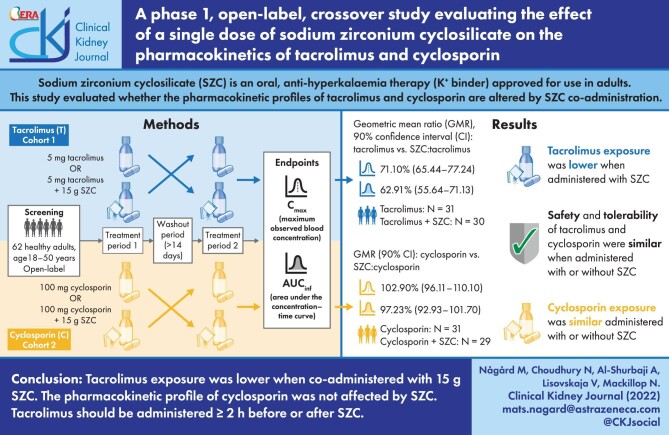
ABSTRACT
Background
Sodium zirconium cyclosilicate (SZC) is an oral, highly selective potassium binder approved for the treatment of hyperkalaemia in adults. SZC may change the absorption of co-administered drugs that exhibit pH-dependent bioavailability. This study evaluated whether the pharmacokinetic (PK) profiles of tacrolimus and cyclosporin were altered by concomitant SZC administration in healthy participants.
Methods
This was an open-label, randomised sequence, two-cohort crossover, single-centre study. Healthy adults were assigned to one of two cohorts: Cohort 1 (tacrolimus) received a single dose of tacrolimus 5 mg and tacrolimus 5 mg + SZC 15 g in a random order; Cohort 2 (cyclosporin) received a single dose of cyclosporin 100 mg and cyclosporin 100 mg + SZC 15 g in a random order. Primary PK endpoints were maximum observed blood concentration (Cmax) and area under the concentration–time curve (AUC) from time zero to infinity (AUCinf). Differences in mean Cmax and AUCinf were analysed using a mixed effects model.
Results
Thirty participants in Cohort 1 and 29 in Cohort 2 completed the study. Tacrolimus exposure was lower with tacrolimus + SZC versus tacrolimus alone: Cmax geometric mean ratio (GMR) 71.10% [90% confidence interval (CI) 65.44–77.24], AUCinf 62.91% (55.64–71.13). Cyclosporin exposure was similar with cyclosporin + SZC compared with cyclosporin alone: Cmax GMR 102.9% (90% CI 96.11–110.10), AUCinf 97.23% (92.93–101.70).
Conclusions
Tacrolimus exposure was lower when co-administered with SZC 15 g and should be administered ≥2 h before or after SZC. The PK profile of cyclosporin was not affected by SZC co-administration.
Keywords: cyclosporin, pharmacokinetics, sodium zirconium cyclosilicate, tacrolimus
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Sodium zirconium cyclosilicate (SZC) is an oral, highly selective potassium binder approved for the treatment of hyperkalaemia in adults [1]. Hyperkalaemia is a common condition in patients with chronic kidney disease, even after successful kidney transplantation [2, 3]. Furthermore, comorbid conditions and frequently used medications predispose patients with chronic kidney disease and kidney transplantation recipients to this potentially life-threatening condition [2].
SZC selectively captures potassium ions in exchange for sodium and hydrogen ions in the gastrointestinal tract, thereby reducing the serum potassium concentration and removing potassium from the body through increased faecal excretion [1]. SZC exerts its effect locally and is not absorbed systemically [1]. However, SZC may change the absorption of co-administered drugs that exhibit pH-dependent bioavailability, such as azole antifungals, anti-human immunodeficiency virus drugs and tyrosine kinase inhibitors [4], so it is recommended that SZC should be taken ≥2 h before or after these drugs [5].
Tacrolimus and cyclosporin are important immunosuppressants for kidney transplantation [6]. They have narrow therapeutic indices [7–9], and it is unclear whether their exposure is affected by gastric pH. Tacrolimus and cyclosporin can also cause hyperkalaemia [7, 9] and may therefore be administered with SZC. The aim of this study was to evaluate whether the pharmacokinetic (PK) profiles of tacrolimus (Prograf) and cyclosporin (Sandimmun Optoral) are altered by SZC co-administration in healthy participants.
MATERIALS AND METHODS
This was an open-label, randomised sequence, two-period, two-cohort, two-treatment in each cohort, crossover study in healthy participants, performed at a single study centre in Germany (NCT04788641). The protocol was reviewed and approved by the local regulatory authority and Independent Ethics Committee (Landesamt für Gesundheit und Soziales, Berlin, Germany; study reference number 20/380-IV E 10), and the study was performed in accordance with the ethical principles of the Declaration of Helsinki and was consistent with the International Council for Harmonisation Good Clinical Practice guidelines. Informed, written consent was obtained from the participants prior to the start of the study.
Study participants
Participants who met the following criteria were eligible to participate in the study: healthy adults aged 18–50 years; body mass index 18.5–30.0 kg/m2; and weight ≥50 kg and ≤100 kg.
Participants were not eligible if they had the following: history or presence of a disease or condition known to interfere with the PKs of drugs; systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg, pulse <50 or >90 b.p.m.; clinically important abnormalities in rhythm, conduction or morphology of electrocardiogram (ECG); plasma donation within 1 month of screening visit or >500 mL blood donation/loss during 3 months prior to screening; used drugs with enzyme-inducing properties within 3 weeks prior to screening.
Study design
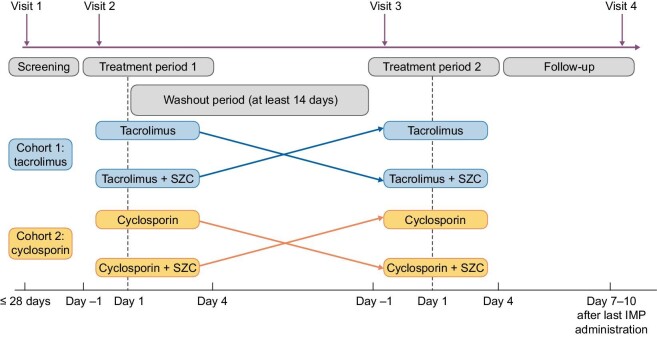
During the screening period (maximum of 28 days), participants were assigned to one of two cohorts, Cohort 1 (tacrolimus) or Cohort 2 (cyclosporin), and participants in each cohort were randomly assigned to one of two treatment sequences (AB|BA or CD|DC) where: Treatment A, tacrolimus 5 mg; Treatment B, tacrolimus 5 mg + SZC 15 g; Treatment C, cyclosporin 100 mg; and Treatment D, cyclosporin 100 mg + SZC 15 g.
Each participant received a single dose of tacrolimus 5 mg or cyclosporin 100 mg oral capsules on two occasions, once alone and once in combination with an oral suspension of SZC 15 g. Drug administrations were planned to occur after a 12-h overnight fast and ≥14-day washout period between treatments. A follow-up visit/early termination visit took place 7–10 days after administration of the last treatment (Fig. 1). No concomitant medications, including herbal remedies, vitamin supplements and over-the-counter products, were allowed apart from paracetamol.
Figure 1:
Study design. Single doses of all IMPs on indicated days: SZC 15 g; tacrolimus 5 mg; cyclosporin 100 mg. IMP, investigational medicinal product.
Treatment period 1: admission to the clinical unit on Day –1, followed by dosing on Day 1 with the assigned treatment (A, B, C or D) as per assigned cohort and treatment sequence.
Treatment period 2: admission to the clinical unit on Day –1, followed by dosing on Day 1 with crossover treatment as per assigned cohort.
Study objectives and endpoints
The primary objective was to assess the effect of co-administered SZC on the PKs of tacrolimus and cyclosporin in healthy participants, based on the primary endpoints of maximum observed blood concentration (Cmax) and area under the concentration-time curve (AUC) from time zero to infinity (AUCinf). The secondary objective was to assess the effect of co-administered SZC on the PKs of tacrolimus and cyclosporin in healthy participants, based on the secondary endpoints of AUC from time zero to the time of the last quantifiable concentration (AUClast), half-life associated with terminal slope (λz) of a semi-logarithmic concentration-time curve (t½λz) and time to reach maximum observed concentration following drug administration (Tmax). The safety and tolerability of co-administration of SZC and tacrolimus/cyclosporin was also compared with tacrolimus/cyclosporin alone, including assessment of adverse events, vital signs, 12-lead ECGs, physical examination and laboratory values.
Analyses
Pharmacokinetic
Blood samples for tacrolimus and cyclosporin PK analyses were collected at: pre-dose, 0.25-h intervals from 0–2.5 and 3 h; 2-h intervals from 4–12-, 16-, 24-, 36-, 48- and 72-h post dose. PK analyses of the whole blood concentration data for tacrolimus/cyclosporin were derived using non-compartmental methods with Phoenix® WinNonlin®, version 8.1 or higher. Cmax and Tmax were derived directly from the plasma concentration-time profiles.
Plasma concentrations that were below the limit of quantification prior to administration of the first dose and up to the quantifiable concentration were set to a value of zero, with certain exceptions shown in the Supplementary Methods. Terminal elimination half-life, calculated as (ln2)/λz, was estimated by log-linear least squares regression of the terminal part of the concentration-time curve. AUCinf was estimated by AUClast + Clast/λz, where Clast was the observed last quantifiable drug concentration. AUCs, including AUCinf and AUClast, were calculated using the linear trapezoidal method when concentrations were increasing, and the logarithmic trapezoidal method when concentrations were decreasing (linear up log down).
Statistical analysis
The PK analysis set included all participants from the safety analysis set and who had received one or more quantifiable whole blood concentration post dose of tacrolimus (Cohort 1) or cyclosporin (Cohort 2), and who had no major protocol deviations or adverse events known to impact the analysis of the PK data.
Mean differences in Cmax and AUCinf for the two treatment cohorts were analysed using a mixed effects model following a natural logarithmic transformation of the individual PK parameters, with period, treatment and sequence as fixed effects, and participant nested within sequence as random effect.
Least squares mean (LSM) for each treatment, the differences and corresponding two-sided 90% confidence intervals (CIs) for the log-transformed values were calculated. Corresponding geometric LSM for each treatment, the ratio thereof and the two-sided 90% CIs were estimated. Limits for the 90% CIs for the geometric mean ratio were compared with the pre-specified interval (80%–125%) for conclusion of no drug–drug interaction. This mixed effects statistical model was repeated for the secondary PK variables of AUClast, t½λz and Tmax, for both treatment cohorts.
The safety analysis set included all participants who had received one or more dose of tacrolimus (Cohort 1) or cyclosporin (Cohort 2) and for whom any post-dose safety data were available. Continuous safety variables were summarised using descriptive statistics (n, mean, standard deviation and median), and categorical variables as frequency and proportion.
RESULTS
A total of 31 participants were enrolled in each treatment cohort; 30 participants in Cohort 1 (tacrolimus) and 29 in Cohort 2 (cyclosporin) completed the study. In Cohort 1, one participant withdrew from the study due to personal reasons and only completed one treatment period. In Cohort 2, two participants were withdrawn, one due to a protocol violation and one due to an adverse event of ventricular extrasystoles (whilst taking cyclosporin 100 mg alone).
In Cohort 1, the median age of participants was 39.0 years (range 19–50 years), and the majority were White (93.5%; 29/31) and male (90.3%; 28/31) (Table 1). In Cohort 2, the median age of participants was 32.0 years (range, 18–48 years), the majority were White (80.6%; 25/31) and all were male (Table 1).
Table 1:
Demographics and baseline characteristics.
| Characteristic | Tacrolimus/tacrolimus + SZC | Tacrolimus + SZC/tacrolimus | Cyclosporin/cyclosporin + SZC | Cyclosporin + SZC/cyclosporin |
|---|---|---|---|---|
| Median age, years (range) | 37.0 (24–50) | 40.0 (19–50) | 32.5 (18–48) | 30.0 (18–45) |
| Male, n (%) | 15 (93.8) | 13 (86.7) | 16 (100) | 15 (100) |
| Race, n (%) | ||||
| White | 14 (87.5) | 15 (100) | 12 (75.0) | 13 (86.7) |
| Black or African American | – | – | 2 (12.5) | – |
| Asian | 1 (6.3) | – | 1 (6.3) | 1 (6.7) |
| American Indian or Alaska Native | – | – | 1 (6.3) | – |
| Other | 1 (6.3) | – | – | 1 (6.7) |
| Mean weight, kg (SD) | 81.36 (13.04) | 79.23 (12.03) | 79.96 (8.98) | 82.09 (8.13) |
| Mean BMI, kg/m2 (SD) | 25.19 (3.39) | 25.00 (2.47) | 24.68 (2.81) | 24.59 (2.82) |
BMI, body mass index; SD, standard deviation.
Pharmacokinetics
Cohort 1 (tacrolimus)
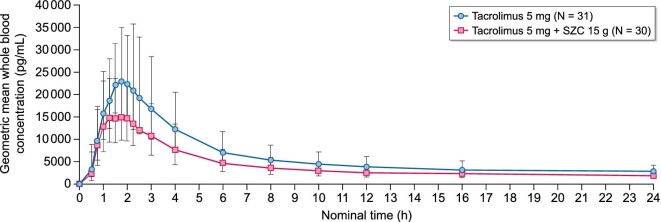
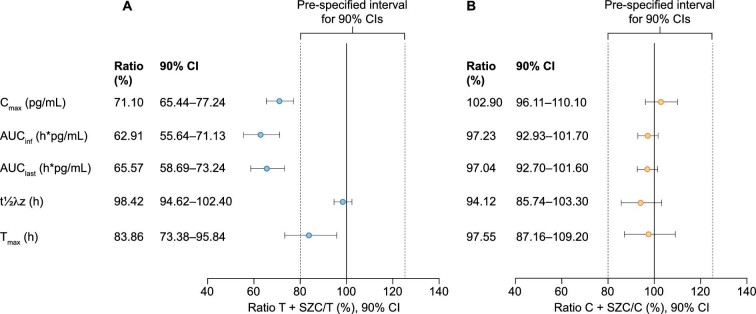
Tacrolimus exposure was lower with tacrolimus + SZC compared with tacrolimus alone (Fig. 2). The geometric LSM ratios were lower for the following PK parameters: Cmax 71.1% (90% CI 65.44–77.24; Fig. 3a; Table 2); AUCinf 62.91% (90% CI 55.64–71.13; Fig. 3a; Table 2); and AUClast 65.57% (90% CI 58.69–73.24; Fig. 3a; Table 3).
Figure 2:
Geometric mean (± geometric standard deviation) whole blood concentration (pg/mL) of tacrolimus with and without SZC versus time—linear scale (0–24 h).
Figure 3:
Forest plot of geometric least squares mean ratios for the pharmacokinetic parameters of (a) tacrolimus + SZC compared with tacrolimus alone and (b) cyclosporin + SZC compared with cyclosporin alone. C, cyclosporin; T, tacrolimus.
Table 2:
Statistical comparison of primary PK parameters for tacrolimus and cyclosporin (PK analysis set).
| Pairwise comparison | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Drug | Parameter (unit) | Treatment | N | n | Geometric LSM | 90% CI | Pair | Ratio (%) | 90% CI |
| 1 | Tacrolimus | Cmax (pg/mL) | T | 31 | 31 | 27 230 | 24 460–30 300 | |||
| T + SZC | 30 | 30 | 19 360 | 17 380–21 560 | T + SZC/T | 71.10 | 65.44–77.24 | |||
| AUCinf (h*pg/mL) | T | 31 | 26 | 279 200 | 239 900–324 900 | |||||
| T + SZC | 30 | 27 | 175 600 | 151 300–203 900 | T + SZC/T | 62.91 | 55.64–71.13 | |||
| 2 | Cyclosporin | Cmax (ng/mL) | C | 31 | 31 | 593.4 | 554.5–635.1 | |||
| C + SZC | 29 | 29 | 610.4 | 569.4–654.4 | C + SZC/C | 102.9 | 96.11–110.10 | |||
| AUCinf (h*ng/mL) | C | 31 | 31 | 1883 | 1746–2031 | |||||
| C + SZC | 29 | 29 | 1831 | 1697–1976 | C + SZC/C | 97.23 | 92.93–101.70 | |||
Treatment T, tacrolimus 5 mg; Treatment T + SZC, tacrolimus 5 mg + SZC 15 g; Treatment C, cyclosporin 100 mg; Treatment C + SZC, cyclosporin 100 mg + SZC 15 g.
Results are based on a mixed effect following a natural logarithmic transformation of the individual PK parameters, with period, treatment and sequence as fixed effects, and subject nested within sequence as random effect.
Geometric LSM ratio and corresponding CI are back-transformed and presented as percentages. Geometric LSM and corresponding 90% CI are also back-transformed.
N, all participants in the PK analysis set; n, all participants included in the statistical comparison analysis.
Table 3:
Statistical comparison of secondary PK parameters for tacrolimus and cyclosporin (PK analysis set).
| Pairwise comparison | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Drug | Parameter (unit) | Treatment | N | n | Geometric LSM | 90% CI | Pair | Ratio (%) | 90% CI |
| 1 | Tacrolimus | AUClast (h*pg/mL) | T | 31 | 31 | 241 500 | 209 100–278 800 | |||
| T + SZC | 30 | 30 | 158 300 | 136 900–183 000 | T + SZC/T | 65.57 | 58.69–73.24 | |||
| t½λz (h) | T | 31 | 31 | 33.65 | 31.71–35.71 | |||||
| T + SZC | 30 | 30 | 33.12 | 31.20–35.16 | T + SZC/T | 98.42 | 94.62–102.40 | |||
| Tmax (h) | T | 31 | 31 | 1.810 | 1.640–1.998 | |||||
| T + SZC | 30 | 30 | 1.518 | 1.373–1.678 | T + SZC/T | 83.86 | 73.38–95.84 | |||
| 2 | Cyclosporin | AUClast (h*pg/mL) | C | 31 | 31 | 1792 | 1659–1935 | |||
| C + SZC | 29 | 29 | 1739 | 1609–1880 | C + SZC/C | 97.04 | 92.70–101.60 | |||
| t½λz (h) | C | 31 | 31 | 9.211 | 8.373–10.13 | |||||
| C + SZC | 29 | 29 | 8.669 | 7.863–9.559 | C + SZC/C | 94.12 | 85.74–103.30 | |||
| Tmax (h) | C | 31 | 31 | 1.417 | 1.303–1.541 | |||||
| C + SZC | 29 | 29 | 1.382 | 1.268–1.507 | C + SZC/C | 97.55 | 87.16–109.20 | |||
Treatment T, tacrolimus 5 mg; Treatment T + SZC, tacrolimus 5 mg + SZC 15 g; Treatment C, cyclosporin 100 mg; Treatment C + SZC, cyclosporin 100 mg + SZC 15 g.
Results are based on a mixed effect following a natural logarithmic transformation of the individual PK parameters, with period, treatment and sequence as fixed effects, and subject nested within sequence as random effect.
Geometric LSM ratio and corresponding CI are back-transformed and presented as percentages. Geometric LSM and corresponding 90% CI are also back-transformed.
N, all participants in the pharmacokinetic analysis set and statistical comparison analysis; n, all participants included in the statistical comparison analysis.
The t½λz of tacrolimus was similar between tacrolimus + SZC and tacrolimus alone (33.65 h and 33.12 h, respectively; geometric LSM ratio: 98.42%; 90% CI 94.62–102.40; Fig. 3a; Table 3). Tacrolimus was rapidly absorbed with the median Tmax at 1.52 h and 1.81 h in the tacrolimus + SZC and tacrolimus alone groups, respectively (geometric LSM ratio: 83.86%; 90% CI 73.38–95.84; Fig. 3a; Table 3).
Geometric mean and geometric coefficient of variation PK parameters of tacrolimus are presented in Supplementary data, Table S1.
Cohort 2 (cyclosporin)
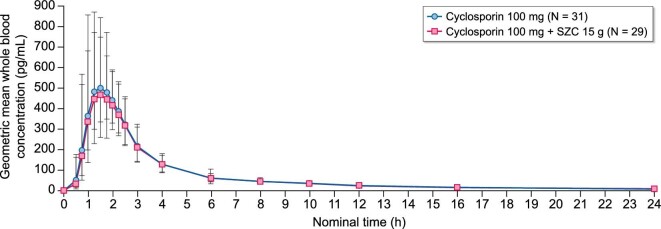
Cyclosporin exposure was similar with cyclosporin + SZC compared with cyclosporin alone (Fig. 4). The geometric LSM ratios were similar for the following PK parameters: Cmax 102.9% (90% CI 96.11–110.10; Fig. 3b; Table 2); AUCinf 97.23% (90% CI 92.93–101.70; Fig. 3b; Table 2); and AUClast 97.04% (90% CI 92.70–101.60; Fig. 3b; Table 3).
Figure 4:
Geometric mean (± geometric standard deviation) whole blood concentration (ng/mL) of cyclosporin with and without SZC versus time—linear scale (0–24 h).
The t½λz of cyclosporin was comparable with cyclosporin + SZC and cyclosporin alone (geometric LSM ratio: 94.12%; 90% CI 85.74–103.30; Fig. 3b; Table 3). Median Tmax of cyclosporin was 1.38 h with cyclosporin + SZC and 1.42 h with cyclosporin alone (geometric LSM ratio: 97.55; 90% CI 87.16–109.20; Fig. 3b; Table 3).
Geometric mean and geometric coefficient of variation PK parameters of cyclosporin are presented in Supplementary data, Table S2.
Safety
Adverse events were generally mild in intensity in all groups. There were no serious adverse events and no systematic pattern in preferred terms. A total of 15 adverse events were reported in 13 participants with tacrolimus + SZC and 12 adverse events in seven participants with tacrolimus alone (Table 4). A total of nine adverse events were reported in seven participants with cyclosporin + SZC and eight adverse events in eight participants with cyclosporin alone (Table 4). One participant in the cyclosporin group (Treatment C) discontinued treatment due to ventricular extrasystoles, which occurred 2 days after cyclosporin 100 mg administration (patient did not receive SZC). The ventricular extrasystoles were of mild intensity and considered by the investigator not to be related to study treatment. A total of four (6.5%) participants had outlier ECG values, all during Treatment B (tacrolimus + SZC): one had QT interval corrected for heart rate using Fridericia's formula (QTcF) value >450 ms; three had QTcF increase from baseline of >30 ms. No clinically relevant trends were observed for laboratory values or vital signs.
Table 4:
Number of participants with AEs by system organ class (safety analysis set).
| Participants, n (%) | ||||
|---|---|---|---|---|
| System organ class | Tacrolimus (N = 31) | Tacrolimus + SZC (N = 30) | Cyclosporin (N = 31) | Cyclosporin + SZC (N = 29) |
| Participants with any AE | 7 (22.6) | 13 (43.3) | 8 (25.8) | 7 (24.1) |
| Infections and infestations | 0 | 2 (6.7) | 1 (3.2) | 0 |
| Nervous system disorders | 3 (9.7) | 4 (13.3) | 1 (3.2) | 2 (6.9) |
| Eye disorders | 1 (3.2) | 0 | 1 (3.2) | 0 |
| Cardiac disorders | 0 | 0 | 1 (3.2) | 0 |
| Respiratory, thoracic and mediastinal disorders | 0 | 2 (6.7) | 0 | 0 |
| Gastrointestinal disorders | 2 (6.5) | 3 (10.0) | 2 (6.5) | 2 (6.9) |
| Musculoskeletal and connective tissue disorders | 1 (3.2) | 2 (6.7) | 1 (3.2) | 2 (6.9) |
| Renal and urinary disorders | 0 | 0 | 1 (3.2) | 0 |
| General disorders and administration site conditions | 1 (3.2) | 0 | 0 | 2 (6.9) |
| Injury, poisoning and procedural complications | 1 (3.2) | 1 (3.3) | 0 | 1 (3.4) |
AE, adverse event.
DISCUSSION
In this Phase 1 study, tacrolimus exposure was reduced by approximately one-third when it was co-administered with SZC 15 g in healthy participants. The 90% CI for the exposure parameters (Cmax, AUCinf and AUClast) were below the range of 80%–125%. As t½λz was unchanged when tacrolimus was co-administered with SZC, it appears that the reduction in tacrolimus exposure is attributable to a reduction in its absorption rather than a change in clearance, and it is likely due to SZC transiently increasing gastric pH. SZC (15 g) has approximately half the acid-neutralising capacity as over-the-counter antacids (unpublished observation). The duration of the increase in gastric pH by antacids is reported as approximately 2 h [4]. As SZC has a lower acid-neutralising capacity than antacids, it is unlikely that the effect of SZC on gastric pH would extend beyond 2 h. Therefore, this time window is deemed adequate to avoid any pH-related drug–drug interactions [5]. The reduction in tacrolimus exposure when administered together with SZC has the potential to be a clinically relevant drug interaction. While the reduction in exposure was modest, it is important to maintain regular monitoring of tacrolimus concentration and dose separation with SZC, ≥2 h before or after SZC [1], given the narrow therapeutic index of tacrolimus [7]. It should also be noted that this study was conducted with an immediate-release formulation of tacrolimus and the results may be different for an extended-release formulation. Co-administration of SZC had no meaningful effect on the PK profile of cyclosporin in healthy participants, therefore no dose separation is required.
In this study, the safety and tolerability of tacrolimus and cyclosporin were similar when administered alone and in combination with SZC. The safety data do not indicate any change in the safety and tolerability profile of tacrolimus or cyclosporin when co-administered with SZC in healthy participants compared with administration alone.
The dosing regimen of SZC for the treatment of hyperkalaemia includes a correction phase using a dose of 10 g three times daily (typically, normokalaemia is achieved within 24 to 48 h), followed by a maintenance dose during which the minimal effective dose of SZC should be used [1, 5]. The maximum maintenance dose of SZC is 10 g once daily in Europe [5] and 15 g daily in the USA [1]. To maximise the possibility of identifying a drug–drug interaction in this PK study, the maximum dose of SZC (15 g) was used.
In conclusion, tacrolimus exposure was lower when co-administered with SZC 15 g, which has the potential to reduce its immunosuppressive efficacy. As for other oral drugs with drug–drug interactions with SZC, tacrolimus should be administered ≥2 h before or after SZC. The PK profile of cyclosporin was not affected by SZC co-administration, and dose separation is therefore not warranted.
Supplementary Material
ACKNOWLEDGEMENTS
Medical writing support was provided by Vicky Hinstridge, and editorial support was provided by Sharmin Saleque, all of Core, London, UK, in accordance with Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M22-1460), and funded by AstraZeneca. Data collection and the PK sample analyses was performed by Covance Laboratories Inc., Wisconsin, USA.
Contributor Information
Mats Någård, Clinical Pharmacology and Quantitative Pharmacology, Clinical Pharmacology and Safety Sciences, Research and Development, AstraZeneca, Gaithersburg, MD, USA.
Nurul Choudhury, Department of Patient Safety, Research and Development, AstraZeneca, Gothenburg, Sweden.
Ayman Al-Shurbaji, BioPharmaceuticals Research and Development, AstraZeneca, Gothenburg, Sweden.
Vera Lisovskaja, Department of Biostatistics, Research and Development, AstraZeneca, Gothenburg, Sweden.
Neil Mackillop, Late Clinical Development, Cardiovascular, Renal and Metabolism, AstraZeneca, Cambridge, UK.
FUNDING
This study was funded by AstraZeneca.
AUTHORS’ CONTRIBUTIONS
All authors were involved in the study design, analysis and data checking of information provided in the manuscript. All authors contributed to the data interpretation, critically reviewed the manuscript, approved the final version and accept accountability for the overall work. Ultimate responsibility for opinions, conclusions and data interpretation lies with the authors.
DATA AVAILABILITY STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagroup-dt.pharmacm.com/DT/Home.
CONFLICT OF INTEREST STATEMENT
M.N., N.C., V.L., A.A.-S. and N.M. are employees and hold shares in AstraZeneca. The results presented in this paper have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. AstraZeneca . US Prescribing Information for Lokelma (sodium zirconium cyclosilicate). https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/6de8f71b-d3af-4f76-9600-907c98616be6/6de8f71b-d3af-4f76-9600-907c98616be6_viewable_rendition__v.pdf (27 September 2022, date last accessed). [Google Scholar]
- 2. Smyrli M, Sarafidis PA, Loutradis Cet al. Prevalence and factors associated with hyperkalaemia in stable kidney transplant recipients. Clin Kidney J 2022;15:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaplan B, Wang Z, Abecassis MMet al. Frequency of hyperkalemia in recipients of simultaneous pancreas and kidney transplants with bladder drainage. Transplantation 1996;62:1174–5. [DOI] [PubMed] [Google Scholar]
- 4. Patel D, Bertz R, Ren Set al. A systematic review of gastric acid-reducing agent-mediated drug-drug interactions with orally administered medications. Clin Pharmacokinet 2020;59:447–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. European Medicines Agency . Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/lokelma-epar-product-information_en.pdf (27 September 2022, date last accessed). [Google Scholar]
- 6. Kim KI, Bae J, Kang HJet al. Three-year clinical follow-up results of intracoronary radiation therapy using a rhenium-188-diethylene-triamine-penta-acetic-acid-filled balloon system. Circ J 2004;68:532–7. [DOI] [PubMed] [Google Scholar]
- 7. Astellas Pharma Inc . US Prescribing Information for PROGAF (tacrolimus). https://www.astellas.us/docs/prograf.pdf (27 September 2022, date last accessed). [Google Scholar]
- 8. Johnston A. Equivalence and interchangeability of narrow therapeutic index drugs in organ transplantation. Eur J Hosp Pharm 2013;20:302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corporation NP. US prescribing information for sandimmune (cyclosporine). https://www.novartis.us/sites/www.novartis.us/files/sandimmune.pdf (27 September 2022, date last accessed). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagroup-dt.pharmacm.com/DT/Home.