelines during immediate transition and resuscitation increases survival without cerebral injury of premature infants compared with standard care alone.
Design
Multicentre, multinational, randomised controlled phase 3 trial.
Setting
11 tertiary neonatal intensive care units in six countries in Europe and in Canada.
Participants
1121 pregnant women (<32 weeks’ gestation) were screened prenatally. The primary outcome was analysed in 607 of 655 randomised preterm neonates: 304 neonates in the near infrared spectroscopy group and 303 in the control group.
Intervention
Preterm neonates were randomly assigned to either standard care (control group) or standard care plus monitoring of cerebral oxygen saturation with a dedicated treatment guideline (near infrared spectroscopy group) during immediate transition (first 15 minutes after birth) and resuscitation.
Main outcome measure
The primary outcome, assessed using all cause mortality and serial cerebral ultrasonography, was a composite of survival without cerebral injury. Cerebral injury was defined as any intraventricular haemorrhage or cystic periventricular leukomalacia, or both, at term equivalent age or before discharge.
Results
Cerebral tissue oxygen saturation was similar in both groups. 252 (82.9%) out of 304 neonates (median gestational age 28.9 (interquartile range 26.9-30.6) weeks) in the near infrared spectroscopy group survived without cerebral injury compared with 238 (78.5%) out of 303 neonates (28.6 (26.6-30.6) weeks) in the control group (relative risk 1.06, 95% confidence interval 0.98 to 1.14). 28 neonates died (near infrared spectroscopy group 12 (4.0%) v control group 16 (5.3%): relative risk 0.75 (0.33 to 1.70).
Conclusion
Monitoring of cerebral tissue oxygen saturation in combination with dedicated interventions in preterm neonates (<32 weeks’ gestation) during immediate transition and resuscitation after birth did not result in substantially higher survival without cerebral injury compared with standard care alone. Survival without cerebral injury increased by 4.3% but was not statistically significant.
Trial registration
ClinicalTrials.gov NCT03166722.
Introduction
The immediate fetal-neonatal transition period is a complex physiological process encompassing initiation of spontaneous breathing, lung aeration, and switching from intrauterine to extra-uterine circulation. About 10% of newborn infants require respiratory support, including supplemental oxygen, immediately after birth.1 In neonatal resuscitation guidelines, the recommended targets for guiding respiratory support and titration of fractional inspired oxygen after birth against arterial oxygen saturation (SpO2) are derived from healthy spontaneously breathing infants.2 3 A recent individual participant analysis of preterm infants (<32 weeks’ gestation) reported that only 23% met these SpO2 targets, with the risk of intraventricular haemorrhage and death increased in those who did not.4
After initiation of spontaneous breathing the partial pressure of oxygen rapidly increases, and delivery of oxygen to cerebral tissue normalises the cerebral regional tissue oxygen saturation (crSO2) faster than that of SpO2.5 6 This preferential oxygen delivery to the brain suggests an increasing cerebral blood flow during the first minutes after birth.7 8 9 10 Observational studies in delivery rooms reported that the crSO2 values in preterm infants who experienced intraventricular haemorrhage11 12 were significantly lower during the first 15 minutes after birth compared with neonates without cerebral injury,13 14 whereas the SpO2 levels of these neonates reached the targets and were comparable to those of neonates without cerebral injury. In the COSGOD phase I/II trial, targeting crSO2 using specified clinical treatment guidelines during the immediate transition period (first 15 minutes) after birth in addition to routine monitoring (SpO2 and heart rate) achieved a relative reduction of 55.4% in the burden of cerebral hypoxia within the first 15 minutes.15 The effects, however, of crSO2 monitoring with dedicated interventions during the neonatal transition period on clinically relevant outcomes remain unknown.16
We investigated whether targeting crSO2 using specified clinical treatment guidelines during the immediate transition period in addition to routine monitoring (SpO2 and heart rate) has an effect on mortality and cerebral injury in preterm neonates (<32 weeks’ gestation).17 We hypothesised that crSO2 in addition to routine monitoring with defined treatment guidelines during immediate transition and resuscitation compared with routine monitoring and standard care alone in premature infants would increase survival without cerebral injury.
Methods
For this randomised, multicentre, multinational, phase 3 clinical trial we recruited infants from 11 neonatal intensive care units in Europe and Canada from 1 October 2017 to 30 October 2021. Written informed parental consent was obtained for all participating infants: antenatally in nine European centres and postnatally in one European centre (Trieste) and in Canada (deferred consent).
Participants
Preterm neonates were eligible for inclusion in the study if they were born before 32 completed weeks of gestation. Inclusion criteria were written informed parental consent (for measurement and analyses in centres with antenatal consent and for analyses in centres with deferred consent) and decision to provide full life support. Exclusion criteria were severe congenital malformation, including of the brain, heart, or lung, or the presence of prenatal cerebral injury. For each included neonate we collected information on antenatal medical history, birth history, and data on interventions during the immediate transition period.
Randomisation
The neonates were randomised before birth to either the near infrared spectroscopy group or the control group using a web based randomisation service (https://www.randomizer.at/random/) and a block size of 10. The ratio of allocation was 1:1 and neonates were stratified according to trial site. For multiple births we randomised only the first infant because inclusion of two neonates immediately after birth and during resuscitation was not feasible owing to availability of research staff and devices.
Masking
The allocation sequence remained concealed throughout the trial. The resuscitation team was masked to crSO2 in the control group (a member of the research team was present during the 15 minute measurement period and either covered the near infrared spectroscopy monitor or turned the monitor away). Efforts were made to mask the clinical team in the neonatal intensive care unit—especially to avoid recording the allocation group in the neonate’s chart. Group allocation was concealed during statistical analysis.
Monitoring
Pulse oximetry was used to monitor SpO2 and heart rate, and electrocardiography was optionally performed according to local guidelines. The pulse oximetry sensor was applied to the neonate’s right palm or wrist and the electrocardiography electrodes to the chest. The crSO2 sensor was applied within three minutes after birth and continued until minute 15 after birth using a neonatal sensor connected to an Invos 5100 Cerebral/Somatic Oximeter monitor (Medtronic, Minneapolis, MN). The near infrared spectroscopy sensor was placed on the left side of the neonate’s forehead and secured using a cap or self-adhesive elastic bandage. The resuscitation teams were trained to apply the near infrared spectroscopy sensors, interpret the spectroscopy values and the crSO2 targets, and apply the trial interventions. In the intervention group, the crSO2 targets for every minute were displayed in the field of view of the healthcare providers during resuscitation. A member of the research team documented the crSO2 values for every minute, either during resuscitation (near infrared spectroscopy group) or from the device’s storage after resuscitation (control group).
Interventions
All delivery room interventions (except targeting of crSO2 in the near infrared spectroscopy group) were performed in accordance with local hospital guidelines and the neonatal resuscitation guidelines.2 3
Control group
During the study period, targeting of SpO2 had to be performed in accordance with local hospital guidelines and published neonatal resuscitation guidelines,2 3 whereby SpO2 had to be targeted independently of local guidelines to be at least between the 10th and 90th centile of published reference ranges.18 As was routine in all participating centres, the lower local limit of SpO2 was targeted according to the resuscitation guidelines.2 3
If SpO2 remained <10th centile or below the local lower limit, fractional inspired oxygen (FiO2) was increased by 10-20% every 60 seconds or respiratory support was started or increased. If SpO2 remained stable >10th centile18 or above the local lower limit for >60 seconds, or if SpO2 was >90th centile,18 FiO2 was reduced by 10-20% or respiratory support was adjusted accordingly.
Near infrared spectroscopy group
CrSO2 monitoring was visible to the clinical team with the same SpO2 target as in the control group. If SpO2 remained between the 10th and 90th centiles and within local limits, and crSO2 was <10th centile according to published reference ranges,13 FiO2 was increased by 10-20% every 60 seconds or respiratory support was started or increased. If crSO2 remained >10th centile13 for >60 seconds or if crSO2 was >90th centile,13 FiO2 was reduced by 10-20% or respiratory support was adjusted accordingly. If there was a history of blood loss or clinical signs of blood loss, intravenous fluids (10 mL/kg) were considered.2 3
Outcome measures
The primary composite outcome was survival without cerebral injury, assessed using all cause mortality and by cerebral ultrasonography, the routine assessment method at all sites. Cerebral injury was defined as any grade of intraventricular haemorrhage or cystic periventricular leukomalacia. The local team performed cerebral ultrasonography according to routine clinical care at 2-24 hours (optional), 2-5 days, 6-8 days, 12-16 days, and before discharge or at term equivalent age, depending on whichever came first.
The secondary outcome measures were individual components of the primary outcome (mortality and cerebral injury), culture proven infection or sepsis, necrotising enterocolitis, bronchopulmonary dysplasia, retinopathy of prematurity, and persistent ductus arteriosus requiring intervention. The medical record of each neonate was reviewed for assessment of secondary outcomes.
Data management and safety
Data were entered in a central database (Medical Informatics, Statistics and Documentation, Medical University of Graz, Austria) using a standard web based electronic case report form. Data entry was the responsibility of local investigators, and at each trial site a locally appointed external monitoring committee monitored data flow according to good clinical practice principles. An independent data safety monitoring committee reviewed the data after inclusion of 20% of the neonates. This was done to evaluate safety and efficacy (primary and secondary outcome variables) of the intervention with a certain or a probably or likely relationship with the cerebral near infrared spectroscopy oximeter or the application of the treatment guidelines.
Statistical analyses
From our sample size calculation, we determined that 329 neonates would need to be enrolled in each group to provide a power of 80% to detect a difference of 10% increase in survival without cerebral injury (65% in control group v 75% in near infrared spectroscopy group), considering a significance level of 5%. Assuming a dropout rate of 10%, we planned to enrol a total of 724 neonates. The sample size calculation was based on data from two European centres (Medical University of Graz, Austria, and Erasmus Medical Center, Rotterdam, Netherlands) and one Canadian centre (Royal Alexandra Hospital, Edmonton, Canada). In these centres the percentage of neonates surviving without cerebral injury ranged from 56% to 77%, with an average of 65%.
To investigate the primary hypothesis of whether the frequency of survival without cerebral injury in preterm infants (<32 weeks’ gestation) differed between the two groups, we used generalised linear models (probability distribution: binomial; link function: log) adjusted for trial site relative risk and corresponding 95% confidence interval. For sensitivity analysis, we also performed this analysis adjusted for gestational age. Furthermore, we performed two separate analyses: for neonates born at <28 weeks of gestation and at ≥28 weeks of gestation and for neonates born by vaginal delivery and caesarean section. Secondary outcomes including individual primary outcome measures (death, cerebral injury) were analysed in the same way. Data including personal, baseline, and interventions were compared using χ2 test or Fisher’s exact test for categorical variables and t test or Mann-Whitney U test for continuous variables, depending on whether the data were normally distributed or skewed. Trends for cerebral oxygenation (crSO2) during the first 15 minutes after birth were analysed using linear mixed models with fixed effects for group (near infrared spectroscopy group v control group), and time and random effect for centre. A first order autoregressive covariance structure was used. Estimated mean scores with 95% confidence intervals are given for the analysed variables. A P value <0.05 was considered statistically significant. Statistical analyses were performed using SAS 9.4 (2002-12, SAS Institute, Cary, NC).
Patient and public involvement
No parents were involved in setting the research question or the outcome measures, nor were they involved in developing plans for recruitment, design, or implementation of the study. No patients were asked to advise on interpretation or writing up of results. It was not the policy of the involved institutions to include parents or members of the public in planning or decision making processes at the time when the study was planned, submitted to ethical committees and funding agencies, and started.
Results
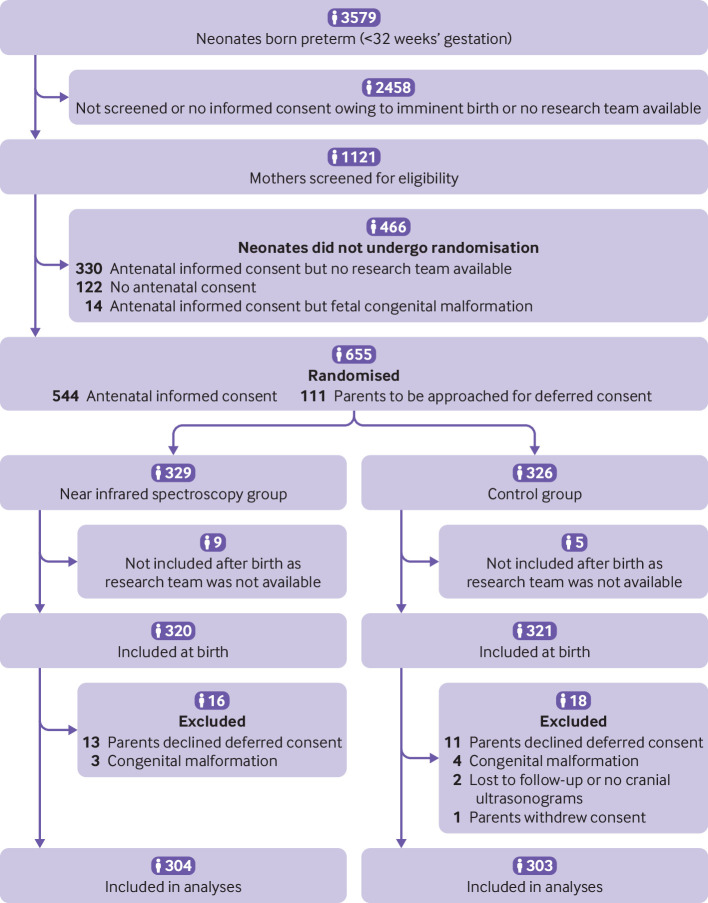
Of 655 infants who underwent randomisation (329 in the near infrared spectroscopy group, 326 in the control group) during the study period, 607 (304 and 303, respectively) were included in the final analysis (fig 1). Two neonates were excluded as no cerebral ultrasonography data were available (lost to follow-up). The personal and clinical characteristics of the mothers and infants did not differ between the groups (table 1), except for a higher number of fetuses with bradycardia in the near infrared spectroscopy group and a higher number of male infants in the control group, although both were of borderline significance (P=0.05 and P=0.06, respectively).
Fig 1.
Screening, randomisation, intervention, and follow-up of participants through study
Table 1.
Maternal, fetal, and neonatal baseline characteristics. Values are numbers (percentages) unless stated otherwise
| Characteristics | Near infrared spectroscopy group (n=304) | Control group (n=303) |
|---|---|---|
| Maternal cause of preterm birth | ||
| Antepartum bleeding (n=300/302) | 38 (12.7) | 44 (14.6) |
| Chorioamnionitis (n=300/302) | 57 (19.0) | 74 (24.5) |
| Premature rupture of membranes (n=300/303) | 97 (32.3) | 85 (28.1) |
| Pre-eclampsia (n=301/302) | 57 (18.9) | 59 (19.5) |
| Gestational diabetes (n=300/302) | 7 (2.3) | 14 (4.6) |
| Other (n=299/301) | 64 (21.4) | 72 (23.9) |
| Fetal related preterm birth | ||
| Intrauterine growth restriction (n=300/301) | 50 (16.7) | 54 (17.9) |
| Fetal bradycardia (n=301/301) | 69 (22.9) | 50 (16.6) |
| Doppler sonography detected disease (n=300/301) | 61 (20.3) | 46 (15.3) |
| Multiple birth (n=299/300) | 36 (12.0) | 31 (10.3) |
| Other (n=298/301) | 13 (4.4) | 23 (7.6) |
| Antenatal steroids (n=297/300) | 290 (97.6) | 291 (97.0) |
| Mode of delivery (n=303/302) | ||
| Spontaneous vaginal | 49 (16.2) | 43 (14.2) |
| Caesarean section | 253 (83.5) | 258 (85.4) |
| Instrumental | 1 (0.3) | 1 (0.3) |
| Time to cord clamping (n=291/281) | ||
| <30 seconds | 176 (60.5) | 163 (58.0) |
| 30-60 seconds | 86 (29.6) | 78 (27.8) |
| >60 seconds | 29 (10.0) | 40 (14.2) |
| Neonatal characteristics (n=304/303) | ||
| Median (IQR) gestational age (weeks) | 28.9 (26.9-30.6) | 28.6 (26.6-30.6) |
| Gestational age: | ||
| <28 weeks | 110 (36.2) | 118 (38.9) |
| >28 weeks | 194 (63.8) | 185 (61.0) |
| Median (IQR) birth weight (g) | 1123 (860-1405) | 1075 (820-1360) |
| Male/female (n=302/301) | 148/154 (49.0/51.0) | 171/130 (56.8/43.2) |
| Median (IQR) umbilical artery pH | 7.32 (7.28-7.36) | 7.32 (7.28-7.37) |
| Median (IQR) Apgar score: | ||
| 1 minute | 7.0 (5.0-8.0) | 7.0 (5.0-8.0) |
| 5 minutes | 8.0 (7.0-9.0) | 8.0 (7.0-9.0) |
| 10 minutes | 9.0 (8.0-9.0) | 9.0 (8.0-9.0) |
IQR=interquartile range.
The incidence of delivery room interventions within the first 15 minutes after birth did not differ between the groups except for a significantly higher number of neonates receiving intravenous fluids in the near infrared spectroscopy group (12 (4.0%) v 2 (0.7%), P=0.007; table 2); in four neonates administration of intravenous fluids was indicated by crSO2. Respiratory support by intubation was indicated in nine neonates by crSO2. CrSO2 values at each minute during the first 15 minutes after birth did not differ between the groups (see supplementary figures S1 and S2). FiO2 was slightly higher in the near infrared spectroscopy group than control group in the first minutes after birth (see supplementary table 3), and SpO2 values during the first 15 minutes after birth were similar in both groups (see supplementary table 3).
Table 2.
Interventions during first 15 minutes and first 24 hours after birth
| Interventions | Near infrared spectroscopy group (n=304) | Control group (n=303) | P value |
|---|---|---|---|
| First 15 minutes after birth | |||
| Supplemental oxygen (n=303/303) | 297 (98.0) | 290 (95.7) | 0.1 |
| Respiratory support (n=304/303): | |||
| None | 5 (1.6) | 8 (2.6) | 0.55 |
| Mask continuous positive pressure | 103 (33.9) | 106 (35.0) | |
| Mask positive pressure ventilation | 162 (53.3) | 164 (54.1) | |
| Intubation | 34 (11.2) | 25 (8.3) | |
| Chest compressions (n=301/299) | 4 (1.3) | 5 (1.7) | 0.75 |
| Caffeine (n=299/302) | 71 (23.8) | 82 (27.2) | 0.34 |
| Adrenaline (n=302/303) | 2 (0.7) | 1 (0.3) | 0.56 |
| Surfactant (n=303/303) | 23 (7.6) | 27 (8.9) | 0.56 |
| Intravenous fluids (n=303/303) | 12 (4.0) | 2 (0.7) | 0.007 |
| Others (n=303/301) | 6 (2.0) | 3 (1.0) | 0.5 |
| First 24 hours after birth | |||
| Surfactant (n=303/302) | 169 (55.8) | 157 (52.0) | 0.35 |
| Respiratory support (n=304/303): | |||
| None | 22 (7.2) | 20 (6.6) | 0.19 |
| Non-invasive ventilation | 193 (63.5) | 213 (70.3) | |
| Mechanical ventilation | 89 (29.3) | 70 (23.1) |
Primary outcome
The primary outcome was assessed in 607 infants (table 3). Overall, 252 (82.9%) out of 304 neonates in the near infrared spectroscopy group survived without cerebral injury compared with 238 (78.5%) out of 303 in the control group (relative risk 1.06, 95% confidence interval 0.98 to 1.14). Twenty eight neonates died (12 (4.0%) in the near infrared spectroscopy group v 16 (5.3%) in the control group (relative risk 0.75, 0.33 to 1.70; table 3). After controlling for age, the primary outcome also showed no significant difference between the two groups (1.04, 0.98 to 1.12). The primary outcome was also similar between neonates born at <28 gestational weeks and those born at ≥28 gestational weeks, and in neonates born by caesarean section compared with vaginal delivery (see supplementary tables 1 and 2). The incidence of all components of the primary outcome were not significantly different between the two groups (table 3).
Table 3.
Primary composite outcome measure, primary outcome measures, and secondary outcome variables at term age or before discharge, adjusted for trial site
| Outcome measures and variables | Near infrared spectroscopy group (n=304) | Control group (n=303) | Relative risk (95% CI) | P value |
|---|---|---|---|---|
| Primary composite outcome | ||||
| Survival without cerebral injury* | 252 (82.9) | 238 (78.5) | 1.06 (0.98 to 1.14) | |
| Primary outcome measures | ||||
| Death or cerebral injury, or both* | 52 (17.1) | 65 (21.5) | 0.80 (0.57 to 1.11) | |
| Death | 12 (4.0) | 16 (5.3) | 0.75 (0.33 to 1.70) | |
| Intraventricular haemorrhage: | ||||
| Any grade | 41 (13.5) | 55 (18.2) | 0.74 (0.52 to 1.06) | |
| Absent | 263 (86.5) | 248 (81.9) | 0.29 | |
| Grade I-II | 29 (9.5) | 39 (12.9) | ||
| Grade III-IV | 12 (4.0) | 16 (5.3) | ||
| Cystic periventricular leukomalacia: | ||||
| Any grade | 8 (2.6) | 3 (1.0) | 2.66 (0.80 to 8.80) | |
| Absent | 296 (97.4) | 300 (99.0) | 0.24 | |
| Grade II | 3 (1.0) | 2 (0.7) | ||
| Grade III | 5 (1.6) | 1 (0.3) | ||
| Secondary outcome measures (morbidities) | ||||
| Respiratory distress syndrome: | ||||
| Present | 248 (81.6) | 244 (80.5) | 1.01 (0.96 to 1.07) | |
| Absent | 56 (18.4) | 59 (19.5) | 0.92 | |
| Not defined | 26 (8.6) | 28 (9.2) | ||
| Grade 1-2 | 163 (53.6) | 163 (53.8) | ||
| Grade 3-4 | 59 (19.4) | 53 (17.5) | ||
| Culture proven sepsis | 91 (29.9) | 111 (36.6) | 0.82 (0.67 to 1.00) | |
| Necrotising enterocolitis any grade | 17 (5.6) | 15 (5.0) | 1.13 (0.57 to 2.24) | |
| Bronchopulmonary dysplasia† | 54 (17.8) | 57 (18.8) | 0.94 (0.67 to 1.34) | |
| Retinopathy of prematurity ≥grade 2 | 35 (11.5) | 33 (10.9) | 1.06 (0.68 to 1.65) | |
| Persistent ductus arteriosus with intervention‡ | 41 (13.5) | 54 (17.8) | 0.76 (0.57 to 1.01) |
CI=confidence interval.
Any intraventricular haemorrhage or cystic periventricular leukomalacia, or both, at term age or before discharge.
Oxygen dependency or need for respiratory support at 36 weeks corrected age.
Medical intervention or surgical intervention, or both.
Secondary outcomes
The incidence of predefined secondary neonatal outcomes did not differ significantly between the two groups (table 3). No serious adverse reactions or serious adverse device related events were observed.
Discussion
In preterm neonates with <32 weeks’ gestation, monitoring of cerebral tissue oxygen saturation combined with dedicated interventions during immediate transition and resuscitation in the first 15 minutes after birth compared with standard care increased survival without cerebral injury by 4.3% (95% confidence interval −1.9% to 10.6%) and decreased the risk of mortality by 1.3% (−2.0% to 4.7%), although both findings were not statistically significant. Monitoring using near infrared spectroscopy was not associated with serious adverse reactions or serious adverse device related events.
Preterm birth has lifelong effects on neurodevelopmental outcomes, including an increased risk of cerebral palsy or impaired learning, which result in high economic cost for families and healthcare systems.19 An estimated 15 million neonates are born worldwide at <37 weeks of gestation annually and account for 35% of the world’s 3.1 million neonatal deaths each year.19
Comparison with other studies
In the initial (COSGOD phase I/II) pilot feasibility trial,15 near infrared spectroscopy in addition to routine monitoring to guide medical support during immediate transition resulted in a 55.4% relative reduction in risk of cerebral hypoxia. Although in the present trial more than 50% of neonates in the near infrared spectroscopy group received interventions because of cerebral hypoxia, there was no significant improvement in crSO2. This finding must be interpreted with caution, however, as near infrared spectroscopy monitoring was masked in the control group and therefore not evaluated for signal quality and artefacts, whereas in the near infrared spectroscopy group the signal and its quality were visible, and, when necessary, manoeuvres such as repositioning of the sensor were performed to improve the signal. Nevertheless, similar confidence intervals of crSO2 values in both groups suggest similar variations and artefacts between the groups. The absence of an improvement in the crSO2 during the first 15 minutes in the near infrared spectroscopy group might also reflect the lack of current effective interventions to improve crSO2 in this trial. Cerebral oxygenation is influenced by three main components: the oxygen content of blood, cerebral perfusion, and oxygen consumption. Each of these factors are influenced by several other variables, such as SpO2, blood glucose level, partial pressure of carbon dioxide, blood pressure, and haemoglobin level.20 21 22 The SafeBoosC 2 trial examined monitoring using near infrared spectroscopy and dedicated interventions within the first 72 hours after birth in preterm neonates and reported a reduced cerebral burden of hypoxia.23 Although ample opportunities exist to assess and correct factors that might influence crSO2 within the first 72 hours after birth, limited information is available for resuscitation within the first 15 minutes after birth. Interventions in the present trial were therefore limited to oxygen titration, respiratory support, or intravenous fluids, or a combination of these factors. Changes in cardiovascular variables, including arterial blood pressure and cardiac output have been associated with changes in cerebral oxygenation during immediate transition9 10 24 and have been described as predictors of cerebral injury, including intraventricular haemorrhage and cystic periventricular leukomalacia.25 26 27 In the present trial, significantly more neonates in the near infrared spectroscopy group than control group received intravenous fluids (table 2), in part indicated by crSO2 monitoring to improve cardio-circulation and cerebral perfusion. Furthermore, a trend to a higher rate of mechanical ventilation was observed after resuscitation during the first day after birth in the near infrared spectroscopy group. This might be explained by the higher rate of intubations during resuscitation in the first minutes after birth, which were also in part indicated by crSO2 monitoring.
Cerebral injury was determined by cerebral ultrasonography and included all grades of intraventricular haemorrhage (grades I-IV) as well as cystic periventricular leukomalacia. The lower grades of intraventricular haemorrhage were included because grades I and II have been associated with potential impairment of cognitive and motor abilities in preterm infants.28 Furthermore, on cranial ultrasonography no abnormality is associated with the lowest probability of impaired development.29 Although magnetic resonance imaging may have provided a more precise assessment,30 31 this was not available and feasible in many units and is often difficult to perform in critically ill preterm neonates. Furthermore, cranial ultrasonography is routinely used in most neonatal intensive care units thereby making the results of this trial more generalisable. Therefore, cranial ultrasonography was used as the standard method to determine brain injury such as intraventricular haemorrhage or cystic periventricular leukomalacia.
Limitations of this study
Several limitations of this study are worth noting. More than two thirds of eligible neonates were not screened as a result of imminent birth or unavailability of the research team as approximately 75% of deliveries occurred after hours or during weekends.32 The included sample size was about 10% below the calculated sample size, because recruitment ended after four years. More than 80% of included neonates were born by caesarean section, which was higher than recently reported rates for caesarean section.33 The higher number of neonates included after caesarean section might be due to a wider window of opportunity to obtain consent as mothers are not in labour. Cerebral ultrasonography was performed locally, and therefore despite every effort, full masking to group allocation could not be guaranteed. Since the primary outcome included all grades of intraventricular haemorrhage the high interobserver variability in grading of intraventricular haemorrhage should not have influenced the results. The survival without cerebral injury was probably related to overall improvements in outcomes for preterm neonates compared with the pre-trial era. The number of neonates who survived without cerebral injury was high in both groups in our study and therefore should not have influenced the findings.
Conclusions
Monitoring of cerebral tissue oxygen saturation in combination with dedicated interventions during immediate transition and resuscitation after birth in preterm neonates <32 weeks’ gestation did not result in substantially higher survival without cerebral injury compared with standard care alone. No serious adverse reactions or serious adverse device related events were observed when using near infrared spectroscopy. Further trials are warranted to explore if different groups of preterm neonates may have a substantial benefit and which near infrared spectroscopy guided interventions might improve cerebral oxygenation the most.
What is already known on this topic
Preterm neonates who developed intraventricular haemorrhage have been found to have significantly lower cerebral tissue oxygen saturation during the first minutes after birth than neonates without intraventricular haemorrhage
Guiding respiratory support and titrating fractional inspired oxygen using cerebral tissue oxygen saturation in addition to arterial oxygen saturation monitoring (COSGOD phase I/II trial) achieved a relative reduction of 55.4% in the burden of cerebral hypoxia within the first 15 minutes after birth
What this study adds
In preterm neonates <32 weeks’ gestation, the primary outcome of survival without cerebral injury was similar in infants who received monitoring of cerebral tissue oxygen saturation combined with dedicated interventions during immediate transition and resuscitation in the first 15 minutes after birth and those who received standard care
Acknowledgments
We thank the parents, who agreed for their infants to take part in the trial; Tom Goos (Department of Pediatrics, Division of Neonatology, Erasmus MC-Sophia Children’s Hospital, Rotterdam, Netherlands) who helped to perform the sample size calculation; and the public for donating money to our funding agencies (see funding section); and the Independent Data Monitoring and Safety Committee: Petra Lemmers (associate professor of neonatology, Division Woman and Baby, University Medical Center, Utrecht, Netherlands) and Maximo Vento (professor of paediatrics, scientific director, Health Research Institute and University and Polytechnic Hospital La Fe, Valencia, Spain).
Collaborators of the COSGOD III study group:
Division of Neonatology, Department of Pediatrics, Medical University of Graz, Graz, Austria: Christina Wolfsberger, Nariae Baik-Schneditz, Marlies Bruckner, Corinna Binder-Heschl, Daniel Pfurtscheller, Johann Martensen, Nina Höller, Evelyn Ziehenberger, Lukas Mileder; Comprehensive Center for Pediatrics, Department of Pediatrics and Adolescent Medicine, Division of Neonatology, Intensive Care and Neuropediatrics, Medical University of Vienna, Vienna, Austria: Angelika Berger (local principal investigator), Sigrid Baumgartner, Agnes Grill, Michaela Mayr, Judith Rittenschober-Boehm, Michael Schneider; Department of Pediatrics II, Neonatology, Medical University of Innsbruck, Innsbruck, Austria: Christina Schreiner, Elke Griesmaier, Vera Neubauer, Peter Wöckinger, Anna Posod; Neonatal Intensive Care Unit, Department for Perinatology, Division of Gynaecology and Obstetrics, University Medical Centre Ljubljana, Slovenia: Anja Marolt, Ana Dimnik, Vlasta Lubej Kurtovič; Infant Centre, University College Cork, Cork University Maternity Hospital, Cork, Ireland: Garvey Aisling, Jurate Panaviene, David Healy, Nahla Ahmed, Ita Herlihy; Department of Neonatology, University Children’s Hospital of Tübingen, Germany: Axel Franz; Neonatologia e Terapia Intensiva Neonatale (TIN) Ospedale dei Bambini “V.Buzzi,” Milano, Italia: Francesca Castoldi, Francesco Cavigioli; Ginekologiczno Położniczy Szpital Kliniczny Uniwersytetu Medycznego im. Karola Marcinkowskiego w Poznańiu, Poznań, Poland: Zuzanna Kozłowska, Marcin Minta, Zuzanna Owsiańska, Sonia Kahtan, Natalia Neumann-Klimasińska, Karolina Wróbel, Agata Kubiaczyk, Katarzyna Kosik, Katarzyna Olek, Michalina Bugiera, Julita Porwolik, Agnieszka Basiukajć, Elzbieta Czapla, Wojciech Łukaszuk, Katarzyna Gryczka, Dobrochna Naskręcka, Jan Mazela, Marta Szymankiewicz-Bręborowicz; Division of Neonatology and Pediatric Intensive Care Medicine, Center for Pediatrics and Adolescent Medicine, Medical Center – University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany: Daniel Klotz, Jana Baumgartner; Institute for Maternal and Child Health, “IRCCS Burlo Garofolo,” Trieste, Italy: Jana Bembich, Laura Travan; Department of Pediatrics, University of Alberta, Edmonton, Alberta, Canada: Po-Yin Cheung.
Web extra.
Extra material supplied by authors
Supplementary information: Supplementary figures 1 and 2 and supplementary tables 1-3
Contributors: GP and GMS conceptualised and designed the study. GP, KG, EMD, LS, LG, TS, HF, LK, JB, UKK, LKC, and GMS supervised and coordinated the study at different sites. All authors contributed to the acquisition and preparation of study data. AV and GP performed the statistical analyses. All authors contributed to interpretation of the findings. GP, AV, and GMS drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors approved the final manuscript. GP is guarantor of the study. GP, EMD, and GMS obtained funding. The corresponding author GP attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This trial was funded by the Austrian Science Fund (Fonds zur Förderung der wissenschaftlichen Forschung (FWF) Austria) through an unconditional and unrestricted grant (KLI 586-B31). HRB Clinical Research Facility at University College Cork supported the study at the Infant Centre, University College Cork, Cork University Maternity Hospital, Cork, Ireland. GMS was a recipient of the Heart and Stroke Foundation/University of Alberta Professorship of Neonatal Resuscitation, a National New Investigator of the Heart and Stroke Foundation Canada, and an Alberta New Investigator of the Heart and Stroke Foundation Alberta. This research was facilitated by the Women and Children’s Health Research Institute through the support of the Stollery Children's Hospital Foundation. The trial sponsors and funders had no influence in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the Austrian Science Fund and the HRB Clinical Research Facility at the University of Cork for the submitted work, and from the Stollery Children’s Hospital Foundation facilitated by the Women and Children’s Health Research Institute; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The study guarantor (GP) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The authors plan on presenting the results of the trial at national and international congresses. Results will be made available to the public on the homepages of the participating institutions, and press releases will be sought in cooperation with each institution’s press department.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
Collaborators: COSGOD III study group, Christina Wolfsberger, Nariae Baik-Schneditz, Marlies Bruckner, Corinna Binder-Heschl, Daniel Pfurtscheller, Johann Martensen, Nina Höller, Evelyn Ziehenberger, Angelika Berger, Sigrid Baumgartner, Agnes Grill, Michaela Mayr, Judith Rittenschober-Boehm, Michael Schneider, Christina Schreiner, Elke Griesmaier, Vera Neubauer, Peter Wöckinger, Anna Posod, Anja Marolt, Ana Dimnik, Vlasta Lubej Kurtovič, Garvey Aisling, Jurate Panaviene, David Healy, Nahla Ahmed, Ita Herlihy, Axel Franz, Francesca Castoldi, Francesco Cavigioli, Zuzanna Kozłowska, Marcin Minta, Zuzanna Owsiańska, Sonia Kahtan, Natalia Neumann-Klimasińska, Karolina Wróbel, Agata Kubiaczyk, Katarzyna Kosik, Katarzyna Olek, Michalina Bugiera, Julita Porwolik, Agnieszka Basiukajć, Elzbieta Czapla, Wojciech Łukaszuk, Katarzyna Gryczka, Dobrochna Naskręcka, Jan Mazela, Marta Szymankiewicz-Bręborowicz, Daniel Klotz, Jana Baumgartner, Stefano Bembich, Laura Travan, and Po-Yin Cheung
Ethics statements
Ethical approval
The trial was approved by the human research ethics committee at each participating site: Graz: institutional ethical board of the Medical University of Graz (reference 28 456 ex 15/16); Vienna: institutional review board of the Medical University Vienna (reference 1823/2017); Innsbruck: institutional ethical board of the Medical University of Innsbruck (reference 1048/2017); Tübingen: Ethics committee of the Medical Institute of the Eberhard Karls University and the University Hospital Tübingen (reference 560/2017BO1); Freiburg: Ethics committee of the University Freiburg (reference 612/17); Cork: Cork research ethics committee (reference ECM 4(1) 17/1/18); Poznan: Bioethics committee at Poznan University of Medical Sciences (reference 484/20); Milan: Ethics committee Milano Area 1 (protocol No 19140/2018); Trieste: Ethics committee Unico Regionale del Friuli Venezia Giulia (reference PARERE CEUR-2020-Sper-061); Ljubljana: Commission of the Republic of Slovenia for Medical Ethics (reference 0120-529/2017/4); Edmonton, Canada: human ethics research board (reference Pro00065767).
Data availability statement
Deidentified data are available on request from the corresponding author (gerhard.pichler@medunigraz.at) on approval and with a signed data access agreement.
References
- 1. Aziz K, Chadwick M, Baker M, Andrews W. Ante- and intra-partum factors that predict increased need for neonatal resuscitation. Resuscitation 2008;79:444-52. 10.1016/j.resuscitation.2008.08.004 [DOI] [PubMed] [Google Scholar]
- 2. Wyllie J, Bruinenberg J, Roehr CC, Rüdiger M, Trevisanuto D, Urlesberger B. European Resuscitation Council Guidelines for Resuscitation 2015: Section 7. Resuscitation and support of transition of babies at birth. Resuscitation 2015;95:249-63. 10.1016/j.resuscitation.2015.07.029 [DOI] [PubMed] [Google Scholar]
- 3. Madar J, Roehr CC, Ainsworth S, et al. European Resuscitation Council Guidelines 2021: Newborn resuscitation and support of transition of infants at birth. Resuscitation 2021;161:291-326. 10.1016/j.resuscitation.2021.02.014 [DOI] [PubMed] [Google Scholar]
- 4. Oei JL, Finer NN, Saugstad OD, et al. Outcomes of oxygen saturation targeting during delivery room stabilisation of preterm infants. Arch Dis Child Fetal Neonatal Ed 2018;103:F446-54. 10.1136/archdischild-2016-312366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pichler G, Schmölzer GM, Urlesberger B. Cerebral Tissue Oxygenation during Immediate Neonatal Transition and Resuscitation. Front Pediatr 2017;5:29. 10.3389/fped.2017.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bruckner M, Pichler G, Urlesberger B. NIRS in the fetal to neonatal transition and immediate postnatal period. Semin Fetal Neonatal Med 2020;25:101079. 10.1016/j.siny.2020.101079 [DOI] [PubMed] [Google Scholar]
- 7. Peebles DM, Edwards AD, Wyatt JS, Cope M, Delpy DT, Reynold EO. Changes in human fetal cerebral oxygenation and blood volume during delivery. Am J Obstet Gynecol 1992;167:1916-7. 10.1016/0002-9378(92)91808-N [DOI] [PubMed] [Google Scholar]
- 8. Urlesberger B, Kratky E, Rehak T, et al. Regional oxygen saturation of the brain during birth transition of term infants: comparison between elective cesarean and vaginal deliveries. J Pediatr 2011;159:404-8. 10.1016/j.jpeds.2011.02.030 [DOI] [PubMed] [Google Scholar]
- 9. Noori S, Wlodaver A, Gottipati V, McCoy M, Schultz D, Escobedo M. Transitional changes in cardiac and cerebral hemodynamics in term neonates at birth. J Pediatr 2012;160:943-8. 10.1016/j.jpeds.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 10. Bresesti I, Avian A, Bruckner M, et al. Impact of bradycardia and hypoxemia on oxygenation in preterm infants requiring respiratory support at birth. Resuscitation 2021;164:62-9. 10.1016/j.resuscitation.2021.05.004 [DOI] [PubMed] [Google Scholar]
- 11. Baik N, Urlesberger B, Schwaberger B, Schmölzer GM, Avian A, Pichler G. Cerebral haemorrhage in preterm neonates: does cerebral regional oxygen saturation during the immediate transition matter? Arch Dis Child Fetal Neonatal Ed 2015;100:F422-7. 10.1136/archdischild-2014-307590 [DOI] [PubMed] [Google Scholar]
- 12. Fuchs H, Lindner W, Buschko A, Almazam M, Hummler HD, Schmid MB. Brain oxygenation monitoring during neonatal resuscitation of very low birth weight infants. J Perinatol 2012;32:356-62. 10.1038/jp.2011.110 [DOI] [PubMed] [Google Scholar]
- 13. Pichler G, Binder C, Avian A, Beckenbach E, Schmölzer GM, Urlesberger B. Reference ranges for regional cerebral tissue oxygen saturation and fractional oxygen extraction in neonates during immediate transition after birth. J Pediatr 2013;163:1558-63. 10.1016/j.jpeds.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 14. Baik N, Urlesberger B, Schwaberger B, et al. Reference Ranges for Cerebral Tissue Oxygen Saturation Index in Term Neonates during Immediate Neonatal Transition after Birth. Neonatology 2015;108:283-6. 10.1159/000438450 [DOI] [PubMed] [Google Scholar]
- 15. Pichler G, Urlesberger B, Baik N, et al. Cerebral Oxygen Saturation to Guide Oxygen Delivery in Preterm Neonates for the Immediate Transition after Birth: A 2-Center Randomized Controlled Pilot Feasibility Trial. J Pediatr 2016;170:73-8.e1, 4. 10.1016/j.jpeds.2015.11.053 [DOI] [PubMed] [Google Scholar]
- 16. Hansen ML, Hyttel-Sørensen S, Jakobsen JC, et al. European Society for Paediatric Research Special Interest Group ‘NearInfraRed Spectroscopy’ (NIRS). Cerebral near-infrared spectroscopy monitoring (NIRS) in children and adults: a systematic review with meta-analysis. Pediatr Res 2022; published online 22 February. 10.1038/s41390-022-01995-z. [DOI] [Google Scholar]
- 17. Pichler G, Baumgartner S, Biermayr M, et al. Cerebral regional tissue Oxygen Saturation to Guide Oxygen Delivery in preterm neonates during immediate transition after birth (COSGOD III): an investigator-initiated, randomized, multi-center, multi-national, clinical trial on additional cerebral tissue oxygen saturation monitoring combined with defined treatment guidelines versus standard monitoring and treatment as usual in premature infants during immediate transition: study protocol for a randomized controlled trial. Trials 2019;20:178. 10.1186/s13063-019-3258-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dawson JA, Kamlin COF, Vento M, et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics 2010;125:e1340-7. 10.1542/peds.2009-1510 [DOI] [PubMed] [Google Scholar]
- 19. Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379:2162-72. 10.1016/S0140-6736(12)60820-4 [DOI] [PubMed] [Google Scholar]
- 20. Greisen G, Hansen ML, Rasmussen MIS, Vestager M, Hyttel-Sørensen S, Hahn GH. Cerebral Oximetry in Preterm Infants-To Use or Not to Use, That Is the Question. Front Pediatr 2022;9:747660. 10.3389/fped.2021.747660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hansen ML, Pellicer A, Gluud C, et al. Cerebral near-infrared spectroscopy monitoring versus treatment as usual for extremely preterm infants: a protocol for the SafeBoosC randomised clinical phase III trial. Trials 2019;20:811. 10.1186/s13063-019-3955-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pellicer A, Greisen G, Benders M, et al. The SafeBoosC phase II randomised clinical trial: a treatment guideline for targeted near-infrared-derived cerebral tissue oxygenation versus standard treatment in extremely preterm infants. Neonatology 2013;104:171-8. 10.1159/000351346 [DOI] [PubMed] [Google Scholar]
- 23. Hyttel-Sorensen S, Pellicer A, Alderliesten T, et al. Cerebral near infrared spectroscopy oximetry in extremely preterm infants: phase II randomised clinical trial. BMJ 2015;350:g7635. 10.1136/bmj.g7635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baik N, Urlesberger B, Schwaberger B, et al. Foramen ovale (FO) - The underestimated sibling of ductus arteriosus (DA): Relevance during neonatal transition. Early Hum Dev 2016;103:137-40. 10.1016/j.earlhumdev.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 25. El-Dib M, Aly S, Govindan R, Mohamed M, du Plessis A, Aly H. Brain maturity and variation of oxygen extraction in premature infants. Am J Perinatol 2016;33:814-20. 10.1055/s-0036-1572542 [DOI] [PubMed] [Google Scholar]
- 26. Massa-Buck B, Amendola V, McCloskey R, Rais-Bahrami K. Significant correlation between regional tissue oxygen saturation and vital signs of critically ill infants. Front Pediatr 2017;5:276. 10.3389/fped.2017.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soul JS, Hammer PE, Tsuji M, et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res 2007;61:467-73. 10.1203/pdr.0b013e31803237f6 [DOI] [PubMed] [Google Scholar]
- 28. Briana DD, Malamitsi-Puchner A. Low-grade intraventricular hemorrhage of preterm infants: neurodevelopmental and motor outcome. J Matern Fetal Neonatal Med 2021;34:646-52. 10.1080/14767058.2019.1610741 [DOI] [PubMed] [Google Scholar]
- 29. O’Shea TM, Kuban KC, Allred EN, et al. Extremely Low Gestational Age Newborns Study Investigators . Neonatal cranial ultrasound lesions and developmental delays at 2 years of age among extremely low gestational age children. Pediatrics 2008;122:e662-9. 10.1542/peds.2008-0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Whyte HE, Blaser S. Limitations of routine neuroimaging in predicting outcomes of preterm infants. Neuroradiology 2013;55(Suppl 2):3-11. 10.1007/s00234-013-1238-6 [DOI] [PubMed] [Google Scholar]
- 31. Plomgaard AM, Hagmann C, Alderliesten T, et al. Brain injury in the international multicenter randomized SafeBoosC phase II feasibility trial: cranial ultrasound and magnetic resonance imaging assessments. Pediatr Res 2016;79:466-72. 10.1038/pr.2015.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reif P, Pichler G, Griesbacher A, et al. Do time of birth, unit volume, and staff seniority affect neonatal outcome in deliveries at ≥34+0 weeks of gestation? BJOG 2018;125:884-91. 10.1111/1471-0528.15000 [DOI] [PubMed] [Google Scholar]
- 33. Costa STB, Costa P, Graça AM, Abrantes M, Portuguese National Registry of very low birth weight infants . Delivery mode and neurological complications in very low birth weight infants. Am J Perinatol 2022; published online 23 June. 10.1055/a-1815-1842 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Supplementary figures 1 and 2 and supplementary tables 1-3
Data Availability Statement
Deidentified data are available on request from the corresponding author (gerhard.pichler@medunigraz.at) on approval and with a signed data access agreement.